Abstract
Background/Objectives: Some patients undergoing vitreoretinal surgery (VRS) require general anesthesia (GA), despite the possibility of developing intolerable postoperative pain perception (IPPP). Intraoperative rescue opioid analgesia (IROA) administration during GA poses a risk of perioperative nausea and vomiting (PONV), which may result in suprachoroidal hemorrhage with permanent visual impairment. Adequacy of Anesthesia (AoA) optimizes intraoperative IROA titration. Intravenous preemptive analgesia (IPA) with cyclooxygenase-3 (COX-3) inhibitors is added to GA to reduce the IROA dose. In this additional analysis, we assessed the impact of preemptive analgesia with COX-3 inhibitors, administered alongside GA with AoA-guided IROA, on the incidence of PONV, oculocardiac reflex (OCR), and oculoemetic reflex (OER) in patients undergoing VRS as secondary outcomes. Methods: A total of 165 patients scheduled for VRS were randomly assigned to receive AoA-guided GA combined with IPA at a single dose of 1 g of paracetamol (acetaminophen) or 2.5 g of metamizole or both. A total of nine patients were excluded due to technical problems with the intraoperative surgical pleth index (SPI) measurement, inability to report postoperative pain, and postoperative arousal resulting in a loss of follow-up in Stage 5. Results: Regardless of the group assignment, AoA guidance of GA resulted in PONV in 4%, OCR in 10%, and OER in 0% of the 153 analyzed patients undergoing VRS. No significant differences were observed between the groups regarding the type of IPA. PONV was observed in 2.11% (3/142) of patients with zero, one, or two risk factors of PONV, as compared to 27% (3/11) of patients with at least three PONV risk factors, assessed using the Apfel score. Conclusions: IPA with both paracetamol and metamizole did not demonstrate a benefit in reducing the analyzed adverse events compared with their single use in patients undergoing VRS under AoA guidance during GA. Surprisingly, PONV was hardly observed in patients with zero, one, or two PONV risk factors assessed by the Apfel score who underwent AoA-guided VRS during GA with IPA using one or two COX-3 inhibitors.
1. Introduction
Postoperative nausea and vomiting (PONV) constitutes one of the most serious complications following vitreoretinal surgery (VRS) and may lead to suprachoroidal hemorrhage, regardless of the use of regional anesthesia (RA) with monitored anesthesia care (MAC) [1] or general anesthesia (GA) due to contraindications for RA alone [2,3]. Rupture of the posterior ciliary arteries or vortex veins after PONV can result in suprachoroidal hemorrhage, leading to permanent visual impairment, with an incidence of up to 1% after VRS [4].
Strategies to reduce the adverse events of PONV include pharmacological approaches, such as antiemetic prophylaxis [5], opioid-free anesthesia [6], premedication with benzodiazepines [7,8] or anxiolytics [9], and the use of a muscle relaxant antagonist for smooth recovery from GA [10], as well as non-pharmacological interventions, including aromatherapy [11], chewing gum [12], cheek acupuncture [13], or the intraoperative use of noise-canceling headphones [14]. RA has been shown to reduce the cumulative dose of intravenous rescue opioid analgesia (IROA), an independent risk factor for PONV after VRS [15]. The employment of different preoperative RA techniques or intravenous preventive analgesia (IPA) added to GA in patients undergoing VRS decreases IROA requirements [16,17,18], enhances the effectiveness of postoperative analgesia [19], and reduces the incidence of PONV and oculocardiac reflex (OCR) [16].
Surgical maneuvers under GA induce painful afferent stimulation (nociception). Insufficient intraoperative analgesia (anti-nociception) leads to the release of stress hormones, which increases heart rate and arterial blood pressure, while IROA administration attenuates these effects. Underdosing IROA during GA may evoke intolerable postoperative pain perception (IPPP) [20] due to central sensitization [21], whereas overdosing may result in hemodynamic instability, including bradycardia and hypotension. Volatile anesthetics can impair hemodynamic responses to painful stimuli, particularly in patients with diabetic and elderly patients [22], often requiring VRS under GA. Consequently, the use of digital devices for the intraoperative monitoring of nociception/anti-nociception balance is steadily increasing [23].
Adequacy of Anesthesia (AoA) guidance for GA facilitates intraoperative monitoring [24]. The surgical pleth index (SPI; 0–100) reflects nociceptive stimulation and recovery after affective anti-nociception. Response entropy (RE) and state entropy (SE), ranging from 45 to 60, indicate the depth of surgical anesthesia. The SPI signal is derived from finger photoplethysmography, while SE and RE signals are derived from a forehead sensor, eliminating time-consuming preparation prior to GA induction [25]. The SPI correlates with IROA serum concentrations [26] and allows a more reliable titration than hemodynamic monitoring alone [27,28,29], reducing cumulative IROA during GA [30] and the IPPP incidence assessed by the numeric pain rating scale (NPRS) [31]. The SPI guidance in terms of the nociception/anti-nociception balance, together with the depth of anesthesia monitored with the bispectral index, has been reported to halve the PONV incidence after thoracoscopic lung resection, albeit with a longer stay in the postoperative care unit (PACU) [32].
In our previous study, preventive analgesia did not affect the overall IPPP incidence in patients undergoing VRS when IROA was titrated under the SPI guidance, suggesting that AoA guidance attenuates the effect of preventive analgesia [33]. However, we also observed reduced PONV incidence in patients receiving IPA compared with preventive RA, topical anesthesia, and the control group, with decreased IROA requirements in patients receiving paracetamol and peribulbar block compared with metamizole, topical anesthesia, and the control group [34].
To date, no study has investigated the effect of single-dose IPA with paracetamol (1 g), metamizole (2.5 g), or their combination on the adverse event incidence in patients undergoing VRS under AoA guidance during GA, in line with the recent guidelines for postoperative pain management after head and neck surgery [35].
A previous analysis of primary outcomes showed a reduced IPPP incidence without affecting hemodynamic stability in patients receiving both cyclooxygenase-3 (COX-3) inhibitors compared to metamizole alone, and lower IROA requirements in the paracetamol group compared to the metamizole group [36]. Based on these findings and the current literature [37], we analyzed secondary outcomes to evaluate the effect of combined or single IPA on OCR, PONV, and oculoemetic reflex (OER) in patients undergoing VRS under AoA guidance.
2. Materials and Methods
Patients scheduled for VRS at the Department of Ophthalmology (DO) of the 5th Regional Hospital in Sosnowiec, who met the inclusion criteria, were invited to participate in this study. After obtaining written informed consent, 165 patients classified as ASA I-III according to the American Society of Anaesthesiologists were enrolled. Patients were then randomly assigned to groups using the sealed envelopes method.
In compliance with the Declaration of Helsinki, the Bioethical Committee of Medical University of Silesia (Chairman Ph. Dr. B. Okopień) issued approval for this study (KNW/0022/KB1/122/17; 5 December 2017). This study was recorded in the Clinical Trial Registry (Silesian MUKOAiIT7, NCT03389243; 27 December 2017), and data were collected from 15 January 2018 to 16 December 2022.
Exclusion criteria included the following: acute or chronic pain, drug or alcohol abuse, pregnancy, history of pulmonary disease, neurological disorders or prior neurosurgical procedures that could interfere with EEG entropy monitoring, allergy or abuse of metamizole or paracetamol, liver dysfunction, predictors of difficult laryngeal mask placement, and cardiac arrhythmia on ECG that could complicate the SPI monitoring.
Patients were randomly assigned to three groups. In the P group, AoA-guided GA combined with single-dose IPA of 1 g of paracetamol in 100 mL saline (Paracetamol Kabi 10 mg/mL; Fresenius Kabi, Błonie, Poland) was administered 30 min before arrival in the operating room. In the M group, AoA-guided GA combined with a single-dose IPA of 2.5 g of metamizole in 100 mL saline (Pyralgin 0.5 g/mL; Polpharma S.A., Gdansk, Poland) was administered 30 min before arrival in the operating room. In the PM group, patients received AoA-guided GA combined with both IPA interventions as described for the P and M groups, in accordance with recent postoperative pain management guidelines [35].
During the preoperative visit, patients were educated on postoperative pain and trained to report pain using the NPRS (zero corresponded to no pain and 10 to the worst pain they could imagine).
All patients fasted for at least 12 h. On the day of surgery, patients received age- and weight-adjusted dose of midazolam (3.75–7.5 mg/kg; Dormicum, Roche, Warsaw, Poland) prior to the induction of anesthesia [38]. Patients were preoxygenated with 100% oxygen for 5 min and received intravenous Ringer’s Solution at 10 mL/kg. GA was induced intravenously with fentanyl (FNT) at 1 mcg/kg and a single dose of 2 mg/kg of propofol (Etomidate Lipuro, Braun, Germany). After loss of consciousness, 0.6 mg/kg of rocuronium (Esmeron, Fresenius, Błonie, Poland) was administered intravenously to paralyze patients, and after 45 s, a laryngeal mask was placed. CO2 was maintained at 35–37 mmHg. After laryngeal mask installation, before VRS started, the sevoflurane concentration was maintained at approximately 40–45 SE. Standard monitoring included the monitoring of non-invasive blood pressure (NIBP), heart rate (HR), standard electrocardiography (ECG) II, arterial oxygen saturation (SaO2), fraction of inspired oxygen (FiO2), end-tidal carbon dioxide (etCO2), minimum alveolar concentration of sevoflurane, and fraction of inspired and expired sevoflurane (FiAA and FeAA).
Entropy EEG (SE, RE) was used to monitor anesthesia depth, SPI guided the management of intraoperative analgesia, and muscle relaxation was ensured via neuromuscular transmission monitoring (Carescape B650, GE Healthcare, Helsinki, Finland).
2.1. Stage 1
On arrival in the operating room, patients were fitted with EEG entropy sensors (SE, RE) on their forehead, the SPI sensors on their finger opposite to the venous access, and an NIBP cuff on their right arm, while standard ECG electrodes were placed on the patients’ backs. First values were then recorded.
2.2. Stage 2
The mean SPI value was calculated from 5 min following laryngeal mask placement up to the start of orbital sterilization, allowing for sensor calibration.
2.3. Stage 3—Intraoperative Analgesia
The SPI was recorded every minute. A rescue dose of 1 mcg/kg of FNT was administered intravenously every 5 min when ∆SPI exceeded 15 points above the Stage 2 baseline until the SPI returned to the baseline. VRS duration was measured from the installation of the speculum to its removal.
We assumed that an initial FNT dose of 1 mcg/kg would provide sufficient analgesia before speculum installation. Furthermore, Gruenewald et al. [23] suggested that insufficient analgesia could be predicted by either a ∆SPI greater than 10 or an absolute SPI value above 50. In some studies, only an absolute ∆SPI value > 50 has been used as an indication for rescue analgesia [39]. To account for SPI variability and avoid a potentially dangerous overdose of FNT, a ∆SPI > 15 compared with the Stage 2 baseline, maintained for a minimum of one minute, served as an indicator of rescue analgesia. All surgeries were conducted by the ophthalmic surgeon (A. L-B) with >10 years of experience and >400 procedures annually.
The incidence of intraoperative OCR, which typically occurs when HR decreases by 20% during ocular manipulation, was recorded. When OCR was observed, stimulation in the surgical field was temporarily halted, and a single intravenous dose of 0.5 mg of atropine was administered if HR did not normalize. Persisting hypotension was managed with 5 mL/kg of crystalloid. A single dose of 5 mL of ephedrine (Ephedrinum hydrochloricum WZF 25 mg/mL, Polfa Warszawa S.A., Warsaw, Poland) was administered if the infusion did not raise the mean arterial pressure (MAP) above 65 mmHg.
2.4. Stage 4—Emergence from GA
During recovery from GA, the anesthesia team continued to monitor patients for their SPI, HR, SAP, MAP, DAP, and SaO2.
2.5. Stage 5—Postoperative Monitoring
In the PACU, patients were further monitored (SPI, HR, SAP, MAP, DAP, SaO2) by the anesthesiology team, blinded to the patient group assignment. In addition to postoperative hemodynamic parameters, each patient was monitored for adverse effects such as nausea, vomiting (PONV), allergic reactions, and sedation level, along with pain assessment for 24 h. For the management of PONV, patients received 4 mg of ondansetron (Ondansetron Accord, Accord Healthcare Limited, Harrow, UK) intravenously. If ondansetron was ineffective, a single dose of 4 mg of dexamethasone (Dexaven, Jelfa, Jelenia Góra, Poland) was administered intravenously. A 5 mL/kg quantity of Optilyte solution was infused when the MAP was <65 mmHg. Patients were provided with oxygen at 3 L/min through a nasal cannula. Patients were queried about their perception of pain intensity using the NPRS every 10 min. For an NPRS of >3, a standard dose of non-steroidal anti-inflammatory drugs (NSAIDs), such as paracetamol or metamizole, was provided, according to the current guidelines for acute pain management issued by the Polish Society of Anaesthesiologists [20]. The SPI was recorded every minute. The NPRS and SPI were recorded for mild pain (NPRS 0–3), average pain (NPRS 4–6), and acute pain (NPRS 7–10). Patients were monitored in the PACU for at least 30 min until transfer to the DO. Monitoring and data recording were discontinued, except for the PONV incidence, which was recorded until discharge from the DO.
The Apfel scores were calculated preoperatively to ensure group homogeneity. PONV risk factors include female gender, history of motion sickness or PONV, non-smoking status, and postoperative opioid administration. The estimated PONV incidence was 10%, 21%, 39%, 61%, and 79% corresponding to the presence of zero, one, two, three, or four risk factors, respectively [40,41]. PONV was categorized as early when it occurred in the PACU and late when it occurred in the DO. Overall PONV was defined as the presence of either early or late PONV, or both.
2.6. Statistical Analysis
Statistical analyses were conducted with STATISTICA 13.3 (StatSoft, Kraków, Poland) and R (ver. 4.4.0) [42]. Continuous data were presented as mean with standard deviation (X ± SD) and median (Me) with interquartile range (IQR). First, normality was determined with the Shapiro–Wilk test. The Kruskal–Wallis test, followed by Dunn’s post hoc test, was then employed to compare continuous variables across groups. Nominal variables were reported as percentages and compared between groups using the Chi-square test, or Fisher’s exact test in cases where any expected frequency was under 5. Bonferroni correction was applied for multiple testing. To account for potential imbalances between groups in gender, the BMI, and the Apfel score, multivariable logistic regression was conducted. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for each predictor. Post hoc pairwise comparisons between all three treatment groups were performed using estimated marginal means with Tukey adjustment for multiple comparisons. Statistical significance was set at p < 0.05.
G*Power (ver. 3.1.9.7; Heinrich Heine University, Düsseldorf, Germany) was used to calculate group size. One-way ANOVA (f = 0.25, α = 0.05, power = 0.8) for 3 groups determined the total sample size of 159. As data from 153 patients was analyzed, a post hoc test showed a power of 0.79.
3. Results
A total of nine patients were excluded from this study due to technical problems with the intraoperative SPI measurement, inability to report postoperative pain, and postoperative arousal resulting in loss of follow-up in Stage 5. The final analysis included 153 patients (Figure 1).
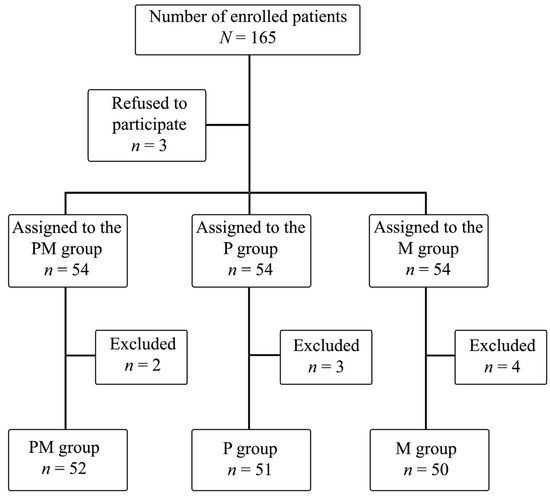
Figure 1.
Randomization scheme for study participants.
Significant differences between groups were observed in height, weight, the BMI, and HA. The PM group had a significantly higher proportion of men compared to the P group (Table 1).

Table 1.
Patient anthropometric characteristics.
Intraoperative FNT consumption was significantly higher in the M group than in the P group (Table 2).

Table 2.
Characteristics of the performed treatments.
Although the PM group had a significantly higher Apfel score (%) than the P group, no significant differences were found in their patients’ medical histories (Table 3).

Table 3.
Distribution of Apfel scores and patient case histories across study groups.
Postoperative pain intensity, assessed using the NPRS, did not differ significantly between groups. Mild pain was more frequently reported in the PM group compared to the M group, while acute pain was observed only in the PM and P groups (Table 4).

Table 4.
Postoperative pain occurrence in the study groups.
The IPPP incidence was higher in the M group compared to the PM group. The incidence of PONV and OCR was not affected by the use of various preemptive analgesia methods (Table 5).

Table 5.
Incidence of adverse events after vitrectomy/phacovitrectomy in patients depending on their group allocation.
In addition, multivariable logistic regression analyses with Tukey-adjusted estimated marginal means were performed to evaluate the incidence of overall PONV and OCR while adjusting for potential confounders, including gender, BMI, and the Apfel score (%), accounting for observed group imbalances. For PONV, no statistically significant differences were observed between any groups (M vs. MP OR = 0.17, 95% CI: 0.009–3.21, p = 0.33; M vs. P OR = 0.86, 95% CI: 0.03–24.68, p = 0.99; MP vs. P OR = 5.08, 95% CI: 0.27–95.93, p = 0.40). Similarly, for OCR, no significant differences were observed: M vs. MP OR = 1.75 (95% CI: 0.29–10.41, p = 0.74), M vs. P OR = 1.19 (95% CI: 0.28–5.19, p = 0.96), MP vs. P OR = 0.68 (95% CI: 0.11–4.10, p = 0.87). The Apfel score, the BMI, and gender did not significantly influence the incidence of PONV or OCR. The wide confidence intervals, particularly for PONV, reflect the low number of events and increased uncertainty around the estimated odds ratios.
Further analysis examined the correlation between the overall PONV incidence and the number of PONV risk factors according to the Apfel score. Significantly more patients were at risk due to the presence of one or two separate risk factors than zero or three risk factors. Despite this, no statistically significant difference was observed in the overall PONV incidence based on the number of V risk factors. There were no patients with an Apfel score of 4, as opioid analgesics were not required to alleviate postoperative pain (Table 6).

Table 6.
Apfel score of patients depending on the presence or absence of PONV in the postoperative period.
4. Discussion
The growing prevalence of diabetic and geriatric populations has increased the requirements for VRS, while comorbidities, such as diabetes, severe hepatic insufficiency, the chronic dysfunction of the respiratory, hepatic, renal, or other major organs, antiplatelet therapy, and the risk of globe perforation due to impaired patient cooperation during prolonged procedures caused by psychotic illness, depression, chronic pain, or other disabilities, may potentially preclude VRS under RA alone with MAC [1]. Consequently, the necessity for GA regimens involving IROA encouraged the exploration of IPAs, which have been reported to reduce IROA requirements and, in turn, decrease the incidence of perioperative adverse events such as PONV, OCR, IPPP, similar to RA techniques used adjunctively with GA for preventive analgesia [36].
Paracetamol (acetaminophen) and metamizole are among the most widely used non-opioid analgesics, exerting both central (COX-3 inhibition) and peripheral effects [43,44,45,46,47]. Paracetamol has been reported to act via cannabinoid receptor CB1, activating internal cannabinoids [48], potentially contributing to the sparing effects on IROA [49]. Metamizole acts through the PI3Kγ/AKT signaling cascade, a well-established molecular mechanism promoting nitric oxide production in sensory neurons [50]. Their analgesic efficacy in managing IPPP, even when administered as a single IPA dose, has been widely reported [51,52,53,54,55,56]. These agents also reduce the PONV incidence [52,54,57], and their relatively good safety profile is consistently emphasized [53,55,56,58]. Therefore, their use is preferred over standard NSAIDs, such as ketorolac, particularly in patients with upper gastrointestinal and kidney diseases that increase the risk of NSAID-related complications [53,59,60].
In this study, despite the group allocation, an overall PONV rate of 4% (in 6 out of 153 patients) was observed, which is markedly lower than that reported in the current literature. In the study by Eberhart et al. [61], a standardized GA was performed, including premedication with benzodiazepine, propofol, atracurium, or vecuronium for GA induction and desflurane in N(2)O/O(2) with a continuous remifentanil infusion for GA in patients undergoing VRS (standard three-port pars plana vitrectomy for proliferative diabetic vitreoretinopathy, complicated retinal detachment, or macular disease). The reported PONV incidence was 56% (placebo), 40% (dolasetron), 28% (droperidol), and 18% (dolasetron and droperidol).
Similarly, low PONV rates after VRS, comparable to our findings, were reported by Reibaldi et al. [1] in awake patients receiving both ondansetron and dexamethasone for PONV prophylaxis prior to VRS under peribulbar block using ropivacaine with MAC and without IROA. Double prophylaxis resulted in no PONV in 95.96% of the patients, whereas dexamethasone alone prevented PONV in 80.79%, ondansetron alone in 80.38%, and placebo in 71.96% of the patients. Notably, 186 of 1937 patients (about 9.6%) were excluded due to diabetes, whereas patients with diabetes in whom fasting-related nausea is a recognized effect of diabetic gastroparesis [62] were included in this study. Our previous work also identified diabetes as an independent PONV risk factor in patients undergoing AoA-guided GA for VRS [63]. Consequently, AoA-guided GA with IPA using COX-3 inhibitors may represent the anesthetic regimen of choice for patients with diabetes excluded from VRS under RA with MAC.
Similarly to diabetes, underhydration with preoperative morning fasting have been reported to increase the PONV incidence via enhanced serotonin secretion [64]. Thus, intraoperative intravenous crystalloid administration may serve as an important preventive measure against PONV, representing a low-cost, non-pharmacological option to reduce its incidence, free from the side effects of pharmacological therapy [40]. In this study, no statistically significant differences in intraoperative fluid requirements were found between groups. We hypothesize that AoA guidance resulted in an indirectly comparable demand for intraoperative fluid administration due to a directly comparable requirement for IROA, as well as for intravenous and volatile anesthetics for the co-induction of GA; all of these factors proportionally impaired the systemic vascular resistance, creating a demand for intravenous crystalloids, while avoiding overhydration, which is a separate PONV risk factor. Overall, balanced mesenteric perfusion, which prevents gut ischemia or edema due to overhydration, may further reduce serotonin-mediated PONV [64].
Numerous anesthetic regimens have long been employed to reduce the need for IROA and, consequently, the PONV incidence, including the use of IPA to decrease IPPP and, thus, possibly reduce PONV. In cases where GA is required for VRS, and concomitant RA contraindications have a common limiting effect on the IROA dosage [16], an IPA regimen with the potential to achieve a similar effect is sought as a reasonable alternative to antiemetics. Both metamizole [52] and paracetamol [54] have demonstrated the aforementioned antiemetic potency.
In this study, PONV occurred in one patient assigned to the P group (2%), two patients assigned to the M group (4%), and three patients assigned to the MP group (6%), with no significant differences between the groups. This contrasts with the observation of a previous project, which formed the basis of this study, where IPA using either of the two COX-3 inhibitors, metamizole and paracetamol, reduced the PONV incidence compared to the regional techniques and control group, despite no preventive analgesic regimen reducing the IPPP incidence [34].
In one of the first studies employing AoA guidance, Bergmann et al. [30] observed a reduction in the cumulative IROA requirements during the SPI-guided GA. Consequently, our initial assumption that the combined limiting effect of IPA and AoA guidance on the IROA requirement would synergistically reduce both the IROA demand and PONV incidence was not confirmed. To sum up, IPA using either a paracetamol/metamizole combination or metamizole alone reduced the IPPP incidence but did not reduce PONV to a degree lower than that achieved with COX-3 inhibitors alone in the previous project under AoA guidance.
Mandelcorn et al. [15] observed postoperative nausea occurring almost three times more frequently in patients undergoing VRS under RA with MAC. Their patients required IROA due to incomplete RA block compared with those who did not need it due to a complete block. The authors determined that the use of IROA was the only variable associated with postoperative nausea. In this study, IPA with metamizole or paracetamol or their combination as an anesthetic modality led to a statistically significant difference in the cumulative IROA dose between the M and P groups, although it did not result in a proportional reduction in the PONV rate in the M group. Similar observations were made by Ohnesorge et al. [65] in their study on breast tumor surgery and by Sener et al. [66] in their study on tonsillectomy in children.
Regardless of these findings, the PONV incidence has been shown to be influenced by a number of other factors, including type of surgery, gender, smoking status, motion sickness, body weight, and history of PONV [40]. Its estimated incidence is approximately 30%, with nausea occurring in approximately 50% of patients, and in high-risk patients, the incidence of PONV may reach up to 80% [41,67,68,69]. Nitahara et al. [70] reported one or more episodes of PONV in 24% of adult patients undergoing VRS. Female gender, a lower BMI, and GA with inhalational anesthetics, also used in this study, were significantly associated with nausea within the first 2 h after VRS, whereas smoking did not influence the risk of PONV. Intravenous opioid analgesia, especially continuous oxycodone infusion, may elevate nausea incidence to 94% and vomiting to 26% [71]. Therefore, any IPA strategy aiming to reduce or avoid IROA is valuable.
According to the latest guidelines, standard prophylaxis with two antiemetics, such as dexamethasone in combination with a 5-HT3 receptor antagonist, should be the basis for treating PONV in every adult patient. In high-risk patients, the standard prophylaxis regimen should include a third and potentially a fourth antiemetic with a different mechanism of action [68]. The recent consensus guidelines advocate for assessing risk factors such as female gender, prior PONV or motion sickness, non-smoking status, postoperative opioid administration, younger age, surgery type, prolonged anesthesia, and volatile anesthetics, as well as reducing the patient’s baseline risk (e.g., via RA or employing non-opioid analgesics as a component of a multimodal strategy).
In this study, patients were assessed for the risk of PONV using the most popular method, i.e., the Apfel score. Among patients with zero or one PONV risk factor (68 patients), representing 44% of the analyzed patients (21% risk of PONV), no cases of PONV were observed. In patients with two risk factors (39% risk of PONV), PONV was observed in 3 of 74 patients (4.05%), whereas in patients with three risk factors (61% risk of PONV), it was observed in 3 of 11 patients (27%). On the one hand, liberal PONV prophylaxis is currently recommended in adult patients and children, which should be used even when risk evaluation has not been performed [5]. On the other hand, in this study, among 142 patients (with zero, one, or two risk factors for PONV), the occurrence of PONV was observed only three times (2.11%), i.e., two and a half times lower than that in the study performed by Reibaldi et al., who observed a 5% incidence of PONV in patients receiving VRS under RA and MAC with antiemetic prophylaxis using dexamethasone and ondansetron [1]. Therefore, we hypothesize that the anesthetic regimen based on AoA guidance of GA with IPA using COX-3 inhibitors almost eradicated PONV in low-risk patients. This raises the question of whether following the current guidelines in patients with co-morbidities undergoing VRS would have brought more benefits than harm to these 142 patients in view of possible complications associated with the administration of drugs used for PONV prophylaxis, such as the exemplary QT interval prolongation in healthy individuals, evident even at low ondansetron dosages [72]. A 27% PONV incidence after VRS under AoA-guided GA with IPA using COX-3 inhibitors in patients with at least three risk factors, with a basic 61% risk of PONV, is in concordance with a similar observation made by Feng CD et al., who reported that the SPI guidance for opioid-free anesthesia halved the PONV incidence in patients undergoing thoracoscopic lung resection when compared to opioid-based anesthesia with antiemetic prophylaxis using dexamethasone and ondansetron, despite the group allocation [32]. Further studies in this area are needed on larger groups, including those analyzing a higher percentage of high-risk patients.
In this study, it is hypothesized that the precision of IROA administration using the SPI-guided FNT, along with a stable depth of GA under SE control as AoA guidance, could likely personalize anesthesia for each patient undergoing VRS, in terms of the optimal IROA demand reduced by IPA with intrinsic antiemetic potency and the optimal fluid challenge pertaining to the appropriate depth of volatile anesthesia, an overdose of which is an independent risk factor for PONV.
OCR is a trigeminovagal reflex that is triggered intraoperatively by applying pressure to the eyeball and/or traction of extraocular muscles. Clinically, OCR is defined and, thus, observed as a rapid decrease in heart rate of more than 20% [73]. Its occurrence is a significant intraoperative adverse event, as it can inevitably lead to even serious cardiac arrhythmias, resulting in hemodynamic instability (bradycardia, ectopic beats, nodal rhythm, ventricular fibrillation, or asystole), thus significantly influencing further intraoperative management. According to the current literature, the incidence of OCR is estimated to be 7–70%, depending on the type of ophthalmic surgery and the anesthetic regimen [16,74]. Ghali et al. reported an OCR incidence rate of 27% in patients receiving GA for VRS [16]. In turn, Abdeldayem et al. [75] achieved a 50% decrease in the incidence of OCR by adding sub-Tenon’s block using levobupivacaine for GA in patients undergoing retinal surgery, compared with GA alone. In the current study, OCR was observed in 10% of cases despite group assignment, demonstrating no statistically significant differences between groups. Therefore, the type of IPA has little impact on the incidence of OCR, as observed in our previous project, on which this study was based, and the incidence of OCR was 12% regardless of the group assignment.
In our opinion, the perioperative effects observed in our study can probably also be explained by the employment of SPI-guided IROA titration during AoA-guided GA. The precision of IROA administration, only in the case of ineffective intraoperative analgesia, defined as ∆SPI >15, likely led to stable serum FNT concentration, as changes in the SPI values have been shown to correspond to serum opioid concentrations [26] and have supposedly resulted in the effective suppression of afferent nociceptive stimulation responsible for inducing IPPP. Similarly, we hypothesized that AoA guidance resulted in stable intraoperative analgesia achieved by IROA administration titrated under the observance of SPI values, along with a stable depth of anesthesia reflecting limbic suppression monitored by SE, which attenuated potentially induced OCR by surgical manipulation during the stage of indentation in patients undergoing VRS, as we also demonstrated in our previous analysis of SPI value observations during the detailed stages of AoA-guided GA for VRS [76]. The SPI-guided IROA dosing during GA suppressed afferent nociceptive stimulation evoked by intraoperative muscle traction, which could also prevent central hyperexcitability [21], reducing the OCR rate to a rate close to the lowest rates observed in the literature.
OER was described by Van den Berg et al. as an afferent pathway sharing its limb of the reflex arc with OCR [77] produced by squint surgery in children. Their hypothesis that there is an association between the occurrence of OCR and PONV, particularly early PONV [78], was not proven, as in no case did intraoperative OCR cause PONV in the current study, as well as in our previous project [34]. In further studies, again in our view, the AoA guidance, expressed by stable SE guidance for inhalational anesthetic agent administration and the SPI guidance for IROA titration, may lead to personalized GA and, therefore, result in low rates of OCR and PONV, without any evidence of OER. Similarly, the PONV incidence was not observed to be correlated with the IPPP incidence in this study, although a relationship between IPPP and PONV has been reported in the current literature [54].
This study has several limitations. First, we were unable to examine late PONV in all patients, usually assessed up to 48 h after emergence from GA, because up to half of the patients were enrolled in this study during the COVID-19 pandemic period between lockdowns, and they were discharged from the DO as soon as the effects of anesthetics had subsided. Nevertheless, the incidence of late PONV may have little influence on the final results of overall PONV calculated as early PONV in the PACU and late PONV in the DO, as in our previous project, and no late PONV was observed in patients receiving intravenous preventive analgesia; only cases of late PONV were observed in patients receiving regional analgesia techniques at a similar rate to our current observations.
The presence of IPPP is a subjective phenomenon, the quantification of which is burdensome [79]. This study did not include a control group without AoA guidance on purpose, as studies with such control groups have already been conducted and their results are widely available. Moreover, the digital monitoring of nociception/anti-nociception balance has become a standard anesthetic modality in many centers; thus, obtaining consent for the study from a Bioethical Committee in view of strict regulations, requiring potential benefits from participation in the study, despite the group allocation, may no longer be possible. We also did not deliberately analyze the IPPP incidence after discharge from the recovery room to the DO, because this study included the monitoring of NPRS and SPI at Stage 5, while patient arousal (changing position in bed, cough, etc.) has been shown to significantly interfere with the SPI monitoring [80], thus making such a comparison neither clinically nor scientifically meaningful. Finally, a single dose of intravenous metamizole or paracetamol is known to have an effect for up to several hours; thus, the comparison of NPRS values over a period longer than four hours was found to be of little clinical relevance [81]. All patients received oral midazolam preoperatively, which has been shown to have antiemetic properties in patients undergoing VRS [82], which may have proportionally influenced the final total PONV incidence and the final outcomes across all groups.
In summary, in this study, we observed no advantage of combining IPA with 1 g of paracetamol and 2.5 g of metamizole before VRS in terms of the incidence of OCR and PONV compared to their use alone and to the findings from previous studies in which we observed that a single dose of IPA with any COX-3 inhibitor overweighed RA and control techniques without affecting the efficacy of preventive analgesia when AoA guidance was used. The stability of personalized GA using AoA guidance for VRS resulted in an overall rate of 4% PONV and 10% OCR, regardless of the group allocation, and the requirement for IROA had no influence on secondary outcomes, including perioperative adverse events.
5. Conclusions
We recommend the employment of AoA guidance for GA in patients undergoing VRS with IPA using paracetamol or paracetamol/metamizole to achieve low OCR and PONV incidence, thereby optimizing the demand for IROA as a decent alternative to VRS under RA with MAC for carefully selected patients, especially those with zero, one, or two risk factors of PONV, according to the stratification performed using the Apfel score.
Author Contributions
Conceptualization, M.J.S.; data curation, M.J.S. and K.M.; formal analysis, K.M. and N.Z.; investigation, K.M., M.J.S. and A.L.-B.; methodology, M.J.S. and A.L.-B.; supervision, M.J.S.; visualization, N.Z.; writing—original draft preparation, K.M. and M.J.S.; writing—review and editing, K.M., M.J.S. and N.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Medical University of Silesia, grant number: KNW-1-183/N/9/K.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Bioethical Committee of the Medical University of Silesia (KNW/0022/KB1/122/17; 5 December 2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Acknowledgments
The authors sincerely thank Teresa Paczyńska (anesthetic nurse assisting for every GA for PPV) at Regional Hospital no. 5 in Sosnowiec for her enthusiastic cooperation with the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AoA | adequacy of anesthesia |
| COX-3 | cyclooxygenase-3 |
| DM | diabetes mellitus |
| DO | Department of Ophthalmology |
| ECG | Electrocardiography |
| etCO2 | end-tidal carbon dioxide |
| FeAA | fraction of expired sevoflurane |
| FiAA | fraction of inspired sevoflurane |
| FiO2 | fraction of inspired oxygen |
| GA | general anesthesia |
| HA | arterial hypertension |
| HR | heart rate |
| IPA | intravenous preventive analgesia |
| IPPP | intolerable postoperative pain perception |
| IROA | intravenous rescue opioid analgesia |
| M | metamizole |
| MAC | monitored anesthesia care |
| NIBP | non-invasive blood pressure |
| NPRS | numeric pain rating scale |
| OCR | oculocardiac reflex |
| OER | oculoemetic reflex |
| P | paracetamol |
| PACU | postoperative care unit |
| PM | paracetamol/metamizole |
| PONV | perioperative nausea and vomiting |
| RA | regional anesthesia |
| RE | response entropy |
| SaO2 | arterial oxygen saturation |
| SE | state entropy |
| SPI | surgical pleth index |
| VRS | vitreoretinal surgery |
References
- Reibaldi, M.; Fallico, M.; Longo, A.; Avitabile, T.; Astuto, M.; Murabito, P.; Minardi, C.; Bonfiglio, V.; Boscia, F.; Furino, C.; et al. Efficacy of Three Different Prophylactic Treatments for Postoperative Nausea and Vomiting after Vitrectomy: A Randomized Clinical Trial. J. Clin. Med. 2019, 8, 391. [Google Scholar] [CrossRef]
- Licina, A.; Sidhu, S.; Xie, J.; Wan, C. Local versus General Anaesthesia for Adults Undergoing Pars Plana Vitrectomy Surgery. Cochrane Database Syst. Rev. 2016, 9, CD009936. [Google Scholar] [CrossRef] [PubMed]
- Huebert, I.; Heinicke, N.; Kook, D.; Boost, K.A.; Miller, C.V.; Mayer, W.J.; Haritoglou, C.; Kampik, A.; Gandorfer, A.; Hintschich, C.; et al. Dual Platelet Inhibition in Cases of Severe Retrobulbar Hemorrhage Following Retrobulbar and Peribulbar Anesthesia. J. Cataract. Refract. Surg. 2015, 41, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Reibaldi, M.; Longo, A.; Romano, M.R.; Cennamo, G.; Mariotti, C.; Boscia, F.; Bonfiglio, V.; Avitabile, T. Delayed Suprachoroidal Hemorrhage After Pars Plana Vitrectomy: Five-Year Results of a Retrospective Multicenter Cohort Study. Am. J. Ophthalmol. 2015, 160, 1235–1242.e1. [Google Scholar] [CrossRef] [PubMed]
- Kienbaum, P.; Schaefer, M.S.; Weibel, S.; Schlesinger, T.; Meybohm, P.; Eberhart, L.H.; Kranke, P. Update on PONV-What is new in prophylaxis and treatment of postoperative nausea and vomiting?: Summary of recent consensus recommendations and Cochrane reviews on prophylaxis and treatment of postoperative nausea and vomiting. Anaesthesist 2022, 71, 123–128. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Ying, Y.-Y.; Liu, H.-Y.; Qian, L.; Liu, H.; Ji, F.-H.; Peng, K. Opioid-Free Anaesthesia to Reduce Postoperative Nausea and Vomiting after Lower Extremity Wound Surgery: A Randomised Double-Blind Crossover Trial. Ann. Med. 2025, 57, 2517819. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sim, K.M.; Na, H.-S.; Koo, B.-W.; Shin, H.-J. Effect of Remimazolam for General Anesthesia on Postoperative Nausea and Vomiting: A Systematic Review and Meta-Analysis. Anaesthesiologie 2024, 73, 685–693. [Google Scholar] [CrossRef]
- Hussein, A.M.; Yasin, J.A.; Aldalati, A.Y.; Irfan, H.; Qtaishat, F.A.; Tamimi, M.-A.A.; Hageen, A.W.; Odat, R.M.; Albliwi, M. Olanzapine for the Prevention of Postoperative Nausea and Vomiting: An Updated Meta-Analysis With Trial Sequential Analysis. J. Pain Symptom Manag. 2025, 69, e359–e373. [Google Scholar] [CrossRef]
- Jotaki, S.; Murotani, K.; Hiraki, T. Preventive Effect of Hydroxyzine on Postoperative Nausea and Vomiting: A Single-Center, Retrospective, Observational Cohort Study. J. Clin. Med. 2024, 13, 7807. [Google Scholar] [CrossRef]
- Zeng, J.; Cao, Q.; Hong, A.; Gu, Z.; Jian, J.; Liang, X. Comparative Efficacy of Sugammadex and Neostigmine in Postoperative Nausea and Vomiting Management: A Meta-Analysis of Randomized Controlled Trials. J. Anesth. 2025, 39, 637–651. [Google Scholar] [CrossRef]
- Pashaei, S.; Akyüz, N. Effective Role of Aromatherapy in Reducing Big Little Problem-Postoperative Nausea and Vomiting: A Systematic Review. J. Educ. Health Promot. 2024, 13, 298. [Google Scholar] [CrossRef]
- Darvall, J.N.; De Silva, A.P.; von Ungern-Sternberg, B.; Story, D.A.; Davidson, A.J.; Allen, M.L.; Tran-Duy, A.; Schultz-Ferguson, C.; Ha, V.; Braat, S.; et al. Chewing Gum to Treat Postoperative Nausea and Vomiting in Female Patients: A Multicenter Randomized Trial. Anesthesiology 2025, 142, 454–464. [Google Scholar] [CrossRef]
- Wang, L.; Zou, X.; Wu, L.; Jin, Z.; Hu, S.; Zhu, X.; Li, X. Cheek Acupuncture Reduces Postoperative Nausea and Vomiting in Patients Undergoing Laparoscopic Gynecological Surgery: A Randomized Controlled Trial. J. Minim. Invasive Gynecol. 2025, 32, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Xia, F.; Yan, Z.; Xu, H.-B.; Zhao, W.-M.; Lei, Y.-S.; Xu, C.; Huo, W.-W.; Tao, D.-D.; Wang, J.; et al. Effect of Intraoperative Noise Isolation on Postoperative Nausea and Vomiting in Patients Undergoing Gynecological Laparoscopic Surgery: Protocol for a Randomized Controlled Trial. BMC Anesthesiol. 2025, 25, 48. [Google Scholar] [CrossRef] [PubMed]
- Mandelcorn, M.; Taback, N.; Mandelcorn, E.; Ananthanarayan, C. Risk Factors for Pain and Nausea Following Retinal and Vitreous Surgery under Conscious Sedation. Can. J. Ophthalmol. 1999, 34, 281–285. [Google Scholar] [PubMed]
- Ghali, A.M.; El Btarny, A.M. The Effect on Outcome of Peribulbar Anaesthesia in Conjunction with General Anesthesia for Vitreoretinal Surgery. Anaesthesia 2010, 65, 249–253. [Google Scholar] [CrossRef]
- Bayerl, K.; Boost, K.A.; Wolf, A.; Kampik, A.; Schaumberger, M.; Haritoglou, C. A 23-gauge pars plana vitrectomy after induction of general anesthesia: Effect of additional retrobulbar anesthesia on postoperative pain. Ophthalmologe 2014, 111, 1194–1200. [Google Scholar] [CrossRef]
- Schönfeld, C.-L.; Hierneis, S.; Kampik, A. Preemptive Analgesia with Ropivacaine for Pars Plana Vitrectomy: Randomized Controlled Trial on Efficacy and Required Dose. Retina 2012, 32, 912–917. [Google Scholar] [CrossRef]
- Henzler, D.; Müller-Kaulen, B.; Steinhorst, U.H.; Broermann, H.; Piepenbrock, S. The combination of retrobulbar block with general anaesthesia may lead to pre-emptive analgesia in patients undergoing pars plana vitrectomy. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 2002, 37, 267–272. [Google Scholar] [CrossRef]
- Misiołek, H.; Cettler, M.; Woroń, J.; Wordliczek, J.; Dobrogowski, J.; Mayzner-Zawadzka, E. The 2014 Guidelines for Post-Operative Pain Management. Anaesthesiol. Intensive Ther. 2014, 46, 221–244. [Google Scholar] [CrossRef]
- Woolf, C.J.; Chong, M.S. Preemptive Analgesia—Treating Postoperative Pain by Preventing the Establishment of Central Sensitization. Anesth. Analg. 1993, 77, 362–379. [Google Scholar] [CrossRef]
- Bharti, N.; Chari, P.; Kumar, P. Effect of Sevoflurane versus Propofol-Based Anesthesia on the Hemodynamic Response and Recovery Characteristics in Patients Undergoing Microlaryngeal Surgery. Saudi J. Anaesth. 2012, 6, 380–384. [Google Scholar] [CrossRef]
- Gruenewald, M.; Ilies, C. Monitoring the Nociception-Anti-Nociception Balance. Best Pract. Res. Clin. Anaesthesiol. 2013, 27, 235–247. [Google Scholar] [CrossRef]
- Ledowski, T.; Pascoe, E.; Ang, B.; Schmarbeck, T.; Clarke, M.W.; Fuller, C.; Kapoor, V. Monitoring of Intra-Operative Nociception: Skin Conductance and Surgical Stress Index versus Stress Hormone Plasma Levels. Anaesthesia 2010, 65, 1001–1006. [Google Scholar] [CrossRef]
- Ilies, C.; Gruenewald, M.; Ludwigs, J.; Thee, C.; Höcker, J.; Hanss, R.; Steinfath, M.; Bein, B. Evaluation of the Surgical Stress Index during Spinal and General Anaesthesia. Br. J. Anaesth. 2010, 105, 533–537. [Google Scholar] [CrossRef]
- Gruenewald, M.; Meybohm, P.; Ilies, C.; Höcker, J.; Hanss, R.; Scholz, J.; Bein, B. Influence of Different Remifentanil Concentrations on the Performance of the Surgical Stress Index to Detect a Standardized Painful Stimulus during Sevoflurane Anaesthesia. Br. J. Anaesth. 2009, 103, 586–593. [Google Scholar] [CrossRef]
- Struys, M.M.R.F.; Vanpeteghem, C.; Huiku, M.; Uutela, K.; Blyaert, N.B.K.; Mortier, E.P. Changes in a Surgical Stress Index in Response to Standardized Pain Stimuli during Propofol-Remifentanil Infusion. Br. J. Anaesth. 2007, 99, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Wennervirta, J.; Hynynen, M.; Koivusalo, A.-M.; Uutela, K.; Huiku, M.; Vakkuri, A. Surgical Stress Index as a Measure of Nociception/Antinociception Balance during General Anesthesia. Acta Anaesthesiol. Scand. 2008, 52, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Ahonen, J.; Jokela, R.; Uutela, K.; Huiku, M. Surgical Stress Index Reflects Surgical Stress in Gynaecological Laparoscopic Day-Case Surgery. Br. J. Anaesth. 2007, 98, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, I.; Göhner, A.; Crozier, T.A.; Hesjedal, B.; Wiese, C.H.; Popov, A.F.; Bauer, M.; Hinz, J.M. Surgical Pleth Index-Guided Remifentanil Administration Reduces Remifentanil and Propofol Consumption and Shortens Recovery Times in Outpatient Anaesthesia. Br. J. Anaesth. 2013, 110, 622–628. [Google Scholar] [CrossRef]
- Upton, H.D.; Ludbrook, G.L.; Wing, A.; Sleigh, J.W. Intraoperative “Analgesia Nociception Index”-Guided Fentanyl Administration During Sevoflurane Anesthesia in Lumbar Discectomy and Laminectomy: A Randomized Clinical Trial. Anesth. Analg. 2017, 125, 81–90. [Google Scholar] [CrossRef]
- Feng, C.-D.; Xu, Y.; Chen, S.; Song, N.; Meng, X.-W.; Liu, H.; Ji, F.-H.; Peng, K. Opioid-Free Anaesthesia Reduces Postoperative Nausea and Vomiting after Thoracoscopic Lung Resection: A Randomised Controlled Trial. Br. J. Anaesth. 2024, 132, 267–276. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Kawka, M.; Krawczyk, L.; Niewiadomska, E.; Dobrowolski, D.; Rejdak, R.; Król, S.; Żak, J.; et al. Preventive Analgesia, Hemodynamic Stability, and Pain in Vitreoretinal Surgery. Medicina 2021, 57, 262. [Google Scholar] [CrossRef]
- Pluta, A.; Stasiowski, M.J.; Lyssek-Boroń, A.; Król, S.; Krawczyk, L.; Niewiadomska, E.; Żak, J.; Kawka, M.; Dobrowolski, D.; Grabarek, B.O.; et al. Adverse Events during Vitrectomy under Adequacy of Anesthesia-An Additional Report. J. Clin. Med. 2021, 10, 4172. [Google Scholar] [CrossRef]
- Misiołek, H.; Zajączkowska, R.; Daszkiewicz, A.; Woroń, J.; Dobrogowski, J.; Wordliczek, J.; Owczuk, R. Postoperative Pain Management—2018 Consensus Statement of the Section of Regional Anaesthesia and Pain Therapy of the Polish Society of Anaesthesiology and Intensive Therapy, the Polish Society of Regional Anaesthesia and Pain Therapy, the Polish Association for the Study of Pain and the National Consultant in Anaesthesiology and Intensive Therapy. Anaesthesiol. Intensive Ther. 2018, 50, 173–199. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Lyssek-Boroń, A.; Zmarzły, N.; Marczak, K.; Grabarek, B.O. The Adequacy of Anesthesia Guidance for Vitreoretinal Surgeries with Preemptive Paracetamol/Metamizole. Pharmaceuticals 2024, 17, 129. [Google Scholar] [CrossRef]
- Carron, M.; Tamburini, E.; Linassi, F.; Pettenuzzo, T.; Boscolo, A.; Navalesi, P. Efficacy of Nonopioid Analgesics and Adjuvants in Multimodal Analgesia for Reducing Postoperative Opioid Consumption and Complications in Obesity: A Systematic Review and Network Meta-Analysis. Br. J. Anaesth. 2024, 133, 1234–1249. [Google Scholar] [CrossRef] [PubMed]
- Owczuk, R. Guidelines for General Anaesthesia in the Elderly of the Committee on Quality and Safety in Anaesthesia, Polish Society of Anaesthesiology and Intensive Therapy. Anaesthesiol. Intensive Ther. 2013, 45, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Ilies, C.; Ludwigs, J.; Gruenewald, M.; Thee, C.; Hanf, J.; Hanss, R.; Steinfath, M.; Bein, B. The Effect of Posture and Anaesthetic Technique on the Surgical Pleth Index. Anaesthesia 2012, 67, 508–513. [Google Scholar] [CrossRef]
- Apfel, C.C.; Läärä, E.; Koivuranta, M.; Greim, C.A.; Roewer, N. A Simplified Risk Score for Predicting Postoperative Nausea and Vomiting: Conclusions from Cross-Validations between Two Centers. Anesthesiology 1999, 91, 693–700. [Google Scholar] [CrossRef]
- Darvall, J.; Handscombe, M.; Maat, B.; So, K.; Suganthirakumar, A.; Leslie, K. Interpretation of the Four Risk Factors for Postoperative Nausea and Vomiting in the Apfel Simplified Risk Score: An Analysis of Published Studies. Can. J. Anaesth. 2021, 68, 1057–1063. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Smith, H.S. Potential Analgesic Mechanisms of Acetaminophen. Pain Physician 2009, 12, 269–280. [Google Scholar] [CrossRef]
- Graham, G.G.; Scott, K.F. Mechanism of Action of Paracetamol. Am. J. Ther. 2005, 12, 46–55. [Google Scholar] [CrossRef]
- Raffa, R.B.; Walker, E.A.; Sterious, S.N. Opioid Receptors and Acetaminophen (Paracetamol). Eur. J. Pharmacol. 2004, 503, 209–210. [Google Scholar] [CrossRef]
- Roca-Vinardell, A.; Ortega-Alvaro, A.; Gibert-Rahola, J.; Micó, J.A. The Role of 5-HT1A/B Autoreceptors in the Antinociceptive Effect of Systemic Administration of Acetaminophen. Anesthesiology 2003, 98, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Bujalska, M. Effect of Nitric Oxide Synthase Inhibition on Antinociceptive Action of Different Doses of Acetaminophen. Pol. J. Pharmacol. 2004, 56, 605–610. [Google Scholar] [PubMed]
- Ottani, A.; Leone, S.; Sandrini, M.; Ferrari, A.; Bertolini, A. The Analgesic Activity of Paracetamol Is Prevented by the Blockade of Cannabinoid CB1 Receptors. Eur. J. Pharmacol. 2006, 531, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Freo, U. Paracetamol for Multimodal Analgesia. Pain Manag. 2022, 12, 737–750. [Google Scholar] [CrossRef]
- Cecílio, N.T.; Souza, G.R.; Alves-Filho, J.C.; Cunha, F.Q.; Cunha, T.M. The PI3Kγ/AKT Signaling Pathway Mediates Peripheral Antinociceptive Action of Dipyrone. Fundam. Clin. Pharmacol. 2021, 35, 364–370. [Google Scholar] [CrossRef]
- Brodner, G.; Gogarten, W.; Van Aken, H.; Hahnenkamp, K.; Wempe, C.; Freise, H.; Cosanne, I.; Huppertz-Thyssen, M.; Ellger, B. Efficacy of Intravenous Paracetamol Compared to Dipyrone and Parecoxib for Postoperative Pain Management after Minor-to-Intermediate Surgery: A Randomised, Double-Blind Trial. Eur. J. Anaesthesiol. 2011, 28, 125–132. [Google Scholar] [CrossRef]
- Doleman, B.; Read, D.; Lund, J.N.; Williams, J.P. Preventive Acetaminophen Reduces Postoperative Opioid Consumption, Vomiting, and Pain Scores After Surgery: Systematic Review and Meta-Analysis. Reg. Anesth. Pain Med. 2015, 40, 706–712. [Google Scholar] [CrossRef]
- Toms, L.; McQuay, H.J.; Derry, S.; Moore, R.A. Single Dose Oral Paracetamol (Acetaminophen) for Postoperative Pain in Adults. Cochrane Database Syst. Rev. 2008, 2008, CD004602. [Google Scholar] [CrossRef]
- Apfel, C.C.; Turan, A.; Souza, K.; Pergolizzi, J.; Hornuss, C. Intravenous Acetaminophen Reduces Postoperative Nausea and Vomiting: A Systematic Review and Meta-Analysis. Pain 2013, 154, 677–689. [Google Scholar] [CrossRef]
- Oscier, C.D.; Milner, Q.J.W. Peri-Operative Use of Paracetamol. Anaesthesia 2009, 64, 65–72. [Google Scholar] [CrossRef]
- McNicol, E.D.; Tzortzopoulou, A.; Cepeda, M.S.; Francia, M.B.D.; Farhat, T.; Schumann, R. Single-Dose Intravenous Paracetamol or Propacetamol for Prevention or Treatment of Postoperative Pain: A Systematic Review and Meta-Analysis. Br. J. Anaesth. 2011, 106, 764–775. [Google Scholar] [CrossRef]
- Steffen, P.; Schuhmacher, I.; Weichel, T.; Georgieff, M.; Seeling, W. Differential administration of non-opioids in postoperative analgesia, I. Quantification of the analgesic effect of metamizole using patient-controlled analgesia. Anasthesiol. Intensivmed. Notfallmed. Schmerzther. 1996, 31, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Zukowski, M.; Kotfis, K. Safety of metamizole and paracetamol for acute pain treatment. Anestezjol. Intens. Ter. 2009, 41, 170–175. [Google Scholar]
- Konijnenbelt-Peters, J.; van der Heijden, C.; Ekhart, C.; Bos, J.; Bruhn, J.; Kramers, C. Metamizole (Dipyrone) as an Alternative Agent in Postoperative Analgesia in Patients with Contraindications for Nonsteroidal Anti-Inflammatory Drugs. Pain Pract. 2017, 17, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Pogatzki-Zahn, E.; Chandrasena, C.; Schug, S.A. Nonopioid Analgesics for Postoperative Pain Management. Curr. Opin. Anaesthesiol. 2014, 27, 513–519. [Google Scholar] [CrossRef]
- Eberhart, L.H.J.; Morin, A.M.; Hoerle, S.; Wulf, H.; Geldner, G. Droperidol and Dolasetron Alone or in Combination for Prevention of Postoperative Nausea and Vomiting after Vitrectomy. Ophthalmology 2004, 111, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Parkman, H.P.; Hallinan, E.K.; Hasler, W.L.; Farrugia, G.; Koch, K.L.; Calles, J.; Snape, W.J.; Abell, T.L.; Sarosiek, I.; McCallum, R.W.; et al. Nausea and Vomiting in Gastroparesis: Similarities and Differences in Idiopathic and Diabetic Gastroparesis. Neurogastroenterol. Motil. 2016, 28, 1902–1914. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Król, S.; Krawczyk, L.; Niewiadomska, E.; Żak, J.; Kawka, M.; Dobrowolski, D.; Grabarek, B.O.; et al. Adverse Events during Vitreoretinal Surgery under Adequacy of Anesthesia Guidance-Risk Factor Analysis. Pharmaceuticals 2022, 15, 237. [Google Scholar] [CrossRef]
- Ali, S.Z.; Taguchi, A.; Holtmann, B.; Kurz, A. Effect of Supplemental Pre-Operative Fluid on Postoperative Nausea and Vomiting. Anaesthesia 2003, 58, 780–784. [Google Scholar] [CrossRef]
- Ohnesorge, H.; Bein, B.; Hanss, R.; Francksen, H.; Mayer, L.; Scholz, J.; Tonner, P.H. Paracetamol versus Metamizol in the Treatment of Postoperative Pain after Breast Surgery: A Randomized, Controlled Trial. Eur. J. Anaesthesiol. 2009, 26, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Sener, M.; Kocum, A.; Caliskan, E.; Yilmaz, I.; Caylakli, F.; Aribogan, A. Administration of Paracetamol versus Dipyrone by Intravenous Patient-Controlled Analgesia for Postoperative Pain Relief in Children after Tonsillectomy. Braz. J. Anesthesiol. 2015, 65, 476–482. [Google Scholar] [CrossRef]
- Koivuranta, M.; Läärä, E.; Snåre, L.; Alahuhta, S. A Survey of Postoperative Nausea and Vomiting. Anaesthesia 1997, 52, 443–449. [Google Scholar] [CrossRef]
- Gan, T.J.; Belani, K.G.; Bergese, S.; Chung, F.; Diemunsch, P.; Habib, A.S.; Jin, Z.; Kovac, A.L.; Meyer, T.A.; Urman, R.D.; et al. Fourth Consensus Guidelines for the Management of Postoperative Nausea and Vomiting. Anesth. Analg. 2020, 131, 411–448. [Google Scholar] [CrossRef]
- Gan, T.; Sloan, F.; de Dear, G.L.; El-Moalem, H.E.; Lubarsky, D.A. How Much Are Patients Willing to Pay to Avoid Postoperative Nausea and Vomiting? Anesth. Analg. 2001, 92, 393–400. [Google Scholar] [CrossRef]
- Nitahara, K.; Sugi, Y.; Shono, S.; Hamada, T.; Higa, K. Risk Factors for Nausea and Vomiting Following Vitrectomy in Adults. Eur. J. Anaesthesiol. 2007, 24, 166–170. [Google Scholar] [CrossRef]
- Gola, W.; Bialka, S.; Owczarek, A.J.; Misiolek, H. Effectiveness of Fascia Iliaca Compartment Block after Elective Total Hip Replacement: A Prospective, Randomized, Controlled Study. Int. J. Environ. Res. Public Health 2021, 18, 4891. [Google Scholar] [CrossRef]
- Doggrell, S.A.; Hancox, J.C. Cardiac Safety Concerns for Ondansetron, an Antiemetic Commonly Used for Nausea Linked to Cancer Treatment and Following Anaesthesia. Expert. Opin. Drug Saf. 2013, 12, 421–431. [Google Scholar] [CrossRef]
- Allison, C.E.; De Lange, J.J.; Koole, F.D.; Zuurmond, W.W.; Ros, H.H.; van Schagen, N.T. A Comparison of the Incidence of the Oculocardiac and Oculorespiratory Reflexes during Sevoflurane or Halothane Anesthesia for Strabismus Surgery in Children. Anesth. Analg. 2000, 90, 306–310. [Google Scholar] [CrossRef]
- Shende, D.; Sadhasivam, S.; Madan, R. Effects of Peribulbar Bupivacaine as an Adjunct to General Anaesthesia on Peri-Operative Outcome Following Retinal Detachment Surgery. Anaesthesia 2000, 55, 970–975. [Google Scholar] [CrossRef]
- Abdeldayem, O.T.; Amer, G.F.; Abdulla, M.G. Postoperative Analgesic Efficacy of Sub-Tenon’s Block with Levobupivacaine in Retinal Surgery under General Anesthesia. Anesth. Essays Res. 2019, 13, 437–440. [Google Scholar] [CrossRef]
- Stasiowski, M.J.; Pluta, A.; Lyssek-Boroń, A.; Niewiadomska, E.; Krawczyk, L.; Dobrowolski, D.; Grabarek, B.O.; Kawka, M.; Rejdak, R.; Szumera, I.; et al. Adequacy of Anaesthesia for Nociception Detection during Vitreoretinal Surgery. Life 2023, 13, 505. [Google Scholar] [CrossRef]
- Van den Berg, A.A.; Lambourne, A.; Yazji, N.S.; Laghari, N.A. Vomiting after Ophthalmic Surgery. Effects of Intra-Operative Antiemetics and Postoperative Oral Fluid Restriction. Anaesthesia 1987, 42, 270–276. [Google Scholar] [CrossRef]
- Van den Berg, A.A.; Lambourne, A.; Clyburn, P.A. The Oculo-Emetic Reflex. A Rationalisation of Postophthalmic Anaesthesia Vomiting. Anaesthesia 1989, 44, 110–117. [Google Scholar] [CrossRef]
- Krebs, E.E.; Carey, T.S.; Weinberger, M. Accuracy of the Pain Numeric Rating Scale as a Screening Test in Primary Care. J. Gen. Intern. Med. 2007, 22, 1453–1458. [Google Scholar] [CrossRef]
- Ledowski, T.; Burke, J.; Hruby, J. Surgical Pleth Index: Prediction of Postoperative Pain and Influence of Arousal. Br. J. Anaesth. 2016, 117, 371–374. [Google Scholar] [CrossRef]
- Sadrolsadat, S.H.; Yousefshahi, F.; Ostadalipour, A.; Mohammadi, F.Z.; Makarem, J. Effect of Intravenous Acetaminophen on Postoperative Pain in Vitrectomy: A Randomized, Double-Blind, Clinical Trial. Anesth. Pain Med. 2017, 7, e13639. [Google Scholar] [CrossRef]
- Xiang, Y.; Ye, W.; Sun, N.; Jin, X. Analgesic and Sedative Effects of Dezocine and Midazolam During Vitrectomy. Curr. Eye Res. 2016, 41, 1460–1464. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).