Changes in Body Composition Compartments After Kidney Transplantation: A One-Year Prospective Study
Abstract
1. Introduction
Background
2. Materials and Methods
2.1. Study Design and Participants
2.2. Variables, Data Collection and Measuring Instruments
2.3. Data Analisys
2.4. Ethical Considerations
3. Results
3.1. Descriptive Analysis
3.2. Body Composition Changes over Time
3.2.1. Body Weight Changes over Time
3.2.2. Evolution of Muscle Mass (MM) According to Independent Variables
3.2.3. Evolution of Fat Mass (FM) According to Independent Variables
3.2.4. Evolution of Visceral Fat (VF) According to Independent Variables
3.2.5. Evolution of Total Body Water Percentage (%TBW) According to Independent Variables
3.2.6. Weight Gain According to Comorbidities
4. Discussion
4.1. Sociodemographic Characteristics
4.2. Cardiovascular Risk Factors
4.3. Weight Evolution
4.4. Body Composition Changes
4.5. Clinical Implications
4.6. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BIA | Bioelectrical Impedance Analysis |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| CKD | Chronic Kidney Disease |
| CVD | Cardiovascular Disease |
| DLP | Dyslipidaemia |
| DM | Diabetes mellitus |
| FM | Fat mass |
| HD | Haemodialysis |
| HT | Hypertension |
| IQR | Interquartile Range |
| KRT | Kidney Replacement Therapy |
| LDKT | Living Donor Kidney Transplant |
| MM | Muscle Mass |
| NODAT | New Onset Diabetes After Transplantation |
| PD | Peritoneal Dialysis |
| PTDM | Post-Transplant Diabetes Mellitus |
| RRT | Renal Replacement Therapy |
| SD | Standard Deviation |
| SGLT2 Inhibitors | Sodium–glucose cotransporter 2 |
| VF | Visceral Fat |
| WG | Weight Gain |
| WHO | World Health Organisation |
| %TBW | Total Body Water Percentage |
References
- Caamiña, L.; Pietropaolo, A.; Basile, G.; Dönmez, M.I.; Uleri, A.; Territo, A.; Fraile-Gómez, P. Evaluación del impacto de la obesidad en los resultados del trasplante renal. Actas Urológicas Españolas 2024, 48, 125–133. [Google Scholar] [CrossRef]
- Scheuermann, U.; Babel, J.; Pietsch, U.C.; Weimann, A.; Lyros, O.; Semmling, K.; Sucher, R. Recipient obesity as a risk factor in kidney transplantation. BMC Nephrol. 2022, 23, 37. [Google Scholar] [CrossRef]
- Moreau, K.; Desseix, A.; Germain, C.; Merville, P.; Couzi, L.; Thiébaut, R.; Chauveau, P. Evolution of Body Composition Following Successful Kidney Transplantation Is Strongly Influenced by Physical Activity: Results of the CORPOS Study. BMC Nephrol. 2021, 22, 31. [Google Scholar] [CrossRef]
- Ferreira, T.D.S.; Barreto Silva, M.I.; Costa, M.S.; Pontes, K.S.D.S.; Castro, F.G.; Antunes, V.P.; Klein, M.R.S.T. High Abdominal Adiposity and Low Phase Angle in Overweight Renal Transplant Recipients. Clin. Transplant. 2019, 33, 13654. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, T.J.; Clarke, A.L.; Nixon, D.G.D.; Hull, K.L.; Song, Y.; Burton, J.O.; Yates, T.; Smith, A.C. Prevalence and Correlates of Physical Activity across Kidney Disease Stages: An Observational Multicentre Study. Nephrol. Dial. Transplant. 2021, 36, 641–649. [Google Scholar] [CrossRef]
- Quero, M.; Montero, N.; Rama, I.; Codina, S.; Couceiro, C.; Cruzado, J.M. Obesity in renal transplantation. Nephron 2021, 145, 614–623. [Google Scholar] [CrossRef]
- Quint, E.E.; Schopmeyer, L.; Banning, L.B.; Moers, C.; El Moumni, M.; Nieuwenhuijs-Moeke, G.J.; Pol, R.A. Transiciones en estado de fragilidad después del trasplante de riñón. Langenbeck’s Arch. Surg. 2020, 405, 843–850. [Google Scholar] [CrossRef]
- Gómez-Ambrosi, J.; Catalán, V. Prevalencia de diabesidad en España: Depende de cómo se defina la obesidad. An. Del Sist. Sanit. De Navar. 2022, 45, e0993. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodrigo, C.; Bárbara, G.H.; Citores, M.G.; Aranceta-Bartrina, J. Prevalencia de obesidad y factores de riesgo cardiovascular asociados en la población general española: Estudio ENPE. Rev. Española De Cardiol. 2022, 75, 232–241. [Google Scholar] [CrossRef]
- Dienemann, T.; Ziolkowski, S.L.; Bender, S.; Goral, S.; Long, J.; Baker, J.F.; Leonard, M.B. Changes in Body Composition, Muscle Strength, and Fat Distribution Following Kidney Transplantation. Am. J. Kidney Dis. 2021, 78, 816–825. [Google Scholar] [CrossRef]
- Erturk, T.; Berber, I.; Cakir, U. Effect of Obesity on Clinical Outcomes of Kidney Transplant Patients. Transplant. Proc. 2019, 51, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Ladhani, M.; Craig, J.C.; Irving, M.; Clayton, P.A.; Wong, G. Obesity and the Risk of Cardiovascular and All-Cause Mortality in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Nephrol. Dial. Transplant. 2017, 32, 439–449. [Google Scholar] [CrossRef]
- Brilleman, S.L.; Moreno-Betancur, M.; Polkinghorne, K.R.; McDonald, S.; Crowther, M.J.; Thomson, J.; Wolfe, R. Changes in Body Mass Index and Rates of Death and Transplant in Hemodialysis Patients: A Latent Class Joint Modeling Approach. Epidemiology 2019, 30, 38–47. [Google Scholar] [CrossRef]
- Bellini, M.I.; Deurloo, E.; Consorti, F.; Herbert, P.E. Body Mass Index Affects Kidney Transplant Outcomes: A Cohort Study over 5 Years Using a Steroid Sparing Protocol. Front. Endocrinol. 2023, 14, 1106087. [Google Scholar] [CrossRef]
- Chang, J.H.; Mushailov, V.; Mohan, S. Obesity and Kidney Transplantation. Curr. Opin. Organ Transplant. 2023, 28, 149–155. [Google Scholar] [CrossRef]
- Azhar, A.; Hassan, N.; Tapolyai, M.; Molnar, M.Z. Obesity, chronic kidney disease, and kidney transplantation: An evolving relationship. Semin. Nephrol. 2021, 41, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Kostakis, I.D.; Kassimatis, T.; Bianchi, V.; Paraskeva, P.; Flach, C.; Callaghan, C.; Loukopoulos, I. UK Renal Transplant Outcomes in Low and High BMI Recipients: The Need for a National Policy. J. Nephrol. 2020, 33, 371–381. [Google Scholar] [CrossRef]
- Wu, D.A.; Robb, M.L.; Forsythe, J.L.; Bradley, C.; Cairns, J.; Draper, H.; Oniscu, G.C. Recipient Comorbidity and Survival Outcomes after Kidney Transplantation: A UK-Wide Prospective Cohort Study. Transplantation 2020, 104, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Arenas, M.D.; Collado, S.; Fernández-Chamarro, M. Pautas de derivación a la Unidad de Enfermedad Renal Crónica Avanzada (ERCA). In Nefrología al Día; Lorenzo, V., López Gómez, J.M., Eds.; Elsevier España S.L.U.: Barcelona, Spain, 2024. [Google Scholar]
- Rangaswami, J.; Mathew, R.O.; Parasuraman, R.; Tantisattamo, E.; Lubetzky, M.; Rao, S.; Yaqub, M.S.; Birdwell, K.A.; Bennett, W.; Dalal, P.; et al. Cardiovascular Disease in the Kidney Transplant Recipient: Epidemiology, Diagnosis and Management Strategies. Nephrol. Dial. Transplant. 2019, 34, 760–773. [Google Scholar] [CrossRef]
- Dashti-Khavidaki, S.; Saidi, R.; Lu, H. Current Status of Glucocorticoid Usage in Solid Organ Transplantation. World J. Transplant. 2021, 11, 443–465. [Google Scholar] [CrossRef]
- Melek, K.; Lilia, B.F.; Hela, J.; Rania, K.; Lamia, R.; Ikram, M.; Madiha, K.; Wided, S.; Soumaya, B.; Karim, Z. Incidence of Cardiovascular Events and Associated Risk Factors in Kidney Transplant Patients. Transplantation 2018, 102, S637. [Google Scholar] [CrossRef]
- Gaillard, F.; Ould Rabah, M.; Garcelon, N.; Touam, M.; Neuraz, A.; Legendre, C.; Anglicheau, D.; Prié, D.; Bienaimé, F. Allograft Function and Muscle Mass Evolution after Kidney Transplantation. J. Cachexia Sarcopenia Muscle 2022, 13, 2875–2887. [Google Scholar] [CrossRef]
- Carbone, S.; Canada, J.M.; Billingsley, H.E.; Siddiqui, M.S.; Elagizi, A.; Lavie, C.J. Obesity Paradox in Cardiovascular Disease: Where Do We Stand? Vasc. Health Risk Manag. 2019, 15, 89–100. [Google Scholar] [CrossRef]
- Magkos, F. Metabolically Healthy Obesity: What–s in a Name? Am. J. Clin. Nutr. 2019, 110, 533–539. [Google Scholar] [CrossRef]
- Nosrati-Oskouie, M.; Salavatizadeh, M.; Ghorban Sabbagh, M.; Aghili-Moghaddam, N.S.; Tarighat-Esfanjani, A.; Sahebkar, A. Current Evidence on Dietary Factors and Kidney Allograft Function in Kidney Transplant Recipients: A Systematic Review. Curr. Med. Chem. 2024, 31, 5818–5836. [Google Scholar] [CrossRef]
- Mascherini, G.; Zappelli, E.; Leone, B.; Musumeci, G.; Totti, V.; Irurtia, A.; Stefani, L. Bioelectrical Impedance Vector Analysis (BIVA) in Renal Transplant Recipients during an Unsupervised Physical Exercise Program. J. Sports Med. Phys. Fit. 2020, 60, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Israni, A.K.; Danovitch, G. Long-term survival after kidney transplantation. N. Engl. J. Med. 2021, 385, 729–743. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Pedreira-Robles, G.; Garcimartín, P.; Pérez-Sáez, M.J.; Bach-Pascual, A.; Crespo, M.; Morín-Fraile, V. Complex Management and Descriptive Cost Analysis of Kidney Transplant Candidates: A Descriptive Cross-Sectional Study. BMC Health Serv. Res. 2024, 24, 763. [Google Scholar] [CrossRef]
- Melk, A.; Babitsch, B.; Borchert-Mörlins, B.; Claas, F.; Dipchand, A.I.; Eifert, S.; Eiz-Vesper, B.; Epping, J.; Falk, C.S.; Foster, B.; et al. Equally Interchangeable? How Sex and Gender Affect Transplantation. Transplantation 2019, 103, 1094–1110. [Google Scholar] [CrossRef] [PubMed]
- Swartling, O.; Rydell, H.; Stendahl, M.; Segelmark, M.; Trolle Lagerros, Y.; Evans, M. CKD Progression and Mortality Among Men and Women: A Nationwide Study in Sweden. Am. J. Kidney Dis. 2021, 78, 190–199.e1. [Google Scholar] [CrossRef]
- Ricardo, A.C.; Yang, W.; Sha, D.; Appel, L.J.; Chen, J.; Krousel-Wood, M.; Manoharan, A.; Steigerwalt, S.; Wright, J.; Rahman, M.; et al. Sex-Related Disparities in CKD Progression. J. Am. Soc. Nephrol. 2019, 30, 137–146. [Google Scholar] [CrossRef]
- Hockham, C.; Schanschieff, F.; Woodward, M. Sex Differences in CKD-Associated Mortality from 1990 to 2019: Data from the Global Burden of Disease Study. Kidney Med. 2022, 4, 100535. [Google Scholar] [CrossRef]
- Mayne, K.J.; Sullivan, M.K.; Lees, J.S. Sex and Gender Differences in the Management of Chronic Kidney Disease and Hypertension. J. Hum. Hypertens. 2023, 37, 649–653. [Google Scholar] [CrossRef]
- Katz-Greenberg, G.; Shah, S. Sex and Gender Differences in Kidney Transplantation. Semin. Nephrol. 2022, 42, 219–229. [Google Scholar] [CrossRef]
- Brar, A.; Markell, M. Impact of Gender and Gender Disparities in Patients with Kidney Disease. Curr. Opin. Nephrol. Hypertens. 2019, 28, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Estupiñán-Bohórquez, A.; Acosta-Reyes, J.; Viasus-Pérez, D.; García-López, A.; Patino-Jaramillo, N.; Girón-Luque, F. Trasplante renal de donantes con criterios expandidos en la región Caribe colombiana. Rev. Nefrol. Latinoam 2021, 18, 119–127. [Google Scholar] [CrossRef]
- Arias-Cabrales, C.; Pérez-Sáez, M.J.; Redondo-Pachón, D.; Buxeda, A.; Burballa, C.; Bermejo, S.; Sierra, A.; Mir, M.; Burón, A.; Zapatero, A.; et al. Usefulness of the KDPI in Spain: A Comparison with Donor Age and Definition of Standard/Expanded Criteria Donor. Nefrología 2018, 38, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Peters-Sengers, H.; Heemskerk, M.B.A.; Geskus, R.B.; Kers, J.; Homan van der Heide, J.J.; Berger, S.P.; Bemelman, F.J. Validation of the Prognostic Kidney Donor Risk Index Scoring System of Deceased Donors for Renal Transplantation in the Netherlands. Transplantation 2018, 102, 162–170. [Google Scholar] [CrossRef]
- Alcázar, R.; Escobar, C.; Palacios, B.; Aranda, U.; Varela, L.; Capel, M.; Botana, M. Risk of Outcomes in a Spanish Population with Chronic Kidney Disease. Clin. Kidney J. 2022, 15, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Agudelo-Botero, M. GBD Chronic Kidney Disease Collaboration: Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Frutos, M.Á.; Crespo, M.; Oliva Valentín, M.; Alonso-Melgar, Á.; Alonso, J.; Fernández, C.; Pascual, J. Recomendaciones para el trasplante renal de donante vivo. Nefrología 2022, 42, 1–128. [Google Scholar] [CrossRef]
- Yagisawa, T.; Mieno, M.; Ichimaru, N.; Morita, K.; Nakamura, M.; Hotta, K.; Yuzawa, K. Trends of Kidney Transplantation in Japan in 2018: Data from the Kidney Transplant Registry. Ren. Replace. Ther. 2019, 5, 1–14. [Google Scholar] [CrossRef]
- Thomas-Fonseca, G.; Cano-Cervantes, H.; Hernández-Estrada, S.; Díaz-Avendaño, O.; Alamilla-Sánchez, M.; García-Macas, V. Variación del peso a los 12 meses postrasplante renal y su efecto en el riesgo cardiovascular. Rev. Mex. De Traspl. 2021, 10, 86–94. [Google Scholar] [CrossRef]
- Liu, Y.; Bendersky, V.A.; Chen, X.; Ghildayal, N.; Harhay, M.N.; Segev, D.L.; McAdams-DeMarco, M. Post-kidney Transplant Body Mass Index Trajectories Are Associated with Graft Loss and Mortality. Clin. Transplant. 2023, 37, 14947. [Google Scholar] [CrossRef]
- Kanbay, M.; Siriopol, D.; Mahmoud Abdel-Rahman, S.; Yilmaz, Z.Y.; Ozbek, L.; Guldan, M.; Copur, S.; Tuttle, K.R. Impact of Weight Change on Kidney Transplantation Outcomes: A Systematic Review and Meta-Analysis. Diabetes Obes. Metab. 2025, 27, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Altheaby, A.; Alajlan, N.; Shaheen, M.F.; Abosamah, G.; Ghallab, B.; Aldawsari, B.; Arabi, Z. Weight Gain after Renal Transplant: Incidence, Risk Factors, and Outcomes. PLoS ONE 2022, 17, 0268044. [Google Scholar] [CrossRef] [PubMed]
- Oliveras, L.; Montero, N. ¿Cómo afectan la obesidad y la pérdida de peso pretrasplante a los pacientes trasplantados renales? Nefrología 2022, 14, 56–58. [Google Scholar]
- Kang, A.W.; Garber, C.E.; Eaton, C.B.; Risica, P.M.; Bostom, A.G. Physical Activity and Cardiovascular Risk among Kidney Transplant Patients. Med. Sci. Sports Exerc. 2019, 51, 1154–1161. [Google Scholar] [CrossRef]
- Skweres, M.; Nieczyporuk, M.; Pucyło, S.; Piotrowska, G.; Ceglarz, K.; Pielaciński, J.; Rudnik, A.; Sikora, K.; Sikora, A. Physical Activity in Kidney Transplant Recipients: Benefits, Barriers, Interventions. Qual. Sport 2024, 25, 56831. [Google Scholar] [CrossRef]
- Forte, C.C.; Pedrollo, E.F.; Nicoletto, B.B.; Lopes, J.B.; Manfro, R.C.; Souza, G.C.; Leitão, C.B. Risk Factors Associated with Weight Gain after Kidney Transplantation: A Cohort Study. PLoS ONE 2020, 15, e0243394. [Google Scholar] [CrossRef]
- Hernández, S.B.; López, Á.Á.; Sabillón, J.A.R.; Arnaldo, C.L.; Gállego, R.H.; Vinuesa Calvo, E.G.; Pérez-Monteoliva, N.R.R. Effect of Weight Change after Renal Transplantation on Outcomes of Graft Survival. Nefrología 2022, 42, 568–577. [Google Scholar] [CrossRef]
- Onofre, T.; Junior, J.F.F.; Amorim, C.F.; Minamoto, S.T.; Moraes Paisani, D.; Chiavegato, L.D. Impact of an Early Physiotherapy Program after Kidney Transplant during Hospital Stay: A Randomized Controlled Trial. J. Bras. Nefrol. 2017, 39, 424–432. [Google Scholar] [CrossRef]
- Osté, M.C.; Gomes-Neto, A.W.; Corpeleijn, E.; Gans, R.O.; Borst, M.H.; Berg, E.; Bakker, S.J. Dietary Approach to Stop Hypertension (DASH) Diet and Risk of Renal Function Decline and All-Cause Mortality in Renal Transplant Recipients. Am. J. Transplant. 2018, 18, 2523–2533. [Google Scholar] [CrossRef]
- Workeneh, B.; Moore, L.W.; Fong, J.V.N.; Shypailo, R.; Gaber, A.O.; Mitch, W.E. Successful kidney transplantation is associated with weight gain from truncal obesity and insulin resistance. J. Ren. Nutr. 2019, 29, 548–555. [Google Scholar] [CrossRef]
- Barreto, A.; Silva, M.; Pontes, K.; Costa, M.; Rosina, K.; Valjalo, E.; Klein, M. Sarcopenia and Its Components in Adult Renal Transplant Recipients: Prevalence and Association with Body Adiposity. Br. J. Nutr. 2019, 122, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Wołoszyk, P.; Małgorzewicz, S.; Chamienia, A.; Dębska-Ślizień, A. Obesity After Successful Kidney Transplantation. Transplant. Proc. 2020, 52, 2352–2356. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Tsai, J.-C.; Chen, S.-C.; Chiu, Y.-W.; Hwang, S.-J.; Hung, C.-C.; Chen, T.-H.; Kuo, M.-C.; Chen, H.-C. Association of Fluid Overload with Kidney Disease Progression in Advanced CKD: A Prospective Cohort Study. Am. J. Kidney Dis. 2014, 63, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Ould Rabah, M.; Morin, L.; Dali-Youcef, N.; Géri, G.; Mazloum, M.; Garcelon, N.; Husson, J.; Touam, M.; Moulin, B.; Caillard, S.; et al. Extracellular Fluid Volume and Mortality after Kidney Transplantation. Kidney360 2024, 5, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Siriopol, D.; Siriopol, M.; Stuard, S.; Voroneanu, L.; Wabel, P.; Moissl, U.; Voiculescu, D.; Covic, A. An Analysis of the Impact of Fluid Overload and Fluid Depletion for All-Cause and Cardiovascular Mortality. Nephrol. Dial. Transplant. 2019, 34, 1385–1393. [Google Scholar] [CrossRef]
- Jia, H.; Huang, F.; Zhang, X.; Cheng, J.; Chen, J.; Wu, J. Early Perioperative Fluid Overload Is Associated with Adverse Outcomes in Deceased Donor Kidney Transplantation. Transpl. Int. 2021, 34, 1862–1874. [Google Scholar] [CrossRef]
| Cause | n (%) |
|---|---|
| Glomerulopathies | 31 (27.70) |
| Unaffiliated | 24 (21.40) |
| Diabetes mellitus | 14 (12.50) |
| Polycystic kidney disease | 14 (12.50) |
| Tubulo-interstitial | 12 (10.70) |
| Hypertension | 8 (8.04) |
| Metabolic-congenital | 5 (4.46) |
| Renovascular | 3 (2.70) |
| Total | 112 (100) |
| n (%) | Men | Women | p | ||
|---|---|---|---|---|---|
| 112 (100%) | 79 (70.5%) | 33 (29.5%) | - | ||
| Age (categorised) | 18–64 | 79 (70.5) | 57 (72.2) | 22 (66.7) | 0562 x |
| ≥65 | 33 (29.5) | 22 (27.8) | 11 (33.3) | ||
| Kidney replacement therapy | Non-dialysis | 9 (8.1) | 6 (7.6) | 3 (9.1) | 0.784 + |
| Peritoneal dialysis | 30 (26.8) | 23 (29.1) | 7 (21.2) | ||
| Haemodialysis | 73 (65.2) | 50 (63.3) | 23 (69.7) | ||
| Transplant Previous | No | 99 (88.4) | 71 (89.9) | 28 (84.8) | 0.449 x |
| Yes | 13 (11.6) | 8 (10.1) | 5 (15.2) | ||
| Vascular Access | No vascular Access | 39 (34.8) | 29 (36.7) | 10 (30.3) | 0.255 + |
| AVF | 50 (44.6) | 37 (46.8) | 13 (39.4) | ||
| Catheter | 23 (20.5) | 13 (16.5) | 10 (30.3) | ||
| BMI | Under weight | 5 (4.5) | 1 (1.3) | 4 (12.1) | 0.007 + |
| Normal weight | 53 (47.3) | 34 (43.0) | 19 (57.5) | ||
| Overweight | 38 (34.0) | 33 (41.8) | 5 (15.2) | ||
| Obesity | 16 (14.3) | 11 (13.9) | 5 (15.2) | ||
| Patients with hypertension | 101 (90.2) | 71 (70.3) | 30 (29.7) | 0.867 x | |
| Patients with dyslipidaemia | 59 (52.7) | 43 (72.9) | 16 (27.1) | 0.566 x | |
| Patients with previous diabetes | 22 (19.6) | 16 (72.7) | 6 (27.3) | 0.801 x | |
| Patients with Ischemic Heart disease | 29 (26.0) | 21 (26.6) | 8 (24.2) | 0.797 x | |
| Patients with respiratory disease | 15 (13.4) | 14 (17.7) | 1 (3.0) | 0.037 x | |
| Patients with cerebrovascular accident | 5 (4.5) | 3 (29.0) | 2 (40.0) | 0.597 x | |
| Patients with toxic habit | 20 (18.0) | 16 (80.0) | 4 (20.0) | 0.306 x | |
| Variable | Pre-Transplant | 12 Months | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | Men | Women | p | Total | Men | Women | p | |
| Ferritin (15–200 ng/mL) | 384.85 | 355 | 387 | 0.632 U | 335 | 287.70 | 381 | 0.390 U |
| (445.20) | (467) | (362) | (550.40) | (502) | (636) | |||
| Urea (17–43 mg/dL) | 107.00 | 112.00 | 99.00 | 0.165 U | 58 | 60 | 54 | 0.278 U |
| (80.00) | (79.50) | (69) | (28.50) | (28.50) | (31.0) | |||
| Creatinine (0.51–0.95 mg/dL) | 6.08 | 6.21 | 5.40 | 0.016 U | 1.48 | 1.54 | 1.25 | 0.001 U |
| (3.03) | (3.78) | (2.83) | (0.66) | (0.57) | (0.59) | |||
| Total. protein (6.6–8.3 g/dL) | 6.60 | 6.70 | 6.60 | 0.196 T | 6.71 | 6.70 | 6.80 | 0.856 T |
| (0.80) | (0.60) | (0.61) | (0.80) | (0.750 | (0.80) | |||
| Albumin (3.5–5.2 g/dL) | 4.00 | 4.03 | 3.90 | 0.128 T | 4.20 | 4.30 | 4.20 | 0.279 T |
| (0.60) | (0.60) | (0.60) | (0.80) | (0.50) | (0.40) | |||
| Haemoglobin (g/dL) | 11.90 | 12.00 | 11.7 | 0.380 T | 13.40 | 13.50 | 12.90 | 0.538 T |
| (1.92) | (1.90) | (2.10) | (2.075) | (1.75) | (2.50) | |||
| Haematocrit (%) | 35.65 | 35.9 | 35.50 | 0.699 T | 39.90 | 40.30 | 38.70 | 0.791 T |
| (5.12) | (4.90) | (5.70) | (6.20) | (5.60) | (0.94) | |||
| Glycated haemoglobin (HbA1c) | 5.30 | 5.20 | 5.30 | 0.924 U | 5.80 | 5.81 | 5.80 | 0.897 U |
| (0.71) | (0.70) | (0.80) | (1.15) | (1.19) | (0.95) | |||
| Variable | 0 Months | 3 Months | 6 Months | 12 Months | p-ANOVA |
|---|---|---|---|---|---|
| Total weight (kg) | 71.3 ± 15.5 | 72.5 ± 15.1 | 73.7 ± 15.0 | 74.5 ± 15.2 | <0.001 |
| (68.4–74.2) | (69.7–75.4) | (70.8–76.5) | (71.7–77.4) | ||
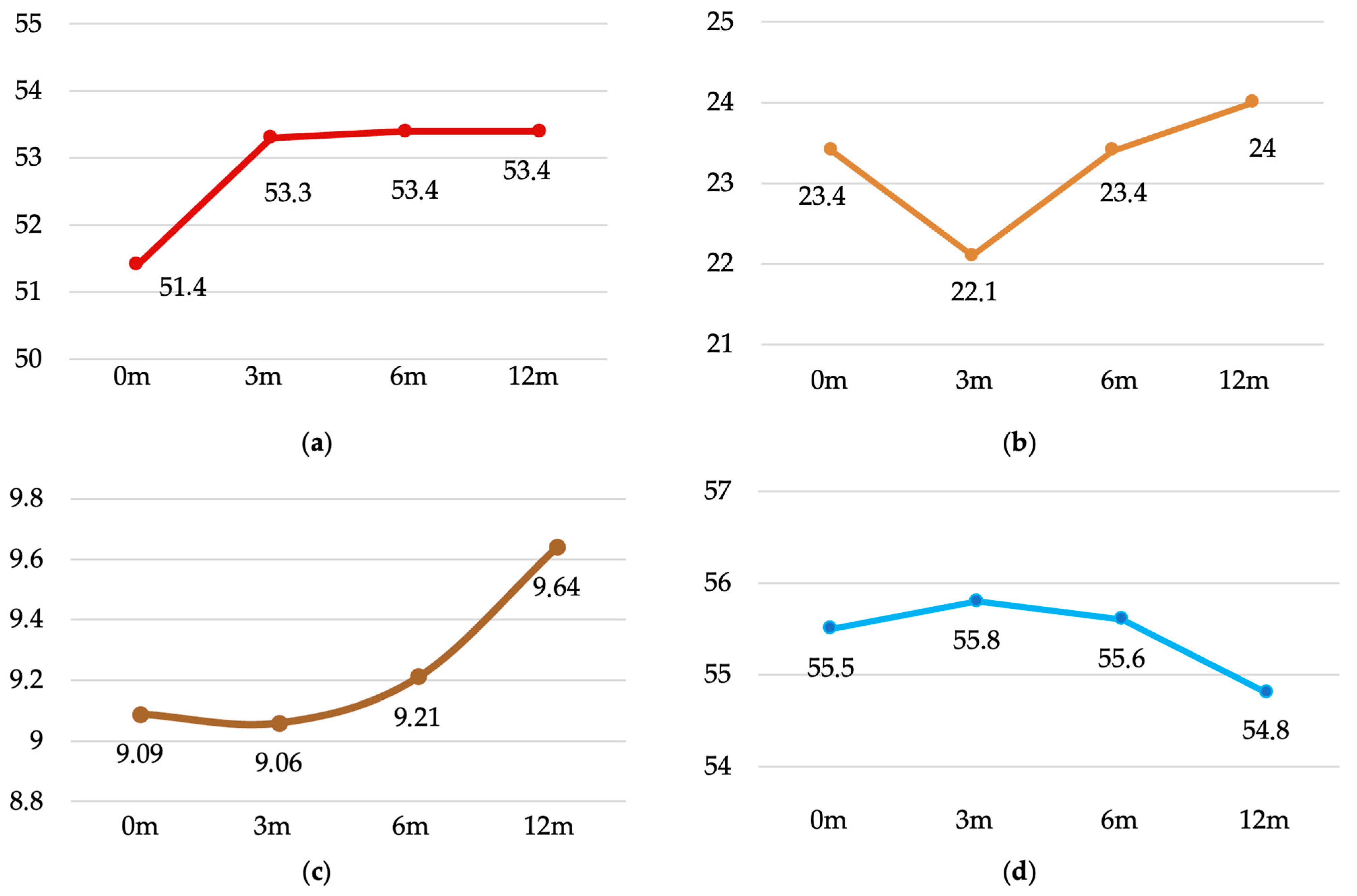
| Muscle Mass (kg) | 51.4 ± 10.7 | 53.3 ± 11.1 | 53.4 ± 11.1 | 53.4 ± 11.0 | <0.001 |
| (49.4–53.4) | (51.2–55.3) | (51.3–55.5) | (51.3–55.4) | ||
| Fat mass (%) | 23.4 ± 9.0 | 22.1 ± 8.8 | 23.4 ± 8.8 | 24.1 ± 9.0 | 0.004 |
| (21.7–25.1) | (20.5–23.8) | (21.8–25.1) | (22.4–25.8) | ||
| Visceral Fat (index) | 9.09 ± 4.77 | 9.06 ± 4.37 | 9.21 ± 4.51 | 9.64 ± 4.47 | 0.023 |
| (8.20–9.98) | (8.24–9.88) | (8.36–10.05) | (8.81–10.48) | ||
| Body water (%) | 55.5 ± 7.4 | 55.8 ± 7.3 | 55.6 ± 6.9 | 54.8 ± 6.6 | 0.190 |
| (54.1–56.8) | (54.4–57.2) | (54.3–56.9) | (53.6–56.0) |
| Time Periods | 0 Months | 3 Months | 6 Months | 12 Months | p-ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Weight | p | Weight | p | Weight | p | Weight | p | ||
| Sex | Men | 75.3 ± 13.8 (72.3–78.4) | <0.001 | 76.4 ± 13.8 (73.3–79.5) | <0.001 | 77.7 ± 13.9 (74.5–80.8) | <0.001 | 78.7 ± 13.8 (75.6–81.7) | <0.001 | <0.001 |
| Women | 61.6 ± 15.4 (56.1–67.1) | 63.2 ± 14.0 (58.2–68.1) | 64.1 ± 13.5 (59.3–68.9) | 64.7 ± 14.1 (59.7–69.7) | 0.002 | |||||
| Age | ≤65 y. | 71.7 ± 16.0 (68.1–75.3) | 0.726 | 72.8 ± 14.9 (69.4–76.1) | 0.954 | 74.0 ± 14.9 (70.7–77.4) | 0.688 | 75.3 ± 15.0 (71.9–78.6) | 0.403 | <0.001 |
| ≥65 y. | 70.3 ± 14.5 (65.1–75.4) | 71.9 ± 15.7 (66.4–77.5) | 72.7 ± 15.6 (67.2–78.3) | 72.8 ± 15.8 (67.2–78.4) | 0.066 | |||||
| KRT | Non- dialysis | 68.8 ± 15.9 (56.6–81.0) | 0.515 | 71.0 ± 16.4 (58.4–83.6) | 0.631 | 71.5 ± 16.2 (59.0–84.0) | 0.639 | 73.1 ± 16.3 (60.6–85.6) | 0.662 | 0.004 |
| PD | 75.0 ± 17.9 (68.3–81.7) | 75.3 ± 16.4 (69.2–81.5) | 76.4 ± 16.6 (70.2–82.6) | 77.1 ± 16.6 (70.9–83.3) | 0.082 | |||||
| HD | 70.1 ± 14.4 (66.7–73.5) | 71.6 ± 14.5 (68.2–74.9) | 72.8 ± 14.3 (69.5–76.1) | 73.7 ± 14.6 (70.3–77.0) | <0.001 | |||||
| Vascular Access | None | 73.3 ± 17.6 (67.6–78.9) | 0.074 | 73.9 ± 16.4 (68.6–79.2) | 0.172 | 74.8 ± 16.6 (69.5–80.2) | 0.250 | 75.7 ± 16.6 (70.3–81.1) | 0.380 | 0.003 |
| AVF | 72.6 ± 12.8 (68.9–76.2) | 73.8 ± 13.4 (70.0–77.6) | 74.7 ± 13.5 (70.9–78.6) | 75.5 ± 13.9 (71.6–79.5) | 0.006 | |||||
| Catheter | 65.2 ± 16.5 (58.1–72.3) | 67.4 ± 15.9 (60.5–74.3) | 69.3 ± 15.3 (62.7–75.9) | 70.4 ± 15.4 (63.7–77.0) | 0.003 | |||||
| BMI | Under weight | 46.6 ± 4.7 (40.7–52.4) | <0.001 | 49.5 ± 5.5 (42.6–56.3) | <0.001 | 50.6 ± 6.1 (43.0–58.1) | <0.001 | 50.4 ± 5.0 (44.1–56.6) | <0.001 | 0.009 |
| Normal weight | 63.9 ± 11.0 (60.8–66.9) | 65.4 ± 10.9 (62.4–68.4) | 66.6 ± 10.6 (63.7–69.5) | 67.5 ± 11.0 (64.5–70.6) | <0.001 | |||||
| Over weight | 76.9 ± 11.0 (73.2–80.5) | 78.2 ± 10.6 (74.7–81.7) | 79.3 ± 10.8 (75.8–82.9) | 80.5 ± 10.0 (77.2–83.8) | <0.001 | |||||
| Obesity | 90.5 ± 13.7 (83.2–97.8) | 89.8 ± 15.3 (81.6–98.0) | 90.7 ± 15.7 (82.3–99.0) | 91.1 ± 17.0 (82.0–100.1) | 0.759 | |||||
| Previous transplant | No | 71.9 ± 15.1 (68.9–74.9) | 0.361 | 72.9 ± 14.7 (70.0–75.9) | 0.540 | 73.8 ± 14.5 (70.9–76.7) | 0.835 | 74.5 ± 19.5 (62.7–86.3) | 0.892 | <0.001 |
| Yes | 66.7 ± 18.4 (55.5–77.8) | 69.5 ± 18.1 (58.6–80.5) | 72.9 ± 19.1 (61.3–84.4) | 75.3 ± 19.4 (62.9–87.6) | <0.001 | |||||
| Type donor | Living | 70.0 ± 21.0 (56.7–83.4) | 0.799 | 72.8 ± 20.4 (59.8–85.7) | 0.899 | 73.9 ± 19.6 (61.5–86.3) | 0.944 | 75.3 ± 19.4 (62.9–87.6) | 0.925 | <0.001 |
| Cadaver | 71.5 ± 14.9 (68.5–74.4) | 72.5 ± 14.5 (69.6–75.4) | 73.6 ± 14.5 (70.7–76.5) | 74.4 ± 14.7 (71.5–77.4) | 0.000 | |||||
| Time Periods | 0 Months | 3 Months | 6 Months | 12 Months | p-ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Weight | p | Weight | p | Weight | p | Weight | p | ||
| Sex | Men | 55.5 ± 9.1 (53.4–57.5) | <0.001 | 57.7 ± 9.5 (55.6–59.8) | <0.001 | 57.8 ± 9.4 (55.7–59.9) | <0.001 | 57.9 ± 8.9 (55.9–59.8) | <0.001 | <0.001 |
| Women | 41.6 ± 7.4 (39.0–44.3) | 42.7 ± 6.5 (40.4–45.0) | 42.9 ± 7.4 (40.3–45.5) | 42.7 ± 7.6 (40.0–45.4) | 0.052 | |||||
| Age | ≤65 y. | 51.7 ± 10.9 (49.2–54.1) | 0.695 | 53.2 ± 10.7 (50.8–55.6) | 1 | 53.2 ± 10.6 (50.8–55.5) | 0.962 | 53.6 ± 10.8 (51.1–56.0) | 0.803 | <0.001 |
| ≥65 y. | 50.7 ± 10.3 (47.1–54.4) | 53.5 ± 12.1 (49.2–57.8) | 54.1 ± 12.5 (49.6–58.5) | 53.0 ± 11.5 (48.9–57.1) | <0.001 | |||||
| KRT | Non- dialysis | 51.5 ± 12.8 (41.7–61.4) | 0.730 | 54.6 ± 11.6 (45.6–63.5) | 0.722 | 54.0 ± 12.3 (44.6–63.5) | 0.865 | 53.2 ± 11.6 (44.3–62.1) | 0.979 | 0.018 |
| PD | 53.4 ± 11.4 (49.2–57.7) | 54.4 ± 12.3 (49.8–59.0) | 54.0 ± 12.0 (49.6–58.5) | 53.9 ± 11.5 (49.6–58.2) | 0.753 | |||||
| HD | 50.5 ± 10.2 (48.2–52.9) | 52.6 ± 10.6 (50.1–55.1) | 53.1 ± 10.8 (50.6–55.6) | 53.2 ± 10.8 (50.7–55.7) | <0.001 | |||||
| Previous trasplant | No | 51.9 ± 10.6 (49.7–54.0) | 0.191 | 53.7 ± 11.2 (51.4–55.9) | 0.388 | 53.6 ± 11.0 (51.4–55.8) | 0.624 | 53.6 ± 10.9 (51.4–55.8) | 0.465 | <0.001 |
| Yes | 47.9 ± 11.2 (41.1–54.6) | 50.3 ± 10.1 (44.1–56.4) | 52.1 ± 12.6 (44.5–59.7) | 51.7 ± 11.9 (44.5–58.8) | 0.043 | |||||
| Type donor | Living | 50.9 ± 13.4 (42.3–59.4) | 0.731 | 52.9 ± 12.7 (44.8–60.9) | 0.836 | 53.1 ± 12.8 (44.9–61.3) | 0.977 | 52.3 ± 11.7 (44.9–59.8) | 0.550 | 0.108 |
| Cadaver | 51.5 ± 10.4 (49.4–53.5) | 53.3 ± 11.0 (51.1–55.5) | 53.5 ± 11.0 (51.3–55.7) | 53.5 ± 10.9 (51.3–55.7) | <0.001 | |||||
| Time Periods | 0 Months | 3 Months | 6 Months | 12 Months | p-ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Weight | p | Weight | p | Weight | p | Weight | p | ||
| Sex | Men | 21.9 ± 8.0 (20.1–23.7) | 0.018 | 20.1 ± 7.6 (18.4–21.8) | 0.001 | 21.4 ± 7.9 (19.6–23.2) | <0.001 | 21.9 ± 8.0 (20.1–23.7) | <0.001 | 0.014 |
| Women | 27.1 ± 10.1 (23.5–30.7) | 27.0 ± 9.5 (23.7–30.4) | 28.3 ± 8.8 (25.2–31.5) | 29.2 ± 9.1 (26.0–32.4) | 0.069 | |||||
| Age | ≤65 y. | 23.3 ± 9.6 (21.1–25.4) | 0.740 | 22.5 ± 9.2 (20.5–24.6) | 0.391 | 23.9 ± 8.8 (21.9–25.9) | 0.410 | 24.5 ± 9.6 (22.4–26.7) | 0.505 | 0.018 |
| ≥65 y. | 23.7 ± 7.3 (21.1–26.3) | 21.1 ± 7.7 (18.4–23.8) | 22.3 ± 8.6 (19.3–25.4) | 23.0 ± 7.2 (20.4–25.5) | 0.053 | |||||
| KRT | Non- dialysis | 20.9 ± 6.6 (15.9–26.0) | 0.543 | 18.3 ± 6.5 (13.3–23.3) | 0.216 | 20.1 ± 7.5 (14.4–25.9) | 0.251 | 22.9 ± 7.0 (17.5–28.3) | 0.447 | 0.050 |
| PD | 24.0 ± 9.3 (20.5–27.5) | 23.7 ± 9.1 (20.3–27.1) | 25.2 ± 8.5 (22.0–28.4) | 25.9 ± 9.8 (22.3–29.6) | 0.159 | |||||
| HD | 23.5 ± 9.1 (21.3–25.6) | 21.9 ± 8.8 (19.9–24.0) | 23.1 ± 9.0 (21.0–25.2) | 23.5 ± 8.8 (21.4–25.5) | 0.069 | |||||
| Previous trasplant | No | 23.5 ± 8.8 (21.7–25.2) | 0.737 | 22.1 ± 8.8 (20.3–23.8) | 1 | 23.4 ± 8.7 (21.7–25.1) | 0.978 | 23.9 ± 8.8 (22.1–25.6) | 0.650 | 0.010 |
| Yes | 22.8 ± 10.8 (16.2–29.3) | 22.6 ± 8.9 (17.2–28.0) | 23.7 ± 9.3 (18.1–29.4) | 25.6 ± 10.2 (19.4–31.8) | 0.180 | |||||
| Type donor | Living | 21.7 ± 9.1 (15.9–27.5) | 0.528 | 22.2 ± 8.7 (16.7–27.8) | 0.843 | 23.4 ± 9.6 (17.3–29.5) | 0.825 | 25.5 ± 10.0 (19.1–31.9) | 0.498 | 0.046 |
| Cadaver | 23.6 ± 9.0 (21.8–25.4) | 22.1 ± 8.8 (20.4–23.9) | 23.4 ± 8.7 (21.7–25.2) | 23.9 ± 8.9 (22.2–25.7) | 0.010 | |||||
| Time Periods | 0 Months | 3 Months | 6 Months | 12 Months | p-ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Weight | p | Weight | p | Weight | p | Weight | p | ||
| Sex | Men | 10.1 ± 4.8 (9.0–11.2) | 0.001 | 9.9 ± 4.4 (9.0–10.9) | 0.001 | 10.1 ± 4.7 (9.0–11.1) | 0.001 | 10.6 ± 4.5 (9.6–11.6) | 0.001 | 0.078 |
| Women | 6.7 ± 3.7 (5.4–8.1) | 6.9 ± 3.6 (5.7–8.2) | 7.2 ± 3.3 (6.0–8.3) | 7.3 ± 3.4 (6.1–8.5) | 0.121 | |||||
| Age | ≤65 y. | 8.0 ± 4.5 (7.0–9.0) | 0.001 | 8.0 ± 4.2 (7.1–9.0) | 0.001 | 8.2 ± 4.4 (7.3–9.2) | 0.001 | 8.7 ± 4.3 (7.7–9.7) | 0.001 | <0.001 |
| ≥65 y. | 11.8 ± 4.3 (10.3–13.3) | 11.5 ± 3.6 (10.3–12.8) | 11.5 ± 3.9 (10.1–12.9) | 11.9 ± 4.0 (10.5–13.3) | 0.751 | |||||
| KRT | Non- dialysis | 7.2 ± 4.1 (4.1–10.3) | 0.391 | 7.9 ± 4.5 (4.4–11.4) | 0.641 | 8.1 ± 4.7 (4.5–11.7) | 0.493 | 9.2 ± 5.0 (5.4–13.1) | 0.779 | 0.012 |
| PD | 9.7 ± 5.7 (7.5–11.8) | 9.4 ± 5.1 (7.5–11.3) | 10.1 ± 5.4 (8.1–12.1) | 10.1 ± 5.3 (8.1–12.1) | 0.323 | |||||
| HD | 9.1 ± 4.4 (8.1–10.1) | 9.1 ± 4.1 (8.1–10.0) | 9.0 ± 4.1 (8.0–9.9) | 9.5 ± 4.1 (8.6–10.5) | 0.160 | |||||
| Previous trasplant | No | 9.2 ± 4.7 (8.3–10.1) | 0.348 | 9.1 ± 4.3 (8.2–9.9) | 0.740 | 9.2 ± 4.5 (8.3–10.1) | 0.899 | 9.7 ± 4.5 (8.8–10.6) | 0.841 | 0.003 |
| Yes | 8.2 ± 5.3 (5.0–11.4) | 8.8 ± 5.1 (5.8–11.9) | 9.3 ± 5.2 (6.2–12.4) | 9.5 ± 4.2 (6.9–1.0) | 0.016 | |||||
| Type donor | Living | 7.3 ± 5.8 (3.6–10.9) | 0.134 | 8.1 ± 5.7 (4.5–11.7) | 0.465 | 8.3 ± 5.6 (4.7–11.8) | 0.534 | 8.4 ± 5.2 (5.1–11.7) | 0.423 | 0.065 |
| Cadaver | 9.3 ± 4.6 (8.4–10.2) | 9.2 ± 4.2 (8.3–10.0) | 9.3 ± 4.4 (8.5–10.2) | 9.8 ± 4.4 (8.9–10.7) | 0.001 | |||||
| Time Periods | 0 Months | 3 Months | 6 Months | 12 Months | p-ANOVA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Weight | p | Weight | p | Weight | p | Weight | p | ||
| Sex | Men | 56.4 ± 7.3 (54.8–58.0) | 0.067 | 57.6 ± 6.1 (56.3–59.0) | <0.001 | 57.1 ± 6.8 (55.6–58.6) | <0.001 | 56.3 ± 6.2 (54.9–57.7) | <0.001 | 0.013 |
| Women | 53.2 ± 7.4 (50.5–55.8) | 51.5 ± 8.3 (48.6–54.5) | 52.0 ± 6.0 (49.8–54.1) | 51.2 ± 6.1 (49.0–53.3) | 0.096 | |||||
| Age | ≤ 65 y. | 55.5 ± 7.9 (53.7–57.2) | 0.898 | 55.5 ± 7.3 (53.9–57.1) | 0.431 | 55.0 ± 6.9 (53.4–56.5) | 0.132 | 54.4 ± 7.0 (52.8–55.9) | 0.285 | 0.007 |
| ≥ 65 y. | 55.4 ± 6.2 (53.2–57.7) | 56.6 ± 7.5 (54.0–59.2) | 57.0 ± 6.8 (54.6–59.5) | 55.8 ± 5.5 (53.9–57.8) | 0.043 | |||||
| KRT | Non- dialysis | 56.7 ± 5.1 (52.8–60.7) | 0.472 | 58.6 ± 6.0 (54.0–63.2) | 0.240 | 57.0 ± 5.7 (52.6–61.4) | 0.200 | 54.9 ± 5.4 (50.8–59.0) | 0.445 | 0.087 |
| PD | 54.8 ± 8.0 (51.8–57.8) | 54.4 ± 8.6 (51.1–57.6) | 54.3 ± 8.1 (51.3–57.3) | 53.6 ± 7.4 (50.8–56.4) | 0.596 | |||||
| HD | 55.6 ± 7.5 (53.8–57.3) | 56.1 ± 6.9 (54–57.7) | 55.9 ± 6.6 (54.4–57.5) | 55.3 ± 6.4 (53.8–56.8) | 0.062 | |||||
| Previous trasplant | No | 55.3 ± 7.2 (53.9–56.7) | 0.507 | 55.9 ± 7.5 (54.4–57.4) | 0.867 | 55.6 ± 6.9 (54.3–57.0) | 0.989 | 54.9 ± 6.5 (53.6–56.2) | 0.696 | 0.013 |
| Yes | 56.6 ± 9.4 (51.0–62.3) | 55.5 ± 6.3 (51.6–59.3) | 55.3 ± 7.4 (50.8–59.7) | 54.1 ± 7.4 (49.6–58.6) | 0.571 | |||||
| Type donor | Living | 56.6 ± 7.4 (51.9–61.3) | 0.410 | 56.1 ± 7.4 (51.4–60.8) | 0.840 | 55.1 ± 7.2 (50.5–59.7) | 0.724 | 53.8 ± 7.4 (49.1–58.5) | 0.475 | 0.182 |
| Cadaver | 55.3 ± 7.5 (53.8–56.8) | 55.8 ± 7.4 (54.3–57.2) | 55.7 ± 6.9 (54.3–57.0) | 54.9 ± 6.5 (53.6–56.2) | 0.013 | |||||
| Comorbidity | n (%) | Mean ± SD | CI 95% | p | |
|---|---|---|---|---|---|
| Hypertension | No | 11 (9.8) | 2.11 ± 5.70 | (−1.72–5.94) | 0.564 |
| Yes | 101 (90.2) | 3.36 ± 6.90 | (2.00–4.72) | ||
| Dyslipidemia | No | 53 (43.3) | 4.07 ± 7.29 | (2.06–6.08) | 0.218 |
| Yes | 59 (52.7) | 2.48 ± 6.25 | (0.85–4.11) | ||
| Previous diabetes | No | 90 (80.4) | 3.35 ± 6.66 | (1.95–4.74) | 0.730 |
| Yes | 22 (19.6) | 2.79 ± 7.39 | (−0.49–6.06) | ||
| Ischemic heart disease | No | 83 (74.1) | 3.16 ± 6.83 | (1.67–4.65) | 0.843 |
| Yes | 29 (25.9) | 3.45 ± 6.76 | (1.67–4.65) | ||
| Respiratory disease | No | 97 (86.6) | 3.28 ± 6.73 | (1.92–4.64) | 0.860 |
| Yes | 15 (13.4) | 2.95 ± 7.34 | (−1.12–7.01) | ||
| Cerebrovascular accident | No | 107 (95.5) | 3.25 ± 6.92 | (1.92–4.58) | 0.916 |
| Yes | 5 (4.5) | 2.92 ± 2.84 | (−0.60–6.44) | ||
| NODAT 1 | No | 90 (80.4) | 4.01 ± 6.54 | (2.65–5.38) | 0.013 |
| Yes | 22 (19.6) | 0.05 ± 6.97 | (−3.04–3.14) | ||
| Toxic habits | No | 92 (82.1) | 2.48 ± 6.47 | (1.14–3.82) | 0.011 |
| Yes | 20 (17.9) | 6.70 ± 7.27 | (3.30–10.10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer-López, E.; López-Blasco, R.; Rubio-Castañeda, F.J.; Cantín-Lahoz, V.; Aguilón-Leiva, J.J.; García-Magán, M.; Navas-Ferrer, C.; Blázquez-Ornat, I.; Fernández-Rodrigo, M.T.; Antón-Solanas, I.; et al. Changes in Body Composition Compartments After Kidney Transplantation: A One-Year Prospective Study. J. Clin. Med. 2025, 14, 7131. https://doi.org/10.3390/jcm14207131
Ferrer-López E, López-Blasco R, Rubio-Castañeda FJ, Cantín-Lahoz V, Aguilón-Leiva JJ, García-Magán M, Navas-Ferrer C, Blázquez-Ornat I, Fernández-Rodrigo MT, Antón-Solanas I, et al. Changes in Body Composition Compartments After Kidney Transplantation: A One-Year Prospective Study. Journal of Clinical Medicine. 2025; 14(20):7131. https://doi.org/10.3390/jcm14207131
Chicago/Turabian StyleFerrer-López, Emilia, Raúl López-Blasco, Francisco Javier Rubio-Castañeda, Víctor Cantín-Lahoz, Juan José Aguilón-Leiva, María García-Magán, Carlos Navas-Ferrer, Isabel Blázquez-Ornat, María Teresa Fernández-Rodrigo, Isabel Antón-Solanas, and et al. 2025. "Changes in Body Composition Compartments After Kidney Transplantation: A One-Year Prospective Study" Journal of Clinical Medicine 14, no. 20: 7131. https://doi.org/10.3390/jcm14207131
APA StyleFerrer-López, E., López-Blasco, R., Rubio-Castañeda, F. J., Cantín-Lahoz, V., Aguilón-Leiva, J. J., García-Magán, M., Navas-Ferrer, C., Blázquez-Ornat, I., Fernández-Rodrigo, M. T., Antón-Solanas, I., & Urcola-Pardo, F. (2025). Changes in Body Composition Compartments After Kidney Transplantation: A One-Year Prospective Study. Journal of Clinical Medicine, 14(20), 7131. https://doi.org/10.3390/jcm14207131






