Integrating Cardiopulmonary Exercise Testing and Stress Echocardiography to Predict Clinical Outcomes in Hypertrophic Cardiomyopathy
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Clinical Assessment
- (a)
- Heart failure (HF) hospitalization, defined as an unplanned, overnight hospital admission with a primary diagnosis of HF requiring intravenous loop diuretics, vasodilators, or inotropes;
- (b)
- Progression to end-stage HCM, operationally defined as transition to the advanced phase characterized by new-onset left ventricular systolic dysfunction with LVEF < 50% persisting for ≥3 months in the absence of alternative causes (e.g., acute myocardial infarction, significant valvular disease), and/or fulfillment of advanced-therapy criteria including listing for heart transplantation or implantation of durable left ventricular assist device (LVAD) due to progressive pump failure. This definition is consistent with contemporary descriptions of the end-stage phenotype and prior cohort studies in HCM [14].
2.2. Cardiopulmonary Exercise Test and Stress Echocardiography
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Echocardiographic Findings
3.3. CPET Results
3.4. Variables Associated with Clinical Events
4. Discussion
5. Strengths and Limitations
6. Conclusions and Clinical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCM | Hypertrophic Cardiomyopathy |
| CPET | Cardiopulmonary Exercise Testing |
| SE | Stress Echocardiography |
| VO2 | Oxygen Consumption |
| VE/VCO2 | Ventilatory Equivalent for Carbon Dioxide |
| AT | Anaerobic Threshold |
| HR | Heart Rate |
| BP | Blood Pressure |
| PETCO2 | Partial Pressure of End-Tidal Carbon Dioxide |
| LVOT | Left Ventricular Outflow Tract |
| LVEF | Left Ventricular Ejection Fraction |
| LA | Left Atrium |
| LV | Left Ventricle |
| LVEDV | Left Ventricular End-Diastolic Volume |
| LVESV | Left Ventricular End-Systolic Volume |
| NYHA | New York Heart Association |
| ESC | European Society of Cardiology |
| SCD | Sudden Cardiac Death |
| ICD | Implantable Cardioverter Defibrillator |
| CAD | Coronary Artery Disease |
| CMR | Cardiac Magnetic Resonance |
| AF | Atrial Fibrillation |
| COPD | Chronic Obstructive Pulmonary Disease |
| PAD | Peripheral Artery Disease |
| BSA | Body Surface Area |
| ACE | Angiotensin-Converting Enzyme |
| ARNI | Angiotensin Receptor–Neprilysin Inhibitor |
| MRA | Mineralocorticoid Receptor Antagonist |
| SGLT2 | Sodium-Glucose Cotransporter 2 |
| SAM | Systolic Anterior Motion (of the mitral valve) |
| PASP | Pulmonary Artery Systolic Pressure |
| TAPSE | Tricuspid Annular Plane Systolic Excursion |
| 4CH | Four-Chamber View |
| MR | Mitral Regurgitation |
References
- Maron, B.J.; Desai, M.Y.; Nishimura, R.A.; Spirito, P.; Rakowski, H.; Towbin, J.A.; Rowin, E.J.; Maron, M.S.; Sherrid, M.V. Diagnosis and Evaluation of Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2022, 79, 372–389. [Google Scholar] [CrossRef]
- Hespe, S.; Waddell, A.; Asatryan, B.; Owens, E.; Thaxton, C.; Adduru, M.-L.; Anderson, K.; Brown, E.E.; Hoffman-Andrews, L.; Jordan, E.; et al. Genes Associated with Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2025, 85, 727–740. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Casey, S.A.; Maron, M.S. How Hypertrophic Cardiomyopathy Became a Contemporary Treatable Genetic Disease with Low Mortality: Shaped by 50 Years of Clinical Research and Practice. JAMA Cardiol. 2016, 1, 98. [Google Scholar] [CrossRef]
- Maron, B.J.; Rowin, E.J.; Udelson, J.E.; Maron, M.S. Clinical Spectrum and Management of Heart Failure in Hypertrophic Cardiomyopathy. JACC Heart Fail. 2018, 6, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Rowin, E.J.; Maron, B.J.; Olivotto, I.; Maron, M.S. Role of Exercise Testing in Hypertrophic Cardiomyopathy. JACC Cardiovasc. Imaging 2017, 10, 1374–1386. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Sietsema, K.E. Cardiopulmonary Exercise Testing in the Clinical Evaluation of Patients With Heart and Lung Disease. Circulation 2011, 123, 668–680. [Google Scholar] [CrossRef]
- Finocchiaro, G.; Haddad, F.; Knowles, J.W.; Caleshu, C.; Pavlovic, A.; Homburger, J.; Shmargad, Y.; Sinagra, G.; Magavern, E.; Wong, M.; et al. Cardiopulmonary Responses and Prognosis in Hypertrophic Cardiomyopathy. JACC Heart Fail. 2015, 3, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Masri, A.; Pierson, L.M.; Smedira, N.G.; Agarwal, S.; Lytle, B.W.; Naji, P.; Thamilarasan, M.; Lever, H.M.; Cho, L.S.; Desai, M.Y. Predictors of long-term outcomes in patients with hypertrophic cardiomyopathy undergoing cardiopulmonary stress testing and echocardiography. Am. Heart J. 2015, 169, 684–692.e1. [Google Scholar] [CrossRef]
- Rodrigues, T.; Raposo, S.C.; Brito, D.; Lopes, L.R. Prognostic relevance of exercise testing in hypertrophic cardiomyopathy. A systematic review. Int. J. Cardiol. 2021, 339, 83–92. [Google Scholar] [CrossRef]
- Magrì, D.; Santolamazza, C. Cardiopulmonary Exercise Test in Hypertrophic Cardiomyopathy. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. S1), S102–S109. [Google Scholar] [CrossRef]
- Bayonas-Ruiz, A.; Muñoz-Franco, F.M.; Ferrer, V.; Pérez-Caballero, C.; Sabater-Molina, M.; Tomé-Esteban, M.T.; Bonacasa, B. Cardiopulmonary Exercise Test in Patients with Hypertrophic Cardiomyopathy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2312. [Google Scholar] [CrossRef]
- Butzner, M.; Kinyik-Merena, C.; Aguiar, M.; Davison, N.; Shreay, S.; Masri, A. The prognostic value of peak oxygen uptake in obstructive hypertrophic cardiomyopathy: A literature review to inform economic model development. J. Med. Econ. 2024, 27, 817–825. [Google Scholar] [CrossRef]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef]
- Arbelo, E.; Protonotarios, A.; Gimeno, J.R.; Arbustini, E.; Barriales-Villa, R.; Basso, C.; Bezzina, C.R.; Biagini, E.; A Blom, N.; A de Boer, R.; et al. 2023 ESC Guidelines for the management of cardiomyopathies. Eur. Heart J. 2023, 44, 3503–3626. [Google Scholar] [CrossRef] [PubMed]
- Maron, M.S.; Rowin, E.J.; Olivotto, I.; Casey, S.A.; Arretini, A.; Tomberli, B.; Garberich, R.F.; Link, M.S.; Chan, R.H.; Lesser, J.R.; et al. Contemporary Natural History and Management of Nonobstructive Hypertrophic Cardiomyopathy. J. Am. Coll. Cardiol. 2016, 67, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Re, F.; Halasz, G.; Moroni, F.; Beltrami, M.; Baratta, P.; Avella, A.; Zachara, E.; Olivotto, I. Exercise-Induced Pulmonary Hypertension in Hypertrophic Cardiomyopathy: A Combined Cardiopulmonary Exercise Test—Echocardiographic Study. Int. J. Cardiovasc. Imaging 2022, 38, 2345–2352. [Google Scholar] [CrossRef]
- Galluzzo, A.; Fiorelli, F.; Rossi, V.A.; Monzo, L.; Montrasio, G.; Camilli, M.; Halasz, G.; Uccello, G.; Mollace, R.; Beltrami, M. Multimodality Imaging in Sarcomeric Hypertrophic Cardiomyopathy: Get It Right…on Time. Life 2023, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. 2016 focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur. Heart J. 2018, 39, 1144–1161. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, P.; Dumitrescu, D. How to perform and report a cardiopulmonary exercise test in patients with chronic heart failure. Int. J. Cardiol. 2019, 288, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270, Erratum in Eur. Heart J. Cardiovasc. Imaging 2016, 17, 412. https://doi.org/10.1093/ehjci/jew041. Erratum in Eur. Heart J. Cardiovasc. Imaging 2016, 17, 969. https://doi.org/10.1093/ehjci/jew124. [Google Scholar] [CrossRef] [PubMed]
- Re, F.; Zachara, E.; Avella, A.; Baratta, P.; di Mauro, M.; Uguccioni, M.; Olivotto, I. Dissecting functional impairment in hypertrophic cardiomyopathy by dynamic assessment of diastolic reserve and outflow obstruction: A combined cardiopulmonary-echocardiographic study. Int. J. Cardiol. 2017, 227, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Halasz, G.; Dei, L.L.; Moroni, F.; Ayers, M.P.; Ciacci, P.; Giacalone, G.; Mistrulli, R.; Redivo, M.; Orellana, S.; Gabrielli, D.; et al. The Impact of Disopyramide on Exercise Capacity Among Patients with Obstructive Hypertrophic Cardiomyopathy: Beyond LVOT Gradient. Eur. J. Prev. Cardiol. 2024, 32, 1122–1124. [Google Scholar] [CrossRef]
- Lumb, R.H.; Benson, B.J.; Clements, J.A. Transfer of phospholipids by a protein fraction obtained from canine pulmonary lavage. Biochim. Biophys. Acta. 1988, 963, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Kostner, K.M. Statin therapy for hypertrophic cardiomyopathy: Too good to be true? Eur. J. Clin. Invest. 2010, 40, 965–967. [Google Scholar] [CrossRef]
- Coats, C.J.; Rantell, K.; Bartnik, A.; Patel, A.; Mist, B.; McKenna, W.J.; Elliott, P.M. Cardiopulmonary Exercise Testing and Prognosis in Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2015, 8, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Mapelli, M.; Romani, S.; Magrì, D.; Merlo, M.; Cittar, M.; Masè, M.; Muratori, M.; Gallo, G.; Sclafani, M.; Carriere, C.; et al. Exercise oxygen pulse kinetics in patients with hypertrophic cardiomyopathy. Heart 2022, 108, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Afana, A.S.; Adam, R.D.; Militaru, S.; Onciul, S.; Andrei, O.; Chirita Emandi, A.; Puiu, M.; Militaru, C.; Jurcut, R. Clinical Characteristics and Prognosis of Patients with End-Stage Hypertrophic Cardiomyopathy from a Tertiary Center Cohort: Systolic Dysfunction and Advanced Diastolic Dysfunction. Diagnostics 2025, 15, 1134. [Google Scholar] [CrossRef]
- Gaar-Humphreys, K.R.; van den Brink, A.; Wekking, M.; Asselbergs, F.W.; van Steenbeek, F.G.; Harakalova, M.; Pei, J. Targeting lipid metabolism as a new therapeutic strategy for inherited cardiomyopathies. Front. Cardiovasc. Med. 2023, 10, 1114459. [Google Scholar] [CrossRef]
- Schoonvelde, S.A.C.; Nollet, E.E.; Zwetsloot, P.P.; Knackstedt, C.; Germans, T.; Hirsch, A.; Schinkel, A.F.L.; van Slegtenhorst, M.A.; Verhagen, J.M.A.; de Boer, R.A.; et al. Genotype-negative hypertrophic cardiomyopathy: Exploring the role of cardiovascular risk factors in disease expression. Int. J. Cardiol. 2025, 437, 133444. [Google Scholar] [CrossRef] [PubMed]
- Landsteiner, I.; Masri, A.; Saberi, S.; Maron, M.S.; McGinnis, S.L.; Griskowitz, C.; Newlands, C.E.; Barriales-Villa, R.; Owens, A.T.; Lewis, G.D. Cardiopulmonary Exercise Testing for Characterization of Hypertrophic Cardiomyopathy: A Meta-Analysis. J. Am. Heart Assoc. 2025, 14, e039551. [Google Scholar] [CrossRef] [PubMed]
| Overall (N = 388) | Non-Event Group (N = 325) | Adverse Event Group (N = 63) | p-Value | |
|---|---|---|---|---|
| Age, years | 48 ± 15 | 47 ± 15 | 51 ± 13.26 | 0.047 |
| Male, n (%) | 245 (63.1) | 206 (63.4) | 39 (61.9) | 0.936 |
| BSA, m2 | 1.86 ± 0.21 | 1.86 ± 0.21 | 1.87 ± 0.20 | 0.694 |
| Comorbidities, n (%) | ||||
| Hypertension | 153 (39.4) | 119 (36.6) | 34 (54.0) | 0.015 |
| Dyslipidaemia | 117 (30.2) | 85 (26.2) | 32 (50.8) | <0.001 |
| Diabetes Mellitus | 30 (7.8) | 24 (7.4) | 6 (9.5) | 0.751 |
| COPD | 7 (1.8) | 6 (1.8) | 1 (1.6) | 1.000 |
| PAD | 9 (2.3) | 6 (1.8) | 3 (4.8) | 0.342 |
| CAD | 19 (4.9) | 14 (4.3) | 5 (7.9) | 0.367 |
| Prior Stroke | 12 (3.1) | 9 (2.8) | 3 (4.8) | 0.661 |
| AF | 51 (13.1) | 31 (9.5) | 20 (31.7) | <0.001 |
| Presenting symptoms, n (%) | ||||
| Chest pain | 121 (31.3) | 103 (31.8) | 18 (28.6) | 0.722 |
| Dyspnoea | 192 (49.5) | 150 (46.2) | 42 (66.7) | 0.004 |
| Palpitations | 99 (25.5) | 79 (24.3) | 20 (31.7) | 0.279 |
| Syncope | 52 (13.4) | 45 (13.8) | 7 (11.1) | 0.703 |
| NYHA functional class, n (%) | <0.001 | |||
| I | 179 (46.3) | 166 (51.2) | 13 (20.6) | |
| II | 181 (46.6) | 140 (43.1) | 41 (65.1) | |
| III | 25 (6.4) | 17 (5.2) | 8 (12.7) | |
| IV | 3 (0.8) | 3 (0.9) | 0 (0.0) | |
| ESC SCD risk score for HCM | 3.7 ± 2.4 | 3.5 ± 2.2 | 4.9 ± 2.6 | <0.001 |
| Medications, n (%) | ||||
| Aspirin | 61 (15.7) | 42 (12.9) | 19 (30.2) | 0.001 |
| Beta-blockers | 236 (60.8) | 190 (58.5) | 46 (73.0) | 0.043 |
| ACE inhibitors | 68 (17.5) | 44 (13.5) | 24 (38.1) | <0.001 |
| Angiotensin II receptor blockers | 86 (22.2) | 72 (22.2) | 14 (22.2) | 1.000 |
| Anticoagulants | 49 (12.7) | 30 (9.3) | 19 (30.2) | <0.001 |
| Calcium channel blockers | 32 (8.2) | 26 (8.0) | 6 (9.5) | 0.879 |
| ARNI | 0 (0) | 0 (0) | 0 (0) | |
| Disopyramide | 39 (10.1) | 29 (9.0) | 10 (15.9) | 0.149 |
| Amiodarone | 33 (8.5) | 20 (6.2) | 13 (20.6) | <0.001 |
| Ranolazine | 14 (3.6) | 10 (3.1) | 4 (6.3) | 0.365 |
| MRA | 14 (3.6) | 6 (1.8) | 8 (12.7) | <0.001 |
| SGLT2 inhibitors | 4 (1.0) | 4 (1.2) | 0 (0.0) | 0.839 |
| Diuretics | 75 (19.4) | 48 (14.9) | 27 (43.5) | <0.001 |
| Overall (N = 388) | Non-Event Group (N = 325) | Adverse Event Group (N = 63) | p-Value | |
|---|---|---|---|---|
| LA diameter, mm | 41.5 [38.0, 46.0] | 41.0 [37.0, 45.0] | 45.0 [41.0, 52.0] | <0.001 |
| LV maximal wall thickness, mm | 20.0 [17.0, 23.0] | 20.0 [17.0, 23.0] | 22.0 [19.0, 25.0] | 0.001 |
| LVEF, % | 66.0 [61.7, 72.5] | 66.0 [62.5, 72.5] | 66.3 [58.9, 71.7] | 0.151 |
| Resting/stress SAM, n (%) | 0.022/0.346 | |||
| Mild | 102 (26.4)/71 (18.5) | 84 (26.0)/ 56 (17.4) | 18 (28.6)/ 15 (23.8) | |
| Moderate | 47 (12.2)/39 (10.2) | 45 (13.9)/ 36 (11.2) | 2 (3.2)/ 3 (4.8) | |
| Severe | 17 (4.4)/49 (12.8) | 11 (3.4)/ 41 (12.8) | 6 (9.5)/ 8 (12.7) | |
| LVEDV, mL | 80.0 [65.0, 100.0] | 80.0 [65.0, 100.0] | 82.0 [64.0, 96.7] | 0.850 |
| LVESV, mL | 26.0 [20.0, 35.0] | 26.0 [19.0, 35.0] | 25.50 [21.00, 36.00] | 0.295 |
| LVEDV index, mL/m2 | 44.8 [34.4, 52.0] | 44.1 [35.3, 51.9] | 45.3 [34.1, 53.5] | 0.852 |
| LVESV index, mL/m2 | 14.1 [10.5, 18.5] | 14.0 [10.2, 18.4] | 14.3 [11.3, 19.8] | 0.344 |
| Resting LA 4CH volume index | 30.4 [24.0, 39.4] | 29.0 [23.0, 37.0] | 36.4 [29.8, 49.0] | <0.001 |
| Stress LA 4CH volume index | 27.5 [0.0, 49.7] | 26.0 [0.0, 44.0] | 43.0 [0.0, 66.0] | 0.046 |
| Resting/stress MR grade, n (%) | 0.09/0.178 | |||
| Grade I | 161 (43.0)/115 (31.4) | 126 (40.3)/ 92 (30.0) | 35 (57.4)/ 23 (39.0) | |
| Grade II | 24 (6.4)/33 (9.0) | 20 (6.4)/ 27 (8.8) | 4 (6.6)/ 6 (10.2) | |
| Grade III | 8 (2.1)/16 (4.4) | 7 (2.2)/ 11 (3.6) | 1 (1.6)/ 5 (8.5) | |
| Grade IV | 0 (0.0)/1 (0.3) | 0 (0.0)/ 1 (0.3) | 0 (0.0)/ 0 (0.0) | |
| Resting PASP, mmHg | 20.0 [12.0, 28.0] | 20.0 [11.5, 26.0] | 25.0 [15.0, 32.0] | 0.027 |
| Stress PASP, mmHg | 34.0 [25.0, 45.0] | 34.0 [25.0, 45.0] | 37.0 [25.7, 54.0] | 0.044 |
| Resting E/A | 1.1 [0.8, 1.5] | 1.08 [0.8, 1.5] | 1.1 [0.8, 1.6] | 0.613 |
| Stress E/A | 1.1 [0.8, 1.6] | 1.1 [0.8, 1.5] | 1.2 [0.9, 1.8] | 0.105 |
| Resting average E/e′ | 9.9 [7.9, 13.1] | 9.8 [7.7, 12.6] | 11.4 [9.0, 17.3] | 0.004 |
| Stress average E/e′ | 10.3 [8.3, 12.9] | 9.9 [8.1, 12.3] | 11.2 [9.6, 15.5] | 0.001 |
| Resting septal E/e′ | 11.8 [9.5, 16.0] | 11.6 [9.3, 15.5] | 13.4 [11.0, 20.0] | 0.001 |
| Stress septal E/e′ | 11.6 [9.5, 15.5] | 11.5 [9.3, 15.0] | 13.7 [11.3, 19.0] | <0.001 |
| Resting lateral E/e′ | 8.0 [6.0, 11.0] | 7.9 [5.9, 10.8] | 9.4 [6.6, 13.7] | 0.025 |
| Stress lateral E/e′ | 8.6 [6.7, 11.0] | 8.2 [6.7, 10.7] | 10.0 [7.3, 13.6] | 0.010 |
| E wave, cm/s | 73.0 [60.5, 90.0] | 73.0 [62.0, 90.0] | 69.0 [58.0, 83.7] | 0.355 |
| A wave, cm/s | 66.0 [52.0, 84.0] | 67.0 [53.0, 84.7] | 60.0 [45.0, 77.2] | 0.077 |
| E wave peak velocity, cm/s | 100.0 [85.0, 120.0] | 100.0 [85.2, 120.0] | 110.0 [82.7, 123.0] | 0.485 |
| A wave peak velocity, cm/s | 89.0 [67.5, 115.0] | 91.0 [68.2, 116.0] | 80.0 [67.0, 109.0] | 0.110 |
| Resting TAPSE, mm | 21.0 [19.0, 23.0] | 21.0 [20.0, 23.0] | 21.0 [19.00, 22.7] | 0.317 |
| Stress TAPSE, mm | 25.0 [22.0, 28.0] | 25.0 [22.0, 28.0] | 24.5 [22.0, 27.0] | 0.271 |
| Resting LVOT gradient, mmHg | 12.0 [8.0, 30.0] | 11.0 [8.0, 30.0] | 14.0 [8.0, 28.5] | 0.527 |
| Provoked LVOT gradient, mmHg | 30.0 [19.0, 70.0] | 30.0 [19.0, 70.0] | 35.0 [19.5, 70.0] | 0.749 |
| Overall (N = 388) | Non-Event Group (N = 325) | Adverse Event Group (N = 63) | p-Value | |
|---|---|---|---|---|
| Workload, watts | 90.0 [70.0, 120.0] | 97.5 [70.0, 120.0] | 90.0 [60.0, 110.0] | 0.015 |
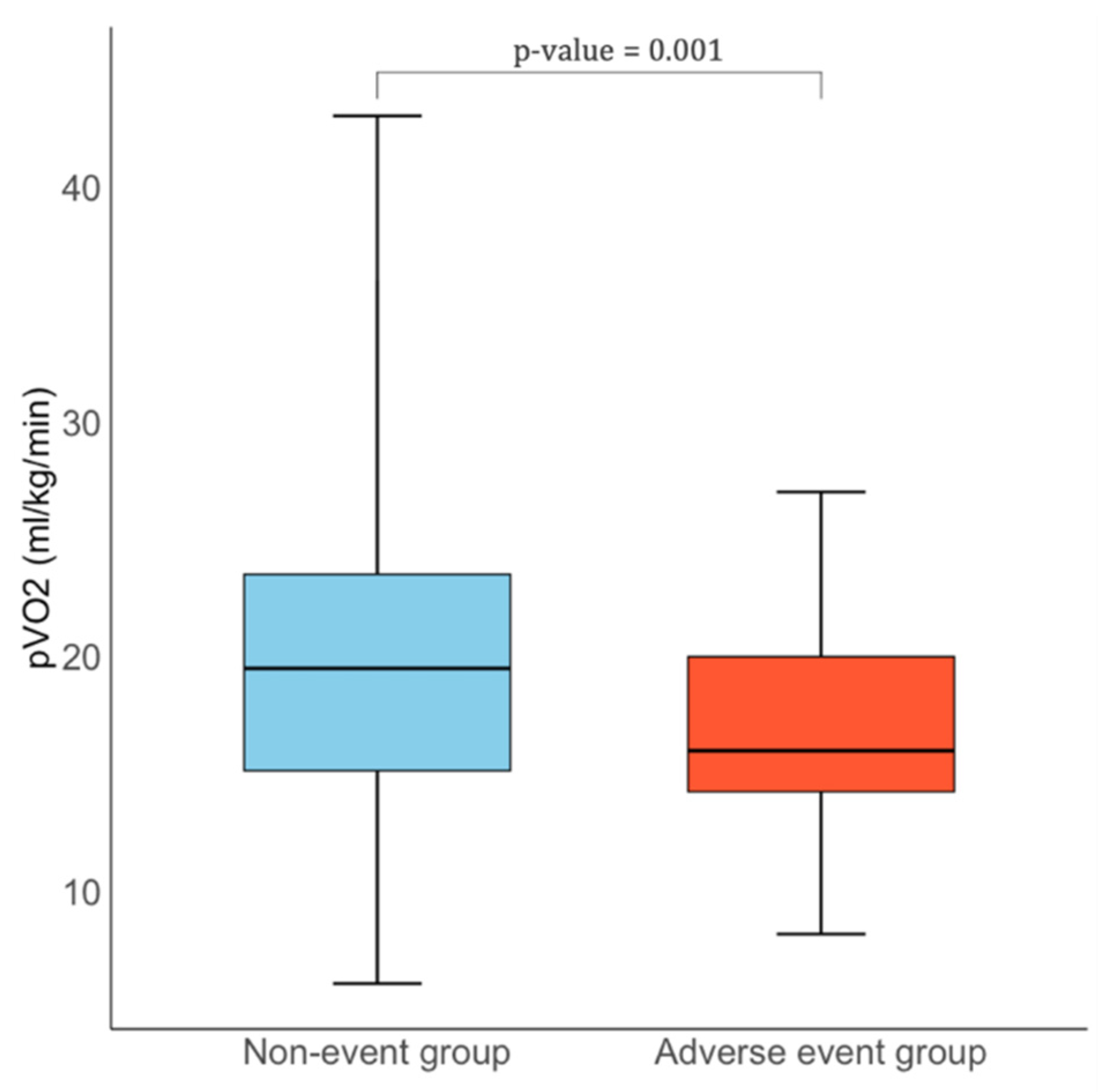
| Peak VO2, mL/kg/min | 19.0 [15.0, 23.0] | 19.5 [15.1–23.5] | 16.0 [14.2–20.0] | 0.001 |
| % VO2 predicted | 66.2 [55.6, 78.1] | 67.1 [56.1, 80.5] | 62.1 [48.1, 73.0] | 0.006 |
| VO2/Watt, mL/min/W | 8.9 [7.7, 10.0] | 9.0 [8.00 10.4] | 8.2 [6.6, 9.0] | 0.026 |
| O2 pulse, VO2/HR | 11.0 [8.6, 14.3] | 11.0 [8.6, 14.2] | 11.0 [8.9, 14.4] | 0.960 |
| VE/VCO2 slope | 27.0 [24.0, 30.2] | 26.6 [24.0, 30.0] | 28.0 [25.0, 33.0] | 0.048 |
| Breathing Reserve, % | 42.0 [32.0, 52.0] | 42.4 [32.0, 53.0] | 41.5 [33.5, 50.2] | 0.793 |
| AT, min | 8.0 [6.0, 9.0] | 8.0 [6.0, 10.0] | 7.0 [5.0, 8.0] | 0.032 |
| % AT predicted | 54.8 [42.7, 64.8] | 55.6 [44.3, 66.1] | 45.6 [34.5, 61.9] | 0.003 |
| AT, mL/kg/min | 15.1 [12.6, 19.0] | 16.0 [13.0, 20.0] | 13.0 [11.1, 15.0] | <0.001 |
| HR rest, bpm | 74.0 [65.0, 86.0] | 75.0 [65.0, 87.0] | 69.0 [63.0, 78.0] | 0.003 |
| HR max, bpm | 128.0 [110.0, 144.0] | 130.0 [112.0, 146.0] | 114.0 [101.5, 129.0] | <0.001 |
| % HR predicted | 79.0 [70.0, 88.0] | 81.0 [71.0, 90.0] | 73.0 [64.0, 80.5] | <0.001 |
| Systolic BP rest, mmHg | 120.0 [110.0, 140.0] | 120.0 [110.0, 140.0] | 120.0 [110.0, 140.0] | 0.831 |
| Systolic BP peak, mmHg | 170.0 [150.0, 190.0] | 170.0 [150.0, 190.0] | 160.0 [142.5, 187.5] | 0.051 |
| Diastolic BP rest, mmHg | 80.0 [70.0, 85.0] | 80.0 [70.0, 85.0] | 75.0 [70.0, 80.0] | 0.050 |
| PETCO2 rest, mmHg | 37.0 [32.0, 41.2] | 37.0 [32.0, 41.0] | 38.0 [35.0, 43.7] | 0.254 |
| PETCO2 peak, mmHg | 42.0 [36.0, 46.0] | 41.0 [36.0, 46.0] | 42.0 [39.2, 46.0] | 0.499 |
| Variable | Univariate Regression | Multivariate Regression | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age (per year) | 1.02 (1.00–1.04) | 0.048 | 0.98 (0.96–1.01) | 0.162 |
| Gender | 0.94 (0.54–1.65) | 0.824 | - | - |
| Dyslipidaemia | 2.91 (1.68–5.08) | <0.0001 | 2.58 (1.68–5.08) | 0.006 |
| Diabetes Mellitus | 1.32 (0.47–3.17) | 0.567 | - | - |
| Resting average E/e′ | 1.09 (1.04–1.14) | 0.011 | 1.06 (1.00–1.12) | 0.042 |
| pVO2 | 0.91 (0.87–0.96) | 0.0006 | 0.92 (0.87–0.98) | 0.016 |
| O2 pulse, VO2/HR | 1.00 (0.94–1.07) | 0.957 | - | - |
| VE/VCO2 slope | 1.03 (0.99–1.08) | 0.145 | - | - |
| PETCO2 peak, mmHg | 1.03 (0.96–1.11) | 0.493 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halasz, G.; Ciacci, P.; Mistrulli, R.; Giacalone, G.; Ferro, A.; Romiti, G.F.; Albi, F.; Gabrielli, D.; Re, F. Integrating Cardiopulmonary Exercise Testing and Stress Echocardiography to Predict Clinical Outcomes in Hypertrophic Cardiomyopathy. J. Clin. Med. 2025, 14, 7231. https://doi.org/10.3390/jcm14207231
Halasz G, Ciacci P, Mistrulli R, Giacalone G, Ferro A, Romiti GF, Albi F, Gabrielli D, Re F. Integrating Cardiopulmonary Exercise Testing and Stress Echocardiography to Predict Clinical Outcomes in Hypertrophic Cardiomyopathy. Journal of Clinical Medicine. 2025; 14(20):7231. https://doi.org/10.3390/jcm14207231
Chicago/Turabian StyleHalasz, Geza, Paolo Ciacci, Raffaella Mistrulli, Guido Giacalone, Aurora Ferro, Giulio Francesco Romiti, Fiammetta Albi, Domenico Gabrielli, and Federica Re. 2025. "Integrating Cardiopulmonary Exercise Testing and Stress Echocardiography to Predict Clinical Outcomes in Hypertrophic Cardiomyopathy" Journal of Clinical Medicine 14, no. 20: 7231. https://doi.org/10.3390/jcm14207231
APA StyleHalasz, G., Ciacci, P., Mistrulli, R., Giacalone, G., Ferro, A., Romiti, G. F., Albi, F., Gabrielli, D., & Re, F. (2025). Integrating Cardiopulmonary Exercise Testing and Stress Echocardiography to Predict Clinical Outcomes in Hypertrophic Cardiomyopathy. Journal of Clinical Medicine, 14(20), 7231. https://doi.org/10.3390/jcm14207231






