Abstract
Background: Previous studies found high but very variable levels of tetranor-PGEM and PGDM (urine metabolites of prostaglandin (PG) E2 and PGD2, respectively) in persons with cystic fibrosis (pwCF). This study aims to assess the role of cyclooxygenase COX-1 and COX-2 genetic polymorphisms in PG production and of PG metabolites as potential markers of symptoms’ severity and imaging findings. Methods: A total of 30 healthy subjects and 103 pwCF were included in this study. Clinical and radiological CF severity was evaluated using clinical scoring methods and chest computed tomography (CT), respectively. Urine metabolites were measured using liquid chromatography/tandem mass spectrometry. Variants in the COX-1 gene (PTGS1 639 C>A, PTGS1 762+14delA and COX-2 gene: PTGS2-899G>C (-765G>C) and PTGS2 (8473T>C) were also analyzed. Results: PGE-M and PGD-M urine concentrations were significantly higher in pwCF than in controls. There were also statistically significant differences between clinically mild and moderate disease and severe disease. Patients with bronchiectasis and/or air trapping had higher PGE-M levels than patients without these complications. The four polymorphisms did not associate with clinical severity, air trapping, bronchiectasis, or urinary PG levels. Conclusions: These results suggest that urinary PG level testing can be used as a biomarker of CF severity. COX genetic polymorphisms are not involved in the variability of PG production.
1. Introduction
Cystic fibrosis (CF) is caused by mutations within the CF transmembrane conductance regulator (CFTR) gene leading to defective epithelial chloride transport in many organs. More than 2114 variants in the CFTR gene have been identified [1]. Various variants can be grouped into different classes based on their known or predicted molecular mechanisms of dysfunction and the functional consequences for the CFTR protein [2].
Class I: pathogenic variants in this category are associated with a lack of biosynthesis or defective biosynthesis, resulting in no CFTR protein. Class II: these gene variants fail to properly process the protein to a mature form and fail to transport the protein to the apical membrane. Class III: variants of this class affect the regulation of CFTR function by preventing ATP binding-induced nucleotide-binding dimerization resulting in the impaired gating of the CFTR channel. Class IV: variants affect the chloride conductance resulting in a normal amount of CFTR with some residual function at the apical membrane. Class V: variants associated with the reduced biosynthesis of a fully active CFTR protein. Class VI: variants destabilize the CFTR protein at the cell surface and produce a high turnover of CFTR. Class VII: large deletions in the CFTR gene resulting in impaired mRNA production.
A graduated risk of developing pancreatic insufficiency and pancreatitis, according to genotype severity, has been reported in various studies [3,4]. Class I, II, and III include pathogenic variants usually associated with pancreatic insufficiency and, thus, are considered severe variants, while class IV, V, and VI variants retain residual function and are usually associated with normal or slightly altered pancreatic function, being classified as mild [3,4]. Numerous studies assessing the impact of CFTR variants on the severity and progression of lung disease have presented discrepant results, some showing correlations between CFTR genotypes and lung disease [5,6,7,8,9], while others did not find any consistent correlation [10,11]. The poor correlation between the severity of the CFTR variants and the severity of the lung disease supports the notion that other cofactors contribute to the modulation of the phenotypic expression of the primary genotype. Due to the direct contact of the lung with microbial pathogens and airborne pollutants, many modulating factors might contribute to the variable lung phenotype observed in patients with the same genotype [12].
An early, sustained, and severe inflammatory process is seen in the airways of pwCF [13], which is characterized by excessive mucus production, chronic bacterial infection, and progressive tissue damage. Airway infection occurs early in the course of the disease; however, there are observations which support that lung inflammation is, at least in part, independent of infections and directly related to defective CFTR [14].
Various CFTR variants related with fatty acid metabolism abnormalities have been reported in CF, such as an abnormally high arachidonic acid (AA) to docosahexaenoic acid (DHA) ratio and a linoleic acid (LA) deficiency, which is directly related to the severity of the CFTR variants [11,15,16,17].
AA released from membrane glycerophospholipids is the rate-limiting step in the enhanced production of eicosanoids such as prostaglandin E2 (PGE2) [18]. There are two cyclooxygenase (COX) enzymes, COX-1 and COX-2, involved in the conversion of AA to PGE2. COX-1 is constitutively expressed in most cells and is involved in the regulation of physiological functions, whereas COX-2 expression is rapidly induced under inflammatory conditions [18]. An increased expression of both COX-1 and COX-2 is found in CF airways [19], which accounts for the increased PGE2 production reported in these patients [20,21].
Several findings support the concept that the enhanced COX-2 expression and increased PGE2 production found in CF are directly related to CFTR dysfunction rather than to the presence of an inflammatory process associated with chronic bacterial infection [22,23]. Chen et al. [23] demonstrated that the COX-2/PGE2 positive feedback loop is negatively regulated by CFTR under normal conditions but augmented with defective CFTR. Borrot et al. showed that CFTR inhibition leads to increased eicosanoid release [24]. Moreover, the absence of CFTR can disrupt cellular signaling networks with broad functional consequences including fatty acid abnormalities [25,26].
Elevated levels of tetranor-PGEM (PGE-M), the PGE2 metabolite detected in urine, have been associated with the severity of the CFTR variants [22]. However, marked differences can be found in urine PGE-M levels among patients with similar CFTR variants, suggesting that PGE2 production is regulated by factors other than the severity of the mutated receptor [22].
Interestingly, one study found that some COX-1 and COX-2 gene polymorphisms were associated with different effects on the severity of lung disease in CF patients with the F508del pathogenic variant [27]. However, it is still unclear whether these polymorphisms play any significant role in PGE2 production and, thereby, in disease severity.
We hypothesized that the severity of lung disease in CF correlates with the amount of PGE2 released, which in turn is related to the severity of the mutated CFTR and further regulated by the presence of some COX polymorphisms. We undertook the present study to test this hypothesis.
2. Subjects and Methods
2.1. Subjects
This study had a cross-sectional design. A total of 30 healthy subjects (12 male, 18 female) aged from 5 to 22 years (11.5 ± 0.75) and 103 pwCF (56 male, 47 female) aged from 4 to 24 years (12.68 ± 0.48) with stable CF were included in this study. PwCF were recruited from a single pediatric CF center (Hospital Universitari Vall d’Hebrón, Barcelona, Spain). The CF diagnosis was established based on clinical data, an abnormal sweat test (sweat chloride > 60 mmol/L), and bi-allelic CFTR pathogenic variants. PwCF were in a stable clinical condition at a regular follow-up visit. Healthy control children were recruited from families of hospital workers. The demographic characteristics of patients and healthy subjects were not statistically different. Clinical and radiological characteristics of pwCF are shown in Table 1. All participants provided informed consent, with parents giving informed consent, prior to enrolment. This study was approved by the institutional Ethics Committee (PR(AMI)143/2013).

Table 1.
Clinical and radiological characteristics of cystic fibrosis patients.
2.2. Methods
The forced expiratory volume in the first second (FEV1) and the forced vital capacity (FVC) were measured by spirometry in pwCF, and the best of three maneuvers, expressed as the percentage of predicted values, was chosen.
Pancreatic sufficiency was defined as the presence of a fecal elastase value > 200 μg/g.
All patients were genotyped using methods reported elsewhere [28]. They were classified into three groups (mild, moderate, and severe) based on the predicted functional consequences of the CFTR protein alteration and on the accepted premise of the dominant phenotypic effect conferred by the milder of the two CFTR pathogenic variants. PwCF carrying Class I, II, and Class III pathogenic variants in their alleles were considered severe, those carrying Class I, II, or III mutations in one allele associated with a Class IV, V, or VI in the second allele were classified as moderate, and those pwCF with any combination of Class IV, V, or VI in both alleles were considered mild. PwCF were divided into three clinical groups according to severity, which was established considering the frequency of upper airway infections, the number of pulmonary exacerbations requiring antibiotic therapy, and the number of pulmonary exacerbations requiring hospitalizations and intravenous antibiotic therapy (Table 1).
Each chest computed tomography (CT) consisted of a volumetric inspiratory and expiratory acquisition. All CTs were scored evaluating the six (lingula as a separate lobe) lung lobes for the presence of central and peripheral bronchiectasis and the extent of trapped air on expiratory CTs. Bronchiectasis was scored as 0 (no bronchiectasis), 1 (one lobe affected), 2 (two lobes affected), or 3 (three or more lobes affected). Similarly, the extension of air trapping was scored from 0 to 3. All scans were scored by a blinded observer radiologist.
Routinely, an airway sample (sputum or cough swab) was collected when pwCF attended the outpatient center every 1–2 months. These samples were incubated in different media for the identification of bacterial and fungal organisms via standard culture protocols.
Urine samples were collected from the participants and stored at −80 °C until analysis. The measurement of PGE-M and PGD-M and final urinary metabolites of PGE2 and prostaglandin D2 (PGD2), respectively, is considered the best method to accurately assess the biosynthesis of PGE2 and PGD2 generated via the COX pathway [20]. Urinary PGE-M and PGD-M levels were measured using liquid chromatography/tandem mass spectrometry (LC/MS) with slight modifications to the method previously described [21]. Briefly, 1 mL of urine was converted to an O-methyloxime derivative and purified by C18 solid phase extraction before LC/MS analysis. LC was performed on a 2.0 × 50 mm 1.7 µm particle Acquity BEH C18 column (Water Corporation, Barcelona, Spain). Mobile phase A was 95:4.9:0.1 (v/v/v) 5 mM ammonium acetate: [22,23] acetonitrile/acetic acid, and mobile phase B was 10.0:89.9:0.1 (v/v/v) 5 mM ammonium acetate/acetonitrile/acetic acid. The samples were separated by a gradient of 85–76% of mobile phase A over 6 min at a flow rate of 900 μL/min prior to delivery to a 6500 QTRAP (Sciex, Framingham, MA, USA) triple quadrupole mass spectrometer. Urinary creatinine (Cr) levels were measured by a Creatinine Colorimetric Assay kit from Cayman Chemical (Ann Arbor, MI, USA).
The DNA extracted from peripheral blood was analyzed for the presence of different functional variants described in the COX-1(PTGS1) and COX-2(PTGS2) genes. Both genes were sequenced by NGS using the Generead DNA seq Targeted Panels V2 technique on MiSeq (Illumina, San Diego, CA, USA) equipment. The following prostaglandin polymorphisms were analyzed: PTGS1 639 C>A, PTGS1 762+14delA, PTGS2-899G>C (-765G>C), and PTGS2 (8473T>C), all the variants were previously reported in both genes [27].
2.3. Statistical Analysis
Parametrical statistical methods were used with variables that satisfied the assumption for parametric statistical testing. With variables which did not satisfy this assumption, non-parametric statistical methods were applied to all data sets. For independent samples, comparisons between two groups were carried out using the Mann–Whitney U test, while the Kruskal–Wallis H test was used for multiple groups. The correlation between clinical scale severity and PG values was expressed as Spearman’s rank correlation coefficient. Statistical significance was established at p ≤ 0.05.
3. Results
The distribution of patients according to CFTR gene pathogenic variants’ severity and clinical and radiological characteristics is shown in Table 1. CFTR variants are depicted in Table 2.

Table 2.
Distribution of CFTR gene variants (N = 103).
3.1. Urinary PGE-M and PGD-M Levels
Urine PGE-M and PGD-M concentrations are expressed as medians and interquartile range (25–75th interquartile). Urine PGE-M concentrations were significantly (p < 0.0001) higher in pwCF patients (18.10; 7.60–30.50 ng/mg Cr) versus healthy controls (5.65; 3.48–11.48 ng/mg Cr). Similarly, PGD-M levels in pwCF (5.10; 2.50–8.30 ng/mg Cr) were significantly (p < 0.01) higher than in healthy controls (2.40; 1.70–5.65 ng/mg Cr).
3.2. Correlations between Urinary PGE-M and PGD-M Levels and CFTR Gene Mutation Severity
When urinary PGE-M levels were compared between healthy controls and pwCF with mild, moderate, or severe phenotypes, there were no differences between healthy controls and patients carrying either the mild or moderate phenotype (Figure 1A). In contrast, there were statistically significant differences between the severe phenotype and both healthy controls (p < 0.0001) and mild pathogenic variants (p < 0.0001). There were no statistically significant differences between mild and moderate phenotypes or moderate and severe phenotypes. Similar results were produced when urinary PGD-M levels were compared, with significant differences only between the severe phenotype and healthy controls (p < 0.001) and patients carrying the mild phenotype (p < 0.01) (Figure 1B).
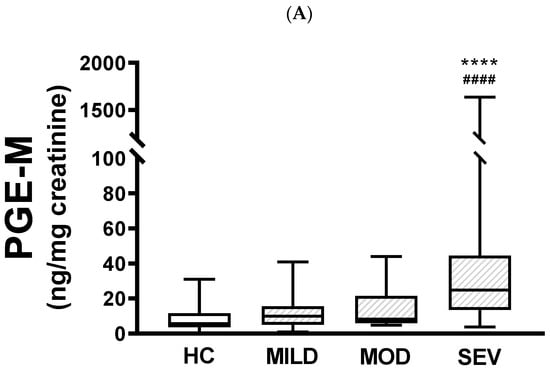
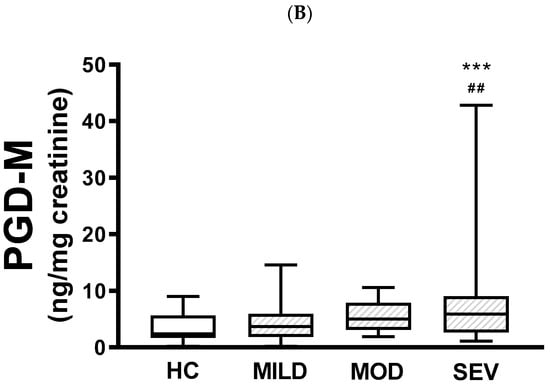
Figure 1.
Urinary PGE-M and PGD-M levels and CFTR gene pathogenic variants severity. PGE-M (Panel A) and PGD-M (Panel B) concentrations were analyzed by liquid chromatography/tandem mass spectrometry (LC/MS) in urine samples from healthy controls (HC, N = 30) and patients with mild (MILD, N = 29), moderate (MOD, N = 7), and severe (SEV, N = 65) CFTR gene phenotypes. The solid line indicates the median, the box indicates 25–75th percentiles, and whiskers represent the minimum and maximum values. An unpaired t-test was used for statistical comparison. **** p ≤ 0.0001 and *** p ≤ 0.001 compared with HC; #### p ≤ 0.0001 and ## p ≤ 0.01 compared with MILD.
3.3. Associations between Urinary PGE-M and PGD-M Levels and CF Severity Parameters
In patients with pancreatic insufficiency (N = 70), PGE-M levels (24.25; 12.48–44.50 ng/mg Cr) were higher than in patients with conserved pancreatic function (N = 33) (8.60; 4.60–16.80 ng/mg Cr, p < 0.0001). Similarly, PGD-M levels (6.15; 3.00–9.02 ng/mg Cr) were higher than in patients with conserved pancreatic function (3.60; 1.75–5.95 ng/mg Cr, p < 0.01).
When urinary PGE-M levels were compared between healthy subjects and pwCF with mild, moderate, or severe clinical severity, there were differences between healthy subjects and pwCF with mild (p < 0.001), moderate (p < 0.0001), and severe disease (p < 0.0001). Also, there were statistically significant differences between mild and moderate disease (p < 0.001) and mild and severe disease (p < 0.001); however, the difference between moderate and severe was not statistically significant (Figure 2A). Similar results were obtained with PGD-M levels, except that there were no statistical differences between healthy controls and pwCF with mild disease nor between moderate and severe disease (Figure 2B).
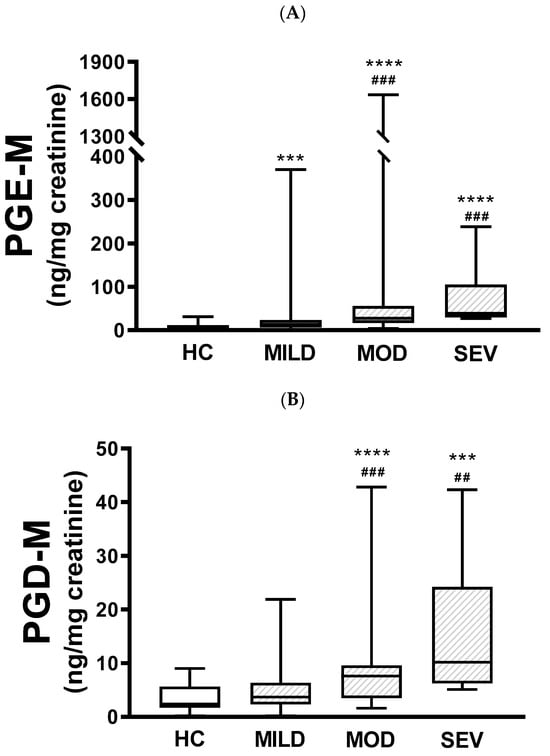
Figure 2.
Urinary PGE-M and PGD-M levels and clinical severity. PGE-M (Panel A) and PGD-M (Panel B) concentrations were analyzed by liquid chromatography/tandem mass spectrometry (LC/MS) in urine samples from healthy controls (HC, N = 30) and patients with mild (MILD, N = 29), moderate (MOD, N = 7), and severe (SEV, N = 65) clinical phenotypes. The solid line indicates the median, the box indicates 25–75th percentiles, and whiskers represent the minimum and maximum values. An unpaired t-test was used for statistical comparison. **** p ≤ 0.0001 and *** p ≤ 0.001 compared with HC; ### p ≤ 0.001 and ## p ≤ 0.01 compared with MILD.
There was no correlation between PGE-M levels with either the FEV1 (r = −0.1235) or the FVC (r = −0.0964). Similarly, PGD-M levels did not correlate with either the FEV1 (r = −0.1901) or the FVC (r = −0.1732).
Since the number of pwCF scoring 2 and 3 was low, the analysis of the presence of bronchiectasis and air trapping was performed binarily (with or without). Patients with bronchiectasis (n = 45) had higher PGE-M (27.80; 16.00–56.30 ng/mg Cr, p < 0.0001) and PGD-M (7.00; 3.70–9.90 ng/mg Cr, p < 0.001) levels than patients without bronchiectasis (n = 57) (PGE-M: 12.70; 6.45–20.35 ng/mg Cr; PGD-M: 3.70; 2.30–6.45 ng/mg Cr).
The presence of air trapping in the CT scan (n = 64) was associated with higher PGE-M levels (22.25; 11.80–41.95 ng/mg Cr) than in those without radiological findings of air trapping (n = 28) (11.65; 6.37–27.68 ng/mg Cr, p < 0.05). In contrast, there were no differences in PGD-M levels between patients with and without air trapping.
3.4. Associations between Urinary PGE-M and PGD-M Levels, Airway Infections, and a Docosahexaenoic (DHA)-Supplemented Diet
PwCF with airways chronically colonized by fungi (n = 15) had similar urinary PGE-M and PGD-M levels as non-colonized patients (n = 87).
Patients with a DHA-supplemented diet (n = 5) had lower urinary PGE-M levels (6.60; 4.90–14.70 ng/mg Cr) than those without a supplemented diet (n = 96) (19.10; 7.80–32.95), but the difference was not statistically significant (p = 0.058). On the other hand, there were no differences in urinary PGD-M levels between the two groups.
3.5. Associations between Prostaglandin Polymorphisms with CF Severity and Urinary Prostaglandin Levels
The four polymorphisms were analyzed in 102 pwCF. The COX1-639 C>A polymorphism was identified as homozygous in 4 patients (3.9%), heterozygous in 17 (16.7%), and negative in 81 (79.4%).
The COX1-762+14delA polymorphism was identified as homozygous in 1 pwCF (1%), heterozygous in 17 (16.7%), and negative in 84 (82.4%).
The COX-2-765G>C polymorphism was identified as homozygous in 4 pwCF (3.9%), heterozygous in 31 (30.4%), and negative in 67 (65.7%).
The COX-2-8473T>C polymorphism was identified as homozygous in 9 pwCF (8.8%), heterozygous in 40 (39.2%), and negative in 53 (52%).
When comparing the four polymorphisms with the different variables: clinical severity, genetic variants, pancreatic function, lung function, and the presence of air trapping and/or bronchiectasis with the chest CT, no significant differences were. In addition, there were no significant differences between the presence of polymorphisms and chronic colonization by Staphyloccocus aureus (SA), Pseudomonas aeruginosa (PA), and PGE-M and PGD-M urinary levels.
4. Discussion
Forty years ago, Charlotte M. Anderson hypothesized that a disturbed PGE2 metabolism could be involved in CF [29]. Supporting this hypothesis, several studies have reported the ability of CFTR to regulate COX-2 expression and, thereby, PGE2 biosynthesis [19,20,21,22,23]. A defective CFTR protein leads to enhanced COX-2 expression resulting in an increased release of PGE2 in CF patients [19,21,23]. Moreover, it was shown that CFTR inhibition leads to membrane destabilization, favoring eicosanoid synthesis [24]. In addition to its role as an ion channel, CFTR also forms complexes with a host of signaling proteins (kinases and phosphatases) involved in fatty acid metabolism [25,26].
Given the direct relationship reported between CFTR dysfunction and increased PG synthesis, we hypothesized that the amount of PG released in CF patients would be a marker of the severity of CFTR dysfunction. According to this hypothesis, PG production should associate with parameters of CF severity.
Our study resulted in several main findings: (a) there is a limited relationship between the severity of CFTR genetic dysfunction and PG production; (b) exocrine pancreatic insufficiency is closely associated with the severity of CFTR dysfunction and PG production; (c) there is no correlation between PG levels and lung function parameters; (d) PG production associates with both clinical status and radiological findings (bronchiectasis and air trapping); and (e) COX-1 and COX-2 gene polymorphisms do not appear to contribute to the regulation of PG synthesis.
Previous studies found moderate or no correlation between CFTR severity and PG production. Moreover, there are notable differences between urinary PG levels in patients with the same pathogenic variants, which suggest that factors other than mutation severity are involved in the regulation of PG metabolism. These factors remain to be elucidated. A previous study suggested the potential role of COX-1 and COX-2 gene polymorphisms in the clinical severity of pwCF harboring the F508del mutation [23]; however, we found no relationship between COX polymorphisms and either severity parameters or urinary levels of PGE-M and PGD-M that reflect systemic prostanoid production.
In keeping with previous reports, our study shows a relationship between the presence of normal or defective exocrine pancreatic function and the severity of CFTR pathogenic variants and urinary PG levels [20].
FEV1 measurement has been a central outcome measurement for clinical management and trials. However, various studies concluded that the FEV1 has limited sensitivity in detecting disease severity and monitoring disease progression [30,31]. Our study replicates previous observations regarding the lack of correlation between lung function parameters (FEV1 and FVC) and PG biosynthesis in CF patients [21]. Based on the concept that PG biosynthesis is associated with the severity of CFTR dysfunction, our results lend further support to studies reporting that lung function assessment has a limited value in the evaluation of CF severity.
In our study, we found that urinary PGE-M levels were associated with the clinical severity of the disease, detecting significant differences between healthy controls and pwCF with mild, moderate, and severe clinical severity. We also found significant differences between mild and moderate CF disease and mild and severe CF disease. Various recent studies support that bronchiectasis detected by chest CT is a sensitive indicator of prognosis, pulmonary exacerbations, and mortality in pwCF patients [32,33,34]. Compared with bronchiectasis, the potential relevance of air trapping as a marker of disease severity remains to be clearly established. Nevertheless, a previous study validated the presence of trapped air in the CT scan as an independent predictor of pulmonary exacerbations [35]. We found marked differences in PG levels between pwCF with and without bronchiectasis in the CT scan. Moreover, the presence of air trapping was also associated with higher levels of PGE-M than in those without radiological findings of air trapping. Taken together, clinical and radiological findings support the hypothesis that the level of PG production is a potential marker of disease severity.
Interestingly, and in accordance with our results, a very recent study found that PGE2 levels in bronchoalveolar lavage fluid collected from the lung of CF patients correlated positively and significantly with disease progression; the higher the PGE2 levels, the faster the progression of the disease [36].
Chronic infection can stimulate COX-2 expression and, therefore, induce PG release. However, in our study, we did not find any difference in urinary PG levels between chronically colonized and non-colonized patients.
A high-dose DHA supplementation diet can improve clinical outcomes, such as disease exacerbations associated with a decrease in inflammatory markers [25]. In our study, we found reduced urinary PG levels in pwCF on a supplemented DHA diet compared with those not on this diet, but the difference only tended towards statistical significance possibly because only a few patients from our cohort were on this therapy. In a very recent study, the effects of a supplemented DHA diet on urinary PGE2 levels were assessed and compared with a control placebo diet. There were no differences in the impact of the supplemented diet and placebo on PGE2 production. The study, however, was carried out in a small sample of pwCF with low baseline urinary PGE2 levels. Thus, a more extensive clinical trial will be required to assess the effects of this therapy on PG production.
A recent study investigated the effects of ivacaftor on urinary PGE-M levels in pwCF with the G551D mutation. Ivacaftor treatment significantly decreased the urine levels of PGE-M, suggesting urinary PGE-M as an interesting marker to assess the efficacy of new CF therapies [37].
The mechanisms underlying the relationship between defective CFTR function and excessive PGE2 synthesis remain to be fully elucidated. We are tempted to speculate that the increased PGE2 release in CF is intended to stimulate the activity of defective CFTR. When CFTR recovers its function, at least partially, with some of the new therapies, the excess PGE2 production is no longer needed.
Our study has some limitations such as not having considered the symptoms of other organs or systems affected in pwCF such as the gastrointestinal tract. Similarly, sweat chloride levels were not taken into account, a test that is related to the severity of the disease and that has been shown to be useful to assess the effectiveness of new therapies with CFTR modulators.
In summary, there is a significant need to develop new, relevant biomarkers to monitor clinical care prognosis and evolution as well as the effects of new therapies in CF. Taken together, the data reported in the present study suggest that measuring urinary PG levels could fulfill this role. On the other hand, we found no relationship between COX polymorphisms with either severity parameters or urinary levels of PGE-M and PGD-M that reflect systemic prostanoid production.
Author Contributions
Conceptualization, S.G. and C.P.; methodology, J.R.-F., P.F.-A., I.L., S.R.-A., E.G.-A. and E.F.T.; validation, S.G., E.F.T. and C.P.; formal analysis, S.G., J.R.-F., E.F.T. and C.P.; investigation, S.G., J.R.-F., P.F.-A., I.L., S.R.-A., E.G.-A., E.F.T. and C.P.; data curation, S.G., J.R.-F. and E.F.T.; writing—original draft preparation, S.G. and C.P.; writing—review and editing S.G. and C.P.; funding acquisition, S.G. All authors have read and agreed to the published version of the manuscript.
Funding
The study was carried out with the support of a grant from the Fundació Catalana de Pneumologia (FUCAP).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Vall d’Hebron Hospital Ethics Committee (PR(AMI)143/2013, 10 October 2013).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is unavailable due to ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Available online: http://www.genet.sickkids.on.ca/ (accessed on 25 April 2011).
- De Boeck, K.; Amaral, M.D. Progress in therapies for cystic fibrosis. Lancet Respir. Med. 2016, 4, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.Y.; Dorfman, R.; Cipolli, M.; Gonska, T.; Castellani, C.; Keenan, K.; Freedman, S.D.; Zielenski, J.; Berthiaume, Y.; Corey, M.; et al. Type of CFRT mutations determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology 2011, 140, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Kristidis, P.; Bozon, D.; Corey, M.; Markiewicz, D.; Rommens, J.; Tsui, L.C.; Durie, P. Genetic determination of exocrine pancreatic function in cystic fibrosis. Am. J. Hum. Genet. 1992, 50, 1178–1184. [Google Scholar] [PubMed]
- Cleveland, R.H.; Zurakowski, D.; Slattery, D.; Collin, A.A. Cystic fibrosis genotype and assessing rates of decline in pulmonary status. Radiology 2009, 253, 813–821. [Google Scholar] [CrossRef] [PubMed]
- de Gracia, J.; Mata, F.; Álvarez, A.; Casals, T.; Gatner, S.; Vendrell, M.; de la Rosa, D.; Guarner, L.; Hermosilla, E. Genotype-phenotype correlation for pulmonary function in cystic fibrosis. Thorax 2005, 60, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Cogen, J.; Emerson, J.; Sanders, D.B.; Ren, C.; Schechter, M.S.; Gibson, R.L.; Morgan, W.; Rosenfeld, M. Risk factors for lung function decline in a large cohort of young cystic fibrosis patients. Pediatr. Pulmonol. 2015, 50, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Gran, K.H.; Geus, W.P.; Bakker, W.; Lamers, C.B.; Heijerman, H.G. Genetic and clinical features of patients with cystic fibrosis diagnosed after the age of 16 years. Thorax 1995, 50, 1301–1304. [Google Scholar]
- Hubert, D.; Bienvenu, T.; Desmazes-Dufeu, N.; Fajac, I.; Lacronique, J.; Matran, R.; Kaplan, J.; Dusser, D. Genotype-phenotype relationships in a cohort of adult cystic fibrosis patients. Eur. Respir. J. 1996, 9, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Borgo, G.; Gasparini, P.; Bonizzato, A.; Cabrini, G.; Mastella, G.; Pignatti, P.F. Cystic fibrosis: The delta508 mutation does not lead to an exceptional severe phenotype. A cohort study. J. Pediatr. 1993, 152, 1006–1011. [Google Scholar] [CrossRef]
- Lai, H.J.; Cheng, Y.; Cho, H.; Kosorok, M.R.; Farrel, P.M. Association between initial disease presentation, lung disease outcomes, and survival in patient with cystic fibrosis. Am. J. Epidemiol. 2004, 159, 537–5446. [Google Scholar] [CrossRef]
- Zielenski, J. Genotype and phenotype in cystic fibrosis. Respiration 2000, 67, 117–133. [Google Scholar] [CrossRef]
- Cantin, A.M.; Hartl, D.; Konstan, M.W.; Chmiel, J. Inflammation in cystic fibrosis lung disease: Pathogenesis and therapy. J. Cyst. Fibros. 2015, 14, 419–430. [Google Scholar] [CrossRef]
- Verhaeghe, K.; Delbecque, K.; de Leval, L.; Oury, C.; Bours, V. Early inflammation in the airways of the cystic fibrosis foetus. J. Cyst. Fibros. 2007, 6, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; McCarron, A.; Rout-Pitt, N.; Donnelley, M.; Parsons, D.W.; Hryciw, D.H. Essential Fatty Acid Deficiency in Cystic Fibrosis Disease Progression: Role of Genotype and Sex. Nutrients 2022, 14, 4666. [Google Scholar] [CrossRef]
- Freedman, S.D.; Blanco, P.G.; Zaman, M.M.; Shea, J.C.; Ollero, M.; Hopper, I.K.; Weed, D.A.; Gelrud, A.; Regan, M.M.; Laposata, M.; et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N. Engl. J. Med. 2004, 350, 560–569. [Google Scholar] [CrossRef]
- Yang, J.; Eiserich, J.P.; Cross, C.E.; Morrisey, B.M.; Hammok, B.C. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free Radic. Biol. Med. 2012, 53, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; Dewitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular and molecular biology. Ann. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef]
- Roca-Ferrer, J.; Pujols, L.; Gartner, S.; Moreno, A.; Pumarola, F.; Mullol, J.; Cobos, N.; Picado, C. Upregulation of COX-1 and COX-2 in nasal polyps in cystic fibrosis. Thorax 2006, 61, 592–596. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.G.; Thomsen, K.; Brown, R.F.; Laposate, M. Elevated prostaglandin E metabolites and abnormal fatty acids at baseline in pediatric patients: A pilot study. Prostaglandins Leukot. Essent. Fat. Acids 2016, 113, 46–49. [Google Scholar] [CrossRef]
- Jabr, S.; Gartner, S.; Milne, G.L.; Roca-Ferrer, J.; Casas, J.; Moreno, A.; Gelpi, E.; Picado, C. Quantification of major urinary metabolites of PGE2 and PGD2 in cystic fibrosis: Correlation with disease severity. Prostaglandins Leukot. Essent. Fat. Acids 2013, 89, 121–126. [Google Scholar] [CrossRef]
- Medjane, S.; Raymon, B.; Wu, Y.; Touqui, L. Impact of CFTR delta508 mutation on prostaglandin E2 production and type IIA phospholipase A2 expression by pulmonary epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 289, L816–L824. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, X.H.; Chen, H.; Guo, J.H.; Tsang, L.L.; Yu, M.K.; Xu, W.M.; Chan, H.C. CFRT negatively regulates cyclooxygenase-2-PGE2 positive feedback loop in inflammation. J. Cell. Physiol. 2012, 227, 2759–2766. [Google Scholar] [CrossRef] [PubMed]
- Borot, F.; Vieu, D.V.; Faure, G.; Fritsch, J.; Colas, J.; Moriceau, S. Eicosanoid Release Is Increased by Membrane Destabilization and CFTR Inhibition in Calu-3 Cells. PLoS ONE 2009, 4, e7116. [Google Scholar] [CrossRef] [PubMed]
- Seegmiller, A.C. Abnormal unsaturated fatty acid metabolism in cystic fibrosis: Biochemical mechanisms and clinical implications. Int. J. Mol. Sci. 2014, 15, 16083–16099. [Google Scholar] [CrossRef] [PubMed]
- Kunzelmann, K.; Mehta, A. CFTR: A hub for kinases and crosstalk of cAMP and Ca2+. FEBS J. 2013, 280, 4417–4429. [Google Scholar] [CrossRef] [PubMed]
- Czerska, K.; Sobczyńska-Tomaszewska, A.; Sands, D.; Nowakowska, A.; Bąk, D.; Wertheim, K.; Poznański, J.; Zielenski, J.; Norek, A.; Bal, J. Prostaglandin-endoperoxide synthase genes COX1 and COX2—Novel modifiers of disease severity in cystic fibrosis patients. J. Appl. Genet. 2010, 51, 323–330. [Google Scholar] [CrossRef]
- Alonso, M.J.; Heine-Suñer, D.; Calvo, M.; Rosell, J.; Giménez, J.; Ramos, M.D.; Telleria, J.J.; Palacio, A.; Estivill, X.; Casals, T. Spectrum of mutations in the CFTR gene in cystic fibrosis patients of Spanish ancestry. Ann. Hum. Genet. 2006, 71, 194–201. [Google Scholar] [CrossRef]
- Anderson, C.M. Hypothesis revisited. Cystic fibrosis: A disturbance of water and electrolyte movement of exocrine secretory tissue associated with altered prostaglandin (PGE2) metabolism. J. Pediatr. Gastroenterol. Nutr. 1984, 3, 15–22. [Google Scholar] [CrossRef]
- de Jong, P.A.; Lindblad, A.; Rubin, L.; Hop, W.C.J.; de Jongste, J.C.; Brink, M.; Tiddens, H.A.W.M. Progression of lung disease on computed tomography and pulmonary function tests in children and adults with cystic fibrosis. Thorax 2006, 61, 80–85. [Google Scholar] [CrossRef]
- Bush, A.; Sly, P.D. Evolution of cystic fibrosis lung function in the early years. Curr. Opin. Pulm. Med. 2015, 21, 602–608. [Google Scholar] [CrossRef]
- McMahon, M.A.; Chotirmall, S.H.; McCullagh, B.; Branagan, P.; McElvaney, N.G.; Logan, P.M. Radiological abnormalities associated with Aspergillus colonization in a cystic fibrosis population. Eur. J. Radiol. 2012, 81, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Loeve, M.; Gerbrandts, K.; Hop, W.C.; Rosenfield, M.; Hartman, I.C.; Tiddens, H.A.L. Bronchiectasis and pulmonary exacerbations in children and young adults with cystic fibrosis. Chest 2011, 140, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Loeve, M.; van Hal, P.T.W.; Robinson, P.; A de Jong, P.; Lequin, M.H.; Hop, W.C.; Williams, T.J.; Nossent, G.D.; Tiddens, H.A. The spectrum of structural abnormalities on CT scans from patients with CF with severe advanced lung disease. Thorax 2009, 64, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Tepper, L.A.; Utens, E.M.; Caudri, D.; Bos, A.C.; Gonzalez-Graniel, K.; Duivenvoorden, H.J.; van der Wiel, E.C.; Quittner, A.L.; Tiddens, H.A. Impact of bronchiectasis and trapped air on quality of life and exacerbations in cystic fibrosis. Eur. Respir. J. 2013, 42, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Horati, H.; Janssens, H.M.; Margaroli, C.; Veltman, M.; Stolarczyk, M.; Kilgore, M.B.; Chou, J.; Peng, L.; Tiddens, H.A.; Chandler, J.D.; et al. Airway profile of bioactive lipids predicts early progression of lung disease in cystic fibrosis. J. Cyst. Fibros. 2020, 19, 902–909. [Google Scholar] [CrossRef]
- O’Connor, M.G.; Seegmiller, A. The effects of ivacaftor on CF fatty acid metabolism: An analysis from the GOAL study. J. Cyst. Fibros. 2017, 16, 132–138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).