Abstract
Background: Hypertrophic cardiomyopathy (HCM) is a heterogeneous myocardial disease in which conventional prognostic models, primarily focused on sudden cardiac death, often fail to identify patients at risk of clinically relevant events such as heart failure progression or rehospitalization. Cardiopulmonary exercise testing (CPET) quantifies functional capacity, while stress echocardiography (SE) provides mechanistic insights into exercise-induced hemodynamic changes. Their combined application (CPET–SE) may enhance risk stratification in patients with HCM. Methods: In this retrospective study, 388 patients with obstructive and non-obstructive HCM (mean age 48 ± 15 years, 63.1% male) underwent baseline CPET–SE between 2010 and 2022 and were followed for a median of 7.4 years [IQR 4.3–10.2]. Echocardiographic parameters were assessed at rest and peak exercise, and CPET indices included peak oxygen consumption (pVO2), ventilatory efficiency, and anaerobic threshold. The primary outcome was a composite of heart failure hospitalization or progression to end-stage HCM. Results: Over a median follow-up of 7.4 years, 63 patients (16.2%) experienced an event of the primary outcome. Patients who developed a primary outcome had greater left atrial diameter (45.0 vs. 41.0 mm, p < 0.001) and indexed volume at rest (36.4 vs. 29.0 mL/m2, p < 0.001), with further dilation during stress (p = 0.046); increased LV wall thickness (p = 0.001); higher average E/e′ at rest and during stress (p ≤ 0.004); and higher pulmonary artery systolic pressure at rest (p = 0.027) and during stress (p = 0.044). CPET findings included lower pVO2 (16.0 vs. 19.5 mL/kg/min, p = 0.001), reduced % predicted pVO2 (p = 0.006), earlier anaerobic threshold (p = 0.032), impaired ventilatory efficiency (p = 0.048), and chronotropic incompetence (p < 0.001) in patients who experienced a primary outcome. Multivariable analysis identified dyslipidemia (OR 2.58), higher E/e′ (OR 1.06), and lower pVO2 (OR 0.92) as independently associated with the primary outcome. Conclusions: CPET–SE provided a comprehensive evaluation of patients with HCM, associating aerobic capacity to its hemodynamic determinants. Reduced pVO2 showed the strongest association with adverse outcomes, while exercise-induced diastolic dysfunction and elevated pulmonary pressures identified a high-risk phenotype. Incorporating CPET–SE into longitudinal management of patients with HCM may enable earlier detection of physiological decompensation and guide personalized therapeutic strategies.
1. Introduction
Hypertrophic cardiomyopathy (HCM) is a primary myocardial disease characterized by unexplained left ventricular hypertrophy in the absence of abnormal loading conditions such as hypertension or valvular disease [1]. About 30–40% of cases have a clear genetic basis, most commonly involving autosomal dominant mutations in sarcomeric protein genes [2]. The clinical expression of HCM is highly heterogeneous, reflecting the interplay of genetic, structural, and functional factors.
Traditionally, the risk of sudden cardiac death (SCD) has been the primary concern in prognostic evaluation, supported by established risk algorithms and criteria for implantable cardioverter-defibrillator (ICD) implantation. However, mortality-based endpoints do not fully capture the disease burden as a significant proportion of patients develop progressive heart failure despite preserved ejection fraction, often culminating in advanced therapies such as heart transplantation. In addition, atrial fibrillation and other arrhythmias are frequent and may anticipate or accelerate clinical decline [3,4]. These limitations highlight the need for tools capable of predicting not only SCD but also non-fatal yet clinically meaningful outcomes, including worsening heart failure, rehospitalization, and transition to end-stage disease.
Cardiopulmonary exercise testing (CPET) provides an integrated assessment of cardiovascular, pulmonary, and muscular responses during effort, offering an objective measure of functional capacity [5,6]. Several CPET-derived parameters have been validated as markers of disease severity and progression in HCM [7]. In particular, reduced peak VO2—defined as <80% of predicted for age and sex or <14 mL/kg/min—has consistently emerged as a robust predictor of adverse outcomes, including clinical deterioration, cardiovascular death, and need for transplantation [7,8,9,10,11,12]. Despite this evidence, CPET is still underutilized in the longitudinal management of HCM.
Stress echocardiography (SE) complements CPET by identifying exercise-induced mechanisms of limitation, such as left ventricular outflow tract obstruction (LVOTO), systolic anterior motion (SAM) of the mitral valve, impaired diastolic reserve (E/e′), exercise-induced pulmonary hypertension, ischemia, and worsening mitral regurgitation [13,14,15,16,17]. For example, an exercise-induced LVOT gradient ≥50 mmHg is independently associated with symptom progression and heart failure hospitalization.
When performed in combination, CPET–SE integrates the prognostically validated metrics of CPET with the mechanistic insights of SE, enabling simultaneous evaluation of functional capacity and its underlying determinants. This comprehensive approach has the potential to refine risk stratification, predict progression to heart failure, and guide long-term management in HCM. Based on these premises, we sought to evaluate the prognostic utility of combined CPET–SE in a single-center cohort of patients with HCM, focusing on clinically meaningful endpoints such as rehospitalization and progression to end-stage disease.
2. Materials and Methods
2.1. Population and Clinical Assessment
We retrospectively evaluated patients with hypertrophic cardiomyopathy (HCM) who were referred to and followed at our dedicated HCM outpatient clinic between 2010 and 2023. Inclusion criteria were age ≥ 18 years, a diagnosis of HCM established according to the most recent guidelines [14], left ventricular ejection fraction (LVEF) > 50%, and the ability to perform cardiopulmonary exercise testing (CPET). Patients with a history of coronary artery disease (CAD) or with alternative diagnoses such as cardiac amyloidosis or Fabry disease (i.e., phenocopies) were excluded. The study complied with the Declaration of Helsinki, and written informed consent was obtained from all participants.
The primary endpoint was the first occurrence of a composite outcome including:
- (a)
- Heart failure (HF) hospitalization, defined as an unplanned, overnight hospital admission with a primary diagnosis of HF requiring intravenous loop diuretics, vasodilators, or inotropes;
- (b)
- Progression to end-stage HCM, operationally defined as transition to the advanced phase characterized by new-onset left ventricular systolic dysfunction with LVEF < 50% persisting for ≥3 months in the absence of alternative causes (e.g., acute myocardial infarction, significant valvular disease), and/or fulfillment of advanced-therapy criteria including listing for heart transplantation or implantation of durable left ventricular assist device (LVAD) due to progressive pump failure. This definition is consistent with contemporary descriptions of the end-stage phenotype and prior cohort studies in HCM [14].
All patients underwent a comprehensive clinical and cardiological evaluation, including detailed medical history, assessment of symptoms and functional status according to the New York Heart Association (NYHA) classification, a standard 12-lead electrocardiogram (ECG), 24 h ambulatory ECG (Holter) monitoring, transthoracic two-dimensional and Doppler echocardiography, cardiac magnetic resonance imaging (CMR), and combined transthoracic echocardiography with cardiopulmonary exercise testing (CPET-SE). Results of genetic testing for HCM-related pathogenic variants, when available, were also collected.
2.2. Cardiopulmonary Exercise Test and Stress Echocardiography
After baseline echocardiography, patients underwent a symptom-limited CPET-SE performed on an upright cycle ergometer with a ramp protocol of incremental load at 10 W/min. A respiratory exchange ratio (RER) > 1.05 was required to define maximal effort; patients who did not achieve this threshold were excluded from the analysis. Exhaled gases were continuously sampled using a mouthpiece-mounted sensor and analyzed to measure oxygen consumption (VO2), carbon dioxide production (VCO2), and minute ventilation (VE). The following exercise parameters were recorded, peak oxygen consumption (pVO2), ventilatory efficiency (VE/VCO2 slope), and oxygen pulse (VO2/heart rate), according to standard methodology [18,19].
Echocardiographic parameters were acquired at three predefined time points: at rest, at peak exercise, and after one minute of recovery. Two-dimensional imaging included standard acquisitions at rest and apical four-, two-, and three-chamber views during exercise to evaluate global and regional left ventricular systolic function. Doppler measurements included transvalvular aortic velocity, left ventricular outflow tract (LVOT) velocity–time integral for stroke volume calculation, peak LVOT velocity for estimation of LVOT gradient, mitral inflow and tissue Doppler imaging of the mitral annulus (e′ velocity) to assess diastolic function, and tricuspid regurgitation velocity. M-mode was used to measure tricuspid annular plane systolic excursion (TAPSE) as an index of right ventricular systolic function [20,21]. All imaging and CPET data were digitally recorded and subsequently analyzed offline by experienced operators.
2.3. Statistical Analysis
The distribution of continuous variables was assessed using the Shapiro–Wilk test. Continuous data are presented as mean ± standard deviation (SD) or as median with interquartile range [IQR], depending on distribution. Categorical variables are expressed as absolute frequencies and percentages. Comparisons between groups were performed using the unpaired Student’s t-test or Mann–Whitney U test for continuous variables, and the Chi-squared or Fisher’s exact test for categorical variables. Univariate logistic regression was applied to identify variables associated with the primary outcome. Variables with a statistically significant association (p < 0.05) were subsequently entered into a multivariable logistic regression model to determine independent predictors. Results are reported as odds ratios (OR) with 95% confidence intervals (CI). A two-sided p-value < 0.05 was considered statistically significant. All statistical analyses were performed using R software (version 4.3.1).
3. Results
3.1. Clinical Characteristics
Baseline characteristics are summarized in Table 1. A total of 388 patients were included (mean age of 48 ± 15 years 63.1% male). Most individuals were mildly symptomatic at baseline, with 92.9% in NYHA class I or II. Cardiovascular comorbidities included hypertension in 39.4% of patients, dyslipidemia in 30.2%, and atrial fibrillation in 13.1%. Patients who experienced adverse events during follow-up were significantly older (51 ± 13 vs. 47 ± 15 years, p = 0.047) and had a higher prevalence of hypertension (54.0% vs. 36.6%, p = 0.015), dyslipidemia (50.8% vs. 26.2%, p < 0.001), and atrial fibrillation (31.7% vs. 9.5%, p < 0.001) compared to those without events. They also reported dyspnea more frequently (66.7% vs. 46.2%, p = 0.004), and were less often in NYHA class I (20.6% vs. 51.2%, p < 0.001), while more commonly in NYHA class II (65.1% vs. 43.1%, p = 0.002). The ESC risk score for sudden cardiac death was also significantly higher in the adverse event group (4.9 ± 2.6 vs. 3.5 ± 2.2, p < 0.001), reflecting a greater baseline risk burden. Regarding pharmacological treatment, patients in the adverse event group were more frequently treated with beta-blockers (73.0% vs. 58.5%, p = 0.04), aspirin (30.2% vs. 12.9%, p = 0.001), ACE inhibitors (38.1% vs. 13.5%, p < 0.001), anticoagulants (30.2% vs. 9.3%, p < 0.001), amiodarone (20.6% vs. 6.2%, p < 0.001), and mineralocorticoid receptor antagonists (12.7% vs. 1.8%, p < 0.001). Diuretics were also more commonly used in the event group (43.5% vs. 14.9%, p < 0.001).

Table 1.
Clinical characteristics.
3.2. Echocardiographic Findings
Structural and functional markers of disease severity were more pronounced in the adverse event group (Table 2). They showed greater maximal LV wall thickness (22.0 [19.0–25.0] mm vs. 20.0 [17.0–23.0] mm, p = 0.001), larger left atrial diameter (45.0 [41.0–52.0] mm vs. 41.0 [37.0–45.0] mm, p < 0.001), and higher resting LA volume index (36.4 [29.8–49.0] vs. 29.0 [23.0–37.0] mL/m2, p < 0.001). LA dilation was further accentuated during stress (43.0 [0.0–66.0] vs. 26.0 [0.0–44.0] mL/m2, p = 0.046). Diastolic function was more impaired in patients with events, with higher average E/e′ ratios both at rest (11.4 [9.0–17.3] vs. 9.8 [7.7–12.6], p = 0.004) and during stress (11.2 [9.6–15.5] vs. 9.9 [8.1–12.3], p = 0.001). Pulmonary artery systolic pressure was also higher at rest (25.0 [15.0–32.0] vs. 20.0 [11.5–26.0] mmHg, p = 0.027) and during stress (37.0 [25.7–54.0] vs. 34.0 [25.0–45.0] mmHg, p = 0.044). No differences were observed in LV volumes, LVEF, or resting/provoked LVOT gradients.

Table 2.
Echocardiographic characteristics between groups.
3.3. CPET Results
CPET findings are summarized in Table 3. Patients with events had lower peak VO2 (16.0 [14.2–20.0] vs. 19.5 [15.1–23.5] mL/kg/min, p = 0.001) [Figure 1] and a reduced percentage of predicted peak VO2 (62.1% vs. 67.1%, p = 0.006). Cardiovascular efficiency was impaired, as reflected by a lower VO2 per Watt (8.2 [6.6–9.0] vs. 9.0 [8.0–10.4] mL/min/W, p = 0.026), while ventilatory efficiency was reduced with a higher VE/VCO2 slope (28.0 [25.0–33.0] vs. 27.0 [24.0–30.0], p = 0.048). Markers of submaximal performance were also altered: anaerobic threshold occurred earlier (7.0 [5.0–8.0] vs. 8.0 [6.0–10.0] min, p = 0.032), with lower oxygen uptake at AT (13.0 [11.1–15.0] vs. 16.0 [13.0–20.0] mL/kg/min, p < 0.001) and a reduced percentage of predicted AT (45.6% vs. 55.6%, p = 0.003).

Table 3.
CPET findings.
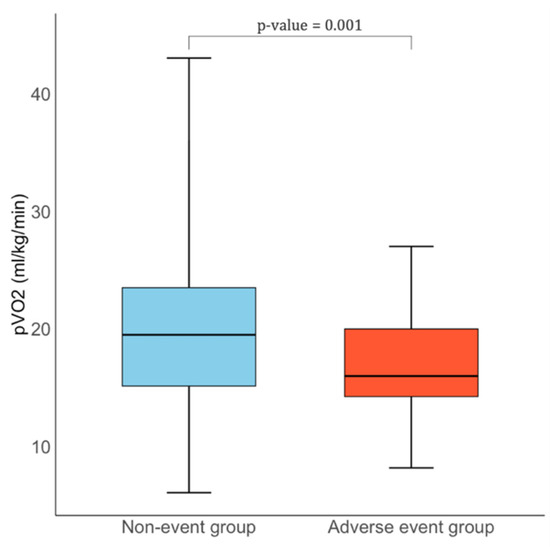
Figure 1.
Comparison of pVO2 levels between HCM patients with and without adverse events.
3.4. Variables Associated with Clinical Events
Over a median follow-up of 7.4 [4.3–10.2] years, 63 patients (16.2%) experienced at least one clinically relevant adverse event, defined as heart failure hospitalization or progression to end-stage HCM. At univariate logistic regression (Table 4), age (OR 1.02, 95% CI 1.00–1.04, p = 0.048), dyslipidemia (OR 2.91, 95% CI 1.68–5.08, p < 0.0001), higher resting E/e′ (OR 1.09, 95% CI 1.04–1.14, p = 0.011), and lower peak VO2 (OR 0.91, 95% CI 0.87–0.96, p = 0.0006) were significantly associated with events. In multivariable analysis, dyslipidemia (OR 2.58, 95% CI 1.68–5.08, p = 0.006), higher resting E/e′ (OR 1.06, 95% CI 1.00–1.12, p = 0.042), and lower peak VO2 (OR 0.92, 95% CI 0.87–0.98, p = 0.016) remained independently associated with the primary outcome.

Table 4.
Univariate and multivariate logistic regression analysis for predictors of adverse events in patients with HCM.
4. Discussion
This study demonstrates that combined assessment with cardiopulmonary exercise testing and stress echocardiography (CPET–SE) provides a comprehensive and synergistic evaluation of patients with HCM, capturing both the quantitative impairment in aerobic capacity and the pathophysiological mechanisms underlying exercise limitation.
From the echocardiographic standpoint, patients who experienced adverse events exhibited a more adverse structural and functional profile, with greater left atrial remodeling, more severe left ventricular hypertrophy, and evidence of impaired diastolic reserve. The rise in E/e′ during exercise, together with higher pulmonary artery systolic pressures, points to a reduced ability to accommodate exercise-related increases in preload and to impaired ventriculo–atrial coupling. These mechanisms may contribute to symptom development and long-term clinical deterioration. Importantly, these differences were observed despite similar LVEF and LVOT gradients at rest and during provocation, indicating that, in many cases, functional limitation was driven more by diastolic and atrial factors than by outflow obstruction [22].
CPET data aligned closely with these imaging findings. The adverse-event group showed markedly reduced peak VO2, earlier anaerobic threshold, impaired ventilatory efficiency, and signs of chronotropic incompetence. The parallel between diastolic dysfunction on stress echocardiography and reduced aerobic capacity suggests that elevated filling pressures during exertion may be a major determinant of exercise intolerance in this population. CPET offers a comprehensive and dynamic assessment of cardiovascular reserve. In contrast to conventional imaging modalities, which are largely static and load-dependent, CPET-derived parameters such as peak VO2 can unmask early physiological deterioration not evident at rest [23]. This is especially relevant in HCM, where patients often maintain preserved ejection fraction and minimal symptoms until advanced stages. Impaired peak VO2 reflects a cumulative deficit in diastolic filling, myocardial energetics, and microvascular perfusion that drive exercise intolerance [24].
Several large cohort studies have consistently shown that patients with peak VO2 values < 14 mL/kg/min or <50% of age- and sex-predicted values face substantially increased risks of progression to advanced heart failure or the need for heart transplantation [25]. For instance, Coats et al. demonstrated in nearly 1900 patients that a peak VO2 ≤ 15.3 mL/kg/min was associated with a 10-year event rate (death or transplant) exceeding 30% [26]. Similarly, Masri et al. reported that a lower percentage of predicted peak VO2 was independently associated with higher incidence of all-cause mortality, ICD shocks, and heart failure hospitalizations [8]. Our results corroborate and extend this evidence by showing that reduced peak VO2 remains strongly associated with clinically meaningful outcomes even when integrated within a multiparametric CPET–SE framework.
We also explored additional CPET parameters, including VE/VCO2 slope, oxygen pulse, and anaerobic threshold. While these variables were associated with adverse outcomes in univariate analyses, they did not remain independently associated in multivariable analysis. This is consistent with prior research indicating that, although a steep VE/VCO2 slope and early anaerobic threshold reflect ventilatory inefficiency and impaired haemodynamics, their prognostic capacity in HCM is generally inferior to that of peak VO2. The primacy of peak VO2 likely reflects its integrative nature, incorporating contributions from diastolic filling, myocardial energetics, microvascular perfusion, chronotropic response, and peripheral oxygen utilization [27].
On the echocardiographic side, our multivariable analysis confirmed that higher E/e′ at rest is independently associated with adverse events. The independent prognostic contribution of stress-derived diastolic indices reinforces the role of dynamic, rather than solely resting, measurements in risk stratification [28].
Another clinically relevant finding is the independent association between dyslipidemia and the composite endpoint. Although at first glance unexpected in a sarcomeric disease, this observation is biologically plausible and supported by emerging evidence. Observational studies have suggested that dyslipidemia may identify patients with blunted improvement in VO2 peak following septal reduction therapy, potentially reflecting adverse interactions between metabolic milieu, microvascular function, and myocardial energetics [29]. More broadly, metabolic dysregulation has been increasingly recognized as a modifier of HCM phenotype and trajectory [30]. Dyslipidemia should therefore be considered a modifiable risk factor and a pragmatic target for optimization while the field awaits high-quality interventional data.
From a clinical perspective, the combined CPET–SE approach enables simultaneous identification of functional limitation, hemodynamic abnormalities, and actionable therapeutic targets. In patients with reduced peak VO2 and stress-induced elevations in E/e′ and pulmonary pressures, closer surveillance and earlier, mechanism-oriented interventions may be warranted—ranging from aggressive risk-factor control (including lipid management), to pharmacologic strategies aimed at diastolic function and heart-rate control, to timely consideration of septal reduction in appropriately selected obstructive phenotypes [31]. Beyond individual parameters, the strength of CPET–SE lies in its integrated read-out: quantifying “how much” capacity is lost (via CPET) while clarifying “why” (via SE), thus refining risk stratification compared with either modality alone.
5. Strengths and Limitations
Key strengths of this study include the large, well-characterized cohort, the long follow-up, and the simultaneous application of multimodal diagnostics. Limitations include the observational, single-center design—potentially limiting generalizability—and the predominantly Caucasian population. A Cox proportional hazards model could not be applied because the exact timing of events was not consistently available, precluding accurate time-to-event estimation and censoring. Consequently, logistic regression was used to identify independent predictors of event occurrence: this approach captures overall associations but does not provide temporal risk estimates. Residual confounding cannot be excluded, and external validation in more diverse cohorts is warranted. Moreover, as an observational study, it cannot determine whether CPET–SE–guided management directly translates into therapeutic changes or improved outcomes, an issue that future prospective studies should address.
6. Conclusions and Clinical Implications
Our study demonstrates that CPET–SE offers a powerful and integrated approach to prognostic assessment in HCM. By linking objective measures of functional capacity with the hemodynamic mechanisms underlying exercise limitation, CPET–SE refines risk stratification beyond either modality alone. Reduced peak VO2 emerged as the strongest predictor of adverse outcomes, while abnormal stress-derived diastolic indices such as elevated E/e′ identified a distinct high-risk phenotype. These findings suggest that impaired exercise capacity should prompt careful evaluation of diastolic reserve, and that stress abnormalities may justify earlier, mechanism-oriented interventions even in patients with mild or borderline symptoms. Early recognition of this combined functional–hemodynamic impairment may support closer surveillance, timely pharmacologic or interventional treatment, and optimal referral for advanced heart failure therapies. Prospective studies are warranted to evaluate whether CPET–SE–guided management can improve clinical outcomes and help personalize long-term care in HCM.
Author Contributions
Conceptualization, G.H., F.R., P.C. and D.G.; methodology, R.M.; formal analysis, R.M. and G.H.; investigation, G.G.; data curation, G.G. and A.F.; writing—original draft preparation, P.C. and R.M.; writing—review and editing, G.F.R., G.H., F.A., D.G. and F.R.; supervision, F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to its retrospective observational design. All patients provided written informed consent at the time of undergoing cardiopulmonary exercise testing and echocardiographic examination, which were performed as part of routine clinical practice. The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
Raffaella Mistrulli has been supported by a research grant provided by the DigiCardiopaTh PhD program.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HCM | Hypertrophic Cardiomyopathy |
| CPET | Cardiopulmonary Exercise Testing |
| SE | Stress Echocardiography |
| VO2 | Oxygen Consumption |
| VE/VCO2 | Ventilatory Equivalent for Carbon Dioxide |
| AT | Anaerobic Threshold |
| HR | Heart Rate |
| BP | Blood Pressure |
| PETCO2 | Partial Pressure of End-Tidal Carbon Dioxide |
| LVOT | Left Ventricular Outflow Tract |
| LVEF | Left Ventricular Ejection Fraction |
| LA | Left Atrium |
| LV | Left Ventricle |
| LVEDV | Left Ventricular End-Diastolic Volume |
| LVESV | Left Ventricular End-Systolic Volume |
| NYHA | New York Heart Association |
| ESC | European Society of Cardiology |
| SCD | Sudden Cardiac Death |
| ICD | Implantable Cardioverter Defibrillator |
| CAD | Coronary Artery Disease |
| CMR | Cardiac Magnetic Resonance |
| AF | Atrial Fibrillation |
| COPD | Chronic Obstructive Pulmonary Disease |
| PAD | Peripheral Artery Disease |
| BSA | Body Surface Area |
| ACE | Angiotensin-Converting Enzyme |
| ARNI | Angiotensin Receptor–Neprilysin Inhibitor |
| MRA | Mineralocorticoid Receptor Antagonist |
| SGLT2 | Sodium-Glucose Cotransporter 2 |
| SAM | Systolic Anterior Motion (of the mitral valve) |
| PASP | Pulmonary Artery Systolic Pressure |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| 4CH | Four-Chamber View |
| MR | Mitral Regurgitation |
References
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef]
- Hespe, S.; Waddell, A.; Asatryan, B.; Owens, E.; Thaxton, C.; Adduru, M.-L.; Anderson, K.; Brown, E.E.; Hoffman-Andrews, L.; Jordan, E.; et al. Genes Associated with Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2025, 85, 727–740. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Maron, M.S. How Hypertrophic Cardiomyopathy Became a Contemporary Treatable Genetic Disease with Low Mortality: Shaped by 50 Years of Clinical Research and Practice. JAMA Cardiol. 2016, 1, 98. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Udelson, J.E.; Maron, M.S. Clinical Spectrum and Management of Heart Failure in Hypertrophic Cardiomyopathy. JACC Heart Fail. 2018, 6, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Rowin, E.J.; Maron, B.J.; Olivotto, I.; Maron, M.S. Role of Exercise Testing in Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2017, 10, 1374–1386. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Sietsema, K.E. Cardiopulmonary Exercise Testing in the Clinical Evaluation of Patients With Heart and Lung Disease. Circulation 2011, 123, 668–680. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Haddad, F.; Knowles, J.W.; Caleshu, C.; Pavlovic, A.; Homburger, J.; Shmargad, Y.; Sinagra, G.; Magavern, E.; Wong, M.; et al. Cardiopulmonary Responses and Prognosis in Hypertrophic Cardiomyopathy. JACC Heart Fail. 2015, 3, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Pierson, L.M.; Smedira, N.G.; Agarwal, S.; Lytle, B.W.; Naji, P.; Thamilarasan, M.; Lever, H.M.; Cho, L.S.; Desai, M.Y. Predictors of long-term outcomes in patients with hypertrophic cardiomyopathy undergoing cardiopulmonary stress testing and echocardiography. Am. Heart J. 2015, 169, 684–692.e1. [Google Scholar] [CrossRef]
- Rodrigues, T.; Raposo, S.C.; Brito, D.; Lopes, L.R. Prognostic relevance of exercise testing in hypertrophic cardiomyopathy. A systematic review. Int. J. Cardiol. 2021, 339, 83–92. [Google Scholar] [CrossRef]
- Magrì, D.; Santolamazza, C. Cardiopulmonary Exercise Test in Hypertrophic Cardiomyopathy. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. S1), S102–S109. [Google Scholar] [CrossRef]
- Bayonas-Ruiz, A.; Muñoz-Franco, F.M.; Ferrer, V.; Pérez-Caballero, C.; Sabater-Molina, M.; Tomé-Esteban, M.T.; Bonacasa, B. Cardiopulmonary Exercise Test in Patients with Hypertrophic Cardiomyopathy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2312. [Google Scholar] [CrossRef]
- Butzner, M.; Kinyik-Merena, C.; Aguiar, M.; Davison, N.; Shreay, S.; Masri, A. The prognostic value of peak oxygen uptake in obstructive hypertrophic cardiomyopathy: A literature review to inform economic model development. J. Med. Econ. 2024, 27, 817–825. [Google Scholar] [CrossRef]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; A Blom, N.; A de Boer, R.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Rowin, E.J.; Olivotto, I.; Casey, S.A.; Arretini, A.; Tomberli, B.; Garberich, R.F.; Link, M.S.; Chan, R.H.; Lesser, J.R.; et al. Contemporary Natural History and Management of Nonobstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Re, F.; Halasz, G.; Moroni, F.; Beltrami, M.; Baratta, P.; Avella, A.; Zachara, E.; Olivotto, I. Exercise-Induced Pulmonary Hypertension in Hypertrophic Cardiomyopathy: A Combined Cardiopulmonary Exercise Test—Echocardiographic Study. Int. J. Cardiovasc. Imaging 2022, 38, 2345–2352. [Google Scholar] [CrossRef]
- Galluzzo, A.; Fiorelli, F.; Rossi, V.A.; Monzo, L.; Montrasio, G.; Camilli, M.; Halasz, G.; Uccello, G.; Mollace, R.; Beltrami, M. Multimodality Imaging in Sarcomeric Hypertrophic Cardiomyopathy: Get It Right…on Time. Life 2023, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. 2016 focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur. Heart J. 2018, 39, 1144–1161. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, P.; Dumitrescu, D. How to perform and report a cardiopulmonary exercise test in patients with chronic heart failure. Int. J. Cardiol. 2019, 288, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270, Erratum in Eur. Heart J. Cardiovasc. Imaging 2016, 17, 412. https://doi.org/10.1093/ehjci/jew041. Erratum in Eur. Heart J. Cardiovasc. Imaging 2016, 17, 969. https://doi.org/10.1093/ehjci/jew124. [Google Scholar] [CrossRef] [PubMed]
- Re, F.; Zachara, E.; Avella, A.; Baratta, P.; di Mauro, M.; Uguccioni, M.; Olivotto, I. Dissecting functional impairment in hypertrophic cardiomyopathy by dynamic assessment of diastolic reserve and outflow obstruction: A combined cardiopulmonary-echocardiographic study. Int. J. Cardiol. 2017, 227, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Halasz, G.; Dei, L.L.; Moroni, F.; Ayers, M.P.; Ciacci, P.; Giacalone, G.; Mistrulli, R.; Redivo, M.; Orellana, S.; Gabrielli, D.; et al. The Impact of Disopyramide on Exercise Capacity Among Patients with Obstructive Hypertrophic Cardiomyopathy: Beyond LVOT Gradient. Eur. J. Prev. Cardiol. 2024, 32, 1122–1124. [Google Scholar] [CrossRef]
- Lumb, R.H.; Benson, B.J.; Clements, J.A. Transfer of phospholipids by a protein fraction obtained from canine pulmonary lavage. Biochim. Biophys. Acta. 1988, 963, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Kostner, K.M. Statin therapy for hypertrophic cardiomyopathy: Too good to be true? Eur. J. Clin. Invest. 2010, 40, 965–967. [Google Scholar] [CrossRef]
- Coats, C.J.; Rantell, K.; Bartnik, A.; Patel, A.; Mist, B.; McKenna, W.J.; Elliott, P.M. Cardiopulmonary Exercise Testing and Prognosis in Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2015, 8, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, M.; Romani, S.; Magrì, D.; Merlo, M.; Cittar, M.; Masè, M.; Muratori, M.; Gallo, G.; Sclafani, M.; Carriere, C.; et al. Exercise oxygen pulse kinetics in patients with hypertrophic cardiomyopathy. Heart 2022, 108, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Afana, A.S.; Adam, R.D.; Militaru, S.; Onciul, S.; Andrei, O.; Chirita Emandi, A.; Puiu, M.; Militaru, C.; Jurcut, R. Clinical Characteristics and Prognosis of Patients with End-Stage Hypertrophic Cardiomyopathy from a Tertiary Center Cohort: Systolic Dysfunction and Advanced Diastolic Dysfunction. Diagnostics 2025, 15, 1134. [Google Scholar] [CrossRef]
- Gaar-Humphreys, K.R.; van den Brink, A.; Wekking, M.; Asselbergs, F.W.; van Steenbeek, F.G.; Harakalova, M.; Pei, J. Targeting lipid metabolism as a new therapeutic strategy for inherited cardiomyopathies. Front. Cardiovasc. Med. 2023, 10, 1114459. [Google Scholar] [CrossRef]
- Schoonvelde, S.A.C.; Nollet, E.E.; Zwetsloot, P.P.; Knackstedt, C.; Germans, T.; Hirsch, A.; Schinkel, A.F.L.; van Slegtenhorst, M.A.; Verhagen, J.M.A.; de Boer, R.A.; et al. Genotype-negative hypertrophic cardiomyopathy: Exploring the role of cardiovascular risk factors in disease expression. Int. J. Cardiol. 2025, 437, 133444. [Google Scholar] [CrossRef] [PubMed]
- Landsteiner, I.; Masri, A.; Saberi, S.; Maron, M.S.; McGinnis, S.L.; Griskowitz, C.; Newlands, C.E.; Barriales-Villa, R.; Owens, A.T.; Lewis, G.D. Cardiopulmonary Exercise Testing for Characterization of Hypertrophic Cardiomyopathy: A Meta-Analysis. J. Am. Heart Assoc. 2025, 14, e039551. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).