Abstract
An increasing amount of research explores the role of race in clinical phenotypes and outcomes in ulcerative colitis (UC). We aimed to investigate racial differences in infliximab (IFX) treatment efficacy in UC. We used aggregate data from IFX trials and evidence synthesis methods to generate race-specific efficacy estimates. Then, we tested the effect modification by race by comparing the race-specific estimates derived from independent evidence syntheses. We computed ratios of relative risks (RRRs) and performed tests of statistical interaction. We analyzed data from five randomized, placebo-controlled trials evaluating IFX as induction and maintenance therapy for adults with moderate-to-severe UC (875 participants; 45% Asians). We found no substantial evidence of racial differences concerning the efficacy of IFX in inducing clinical response (RRR = 0.89, 95% CI: 0.66–1.20; p = 0.44), clinical remission (RRR = 0.58, 95% CI: 0.24–1.44; p = 0.24), and mucosal healing (RRR = 0.99, 95% CI: 0.69–1.41; p = 0.95), or maintaining clinical remission (RRR = 0.81, 95% CI: 0.46–1.42; p = 0.45) and mucosal healing (RRR = 0.84, 95% CI: 0.48–1.46; p = 0.53), between Asian and Caucasian populations. Future clinical studies should expand the participation of racial minorities to comprehensively assess potential racial differences in the effectiveness of advanced therapies, including IFX, in the context of treating UC.
1. Introduction
Ulcerative colitis (UC) is a chronic inflammatory bowel disease affecting the colon and rectum to varying extents and leading to long-term complications and disability [1]. It follows a remitting and relapsing course. Symptoms usually develop over time and often include diarrhea, fever, urgency, tenesmus, abdominal cramps, rectal bleeding, weight loss, and fatigue, which vary in severity based on the extent of inflammation [1,2]. UC’s incidence and prevalence are rising globally, posing a major health challenge [3,4]. Its pathogenesis remains unclear; however, mucosal immune dysregulation, gut microbiota, genetic predisposition, and environmental exposures have been strongly implicated [1,5,6,7]. Its diagnosis relies on clinical symptoms and biologic, endoscopic, and histological findings; however, there is no gold standard [1,2]. UC imposes a substantial global economic burden and severely impacts the quality of life for those affected [8,9].
Treatment decisions, considering factors like disease activity, extent, progression, and patient preferences, play a pivotal role [10,11,12]. While effective for many, a significant proportion of UC patients fail to improve, leading to the emergence of advanced therapies like infliximab (IFX), golimumab, adalimumab, vedolizumab, ustekinumab, tofacitinib and others. These biologic and small-molecule drugs target various points in the inflammatory cascade and constitute the product of our enhanced comprehension of the mechanisms underlying UC. They enabled better control of the disease, leading to increased rates of clinical response and remission, endoscopic healing, and corticosteroid-free remission and, ultimately, an improvement in quality of life [9,13,14]. However, there remains uncertainty regarding the efficacy of many of these therapies in certain patient groups and disease phenotypes.
Among these therapies, IFX was the first biologic to receive United States Food and Drug Administration (FDA; September 2005) and European Medicines Agency (EMA; March 2006) approvals for the treatment of adults with moderately to severely active UC who had an inadequate response to conventional therapy. It is a purified recombinant DNA-derived chimeric IgG monoclonal antibody, with murine and human components, acting to inhibit tumor necrosis factor-alpha (TNF-a) [15,16]. IFX is very useful for neutralizing TNF and inhibiting immune responses in UC [17]. It has demonstrated efficacy and safety [18] and is widely used for treating UC patients, both for induction and maintenance of remission.
In recent years, there has been an expanding body of research on the role that race and ethnicity might play in affecting clinical phenotypes and outcomes in UC. Several studies comparing disease extent revealed distinctive patterns among racial groups. Black patients exhibited higher rates of proctitis and left-sided colitis compared to White patients, who were more likely to suffer extensive colitis [19,20,21]. Hispanic patients showed higher rates of extensive colitis compared with White patients [22]; however, other studies found no differences in disease location [23,24,25]. Asian populations with UC were found to have extensive colitis less frequently than other races [26], while those from South Asia were found to have significantly more pancolitis and less proctitis compared with those from East Asia [27]. On the other hand, examination of disease outcomes revealed interesting findings too: Extraintestinal manifestations, including arthritis and primary sclerosing cholangitis, were more common in Black than among White patients with UC [28]. Hispanic patients showed higher rates of colectomy compared with White patients [22], whereas Asians exhibited a lower likelihood of undergoing surgery for UC compared with Caucasians [27]. This review addresses a crucial research gap, exploring the influence of race on medication efficacy in UC and determining optimal medications based on race. While acknowledging the limitations of using race as a proxy for biological differences, it is important to note that in various diseases, race has proven to be a valuable marker for predicting the response to drug therapy [29].
Recently, Greywoode and colleagues performed the first study assessing the influence of race on the response to biologics [30]. Specifically, they conducted a post hoc analysis of randomized clinical trials in UC to assess the impact of race on response to golimumab. Their study showed that patients from racial minorities, primarily Asians, were less likely to achieve and maintain clinical response, clinical remission, and endoscopic healing when treated with golimumab compared with White/Caucasian patients. However, their analysis did not extend to IFX due to the limited representation of participants from racial and ethnic minority groups in their dataset who had received this treatment.
To address this gap, we utilized aggregate data reported from randomized, placebo-controlled IFX trials and evidence synthesis methodology to generate race-specific efficacy estimates and examine the effect modification by race (i.e., the interaction between treatment and race) to assess the potential influence of race on the efficacy of IFX therapy in UC.
2. Materials and Methods
2.1. Data Sources
To investigate potential racial differences in the efficacy of IFX as induction and maintenance therapy in UC, we used data from randomized, placebo-controlled studies identified through a technical review published recently by our research group [31]. The search was extended up to October 2023, including PubMed, Embase, Scopus, and ClinicalTrials.gov, with search algorithms combining infliximab and ulcerative colitis (limited to randomized clinical trials), yet it yielded no additional IFX studies.
Data were independently extracted in duplicate (A.G.T. and R.S.) and recorded in a Microsoft Excel sheet. This included information such as citation data, trial acronym, first author’s surname, year of publication, geographical setting, duration of follow-up, number of patients, population characteristics, ethnicity/race details, and intervention specifics, including the IFX’s dosage and administration. We exclusively considered efficacy data relating to dosage and administration as specified in the Summary of Product Characteristics (SPC), specifically intravenous infusions of IFX 5 mg/kg at weeks 0, 2, and 6 (for induction) and every 8 weeks thereafter (for maintenance of remission).
2.2. Quality Assessment
Two authors (A.G.T. and R.S.) independently assessed the risk of bias (RoB) for each trial (induction and maintenance phase, separately) using the Cochrane RoB tool [32]. This assessment covered six key domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential sources of bias. Each domain was scrutinized and classified as adequate (low RoB), inadequate (high RoB), or unclear (uncertain RoB). Disagreements in data extraction, or in RoB assessment, were resolved via discussion between the authors, aiming to reach a consensus. If needed, a third author (S.B.) was consulted to facilitate resolution.
2.3. Racial Categories
Race was determined based on information provided in the clinical trials’ published reports. When the vast majority of individuals (>90%) belonged to a specific race, the entire population was categorized as belonging to that particular race. In this analysis, race was categorized as Asian (including Chinese and Japanese patients) vs. Caucasian (encompassing patients from Europe, America, Oceania, and the Middle East). It was not possible to analyze race-specific efficacy for Black and other racial groups.
2.4. Outcomes Assessed
We assessed the following efficacy outcomes: (i) clinical response, clinical remission, and mucosal healing at the end of the induction phase (week 8), and (ii) clinical remission and mucosal healing at the completion of the maintenance phase. Clinical response is defined as a reduction from the baseline in the Mayo Clinic score [33] by a minimum of 3 points and at least 30 percent, along with a reduction in the rectal bleeding subscore of at least 1 point or achieving an absolute rectal bleeding subscore of 0 or 1 point. Clinical remission is defined as a Mayo Clinic score of 2 points or lower, with no individual subscore exceeding 1 point. Mucosal healing is defined as achieving an absolute endoscopy subscore of 0 or 1 point.
2.5. Statistical Methods
The risk ratio (RR) was the metric used to quantify treatment effects. RRs with 95% confidence intervals (CIs) were computed adhering to the intention-to-treat principle. Random-effect models were employed to synthesize evidence. Race-specific pooled RRs were calculated using the Mantel–Haenszel method [34], and estimates of heterogeneity were determined with the restricted maximum likelihood approach [35].
Inconsistency among the trial results was assessed using the I-squared statistic [36]. We interpreted heterogeneity, based on the I-squared, as follows: 0–40%, unimportant heterogeneity; 30–60%, moderate; 50–90%, substantial; and 75–100%, considerable heterogeneity. Due to the limited number of studies included in each evidence synthesis, it was not possible to perform a formal assessment of small-study effects. Generally, tests for funnel plot asymmetry are applied when there are a minimum of ten studies included in the analysis. Otherwise, these tests lack the statistical power to reliably distinguish real asymmetry from chance [37].
To examine for potential racial differences in treatment efficacy of IFX between Asians and Caucasians, we compared the race-specific RRs, derived from independent evidence syntheses, with a test of interaction [38]. We reported the ratio of race-specific RRs, z-score, and respective p-value for each one of these comparisons. A ratio of RRs equal to 1.00 indicates that the estimated treatment effect size is the same in Asians and Caucasians; the further the ratio of RRs is from 1.00, the greater the influence of race on the treatment’s efficacy in UC is. When the ratio of the RRs was >1.00, it indicated a better treatment effect in Asian populations; when it was <1.00, it showed a better treatment effect in Caucasian patients.
We used R statistical software [39], version 4.0.3, for conducting our analysis. All p-values were 2-tailed, and in all tests, a p-value < 0.05 signified statistical significance.
3. Results
We extracted and utilized aggregate data from four publications [40,41,42,43] reporting the results of five multicenter, randomized, placebo-controlled trials (Jiang et al. [40], Xi’an Janssen [41], Kobayashi et al. [42], ACT 1 [43], and ACT 2 [43]; Table 1) that assessed IFX as induction and maintenance therapy for adults with moderate-to-severe UC (i.e., Mayo score of 6 to 12 points, with an endoscopic subscore of at least 2 points) who had not been previously exposed to biologics. Permitted concomitant medications included corticosteroids, aminosalicylates, and immunosuppressants. Their doses remained constant except for corticosteroids, which were tapered. We analyzed data on 875 patients (Asians, 44.5%).

Table 1.
Randomized, placebo-controlled trials assessing IFX therapy in ulcerative colitis.
In these five trials, the mean age of participants spanned a range of 34 to 42 years, while the mean disease duration varied from 4 to 8 years. The percentage of female patients fluctuated between 36% and 40%, and the follow-up ranged from 26 to 54 weeks. Three of the studies [40,41,42] exclusively recruited Asian populations, specifically Chinese [40,41] and Japanese patients [42], while the remaining two studies (ACT 1, ACT 2 [43]) enrolled patients from Argentina, Australia, Austria, Belgium, Canada, Czech Republic, Denmark, England, France, Germany, Israel, Italy, the Netherlands, New Zealand, Scotland, Spain, Switzerland, and the United States, the vast majority of whom (>90%) were Caucasians. The studies exhibited homogeneity in terms of outcomes’ definitions. All studies contributed to all five efficacy outcomes.
3.1. Quality Assessment
RoB assessment of the studies revealed low risk across the induction phases, but high risk across the maintenance phases with regard to the “incomplete outcome data” domain. Though follow-up rates were consistently high in the induction phases and equally balanced between study groups, there was a notable decline in complete follow-up during the maintenance phases. This decline was accompanied by significant imbalances across study groups, and unequal drop-out rates attributed to adverse effects. These disparities in study withdrawals between groups raise the possibility for biased effect estimates. The RoB assessment is presented in Figure 1, for both the induction and maintenance phases.
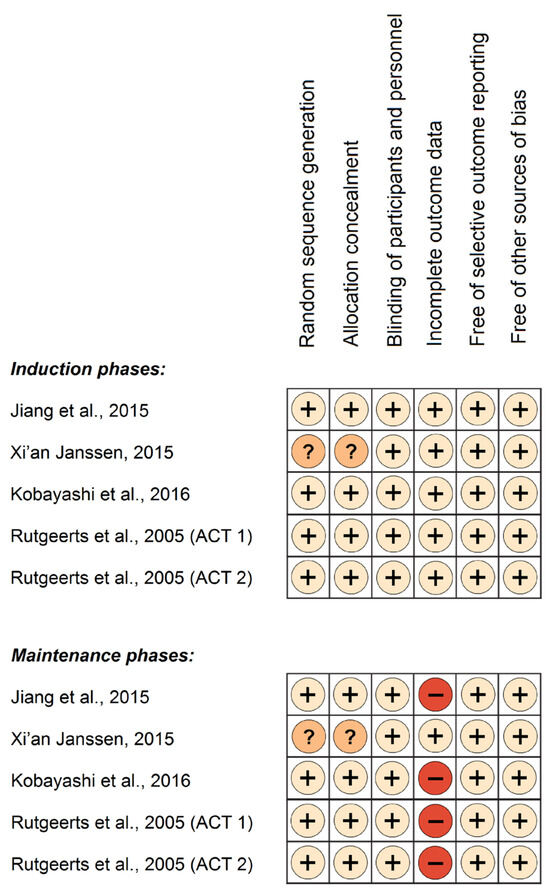
Figure 1.
Risk of bias assessment of clinical trials’ induction and maintenance phases. Symbols: (+), low risk of bias; (−), high risk of bias; (?), unclear risk of bias, [40,41,42,43].
3.2. Assessment of Racial Differences in Treatment Efficacy of IFX
3.2.1. Induction of Clinical Response
IFX (given intravenously at a dose of 5 mg/kg at weeks 0, 2, and 6) showed significant superiority over placebo in inducing clinical response in adult patients with moderately to severely active UC who had not previously received any biologic therapy. This efficacy was observed in both Asian (RR = 1.78, 95% CI: 1.42–2.21) and Caucasian populations (RR = 2.00, 95% CI: 1.64–2.44), as shown in Figure 2.

Figure 2.
Induction of clinical response. Abbreviations: IFX, infliximab; IV, intravenous; RR, risk ratio; CI, confidence interval, [40,41,42,43].
A comparison of the race-specific pooled effect estimates did not reveal any difference (ratio of RRs = 0.89, 95% CI: 0.66–1.20; z-score = −0.77; p-value for interaction = 0.44). Thus, we found no evidence of effect modification by race concerning the efficacy of IFX in inducing clinical response in patients with active UC.
3.2.2. Induction of Clinical Remission
In adults with moderately to severely active UC who were treatment-naive with biologic therapies, IFX (given intravenously at a dose of 5 mg/kg at weeks 0, 2, and 6) displayed efficacy in inducing clinical remission, both in Asian (RR = 2.17, 95% CI: 1.42–3.31) and Caucasian patients (RR = 3.73, 95% CI: 1.68–8.31; Figure 3).
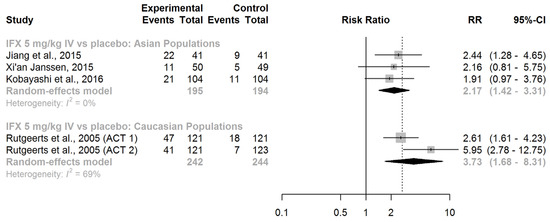
Figure 3.
Induction of clinical remission. Abbreviations: IFX, infliximab; IV, intravenous; RR, risk ratio; CI, confidence interval, [40,41,42,43].
Upon comparing the race-specific pooled RRs, the difference was not significant (ratio of RRs = 0.58, 95% CI: 0.24–1.44; z-score = −1.17; p-value for interaction = 0.24). We found no substantial evidence of effect modification by race regarding the efficacy of IFX in inducing clinical remission in patients with active UC.
3.2.3. Induction of Mucosal Healing
Again, IFX (administered intravenously at a dose of 5 mg/kg at weeks 0, 2, and 6) exhibited notable efficacy in eliciting mucosal healing in UC patients who had not been previously exposed to biological treatments. This effect was similar in Asian (RR = 1.87, 95% CI: 1.40–2.50) and Caucasian populations (RR = 1.89, 95% CI: 1.53–2.32), as shown in Figure 4.

Figure 4.
Induction of mucosal healing. Abbreviations: IFX, infliximab; IV, intravenous; RR, risk ratio; CI, confidence interval, [40,41,42,43].
A comparison of the race-specific pooled effect estimates did not show any difference (ratio of RRs = 0.99, 95% CI: 0.69–1.41; z-score = −0.06; p-value for interaction = 0.95). Thus, we found no evidence of effect modification by race concerning the efficacy of IFX in inducing mucosal healing in patients with active UC.
3.2.4. Maintenance of Clinical Remission
IFX (given intravenously at a dose of 5 mg/kg every 8 weeks) showed significant superiority over placebo in maintaining clinical remission in both Asian (RR = 1.79, 95% CI: 1.17–2.73) and Caucasian patients (RR = 2.22, 95% CI: 1.53–3.21; Figure 5).

Figure 5.
Maintenance of clinical remission. Abbreviations: IFX, infliximab; IV, intravenous; RR, risk ratio; CI, confidence interval; q8w, every 8 weeks, [40,41,42,43].
When we compared the race-specific pooled RRs, no significant difference was noted (ratio of RRs = 0.81, 95% CI: 0.46–1.42; z-score = −0.75; p-value for interaction = 0.45). We found no evidence of effect modification by race regarding the efficacy of IFX in maintaining clinical remission in patients with UC.
3.2.5. Maintenance of Mucosal Healing
IFX (given intravenously at a dose of 5 mg/kg every 8 weeks) demonstrated superiority over placebo in maintaining mucosal healing for both Asian patients (RR = 1.61, 95% CI: 1.21–2.14) and Caucasian patients (RR = 1.92, 95% CI: 1.20–3.09; Figure 6).

Figure 6.
Maintenance of mucosal healing. Abbreviations: IFX, infliximab; IV, intravenous; RR, risk ratio; CI, confidence interval; q8w, every 8 weeks, [40,41,42,43].
A comparison of the race-specific summary effect estimates did not reveal any significant difference (ratio of RRs = 0.84, 95% CI: 0.48–1.46; z-score = −0.63; p-value for interaction = 0.53). We found no evidence of effect modification by race concerning the efficacy of IFX in maintaining mucosal healing in patients with UC.
4. Discussion
In recent years, targeted immune modulators have considerably improved the care of patients with UC; nonetheless, uncertainties persist regarding the optimal selection of these drugs to maximize effectiveness, as well as whether racial disparities exist that could affect treatment outcomes and guide therapeutic choices. In our analysis of high-quality aggregate data from randomized, double-blind, placebo-controlled studies, no substantial evidence of racial differences was found regarding the efficacy of IFX in inducing clinical response, clinical remission, and mucosal healing, or maintaining clinical remission and mucosal healing, between Asian and Caucasian populations with moderate-to-severe UC. To the best of our knowledge, this study is the first to specifically examine whether race modifies the treatment efficacy of IFX in UC.
There is a paucity of research on the interaction between race and the effectiveness of advanced therapies in UC. In the first study conducted in this field [30], Greywoode and colleagues investigated racial differences in the efficacy of golimumab in UC trials [44,45] and concluded that individuals from racial minority groups (Asian, Black, or other races) exhibited a lower likelihood of achieving clinical response, clinical remission, and endoscopic healing compared to White patients. However, the authors neither presented race-specific estimates of the drug efficacy nor provided a ratio of the race-specific estimates for each outcome, nor did they report p-values from tests of interaction. Furthermore, contrary to their results, the efficacy of golimumab in a clinical trial of Japanese patients [46] was shown to be similar to that observed in golimumab trials predominantly involving White participants [44,45].
At this point, the fundamental question is whether one should expect racial differences in drug response, and whether such differences would be reliable for guiding therapeutic decisions. A debate has persisted for several years regarding the use of “race” in clinical research and practice. Some authors have suggested that race holds little or no biological significance and, therefore, should have limited importance in treatment decisions [47,48], while others have insisted that a patient’s race can and should influence physicians’ therapeutic decisions [49]. Current evidence, however, suggests that race is a poor proxy for drug response: Race disappears when examining the human genome. What people consider as racial differences comprise only 0.01% of our genes; geneticists agree that most of the genetic variation occurs within races, not between them [50].
In fact, race lacks a biological definition and has a fluid, political, and social meaning that is independent of science [51]. Accordingly, the American Medical Association has declared that race is a social construct rather than a biological one, recognizing “race as a socially constructed category different from ethnicity, genetic ancestry, or biology” and aiming to “end the misinterpretation of race as a biological category defined by genetic traits or biological differences” [52]. Hence, when research overly emphasizes race, it fails to recognize the social and environmental determinants of health that are the true drivers of racial disparities in health outcomes [53].
Overall, our study findings do not support the hypothesis that race modifies the treatment efficacy of IFX in UC. Yet, our analysis has limitations that merit consideration: First, our assessment of effect modification by race was based on between-study comparisons, which might introduce bias if unmeasured covariates among studies influence relative treatment effects [54]. Nevertheless, all studies included in this analysis exhibited homogeneity in terms of patient characteristics (i.e., adults with moderate to severe UC (defined with a Mayo score of 6–12 points, with endoscopic subscore of 2 or 3) who had not been previously exposed to biologics) and study methods (i.e., randomized, double-blind placebo-controlled design; uniform protocolized IFX treatment across trials; and the use of the Mayo score to define and measure study outcomes). Second, we were unable to assess treatment efficacy for racial groups other than Asian and Caucasian due to the minimal representation of non-White participants in the ACT trials [43], comprising only about 5% of the total, and the lack of reported race-specific efficacy results. Therefore, our findings may not be readily generalizable to Black and other racial groups.
Nonetheless, several strengths support the reliability and robustness of our analysis: First, we synthesized data from studies judged to be at low RoB in all key domains, except for the observed decrease in complete follow-up rates during maintenance phases. Second, we employed random-effect models to synthesize evidence within race-specific groups, thus allowing true effects to differ among studies [54,55]. Third, we used appropriate statistical methods to assess racial differences in the efficacy of IFX treatment between Asian and Caucasian populations. However, it is essential to interpret the results of the current analysis with caution when making clinical and health policy decisions, as “the absence of evidence is not evidence of absence” [56] of an interaction between treatment and race, especially because the statistical power of subgroup analyses in evidence syntheses is rather limited [57].
In conclusion, our study challenges previous assumptions about racial disparities in IFX efficacy for UC through the analysis of high-quality data from randomized trials. This approach provides valuable insights into the interplay of race and treatment outcomes. Our work not only advances scientific understanding, but also underscores the importance of more inclusive research practices. Ensuring the efficacy of advanced therapies for a broad spectrum of patients necessitates research that encompasses diverse populations. However, clinical trials have been criticized for insufficient representation of various racial minorities [58,59,60]. Future research should prioritize expanding the inclusion of racial groups, such as Blacks and Asians, in UC studies. This is crucial for a comprehensive evaluation of racial disparities in the effectiveness of advanced therapies, including but not limited to IFX, in the context of treating UC.
Author Contributions
Conceptualization, S.B., A.E.T., G.K.N. and D.P.; Methodology, S.B., G.K.N. and D.P.; Investigation, S.B., A.G.T. and R.S.; Data Curation, S.B.; Formal Analysis, S.B.; Visualization, S.B.; Writing—Original Draft Preparation, S.B.; Writing—Review and Editing, A.G.T., R.S., A.E.T., G.K.N. and D.P.; Supervision, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are included in the published article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Danese, S.; Fiocchi, C. Ulcerative colitis. N. Engl. J. Med. 2011, 365, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2018, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Bonovas, S. Inflammatory bowel disease: Estimates from the global burden of disease 2017 study. Aliment. Pharmacol. Ther. 2020, 51, 261–270. [Google Scholar] [CrossRef]
- de Lange, K.M.; Moutsianas, L.; Lee, J.C.; Lamb, C.A.; Luo, Y.; Kennedy, N.A.; Jostins, L.; Rice, D.L.; Gutierrez-Achury, J.; Ji, S.G.; et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 2017, 49, 256–261. [Google Scholar] [CrossRef]
- Piovani, D.; Danese, S.; Peyrin-Biroulet, L.; Nikolopoulos, G.K.; Lytras, T.; Bonovas, S. Environmental risk factors for inflammatory bowel diseases: An umbrella review of meta-analyses. Gastroenterology 2019, 157, 647–659. [Google Scholar] [CrossRef]
- Piovani, D.; Pansieri, C.; Kotha, S.R.R.; Piazza, A.C.; Comberg, C.L.; Peyrin-Biroulet, L.; Danese, S.; Bonovas, S. Ethnic differences in the smoking-related risk of inflammatory bowel disease: A systematic review and meta-analysis. J. Crohns Colitis 2021, 15, 1658–1678. [Google Scholar] [CrossRef]
- Cohen, R.D.; Yu, A.P.; Wu, E.Q.; Xie, J.; Mulani, P.M.; Chao, J. Systematic review: The costs of ulcerative colitis in Western countries. Aliment. Pharmacol. Ther. 2010, 31, 693–707. [Google Scholar] [CrossRef]
- Paschos, P.; Katsoula, A.; Salanti, G.; Giouleme, O.; Athanasiadou, E.; Tsapas, A. Systematic review with network meta-analysis: The impact of medical interventions for moderate-to-severe ulcerative colitis on health-related quality of life. Aliment. Pharmacol. Ther. 2018, 48, 1174–1185. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S.; AGA Institute Clinical Guidelines Committee. AGA clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef]
- Singh, S.; Allegretti, J.R.; Siddique, S.M.; Terdiman, J.P. AGA technical review on the management of moderate to severe ulcerative colitis. Gastroenterology 2020, 158, 1465–1496.e17. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, F.S.; Orlando, A.; Papi, C.; Festa, S.; Pugliese, D.; Bonovas, S.; Pansieri, C.; Piovani, D.; Fiorino, G.; Fantini, M.C.; et al. Use of biologics and small molecule drugs for the management of moderate to severe ulcerative colitis: IG-IBD clinical guidelines based on the GRADE methodology. Dig. Liver Dis. 2022, 54, 440–451. [Google Scholar] [CrossRef]
- Pantavou, K.; Yiallourou, A.I.; Piovani, D.; Evripidou, D.; Danese, S.; Peyrin-Biroulet, L.; Bonovas, S.; Nikolopoulos, G.K. Efficacy and safety of biologic agents and tofacitinib in moderate-to-severe ulcerative colitis: A systematic overview of meta-analyses. United Eur. Gastroenterol. J. 2019, 7, 1285–1303. [Google Scholar] [CrossRef] [PubMed]
- Bonovas, S.; Lytras, T.; Nikolopoulos, G.; Peyrin-Biroulet, L.; Danese, S. Systematic review with network meta-analysis: Comparative assessment of tofacitinib and biological therapies for moderate-to-severe ulcerative colitis. Aliment. Pharmacol. Ther. 2018, 47, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.; Vermeire, S.; Van Assche, G. Biological therapies for inflammatory bowel diseases. Gastroenterology 2009, 136, 1182–1197. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.; Van Assche, G.; Vermeire, S. Optimizing anti-TNF treatment in inflammatory bowel disease. Gastroenterology 2004, 126, 1593–1610. [Google Scholar] [CrossRef] [PubMed]
- Cornillie, F.; Shealy, D.; D’Haens, G.; Geboes, K.; Van Assche, G.; Ceuppens, J.; Wagner, C.; Schaible, T.; Plevy, S.; Targan, S.R.; et al. Infliximab induces potent anti-inflammatory and local immunomodulatory activity but no systemic immune suppression in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 2001, 15, 463–473. [Google Scholar] [CrossRef]
- Danese, S.; Fiorino, G.; Peyrin-Biroulet, L.; Lucenteforte, E.; Virgili, G.; Moja, L.; Bonovas, S. Biological agents for moderately to severely active ulcerative colitis: A systematic review and network meta-analysis. Ann. Intern. Med. 2014, 160, 704–711. [Google Scholar] [CrossRef]
- Nguyen, G.C.; LaVeist, T.A.; Harris, M.L.; Wang, M.H.; Datta, L.W.; Brant, S.R. Racial disparities in utilization of specialist care and medications in inflammatory bowel disease. Am. J. Gastroenterol. 2010, 105, 2202–2208. [Google Scholar] [CrossRef]
- Flasar, M.H.; Quezada, S.; Bijpuria, P.; Cross, R.K. Racial differences in disease extent and severity in patients with ulcerative colitis: A retrospective cohort study. Dig. Dis. Sci. 2008, 53, 2754–2760. [Google Scholar] [CrossRef]
- Moore, L.; Gaffney, K.; Lopez, R.; Shen, B. Comparison of the natural history of ulcerative colitis in African Americans and non-Hispanic Caucasians: A historical cohort study. Inflamm. Bowel. Dis. 2012, 18, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.C.; Torres, E.A.; Regueiro, M.; Bromfield, G.; Bitton, A.; Stempak, J.; Dassopoulos, T.; Schumm, P.; Gregory, F.J.; Griffiths, A.M.; et al. Inflammatory bowel disease characteristics among African Americans, Hispanics, and non-Hispanic Whites: Characterization of a large North American cohort. Am. J. Gastroenterol. 2006, 101, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Damas, O.M.; Jahann, D.A.; Reznik, R.; McCauley, J.L.; Tamariz, L.; Deshpande, A.R.; Abreu, M.T.; Sussman, D.A. Phenotypic manifestations of inflammatory bowel disease differ between Hispanics and non-Hispanic whites: Results of a large cohort study. Am. J. Gastroenterol. 2013, 108, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Moore, L.; Wu, X.J.; He, X.S.; Lan, P.; Shen, B. Different clinical characteristics in Hispanic and non-Hispanic whites with ileal pouch-anal anastomosis: A case-control study. Inflamm. Bowel. Dis. 2011, 17, 1003–1007. [Google Scholar] [CrossRef]
- Hou, J.; El-Serag, H.; Sellin, J.; Thirumurthi, S. Inflammatory bowel disease characteristics and treatment in Hispanics and Caucasians. Dig. Dis. Sci. 2011, 56, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.K.; El-Serag, H.; Thirumurthi, S. Distribution and manifestations of inflammatory bowel disease in Asians, Hispanics, and African Americans: A systematic review. Am. J. Gastroenterol. 2009, 104, 2100–2109. [Google Scholar] [CrossRef]
- Shi, H.Y.; Levy, A.N.; Trivedi, H.D.; Chan, F.K.L.; Ng, S.C.; Ananthakrishnan, A.N. Ethnicity influences phenotype and outcomes in inflammatory bowel disease: A systematic review and meta-analysis of population-based studies. Clin. Gastroenterol. Hepatol. 2018, 16, 190–197.e11. [Google Scholar] [CrossRef]
- Simsek, H.; Schuman, B.M. Inflammatory bowel disease in 64 black patients: Analysis of course, complications, and surgery. J. Clin. Gastroenterol. 1989, 11, 294–298. [Google Scholar] [CrossRef]
- Borrell, L.N.; Elhawary, J.R.; Fuentes-Afflick, E.; Witonsky, J.; Bhakta, N.; Wu, A.H.B.; Bibbins-Domingo, K.; Rodríguez-Santana, J.R.; Lenoir, M.A.; Gavin, J.R., 3rd; et al. Race and genetic ancestry in medicine—A time for reckoning with racism. N. Engl. J. Med. 2021, 384, 474–480. [Google Scholar] [CrossRef]
- Greywoode, R.; Petralia, F.; Ullman, T.A.; Frederic Colombel, J.; Ungaro, R.C. Racial difference in efficacy of golimumab in ulcerative colitis. Inflamm. Bowel Dis. 2023, 29, 843–849. [Google Scholar] [CrossRef]
- Bonovas, S.; Pansieri, C.; Piovani, D.; Macaluso, F.S.; Orlando, A.; Festa, S.; Papi, C.; Pugliese, D.; Armuzzi, A. Use of biologics and small molecule drugs for the management of moderate to severe ulcerative colitis: IG-IBD technical review based on the GRADE methodology. Dig. Liver Dis. 2022, 54, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomised study. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- Harville, D.A. Maximum likelihood approaches to variance component estimation and to related problems. J. Am. Stat. Assoc. 1977, 72, 320–338. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Sterne, J.A.; Sutton, A.J.; Ioannidis, J.P.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Altman, D.G.; Bland, J.M. Interaction revisited: The difference between two estimates. BMJ 2003, 326, 219. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing; R Development Core Team: Vienna, Austria, 2014. [Google Scholar]
- Jiang, X.L.; Cui, H.F.; Gao, J.; Fan, H. Low-dose infliximab for induction and maintenance treatment in Chinese patients with moderate to severe active ulcerative colitis. J. Clin. Gastroenterol. 2015, 49, 582–588. [Google Scholar] [CrossRef]
- ClinicalTrials.gov, Xi’an Janssen. 2015. A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Efficacy and Safety of Infliximab in Chinese Subjects with Active Ulcerative Colitis. Available online: https://clinicaltrials.gov/study/NCT01551290 (accessed on 17 November 2023).
- Kobayashi, T.; Suzuki, Y.; Motoya, S.; Hirai, F.; Ogata, H.; Ito, H.; Sato, N.; Ozaki, K.; Watanabe, M.; Hibi, T. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis-results from a multicenter prospective randomized controlled trial and its post hoc analysis. J. Gastroenterol. 2016, 51, 241–251. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.F.; Reinisch, W.; et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.F.; Reinisch, W.; et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 96–109.e1. [Google Scholar] [CrossRef] [PubMed]
- Hibi, T.; Imai, Y.; Senoo, A.; Ohta, K.; Ukyo, Y. Efficacy and safety of golimumab 52-week maintenance therapy in Japanese patients with moderate to severely active ulcerative colitis: A phase 3, double-blind, randomized, placebo-controlled study (PURSUIT-J study). J. Gastroenterol. 2017, 52, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Osborne, N.G.; Feit, M.D. The use of race in medical research. JAMA 1992, 267, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.S. Racial profiling in medical research. N. Engl. J. Med. 2001, 344, 1392–1393. [Google Scholar] [CrossRef] [PubMed]
- Satel, S.L. PC, MD: How Political Correctness Is Corrupting Medicine; Basic Books: New York, NY, USA, 2000. [Google Scholar]
- Owens, K.; King, M.C. Genomic views of human history. Science 1999, 286, 451–453. [Google Scholar] [CrossRef]
- Hahn, R.A. The state of federal health statistics on racial and ethnic groups. JAMA 1992, 267, 268–271. [Google Scholar] [CrossRef]
- American Medical Association. New AMA Policies Recognize Race as a Social, Not Biological, Construct. 16 November 2020. Available online: https://www.ama-assn.org/press-center/press-releases/new-ama-policies-recognize-race-social-not-biological-construct (accessed on 17 November 2023).
- Sherin, K.; Adebanjo, T.; Jani, A. Social determinants of health: Family physicians’ leadership role. Am. Fam. Physician 2019, 99, 476–477. [Google Scholar]
- Schandelmaier, S.; Briel, M.; Varadhan, R.; Schmid, C.H.; Devasenapathy, N.; Hayward, R.A.; Gagnier, J.; Borenstein, M.; van der Heijden, G.J.M.G.; Dahabreh, I.J.; et al. Development of the instrument to assess the credibility of effect modification analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ 2020, 192, E901–E906. [Google Scholar] [CrossRef]
- Borenstein, M.; Higgins, J.P. Meta-analysis and subgroups. Prev. Sci. 2013, 14, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.G.; Bland, J.M. Absence of evidence is not evidence of absence. BMJ 1995, 311, 485. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Griffin, J.W.; Furukawa, T.A. The lack of statistical power of subgroup analyses in meta-analyses: A cautionary note. Epidemiol. Psychiatr. Sci. 2021, 30, e78. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.H.; Krumholz, H.M.; Gross, C.P. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 2004, 291, 2720–2726. [Google Scholar] [CrossRef]
- Sardar, M.R.; Badri, M.; Prince, C.T.; Seltzer, J.; Kowey, P.R. Under-representation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA Intern. Med. 2014, 174, 1868–1870. [Google Scholar] [CrossRef]
- Oh, S.S.; Galanter, J.; Thakur, N.; Pino-Yanes, M.; Barcelo, N.E.; White, M.J.; de Bruin, D.M.; Greenblatt, R.M.; Bibbins-Domingo, K.; Wu, A.H.; et al. Diversity in clinical and biomedical research: A promise yet to be fulfilled. PLoS Med. 2015, 12, e1001918. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).