Abstract
Anemia is a common hematological disorder that affects 12% of the community-dwelling population, 40% of hospitalized patients, and 47% of nursing home residents. Our understanding of the impact of inflammation on iron metabolism and erythropoiesis is still lacking. In older adults, anemia can be divided into nutritional deficiency anemia, bleeding anemia, and unexplained anemia. The last type of anemia might be caused by reduced erythropoietin (EPO) activity, progressive EPO resistance of bone marrow erythroid progenitors, and the chronic subclinical pro-inflammatory state. Overall, one-third of older patients with anemia demonstrate a nutritional deficiency, one-third have a chronic subclinical pro-inflammatory state and chronic kidney disease, and one-third suffer from anemia of unknown etiology. Understanding anemia’s pathophysiology in people aged 65 and over is crucial because it contributes to frailty, falls, cognitive decline, decreased functional ability, and higher mortality risk. Inflammation produces adverse effects on the cells of the hematological system. These effects include iron deficiency (hypoferremia), reduced EPO production, and the elevated phagocytosis of erythrocytes by hepatic and splenic macrophages. Additionally, inflammation causes enhanced eryptosis due to oxidative stress in the circulation. Identifying mechanisms behind age-related inflammation is essential for a better understanding and preventing anemia in older adults.
1. Introduction
The world population is rapidly aging, and this demographic shift is expected to continue over the coming decades. This phenomenon is characterized by an increase in both the number and the percentage of older adults worldwide. Currently, 10% of the world population is aged 65 years or older, but this figure is expected to reach 16% by 2050. Developing countries are particularly affected by this trend due to the declining levels of mortality, as reflected in the increased levels of life expectancy at birth [1]. As individuals grow older, their organic functionality naturally declines over time (aging), ultimately resulting in death. Aging is also associated with an increased likelihood of common conditions such as cardiovascular diseases, cancer, diabetes, or neurodegenerative diseases, which, in turn, elevate the risk of mortality [2].
Anemia, a condition that frequently occurs in older patients, has no standard definition. The World Health Organization (WHO) established the diagnostic criteria for anemia, which was defined as a hemoglobin (Hb) level < 13.0 g/dL for men and <12.0 g/dL for women [3]. Since the WHO definition of anemia was established more than five decades ago on the basis of a limited population sample and without proper documentation of the methodology used, understandably, there are now certain restrictions related to these thresholds. Nevertheless, the WHO definition continues to be the standard for anemia classification in older adults, despite suggestions from various studies that the definition be revised. Higher Hb reference values to define anemia were suggested after the analyses of American databases including the National Health and Nutrition Examination Survey III [4] and the Scripps-Kaiser database [5]. The Cardiovascular Health Study [6] identified optimal Hb levels of ≥13.7 g/dL for men and ≥12.6 g/dL for women, which were recorded to be associated with improved survival. The population study by Culleton et al. [7] determined that optimal Hb values of 13.0 to 15.0 g/dL for women and 14.0 to 17.0 g/dL for men could help avoid hospitalization and reduce the risk of mortality in old age. Wouters et al. recommended modifying Hb values to <13.0 g/dL for women over 60 years of age to align with the definition used for men [8].
Age-related, chronic, low-grade inflammation is not only a consequence of increasing chronological age, but also a marker of biological aging, multimorbidity, and mortality risk [9]. Systemic inflammation can significantly exacerbate health status and lead to a decline in overall well-being [10]. As the immune system ages, its ability to effectively respond to and manage inflammation diminishes, which renders the elderly more susceptible to a range of diseases such as anemia [11,12]. Therefore, the objective of this review was to explore the pathophysiological causes of anemia in the elderly, particularly those associated with inflammation, and to elucidate the underlying mechanisms and contributing factors for anemia in this age group.
Prevalence of Anemia
The prevalence of anemia varies across age groups, genders, and races, and the condition is more common in older individuals, with higher rates observed in men compared to women and in black individuals compared to white ones. However, it is noteworthy that most individuals classified as anemic according to the WHO criteria demonstrated anemia of a mild degree [3].
A systematic review of 34 studies showed that in people aged >65 years, the prevalence of anemia was recorded in 12% of community-dwelling persons, 40% of hospitalized patients, and 47% of nursing home residents [13], with the overall mean prevalence of 17% [14]. The increased prevalence of anemia among nursing home residents was often attributed to poorer health status and the higher occurrence of comorbidities in the elderly residents of these facilities compared to the community-dwelling age-matched population [15]. Insights into the prevalence of anemia across different populations and its findings, based on selected studies, are summarized in Table 1.

Table 1.
Selected studies examining the prevalence of anemia across various populations and its findings.
The severity of anemia was found to be higher in skilled nursing facilities compared to community-based settings, as revealed in a survey of five such facilities where a hemoglobin level ≤ 10 g/dL was detected in 11.4% of the residents [19]. In hospitalized patients aged ≥65 years, the prevalence of anemia reached up to 48%, with 65% of patients exhibiting mild (Hb > 10 g/dL) to moderate (Hb 8–10 g/dL) anemia [20]. Interestingly, it was observed that the recognition and investigation of anemia were rarely undertaken [21]. These findings highlight the increased severity of anemia in skilled nursing facilities and the need to raise the awareness of the staff and the management of this condition in both health care settings.
It is evident that the analyzed issue differs depending on the geographical location and the economic status of various countries.
The identification of the putative factors underlying anemia of inflammation in older adults poses a considerable challenge as this age group is affected by a tremendous extent of subclinical and clinical morbidities as well as an age-related increase in the levels of proinflammatory cytokines. It is therefore hardly surprising that nearly a fifth of anemia cases (19.7%) in older adults have been classified as anemia of inflammation, also known as anemia of chronic disease [1]. However, distinguishing anemia of chronic inflammation from iron deficiency anemia is particularly challenging in older adults due to the comorbid effects of gastrointestinal bleeding and the effects of medications [22]. Serum ferritin levels can still fall within the reference range when both types of anemia are present, which might potentially have led to an overestimation of anemia of chronic inflammation prevalence in the NHANES III study [1] at the expense of iron deficiency anemia. Furthermore, even distinguishing anemia of chronic inflammation from anemia of chronic kidney disease is somewhat tenuous, given the emerging evidence of increased inflammation associated with renal function in older adults without chronic kidney disease [23,24].
2. Causes of Anemia in Older Adults
The processes responsible for the maintenance of homeostasis diminish with increasing age, and one of these processes involves a decrease in hematopoietic potential. However, there are no adequate hemoglobin reference values below which anemia can be diagnosed in adults aged over 65 years, so the referential range for the general population is still applied [25]. Figure 1 provides a visual representation of the main factors contributing to the development of aging-related inflammation that can further contribute to the development of anemia in the elderly.
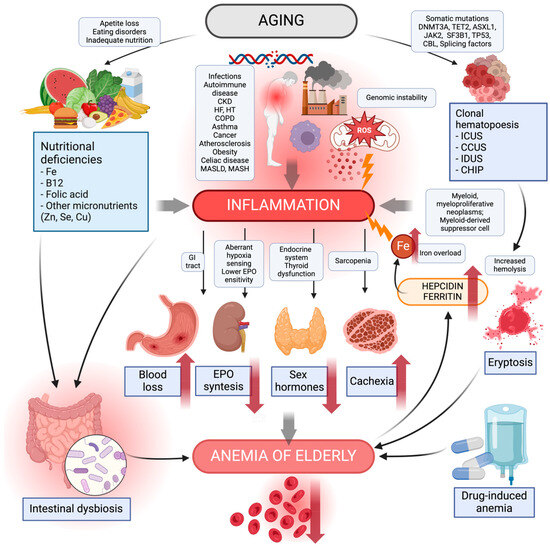
Figure 1.
Causes of anemia in the elderly. The diagram shows the main causes of the development of aging-related inflammation that can contribute to anemia in the elderly. Aging processes such as genome instability, reactive oxygen species in the mitochondria, synthesis of pro-inflammatory cytokines, negative environmental factors, and chronic diseases lead to inflammation. Inadequate nutrition, eating disorders, and loss of appetite contribute to an increased risk of nutritional deficiencies—iron, vitamin B12, folic acid, zinc, selenium, and copper. Their deficiency leads to inflammation and modulation of the intestinal microbiota to its detriment, increasing the risk of intestinal dysbiosis. The number of somatic mutations increasing with age can lead to clonal hematopoiesis. This, in turn, increases the incidence of myeloid myeloproliferative neoplasms, myeloid-derived suppressor cells causing inflammation. Clonal hematopoiesis shortens the lifespan and durability of erythrocytes, increasing their risk of hemolysis, the process of eryptosis. Chronic inflammatory processes further contribute to gastrointestinal inflammatory disease and blood loss; a decrease in sensitivity to hypoxia and EPO, thereby causing a reduction in EPO synthesis; endocrine dysfunction causing a decrease in sex hormones; and a decrease in muscle mass to sarcopenia, leading to the risk of cachexia. Pharmacotherapy with drug–drug interactions can produce adverse effects potentially contributing to anemia in older adults. Lately, anemia in the elderly has been reported to cause an increase in hepcidin, a plasma ferritin causing pro-inflammatory iron overload. CCUS, clonal cytopenia of unknown significance; CHIP, clonal hematopoiesis of indeterminate potential; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; EPO, erythropoietin; GI, gastrointestinal; HF, heart failure; HT, hypertensive; ICUS, idiopathic cytopenia of undetermined significance; IDUS, idiopathic dysplasia of undetermined significance; MASLD, metabolic dysfunction-associated steatotic liver disease; MASH, metabolic dysfunction-associated steatohepatitis; ROS, reactive oxygen species. Created with BioRender.com (accessed on 9 March 2024).
Genomic instability is a fundamental cause of the progressive aging process [26]. The factors favoring genome instability include environmental factors [27], the influence of chemicals [28], oxidative stress [29], hypoxia progression with aging [30], and chronic inflammation [31]. An increase in DNA instability results in elevated numbers of somatic mutations in the resulting cells [32]. With age, somatic mutations also affect all cells involved in hematopoiesis, resulting in clonal hematopoiesis [33]. The incidence of progressive clonal hematopoiesis increases with age [34]. The number of clonal abnormalities is correlated with the risk of developing myeloid and myeloproliferative neoplasms including acute myeloid leukemia, myelodysplastic syndromes, myeloproliferative syndromes, and mixed (myelodysplastic-myeloproliferative) syndromes. The list of the above-mentioned diseases also includes anaplastic anemia.
Increased mitochondrial damage in stem cells [35] and impaired mitochondrial function observed in chronic diseases during hematopoiesis [36] are further elements of the hypothesis concerning the etiology of anemia in old age. Accumulating damage to telomeres leads to aging of the mitochondria and the increased production of reactive oxygen species (ROS), which results in generalized hypoxia as a consequence [37,38]. In the longer term, a persistent increase in hypoxia activates systemic compensatory and adaptive mechanisms [39].
3. Pathophysiology of Inflammation Causing Anemia in Older Adults
As individuals age, the phenomenon known as inflammaging becomes increasingly prevalent. Inflammaging is characterized by chronic low-grade inflammation, and it is considered a significant contributor to the aging process. The underlying mechanism involves the release of a multitude of inflammatory mediators that are produced in response to tissue damage and stress. The key players in chronic inflammation include a variety of interleukins such as IL-1, IL-1b IL-2, IL-6, IL-8, IL-12, IL-13, IL-15, IL-18, IL-22, and IL-23. The pro-inflammatory activity of these cytokines initiates and amplifies the inflammatory response. Additionally, tumor necrosis factor α (TNFα) and interferon-γ (IFN-γ) are also prominent pro-inflammatory cytokines. Variations in the genetic sequences within the promoter regions of proinflammatory and controlled cytokine genes can influence the processes of inflammaging and vulnerability to age-related diseases [40].
On the other hand, attempts are made by anti-inflammatory cytokines including IL-1Ra, IL-4, IL-10, and transforming growth factor (TGF-β1) to counterbalance the pro-inflammatory response. These cytokines, in turn, are engaged in the suppression of inflammation and they promote a more balanced immune response. Along with the cytokines, a range of other molecules contribute to the complex network of inflammaging. For instance, lipoxin A4 plays the role of a lipid mediator with potent anti-inflammatory properties. Heat shock proteins are also involved in the regulation of inflammation, acting like chaperones that help to protect cells from stress-induced damage [41,42,43]. According to Minciullo et al. [43], inflammaging is a key to our understanding of the aging process, and anti-inflammaging may be one of the secrets of longevity. Therefore, anemia caused by inflammation is an important issue to be tackled more quickly and multidimensionally.
3.1. Iron Restriction (Hypoferremia)
During infection or inflammatory events, hypoferremia occurs quickly with a decrease in plasma iron level and transferrin saturation, which prevents the formation of nontransferrin-bound iron, a powerful trigger for the pathogenicity of Gram-negative bacteria and potentially also other microorganisms [44,45]. Iron consumption by erythropoiesis and the turnover of senescent or damaged erythrocytes by macrophages are the primary factors affected by various inflammatory processes. Therefore, maintaining strict control over iron levels during inflammation is crucial for host defense.
Hepcidin, a 25-amino-acid peptide released by liver cells, circulates in the blood and is expelled in the urine. Hepcidin serves as the primary governing factor for both iron absorption and distribution across different tissues [46,47]. Elevated levels of circulating hepcidin, induced by IL-6, inhibit the release of cellular iron into plasma by binding to the cellular iron efflux channel ferroportin [48]. Ferroportin occurs on the cells that serve as specialized iron managers within the body, and these cells include duodenal enterocytes that are responsible for the absorption of dietary iron, hepatic and splenic macrophages that recycle senescent erythrocytes, hepatocytes that are engaged in iron storage, and placental trophoblasts that facilitate iron transfer to the developing fetus during pregnancy [49].
Macrophages play a crucial role in recycling iron from aging red blood cells and once recycled, the iron is released into the bloodstream through ferroportin. Inflammation triggers an increase in hepcidin levels, thereby leading to enhanced internalization and the breakdown of ferroportin [50]. As a result, the release of ferrous iron from key iron transport tissues such as duodenal enterocytes, iron-recycling macrophages, and iron-storing hepatocytes into the bloodstream is reduced. This leads to the accumulation of iron within their cellular ferritin. Subsequently, the continuous utilization of iron by erythropoiesis depletes the extracellular iron pool, which results in low levels of iron and restricted erythropoiesis.
Anemia of inflammation is characterized by hypoferremia accompanied by increased plasma ferritin and hepcidin levels, whereas iron-deficiency anemia manifests itself in hypoferremia accompanied by low levels of plasma ferritin and hepcidin. Inflammatory hypoferremia, similar to hypoferremia in systemic iron deficiency, inhibits erythropoiesis, however, the inhibitory effect is detected at a relatively high threshold (transferrin saturation of 15 to 20%), which may suggest a protective function of this mechanism to ensure an adequate iron supply for other tissues such as muscles, the central nervous system, and nonerythroid marrow, which are less affected by decreased plasma iron levels (Figure 2) [51].
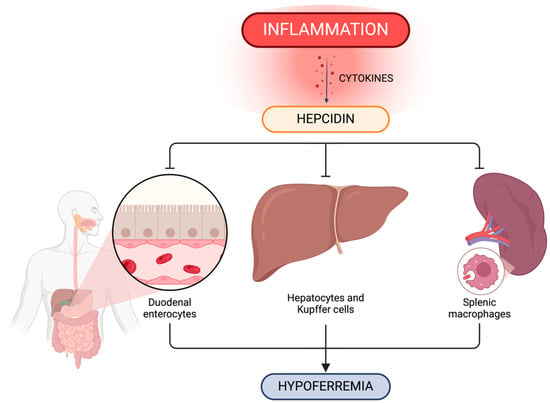
Figure 2.
Inflammation impact on the regulation of systemic iron metabolism. Hepcidin plays a crucial role in systemic iron level control via ferroportin concentration in iron-exporting cells including duodenal enterocytes, hepatic and splenic iron-recycling macrophages and hepatocytes. Created with BioRender.com (accessed on 9 March 2024).
Our previous study demonstrated higher hepcidin levels in the group with anemia compared to non-anemic participants [52], which is consistent with other reports, for instance, the Leiden 85-plus Study, which also revealed elevated serum hepcidin levels in older adults with anemia of inflammation. However, the InCHIANTI study did not find an increase in urinary hepcidin levels [48,53]. The available studies also reported differences in hepcidin levels across the compared genders. On average, approximately 50% lower hepcidin levels were observed in premenopausal women than in the age-matched male groups. However, post-menopause hepcidin levels tend to become comparable in both gender groups, which was reported in the Val Borbera study [54] and the Nijmegen Biomedical Studies [55].
The impact of hepcidin–ferroportin interaction in iron homeostasis is shown in Figure 2.
3.2. Erythropoiesis Suppression
Pro-inflammatory cytokines, especially interferons and TNFα, appear to inhibit the proliferation and differentiation of erythroid progenitor cells, leading to ineffective erythropoiesis as a result [56].
Early inflammatory responses include leukocytosis and the increased production of leukocytes in the marrow, which is manifested by an increased number of myeloid precursors (>4:1 myeloid to erythroid precursors ratio). Inflammatory cytokines such as TNF-α [57] and interferon-γ [58] activate the transcription factor PU.1 and trigger bone marrow reprogramming, which promotes myelopoiesis and lymphopoiesis while suppressing erythropoiesis. Inflammatory cytokines also inhibit the ability of BFU-E to generate more differentiated erythroid cells [59,60].
Another bone marrow-reprogramming mechanism involves inflammation-induced suppression of erythropoietin production, the primary hormone responsible for erythropoiesis. In patients with systemic inflammation, serum erythropoietin levels are lower compared to the individuals with a similar degree of iron-deficiency anemia [61,62]. Inflammation also impairs the responsiveness of erythroid precursors to erythropoietin, as evidenced by increased exogenous erythropoietin requirements in end-stage kidney disease patients with inflammation [63,64]. Resistance to erythropoietin is partly mediated by a decrease in the number of erythropoietin receptors on erythroid progenitors, whose proliferative capacity is therefore reduced, which is a recently discovered effect of hypoferremia [65].
The Klotho enzyme, which is mainly expressed in humans in the kidney and brain by the KL gene, especially its alpha-Klotho variant activated by fibroblast growth factor 23 (FGF23), has been indicated as another potential cause of inflammatory anemia in old age [66,67]. Most of the early as well as current studies have focused on the role of alpha-Klotho in chronic kidney disease (CKD) in elderly populations, and reported an age-related Klotho reduction and association with increased likelihood of anemia [68]. Klotho is involved in hematopoiesis regulation through its impact on the hypoxia-inducible factor (HIF1α) pathway. Its deficiency interferes with hematopoietic stem cell development and erythropoiesis [69]. Klotho has the ability to modulate inflammation and oxidative stress through various mechanisms. As a suppressor gene of aging, Klotho protein expression, among other things, reduces phosphorus, ROS, and slows age-related renal fibrosis [70,71]. Increased Klotho levels could also potentially contribute to inflammation and anemia reduction in the elderly [70].
The production of erythropoietin, a major cytokine that affects the development of red blood cells, is triggered by a mechanism that detects low oxygen levels in anemia conditions. The relationship between the impaired response of hematopoietic stem cells to EPO and the development of anemia was observed in elderly patients [72]. The Baltimore Longitudinal Study on Aging [73] reported that EPO levels increased with age in healthy individuals without anemia, particularly in those without diabetes or hypertension. Conversely, individuals with anemia demonstrated a lower rate of EPO increase, suggesting that anemia is linked to a failure in the normal compensatory rise of EPO levels during aging. Although low EPO levels have specifically been associated with unexplained anemia in the elderly population, the exact cause of the inadequate EPO response is still unknown. Therefore, further studies on larger samples of elderly patients are necessary to confirm these findings [73]. A study by Chencheng et al. [68] suggested that low serum Klotho levels were associated with an increased likelihood of anemia in middle-aged and older adults regardless of kidney disease.
Overall, the age-dependent impairment of EPO response suggests a progressive resistance of hematopoietic stem cells to EPO as individuals age. The underlying reasons are yet to be determined, however, they could be attributed to impairments in normal EPO-dependent pathways caused by inflammatory cytokines, age-related comorbidities, declines in renal function, or a combination of these factors [74].
3.3. Shorter Erythrocyte Lifespan
The available studies on anemia of inflammation have consistently reported a moderate reduction (approximately 2–5%) in the lifespan of red blood cells with a decrease to approximately 90 days. However, a shortened erythrocyte lifespan was also observed in many cases of non-anemic inflammation, which indicates that anemia develops only when the compensatory response of red blood cell production is impaired [75].
The shortened lifespan of erythrocytes during inflammation has been ascribed to macrophage activation triggered by inflammatory cytokines, which results in premature phagocytosis and erythrocyte elimination. Macrocytic anemia and heightened erythrophagocytosis are prominent manifestations observed in macrophage activation syndromes, particularly those associated with systemic juvenile rheumatoid arthritis [76]. Multiple cytokines including interferon-γ and IL-4 have been implicated in macrophage activation for erythrophagocytosis in mouse models [57,77].
Except for rare cases of fulminant hemophagocytic states, erythrophagocytosis in anemia of inflammation exhibits only a mild increase, and the increase could easily be compensated if the production of erythrocytes is unimpaired [78,79].
Furthermore, the inflammatory cascade involves the generation of reactive oxygen and nitrogen species, shaping the intricate interrelationship between inflammation and the behavior of erythrocytes in the circulatory system. The oxidative stress within the vascular bed exerts multifaceted effects on the structural integrity of red blood cells, characterized by lipid peroxidation and the oxidation of membrane skeletal proteins. These biochemical alterations do not only compromise the molecular architecture of red blood cell membranes, but they also have profound implications for their functional properties [80,81,82,83].
The consequence of oxidative stress-induced modifications extends beyond mere structural compromise, significantly impacting the physiological characteristics of red blood cells. Notably, the reduction in osmotic resistance and deformability of red blood cells has emerged as a pivotal outcome of oxidative stress in the vascular microenvironment [84]. The compromised osmotic resistance renders erythrocytes more susceptible to premature removal from circulation as their resilience to environmental challenges diminishes [81,82,85,86].
The intricate relationship between oxidative stress and red blood cell dynamics underscores the accelerated elimination of these cells from the circulatory system. This phenomenon, although crucial in our understanding of broader implications of inflammation-induced alterations, unfortunately remains unexplored in the current discourse. An in-depth exploration of this aspect of oxidative stress is imperative for our thorough comprehension of the intricate mechanisms underpinning anemia in the context of inflammation.
Figure 3 provides a comprehensive overview of the interconnected processes contributing to inflammation-induced anemia. The schematic representation serves as a visual guide to illustrating the complex dynamics involving the key elements in the pathogenesis of inflammation-associated anemia.
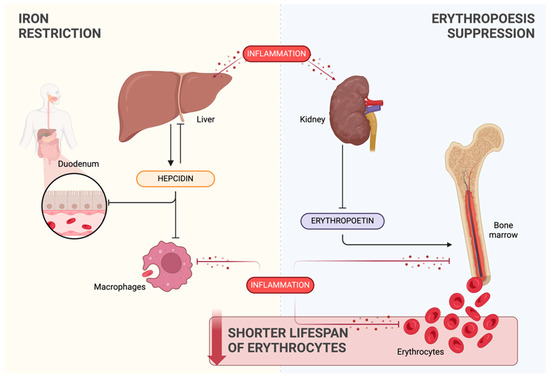
Figure 3.
Overview of the processes linked to inflammation-induced anemia: iron restriction (yellow background), erythropoiesis suppression (blue background), and shortened erythrocyte lifespan (red background). Created with BioRender.com (accessed on 9 March 2024).
4. Diseases Associated with Anemia in Older Adults
Anemia in older adults has a multifactorial cause. Consequently, there are no and major and clear-cut contributors to anemia in the elderly. Overlapping diseases leading to multimorbidity and an increased risk of frailty syndrome make the identification of the causes of anemia even more challenging [87,88]. Nonetheless, the disease entities that are associated with reduced synthesis, disruption of normal hematopoiesis to achieve the desired volume and number of red blood cells and hemoglobin content, can be included in the list of potential anemia-inducing conditions. This section discusses the most common diseases associated with anemia in older adults. As depicted in Table 2, there are numerous common diseases prevalent among the elderly that have the potential to lead to anemia. Understanding these diseases is vital for health care professionals to accurately diagnose and manage anemia in this population.

Table 2.
Examples of common diseases potentially leading to anemia in older adults.
The commonly reported reasons include the increased risk of nutritional disorders due to an excessive intake or negative balance in dietary supply of energy, nutrients, vitamins, and the inability to replenish the effects of catabolic processes [89]. Cachexia, defined as disease-related malnutrition (DRM) with inflammation in the ESPEN guidelines on definitions and terminology of clinical nutrition [90], has been reported in relevant analyses.
The process of aging is associated with epigenetic changes that lead to somatic mutations of cells beginning with pluripotent hematopoietic stem cells (PHSCs), which are further growth pathways in hematopoiesis. These alterations result in shorter erythrocyte lifespan and increased eryptosis rate. As a consequence, augmented oxidative stress increases inflammation and the risk of age-related chronic diseases as well as hematopoietic disorders [91,92]. The progressive deterioration of kidney and liver function in elderly populations is a key element in the development of anemia and it involves: firstly, macroscopic changes, mainly a lower mass of the organs and, secondly, microscopic pathological tissue and cells changes such as atherosclerosis of capillaries, atrophy, fibrosis, and collagen deposition. Functional and structural changes in the aging organs increase the probability of erythropoiesis suppression [93,94,95].
4.1. Hematopoietic Disorders
Hematopoiesis-regulating mechanisms that involve players such as cytokines, chemokines, hormones, adhesion molecules, and transcription factors occur at each stage of the cell lines, ranging from the process of renewal, through differentiation to maturation of blood cells. However, the process of aging is linked to the impairment to the processes of self-renewal, differentiation, proliferation, and maturation of cells involved in hematopoiesis.
Age-related progressive changes such as epigenetic alterations, genetic instability, telomere shortening, and accumulation of p53 damage have all been reported to affect cellular aging [96,97]. Progressive somatic mutations cause an increase in clonal hematopoiesis of indeterminate potential (CHIP) in an average of 25% of the human population aged over 65 years, with a further increase observed with aging. The most common mutations concern the following genes: DNMT3A, TET2, ASXL1, JAK2, SF3B1, and TP53 [33,98,99,100]. Clonal changes correlate with increased heterogeneity of the cell size indices such as the red cell distribution width (RDW) [100]. These findings have been supported by recent genome-wide association studies. Furthermore, studies have also demonstrated correlations with an increase in inflammatory markers such as CRP, IL-1b, and IL-18, but with a decrease in hemoglobin [98,99,101]. These somatic mutations result in an increased risk of chronic diseases typical of old age, myeloproliferative diseases, and mortality.
Changes associated with clonal hematopoiesis can also lead to aplastic anemias [102]. Along with changes in the cell population in the hematopoietic lineage, the bone marrow undergoes conversion from hematopoietically active red marrow to hematopoietically inactive yellow bone marrow [103]. A decrease in bone density and disturbances to homeostasis in osteoblast–osteoclast communication are also correlated with an increased risk of anemia in elderly people [104].
Consequently, the balance between new erythrocyte formation and their erythrophagocytosis and hemolysis is disrupted and can lead to a decrease in the number of erythrocytes in the bloodstream. Since oxidative stress is known to affect erythrocyte lifespan, one of the hypotheses for anemia involves the activity of reactive oxygen species on erythrocytes in the course of chronic inflammation in a progressive process of aging.
In the 1980s, Tozzi-Ciancarelli and Fedele showed that the structural properties of erythrocytes differed between older and young adults [105]. The age-related accumulation of defective proteins has further consequences for hematopoiesis, ultimately creating defects in the structure of reticulocytes and erythrocytes such as changes in the spectrin-4.1-actin complex, cytoskeleton structure, glycocalyx, and band protein III, which causes hemolysis [106,107,108,109,110].
4.2. Kidney Disease
Chronic kidney disease was defined by the KDIGO (Kidney Disease; Improving Global Outcomes) 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease as a progressive abnormality of kidney structure and function, a condition leading to end-stage renal disease requiring renal replacement therapy [111]. Uremia progression is accompanied by an increase in chronic inflammation and oxidative stress. The processes of hematopoiesis and eryptosis are therefore disrupted by the accumulation of pro-inflammatory molecules such as CRP, NOS, ROS, IL-1, IL-6, TNF-α, and other inflammatory mediators. Normal nephron functioning deteriorates with age, as shown by a decrease in the glomerular filtration rate (GFR) in the aging population. In a population study of 12,381 Germans, Waas and Schulz [112] recorded a decrease by 1 mL/min/m2 estimated GFR (eGFR) per year from the third decade of life. The increased risk of developing progressive nephropathy includes age-related chronic diseases such as hypertension, diabetes, and glomerulonephritis [113]. An epidemiological study by Kovesda et al. [114] recorded 25.3% prevalence of anemia in people with stages 3–5 CKD (eGFR < 60 mL/min/1.73 m2). With regard to age groups, anemia was more likely to develop in patients aged ≥75 years, who also demonstrated a significant correlation with lower mean hemoglobin concentrations. According to Stauffer et al. [115], anemia was twice as prevalent in people with CKD as in the general population (15.4% vs. 7.6%, respectively).
The phenomena of polypharmacy and polypragmasy, which belong to the risk factors for kidney disease, are frequently recorded in the elderly [116]. Increased use of drugs available without prescription, primarily, non-steroidal anti-inflammatory drugs (NSAIDs), herbal remedies, and dietary supplements can lead to drug-induced nephrotoxicity [117] with potential consequences being acute kidney injury (AKI), possibly leading to irreversible CKD [118].
Other risk factors of AKI and CKD progression include water–electrolyte disturbances. Older adults belong to the age group at increased risk of disruption of physiological homeostasis such as thirst control disturbances and water–electrolyte imbalance. Dysfunction of central nervous system mechanisms of thirst control results in reduced thirst in response to current water–electrolyte needs [119,120]. These tendencies toward dehydration are the risk factors for hemodynamic weakness, which significantly contributes to the increased risk of kidney damage [121,122].
Erythropoietin, the major regulator in erythropoiesis renewal, is prenatally synthesized in the liver, but hepatic EPO production is switched to the kidneys and taken over perinatally by peritubular interstitial fibroblasts (which belong to renal erythropoietin-producing cells (REPCs)) [123]. In chronic kidney disease, the number of REPCs is reduced due to their differentiation into myofibroblasts, which lose their ability to produce EPO. Therefore, an insufficient number of hypoxia-inducible factor (HIF)-sensitive REPCs in response to hypoxic stimuli causes an EPO decrease and erythropoiesis, leading to the development of anemia [124].
Vlasschaert et al. [125] observed more rapid development of progressive chronic kidney disease and greater severity of anemia in patients with previous CKD and current CHIP.
4.3. Hormonal Factors
Hormonal regulation is also affected by age-related changes, which pertains to both genders: menopausal and postmenopausal changes in women and andropausal transition in men have been widely investigated. In both genders, a reduction in circulating estrogen and testosterone plasma levels have been recorded. An age-related decrease in muscle mass due to lower testosterone levels also results in reduced sensitivity of erythropoietin forming [126,127]. Significant correlations between sarcopenia and anemia were detected in population-based studies [128,129,130]. A reduction in erythropoiesis may also result from decreased thyroid hormonal activity, which slows down the anabolic hormone level. Fatigue, weakness, and loss of appetite are among the significant effects of decreased numbers of blood erythrocytes [131].
With aging, the endocrine abnormalities decrease the production of hormones and consequently affect red blood cell homeostasis. A potential impact of anabolic hormones (IGF-1, testosterone, TSH, T3, T4) on hepcidin regulation and the expression of progenitor cells involved in hematopoiesis has been recorded [132] The late-onset hypogonadism in andropausal elderly males and hypoestrogenism in postmenopausal elderly females are the hormonal factors that potentially contribute to anemia development. Adequate plasma testosterone levels modulate pro-inflammatory cytokines, mainly IL-6, which ensure appropriate hepcidin levels and proper hematopoietic cell differentiation without clonal cells. These are the elements that favorably affect the hemoglobin and hematocrit levels in older men [133,134]. Reduced testosterone levels correlate with a negative response to erythropoiesis-stimulating factors [135].
It should be noted, however, that in elderly patients with prostate cancer treated with hormone replacement therapy (androgen deprivation), radiotherapy, and brachytherapy procedures, their hemoglobin levels were observed to fall by an average of 1–2.5 g/dL, which should not be directly linked to inflammatory processes and anemia [132,136].
Progenitor cells contain estrogen receptors (ER-α and ER-β) that are influenced by estrogen during differentiation [137]. Zhou and Tseng [138] showed that estrogen regulated erythropoiesis by ROS and NOS modulation on the progenitor erythroid cells, which affected proliferation and differentiation. The research conducted to date has also confirmed the protective cardiovascular effects of estrogens in women as their NOS and ROS-modulating activity produces anti-inflammatory effects [139]. Moreover, estrogen has an erythropoiesis-stimulating effect on bone marrow stem cells, which has been proven to support erythrocyte count and hemoglobin levels in pregnant women [140].
Estrogens participate in the estrogen–iron axis through their ability to inhibit hepcidin formation. A decrease in hepcidin results in an increase in iron storage, while an inflammation-induced increase of hepcidin levels, among other factors, negatively affects iron metabolism.
4.4. Gastrointestinal Diseases
Gastrointestinal (GI) changes are common in the elderly, with some GI disorders being more prevalent in this age group such as changes in the oral cavity, esophagitis, gastroesophageal reflux disease (GERD), chronic atrophic gastritis, Clostridioides difficile and Helicobacter pylori infection, peptic ulcer disease, celiac disease, small bowel bleeding, angiodysplasias, small bowel ulcers, inflammatory bowel disease (IBD), small intestinal bacterial overgrowth (SIBO), abdominal hernia, constipation, and diarrheal illnesses [141].
Decreased production of pepsin and hydrochloric acid limits the bioavailability of dietary and supplementary vitamin B12 [141,142]. Examples of bioavailability limitations include the use of acid suppressants to protect against medication side-effects (mainly NSAIDs), the presence of gastritis and/or duodenal inflammation, and esophageal disorders. Age-associated changes to intestinal epithelial cells and enterocyte function may result in insufficient nutrient absorption [143]. These limitations are also related to the higher prevalence of celiac disease in the elderly, reaching from 4 to about 25 percent [144]. In fact, this difference may be due to delayed diagnosis for celiac disease, mainly because of the atypical clinical manifestations of this enteropathy [141,143,145]. A higher incidence of hernias, adhesions, diverticulosis, and risk of obstruction may also contribute to bacterial overgrowth (SIBO) and chronic intestinal inflammation. Diverticular disease is rare in the general population, but it was found to affect 65% of people aged ≥65 years [141,146,147].
Abnormal hematological indicators such as inflammation-related normocytic anemia occur in approximately half of patients with hepatic cirrhosis [148]. Hepcidin plays a major role in hepatic disorders due to iron restriction. On the other hand, information regarding patients with cirrhosis is limited, and there is debate about the plasma erythropoietin (EPO) levels in these individuals. It is plausible that EPO elevation could be a result of renal hypoperfusion, hypoxia, anemia, or a hepato-protective and regenerative mechanism mediated by EPO. In contrast, inadequate EPO response in advanced cirrhosis might be attributed to poor hepatic synthesis capacity, decreasing co-factor levels, and inflammatory feedback mechanisms. Ultimately, the source of a potential increase in EPO production during certain stages of cirrhosis—whether from the kidney or liver—remains a lingering question [149].
While some changes associated with an aging GI system are physiologic, others are pathological and particularly more prevalent among those above 65 years of age [141]. Such GI diseases increase the risk of gastrointestinal bleeding.
Diseases of the gastrointestinal tract in old age are among the main causes of anemia due to the reduced absorption of micro- and macronutrients necessary for cell synthesis in erythropoiesis up to the erythrocytes themselves. It is challenging to unequivocally demonstrate anemia in the elderly as a result of chronic inflammation due to the overlapping causes of anemia such as disorders, impaired iron absorption, and the intake of other micronutrients.
4.5. Intestinal Dysbiosis
Our microbiota is subject to constant variation over our life course. The risk of intestinal dysbiosis (i.e., a significant reduction in beneficial microorganisms and an increase in opportunistic or pathobiont microbes in the gastrointestinal tract) is on the increase in old age depending on our health status, lifestyle, previous illnesses, and general inflammation [150,151]. Intestinal epithelial barrier dysfunction and increased permeability with aging, previously confirmed only in patients with inflammatory bowel diseases, have raised particular concerns. This is especially relevant for individuals with inflammatory bowel disease, nutritional deficiencies, overweight, metabolic syndrome, or those undergoing antibiotic therapy [152].
Josefsdottir et al. [153] demonstrated that the gut microbiota supports adequate hematopoiesis. Previous hypotheses suggested a signaling model involving the gut microbiome and the bone marrow.
The gut microbiome–macrophage–iron axis recently discovered by Zhang and Gao [154] shows that microbiota-derived metabolites increase iron availability in a manner dependent on bone marrow macrophage erythrophagocytosis, which affects hematopoietic differentiation and blood regeneration. These include STAT1 signaling, type I IFN signaling in hematopoietic cells [155]. The presence of a favorable gut microbiota population ensuring adequate synthesis of short-chain fatty acids (SCFAs) such as acetate, butyrate, and propionate may contribute to erythrophagocytosis and favorable hematopoietic regeneration [156,157,158]. Soriano-Lerma and García-Burgos [159] reported a potential mediatory function of SCFAs in iron absorption, and possibly in anemia status modulation. A gradual increase in unfavorable intestinal microbiota contributes to an increased risk of intestinal absorption disorders in the elderly [143,160].
4.6. Autoimmune Diseases
The risk of chronic inflammation including autoimmunization increases with age. Paradoxically, an increase in the incidence of new autoimmune pathologies in all autoimmune diseases has not been recorded in older adults [161]. A recent population-based study by Conrad et al. [162] confirmed that they occurred almost twice as often in women as in men, and the mean age of diagnosis was 54 years. Only in entities such as Graves’ disease, pernicious anemia, and rheumatoid arthritis (RA) did the risk increase with age. For the other diseases (coeliac disease, inflammatory bowel disease and vasculitis), the incidence reached three different peaks: in childhood, early adulthood, and old age.
Even if we assume that the components of immunescence such as chronic inflammation, increased production of autoantibodies, and a decline in the immune response are most likely to be chronic autoimmunity rather than autoimmune diseases, they are still potential contributors to anemia in the elderly [163,164]. Some scientific reports support the link between anemia and autoimmune diseases, however, other causes of anemia should be considered such as anti-inflammatory drugs, glucocorticosteroids, biologic drugs that reduce iron-bioavailability and other essential micro- and macro-nutrients for erythropoiesis, or increased macrophage release [165]. This hypothesis may be supported by the decrease in Hb levels in RA, and the degree of clinical exacerbation of rheumatic disease. However, more research is needed into the relationships between anemia and autoimmunity and autoimmune diseases in older adults, taking into account confounding factors such as the use of medications that increase the risk of gastrointestinal diseases and limit bioavailability as well as other chronic diseases (e.g., CKD, hematological diseases, malnutrition, or hepatic diseases) [165,166,167,168,169].
Numerous research papers have reported that erythropoiesis-enhancing treatment reduced the severity of autoimmune diseases [170,171,172,173,174]. However, the conclusions should be treated with caution, as in some autoimmune diseases, EPO may produce pro-inflammatory or anti-inflammatory effects [175].
It is possible that the response of B and T lymphocytes in the autoimmunity process is potentially related to the increased risk of stress erythropoiesis, which does not allow older people to keep pace with the demand for erythrocytes [176,177].
5. Summary
Extensive studies on anemia in older adults have revealed significant outcomes including increased mortality, hospitalization rates, frailty, falls, mobility limitations, cognitive decline, dementia, functional dependence, and reduced quality of life. These findings are consistent across major cohort studies that excluded individuals with other conditions [6,178,179,180,181]. The multifactorial and highly prevalent nature of anemia in older adults is directly correlated with age. While the degree of anemia is mostly mild in the ambulatory setting, institutionalized patients exhibit higher rates of anemia of increased severity.
Aging-related changes are evident in the frequent occurrence of both anemia and indicators of inflammation in the elderly. It is crucial to recognize the interconnectedness of anemia and inflammaging, as they are, to some extent, manifestations of the same biological processes such as elevated levels of various proinflammatory mediators. Both conditions, anemia and inflammation, contribute to a heightened mortality risk, with the underlying causes of decreased survival likely stemming from a variety of factors. To enhance the clinical management of individual patients, a deeper understanding of the molecular mechanisms driving anemia and inflammation is essential. Our perspective emphasizes the need for personalized care based on the comprehensive clinical context when dealing with elderly patients.
A critical facet involves recalibrating reference values for older adults for key hematological parameters such as hemoglobin and hematocrit to align with the unique physiological changes associated with aging. This should make the diagnostic process more accurate and reflective of the health status of older individuals. Moreover, a diagnostic panel should be devised to encompass a spectrum of markers essential for comprehensive anemia assessment in older age. In addition to conventional indicators, special attention should be directed to hepcidin and inflammatory biomarkers such as CRP and cytokines such as IL-1β and TNF-α [52]. These indicators provide a more nuanced insight into the underlying causes of anemia, enabling tailored interventions that could address specific clinical conditions common in the elderly population.
Stratifying the elderly population into distinct age groups is another crucial modification proposal in the diagnostic approach. While anemia can affect individuals at various life stages, placing emphasis on those aged 65 and above recognizes the increased susceptibility to anemia-related issues in this demographic. Moreover, pinpointing high-risk groups such as individuals with specific chronic diseases ensures targeted and more frequent screenings for those who need it the most. Notably, persons aged 80 and above as well as institutionalized patients require even more vigilant monitoring, considering their advanced age and higher vulnerability to potential anemia-associated complications.
Moving beyond diagnostic measures, the multifaceted nature of combating anemia in the elderly necessitates the consideration of both non-pharmacological and pharmacological interventions. Non-pharmacological strategies, particularly dietary adjustments, have emerged as a cornerstone in anemia management.
However, when pharmacological interventions are warranted, a judicious approach is essential. Considering the likelihood of elderly individuals already being on a myriad of medications, potential interactions must be carefully evaluated.
In essence, addressing anemia in the elderly requires a meticulous balance between comprehensive diagnostic measures and tailored interventions. By embracing these modifications in diagnostic testing and intervention strategies, health care professionals can navigate the complexities of anemia management in older individuals with greater precision and efficacy.
We ought to incorporate every accessible data found in the Clinical Practice Guidelines, which shall support the comprehensive management of these patients through a multidisciplinary and multimodal approach.
This review provides valuable insights that can guide future endeavors in refining the approach to anemia in the elderly, ultimately contributing to improved health care outcomes for this demographic population.
Author Contributions
Conceptualization, E.W.; Writing—Original Draft Preparation, E.W. and J.N.; Visualization, E.W. and J.N.; Supervision, A.Z.-L. and P.J.; Funding Acquisition, A.Z.-L. and P.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funds from the University of Zielona Gora (No. 2023/2024 Ministry of Science and High Education, Poland).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guralnik, J.M.; Eisenstaedt, R.S.; Ferrucci, L.; Klein, H.G.; Woodman, R.C. Prevalence of Anemia in Persons 65 Years and Older in the United States: Evidence for a High Rate of Unexplained Anemia. Blood 2004, 104, 2263–2268. [Google Scholar] [CrossRef]
- Aunan, J.R.; Watson, M.M.; Hagland, H.R.; Søreide, K. Molecular and Biological Hallmarks of Ageing. Br. J. Surg. 2016, 103, e29–e46. [Google Scholar] [CrossRef]
- WHO Scientific Group on Nutritional Anaemias; World Health Organization. Nutritional Anaemias: Report of a WHO Scientific Group [Meeting Held in Geneva from 13 to 17 March 1967]; World Health Organization: Geneva, Switzerland, 1968.
- Shavelle, R.M.; MacKenzie, R.; Paculdo, D.R. Anemia and Mortality in Older Persons: Does the Type of Anemia Affect Survival? Int. J. Hematol. 2012, 95, 248–256. [Google Scholar] [CrossRef]
- Beutler, E.; Waalen, J. The Definition of Anemia: What Is the Lower Limit of Normal of the Blood Hemoglobin Concentration? Blood 2006, 107, 1747–1750. [Google Scholar] [CrossRef]
- Zakai, N.A.; Katz, R.; Hirsch, C.; Shlipak, M.G.; Chaves, P.H.M.; Newman, A.B.; Cushman, M. A Prospective Study of Anemia Status, Hemoglobin Concentration, and Mortality in an Elderly Cohort: The Cardiovascular Health Study. Arch. Intern. Med. 2005, 165, 2214–2220. [Google Scholar] [CrossRef]
- Culleton, B.F.; Manns, B.J.; Zhang, J.; Tonelli, M.; Klarenbach, S.; Hemmelgarn, B.R. Impact of Anemia on Hospitalization and Mortality in Older Adults. Blood 2006, 107, 3841–3846. [Google Scholar] [CrossRef]
- Wouters, H.J.C.M.; van der Klauw, M.M.; de Witte, T.; Stauder, R.; Swinkels, D.W.; Wolffenbuttel, B.H.R.; Huls, G. Association of Anemia with Health-Related Quality of Life and Survival: A Large Population-Based Cohort Study. Haematologica 2019, 104, 468–476. [Google Scholar] [CrossRef]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells 2022, 11, 359. [Google Scholar] [CrossRef]
- Brognara, L.; Luna, O.C.; Traina, F.; Cauli, O. Inflammatory Biomarkers and Gait Impairment in Older Adults: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 1368. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Anemia of Inflammation. Hematol./Oncol. Clin. N. Am. 2014, 28, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Badimon, L.; Montecucco, F.; Lüscher, T.F.; Libby, P.; Camici, G.G. Inflammation, Aging, and Cardiovascular Disease. J. Am. Coll. Cardiol. 2022, 79, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Gaskell, H.; Derry, S.; Andrew Moore, R.; McQuay, H.J. Prevalence of Anaemia in Older Persons: Systematic Review. BMC Geriatr. 2008, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- Bach, V.; Schruckmayer, G.; Sam, I.; Kemmler, G.; Stauder, R. Prevalence and Possible Causes of Anemia in the Elderly: A Cross-Sectional Analysis of a Large European University Hospital Cohort. Clin. Interv. Aging 2014, 9, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.; Tasar, P.T.; Simsek, H.; Çicek, Z.; Eskiizmirli, H.; Aykar, F.S.; Sahin, F.; Akcicek, F. Prevalence of Anemia and Malnutrition and Their Association in Elderly Nursing Home Residents. Aging Clin. Exp. Res. 2016, 28, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Robalo Nunes, A.; Fonseca, C.; Marques, F.; Belo, A.; Brilhante, D.; Cortez, J. Prevalence of Anemia and Iron Deficiency in Older Portuguese Adults: An EMPIRE Substudy. Geriatr. Gerontol. Int. 2017, 17, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Zaninetti, C.; Klersy, C.; Scavariello, C.; Bastia, R.; Balduini, C.L.; Invernizzi, R. Prevalence of Anemia in Hospitalized Internal Medicine Patients: Correlations with Comorbidities and Length of Hospital Stay. Eur. J. Intern. Med. 2018, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, M.; Laso-Morales, M.J.; Gómez-Ramírez, S.; Cadellas, M.; Núñez-Matas, M.J.; García-Erce, J.A. Pre-Operative Haemoglobin Levels and Iron Status in a Large Multicentre Cohort of Patients Undergoing Major Elective Surgery. Anaesthesia 2017, 72, 826–834. [Google Scholar] [CrossRef]
- Artz, A.S.; Fergusson, D.; Drinka, P.J.; Gerald, M.; Gravenstein, S.; Lechich, A.; Silverstone, F.; Finnigan, S.; Janowski, M.C.; McCamish, M.A.; et al. Prevalence of Anemia in Skilled-Nursing Home Residents. Arch. Gerontol. Geriatr. 2004, 39, 201–206. [Google Scholar] [CrossRef]
- Migone De Amicis, M.; Poggiali, E.; Motta, I.; Minonzio, F.; Fabio, G.; Hu, C.; Cappellini, M.D. Anemia in Elderly Hospitalized Patients: Prevalence and Clinical Impact. Intern. Emerg. Med. 2015, 10, 581–586. [Google Scholar] [CrossRef]
- Nathavitharana, R.L.; Murray, J.A.; D’Sousa, N.; Sheehan, T.; Frampton, C.M.; Baker, B.W. Anaemia Is Highly Prevalent among Unselected Internal Medicine Inpatients and Is Associated with Increased Mortality, Earlier Readmission and More Prolonged Hospital Stay: An Observational Retrospective Cohort Study. Intern. Med. J. 2012, 42, 683–691. [Google Scholar] [CrossRef]
- Weiss, G.; Goodnough, L.T. Anemia of Chronic Disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef]
- Keller, C.R.; Odden, M.C.; Fried, L.F.; Newman, A.B.; Angleman, S.; Green, C.A.; Cummings, S.R.; Harris, T.B.; Shlipak, M.G. Kidney Function and Markers of Inflammation in Elderly Persons without Chronic Kidney Disease: The Health, Aging, and Body Composition Study. Kidney Int. 2007, 71, 239–244. [Google Scholar] [CrossRef]
- Patel, K.V. Epidemiology of Anemia in Older Adults. Semin. Hematol. 2008, 45, 210–217. [Google Scholar] [CrossRef]
- Stauder, R.; Valent, P.; Theurl, I. Anemia at Older Age: Etiologies, Clinical Implications, and Management. Blood 2018, 131, 505–514. [Google Scholar] [CrossRef]
- Melzer, D.; Pilling, L.C.; Ferrucci, L. The Genetics of Human Ageing. Nat. Rev. Genet. 2020, 21, 88–101. [Google Scholar] [CrossRef]
- Wang, H.; Lou, D.; Wang, Z. Crosstalk of Genetic Variants, Allele-Specific DNA Methylation, and Environmental Factors for Complex Disease Risk. Front. Genet. 2018, 9, 695. [Google Scholar] [CrossRef]
- Langie, S.A.S.; Koppen, G.; Desaulniers, D.; Al-Mulla, F.; Al-Temaimi, R.; Amedei, A.; Azqueta, A.; Bisson, W.H.; Brown, D.G.; Brunborg, G.; et al. Causes of Genome Instability: The Effect of Low Dose Chemical Exposures in Modern Society. Carcinogenesis 2015, 36 (Suppl. S1), S61–S88. [Google Scholar] [CrossRef]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA Damage: Mechanisms, Mutation, and Disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Wei, Y.; Giunta, S.; Xia, S. Hypoxia in Aging and Aging-Related Diseases: Mechanism and Therapeutic Strategies. Int. J. Mol. Sci. 2022, 23, 8165. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-Induced DNA Damage, Mutations and Cancer. DNA Repair 2019, 83, 102673. [Google Scholar] [CrossRef]
- Liu, W.; Deng, Y.; Li, Z.; Chen, Y.; Zhu, X.; Tan, X.; Cao, G. Cancer Evo-Dev: A Theory of Inflammation-Induced Oncogenesis. Front. Immunol. 2021, 12, 768098. [Google Scholar] [CrossRef]
- Jaiswal, S.; Ebert, B.L. Clonal Hematopoiesis in Human Aging and Disease. Science 2019, 366, eaan4673. [Google Scholar] [CrossRef]
- Mitchell, E.; Spencer Chapman, M.; Williams, N.; Dawson, K.J.; Mende, N.; Calderbank, E.F.; Jung, H.; Mitchell, T.; Coorens, T.H.H.; Spencer, D.H.; et al. Clonal Dynamics of Haematopoiesis across the Human Lifespan. Nature 2022, 606, 343–350. [Google Scholar] [CrossRef]
- Morganti, C.; Ito, K. Mitochondrial Contributions to Hematopoietic Stem Cell Aging. Int. J. Mol. Sci. 2021, 22, 11117. [Google Scholar] [CrossRef]
- Zampino, M.; Brennan, N.A.; Kuo, P.-L.; Spencer, R.G.; Fishbein, K.W.; Simonsick, E.M.; Ferrucci, L. Poor Mitochondrial Health and Systemic Inflammation? Test of a Classic Hypothesis in the Baltimore Longitudinal Study of Aging. Geroscience 2020, 42, 1175–1182. [Google Scholar] [CrossRef]
- Yeo, E.-J. Hypoxia and Aging. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular Adaptation to Hypoxia through Hypoxia Inducible Factors and Beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef]
- Tojo, Y.; Sekine, H.; Hirano, I.; Pan, X.; Souma, T.; Tsujita, T.; Kawaguchi, S.; Takeda, N.; Takeda, K.; Fong, G.-H.; et al. Hypoxia Signaling Cascade for Erythropoietin Production in Hepatocytes. Mol. Cell. Biol. 2015, 35, 2658–2672. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J. Immunol. Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Wawrzyniak-Gramacka, E.; Hertmanowska, N.; Tylutka, A.; Morawin, B.; Wacka, E.; Gutowicz, M.; Zembron-Lacny, A. The Association of Anti-Inflammatory Diet Ingredients and Lifestyle Exercise with Inflammaging. Nutrients 2021, 13, 3696. [Google Scholar] [CrossRef] [PubMed]
- Minciullo, P.L.; Catalano, A.; Mandraffino, G.; Casciaro, M.; Crucitti, A.; Maltese, G.; Morabito, N.; Lasco, A.; Gangemi, S.; Basile, G. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch. Immunol. Ther. Exp. 2016, 64, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, D.; Raychev, A.; Deville, J.; Humphries, R.; Campeau, S.; Ruchala, P.; Nemeth, E.; Ganz, T.; Bulut, Y. Hepcidin Protects against Lethal Escherichia Coli Sepsis in Mice Inoculated with Isolates from Septic Patients. Infect. Immun. 2018, 86, e00253-18. [Google Scholar] [CrossRef]
- Stefanova, D.; Raychev, A.; Arezes, J.; Ruchala, P.; Gabayan, V.; Skurnik, M.; Dillon, B.J.; Horwitz, M.A.; Ganz, T.; Bulut, Y.; et al. Endogenous Hepcidin and Its Agonist Mediate Resistance to Selected Infections by Clearing Non-Transferrin-Bound Iron. Blood 2017, 130, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. The Role of Hepcidin in Iron Metabolism. Acta Haematol. 2009, 122, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Valore, E.V.; Waring, A.J.; Ganz, T. Hepcidin, a Urinary Antimicrobial Peptide Synthesized in the Liver. J. Biol. Chem. 2001, 276, 7806–7810. [Google Scholar] [CrossRef] [PubMed]
- den Elzen, W.P.J.; de Craen, A.J.M.; Wiegerinck, E.T.; Westendorp, R.G.J.; Swinkels, D.W.; Gussekloo, J. Plasma Hepcidin Levels and Anemia in Old Age. The Leiden 85-Plus Study. Haematologica 2013, 98, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Donovan, A.; Lima, C.A.; Pinkus, J.L.; Pinkus, G.S.; Zon, L.I.; Robine, S.; Andrews, N.C. The Iron Exporter Ferroportin/Slc40a1 Is Essential for Iron Homeostasis. Cell Metab. 2005, 1, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Tuttle, M.S.; Powelson, J.; Vaughn, M.B.; Donovan, A.; Ward, D.M.; Ganz, T.; Kaplan, J. Hepcidin Regulates Cellular Iron Efflux by Binding to Ferroportin and Inducing Its Internalization. Science 2004, 306, 2090–2093. [Google Scholar] [CrossRef]
- Ganz, T. Anemia of Inflammation. N. Engl. J. Med. 2019, 381, 1148–1157. [Google Scholar] [CrossRef]
- Wacka, E.; Wawrzyniak-Gramacka, E.; Tylutka, A.; Morawin, B.; Gutowicz, M.; Zembron-Lacny, A. The Role of Inflammation in Age-Associated Changes in Red Blood System. Int. J. Mol. Sci. 2023, 24, 8944. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Semba, R.D.; Guralnik, J.M.; Ershler, W.B.; Bandinelli, S.; Patel, K.V.; Sun, K.; Woodman, R.C.; Andrews, N.C.; Cotter, R.J.; et al. Proinflammatory State, Hepcidin, and Anemia in Older Persons. Blood 2010, 115, 3810–3816. [Google Scholar] [CrossRef]
- Campostrini, N.; Traglia, M.; Martinelli, N.; Corbella, M.; Cocca, M.; Manna, D.; Castagna, A.; Masciullo, C.; Silvestri, L.; Olivieri, O.; et al. Serum Levels of the Hepcidin-20 Isoform in a Large General Population: The Val Borbera Study. J. Proteom. 2012, 76, 28–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Galesloot, T.E.; Vermeulen, S.H.; Geurts-Moespot, A.J.; Klaver, S.M.; Kroot, J.J.; van Tienoven, D.; Wetzels, J.F.M.; Kiemeney, L.A.L.M.; Sweep, F.C.; den Heijer, M.; et al. Serum Hepcidin: Reference Ranges and Biochemical Correlates in the General Population. Blood 2011, 117, e218–e225. [Google Scholar] [CrossRef] [PubMed]
- Morceau, F.; Dicato, M.; Diederich, M. Pro-Inflammatory Cytokine-Mediated Anemia: Regarding Molecular Mechanisms of Erythropoiesis. Mediat. Inflamm. 2009, 2009, 405016. [Google Scholar] [CrossRef] [PubMed]
- Orsini, M.; Chateauvieux, S.; Rhim, J.; Gaigneaux, A.; Cheillan, D.; Christov, C.; Dicato, M.; Morceau, F.; Diederich, M. Sphingolipid-Mediated Inflammatory Signaling Leading to Autophagy Inhibition Converts Erythropoiesis to Myelopoiesis in Human Hematopoietic Stem/Progenitor Cells. Cell Death Differ. 2019, 26, 1796–1812. [Google Scholar] [CrossRef]
- Libregts, S.F.; Gutiérrez, L.; de Bruin, A.M.; Wensveen, F.M.; Papadopoulos, P.; van Ijcken, W.; Ozgür, Z.; Philipsen, S.; Nolte, M.A. Chronic IFN-γ Production in Mice Induces Anemia by Reducing Erythrocyte Life Span and Inhibiting Erythropoiesis through an IRF-1/PU.1 Axis. Blood 2011, 118, 2578–2588. [Google Scholar] [CrossRef] [PubMed]
- Means, R.T.; Dessypris, E.N.; Krantz, S.B. Inhibition of Human Erythroid Colony-Forming Units by Interleukin-1 Is Mediated by Gamma Interferon. J. Cell Physiol. 1992, 150, 59–64. [Google Scholar] [CrossRef]
- Means, R.T.; Krantz, S.B. Inhibition of Human Erythroid Colony-Forming Units by Gamma Interferon Can Be Corrected by Recombinant Human Erythropoietin. Blood 1991, 78, 2564–2567. [Google Scholar] [CrossRef]
- Miller, C.B.; Jones, R.J.; Piantadosi, S.; Abeloff, M.D.; Spivak, J.L. Decreased Erythropoietin Response in Patients with the Anemia of Cancer. N. Engl. J. Med. 1990, 322, 1689–1692. [Google Scholar] [CrossRef]
- Cazzola, M.; Ponchio, L.; de Benedetti, F.; Ravelli, A.; Rosti, V.; Beguin, Y.; Invernizzi, R.; Barosi, G.; Martini, A. Defective Iron Supply for Erythropoiesis and Adequate Endogenous Erythropoietin Production in the Anemia Associated with Systemic-Onset Juvenile Chronic Arthritis. Blood 1996, 87, 4824–4830. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Cooper, A.C. Erythropoietin Resistance: The Role of Inflammation and pro-Inflammatory Cytokines. Nephrol. Dial. Transplant. 2002, 17 (Suppl. S11), 39–43. [Google Scholar] [CrossRef]
- Kimachi, M.; Fukuma, S.; Yamazaki, S.; Yamamoto, Y.; Akizawa, T.; Akiba, T.; Saito, A.; Fukuhara, S. Minor Elevation in C-Reactive Protein Levels Predicts Incidence of Erythropoiesis-Stimulating Agent Hyporesponsiveness among Hemodialysis Patients. Nephron 2015, 131, 123–130. [Google Scholar] [CrossRef]
- Khalil, S.; Delehanty, L.; Grado, S.; Holy, M.; White, Z.; Freeman, K.; Kurita, R.; Nakamura, Y.; Bullock, G.; Goldfarb, A. Iron Modulation of Erythropoiesis Is Associated with Scribble-Mediated Control of the Erythropoietin Receptor. J. Exp. Med. 2018, 215, 661–679. [Google Scholar] [CrossRef]
- Buchanan, S.; Combet, E.; Stenvinkel, P.; Shiels, P.G. Klotho, Aging, and the Failing Kidney. Front. Endocrinol. 2020, 11, 560. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, Z. Molecular Basis of Klotho: From Gene to Function in Aging. Endocr. Rev. 2015, 36, 174–193. [Google Scholar] [CrossRef]
- An, C.; Chen, X.; Zheng, D. Association between Anemia and Serum Klotho in Middle-Aged and Older Adults. BMC Nephrol. 2023, 24, 38. [Google Scholar] [CrossRef]
- Vadakke Madathil, S.; Coe, L.M.; Casu, C.; Sitara, D. Klotho Deficiency Disrupts Hematopoietic Stem Cell Development and Erythropoiesis. Am. J. Pathol. 2014, 184, 827–841. [Google Scholar] [CrossRef]
- Kanbay, M.; Copur, S.; Ozbek, L.; Mutlu, A.; Cejka, D.; Ciceri, P.; Cozzolino, M.; Haarhaus, M.L. Klotho: A Potential Therapeutic Target in Aging and Neurodegeneration beyond Chronic Kidney Disease—A Comprehensive Review from the ERA CKD-MBD Working Group. Clin. Kidney J. 2024, 17, sfad276. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-C.; Fogo, A.B. Fibrosis and Renal Aging. Kidney Int. Suppl. 2014, 4, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Tsiftsoglou, A.S. Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine. Cells 2021, 10, 2140. [Google Scholar] [CrossRef]
- Ershler, W.B.; Sheng, S.; McKelvey, J.; Artz, A.S.; Denduluri, N.; Tecson, J.; Taub, D.D.; Brant, L.J.; Ferrucci, L.; Longo, D.L. Serum Erythropoietin and Aging: A Longitudinal Analysis. J. Am. Geriatr. Soc. 2005, 53, 1360–1365. [Google Scholar] [CrossRef]
- Macciò, A.; Madeddu, C. Management of Anemia of Inflammation in the Elderly. Anemia 2012, 2012, 563251. [Google Scholar] [CrossRef]
- Mitlyng, B.L.; Singh, J.A.; Furne, J.K.; Ruddy, J.; Levitt, M.D. Use of Breath Carbon Monoxide Measurements to Assess Erythrocyte Survival in Subjects with Chronic Diseases. Am. J. Hematol. 2006, 81, 432–438. [Google Scholar] [CrossRef]
- Correll, C.K.; Binstadt, B.A. Advances in the Pathogenesis and Treatment of Systemic Juvenile Idiopathic Arthritis. Pediatr. Res. 2014, 75, 176–183. [Google Scholar] [CrossRef]
- Milner, J.D.; Orekov, T.; Ward, J.M.; Cheng, L.; Torres-Velez, F.; Junttila, I.; Sun, G.; Buller, M.; Morris, S.C.; Finkelman, F.D.; et al. Sustained IL-4 Exposure Leads to a Novel Pathway for Hemophagocytosis, Inflammation, and Tissue Macrophage Accumulation. Blood 2010, 116, 2476–2483. [Google Scholar] [CrossRef]
- Cartwright, G.E.; Lee, G.R. The Anaemia of Chronic Disorders. Br. J. Haematol. 1971, 21, 147–152. [Google Scholar] [CrossRef]
- Freireich, E.J.; Ross, J.F.; Bayles, T.B.; Emerson, C.P.; Finch, S.C. Radioactive Iron Metabolism and Erythrocyte Survival Studies of the Mechanism of the Anemia Associated with Rheumatoid Arthritis. J. Clin. Investig. 1957, 36, 1043–1058. [Google Scholar] [CrossRef]
- Kvietys, P.R.; Granger, D.N. Role of Reactive Oxygen and Nitrogen Species in the Vascular Responses to Inflammation. Free Radic. Biol. Med. 2012, 52, 556–592. [Google Scholar] [CrossRef]
- Orrico, F.; Laurance, S.; Lopez, A.C.; Lefevre, S.D.; Thomson, L.; Möller, M.N.; Ostuni, M.A. Oxidative Stress in Healthy and Pathological Red Blood Cells. Biomolecules 2023, 13, 1262. [Google Scholar] [CrossRef]
- Williams, A.; Bissinger, R.; Shamaa, H.; Patel, S.; Bourne, L.; Artunc, F.; Qadri, S. Pathophysiology of Red Blood Cell Dysfunction in Diabetes and Its Complications. Pathophysiology 2023, 30, 327–345. [Google Scholar] [CrossRef]
- Barshtein, G. Biochemical and Biophysical Properties of Red Blood Cells in Disease. Biomolecules 2022, 12, 923. [Google Scholar] [CrossRef]
- Chaudhary, R.; Katharia, R. Oxidative Injury as Contributory Factor for Red Cells Storage Lesion during Twenty Eight Days of Storage. Blood Transfus. 2012, 10, 59. [Google Scholar] [CrossRef]
- Huisjes, R.; Bogdanova, A.; Van Solinge, W.W.; Schiffelers, R.M.; Kaestner, L.; Van Wijk, R. Squeezing for Life—Properties of Red Blood Cell Deformability. Front. Physiol. 2018, 9, 656. [Google Scholar] [CrossRef]
- Ammendolia, D.A.; Bement, W.M.; Brumell, J.H. Plasma Membrane Integrity: Implications for Health and Disease. BMC Biol. 2021, 19, 71. [Google Scholar] [CrossRef]
- Skou, S.T.; Mair, F.S.; Fortin, M.; Guthrie, B.; Nunes, B.P.; Miranda, J.J.; Boyd, C.M.; Pati, S.; Mtenga, S.; Smith, S.M. Multimorbidity. Nat. Rev. Dis. Primers 2022, 8, 48. [Google Scholar] [CrossRef]
- Steinmeyer, Z.; Delpierre, C.; Soriano, G.; Steinmeyer, A.; Ysebaert, L.; Balardy, L.; Sourdet, S. Hemoglobin Concentration; a Pathway to Frailty. BMC Geriatr. 2020, 20, 202. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Imbimbo, G.; Jager-Wittenaar, H.; Cederholm, T.; Rothenberg, E.; Di Girolamo, F.G.; Amabile, M.I.; Sealy, M.; Schneider, S.; Barazzoni, R.; et al. Disease-Related Malnutrition with Inflammation and Cachexia. Clin. Nutr. 2023, 42, 1475–1479. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN Guidelines on Definitions and Terminology of Clinical Nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Alghareeb, S.A.; Alfhili, M.A.; Fatima, S. Molecular Mechanisms and Pathophysiological Significance of Eryptosis. Int. J. Mol. Sci. 2023, 24, 5079. [Google Scholar] [CrossRef]
- Bissinger, R.; Bhuyan, A.A.M.; Qadri, S.M.; Lang, F. Oxidative Stress, Eryptosis and Anemia: A Pivotal Mechanistic Nexus in Systemic Diseases. FEBS J. 2019, 286, 826–854. [Google Scholar] [CrossRef]
- O’Sullivan, E.D.; Hughes, J.; Ferenbach, D.A. Renal Aging: Causes and Consequences. J. Am. Soc. Nephrol. 2017, 28, 407–420. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Li, Q.; Li, J. Understanding the Unique Microenvironment in the Aging Liver. Front. Med. 2022, 9, 842024. [Google Scholar] [CrossRef]
- Bolignano, D.; Mattace-Raso, F.; Sijbrands, E.J.G.; Zoccali, C. The Aging Kidney Revisited: A Systematic Review. Ageing Res. Rev. 2014, 14, 65–80. [Google Scholar] [CrossRef]
- Ou, H.-L.; Schumacher, B. DNA Damage Responses and P53 in the Aging Process. Blood 2018, 131, 488–495. [Google Scholar] [CrossRef]
- Walsh, K.; Raghavachari, N.; Kerr, C.; Bick, A.G.; Cummings, S.R.; Druley, T.; Dunbar, C.E.; Genovese, G.; Goodell, M.A.; Jaiswal, S.; et al. Clonal Hematopoiesis Analyses in Clinical, Epidemiologic, and Genetic Aging Studies to Unravel Underlying Mechanisms of Age-Related Dysfunction in Humans. Front. Aging 2022, 3, 841796. [Google Scholar] [CrossRef]
- Bick, A.G.; Weinstock, J.S.; Nandakumar, S.K.; Fulco, C.P.; Bao, E.L.; Zekavat, S.M.; Szeto, M.D.; Liao, X.; Leventhal, M.J.; Nasser, J.; et al. Inherited Causes of Clonal Haematopoiesis in 97,691 Whole Genomes. Nature 2020, 586, 763–768. [Google Scholar] [CrossRef]
- Kar, S.P.; Quiros, P.M.; Gu, M.; Jiang, T.; Mitchell, J.; Langdon, R.; Iyer, V.; Barcena, C.; Vijayabaskar, M.S.; Fabre, M.A.; et al. Genome-Wide Analyses of 200,453 Individuals Yield New Insights into the Causes and Consequences of Clonal Hematopoiesis. Nat. Genet. 2022, 54, 1155–1166. [Google Scholar] [CrossRef]
- Hoermann, G. Clinical Significance of Clonal Hematopoiesis of Indeterminate Potential in Hematology and Cardiovascular Disease. Diagnostics 2022, 12, 1613. [Google Scholar] [CrossRef]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef]
- Mangaonkar, A.A.; Patnaik, M.M. Clonal Hematopoiesis of Indeterminate Potential and Clonal Cytopenias of Undetermined Significance: 2023 Update on Clinical Associations and Management Recommendations. Am. J. Hematol. 2023, 98, 951–964. [Google Scholar] [CrossRef]
- Kovtonyuk, L.V.; Fritsch, K.; Feng, X.; Manz, M.G.; Takizawa, H. Inflamm-Aging of Hematopoiesis, Hematopoietic Stem Cells, and the Bone Marrow Microenvironment. Front. Immunol. 2016, 7, 502. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Yoo, D.-M.; Min, C.; Choi, H.-G. Association between Osteoporosis and Low Hemoglobin Levels: A Nested Case–Control Study Using a National Health Screening Cohort. Int. J. Environ. Res. Public Health 2021, 18, 8598. [Google Scholar] [CrossRef]
- Tozzi-Ciancarelli, M.G.; Fedele, F.; Tozzi, E.; Di Massimo, C.; Oratore, A.; De Matteis, G.; D’Alfonso, A.; Troiani-Sevi, E.; Gallo, P.; Prencipe, M. Age-Dependent Changes in Human Erythrocyte Properties. CH 2016, 9, 999–1007. [Google Scholar] [CrossRef]
- Higuchi-Sanabria, R.; Paul, J.W.; Durieux, J.; Benitez, C.; Frankino, P.A.; Tronnes, S.U.; Garcia, G.; Daniele, J.R.; Monshietehadi, S.; Dillin, A. Spatial Regulation of the Actin Cytoskeleton by HSF-1 during Aging. MBoC 2018, 29, 2522–2527. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Chu, T.T.; Naidu, R.; Lu, L.; Chandramohanadas, R.; Dao, M.; Karniadakis, G.E. Cytoskeleton Remodeling Induces Membrane Stiffness and Stability Changes of Maturing Reticulocytes. Biophys. J. 2018, 114, 2014–2023. [Google Scholar] [CrossRef]
- Ciana, A.; Achilli, C.; Minetti, G. Spectrin and Other Membrane-Skeletal Components in Human Red Blood Cells of Different Age. Cell Physiol. Biochem. 2017, 42, 1139–1152. [Google Scholar] [CrossRef]
- Kim, Y.J.; Cho, M.J.; Yu, W.D.; Kim, M.J.; Kim, S.Y.; Lee, J.H. Links of Cytoskeletal Integrity with Disease and Aging. Cells 2022, 11, 2896. [Google Scholar] [CrossRef]
- Remigante, A.; Morabito, R.; Marino, A. Band 3 Protein Function and Oxidative Stress in Erythrocytes. J. Cell. Physiol. 2021, 236, 6225–6234. [Google Scholar] [CrossRef]
- Levin, A.S.; Bilous, R.W.; Coresh, J. Chapter 1: Definition and Classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [Google Scholar] [CrossRef]
- Waas, T.; Schulz, A.; Lotz, J.; Rossmann, H.; Pfeiffer, N.; Beutel, M.E.; Schmidtmann, I.; Münzel, T.; Wild, P.S.; Lackner, K.J. Distribution of Estimated Glomerular Filtration Rate and Determinants of Its Age Dependent Loss in a German Population-Based Study. Sci. Rep. 2021, 11, 10165. [Google Scholar] [CrossRef] [PubMed]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, Regional, and National Burden of Chronic Kidney Disease, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Davis, J.R.; Duling, I.; Little, D.J. Prevalence of Anaemia in Adults with Chronic Kidney Disease in a Representative Sample of the United States Population: Analysis of the 1999–2018 National Health and Nutrition Examination Survey. Clin. Kidney J. 2023, 16, 303–311. [Google Scholar] [CrossRef]
- Stauffer, M.E.; Fan, T. Prevalence of Anemia in Chronic Kidney Disease in the United States. PLoS ONE 2014, 9, e84943. [Google Scholar] [CrossRef] [PubMed]
- Sutaria, A.; Liu, L.; Ahmed, Z. Multiple Medication (Polypharmacy) and Chronic Kidney Disease in Patients Aged 60 and Older: A Pharmacoepidemiologic Perspective. Ther. Adv. Cardiovasc. Dis. 2016, 10, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Fusco, S.; Garasto, S.; Corsonello, A.; Vena, S.; Mari, V.; Gareri, P.; Ruotolo, G.; Luciani, F.; Roncone, A.; Maggio, M.; et al. Medication-Induced Nephrotoxicity in Older Patients. CDM 2016, 17, 608–625. [Google Scholar] [CrossRef]
- Khan, S.; Loi, V.; Rosner, M.H. Drug-Induced Kidney Injury in the Elderly. Drugs Aging 2017, 34, 729–741. [Google Scholar] [CrossRef]
- Luckey, A.E.; Parsa, C.J. Fluid and Electrolytes in the Aged. Arch. Surg. 2003, 138, 1055–1060. [Google Scholar] [CrossRef]
- Begg, D.P. Disturbances of Thirst and Fluid Balance Associated with Aging. Physiol. Behav. 2017, 178, 28–34. [Google Scholar] [CrossRef]
- Docherty, N.G.; Delles, C.; D’Haese, P.; Layton, A.T.; Martínez-Salgado, C.; Vervaet, B.A.; López-Hernández, F.J. Haemodynamic Frailty—A Risk Factor for Acute Kidney Injury in the Elderly. Ageing Res. Rev. 2021, 70, 101408. [Google Scholar] [CrossRef]
- El-Sharkawy, A.M.; Devonald, M.A.J.; Humes, D.J.; Sahota, O.; Lobo, D.N. Hyperosmolar Dehydration: A Predictor of Kidney Injury and Outcome in Hospitalised Older Adults. Clin. Nutr. 2020, 39, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Fandrey, J. Why Not the Liver Instead of the Kidney? Blood 2012, 120, 1760–1761. [Google Scholar] [CrossRef][Green Version]
- Haase, V.H. Regulation of Erythropoiesis by Hypoxia-Inducible Factors. Blood Rev. 2013, 27, 41–53. [Google Scholar] [CrossRef]
- Vlasschaert, C.; McNaughton, A.J.M.; Chong, M.; Cook, E.K.; Hopman, W.; Kestenbaum, B.; Robinson-Cohen, C.; Garland, J.; Moran, S.M.; Paré, G.; et al. Association of Clonal Hematopoiesis of Indeterminate Potential with Worse Kidney Function and Anemia in Two Cohorts of Patients with Advanced Chronic Kidney Disease. JASN 2022, 33, 985–995. [Google Scholar] [CrossRef]
- Takata, T.; Mae, Y.; Yamada, K.; Taniguchi, S.; Hamada, S.; Yamamoto, M.; Iyama, T.; Isomoto, H. Skeletal Muscle Mass Is Associated with Erythropoietin Response in Hemodialysis Patients. BMC Nephrol. 2021, 22, 134. [Google Scholar] [CrossRef] [PubMed]
- Rundqvist, H.; Rullman, E.; Sundberg, C.J.; Fischer, H.; Eisleitner, K.; Ståhlberg, M.; Sundblad, P.; Jansson, E.; Gustafsson, T. Activation of the Erythropoietin Receptor in Human Skeletal Muscle. Eur. J. Endocrinol. 2009, 161, 427–434. [Google Scholar] [CrossRef]
- Zeng, F.; Huang, L.; Zhang, Y.; Hong, X.; Weng, S.; Shen, X.; Zhao, F.; Yan, S. Additive Effect of Sarcopenia and Anemia on the 10-Year Risk of Cardiovascular Disease in Patients with Type 2 Diabetes. J. Diabetes Res. 2022, 2022, 2202511. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.-H.; Lee, W.-J.; Peng, L.-N.; Lin, M.-H.; Chen, L.-K. Associations between Hemoglobin Levels and Sarcopenia and Its Components: Results from the I-Lan Longitudinal Study. Exp. Gerontol. 2021, 150, 111379. [Google Scholar] [CrossRef] [PubMed]
- Hirani, V.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Seibel, M.J.; Waite, L.M.; Handelsman, D.J.; Hsu, B.; Cumming, R.G. Low Hemoglobin Concentrations Are Associated with Sarcopenia, Physical Performance, and Disability in Older Australian Men in Cross-Sectional and Longitudinal Analysis: The Concord Health and Ageing in Men Project. Gerona 2016, 71, 1667–1675. [Google Scholar] [CrossRef]
- Boelaert, K. Thyroid Dysfunction in the Elderly. Nat. Rev. Endocrinol. 2013, 9, 194–204. [Google Scholar] [CrossRef]
- Maggio, M.; De Vita, F.; Fisichella, A.; Lauretani, F.; Ticinesi, A.; Ceresini, G.; Cappola, A.; Ferrucci, L.; Ceda, G.P. The Role of the Multiple Hormonal Dysregulation in the Onset of “Anemia of Aging”: Focus on Testosterone, IGF-1, and Thyroid Hormones. Int. J. Endocrinol. 2015, 2015, 292574. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone Therapy in Men with Hypogonadism: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef] [PubMed]
- Al-Sharefi, A.; Mohammed, A.; Abdalaziz, A.; Jayasena, C.N. Androgens and Anemia: Current Trends and Future Prospects. Front. Endocrinol. 2019, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Bárány, P.; Yilmaz, M.I.; Qureshi, A.R.; Sonmez, A.; Heimbürger, O.; Ozgurtas, T.; Yenicesu, M.; Lindholm, B.; Stenvinkel, P. Testosterone Deficiency Is a Cause of Anaemia and Reduced Responsiveness to Erythropoiesis-Stimulating Agents in Men with Chronic Kidney Disease. Nephrol. Dial. Transplant. 2012, 27, 709–715. [Google Scholar] [CrossRef]
- Magné, N.; Daguenet, E.; Bouleftour, W.; Conraux, L.; Tinquaut, F.; Grangeon, K.; Moreno-Acosta, P.; Suchaud, J.-P.; Rancoule, C.; Guy, J.-B. Impact of Radiation Therapy on Biological Parameters in Cancer Patients: Sub-Analysis from the RIT Prospective Epidemiological Study. Cancer Investig. 2023, 41, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-R.; Lee, J.-H.; Heo, H.-R.; Yang, S.-R.; Ha, K.-S.; Park, W.S.; Han, E.-T.; Song, H.; Hong, S.-H. Improved Hematopoietic Differentiation of Human Pluripotent Stem Cells via Estrogen Receptor Signaling Pathway. Cell Biosci. 2016, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Gao, Z.; Wang, G.; Li, H.; Zheng, J. Estrogen Potentiates Reactive Oxygen Species (ROS) Tolerance to Initiate Carcinogenesis and Promote Cancer Malignant Transformation. Tumour Biol. 2016, 37, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Guajardo-Correa, E.; Silva-Agüero, J.F.; Calle, X.; Chiong, M.; Henríquez, M.; García-Rivas, G.; Latorre, M.; Parra, V. Estrogen Signaling as a Bridge between the Nucleus and Mitochondria in Cardiovascular Diseases. Front. Cell Dev. Biol. 2022, 10, 968373. [Google Scholar] [CrossRef] [PubMed]
- Nakada, D.; Oguro, H.; Levi, B.P.; Ryan, N.; Kitano, A.; Saitoh, Y.; Takeichi, M.; Wendt, G.R.; Morrison, S.J. Oestrogen Increases Haematopoietic Stem-Cell Self-Renewal in Females and during Pregnancy. Nature 2014, 505, 555–558. [Google Scholar] [CrossRef]
- Dumic, I.; Nordin, T.; Jecmenica, M.; Stojkovic Lalosevic, M.; Milosavljevic, T.; Milovanovic, T. Gastrointestinal Tract Disorders in Older Age. Can. J. Gastroenterol. Hepatol. 2019, 2019, 6757524. [Google Scholar] [CrossRef]
- Porter, K.M.; Hoey, L.; Hughes, C.F.; Ward, M.; Clements, M.; Strain, J.; Cunningham, C.; Casey, M.C.; Tracey, F.; O’Kane, M.; et al. Associations of Atrophic Gastritis and Proton-Pump Inhibitor Drug Use with Vitamin B-12 Status, and the Impact of Fortified Foods, in Older Adults. Am. J. Clin. Nutr. 2021, 114, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Hohman, L.S.; Osborne, L.C. A Gut-Centric View of Aging: Do Intestinal Epithelial Cells Contribute to Age-Associated Microbiota Changes, Inflammaging, and Immunosenescence? Aging Cell 2022, 21, e13700. [Google Scholar] [CrossRef]
- Cappello, M.; Morreale, G.C.; Licata, A. Elderly Onset Celiac Disease: A Narrative Review. Clin. Med. Insights Gastroenterol. 2016, 9, CGast.S38454. [Google Scholar] [CrossRef]
- Ching, C.K.; Lebwohl, B. Celiac Disease in the Elderly. Curr. Treat. Options Gastro 2022, 20, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Sopeña, F.; Lanas, A. Management of Colonic Diverticular Disease with Poorly Absorbed Antibiotics and Other Therapies. Ther. Adv. Gastroenterol. 2011, 4, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Efremova, I.; Maslennikov, R.; Poluektova, E.; Vasilieva, E.; Zharikov, Y.; Suslov, A.; Letyagina, Y.; Kozlov, E.; Levshina, A.; Ivashkin, V. Epidemiology of Small Intestinal Bacterial Overgrowth. World J. Gastroenterol. 2023, 29, 3400–3421. [Google Scholar] [CrossRef] [PubMed]
- Manrai, M.; Dawra, S.; Kapoor, R.; Srivastava, S.; Singh, A. Anemia in Cirrhosis: An Underestimated Entity. WJCC 2022, 10, 777–789. [Google Scholar] [CrossRef]
- Risør, L.M.; Fenger, M.; Olsen, N.V.; Møller, S. Hepatic Erythropoietin Response in Cirrhosis. A Contemporary Review. Scand. J. Clin. Lab. Investig. 2016, 76, 183–189. [Google Scholar] [CrossRef]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The Gut Microbiome as a Modulator of Healthy Ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and Consequences of Intestinal Dysbiosis. Cell Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Wilms, E.; Troost, F.J.; Elizalde, M.; Winkens, B.; De Vos, P.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M. Intestinal Barrier Function Is Maintained with Aging—A Comprehensive Study in Healthy Subjects and Irritable Bowel Syndrome Patients. Sci. Rep. 2020, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Josefsdottir, K.S.; Baldridge, M.T.; Kadmon, C.S.; King, K.Y. Antibiotics Impair Murine Hematopoiesis by Depleting the Intestinal Microbiota. Blood 2017, 129, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tian, Y.; Yuan, Z.; Zeng, Y.; Wang, S.; Fan, X.; Yang, D.; Yang, M. Iron Metabolism in Aging and Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 3612. [Google Scholar] [CrossRef]
- Yan, H.; Walker, F.C.; Ali, A.; Han, H.; Tan, L.; Veillon, L.; Lorenzi, P.L.; Baldridge, M.T.; King, K.Y. The Bacterial Microbiota Regulates Normal Hematopoiesis via Metabolite-Induced Type 1 Interferon Signaling. Blood Adv. 2022, 6, 1754–1765. [Google Scholar] [CrossRef]
- Manzo, V.E.; Bhatt, A.S. The Human Microbiome in Hematopoiesis and Hematologic Disorders. Blood 2015, 126, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Rackerby, B.; Kim, H.J.; Dallas, D.C.; Park, S.H. Understanding the Effects of Dietary Components on the Gut Microbiome and Human Health. Food Sci. Biotechnol. 2020, 29, 1463–1474. [Google Scholar] [CrossRef]
- Caiado, F.; Manz, M.G. A Microbiome-Macrophage-Iron Axis Guides Stressed Hematopoietic Stem Cell Fate. Cell Stem Cell 2022, 29, 177–179. [Google Scholar] [CrossRef]
- Soriano-Lerma, A.; García-Burgos, M.; Alférez, M.J.M.; Pérez-Carrasco, V.; Sanchez-Martin, V.; Linde-Rodríguez, Á.; Ortiz-González, M.; Soriano, M.; García-Salcedo, J.A.; López-Aliaga, I. Gut Microbiome-Short-Chain Fatty Acids Interplay in the Context of Iron Deficiency Anaemia. Eur. J. Nutr. 2022, 61, 399–412. [Google Scholar] [CrossRef]
- Ragonnaud, E.; Biragyn, A. Gut Microbiota as the Key Controllers of “Healthy” Aging of Elderly People. Immun. Ageing 2021, 18, 2. [Google Scholar] [CrossRef]
- Miller, F.W. The Increasing Prevalence of Autoimmunity and Autoimmune Diseases: An Urgent Call to Action for Improved Understanding, Diagnosis, Treatment, and Prevention. Curr. Opin. Immunol. 2023, 80, 102266. [Google Scholar] [CrossRef]
- Conrad, N.; Misra, S.; Verbakel, J.Y.; Verbeke, G.; Molenberghs, G.; Taylor, P.N.; Mason, J.; Sattar, N.; McMurray, J.J.V.; McInnes, I.B.; et al. Incidence, Prevalence, and Co-Occurrence of Autoimmune Disorders over Time and by Age, Sex, and Socioeconomic Status: A Population-Based Cohort Study of 22 Million Individuals in the UK. Lancet 2023, 401, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Moskalec, O.V. Characteristics of immune response in elderly and autoimmunity. Adv. Gerontol. 2020, 33, 246–255. [Google Scholar] [PubMed]
- Watad, A.; Bragazzi, N.L.; Adawi, M.; Amital, H.; Toubi, E.; Porat, B.-S.; Shoenfeld, Y. Autoimmunity in the Elderly: Insights from Basic Science and Clinics—A Mini-Review. Gerontology 2017, 63, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; Xu, S.-Q.; Xu, Y.-C.; Li, W.-J.; Chen, K.-M.; Cai, J.; Li, M. Inflammatory Anemia May Be an Indicator for Predicting Disease Activity and Structural Damage in Chinese Patients with Rheumatoid Arthritis. Clin. Rheumatol. 2020, 39, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Vadasz, Z.; Haj, T.; Kessel, A.; Toubi, E. Age-Related Autoimmunity. BMC Med. 2013, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Imanuel, C.A.; Sivatheesan, S.; Koyanagi, A.; Smith, L.; Konrad, M.; Kostev, K. Associations between Rheumatoid Arthritis and Various Comorbid Conditions in Germany-A Retrospective Cohort Study. J. Clin. Med. 2023, 12, 7265. [Google Scholar] [CrossRef] [PubMed]
- Shadick, N.; Hagino, O.; Praestgaard, A.; Fiore, S.; Weinblatt, M.; Burmester, G. Association of Hemoglobin Levels with Radiographic Progression in Patients with Rheumatoid Arthritis: An Analysis from the BRASS Registry. Arthritis Res. Ther. 2023, 25, 88. [Google Scholar] [CrossRef] [PubMed]
- Günther, F.; Straub, R.H.; Hartung, W.; Fleck, M.; Ehrenstein, B.; Schminke, L. Usefulness of Soluble Transferrin Receptor in the Diagnosis of Iron Deficiency Anemia in Rheumatoid Arthritis Patients in Clinical Practice. Int. J. Rheumatol. 2022, 2022, 7067262. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Kong, G.; Yang, C.; Ming, Y. Erythropoietin and Its Derivatives: From Tissue Protection to Immune Regulation. Cell Death Dis. 2020, 11, 79. [Google Scholar] [CrossRef]
- Nairz, M.; Schroll, A.; Moschen, A.R.; Sonnweber, T.; Theurl, M.; Theurl, I.; Taub, N.; Jamnig, C.; Neurauter, D.; Huber, L.A.; et al. Erythropoietin Contrastingly Affects Bacterial Infection and Experimental Colitis by Inhibiting Nuclear Factor-κB-Inducible Immune Pathways. Immunity 2011, 34, 61–74. [Google Scholar] [CrossRef]
- Eswarappa, M.; Cantarelli, C.; Cravedi, P. Erythropoietin in Lupus: Unanticipated Immune Modulating Effects of a Kidney Hormone. Front. Immunol. 2021, 12, 639370. [Google Scholar] [CrossRef]
- Moransard, M.; Bednar, M.; Frei, K.; Gassmann, M.; Ogunshola, O.O. Erythropoietin Reduces Experimental Autoimmune Encephalomyelitis Severity via Neuroprotective Mechanisms. J. Neuroinflamm. 2017, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Fattizzo, B.; Pedone, G.L.; Brambilla, C.; Pettine, L.; Zaninoni, A.; Passamonti, F.; Barcellini, W. Recombinant Erythropoietin in Autoimmune Hemolytic Anemia with Inadequate Bone Marrow Response: A Prospective Analysis. Blood Adv. 2023, 8, 1322–1327. [Google Scholar] [CrossRef]
- Wu, G.; Cao, B.; Zhai, H.; Liu, B.; Huang, Y.; Chen, X.; Ling, H.; Ling, S.; Jin, S.; Yang, X.; et al. EPO Promotes the Progression of Rheumatoid Arthritis by Inducing Desialylation via Increasing the Expression of Neuraminidase 3. Ann. Rheum. Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Paulson, R.F.; Ruan, B.; Hao, S.; Chen, Y. Stress Erythropoiesis Is a Key Inflammatory Response. Cells 2020, 9, 634. [Google Scholar] [CrossRef] [PubMed]
- Bennett, L.F.; Liao, C.; Quickel, M.D.; Yeoh, B.S.; Vijay-Kumar, M.; Hankey-Giblin, P.; Prabhu, K.S.; Paulson, R.F. Inflammation Induces Stress Erythropoiesis through Heme-Dependent Activation of SPI-C. Sci. Signal. 2019, 12, eaap7336. [Google Scholar] [CrossRef]
- Denny, S.D.; Kuchibhatla, M.N.; Cohen, H.J. Impact of Anemia on Mortality, Cognition, and Function in Community-Dwelling Elderly. Am. J. Med. 2006, 119, 327–334. [Google Scholar] [CrossRef]
- Izaks, G.J.; Westendorp, R.G.; Knook, D.L. The Definition of Anemia in Older Persons. JAMA 1999, 281, 1714–1717. [Google Scholar] [CrossRef]
- Patel, K.V.; Harris, T.B.; Faulhaber, M.; Angleman, S.B.; Connelly, S.; Bauer, D.C.; Kuller, L.H.; Newman, A.B.; Guralnik, J.M. Racial Variation in the Relationship of Anemia with Mortality and Mobility Disability among Older Adults. Blood 2007, 109, 4663–4670. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Pahor, M.; Woodman, R.C.; Guralnik, J.M. Anemia in Old Age Is Associated with Increased Mortality and Hospitalization. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 474–479. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).