Abstract
Background: The effectiveness of metformin in preventing Type-2 Diabetes Mellitus (T2DM) is examined. There are new available data. Currently, there are no available analyses classifying its effectiveness compared to placebo, standard care, or lifestyle interventions, and there is limited evidence on the combined action of metformin and lifestyle interventions in preventing T2DM. Objective: To calculate the updated overall effectiveness of metformin in preventing T2DM using all available and most recent data, and to explore the effectiveness of metformin and lifestyle interventions in preventing T2DM. Materials and Methods: A search was performed in PubMed and the Cochrane Library Central Register of Controlled Trials (CENTRAL) (from inception to 24 May 2025). A systematic review (SR) and meta-analysis (MA) of randomized controlled trials (RCTs) was carried out, including metformin-naïve adults with any identified diabetes risk factors. The overall effectiveness of metformin was estimated by combining studies that compare metformin against placebo, metformin and standard care against standard care, and metformin plus lifestyle interventions and the same lifestyle interventions. The combined action of metformin and lifestyle interventions was evaluated against standard care. We performed a GRADE assessment of the overall evidence. Results: Overall, metformin may reduce the incidence of T2DM by 23% in high-risk adults (OR 0.77, 95% CI 0.67, 0.88, p-value 0.0001) and 25% in patients with prediabetes (OR 0.75, 95%CI 0.66, 0.86, p-value < 0.0001). It is also effective in both obese and normal-weight patients, in Caucasians, in studies with female predominance, in studies with a mean age over 60 years, at 1700 mg daily, and after 18 months of administration. Effectiveness weakens after interruption of administration. Metformin is more effective compared to placebo and when combined with standard care than standard care alone, but not when combined with lifestyle interventions against lifestyle interventions alone. Metformin and lifestyle interventions reduce the incidence of diabetes in patients with prediabetes by 52% compared to standard care (OR 0.48, 95% CI 0.30, 0.77; p-value 0.002). There are effectiveness concerns in studies with more men than women, Asian Indians and Pakistanis, a mean age below 60 years, 500 mg of metformin daily, and after six months. The effect is reduced during post-intervention. Finally, metformin alone is more effective than standard care (OR 0.56, 95% CI 0.34, 0.90, p-value 0.02). The quality of evidence was moderate for the overall effectiveness of metformin and metformin combined with lifestyle interventions, and low for metformin against standard care. Conclusions: A 1700 mg dose of metformin daily is effective in preventing T2DM, especially in Caucasians, in women over 60 years, in prediabetes, and independent of obesity. Lifestyle interventions and 500 mg of metformin daily may prevent T2DM in patients with prediabetes, especially in men and Asian Indians or Pakistanis under 60 years. The effectiveness of complex interventions is more pronounced than that of metformin alone in patients with prediabetes. Further research is needed for post-intervention effectiveness, patients with any diabetes risk factors, patients from different regions, and women in complex interventions.
1. Introduction
Type-2 Diabetes Mellitus (T2DM) is the result of genetic predisposition, inflammation, and metabolic stress that lead to progressive pancreatic β-cell dysfunction with insufficient insulin secretion, often combined with insulin resistance and metabolic syndrome, resulting in hyperglycemia [1]. It is the most common type of DM, representing 90–95% of all diabetes cases [2]. The median estimated age of diabetes onset is 42.5 years [3]. The incidence of DM is distributed equally among females and males, affecting 8.2% of people across the world [3]. DM’s prevalence is increasing worldwide [4]. Globally, 642 million people will have T2DM by 2040 [5].
Currently, prediabetes, defined as either impaired fasting glucose (IFG), impaired glucose tolerance (IGT), or elevated hemoglobin A1c (HbA1c) between 5.7% and 6.4%, does not represent a separate metabolic disorder, constituting an independent risk factor for T2DM [1]. Additional risk factors include a history of gestational DM (GDM) [1], history of polycystic ovary syndrome (PCOS) [1], family history of DM in first-degree relatives [1], age greater than 45 years [6], high-risk ethnicities (i.e., Hispanic, Latino, Native American, African American, Asian American, Alaska Native, Pacific islander) [1,6], unhealthy nutrition [4], physical inactivity [1] or physical activity (PA) less than three times weekly [6], overweight or obesity [6], central adiposity [7], non-alcoholic fatty liver disease (NAFLD) [6], hypertension (HY) or anti-hypertensive treatment [1], hypertriglyceridemia (>250 mg/dL) and/or low high-density lipoprotein (HDL) cholesterol (<35 mg/dL) [1], cardiovascular disease (CVD) [1], insulin resistance-related acanthosis nigricans [1], and human immunodeficiency virus (HIV) infection [1].
Metformin is a cornerstone in T2DM treatment either alone [8] or combined with another antidiabetic drug [9], and its administration during pregnancy may decrease the incidence of GDM in women with PCOS [10,11,12].
For high-risk patients, two previously published meta-analyses (MAs), one in 2008 and the other in 2019, reported significant results for metformin in preventing T2DM [13,14]. For patients with prediabetes, metformin is also effective in preventing T2DM, according to four other MAs [15,16,17,18]. Two of them are published in the Chinese language [15,18], and the remaining two include data published before 2021. Finally, a MA published in 2023 included data published before 2016 and demonstrated a decreased incidence of T2DM in both high-risk and prediabetic patients [19].
Systematic reviews (SRs) and MAs have also declared the effectiveness of lifestyle interventions alone in preventing T2DM in high-risk [20] and prediabetic patients [21]. An antidiabetic regimen including metformin in addition to lifestyle modifications should be considered in real life but needs ongoing healthcare support [22].
At present, the overall effectiveness of metformin is not clearly classified in terms of placebo, standard care, and lifestyle interventions. The key clinical questions that we aim to address in the present MA are (1) which patient population could metformin benefit more, (2) what should be the optimal dose, (3) what should be the length of treatment, and (4) whether there is a post-intervention benefit. Moreover, we intend to assess (1) if metformin administration is more effective than standard care or lifestyle interventions, and (2) whether the combination of metformin and lifestyle interventions is more effective in preventing diabetes. Finally, the most recently published data have not been systematically analyzed, as they were not included in previous MAs [23,24,25].
2. Materials and Methods
Our initial protocol included a search for pharmaceutical and lifestyle interventions for diabetes prevention between 1 January 2000 and 31 July 2024 and was pre-registered in the Open Science Framework (OSF) (Registration DOI 10.17605/OSF.IO/8XH4G). To broaden the date of search (from inception to 24 May 2025) and specify the topic of the present study, we created an updated protocol in the OSF (Registration DOI 10.17605/OSF.IO/D4MRH [26]). The SR was performed according to PRISMA extension guidelines for complex interventions [27].
2.1. Search Strategy
The search for eligible RCTs was performed in PubMed and the Cochrane Library Central Register of Controlled Trials (CENTRAL) (from the inception of data to 24 May 2025). The keywords were related to diabetes, metformin, diet or nutrition, exercise or physical activity (PA), and lifestyle (Supplemental Table S1). The search in PubMed was applied combined with the Cochrane collaboration search algorithm for RCTs (Supplemental Table S1). The systematic search was completed by using the same keywords in CENTRAL. After removing duplicates using EndNote 21.5 software, four researchers (GIT, VT, GZ, VR) screened all databases. Potentially eligible studies based on titles and/or abstracts were retrieved, and the full text was checked. A fifth contributor (AB) checked the studies that the four investigators (GIT, VT, GZ, VR) could not decide on. Discrepancies were solved through consensus.
2.2. Eligibility Criteria
We accepted RCTs in the English language. Pilot or feasibility RCTs and studies reporting secondary analyses of RCTs were not accepted. Conference proceedings were also excluded. The PICO (population, intervention, control, outcome) approach was implemented to select trials. RCTs with adult participants who had any identified risk factor for T2DM that received metformin (alone or combined with standard care or lifestyle interventions) versus placebo, standard care, or lifestyle interventions, and reporting new occurrences of T2DM were considered eligible. Studies assessing the incidence of T2DM either as the primary or secondary outcome were considered eligible. RCTs that included patients who had previously received metformin and did not report new cases of T2DM in their outcomes were excluded. Overweight and obesity were also considered as risk factors. The definition of standard care was accepted as reported in each study. Any diagnostic modality for T2DM was accepted. If studies included more than one diagnostic test and reported different results, we accepted the laboratory exam with the most T2DM occurrences. For studies reporting the incidence of diabetes in different time periods, we accepted diagnoses in the final period of interventions.
The overall effect of metformin was evaluated in studies comparing metformin versus placebo, and in studies applying standard care or lifestyle intervention in the control group and metformin with the same standard care or lifestyle intervention in the experimental group.
2.3. Data Extraction
The data were extracted by four investigators (GIT, VT, GZ, VR). Where necessary, a fifth investigator (AB) contributed to the final decision. Data extraction included the name of the first author, publication year, country, type of RCT with number of clusters or centers if clustered or multicenter, number of arms if multiarm, study duration, and drop-out rate. We also extracted potential follow-up durations for studies assessing post-intervention outcomes. Data extraction also included the total sample size, characteristics of participants (gender, age, risk factors for T2DM), characteristics of interventions (dosage, duration), characteristics of the comparator arm, the number of patients that were analyzed for T2DM, events of T2DM, diagnostic tests for diabetes, and potential adverse events.
2.4. Quality Assessment of the Studies and Rating of Overall Evidence
Regarding the quality of eligible trials, the data were also extracted by four researchers (GIT, VT, GZ, VR). If required, a fifth researcher (AB) checked the items. The risk of bias tool proposed by the Cochrane Collaboration was used for quality assessment of eligible RCTs [28]. To rate overall evidence, we used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) tool (GRADEpro, version 3.6.1 McMaster University, 2011).
2.5. Statistical Analysis
For our analyses, we used the Review Manager software version 5.4.1 (Cochrane Collaboration, London, UK) and the Statistical Package for the Social Sciences (SPSS) software version 29.0 (SPSS, Inc., Chicago, IL, USA).
The main analyses included all available data. The statistically significant level was generally set at p-value < 0.05 [26]; for Cochran’s Q statistic, it was set at p-value < 0.1 [26]. MAs were performed to combine the events of T2DM. Heterogeneity across studies was assessed by Cochran’s Q statistic (statistically significant for p-value < 0.1) [29] and measured by the I2 index (<25%, low; 25–49%, moderate; 50–75%, large; >75%, very large) [30,31]. Both fixed effects (FE) and random effects (RE) models of MA were created. If heterogeneity was large, synthesis was performed by the RE model [30,31].
Subgroup analyses were performed based on the studies’ characteristics (similar countries, follow-up durations), population characteristics (gender, mean age, diabetes risk factors), and intervention characteristics (dosage, duration, T2DM assessed as the primary or secondary outcome). Sensitivity analyses were also performed to assess the effect of the RCT with the largest sample size, studies with post-intervention follow-up of participants, and studies with drop-outs. Finally, we performed meta-regression analyses with study duration and baseline risk as covariates of the T2DM odds ratio (OR) [32].
Publication bias was assessed optically by funnel plots (a symmetrical inverted funnel in the absence of bias), and statistically by Egger’s test [33].
3. Results
The initial search yielded 74,576 items. After removing 11,600 duplicates, 62,976 items remained to be assessed for potential eligibility. A total of 62,923 papers were excluded based on their title and/or abstract. Then, we retrieved the full text of the remaining 53 papers. Two studies had non-RCT designs, one was a pilot RCT, and 34 did not report the outcome of T2DM and were excluded. Finally, 16 RCTs were accepted (Figure 1).
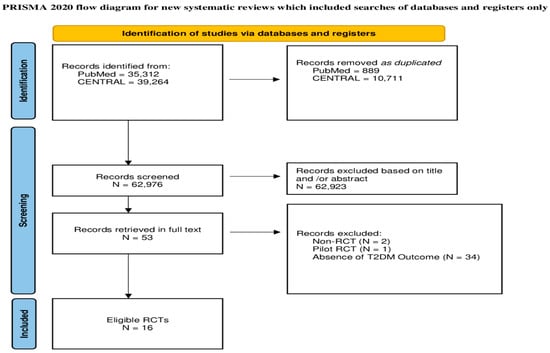
Figure 1.
Flowchart of the study selection procedure.
3.1. Characteristics of Eligible Studies
The eligible RCTs were published between 1996 and 2023, and they all had a parallel design [23,24,25,34,35,36,37,38,39,40,41,42,43,44,45,46]. Seven were conducted in Asia [23,24,35,38,39,40,43] (two in India [38,43], two in China [24,35], one in Thailand [23], and one in Pakistan [40]), six trials in Europe [34,36,41,42,45,46] (two in Scotland, United Kingdom (UK) [41,46]; two in The Netherlands [42,45]; one in France [34]; and one in Finland [36]), two in north America (in the United States of America (USA)) [37,44], and one in Africa (in Tanzania) [25]. Four studies were multiarm [37,38,40,44] (one study with four arms [38], and the other three with three arms [37,40,44]). Thirteen RCTs were single-centered [23,24,35,36,38,39,40,41,42,43,44,45,46], and three studies were multicentered [25,34,37] (one of them included 27 centers [37], while two did not report the number of included centers [25,34]). Study duration varied between 4 and 48 months [23,24,25,34,35,36,37,38,39,40,41,42,43,44,45,46]. Two studies reported participants’ post-intervention follow-up: a 30-month duration in one of them [43] and a 24-month duration in the other one [45]. Five trials had no drop-outs [23,36,38,42,45]. Six studies had a drop-out rate up to 10% (2% [44], 5% [24,43], 7% [46], 8% [41], 10% [37]), three between 10% and 20% (13% [39], 14% [40], 19% [25]), and two more than 20% (22% [25], 29% [34]) (Table 1).

Table 1.
Characteristics of eligible RCTs.
3.2. Characteristics of Participants
A total of 8529 adults were included in eligible trials [23,24,25,34,35,36,37,38,39,40,41,42,43,44,45,46]. Male and female genders represented 54% and 46% of the studies’ participants [23,24,25,34,35,36,37,38,39,40,41,42,43,44,45,46]. Their mean age varied between 44.4 [43] and 67.5 years [37]. The ethnicities included 3515 Asians (41%) [1952 Chinese and Thailand Asians (56%), 1106 Indians (31%), 317 Pakistanis (9%), 124 Asian Americans including Pacific Islanders (4%), and 14 Asians living in Europe (<1%)], 3227 Caucasians (38%) [1769 (55%) from North America and 1458 from Europe (45%)], 1017 Africans (12%) [644 African Americans (63%), 364 Sub-Saharan Africans (36%), and 9 Africans living in Europe (<1%)], 600 Hispanics (7%), and 171 Indian Americans (2%) [23,24,25,35,36,37,38,39,40,41,42,43,44,45,46]. One study did not report data about ethnicities [34] (Table 2).

Table 2.
Characteristics of participants.
Risk Factors
Several T2DM risk factors were evaluated in eligible trials [23,24,25,35,36,37,38,39,40,41,42,43,44,45,46]. Probable concurrence of risk factors that may affect a portion of individuals was reported in eight RCTs [23,25,33,34,35,37,38,46]. Prediabetes was the most common overall risk factor, considered in eleven RCTs [23,24,25,35,36,37,38,39,40,43,44], overweight/obesity in four trials [34,36,37,43], CVD in four RCTs [42,43,45,46], central adiposity in three studies [34,41,43], HIV infection in another two [23,25], and family history of T2DM in first-degree relatives in one study [36]. The overall number of T2DM risk factors included was one in seven studies [24,35,38,40,42,45,46], two in five studies [23,25,34,37,41], and three in two studies [36,43]. Probable coexistent risk factors were included in eight studies, comprising prediabetes, family history of T2DM or GDM, HY, elevated triglycerides, and low HDL cholesterol [23,25,34,35,37,38,43,46] (Table 2).
3.3. Characteristics of Interventions and Comparators
The components of comparator arms vary across studies [23,24,25,35,36,37,38,39,40,41,42,43,44,45,46]. They include either single or combined elements [23,24,25,35,36,37,38,39,40,41,42,43,44,45,46]. Single-part arms may be composed of an active intervention (i.e., metformin or lifestyle), standard care, or placebo, and combined-part arms may contain mixed items such as two active interventions (i.e., metformin plus lifestyle), metformin in addition to standard care, or standard care with placebo [23,24,25,35,36,37,38,39,40,41,42,43,44,45,46]. Interventions’ duration varied between 4 and 33.6 months [23,24,25,35,36,37,38,39,40,41,42,43,44,45,46] (Table 3).

Table 3.
Characteristics of interventions and comparators.
3.3.1. Metformin
The total daily intake of metformin also varied between 500 milligrams (mg) and 2000 mg [23,24,25,35,36,37,38,39,40,41,42,43,44,45,46]. One trial included 500 mg for most participants and 1000 mg for part of them during the last few months of the intervention [38], two studies 750 mg [35,39], six 1000 mg [23,36,40,42,43,45], four 1700 mg [34,37,41,44], and two 2000 mg [25,46]. Titration of the drug’s initial dose is reported in four RCTs [24,37,41,46]. The administration of metformin is distributed in equal doses twice daily in thirteen studies [23,24,34,36,37,38,40,41,42,43,44,45,46], three times daily in two studies [35,39], and one study reported the daily distribution of a total amount of 2000 mg in tablets of 500 mg [25] (Table 3).
Eight RCTs reported the assessment of medicine adherence [23,25,37,38,40,41,43,44]. Six of them adopted pill count as the medicine adherence measure [23,37,38,41,43,44]. One study described that tablets were counted every three months, considering a percentage of consumed tablets over 50% as acceptable [38]. This percentage was estimated by diaries where patients recorded consumed tablets and missed doses [38]. Patients received numbered bottles in another trial, and the acceptable percentage after counting tablets was set at more than 80% [41]. Four trials did not provide details concerning pill count [23,37,43,44]. One of them reported interviews in addition to pill count to assess adherence [37], and another reported pill count during every visit [43]. Two trials reported the assessment of metformin adherence at every visit; however, adherence assessment tools were not reported [25,40]. Additionally, one reported that medical doctors (MDs) assessed adherence [40]. Seven studies did not report data regarding medicine adherence [34,35,36,39,42,45,46] (Table 3).
3.3.2. Lifestyle
Lifestyle interventions were applied in seven RCTs and included both dietary and exercise components [24,37,38,39,40,43,44]. The initial lifestyle arm of the United States (US) Diabetes Prevention Program (DPP) [37] was also adopted by two other studies [43,44]. Zhang et al. used the Chinese Diabetes Prevention Program (CDPP) in 2023 [24]. Some lifestyle interventions included motivation strategies [24,37,38,39,40,43,44] (Table 3).
Six trials reported adherence assessment [24,37,38,40,43,44]. Self-reported adherence was assessed in five lifestyle programs [24,37,38,43,44]. Moreover, three of them used questionnaires [24,37,43]. In one study, the adherence was assessed by MDs during visits [40]. Finally, one RCT did not report assessment of lifestyle intervention adherence [39] (Table 3).
3.3.3. Standard Care, Placebo
Standard care was reported in 10 RCTs [23,25,34,37,38,39,40,42,43,45]. In eight RCTs, it consisted mainly of general lifestyle counseling [23,25,34,37,38,39,40,43]. Particularly, two studies reported diet and exercise counseling [23,34]. One trial described healthcare advice [38]. Another RCT applied diabetic education with follow-up education of patients annually [39]. Two studies provided written information, in addition to general diet and exercise counseling [37,44]. They both included an annual individual session for all participants to enhance the adoption of local dietary guidelines for weight loss and increase PA [37,44]. Moreover, one of them included educational materials for T2DM prevention that were provided during sessions [44]. The definition of standard care in another study included one individual session with a physician, a dietitian and a fitness instructor and one group session where patients were given general advice on a healthy diet and increasing PA [43]. Finally, two trials reported standard care for patients with CVD [42,45] (Table 3).
3.4. Effectiveness and Safety of Interventions
T2DM was assessed as the primary outcome in eight RCTs which evaluated metformin’s overall effect [23,24,25,35,37,38,40,45], and as the secondary outcome in the other four [34,36,41,42,46]. The primary outcomes in five studies that evaluated the incidence of diabetes as the secondary outcome were changes in body weight and in metabolic and lipidemic profile in patients with overweight/obesity and central adiposity [34], metformin sensitivity and glucose tolerance in patients with prediabetes [36], the progression of mean distal carotid intima–media thickness in patients with coronary heart disease (CHD) [41], cardiovascular risk profile in patients with CHD [42], and left ventricular hypertrophy in patients with CHD and prediabetes [46] (Supplemental Table S2).
T2DM was the primary outcome of four RCTs that evaluated metformin plus lifestyle interventions versus standard care [38,39,40,43]. For two studies including comparators for metformin versus standard care, T2DM was reported as either the primary [38] or secondary outcome [44]. The study that reported the incidence of diabetes as the secondary outcome considered participants’ weight as the primary outcome [44] (Supplemental Table S2).
The diagnosis of T2DM was set by a combined adoption of fasting plasma glucose (FPG), HbA1c, and the two-hour 75 g oral glucose tolerance test (2 h 75 g OGTT) in four RCTs [23,24,25,42], by FPG and 75 g OGTT in four RCTs [37,38,40,43], by FPG and HbA1c in two RCTs [44,46], by 75 g OGTT alone in four studies [34,35,36,39], and by HbA1c alone in two trials [41,45] (Supplemental Table S2).
3.4.1. Effectiveness
Overall Effectiveness of Metformin
A total of 5329 high-risk individuals (2603 in the intervention and 2726 in the control arm) were analyzed for T2DM in 13 RCTs that evaluated overall effect of metformin in preventing diabetes [31,32,33,34,35,37,38,39,42,43,44,45,46] (Supplemental Table S2) (Figure 2). Among them, 1412 (26.5%) [(621 (23.9%) in the intervention, and 791 (29%) in the control group)] developed T2DM (Supplemental Table S2) (Figure 2). Although the Q statistic was non-significant for heterogeneity (Q 12.22, p-value 0.43), the upper limit of the I2 index for variability measurements was more than 50% (I2 2%, 95% CI 0, 57%) (Figure 2). Thus, the RE model was used for synthesis (Figure 2). The MA revealed statistically significant results for the overall effect of metformin in preventing T2DM among high-risk adults (OR 0.77, 95% CI 0.67, 0.88, p-value 0.0001) (Figure 2).
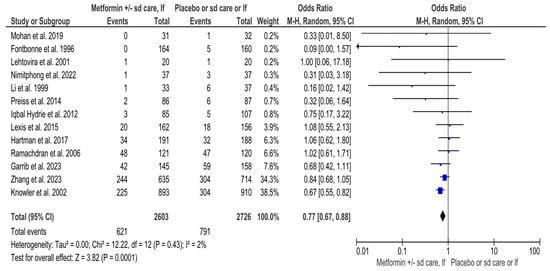
Figure 2.
Interventions including metformin and the risk of developing Type-2 Diabetes Mellitus. Sd, standard; lf, lifestyle; Events, number of participants with Type-2 Diabetes Mellitus; Total, number of participants at risk for Type-2 Diabetes Mellitus; blue dots, weight of studies; black blocks, 95% confidence interval of studies; diamond, estimate with 95% confidence interval. Studies are presented in ascending weight [23,24,25,28,34,35,36,37,40,41,42,45,46].
3.4.2. Subgroup and Sensitivity Analyses, and Meta-Regressions for Overall Effect of Metformin
Due to potential heterogeneity, subgroup and sensitivity analyses were also performed with the RE model. The results remained significant in the subgroup analysis for the study that was conducted in the USA (OR 0.67, 95%CI 0.55, 0.82, p-value 0.0001) (Figure S1), and for the following studies: where women comprised the majority of participants (OR 0.76, 95%CI 0.61, 0.95, p-value 0.02) (Figure S2); with participants’ mean age greater than 60 years (OR 0.66, 95%CI 0.54, 0.81, p-value < 0.0001) (Figure S3); assessing prediabetes as the main risk factor (OR 0.75, 95%CI 0.66, 0.86, p-value < 0.0001) (Figure S4); including overweight/obesity as the main risk factor (OR 0.66, 95%CI 0.53, 0.84, p-value 0.0006) and for studies not considering overweight/obesity (OR 0.78, 95%CI 0.66, 0.92, p-value 0.003) (Figure S5); for studies with a daily metformin dosage of 1700 mg (OR 0.81, 95%CI 0.72, 0.92, p-value 0.001) (Figure S8); implementing interventions longer than 18 months (OR 0.78, 95%CI 0.63, 0.96, p-value 0.02); (Figure S9); without a post-intervention follow-up (OR 0.75, 95%CI 0.66, 0.86, p-value < 0.0001) (Figure S10); and considering the incidence of T2DM as the primary outcome (OR 0.77, 95%CI 0.66, 0.90, p-value 0.0007) (Figure S11). On the contrary, a statistically significant result was not found for studies including high-risk patients with either CAD (Figure S6); or HIV infection (Figure S7); [(OR 0.97, 95%CI 0.65, 1.45, p-value 0.89) and (OR 0.66, 95%CI 0.41, 1.06, p-value 0.09), respectively]. Finally, metformin alone was more effective compared to placebo (OR 0.32, 95%CI 0.11, 0.98, p-value 0.05), and when combined with standard care, compared to standard care alone (OR 0.75, 95%CI 0.58, 0.97, p-value 0.03) (Figure S12). However, no differences were found for metformin combined with lifestyle interventions against lifestyle interventions alone (OR 0.86, 95%CI 0.71, 1.05, p-value 0.15) (Figure S12). Subgroup differences were non-significant in all analyses (Supplemental Table S3).
The results also remained significant in sensitivity analyses exploring the effect of the RCT with the largest sample size (OR 0.84, 95%CI 0.71, 0.99, p-value 0.04) (Figure S18), with a post-intervention follow-up (OR 0.75, 95%CI 0.66, 0.86, p-value < 0.0001) (Figure S19), and RCTs with a drop-out rate more than 10% (OR 0.78, 95%CI 0.68, 0.89, p-value 0.0003) (Figure S20) (Supplemental Table S3). Finally, meta-regression analyses with the intervention’s duration and baseline risk as covariates did not affect the summary OR (Supplemental Table S4).
Effectiveness of Metformin Plus Lifestyle Interventions Versus Standard Care
Fewer occurrences were identified for patients who received metformin plus lifestyle interventions versus standard care. Particularly, 74 (20.8%) out of 356 participants in mixed interventions developed T2DM versus 114 (34.4%) out of 331 patients in the standard care arm (Supplemental Table S2) (Figure 3). In total, 188 (27.4%) patients were diagnosed with diabetes out of 687 participants (Supplemental Table S2) (Figure 3). The result of the MA was statistically significant (OR 0.48, 95%CI 0.30, 0.77; p-value 0.002) (Figure 3). Heterogeneity across studies was non-significant (Q 3.79, p-value 0.28) (Figure 3). However, the RE model applied in the MA found the upper limit of I2 and CI to be more than 75%, indicating very high variability (I2 21%, 95%CI 0, 90%) (Figure 3).
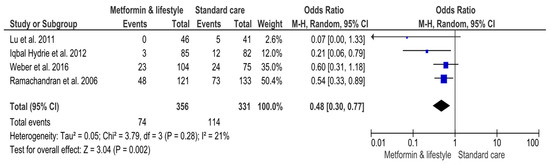
Figure 3.
Effects of interventions including metformin and lifestyle interventions on the risk of developing Type-2 Diabetes Mellitus. Events, number of participants with Type-2 Diabetes Mellitus; Total, number of participants at risk for Type-2 Diabetes Mellitus; blue dots, weight of studies; black blocks, 95% confidence interval of studies; diamond, estimate with 95% confidence interval. Studies are presented in ascending weight [38,39,40,43].
3.4.3. Subgroup and Sensitivity Analyses, and Meta-Regressions for Metformin Plus Lifestyle Interventions Versus Standard Care
Analyses were also conducted with the RE model. Significant results were found for studies that were performed in India (OR 0.56, 95%CI 0.38, 0.84, p-value 0.005) (Figure S13); for studies including participants with a mean age below 60 years (OR 0.52, 95%CI 0.35, 0.76, p-value 0.0008) (Figure S14); and for studies with a metformin dosage of 500 mg daily (OR 0.54, 95%CI 0.33, 0.89, p-value 0.02) (Figure S15). In subgroup analyses based on study duration and post-intervention follow-up, a significant effect was found for studies that lasted more than six months (Figure S16) and adopted a continuous intervention without post-intervention follow-up (Figure S17) (OR 0.34, 95%CI 0.14, 0.86; p-value 0.02). The result was non-significant for the study that implemented interventions up to six months (Figure S16) and assessed post-intervention outcomes (Figure S17) (OR 0.60, 95%CI 0.31, 1.18; p-value 0.14). All subgroup analyses had non-statistically significant differences (Supplemental Table S3).
In sensitivity analyses, the studies with the largest sample size (Figure S21), post-intervention duration (Figure S22), and drop-out rate more than 10% (Figure S23) did not have significant results [(OR 0.34, 95%CI 0.12, 0.95, p-value 0.04), (OR 0.34, 95%CI 0.14, 0.86, p-value 0.02), and OR (0.56, 95%CI 0.38, 0.84, p-value 0.005), respectively] (Supplemental Table S3). Finally, meta-regressions for T2DM OR with covariates of intervention duration and baseline risk were non-significant (Supplemental Table S4).
Effectiveness of Metformin Versus Standard Care
Two RCTs were included in the analysis comparing metformin versus standard care (Supplemental Table S2) (Figure 4). A total of 155 participants received metformin and 161 received standard care (Supplemental Table S2) (Figure 4). Exploring the comparative effect of metformin versus standard care, fewer diabetes cases were detected in the metformin group compared to the standard care group [52 (33.5%)/74 (46%), respectively]. In total, 126 (39.9%) participants were diagnosed with T2DM (Supplemental Table S2) (Figure 4). The result of the MA was significant (OR 0.56, 95%CI 0.34, 0.90; p-value 0.02) (Figure 4). Despite non-significant heterogeneity (Q 0,10, p-value 0.75; I2 0), variability across studies cannot be excluded due to the low number of studies and inapplicable 95%CI of I2 (Figure 4). Therefore, the data were combined with the RE model (Figure 4).
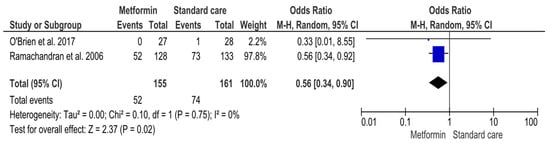
Figure 4.
Interventions including metformin compared to standard care and the risk of developing Type-2 Diabetes Mellitus. Events, number of participants with Type-2 Diabetes Mellitus; Total, number of participants at risk for Type-2 Diabetes Mellitus; blue dots, weight of studies; black blocks, 95% confidence interval of studies; diamond, estimate with 95% confidence interval. Studies are presented in ascending weight [38,44].
3.4.4. Safety
Adverse events, when reported, were mainly mild and related to gastrointestinal manifestations and hypoglycemia symptoms [24,25,34,35,36,37,38,39,40,41,44,46]. Other reported events, probably not associated with metformin, included musculoskeletal disorders [37], vertigo [44], headache [34,44], and rash [34,43]. Severe adverse events (i.e., CVD events [38,41,46], neoplasms [41]) or deaths [24,25,38,41] were very rare and were not related to interventions [25] (Supplemental Table S2).
4. Quality of Reporting, Potential Bias, and Quality of Evidence
Optically assessing the funnel plots of our four analyses, the obvious asymmetry found cannot exclude publication bias (Figure 5, Figure 6 and Figure 7). Performing Egger’s test for every separate analysis, the p-value was identified as 0.299 in the analysis of metformin’s overall effect, and 0.079 in the analysis of metformin plus lifestyle versus standard care, indicating a non-statistically significant bias.
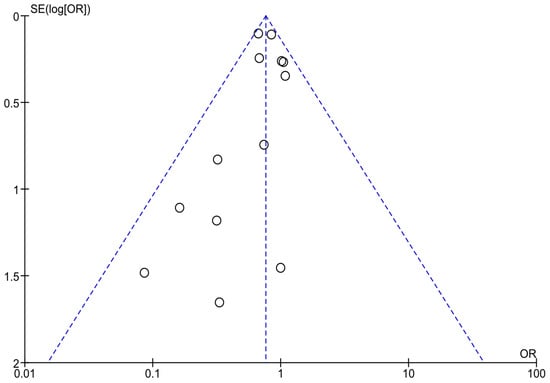
Figure 5.
Funnel plots of analyses of studies including metformin.
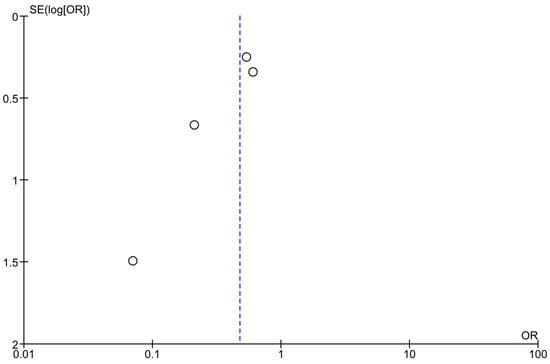
Figure 6.
Funnel plots of analyses of studies including metformin and lifestyle interventions.
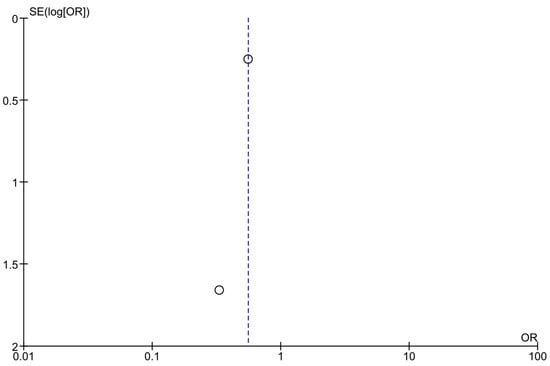
Figure 7.
Funnel plots of analyses of studies comparing metformin and standard care.
The funnel plot analyses included studies on the overall effectiveness of metformin in preventing diabetes in high-risk adults [23,24,25,28,34,35,36,37,40,41,42,45,46]; Egger’s test p-value = 0.299.
The funnel plot analyses included studies assessing the effectiveness of metformin and lifestyle interventions in preventing diabetes in high-risk adults [38,39,40,43]; Egger’s test p-value = 0.079.
The funnel plot analyses included studies comparing metformin versus standard care in preventing diabetes in high-risk adults [38,44].
All eligible trials assessed had a low risk of selection, reporting, and other bias [23,24,25,35,36,37,38,39,40,41,42,43,44,45,46]. Evaluating detection bias, 15 trials were judged as low risk [23,24,25,34,35,36,37,38,39,41,42,43,44,45,46], and one did not provide related information, raising an unclear risk [40]. Regarding attrition bias, 14 RCTs were judged as low risk [23,24,25,36,37,38,39,40,41,42,43], and two as high risk [34,35]. Finally, regarding performance bias, the quality characteristics revealed a low risk in ten studies [25,34,35,36,37,40,41,42,45,46], a high risk in four studies [24,38,43,44], and two studies with an unclear risk [23,39] (Figure 8).
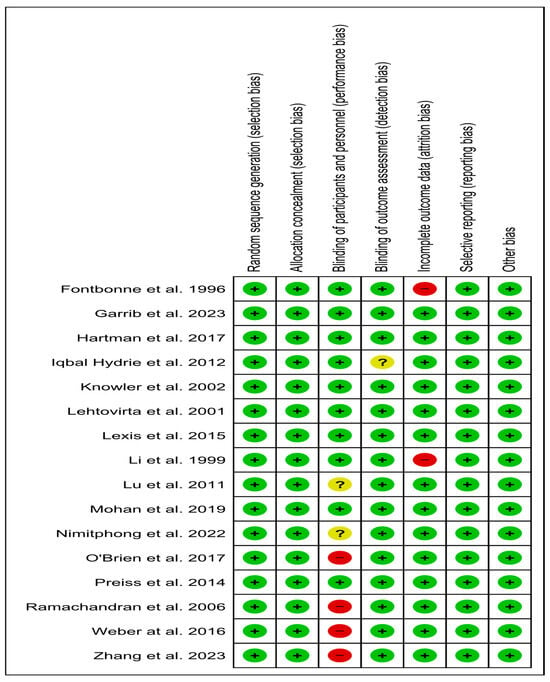
Figure 8.
Risk of bias of eligible trials [23,24,25,34,35,36,37,38,39,40,41,42,43,44,45,46].; green color, low risk; red color, high risk; yellow color, unclear risk.
The moderate overall quality of evidence demonstrated that persons at risk for T2DM may benefit by reducing diabetes risk with metformin, and metformin combined with lifestyle interventions. The quality of evidence was very low for metformin against standard care (Table 4).

Table 4.
GRADE evaluation of the overall evidence of studies according to analyses.
5. Discussion
In our MA, the results support the beneficial effect of metformin in preventing T2DM among high-risk adults. Interestingly, the beneficial effects concern patients with prediabetes, obese and normal-weight patients, Caucasians, women, and patients over 60 years old. Moreover, it was demonstrated that metformin is effective at a daily dosage of 1700 mg, and after 18 months of administration, while the protective effect weakens after cessation of metformin. Metformin, irrespective of its addition to standard care, is more effective than placebo or standard care alone. Finally, our MA found that metformin and lifestyle interventions are more effective compared to standard care in preventing T2DM in patients with prediabetes. This action is more significant in men and patients younger than 60 years. Interestingly, when combined with lifestyle interventions, metformin’s effective dose is lower than when used alone to prevent T2DM. During the post-intervention period, the benefit weakens. Heterogeneity cannot be excluded in analyses. The quality of evidence is moderate for metformin’s overall effectiveness and for metformin combined with lifestyle interventions, and low for metformin against standard care. Figure 9 summarizes metformin’s overall effectiveness and the effectiveness of metformin combined with lifestyle interventions in preventing T2DM.
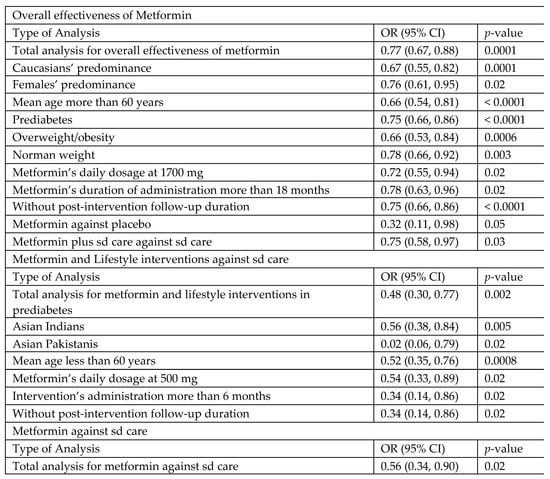
Figure 9.
Summary of results for metformin and lifestyle interventions for diabetes prevention in high-risk and metformin-naïve adults. OR, odds ratio; CI, confidence interval; mg, milligram; sd, standard.
5.1. Rationale for Eligible Population
In the present MA, we chose to include metformin-naïve patients. Analyzing patients receiving long-term metformin therapy together with metformin-naïve patients might be confusing. We believe that patients receiving metformin or lifestyle interventions on a long-term basis might have different effects concerning the prevention of T2DM compared to patients evaluated from the onset of the intervention. This might hinder the accuracy of comparisons between treatments. To specifically highlight the effect of metformin in preventing T2DM, in our study, we chose to include only drug-naïve patients.
5.2. Existing Literature and Comparisons
To our knowledge, this is the first analysis that defines the overall effectiveness of metformin, assessing interactions with placebo, standard care, and lifestyle interventions with special emphasis on studies’ and participants’ characteristics. In the present study, we found that metformin combined with lifestyle interventions is not superior to lifestyle interventions alone, setting lifestyle interventions as a strong factor for diabetes prevention. On the contrary, a previously published MA demonstrated the effectiveness of combined metformin and lifestyle interventions against lifestyle interventions for patients with prediabetes [47]. However, this analysis included pilot RCTs, mixed adolescent and adult populations, and interventions not considered lifestyle adjuncts to metformin (i.e., metformin with placebo, metformin with standard care, or metformin with only exercise not including dietary intervention), reaching a highly biased risk ratio (RR) (RR 0.85) [47]. As far as we know, this is the first study that evaluates metformin and lifestyle interventions versus standard care. We found that the combination of metformin and lifestyle interventions is superior to standard care in patients with prediabetes. Additionally, we found that combined interventions are more effective than metformin alone in preventing T2DM in patients with prediabetes. The effectiveness of metformin alone was confirmed for high-risk patients, as was previously found [13,14,19]. However, in our study, we broadened the inclusion criteria, also considering patients with any diabetes risk factor. In the same line as previous MAs, a protective effect was found for patients with prediabetes [15,16,17,18,19]. Contrariwise to the most recent MA, we excluded RCTs evaluating the effects of other hypoglycemic agents in addition to metformin, and publications reporting secondary analyses of RCTs, which probably conflict with estimations of effectiveness [19]. It should be pointed out that we have included all recently published data.
5.3. Interpretation of the Results
5.3.1. Metformin’s Overall Effectiveness
RCTs following patients under metformin and lifestyle interventions for years have declared significant results for both interventions [48,49]. However, the effectiveness of metformin compared to lifestyle interventions is unclear, as the long-term effects of each treatment may have obscured the superiority of one intervention over the other [50,51,52]. In our MA, it was shown that the combination of lifestyle interventions with metformin is not superior to lifestyle interventions alone in preventing T2DM. The combined effect of both interventions was also superior compared to standard care. We have also found that metformin alone is more effective than standard care and placebo. This action might be useful for patients with disabilities, underserved patients, and patients unwilling to adopt lifestyle modifications.
5.3.2. Metformin and Prediabetes
The preventive effect of metformin alone in patients with prediabetes may be attributed to reduced insulin resistance [23,24]. Metformin can also improve endothelial integrity, depicted by a decrease in endothelial dysfunction markers, including soluble intercellular adhesion molecules, soluble vascular cell adhesion molecules, and von Willebrand factors [53]. Long-term use of metformin in patients with prediabetes ameliorates the metabolic profile by reducing systolic and diastolic blood pressure (BP), LDL cholesterol, and triglycerides, and elevating HDL cholesterol levels [16]. Lifestyle interventions alone may also prevent progression to diabetes in patients with prediabetes and reduce participants’ weight [54]. Thus, the combined actions of metformin and lifestyle interventions may be more effective than any intervention alone, explaining our findings.
5.3.3. Metformin and Participants’ Weight
In the present study, we also found that metformin is effective in preventing T2DM in patients with obesity. A recent SR and MA revealed that metformin may significantly reduce body mass index (BMI) in non-diabetic patients with obesity [55]. This action may explain the preventive effect of metformin in obese patients. Additionally, glucagon-like peptide 1 receptor agonists (GLP-1 RAs) may also reduce weight and prevent T2DM in patients with obesity [56,57]. Furthermore, a statistically significant result was also found for patients without obesity. Thus, metformin might be optimal in preventing T2DM in normal-weight patients as well.
5.3.4. Metformin and Cardiovascular Disease
In our study, metformin could not prevent T2DM in patients with CVD. However, it has been estimated that metformin initiated early in patients with prediabetes may reduce total mortality and cardiovascular 10-year risk > 10% in patients without established CVD [58]. Moreover, dapagliflozin is effective in preventing diabetes in patients with CVD [59], and so is semaglutide in patients with obesity and CVD [60]. One RCT with CVD patients that was included in our analysis reported that the treatment was administered for a very short period (four months) [45], while in another three RCTs, the incidence of T2DM was a secondary outcome [41,42,46]. We believe that the treatment duration might have obscured the beneficial effects of metformin in preventing T2DM in CVD patients, while considering the prevention as a primary outcome is also of great importance. In our MA, we found that effectiveness was significant when T2DM was the primary outcome of the studies. Further research is needed for those patients.
5.3.5. Post-Intervention Effectiveness
Investigating the post-intervention effectiveness of both metformin alone and metformin with lifestyle interventions, we found that it might be weakened. Particularly, according to sensitivity analyses on the effect of RCTs that investigated patients who received metformin for 4 months in a 24-month follow-up post-intervention period [45], the overall effectiveness of metformin changed to non-significant. This result may explain potential heterogeneity. Additionally, the secondary analysis of the RCT with the largest sample size [37], analyzing metformin’s post-intervention effects, reported a higher incidence of diabetes [61]. These findings raise a high suspicion that effectiveness decreases after cessation of interventions.
5.4. Cost-Effectiveness
Lifestyle interventions have been assessed as cost-effective for quality of life (QUALY), increased survival, disability, and diabetes complications compared to usual care for patients with prediabetes [54]. Additionally, the benefits of lifestyle interventions versus standard care are value for money for diabetes prevention in patients with prediabetes, according to an economic evaluation study [62]. Metformin administration is also cost-effective for QUALY and healthcare systems [63]; however, it is not widely adopted in real life for diabetes prevention, enhancing unfavorable circumstances for both patients and healthcare systems [63]. Moreover, metformin combined with lifestyle interventions against standard care is also cost-effective for patients with prediabetes in India [64]. Incremental cost-effectiveness ratios (ICERs) of USD 145 per percentage point of diabetes risk reduction were found using screening, and USD 14,539 was saved per prevented T2DM case [64]. Finally, either lifestyle interventions or metformin is cost-effective compared to standard care for patients with prediabetes and overweight or obesity in Australia [65]. Particularly, the ICERs for metformin compared to standard care were USD 17,767 and, for lifestyle interventions versus standard care, USD 2702 per quality-adjusted life year gained [65].
5.5. Clinical Recommendations and Differences
According to the results of the present study, metformin may have a protective effect at a daily dosage of 1700 mg and after 18 months of administration in high-risk adults with a mean age over 60 years. Concerning specific subgroups of patients that were found to benefit from metformin in the prevention of T2DM, our MA identified Caucasians, women, and patients with prediabetes, obesity, and normal weight.
Metformin, when combined with lifestyle interventions, is effective at a lower dosage of 500 mg daily. The combined action is effective in younger participants with a mean age less than 60 years and when adopted for more than six months. The combination of metformin with lifestyle interventions is effective in Asian Indians, Asian Pakistanis, and men. Metformin and lifestyle interventions may prevent diabetes in patients with prediabetes faster compared to metformin alone. The metformin dosage is lower when combined with lifestyle interventions in younger patients, while the dosage should be increased in older persons. Those differences may be attributed to lower adherence and ability to follow lifestyle programs in older populations. Moreover, lifestyle interventions need ongoing support so that the beneficial effects are maintained in the long term [22].
5.6. Strengths and Limitations
To date, our MA is the most comprehensive as it includes updated data. We evaluated patients with any diabetes risk factors and aimed to answer clinical questions that arise in everyday clinical practice. In addition, we included only studies concerning metformin-naïve patients, in order to evaluate and emphasize the true effects of metformin on the prevention of T2DM. In this way, the results are not biased by the time period of metformin treatment. Moreover, the quality of evidence was moderate for the overall effectiveness of metformin and for the combination of metformin with lifestyle interventions. The study also has several limitations. The entire spectrum of patients with any predisposing risk factor (i.e., family history of diabetes, history of GDM, HY, dyslipidemia) is not entirely represented. Additionally, women in metformin and lifestyle interventions are underestimated; therefore, the true effect on women might have been underappreciated. Yet, we have included all the available data of patient populations that have been evaluated in existing RCTs. The combination of metformin with other interventions has not been widely assessed in RCTs across the world. Regarding heterogeneity, it could not be excluded. Subgroup differences may explain heterogeneity; however, sensitivity analyses failed to explain heterogeneity. Additionally, in sensitivity analyses, heterogeneity is also probably present. Finally, the comparison of metformin and standard care had a low sample size with only two RCTs, and the quality of evidence was low.
6. Conclusions
In conclusion, metformin alone or combined with lifestyle interventions may prevent T2DM in patients with prediabetes. The combined intervention is more effective in those patients. Additionally, metformin may prevent diabetes in patients with obesity and normal-weight patients as well. Metformin alone is more effective in Caucasian women, patients older than 60 years, and metformin with lifestyle interventions in Asian Indian men and those younger than 60 years. The combined intervention reduces metformin dosage and may act faster than metformin alone. Future research is needed to investigate its effectiveness in patients with more diabetes risk factors worldwide.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jcm14144947/s1: Figure S1:Subgroup Analysis for overall effectiveness of metformin based on same performance countries; Figure S2: Subgroup Analysis for overall effectiveness of metformin based on gender predominance; Figure S3: Subgroup Analysis for overall effectiveness of metformin based on participants’ mean age; Figure S4: Subgroup Analysis for overall effectiveness of metformin based on prediabetes as diabetes’ risk factor; Figure S5: Subgroup Analysis for overall effectiveness of metformin based on overweight/obesity as diabetes’ risk factor; Figure S6: Subgroup Analysis for overall effectiveness of metformin based on cardiovascular disease as diabetes’ risk factor; Figure S7: Subgroup Analysis for overall effectiveness of metformin based on Human Immunodeficiency Virus as diabetes’ risk factor; Figure S8: Subgroup Analysis for overall effectiveness of metformin based on metformin’s daily dosage; Figure S9: Subgroup Analysis for overall effectiveness of metformin based on metformin’s intervention duration.; Figure S10: Subgroup Analysis for overall effectiveness of metformin based on metformin’s post-intervention duration; Figure S11: Subgroup Analysis for overall effectiveness of metformin based on diabetes’ outcome assessment; Figure S12: Subgroup Analysis for overall effectiveness of metformin based on studies’ compared arms characteristics.; Figure S13: Subgroup Analysis for effectiveness of metformin and lifestyle interventions based on same performance countries; Figure S14: Subgroup Analysis for effectiveness of metformin and lifestyle interventions based on participants’ mean age; Figure S15: Subgroup Analysis for effectiveness of metformin and lifestyle interventions based on metformin’s daily dosage; Figure S16: Subgroup Analysis for effectiveness of metformin and lifestyle interventions based on metformin’s intervention duration; Figure S17: Subgroup Analysis for effectiveness of metformin and lifestyle interventions based on metformin’s post-intervention duration; Figure S18: Sensitivity Analysis for RCT with the largest sample size in overall effectiveness of metformin; Figure S19: Sensitivity Analysis for RCT with post-intervention in overall effectiveness of metformin; Figure S20: Sensitivity Analysis for RCTs with drop-out rate more than 10% in overall effectiveness of metformin; Figure S21: Sensitivity Analysis for RCT with the largest sample size in effectiveness of metformin and lifestyle interventions; Figure S22: Sensitivity Analysis for RCT with post-intervention in effectiveness of metformin and lifestyle interventions; Figure S23: Sensitivity Analysis for RCTs with drop-out rate more than 10% in effectiveness of metformin and lifestyle interventions; Table S1: Search strategy; Table S2: Efficacy and safety of Metformin’s included interventions in preventing Type-2 Diabetes; Table S3: Subgroup and sensitivity analyses for metformin’s overall effectiveness and metformin with lifestyle interventions compared to standard care; Table S4. Meta-regression for T2DM OR in metformin’s overall effect and metformin combined with lifestyle interventions.
Author Contributions
Conceptualization, G.I.T. and A.B.; methodology, G.I.T., V.T., G.E.Z., V.R., D.K., T.A., E.Z. and A.B.; software, G.I.T.; validation, G.I.T. and A.B.; formal analysis, G.I.T., V.T. and A.B.; investigation, G.I.T., V.T., G.E.Z., V.R. and A.B.; resources, G.I.T., V.T., G.E.Z., V.R. and A.B.; data curation, G.I.T., V.T., G.E.Z., V.R. and A.B.; writing—original draft preparation, G.I.T., V.T., G.E.Z., V.R., D.K., T.A., E.Z. and A.B.; writing—review and editing, G.I.T., V.T., E.Z. and A.B.; investigation, G.I.T., V.T., G.E.Z., V.R. and A.B.; visualization, G.I.T.; supervision, E.Z. and A.B.; project administration, G.I.T. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Gaglia, J.L.; Hilliard, M.E.; Isaacs, D.; et al. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. 1), S19–S40. [Google Scholar] [CrossRef]
- Joseph, J.J.; Deedwania, P.; Acharya, T.; Aguilar, D.; Bhatt, D.L.; Chyun, D.A.; Di Palo, K.E.; Golden, S.H.; Sperling, L.S.; on behalf of the American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health; et al. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: A scientific statement from the American Heart Association. Circulation 2022, 145, e722–e759. [Google Scholar] [CrossRef]
- Tao, Z.; Shi, A. Epidemiological perspectives of diabetes. Cell Biochem. Biophys. 2015, 73, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes—Global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Available online: https://www.cdc.gov/diabetes/risk-factors/?CDC_AAref_Val (accessed on 1 February 2025).
- Moore, S.M.; Hardie, E.A.; Hackworth, N.J.; Critchley, C.R.; Kyrios, M.; Buzwell, S.A.; Crafti, N.A. Can the onset of type 2 diabetes be delayed by a group-based lifestyle intervention? A randomised control trial. Psychol. Health 2011, 26, 485–499. [Google Scholar] [CrossRef]
- Jia, Y.; Lao, Y.; Zhu, H.; Li, N.; Leung, S. Is metformin still the most efficacious first-line oral hypoglycaemic drug in treating type 2 diabetes? A network meta-analysis of randomized controlled trials. Obes. Rev. 2019, 20, 1–12. [Google Scholar]
- Hung, W.T.; Chen, Y.J.; Cheng, C.-Y.; Ovbiagele, B.; Lee, M.; Hsu, C.-Y. Metformin plus a low hypoglycemic risk antidiabetic drug vs. metformin monotherapy for untreated type 2 diabetes mellitus: A meta-analysis of randomized controlled trials. Diabetes Res. Clin. Pract. 2022, 189, 109937. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, X.; Zhang, W. The effect of metformin therapy for preventing gestational diabetes mellitus in women with polycystic ovary syndrome: A meta-analysis. Exp. Clin. Endocrinol. Diabetes 2020, 128, 199–205. [Google Scholar] [CrossRef]
- Pani, A.; Gironi, I.; Di Vieste, G.; Mion, E.; Bertuzzi, F.; Pintaudi, B. From prediabetes to type 2 diabetes mellitus in women with polycystic ovary syndrome: Lifestyle and pharmacological management. Int. J. Endocrinol. 2020, 2020, 6276187. [Google Scholar] [CrossRef]
- Yu, H.; Sun, J.; Hu, H. Prophylactic administration of metformin reduces gestational diabetes mellitus incidence in high-risk populations: A meta-analysis. Ir. J. Med. Sci. 2024, 193, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Salpeter, S.R.; Buckley, N.S.; Kahn, J.A.; Salpeter, E.E. Meta-analysis: Metformin treatment in persons at risk for diabetes mellitus. Am. J. Med. 2008, 121, 149–157.e2. [Google Scholar] [CrossRef]
- Yamaoka, K.; Nemoto, A.; Tango, T. Comparison of the effectiveness of lifestyle modification with other treatments on the incidence of type 2 diabetes in people at high risk: A network meta-analysis. Nutrients 2019, 11, 1373. [Google Scholar] [CrossRef]
- Pang, B.; Zhao, L.H.; Li, X.; Song, J.; Li, Q.; Liao, X.; Feng, S.; Zhao, X.; Zheng, Y.; Gou, X.; et al. Different intervention strategies for preventing type 2 diabetes mellitus in China: A systematic review and network meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2018, 20, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Cao, J.Y.; Pang, Y.-C.; Xu, H.-C.; Chen, J.-W.; Yuan, J.-H.; Wang, R.; Zhang, C.-S.; Wang, L.-X.; Dong, J. Effects of lifestyle modification and anti-diabetic medicine on prediabetes progress: A systematic review and meta-analysis. Front. Endocrinol 2019, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Jonas, D.E.; Crotty, K.; Yun, J.D.Y.; Middleton, J.C.; Feltner, C.; Taylor-Phillips, S.; Barclay, C.; Dotson, A.; Baker, C.; Balio, C.P.; et al. Screening for prediabetes and type 2 diabetes: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2021, 326, 744–760. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Y.; Mu, Y.M.; Huang, Y.; Xuan, J. Network meta-analysis of the therapeutic effects of hypoglycemic drugs and intensive lifestyle modification on impaired glucose tolerance. Clin. Ther. 2021, 43, 1524–1556. [Google Scholar] [CrossRef]
- Patel, D.; Ayesha, I.E.; Monson, N.R.; Klair, N.; Saxena, A. The Effectiveness of Metformin in Diabetes Prevention: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e46108. [Google Scholar] [CrossRef]
- Schellenberg, E.S.; Dryden, D.M.; Vandermeer, B.H.C.; Korownyk, C. Lifestyle interventions for patients with and at risk for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2013, 159, 543–551. [Google Scholar] [CrossRef]
- Gillies, C.L.; Abrams, K.R.; Lambert, P.C.; Cooper, N.J.; Sutton, A.J.; Hsu, R.T.; Khunti, K. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: Systematic review and meta-analysis. BMJ 2007, 334, 299. [Google Scholar] [CrossRef]
- Chatterjee, S.; Davies, M.; Khunti, K. Pharmaceutical interventions for diabetes prevention in patients at risk. Am. J. Cardiovasc. Drugs 2018, 18, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Nimitphong, H.; Jiriyasin, S.; Kasemasawachanon, P.; Sungkanuparph, S. Metformin for preventing progression from prediabetes to diabetes mellitus in people living with human immunodeficiency virus. Cureus 2022, 14, e24540. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Shen, S.; Wang, X.; Dong, L.; Li, Q.; Ren, W.; Li, Y.; Bai, J.; Gong, Q.; et al. Safety and effectiveness of metformin plus lifestyle intervention compared with lifestyle intervention alone in preventing progression to diabetes in a Chinese population with impaired glucose regulation: A multicentre, open-label, randomised controlled trial. Lancet Diabetes Endocrinol. 2023, 11, 567–577. [Google Scholar] [PubMed]
- Garrib, A.; Kivuyo, S.; Bates, K.; Ramaiya, K.; Wang, D.; Majaliwa, E.; Simbauranga, R.; Charles, G.; van Widenfelt, E.; Luo, H.; et al. Metformin for the prevention of diabetes among people with HIV and either impaired fasting glucose or impaired glucose tolerance (prediabetes) in Tanzania: A Phase II randomised placebo-controlled trial. Diabetologia 2023, 66, 1882–1896. [Google Scholar] [CrossRef]
- Open Science Framework. Available online: https://osf.io/d4mrh (accessed on 24 May 2025).
- Guise, J.M.; Butler, M.E.; Chang, C.; Viswanathan, M.; Pigott, T.; Tugwell, P. AHRQ series on complex intervention systematic reviews—Paper 6: PRISMA-CI extension statement and checklist. J. Clin. Epidemiol. 2017, 90, 43–50. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Engels, E.A.; Schmid, C.H.; Terrin, N.; Olkin, I.; Lau, J. Heterogeneity and statistical significance in meta-analysis: An empirical study of 125 meta-analyses. Stat. Med. 2000, 19, 1707–1728. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V. Introduction to Meta-Analysis; John Wiley & Sons: West Sussex, UK, 2009; pp. 124–125, 192–200. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Controlling the risk of spurious findings from meta-regression. Stat. Med. 2004, 23, 1663–1682. [Google Scholar] [CrossRef]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef]
- Fontbonne, A.; Charles, M.A.; Juhan-Vague, I.; Bard, J.M.; André, P.; Isnard, F.; Cohen, J.M.; Grandmottet, P.; Vague, P.; Safar, M.E.; et al. The effect of metformin on the metabolic abnormalities associated with upper-body fat distribution. BIGPRO Study Group. Diabetes Care 1996, 19, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Pan, C.Y.; Lu, J.M.; Zhu, Y.; Wang, J.H.; Deng, X.X.; Xia, F.C.; Wang, H.Y. Effect of metformin on patients with impaired glucose tolerance. Diabet. Med. 1999, 16, 477–481. [Google Scholar] [CrossRef]
- Lehtovirta, M.; Forsén, B.; Gullström, M.; Häggblom, M.; Eriksson, J.G.; Taskinen, M.; Groop, L. Metabolic effects of metformin in patients with impaired glucose tolerance. Diabet. Med. 2001, 18, 578–583. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Snehalatha, C.; Mary, S.; Mukesh, B.; Bhaskar, A.D.; Vijay, V.; Indian Diabetes Prevention Programme (IDPP). The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006, 49, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Lu, J.M.; Wang, S.-Y.; Li, C.-L.; Zheng, R.-P.; Tian, H.; Wang, X.-L. Outcome of intensive integrated intervention in participants with impaired glucose regulation in China. Adv. Ther. 2011, 28, 511–519. [Google Scholar] [CrossRef]
- Iqbal Hydrie, M.Z.; Basit, A.; Shera, A.S.; Hussain, A. Effect of intervention in subjects with high risk of diabetes mellitus in Pakistan. J. Nutr. Metab. 2012, 2012, 867604. [Google Scholar] [CrossRef]
- Preiss, D.; Lloyd, S.M.; Ford, I.; McMurray, J.J.; Holman, R.R.; Welsh, P.; Fisher, M.; Packard, C.J.; Sattar, N. Metformin for non-diabetic patients with coronary heart disease (the CAMERA study): A randomised controlled trial. Lancet Diabetes Endocrinol. 2014, 2, 116–124. [Google Scholar] [CrossRef]
- Lexis, C.P.; van der Horst-Schrivers, A.N. The effect of metformin on cardiovascular risk profile in patients without diabetes presenting with acute myocardial infarction: Data from the Glycometabolic Intervention as adjunct to Primary Coronary Intervention in ST Elevation Myocardial Infarction (GIPS-III) trial. BMJ Open Diabetes Res. Care 2015, 3, e000090. [Google Scholar]
- Weber, M.B.; Ranjani, H.; Staimez, L.R.; Anjana, R.M.; Ali, M.K.; Narayan, K.V.; Mohan, V. The stepwise approach to diabetes prevention: Results from the D-CLIP randomized controlled trial. Diabetes Care 2016, 39, 1760–1767. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Perez, A.; Scanlan, A.B.; Alos, V.A.; Whitaker, R.C.; Foster, G.D.; Ackermann, R.T.; Ciolino, J.D.; Homko, C. PREVENT-DM comparative effectiveness trial of lifestyle intervention and metformin. Am. J. Prev. Med. 2017, 52, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.H.T.; Prins, J.K.B.; Schurer, R.A.J.; Lipsic, E.; Lexis, C.P.H.; van der Horst-Schrivers, A.N.A.; van Veldhuisen, D.J.; van der Horst, I.C.C.; van der Harst, P. Two-year follow-up of 4 months metformin treatment vs. placebo in ST-elevation myocardial infarction: Data from the GIPS-III RCT. Clin. Res. Cardiol. 2017, 106, 939–946. [Google Scholar] [CrossRef]
- Mohan, M.; Al-Talabany, S.; McKinnie, A.; Mordi, I.R.; Singh, J.S.S.; Gandy, S.J.; Baig, F.; Hussain, M.S.; Bhalraam, U.; Khan, F.; et al. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: The MET-REMODEL trial. Eur. Heart J. 2019, 40, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Amer, B.E.; Abdelgalil, M.S.; Hamad, A.A.; Abdelsayed, K.; Elaraby, A.; Abozaid, A.M.; Abd-ElGawad, M. Metformin plus lifestyle interventions versus lifestyle interventions alone for the delay or prevention of type 2 diabetes in individuals with prediabetes: A meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2024, 16, 273. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Fowler, S.E. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar]
- Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: The Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015, 3, 866–875. [Google Scholar] [CrossRef]
- Madsen, K.S.; Chi, Y.; Metzendorf, M.-I.; Richter, B.; Hemmingsen, B.; Cochrane Metabolic and Endocrine Disorders Group. Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2019, 12, CD008558. [Google Scholar]
- Mousavi, S.S.; Namayandeh, S.M.; Fallahzadeh, H.; Rahmanian, M.; Mollahosseini, M. Comparing the effectiveness of metformin with lifestyle modification for the primary prevention of type 2 diabetes: A systematic review and meta-analysis. BMC Endocr. Disord. 2023, 23, 198. [Google Scholar] [CrossRef]
- Vajje, J.; Khan, S.; Kaur, A.; Kataria, H.; Sarpoolaki, S.; Goudel, A.; Bhatti, A.H.; Allahwala, D. Comparison of the efficacy of metformin and lifestyle modification for the primary prevention of type 2 diabetes: A meta-analysis of randomized controlled trials. Cureus 2023, 15, e47105. [Google Scholar] [CrossRef]
- Caballero, A.E.; Delgado, A.; Aguilar-Salinas, C.A.; Herrera, A.N.; Castillo, J.L.; Cabrera, T.; Gomez-Perez, F.J.; Rull, J.A. The differential effects of metformin on markers of endothelial activation and inflammation in subjects with impaired glucose tolerance: A placebo-controlled, randomized clinical trial. J. Clin. Endocrinol. Metab. 2004, 89, 3943–3948. [Google Scholar] [CrossRef]
- Glechner, A.; Keuchel, L.; Affengruber, L.; Titscher, V.; Sommer, I.; Matyas, N.; Wagner, G.; Kien, C.; Klerings, I.; Gartlehner, G. Effects of lifestyle changes on adults with prediabetes: A systematic review and meta-analysis. Prim. Care Diabetes 2018, 12, 393–408. [Google Scholar] [CrossRef]
- Haber, R.; Zarzour, F.; Ghezzawi, M.; Saadeh, N.; Bacha, D.S.; Al Jebbawi, L.; Chakhtoura, M.; Mantzoros, C.S. The impact of metformin on weight and metabolic parameters in patients with obesity: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2024, 26, 1850–1867. [Google Scholar] [CrossRef]
- Wang, W.; Wei, R.; Huang, Z.; Luo, J.; Pan, Q.; Guo, L. Effects of treatment with Glucagon-like peptide-1 receptor agonist on prediabetes with overweight/obesity: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2023, 39, e3680. [Google Scholar] [CrossRef] [PubMed]
- Salamah, H.M.; Marey, A.; Abugdida, M.; Abualkhair, K.A.; Elshenawy, S.; Elhassan, W.A.F.; Naguib, M.M.; Malnev, D.; Durrani, J.; Bailey, R.; et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists on prediabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2024, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Zhang, Y.; Sun, W.; Kang, X.; Ji, H.; Sun, Y.; Jiang, L.; Zhao, X.; Gao, Q.; Lian, F.; et al. Early effective intervention can significantly reduce all-cause mortality in prediabetic patients: A systematic review and meta-analysis based on high-quality clinical studies. Front. Endocrinol. 2024, 15, 1294819. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Erlinge, D.; Storey, R.F.; McGuire, D.K.; de Belder, M.; Eriksson, N.; Andersen, K.; Austin, D.; Arefalk, G.; Carrick, D.; et al. Dapagliflozin in Myocardial Infarction without Diabetes or Heart Failure. NEJM Evid. 2024, 3, EVIDoa2300286. [Google Scholar] [CrossRef]
- Kahn, S.E.; Deanfield, J.E.; Jeppesen, O.K.; Emerson, S.S.; Boesgaard, T.W.; Colhoun, H.M.; Kushner, R.F.; Lingvay, I.; Burguera, B.; Gajos, G.; et al. Effect of Semaglutide on Regression and Progression of Glycemia in People with Overweight or Obesity but Without Diabetes in the SELECT Trial. Diabetes Care 2024, 47, 1350–1359. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. Effects of withdrawal from metformin on the development of diabetes in the diabetes prevention program. Diabetes Care 2003, 26, 977–980. [Google Scholar] [CrossRef]
- Gillett, M.; Royle, P.; Snaith, A.; Scotland, G.; Imamura, M.; Black, C.; Jick, S.; Wyness, L.; McNamee, P.; Waugh, N. Non-pharmacological interventions to reduce the risk of diabetes in people with impaired glucose regulation: A systematic review and economic evaluation. Health Technol. Assess. 2012, 16, 1–236. [Google Scholar] [CrossRef]
- Moin, T.; Schmittdiel, J.A. Review of metformin use for type 2 diabetes prevention. Am. J. Prev. Med. 2018, 55, 565–574. [Google Scholar] [CrossRef]
- Islek, D.; Weber, M.B.; Mohan, A.R.; Mohan, V.; Staimez, L.R.; Harish, R.; Narayan, K.M.V.; Laxy, M.; Ali, M.K. Cost-effectiveness of a Stepwise Approach vs. Standard Care for Diabetes Prevention in India. JAMA Netw. Open 2020, 3, e207359. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.J.; Tucker, D.M.D. Cost and clinical implications of diabetes prevention in an Australian setting: A long-term modeling analysis. Prim. Care Diabetes 2012, 6, 109–121. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).