Abstract
Background/Objectives: Ruptured abdominal aortic aneurysm (RAAA) remains a leading cause of vascular death, with mortality rates approaching 90%. Biomarkers capable of identifying the most at-risk population are urgently needed in the clinic. We aimed to identify potential alterations in the urine proteome that can enable non-invasive detection of abdominal aortic aneurysms (AAA) at high risk of rupture. Methods: We used multiplexed kinase inhibitor beads (MIBs) and quantitative mass spectrometry (MIB/MS) to examine potential biomarkers in urine samples. Quantitative proteomic profiling was conducted using iTRAQ labeling and LC-TEMPO MALDI-TOF/TOF analysis, revealing several dysregulated proteins in the urinary proteome between the two groups. MS and MS/MS data were generated using MALDI TOF/TOF instruments (models 5800 or 4800; AB SCIEX). MS/MS spectra were processed with ProteinPilot™ software version 3.0 (AB SCIEX) and matched against the UniProt/Swiss-Prot database for identification of proteins with an Unused ProtScore >1.3. Statistical tests were performed using R/Bioconductor software and bioinformatics analysis using open-source software. Results: We quantitatively measured activity over 130 kinases from various kinase families using MIB/MS with a threshold of 1.5-fold change in expression. Statistical analysis assigned significance to EPHB6, AXL, EPHB4, DDR1, EPHA2 and EPHB3. All were tyrosine kinases, and the Ephrin receptor type was dominant. The reduced expression of specific kinases identified by MIB/MS analysis was validated by Western blot. Conclusions: This pilot study presents a promising breakthrough in the diagnosis and surveillance of AAA. We identified six dysregulated tyrosine kinases in the urine proteome of patients with RAAAs, suggesting their potential as urinary biomarkers for early detection of AAA at high risk of rupture. However, these preliminary findings require confirmation in larger, prospective cohorts to validate their diagnostic utility and generalizability.
Keywords:
abdominal aortic aneurysm; rupture; biomarker; urine; proteome; tyrosine kinase; risk stratification 1. Introduction
Abdominal aortic aneurysms (AAAs) continue to present a significant clinical challenge due to their often silent progression and the potentially fatal outcome associated with rupture [1]. Improved management strategies are essential to minimizing subsequent aneurysm-related mortality and morbidity. Decision-making regarding intervention in patients with AAA is complex, as both repair and rupture carry high morbidity and mortality. Traditionally, decisions have been based largely on aneurysm diameter [2], a parameter that does not always reliably predict rupture risk. Aneurysms vary in expansion rate and risk of rupture, and these characteristics are not necessarily correlated with diameter [3,4]. Although aneurysms exceeding certain size thresholds are generally scheduled for repair, many of these cases may remain stable, whereas some smaller aneurysms can unexpectedly become unstable. In addition, the available surgical procedures—whether open repair or endovascular repair—carry significant risks of complications, highlighting the critical need for more precise risk stratification of AAA rupture.
In response to this clinical dilemma, recent research has increasingly focused on the identification of novel biomarkers that may provide improved prognostic insights [5]. Although much of the biomarker research to date has concentrated on blood-based markers, emerging evidence suggests that urine, as a non-invasive sample, may offer valuable molecular signatures associated with aneurysm instability [6].
In this context, proteomics—the comprehensive study of the proteome—plays a crucial role. Proteomics enables the detailed mapping of proteins across tissues, cells, and fluids such as plasma [7]. The proteome—representing the full complement of proteins within a cell at a given time—encompasses a diverse array of modified protein isoforms generated through alternative splicing and post-translational modifications [8,9]. As proteins are integral to nearly all cellular functions and regulatory mechanisms, alterations in their expression or structure are closely linked to disease pathology [10]. Accordingly, proteomic analysis provides a valuable approach for both quantitative and qualitative mapping of the proteome, and it may offer critical insights into the molecular mechanisms underlying biological processes in AAA.
Our study leverages proteomic analysis of urine to identify proteins associated with AAA rupture, with the aim of refining current risk assessment models. By integrating proteomic data with existing clinical parameters, we seek to more accurately identify patients at true risk of rupture as a guide in the decision of whether treatment should be surveillance or surgical intervention. This integrative approach not only promises to enhance patient outcomes through more tailored surgical planning but also highlights the potential of urinary biomarkers as a valuable supplement to traditional imaging and blood-based tests in the ongoing effort to predict aneurysm behavior with greater precision.
2. Materials and Methods
Urine samples were collected from five patients with RAAA who underwent acute surgery and five patients with non-ruptured AAA who underwent prophylactic surgical treatment. Patient demographics are summarized in Table 1. There were no significant differences between the non-ruptured and ruptured groups in terms of gender, age, aneurysm diameter, body mass index, smoking status, or the use of statins or nonsteroidal anti-inflammatory drugs.

Table 1.
Patient demographics.
Voided middle portion of urine (100 to 150 mL) was collected immediately before surgery from both RAAA and AAA patients. The urine was kept cold overnight and centrifuged at 2000× g for 10 min. The obtained supernatant was then rapidly frozen using liquid nitrogen and preserved at −80 °C for later analysis. Each aliquot contained ∼130 μg of protein, determined by the Bradford assay. Samples of approximately 130 μg were pulverized in liquid nitrogen using a mortar and pestle. The resulting tissue powder was transferred to microcentrifuge tubes and homogenized in a lysis buffer composed of urea (7 M), thiourea (2 M), CHAPS (4% w/v), and dithiothreitol (100 mM).
We employed a previously established methodology that combines multiplexed kinase inhibitor–conjugated beads (MIBs) with quantitative mass spectrometry (MIB/MS) [11] to investigate potential urinary biomarkers in RAAA and AAA patients. Quantitative proteomic profiling was conducted using iTRAQ labeling and LC-TEMPO MALDI-TOF/TOF analysis, revealing several dysregulated proteins in the urinary proteome between the two groups. The bead-immobilized inhibitors in MIBs compete with ATP for binding at the active site of protein kinases, allowing selective capture of activated kinases for later quantification by mass spectrometry. To visualize the trend in kinase abundance changes between RAAA/AAA samples, we applied a fold-change threshold of ±1.5, based on prior technical replicate analysis and criteria outlined by Unwin et al. [12]. The abundance of active protein kinases in RAAA samples was increased in 42 kinases and reduced in 88, compared to AAA samples. Changes in the urine were assessed using Imagene 9.0 Microarray Quantification and Analysis Software. Mass spectrometry data were processed using the MaxQuant software v. 1.6.12.0 and determined protein 25LFQ (Label-free quantification) intensity, showing the relative amount of protein in the sample compared to the results of the AAA patients.
MIBs were sequentially washed with 20 mL of high-salt buffer and 20 mL of low-salt buffer. Both buffers contained HEPES (50 mM, pH 7.5), Triton X-100 (0.5%), EDTA (1 mM), EGTA (1 mM), sodium fluoride (10 mM), and either 1 M NaCl (high-salt) or 150 mM NaCl (low-salt). Columns were given a final wash with 1 mL of 0.1% SDS before protein elution using 1 mL of 0.5% SDS at 100 °C for 5 min. MIBs were incubated with the prepared urine sample, stirring continuously for 1 h at 4 °C. After incubation, the solution with sorbent was centrifuged at 5000× g for 30 s. After discarding the supernatant, the sorbent was washed three times with 500 μL of TBS solution. Proteins were digested using modified trypsin (Promega, Singapore), and the resulting peptides were labeled with iTRAQ reagents (AB SCIEX, Framingham, MA, USA) under light-protected conditions for 2 h, according to the manufacturer’s protocol. Labeled peptides were then purified using PepClean C18 spin columns (Thermo Scientific, Waltham, MA, USA) and fractionated on Tempo™ LC MALDI Spotting System (AB SCIEX) equipped with a Chromolith® CapRod® RP-18e HR analytical column (Merck KGaA, Darmstadt, Germany).
MS and MS/MS data were generated using MALDI TOF/TOF instruments (models 5800 or 4800; AB SCIEX). The MS/MS spectra were processed with ProteinPilot™ software version 3.0 (AB SCIEX) (Paragon algorithm 3.0.0.0, 113442alg3000) and matched against the UniProt/Swiss-Prot database for identification of proteins. Identifications with an Unused ProtScore greater than 1.3 were accepted, corresponding to a confidence level of 95%.
Statistical analyses were performed using R (version 3.6.1)/Bioconductor statistical software (version 3.10) [13,14]. Results were confirmed using STATA (version 16.0) and SAS (version 9.4) statistical software tools. Gene ontology, pathway, and disease analyses were performed using standard databases.
Bioinformatics analysis was performed using open-source software AmiGO (version 2.4.26) [15,16,17], and GeneMANIA (http://www.genemania.org, accessed on 26 May 2025) [18].
A noninterfering assay was used to measure protein concentrations in selected samples (NI Protein Assay, Geno Technology Inc., St. Louis, MO, USA). Equal amounts of protein (5 or 10 µg) from each sample were loaded onto 10–20% or 4–20% Tris-Glycine gels (Invitrogen, Carlsbad, CA, USA) for one-dimensional Western blotting. Following electrophoresis, the separated proteins were transferred onto nitrocellulose membranes. Immunodetection of proteins was performed by incubating the nitrocellulose sheets for a half day at 4 °C in phosphate-buffered saline (PBS), consisting of KCl (2.7 mM), KH2PO4 (1.8 mM), Na2HPO4 (10.1 mM), NaCl, (140 mM; pH 7.3), with 0.05% Tween-20 and 5% skimmed milk. The membranes were rinsed in PBS containing 0.05% Tween-20 to eliminate excess blocking agents. Rabbit polyclonal antibodies against EPHB6 0.05 mg/mL (Abcam, Cambridge, UK) and AXL/UFO 0.5 mg/mL (Thermo Fisher, Waltham, MA, USA) were diluted to 1:50–1:200 and 1:2500–1:5000, respectively. The blots were incubated with primary antibodies for 1 h at room temperature, followed by five washes with PBS containing 0.05% Tween-20. Subsequently, membranes were incubated for 1 h with a 1:1000 dilution of peroxidase-conjugated swine anti-rabbit IgG secondary antibody (Dako, Glostrup, Denmark). After a final series of five washes in PBS-Tween, detection was performed using enhanced chemiluminescence (Amersham Biosciences Inc., Piscataway, NJ, USA). Band intensities were quantified with Imaging Densitometer GS710 and Quantity One software (v4.6.9) (Bio-Rad, Basel, Switzerland).
3. Results
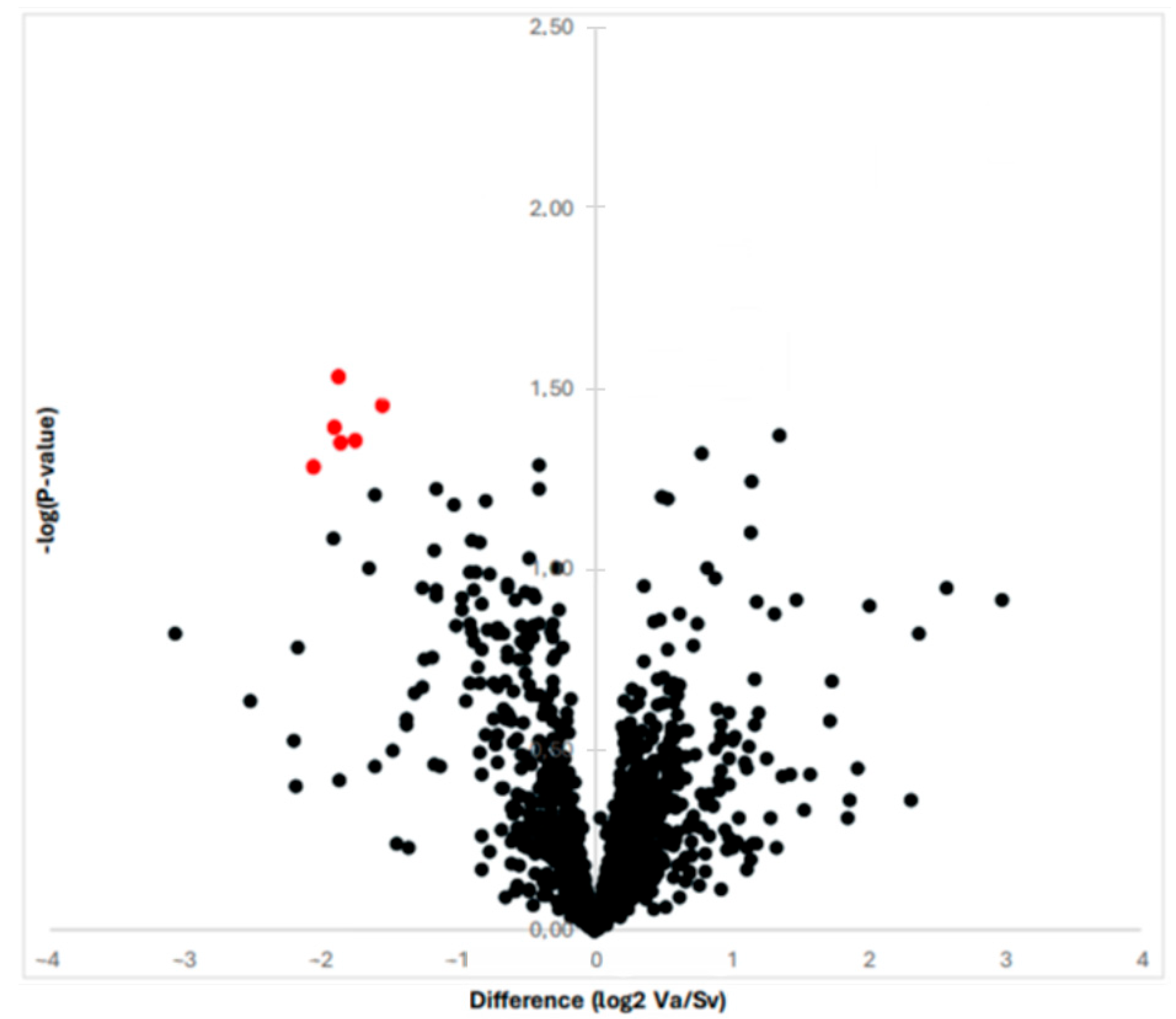
By means of proteomic analysis, we were able to obtain and compare the protein profiles of the RAAA and AAA specimens. The proteomic analysis is illustrated in Figure 1 with a volcano plot. In total, the MS and MS/MS analysis identified 613 proteins over the 10 specimens. The multiplexed kinase inhibitor-conjugated beads detected a total of 130 protein kinases with altered expression (>1.5 fold). The statistical analysis revealed 13 protein kinases with decreased expression in the RAAA compared to AAA specimens.
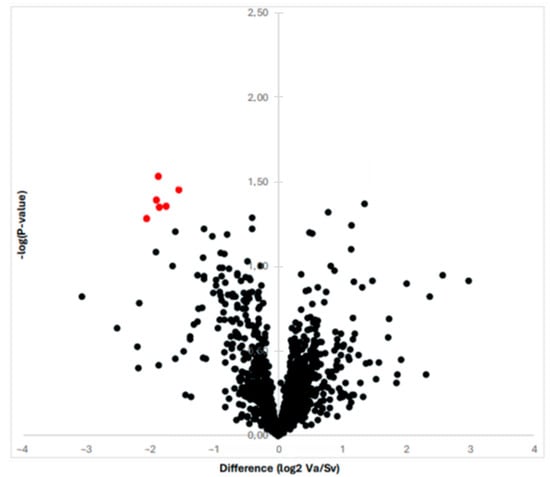
Figure 1.
The graph shows the proteins revealed by proteomic analysis with a Vulcano plot. The six most differentially expressed proteins are shown with red color; they all are down-regulated once to the left to the Y-axis.
A total of six protein kinases were downregulated with statistical significance (p < 0.05). All were tyrosine kinases, and the majority were Ephrin receptor type. The identified protein kinases were Ephrin type-B receptor 6 (EPHB6), Tyrosine-protein kinase receptor UFO (AXL), Ephrin type-B receptor 4 (EPHB4), Epithelial discoidin domain-containing receptor 1 (DDR1), Ephrin type-A receptor 2 (EPHA2), Ephrin type-B receptor 3 (EPHB3) (Table 2).

Table 2.
LFQ Intensity of significant protein kinases.
The other seven protein kinases only reached near-significance (p < 0.10) due to high within-group variability and are shown in Table 3. These were Nucleoside diphosphate kinase A (NME1), Dual specificity mitogen-activated protein kinase 1 (MAP2K1), Casein kinase I isoform alpha (CSNK1A1), Tyrosine-protein kinase Lyn (LYN), Integrin-linked protein kinase (ILK) Serine/threonine-protein kinase MRCK beta (CDC42BPB) and STE20-like serine/threonine-protein kinase (SLK).

Table 3.
LFQ Intensity of near significant protein kinases.
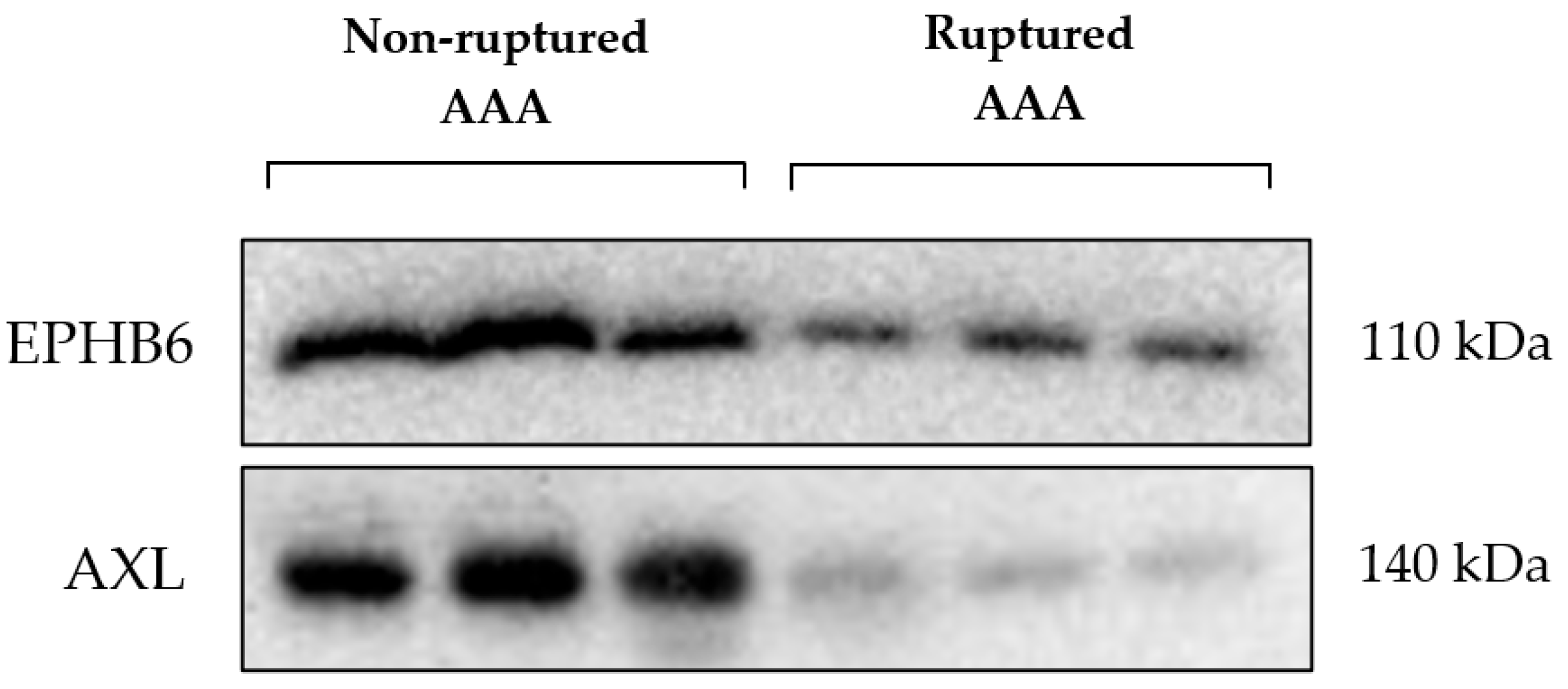
The difference in expression of the kinases EPHB6 and AXL identified by MIB/MS analysis was validated by Western blotting. These kinases were reduced in expression in the RAAA compared to AAA samples, as shown in Figure 2.
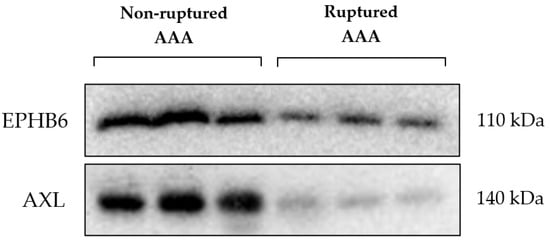
Figure 2.
Ephrin type-B receptor 6 (EPHB6); Tyrosine-protein kinase receptor UFO (AXL); Abdominal aortic aneurysm (AAA).
4. Discussion
The rupture of abdominal aortic aneurysms represents a critical and life-threatening event, demanding a comprehensive understanding of the underlying molecular mechanisms to facilitate the development of effective therapeutic strategies. While the precise etiology of AAA rupture remains multifaceted, involving complex interactions between biomechanical forces and intrinsic biological processes, the dysregulation of intracellular signaling pathways has emerged as a significant area of investigation.
In this study, we have identified six significantly downregulated proteins; all of them were tyrosine kinases and dominated by the ephrin receptor type. It was apparent that some of them could be associated with the formation and development of abdominal aortic aneurysm.
AAA rupture is associated with several molecular and cellular processes, including inflammation, extracellular matrix degradation, and vascular smooth muscle cell (VSMC) dysfunction [19,20,21,22]. Tyrosine kinases are known to be involved in various cellular signaling cascades, including those related to AAA rupture. Several studies have highlighted the involvement of different tyrosine kinase families in vascular pathologies [23,24,25]. The dysregulation of cellular signaling networks, including those involving tyrosine kinases, contributes to the pathogenesis and progression of AAA. Research has shown that vascular smooth muscle cells (VSMCs) derived from patients with AAA exhibit altered tyrosine kinase activity that is crucial for the structural integrity of the aortic wall [26]. VSMC dysfunction plays a role in the weakening of the aortic wall and subsequent risk of rupture [27].
Rombouts et al. identified that the kinase activity of FYN (tyrosine-protein kinase Fyn) was altered in VSMCs derived from patients with AAA compared to healthy controls [26]. This suggests that dysregulation of FYN and potentially other tyrosine kinases may contribute to the pathological changes seen in AAA, such as extracellular matrix remodeling, VSMC dysfunction, and inflammation.
Additionally, the inhibition of Bruton’s tyrosine kinase (BTK) has been shown to ameliorate vascular degeneration, dissection, and rupture in models of aortic aneurysm and dissection (AAD). BTK inhibition reduced macrophage infiltration and modulated macrophage polarization, thereby attenuating vascular inflammation and AAD progression [28]. This indicates that BTK plays a significant role in the inflammatory processes that contribute to aneurysm formation and rupture.
Furthermore, the downregulation of endothelial MerTK (MER proto-oncogene tyrosine kinase) in human aortic aneurysms and dissections (AAAD) has been associated with decreased endothelial cell function and smooth muscle cell phenotypic alterations, promoting aneurysm development [29]. This highlights the importance of maintaining proper tyrosine kinase signaling to prevent pathological changes leading to aneurysm rupture.
Dysregulation of Eph/ephrin signaling has been implicated in various vascular diseases, including AAA. The Ephrin receptor tyrosine kinases, particularly EphB and their ligands ephrin-B2, play significant roles in vascular development and pathology. Ephrin-B2 is crucial for endothelial sprouting and proliferation, as well as supporting vascular smooth muscle cells (VSMCs) [30].
The downregulation of Ephrin receptor tyrosine kinases, particularly ephrin-B2, impacts the progression and potential rupture of abdominal aortic aneurysms (AAA) through several mechanisms. Vascular Smooth Muscle Cell (VSMC) dysfunction. Endothelial cell dysfunction, inflammation, and angiogenesis.
Ephrin-B2 is crucial for the regulation of platelet-derived growth factor receptor β (PDGFRβ) in VSMCs. Downregulation of ephrin-B2 leads to enhanced PDGFRβ internalization and excessive activation of downstream signaling pathways such as MAP kinase and JNK while impairing Tiam1-Rac1 signaling. This results in defective VSMC proliferation and vessel wall integrity, contributing to aneurysm formation and progression [30]. Ephrin-B2 signaling is essential for endothelial cell function, including sprouting, proliferation, and maintaining junctional integrity. Disruption of ephrin-B2 signaling can lead to impaired endothelial cell behavior, contributing to vascular instability and aneurysm development [31,32].
Dysregulation of ephrin-B2 impacts the inflammatory response and angiogenesis, both of which are critical in the pathogenesis of AAA. Altered ephrin-B2 signaling can lead to increased endothelial permeability and pro-inflammatory differentiation, exacerbating vascular inflammation and promoting aneurysm growth [33,34].
In summary, the downregulation of ephrin-B2 disrupts critical signaling pathways in VSMCs and endothelial cells, leading to vascular wall defects, increased inflammation, and impaired angiogenesis, all of which contribute to the progression and potential rupture of AAAs.
The assessment of abdominal aortic aneurysm (AAA) rupture risk traditionally relies on monitoring the maximum diameter of the aneurysm over time, as this has been a primary indicator of potential rupture [35]. However, recent advancements have expanded this approach to incorporate biomechanical modeling and wall stress analysis through computational hemodynamics and solid mechanics simulations, aiming to predict aneurysm-specific wall motion more reliably [36].
Moreover, the integration of novel biomarkers reflecting underlying pathological processes holds promise for enhancing the prediction of rupture risk. Identifying key molecular players involved in aneurysm development and rupture is crucial for improving risk stratification and developing targeted therapies. This approach moves beyond simple diameter-based assessments and aims to capture the complex molecular mechanisms driving aneurysm progression and the weakening of the aortic wall [26].
Although maximum diameter remains a critical factor in assessing AAA rupture risk, incorporating biomechanical modeling and novel biomarkers offers a more comprehensive and accurate prediction of rupture risk.
Urinary biomarkers may serve as early indicators of AAA rupture, and our proteomics discovery might improve disease understanding and risk stratification of AAA. Urine offers a noninvasive and safe source for detecting such biomarkers. Further research would be needed to elucidate any direct connections.
It is important to note that a few limitations apply to the present study. A limited number of patients were included. For this study, we used well-characterized patient samples; however, a key caveat is that only end-stage disease can be assessed, and the inherent variability among patient specimens may affect the findings. Another factor restricting the comprehensive study of the protein repertoire is the current technical constraints. MS/MS and MIB technologies have revolutionized protein analysis and significantly accelerated the process of proteome analysis while maintaining high accuracy and reproducibility. However, the technical complexity of these methods still limits high-throughput capabilities, and lack of sensitivity are aspects that have been restrictive to date. As with omics studies, another issue is that proteins do not act on their own usually but exhibit their activity together with other proteins or ligands, and for the current study, we have focused on the expression levels of the proteins and to further establish the differentially expressed proteins’ putative role in the pathogenesis of RAAA, it is of outermost importance to further investigate those with functional studies. This study is a hypothesis-generating study and aims to identify proteins in the patient’s urine that may have a function in the pathogenesis of AAA rupture, and future studies in larger and independent cohorts will be needed to validate our findings.
5. Conclusions
This pilot study presents a promising breakthrough in the diagnosis and surveillance of AAA. We identified six dysregulated protein kinases in the urine proteome of patients with RAAAs, suggesting their potential as urinary biomarkers for early detection of AAA at high risk of rupture. However, these preliminary findings require confirmation in larger prospective cohorts to validate their diagnostic utility and generalizability.
Author Contributions
Conceptualization, S.U. and E.M.Ö.; methodology, S.U.; software, T.B., validation, S.U.; formal analysis, T.B.; investigation, S.U. and E.M.Ö.; data curation, E.M.Ö.; writing—original draft preparation, E.M.Ö.; writing—review and editing, S.U.; visualization, E.M.Ö.; supervision, S.U.; project administration, N.G.; funding acquisition, N.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. This research was funded by Asta and Rosa Jensen foundation.
Institutional Review Board Statement
This study is approved by the Central Denmark Region Committees on Health Research Ethics as well as the Danish Data Agency (1-10-72-245-13, date of approval: 12 February 2016) and was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish the paper.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AAA | Abdominal aortic aneurysms |
| RAAA | Ruptured abdominal aortic aneurysm |
| MIBs | Multiplexed kinase inhibitor-conjugated beads |
| MIB/MS | Quantitative mass spectrometry |
| NSAID | nonsteroidal anti-inflammatory drugs |
| LFQ | label-free quantification |
| TK | Tyrosine kinase |
| NME1 | Nucleoside diphosphate kinase A |
| MAP2K1 | Dual specificity mitogen-activated protein kinase 1 |
| CSNK1A1 | Casein kinase I isoform alpha |
| LYN | Tyrosine-protein kinase Lyn |
| ILK | Integrin-linked protein kinase |
| CDC42BPB | Serine/threonine-protein kinase MRCK beta |
| SLK | STE20-like serine/threonine-protein kinase |
| EPHB6 | Ephrin type-B receptor 6 |
| AXL | Tyrosine-protein kinase receptor UFO |
| EPHB4 | Ephrin type-B receptor 4 |
| DDR1 | Epithelial discoidin domain-containing receptor 1 |
| EPHA2 | Ephrin type-A receptor 2 |
| EPHB3 | Ephrin type-B receptor 3 |
| LYN | Tyrosine-protein kinase Lyn |
| VSMC | vascular smooth muscle cell |
| AAAD | Aortic aneurysms and dissections |
| PDGFRβ | platelet-derived growth factor receptor β |
| MerTK | MER proto-oncogene tyrosine kinase |
References
- Kent, K.C. Clinical practice. Abdominal aortic aneurysms. N. Engl. J. Med. 2014, 371, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Chaikof, E.L.; Dalman, R.L.; Eskandari, M.K.; Jackson, B.M.; Lee, W.A.; Mansour, M.A.; Mastracci, T.M.; Mell, M.; Murad, M.H.; Nguyen, L.L.; et al. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018, 67, 2–77.e72. [Google Scholar] [CrossRef]
- Spanos, K.; Eckstein, H.H.; Giannoukas, A.D. Small Abdominal Aortic Aneurysms Are Not All the Same. Angiology 2020, 71, 205–207. [Google Scholar] [CrossRef]
- Olson, S.L.; Wijesinha, M.A.; Panthofer, A.M.; Blackwelder, W.C.; Upchurch, G.R., Jr.; Terrin, M.L.; Curci, J.A.; Baxter, B.T.; Matsumura, J.S. Evaluating Growth Patterns of Abdominal Aortic Aneurysm Diameter With Serial Computed Tomography Surveillance. JAMA Surg. 2021, 156, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Abu-Raisi, M.; Feasson, M.; Shaikh, F.; Saposnik, G.; Mamdani, M.; Qadura, M. Current Prognostic Biomarkers for Abdominal Aortic Aneurysm: A Comprehensive Scoping Review of the Literature. Biomolecules 2024, 14, 661. [Google Scholar] [CrossRef]
- Xue, C.; Yang, B.; Fu, L.; Hou, H.; Qiang, J.; Zhou, C.; Gao, Y.; Mao, Z. Urine biomarkers can outperform serum biomarkers in certain diseases. Urine 2023, 5, 57–64. [Google Scholar] [CrossRef]
- Mateos-Cáceres, P.J.; García-Méndez, A.; López Farré, A.; Macaya, C.; Núñez, A.; Gómez, J.; Alonso-Orgaz, S.; Carrasco, C.; Burgos, M.E.; de Andrés, R.; et al. Proteomic analysis of plasma from patients during an acute coronary syndrome. J. Am. Coll. Cardiol. 2004, 44, 1578–1583. [Google Scholar] [CrossRef]
- Loscalzo, J. Proteomics in cardiovascular biology and medicine. Circulation 2003, 108, 380–383. [Google Scholar] [CrossRef]
- Zhou, Y.W.; Xu, P.C.; Cheng, Y. Basic pathogenesis of asthenia of healthy energy and blood stasis in liver cirrhosis studied by serum proteomics. Zhongguo Zhong Xi Yi Jie He Za Zhi 2011, 31, 595–602. [Google Scholar]
- Arrell, D.K.; Neverova, I.; Van Eyk, J.E. Cardiovascular proteomics: Evolution and potential. Circ. Res. 2001, 88, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.J.; Cox, N.J.; Zimmerman, E.I.; Dewar, B.J.; Duncan, J.S.; Whittle, M.C.; Nguyen, T.A.; Jones, L.S.; Ghose Roy, S.; Smalley, D.M.; et al. Application of multiplexed kinase inhibitor beads to study kinome adaptations in drug-resistant leukemia. PLoS ONE 2013, 8, e66755. [Google Scholar] [CrossRef] [PubMed]
- Unwin, R.D.; Griffiths, J.R.; Whetton, A.D. Simultaneous analysis of relative protein expression levels across multiple samples using iTRAQ isobaric tags with 2D nano LC-MS/MS. Nat. Protoc. 2010, 5, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef] [PubMed]
- Carbon, S.; Ireland, A.; Mungall, C.J.; Shu, S.; Marshall, B.; Lewis, S.; AmiGO Hub; Web Presence Working Group. AmiGO: Online access to ontology and annotation data. Bioinformatics 2008, 25, 288–289. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium; Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; et al. The Gene Ontology knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef]
- Warde-Farley, D.; Donaldson, S.L.; Comes, O.; Zuberi, K.; Badrawi, R.; Chao, P.; Franz, M.; Grouios, C.; Kazi, F.; Lopes, C.T.; et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010, 38, W214–W220. [Google Scholar] [CrossRef]
- Chiang, M.-T.; Chen, I.-M.; Hsu, F.-F.; Chen, Y.-H.; Tsai, M.-S.; Hsu, Y.-W.; Leu, H.-B.; Huang, P.-H.; Chen, J.-W.; Liu, F.-T.; et al. Gal-1 (Galectin-1) Upregulation Contributes to Abdominal Aortic Aneurysm Progression by Enhancing Vascular Inflammation. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 331–345. [Google Scholar] [CrossRef]
- Raffort, J.; Lareyre, F.; Clément, M.; Hassen-Khodja, R.; Chinetti, G.; Mallat, Z. Monocytes and macrophages in abdominal aortic aneurysm. Nat. Rev. Cardiol. 2017, 14, 457–471. [Google Scholar] [CrossRef]
- Choke, E.; Cockerill, G.W.; Dawson, J.; Wilson, R.W.; Jones, A.; Loftus, I.M.; Thompson, M.M. Increased angiogenesis at the site of abdominal aortic aneurysm rupture. Ann. N. Y. Acad. Sci. 2006, 1085, 315–319. [Google Scholar] [CrossRef]
- Kugo, H.; Zaima, N.; Tanaka, H.; Mouri, Y.; Yanagimoto, K.; Hayamizu, K.; Hashimoto, K.; Sasaki, T.; Sano, M.; Yata, T.; et al. Adipocyte in vascular wall can induce the rupture of abdominal aortic aneurysm. Sci. Rep. 2016, 6, 31268. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.M.; Jeong, K.; Lim, S.S. FAK Family Kinases in Vascular Diseases. Int. J. Mol. Sci. 2020, 21, 3630. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Src family kinases in chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2017, 313, F721–F728. [Google Scholar] [CrossRef]
- Yin, Z.; Zou, Y.; Wang, D.; Huang, X.; Xiong, S.; Cao, L.; Zhang, Y.; Sun, Y.; Zhang, N. Regulation of the Tec family of non-receptor tyrosine kinases in cardiovascular disease. Cell Death Discov. 2022, 8, 119. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, K.B.; van Merrienboer, T.A.R.; Henneman, A.A.; Knol, J.C.; Pham, T.V.; Piersma, S.R.; Jimenez, C.R.; Bogunovic, N.; van der Velden, J.; Yeung, K.K. Insight in the (Phospho)proteome of Vascular Smooth Muscle Cells Derived From Patients With Abdominal Aortic Aneurysm Reveals Novel Disease Mechanisms. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 2226–2243. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kong, W. Cellular signaling in Abdominal Aortic Aneurysm. Cell. Signal. 2020, 70, 109575. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Cheng, S.; Li, X.; Weng, X.; Yang, J. Pharmacological and genetic inhibition of BTK ameliorates vascular degeneration, dissection, and rupture. Life Sci. 2025, 369, 123533. [Google Scholar] [CrossRef]
- Liu, S.; Wu, J.; Banerjee, O.; Xue, B.; Shi, H.; Ding, Z. Big data analytics and scRNA-seq in human aortic aneurysms and dissections: Role of endothelial MerTK. Theranostics 2025, 15, 202–215. [Google Scholar] [CrossRef]
- Nakayama, A.; Nakayama, M.; Turner, C.J.; Höing, S.; Lepore, J.J.; Adams, R.H. Ephrin-B2 controls PDGFRβ internalization and signaling. Genes Dev. 2013, 27, 2576–2589. [Google Scholar] [CrossRef]
- Oike, Y.; Ito, Y.; Hamada, K.; Zhang, X.Q.; Miyata, K.; Arai, F.; Inada, T.; Araki, K.; Nakagata, N.; Takeya, M.; et al. Regulation of vasculogenesis and angiogenesis by EphB/ephrin-B2 signaling between endothelial cells and surrounding mesenchymal cells. Blood 2002, 100, 1326–1333. [Google Scholar] [CrossRef]
- Liu, H.; Devraj, K.; Möller, K.; Liebner, S.; Hecker, M.; Korff, T. EphrinB-mediated reverse signalling controls junctional integrity and pro-inflammatory differentiation of endothelial cells. Thromb. Haemost. 2014, 112, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, D.; Chmiel, P.; Kołodziej, P.; Borowski, G.; Feldo, M.; Kocki, J.; Bogucka-Kocka, A. Dysregulations of Key Regulators of Angiogenesis and Inflammation in Abdominal Aortic Aneurysm. Int. J. Mol. Sci. 2023, 24, 12087. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, M.G.; Morgan, M.; Woodruff, T.M.; Arumugam, T.V.; Taylor, S.M.; Carpenter, T.C.; Lackmann, M.; Boyd, A.W. Eph/Ephrin signaling in injury and inflammation. Am. J. Pathol. 2012, 181, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hu, T.; Lu, W.; Yu, Y.; Xue, S.; Wu, K.; Liu, Y.; Lin, J.; Bai, H.; Yun, Z.; et al. Morphology and biomechanical index predict the rupture location and rupture risk of abdominal aortic aneurysm. Sci. Rep. 2025, 15, 9604. [Google Scholar] [CrossRef]
- Polzer, S.; Gasser, T.C.; Vlachovský, R.; Kubíček, L.; Lambert, L.; Man, V.; Novák, K.; Slažanský, M.; Burša, J.; Staffa, R. Biomechanical indices are more sensitive than diameter in predicting rupture of asymptomatic abdominal aortic aneurysms. J. Vasc. Surg. 2020, 71, 617–626.e616. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).