Abstract
A pancreatic neuroendocrine tumor (Pan-NET) is a rare neoplasm originating in the neuroendocrine system. Carcinoid syndrome occurs in approximately 19% of patients with functional Pan-NETs, typically when liver metastases occur. In this paper, we describe the case of a patient with a low-grade non-functional Pan-NET, but with a typical clinical presentation of carcinoid syndrome. An 81-year-old male was admitted to our Department of Internal Medicine at Cannizzaro Hospital (Catania, Italy) because of the onset of abdominal pain with nausea, loose stools, and episodic flushing. Firstly, an abdominal contrast-enhanced CT scan showed a small pancreatic hyper-vascular mass; then, a gallium-68 DOTATOC integrated PET/CT revealed an elevated expression of SSTR receptors. Serum chromogranin A and urinary 5-HIAA measurements were negative. We performed an endoscopic ultrasonography (EUS) by a fine-needle biopsy (EUS-FNB), allowing the immunostaining of a small mass (0.8 cm) and the diagnosis of a low-grade (G1) non-functional Pan-NET (NF-Pan-NET). Surgery was waived, while a follow-up strategy was chosen. The early recognition of Pan-NETs, although rare, is necessary to improve the patient’s survival. Although helpful to allow for immunostaining, EUS-FNB needs to be warranted in future studies comparing EUS-FNB to EUS-FNA (fine-needle aspiration), which is, to date, reported as the tool of choice to diagnose Pan-NETs.
1. Introduction
Neuroendocrine neoplasms (NENs) are enigmatic malignancies with an increasing incidence and prevalence [1,2,3,4]. Given their common morphological and immunophenotypical features, all these tumors arise from cells of the diffuse endocrine system.
NENs range from asymptomatic well-differentiated neuroendocrine tumors (NETs) to aggressive neuroendocrine carcinomas (NECs). In fact, nearly 80–90% of NENs are NETs, while the remaining 10–20% are carcinomas [5].
NETs can develop in any tissue of the body. The gastrointestinal tract and pancreas are the most common sites of origin, accounting for approximately 60% of the primary sites [6], followed by the lungs and other sites.
About 40% of NETs can release hormones responsible for symptoms, depending on the secreted hormone. Carcinoid syndrome is characterized by episodic flushing and diarrhea, due to various vasoactive substances (serotonin, histamine, and other amines) released into the systemic circulation [7].
Non-functional NETs may often present with subtle and sporadic symptoms, sometimes with gastrointestinal bleeding, abdominal pain, bowel obstruction, or unexplained weight loss [8].
Treatment and prognosis depend on the grade and stage of the tumor. NETs diagnosis is frequently late, along with symptoms related to hormone hypersecretion, often after metastases occurs in the liver, where bioactive substances fail to be inactivated. An early diagnosis and recognition are necessary to improve the patient’s survival, which has not significantly changed over the last 30 years [9].
In this paper, we present a case of a pauci-symptomatic pancreatic neuroendocrine tumor in a patient with an unspecific clinical presentation (abdominal pain) and mild additional symptoms (nausea and loose stools). This was the occasion for a narrative review of the literature on the diagnosis and management of pancreatic neuroendocrine tumors (Pan-NETs).
2. Case-Report
In May 2023, an 81-year-old man was admitted to the Department of Internal Medicine at Cannizzaro Hospital (Catania, Italy) because of the onset of abdominal pain, especially in the lower abdominal quadrants, with nausea and loose stools (<3 times/day).
The patient’s past medical history included arterial hypertension, type-2 diabetes mellitus, peripheral artery disease (PAD), obesity, hypothyroidism, and depressive syndrome. In the past six months, he complained of abdominal distension and changes in bowel habits (loose stools). There was no relevant family history. He was taking levothyroxine, insulin according to HGT, lansoprazole, acarbose, ezetimibe/simvastatin, and furosemide. He denied the anamnestic consumption of uncooked meat, fish, or unpasteurized dairy products.
On admission, he had no fever, arterial hypertension (177/76 mmHg), had a normal heart rate (86 bpm), glycemia of 102 mg/dL, and normal SaO2 in room air (98%); he presented no sensorium alterations. A physical examination revealed abdominal distension, with colic pain on deep palpation and hypoactive abdomen sounds. Mucous membranes were normally hydrated. The bedside FAST (Focused Assessment with Sonography in Trauma) scan did not detect peritoneal fluid. The digital rectal examination showed blood traces.
Laboratory tests were performed, showing an increase in serum CRP (17.9 mg/dL), moderate leukocytosis, moderate renal dysfunction (serum Cr: 1.33 mg/dL, eGFR: 54 mL/min/1.73 m2), normal serum potassium (3.6 mEq/L), sodium (139 mEq/L) and chloride (100 mEq/L), mild metabolic acidosis (pH: 7.33, HCO3: 21 mmol/L, pCO2: 42 mmHg), and serum procalcitonin < 0.2 ng/mL. Infectious causes of diarrhea were excluded by microbiological and chemical fecal examinations. An abdomen X-ray excluded bowel obstruction or perforation. Moderate intravenous fluid repletion was administered.
A few hours after admission, the patient experienced transient states of agitation, with uncontrolled crying spells and temper tantrums. Due to his past medical history of untreated depression, anxiolytic and antipsychotic therapies were prescribed, followed by poor efficacy. During this altered emotional status, a flushing episode was observed in the face and neck.
A contrast-enhanced abdominal CT scan revealed a pancreatic hypervascular small mass (8 mm) (Figure 1).
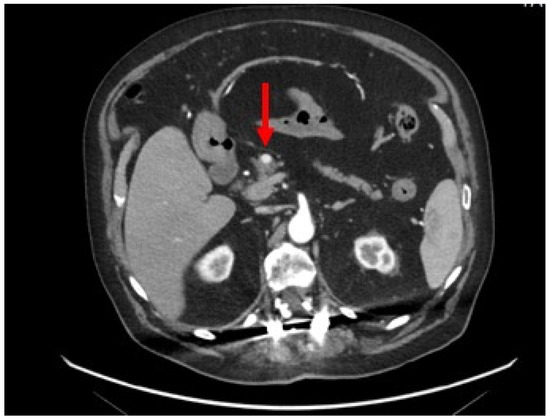
Figure 1.
Contrast-enhanced abdominal CT scan: axial section showing a homogeneous and hypervascular mass of 8 mm (red arrow) on the arterial phase.
On the fifth day of admission, given the suspicion of a pancreatic neuroendocrine tumor (Pan-NET), a gallium-68 DOTATOC integrated PET/CT was performed (Figure 2), confirming a small mass between the head and body of the pancreas, with an elevated expression of SSTR2/5 somatostatin receptors. No other sites of disease were detected.
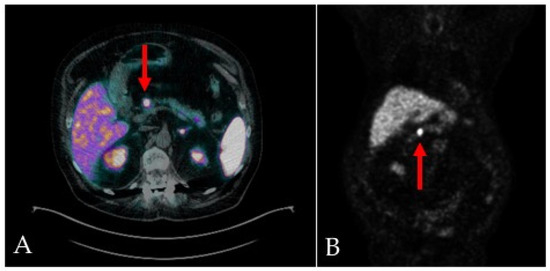
Figure 2.
68Ga-DOTA-TOC integrated PET/CT scans, transaxial (A) and MIP (B), show focal and intense uptake in the primary pancreatic lesion (red arrows), with an elevated expression of SSTR2/5 somatostatin receptors.
The serum chromogranin A (CgA) measurement was within the normal range (98.0 ng/mL, normal values < 101.9 ng/mL); we also performed a urine 5-HIAA test (urinary 5-HIAA: 1.6 mg/24 h; normal values: 1.0–8.2 mg/24 h).
A progressive recovery was observed, with no further abdominal pain. In accordance with the remission of symptoms and the normal laboratory values, the patient was discharged with the prescription to undergo an endoscopic ultrasonography with a fine-needle biopsy (EUS-FNB) for targeted diagnostic and therapeutic management.
In June 2023, an EUS-FNB, performed with a 22-gauge Acquire needle (Boston Scientific, Marlborough, MA, USA) using a slow-pull technique, visualized the presence of an oval hypo-echogenic mass, with a major axis of 8.9 mm (Figure 3), which was sampled for the cyto-histological examination.
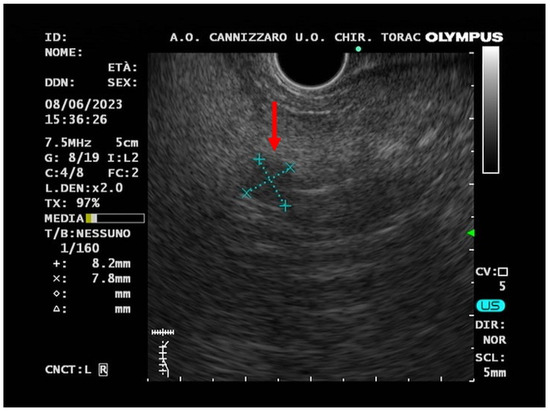
Figure 3.
Endoscopic ultrasound (EUS) image (red arrow) of a small hypo-echogenic lesion with a regular margin and a major axis of 8.9 mm.
Histological and immunohistochemical examinations confirmed the suspicion of Pan-NET (stage WHO G1, well-differentiated, synaptophysin positive, CgA positive, Ki67 1%) (Figure 4). The fine-needle biopsy allowed us to obtain microcores of the sample tissue (Figure 4A). Then, using a pipette, the microcores were picked up to be treated as a traditional biopsy. The microcores were composed of abundant blood and entrapped epithelial elements of pancreatic tissue (Figure 4B). A monomorphic population of epithelial cells, in solid sheets or small nodules, with a granular cytoplasm and nuclei with thickened chromatin was also observed (Figure 4C). Immunochemistry, performed with a Bond-Leica immunostainer, revealed positivity for neuroendocrine markers, such as chromogranin A (Figure 4D) and synaptophysin (Figure 4E), while that of serotonin was negative (Figure 4F). The absence of mitosis and necrosis, together with a low Ki-67 index (Figure 4G), allowed us to determine a low-grade neuroendocrine neoplasm.
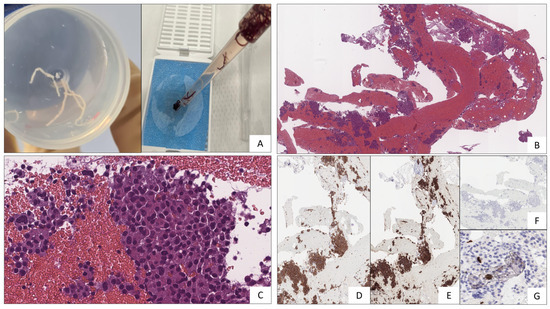
Figure 4.
(A) Microcores of sample tissue. (B) Abundant blood and entrapped epithelial elements of pancreatic tissue stained with Hematoxylin–Eosin. (C) Epithelial cells, with a granular cytoplasm and nuclei with thickened chromatin (Hematoxylin–Eosin staining). (D) Chromogranin A (5H7 clone, immunohistochemical staining). (E) Synaptophysin (27G12 clone, immunohistochemical staining). (F) Serotonin (YC5/45 clone, immunohistochemical staining). (G) Ki67 (MM1 clone, immunohistochemical staining).
In keeping with the current guidelines, these findings suggest the diagnosis of a low-grade (G1) non-functional pancreatic neuroendocrine tumor (NF-Pan-NET) (well-differentiated neoplasm, absence of mythosis, Ki67 ≤ 2%) [10]. This definition of “non-functional”, based only on negative hormone tests, was finalized to a categorical distinction between “secreting” and “non-secreting” tumors, although it underestimated the importance of the clinical presentation.
After the evaluations of the stage, grading, symptoms, and comorbidities, a conservative approach of watchful waiting was chosen by the surgeon, with a radiological follow-up session after one year. We scheduled a clinical follow-up session in order to keep the symptoms under observation.
3. Review of the Literature
Neuroendocrine neoplasms (NENs) are heterogenous neoplasms arising in the secretory cells of the diffuse neuroendocrine system, the so-called APUD (Amine Precursor Uptake and Decarboxylation) System [4]. Characterized by amine and neuropeptide hormone production with dense vesicles, these neuroendocrine cells are specialized to receive neuronal inputs and consequentially release message peptides into circulation for the regulation and modulation of cell proliferation, growth, and development. NENs are distinguished from pheochromocytomas and paragangliomas (neuroendocrine non-epithelial neoplasms) by the expression of keratin in the former ones, given their epithelial origin [11].
Neuroendocrine tumors (NETs) represent only 0.5% of all malignant conditions and 2% of all malignant tumors in the gastrointestinal tract [12]. Given the continued update in the classification of NENs, these epidemiological data are continuously evolving. The prevalence of NETs ranges between 2.5 and 8.35 cases per 10,000, with a recent increase in their incidence rates [1,2,3,4,13,14,15,16], probably due to imaging improvement, leading to an earlier and more frequent diagnosis of the disease [6].
In the 2019 WHO classification of tumors of the digestive system [17], NENs are divided into well-differentiated neuroendocrine tumors (NETs) and poorly differentiated neuroendocrine carcinomas (NECs), based on their molecular differences. In addition, “mixed neuroendocrine–non-neuroendocrine neoplasms” (MiNENs) are better characterized, according to the simultaneous presence of both neuroendocrine and non-neuroendocrine components, typically poorly differentiated (Table 1).

Table 1.
WHO classification (2019) and grading criteria for gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs) [17].
The most frequent primary sites are the gastrointestinal tract (61%), lung (25%), and about 14% remains of an unknown origin [18]. A total of 12 to 22% of patients are metastatic at presentation [6].
Recently, abdominal pain was reported as an unspecific symptom of a small bowel NET [19]. Our case report resembled that very recently described by Daraghmeh et al. [19]; although, in our patient, we found a Pan-NET.
The 2019 WHO classification [17] provided an improved system for determining prognoses and treatments, appliable to all NENs, replacing the previous classification based on cell embryologic origin (foregut, midgut, and hindgut) [20]. In contrast to the 2017 WHO classification of tumors of endocrine organs [21], the last classification included pancreatic tumors in gastroenteropancreatic NENs (GEP-NENs) [17].
Gastroenteropancreatic tumors (GEP-NETs) are most commonly located in the gastric mucosa, the small intestine, the rectum, and the pancreas [4,22]. While a subset of NENs is functional (40%), presenting with characteristic endocrine-related symptoms, most of them are non-functional and do not present with symptoms until later stages.
The distant metastases of NF-PNETs are often found at the time of diagnosis, because symptoms of NF-PNETs develop in an advanced stage. Due to these characteristics, NF-PNETs are usually incidentally diagnosed, like GEP-NETS, thanks to the development of imaging techniques, able to also identify very small lesions. In our patient, the presence of flushing, diarrhea, and neuropsychiatric symptoms, suggesting carcinoid syndrome, was unrelated to a biochemical elevation of hormonal levels. As a matter of fact, small PNETs without metastases can often remain asymptomatic until they reach a significant dimension, or can present with unspecific symptoms, such as abdominal pain, weight loss, anorexia, and nausea.
Up to 90% of Pan-NETs are hormonally silent, a behavior affecting the prognosis as compared to functioning neoplasms, probably because of a late diagnosis [23].
Pan-NETs may produce a large variety of hormones, such as insulin, gastrin, glucagon, vasoactive intestinal peptide (VIP), serotonin, somatostatin, and others [24]. By contrast, non-functional Pan-NETs, without hormone overproduction, may present with unspecific symptoms, such as abdominal pain, weight loss, diarrhea, and gastrointestinal bleeding [8,25]. Most Pan-NENs are sporadic, whereas a minority are inherited, associated with type-1 multiple endocrine neoplasia (MEN-1), von Hippel–Lindau syndrome (VHL), tuberous sclerosis, or neurofibromatosis.
Functional pancreatic neuroendocrine tumors, associated with a variety of clinical syndromes, include [26] insulinomas, the most common functional Pan-NETs; gastrinomas, or Zollinger–Ellison syndrome; pancreatic polypeptide-secreting tumors; VIPomas, or Verner–Morrison syndrome; glucagonomas, exclusively localized in pancreas; and somatostatinomas, the least common NETs.
Carcinoid syndrome is a paraneoplastic syndrome that occurs because of the release of bio-active substances, predominantly serotonin (5-HT), but also histamine, bradykinin, prostaglandins E and F, and tachykinins [27]. The typical symptoms are flushing and diarrhea. Wheezing, palpitations, breathlessness, abdominal pain, telangiectasias, and neuropsychiatric symptoms can also be associated with carcinoid syndrome [27,28]. Recently, Halperin et al. [29] demonstrated in a population-based analysis conducted on the American “Surveillance, Epidemiology, and End Results Medicare” database that 19% of patients with NETs had carcinoid syndrome. In patients harboring Pan-NETs, carcinoid syndrome is even more rare, accounting for approximately 1% [13].
The diagnosis of GEP-NENs is performed on the basis of a tissue histological examination [30]. Radiological and functional imaging is used to evaluate disease extension (staging) and assess the response to therapy, as well as to localize the primary site. Laboratory tests play a diagnostic role only in carcinoid syndrome and hormone-specific syndromes (gastrinomas, insulinomas, and glucagonomas), although the assay of either circulating or urinary hormones fail to be highly sensitive and specific, sometimes because blood sampling and urine collection are not performed closely to the occurrence of typical symptoms.
The current WHO classification emphasizes the role of histological examinations in surgically removed neoplasms, in order to establish the morphological characteristics and grading [17]. Three grades (G1, G2, and G3) are described for GEP-NETs, based on the proliferation activity assessed by the mitotic rate and Ki67 proliferation index [31,32]. For a more specific diagnosis, together with the morphology and grading, the immunohistochemical staining of chromogranin A (CgA) and synaptophysin should be assessed as biomarkers of neuroendocrine tumors.
Although the WHO histological classifications are specifically intended for surgically removed NENs [10,17], recent studies have investigated the role of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) and fine-needle biopsy (EUS-FNB) for the pre-operative evaluation and management of pancreatic NETs (Pan-NETs) [33,34,35,36,37,38,39,40,41,42]. Despite the data for the grading agreement between EUS-FNA and surgical specimens highlighting the significant rate of under- or over-grading [41,42,43,44,45,46,47], the recent introduction of needles for EUS-guided fine-needle biopsies (EUS-FNBs), as for our patient, changed the scenario [37,38]. An EUS-FNB, in fact, allows us to obtain tissue samples on which immunohistochemical examinations can be easily performed, to evaluate the Ki67 proliferation index [39,40,41,42,43,44,45]. As a matter of fact, in patients harboring Pan-NETs smaller than 2 cm, the management remains still controversial, especially for asymptomatic and non-functional Pan-NETs [46,47,48,49]. Endoscopy with a biopsy is already the gold standard for diagnosing NENs of the stomach, duodenum, and colorectum [50,51]. In the diagnosis of pancreatic NENs, EUS is particularly useful in detecting the nature of small lesions. The introduction of EUS-FNB can then overcome the interpretative limits of EUS-FNA, therefore allowing the early characterization of tumors where surgery would destroy healthy tissue [39,40]. However, further prospective, randomized studies are needed to validate these approaches in the specific setting of Pan-NETs [30,52].
The surgical treatment of patients with small low-grade non-functional pancreatic neuroendocrine tumors (<20 mm) is still under debate, according to the ENETS guidelines. In this respect, Sugawara et al. [53], in a recent metanalysis, demonstrated that a surgical resection was recommended in patients with nonmetastatic NF-PNETs measuring between 1.1 and 2.0 cm; alternatively, those patients with a smaller lesion (<1 cm) showed greater prognostic benefits with a conservative approach. JNETS [54] suggests a follow-up strategy, with imaging every 6–12 months of asymptomatic tumors <1 cm without metastases. Moreover, Sadot et al. [55] further reported that, among 104 patients with small, asymptomatic Pan-NETs undergoing non-operative management, no patient developed evidence of metastases or died because of the tumor after a median follow up of 44 months. Several studies suggested that a surgical intervention may not be warranted for very small Pan-NETs, especially in elderly individuals [56,57,58]. It is noteworthy however that all these data were obtained from a population much younger (median age: 60–65 years) than our patient (81 years old).
Paik et al. [59] suggested that patients with Pan-NETs smaller than 1 cm could be managed by observation alone, while Pan-NETs > 1 cm should undergo EUS-FNBs to obtain grading and Ki67 immunostaining, to characterize the tumor according to the WHO classification.
To investigate Pan-NETs, several imaging techniques can be performed, including computed tomography (CT), magnetic resonance (MRI), ultrasonography, and functional imaging with scintigraphy and positron emission tomography (PET). The optimal choice of imaging modality depends on the location of primary and metastatic lesions [60].
Endoscopic ultrasonography (EUS) has become the gold standard technique to evaluate pancreatic neuroendocrine lesions [4,30,61,62]. On an EUS, Pan-NETs typically appear as well-defined, round, hypoechoic, homogenous vascular lesions [63]. As in our case report, the EUS allowed the accurate localization of Pan-NETs, which was crucial for surgical interventions. As mentioned before, the EUS allows the cyto-histological confirmation of neuroendocrine tumors through guided tissue acquisition for histological procedures [33,34,35,36,37,38,39,40,41,42].
The functional imaging of GEP-NENs is based on the typical expression of somatostatin receptors (SSTRs) by neuroendocrine cells [64]. In the past, functional studies were performed with 111indium pentetreotide scintigraphy (Octreoscan®); in recent years, PET/CT with somatostatin analogs tracked with gallium-68 (68Ga-SSA PET/CT) has become the modality of choice for SSTR imaging [10,65,66]. Functional imaging is indicated for staging, the localization of the unknown primary tumor in patients with established neuroendocrine metastases, the in vivo demonstration of SSTR expression in neuroendocrine cells (for therapeutic planning), as well as the extent of disease after treatment. The most common somatostatin analogs used in clinical practice are 68Ga-DOTA-Tyr3-octreotide (68Ga-DOTA-TOC), 68Ga-DOTA-Tyr3-octreotate (68Ga-DOTA-TATE), and 68Ga-DOTA-Nal3-octreotide (68Ga-DOTA-NOC). The mean sensitivity of 68Ga-DOTA-SSA PET/CT for the diagnosis of Pan-NETs was 92%, while the specificity was 83% [67,68]. In advanced, fast-growing G2 and G3 NENs, especially if receptor negativity was evident at 68Ga-SSA PET/CT, 18FDG-PET/CT could be considered in the diagnostic approach [69,70]. The detection of Pan-NETs with functional imaging can be affected by the physiological uptake, especially in the uncinate process, therefore suggesting morphological imaging together with histological confirmation as a specific diagnostic process [71]. However, it still remains under debate whether the combined use of 18FDG-PET/CT and the 68Ga-DOTA-TOC peptide can improve the diagnostic performance of NENs [70]. Of note, a recent retrospective study [72] confirmed the suggestion of the combined use of 68Ga-DOTA peptides and 18F-FDG as radiotracers for a dual-tracer PET/CT to better evaluate tumor aggressiveness before surgery, especially for small masses of doubtful interpretation, when a metabolic confirmation of biopsy grading is needed [73].
At present, the biochemical diagnosis of NENs has been downsized due to the high proportion of non-functioning NENs. Considering the high rates of false-positive and heterogeneous serum determinations, chromogranin A (CgA) should be used in patients with an already documented diagnosis of NEN, in order to establish the treatment response or during the follow up [74,75]; although, the results are less sensitive for the primary diagnosis. On the other hand, neuron-specific enolase (NSE) is considered an unreliable diagnostic biomarker for NETs, due to its low sensitivity and specificity, while no evidence is available regarding its role in the follow up [76].
Laboratory tests for specific biomarkers (gastrin, insulin, glucagon, VIP, and 5-HIAA) are still important tools for certain clinical syndromes. 5-hydroxyindoleacetic acid (5-HIAA), detected in a 24 h urine collection using optimal conditions for the assay, is the specific tumor marker of carcinoid syndrome. 5-HIAA demonstrated a diagnostic sensitivity of 70%, with a specificity of 90% [77]. It is not recommended to use 5-HIAA as a screening test in the presence of diarrhea. Instead, it should be used in patients diagnosed with NENs to confirm carcinoid syndrome and assess its response to therapy [10,77].
Circulating tumor cells, circulating tumor DNA, circulating micro-RNAs, and NETest (the simultaneous measurement of 51 neuroendocrine-specific marker genes in the peripheral blood) are novel biomarkers under validation for NENs. However, this test is not widely available and needs further validation [78].
The treatment of Pan-NENs depends on the functionality, localization, dimension, and disease progression of the tumor. In most cases, surgical resection is the appropriate curative treatment in functioning pancreatic NET syndromes without metastases [49,54]. As for NF-Pan-NETs, surgical treatment, when feasible, is the gold standard [46,79,80], even if, as previously mentioned, a surgical intervention may not be warranted for very small Pan-NETs (<1.0 cm), especially in elderly individuals [53,54,55,56,57,58]. Surgical options include simple enucleation, central pancreatectomy, distal pancreatectomy with or without a splenectomy, and pancreatoduodenectomy (Whipple’s operation), depending on the tumor’s location. Moreover, radiofrequency ablation and trans-arterial chemoembolization are used for liver metastases.
When a macroscopic curative resection is unfeasible, medical treatment is indicated to control hormonal symptoms in F-Pan-NETs and to reduce the tumor’s growth. Since the majority of GEP-NETs express somatostatin receptors (SSTRs), somatostatin analogs are used in F-Pan-NETs, together with adequate treatments for specific clinical syndromes (for example, PPi in ZES) [81]. For tumor growth control, somatostatin analogs, molecular-targeted drugs, and cytotoxic anticancer agents are indicated, regardless of functionality [82]. SSAs are the first choice when a positive expression of SSRT is confirmed. The use of lanreotide and octreotide long-acting release (LAR) were already proven to be effective in reducing a tumor’s progression [81,83,84]. Recently, Wolin et al. [85] reported that the use of pasireotide, a novel SSA, despite a more extensive antiproliferation effect, was associated with more frequent adverse events. Targeted therapy, with everolimus and sunitinib, chemotherapy, and peptide-receptor radionuclide therapy (PRRT) should generally be reserved for SSA-refractory cases [49].
4. Discussion
Our case report described an old patient with an extremely rare pancreatic neuroendocrine tumor (Pan-NET), diagnosed in the presence of unspecific gastrointestinal symptoms and skin flushing. This observation is even rarer in old people. Despite the symptoms suggesting carcinoid syndrome, the tumor was well-differentiated and localized in the pancreas without liver metastases. This presentation is extremely rare, with few cases reported in the literature [86,87,88]. Biochemical testing for serum CgA and urinary 5-HIAA resulted negative. As previously emphasized, laboratory biomarkers were recently downsized due to the high rates of false positivity and their pharmacological interference, leading to low sensitivity and specificity [74,75,76,77,78,89].
We confirmed the Pan-NETs diagnosis through a contrast-enhanced CT, followed by functional imaging with a gallium-68 DOTATOC integrated PET/CT. We decided to perform an EUS-FNB to test immunostaining for the main markers of Pan-NETs and obtain grading. EUS-FNB confirmed the diagnosis of well-differentiated, low-grade (G1) Pan-NET (CgA+, Synaptophysin+, Ki67 1%).
The association of NETs and carcinoid syndrome occurs in approximately 19% of patients [27]. Except for patients with primary ovarian or bronchial neuroendocrine tumors, the evidence of carcinoid syndrome develops when metastases have occurred [26,28]. As a matter of fact, serotonin-producing Pan-NETs account for 0.58–1.4% of all Pan-NETs [90,91]. Only a few cases have been previously reported for Pan-NETs without liver metastases presenting with carcinoid syndrome [87,92]. Some patients with neuroendocrine tumors showed symptoms of flushing with low or normal levels of 5-HIAA [93,94]. Negativity for the immunostaining of serotonin found in our tumor biopsy, while in keeping with the normal values of 5HIAA, may further support the notion that levels of circulating hormones can increase only in the presence of liver metastases [29]. Our patient experienced carcinoid symptoms (diarrhea, flushing, and unresponsive depression) in the absence of documented liver metastases and with negative serum CgA and normal urinary 5-HIAA levels. The guidelines clearly show that negative hormone measurements define NETs as “nonfunctional”, even if presenting with suggestive symptoms or positive hormonal expressions in NET cells on immunohistochemical staining [49]. This may not always true, as can be observed in our case-report, as well as in few other reports [87,92].
It remains unclear why symptoms resembling carcinoid syndrome developed in our patient, with no evidence of any increase in hormone levels. It may well be that a possible, sudden, and transient hormone increase in the circulation failed to be detected. Otherwise, some to date unknown mechanisms might have been responsible for the abdominal pain, diarrhea, and flushing, all together causing us to consider alternative diagnoses regarding bowel diseases, which were excluded by the contrast-enhanced CT scan in our patient. In the presence of this discrepancy between the presence of symptoms and hormone negativity however, our case report emphasized that the clinical presentation should not be disregarded as a presentation of carcinoid-like syndrome, therefore leading to a complete diagnostic work-up for NETs.
Therefore, despite this, the Pan-NET of our patient should be defined as non-functional according to the guidelines [49], because the hormone values were within the normal range; our case report demonstrated that the imaging and histological examinations were useful in the diagnostic work-up of a Pan-NET associated with symptoms of carcinoid syndrome. As we reported, performing an EUS-FNB and assessing the cyto-histological features can help characterize the Pan-NET. Of note, we again underscore the concept that Pan-NET occurrence without metastases in old patients is very rare.
A Pan-NET < 1.0 cm can occur in very old people, without metastases, as in our case report; although, the median age was between 61 and 65 years in a recent metanalysis [50]. A surgical resection in these cases is not warranted. On the contrary, for Pan-NETs between 1.0 and 2.0 cm, surgical resections provided a better survival outcome, but in patients younger than 65 years old, without comorbidities.
The novelties of our case report can be highlighted as follows: (1) the symptoms of carcinoid syndrome can be shown in a Pan-NET < 1.0 cm occurring in very old people, without metastases, and with no evidence of an increase in circulating hormones, in agreement with the negativity of immunostaining for serotonin shown in tumor tissue. To date, the median age range was much lower [52]. (2) The categorical distinction of “functional” and “nonfunctional” NETs suggested by the guidelines [49] on the basis of hormone positivity and clinical presentation can help present Pan-NETs with no evidence of hormone release, as in our case report, thus underscoring the concept that the physician should take into account the possibility that an atypical pattern of apparently “non-functional” Pan-NETs may occur, although rarely. (3) In our patient, the EUS-FNB offered the opportunity of obtaining additional data regarding the immunostaining of the small Pan-NET; although, to date, an EUS-FNA is recognized as the method of choice in the multidisciplinary diagnostic approach of occult primary NETs, as recently reported by Rossi et al. [62]. Further evidence is needed to understand whether an EUS-FNB, as reported by a recent multicenter study [53], can provide physicians with additional details of the diagnostic workup of Pan-NETs.
In conclusion, this case report contributes to the understanding of the clinical spectrum of Pan-NETs, particularly in elderly patients, and highlights the potential challenges in decision making when treating patients with indolent neoplasms, as well as well-differentiated Pan-NETs surgically or even medicinally. It also highlights the role of advanced diagnostic techniques, such as EUS-FNB, in characterizing P-NETs.
Author Contributions
Conceptualization, M.R. and L.M.; methodology, M.R. and L.M.; investigation, M.R., N.C., C.S., M.C., A.L., F.M., S.M., S.P., M.I. and A.G.T.; data curation, M.R., N.C. and C.S.; writing—original draft preparation, M.R., N.C., C.S. and F.M.; writing—review and editing, M.R. and L.M.; visualization, M.R.; supervision, L.M. and M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. Ethical review and approval were waived due to the type of the study that was a case report.
Informed Consent Statement
The patient provided written informed consent to publish this manuscript and for material sampling.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
John Alexander Crosetti, English language fellow at Georgetown University (Washington, DC, USA) and English qualified native expert at Kore University (Enna, Italy), is gratefully acknowledged as the language reviewer of this case report.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| APUD | Amine precursor uptake and decarboxylation |
| CgA | Chromogranin A |
| CHD | Carcinoid heart disease |
| Cr | Creatinine |
| CT | Computed tomography |
| CRP | C-reactive protein |
| eGFR | Estimated glomerular filtration rate |
| ENETS | European neuroendocrine tumor society |
| EUS-FNA | Endoscopic ultrasound with fine-needle aspiration |
| EUS-FNB | Endoscopic ultrasound with fine-needle biopsy |
| FAST | Focused assessment with sonography in trauma |
| 68Ga-DOTA-NOC | Gallium-68-DOTA-Nal3-octreotide |
| 68Ga-DOTA-TATE | Gallium-68-DOTA-Tyr3-octreotate |
| 68Ga-DOTA-TOC | Gallium-68-DOTA-Tyr3-octreotide |
| GEP-NEN | Gastroenteropancreatic neuroendocrine neoplasm |
| GEP-NET | Gastroenteropancreatic neuroendocrine tumor |
| HGT | Hemo-glucose test |
| 5-HIAA | 5-hydroxyindoleacetic acid |
| 5-HT | Serotonin |
| INSM1 | Insulinoma-associated protein 1 |
| JNETS | Japanese neuroendocrine tumor society |
| MEN-1 | Multiple-endocrine neoplasia 1 |
| MiNEN | Mixed neuroendocrine–non-neuroendocrine neoplasm |
| MRI | Magnetic-resonance imaging |
| NF-Pan-NET | Non-functional pancreatic neuroendocrine tumor |
| NEC | Neuroendocrine carcinoma |
| NEN | Neuroendocrine neoplasm |
| NET | Neuroendocrine tumor |
| NSE | Neuron-specific enolase |
| Pan-NET | Pancreatic neuroendocrine tumor |
| PAD | Peripheral artery disease |
| PET | Positive-emission tomography |
| PPi | Proton pomp inhibitor |
| PPRT | Peptide receptor radionuclide therapy |
| SaO2 | Oxygen saturation |
| SSA | Somatostatin analog |
| SSTR | Somatostatin receptor |
| VHL | von Hippel–Lindau syndrome |
| VIP | Vaso-active intestinal peptide |
| WHO | World Health Organization |
References
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef] [PubMed]
- Leoncini, E.; Boffetta, P.; Shafir, M.; Aleksovska, K.; Boccia, S.; Rindi, G. Increased incidence trend of low-grade and high-grade neuroendocrine neoplasms. Endocrine 2017, 58, 368–379. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.E.; Massironi, S. The Increasing Incidence of Neuroendocrine Neoplasms Worldwide: Current Knowledge and Open Issues. J. Clin. Med. 2022, 11, 3794. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A.; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Clement, D.; Ramage, J.; Srirajaskanthan, R. Update on Pathophysiology, Treatment, and Complications of Carcinoid Syndrome. J. Oncol. 2020, 2020, 8341426. [Google Scholar] [CrossRef]
- Metz, D.C.; Jensen, R.T. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology 2008, 135, 1469–1492. [Google Scholar] [CrossRef]
- Garcia-Carbonero, R.; Capdevila, J.; Crespo-Herrero, G.; Díaz-Pérez, J.A.; Martínez Del Prado, M.P.; Alonso Orduña, V.; Sevilla-García, I.; Villabona-Artero, C.; Beguiristain-Gómez, A.; Llanos-Muñoz, M.; et al. Incidence, patterns of care and prognostic factors for outcome of gastroenteropancreatic neuroendocrine tumors (GEP-NETs): Results from the National Cancer Registry of Spain (RGETNE). Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2010, 21, 1794–1803. [Google Scholar] [CrossRef]
- AIOM for Neuroendocrine Tumors Guide Line. 2020. Available online: https://www.aiom.it/wp-content/uploads/2020/10/2020_LG_AIOM_Neuroendocrini.pdf (accessed on 15 July 2023).
- Ordóñez, N.G. Broad-spectrum immunohistochemical epithelial markers: A review. Hum. Pathol. 2013, 44, 1195–1215. [Google Scholar] [CrossRef]
- Moertel, C.G. Karnofsky memorial lecture. An odyssey in the land of small tumors. J. Clin. Oncol. 1987, 5, 1502–1522. [Google Scholar] [CrossRef]
- Ito, T.; Igarashi, H.; Nakamura, K.; Sasano, H.; Okusaka, T.; Takano, K.; Komoto, I.; Tanaka, M.; Imamura, M.; Jensen, R.T.; et al. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: A nationwide survey analysis. J. Gastroenterol. 2015, 50, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.Y.; Kim, J.M.; Sohn, J.H.; Kim, M.J.; Kim, K.M.; Kim, W.H.; Kim, H.; Kook, M.C.; Park, D.Y.; Lee, J.H.; et al. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000–2009: Multicenter Study. Cancer Res. Treat. 2012, 44, 157–165. [Google Scholar] [CrossRef]
- Scherübl, H.; Streller, B.; Stabenow, R.; Herbst, H.; Höpfner, M.; Schwertner, C.; Steinberg, J.; Eick, J.; Ring, W.; Tiwari, K.; et al. Clinically detected gastroenteropancreatic neuroendocrine tumors are on the rise: Epidemiological changes in Germany. World J. Gastroenterol. 2013, 19, 9012–9019. [Google Scholar] [CrossRef] [PubMed]
- White, B.E.; Rous, B.; Chandrakumaran, K.; Wong, K.; Bouvier, C.; Van Hemelrijck, M.; George, G.; Russell, B.; Srirajaskanthan, R.; Ramage, J.K. Incidence and survival of neuroendocrine neoplasia in England 1995–2018: A retrospective, population-based study. Lancet Reg. Health. Eur. 2022, 23, 100510. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef]
- Fernandez, C.J.; Agarwal, M.; Pottakkat, B.; Haroon, N.N.; George, A.S.; Pappachan, J.M. Gastroenteropancreatic neuroendocrine neoplasms: A clinical snapshot. World J. Gastrointest. Surg. 2021, 13, 231–255. [Google Scholar] [CrossRef]
- Daraghmeh, L.; Shbaita, S.; Nassef, O.; Melhem, L.; Maqboul, I. Non-specific Symptoms of Small Bowel Neuroendocrine Tumor and the Diagnostic Challenges: A Case Report. Cureus 2023, 15, e41080. [Google Scholar] [CrossRef]
- Klöppel, G.; Perren, A.; Heitz, P.U. The gastroenteropancreatic neuroendocrine cell system and its tumors: The WHO classification. Ann. N. Y. Acad. Sci. 2004, 1014, 13–27. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017; Volume 10. [Google Scholar]
- Modlin, I.M.; Lye, K.D.; Kidd, M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003, 97, 934–959. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic neuroendocrine tumors. CA A Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Turaga, K.K.; Kvols, L.K. Recent progress in the understanding, diagnosis, and treatment of gastroenteropancreatic neuroendocrine tumors. CA Cancer J. Clin. 2011, 61, 113–132. [Google Scholar] [CrossRef]
- Zerbi, A.; Falconi, M.; Rindi, G.; Delle Fave, G.; Tomassetti, P.; Pasquali, C.; Capitanio, V.; Boninsegna, L.; Di Carlo, V.; AISP-Network Study Group. Clinicopathological features of pancreatic endocrine tumors: A prospective multicenter study in Italy of 297 sporadic cases. Am. J. Gastroenterol. 2010, 105, 1421–1429. [Google Scholar] [CrossRef]
- Jensen, R.T.; Cadiot, G.; Brandi, M.L.; de Herder, W.W.; Kaltsas, G.; Komminoth, P.; Scoazec, J.Y.; Salazar, R.; Sauvanet, A.; Kianmanesh, R.; et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: Functional pancreatic endocrine tumor syndromes. Neuroendocrinology 2012, 95, 98–119. [Google Scholar] [CrossRef]
- Gade, A.K.; Olariu, E.; Douthit, N.T. Carcinoid Syndrome: A Review. Cureus 2020, 12, e7186. [Google Scholar] [CrossRef]
- Mota, J.M.; Sousa, L.G.; Riechelmann, R.P. Complications from carcinoid syndrome: Review of the current evidence. Ecancermedicalscience 2016, 10, 662. [Google Scholar] [CrossRef]
- Halperin, D.M.; Shen, C.; Dasari, A.; Xu, Y.; Chu, Y.; Zhou, S.; Shih, Y.T.; Yao, J.C. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: A population-based study. Lancet. Oncol. 2017, 18, 525–534. [Google Scholar] [CrossRef]
- Rossi, R.E.; Corti, F.; Pusceddu, S.; Milione, M.; Coppa, J.; Masoni, B.; Oldani, S.; Sabella, G.; Cafaro, P.; Repici, A. Multidisciplinary Approach to the Diagnosis of Occult Primary Neuroendocrine Neoplasm: A Clinical Challenge. J. Clin. Med. 2023, 12, 5537. [Google Scholar] [CrossRef]
- Tatsumoto, S.; Kodama, Y.; Sakurai, Y.; Shinohara, T.; Katanuma, A.; Maguchi, H. Pancreatic neuroendocrine neoplasm: Correlation between computed tomography enhancement patterns and prognostic factors of surgical and endoscopic ultrasound-guided fine-needle aspiration biopsy specimens. Abdom. Imaging 2013, 38, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Grillo, F.; Albertelli, M.; Brisigotti, M.P.; Borra, T.; Boschetti, M.; Fiocca, R.; Ferone, D.; Mastracci, L. Grade Increases in Gastroenteropancreatic Neuroendocrine Tumor Metastases Compared to the Primary Tumor. Neuroendocrinology 2016, 103, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Atiq, M.; Bhutani, M.S.; Bektas, M.; Lee, J.E.; Gong, Y.; Tamm, E.P.; Shah, C.P.; Ross, W.A.; Yao, J.; Raju, G.S.; et al. EUS-FNA for pancreatic neuroendocrine tumors: A tertiary cancer center experience. Dig. Dis. Sci. 2012, 57, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Larghi, A.; Capurso, G.; Carnuccio, A.; Ricci, R.; Alfieri, S.; Galasso, D.; Lugli, F.; Bianchi, A.; Panzuto, F.; De Marinis, L.; et al. Ki-67 grading of nonfunctioning pancreatic neuroendocrine tumors on histologic samples obtained by EUS-guided fine-needle tissue acquisition: A prospective study. Gastrointest. Endosc. 2012, 76, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Unno, J.; Kanno, A.; Masamune, A.; Kasajima, A.; Fujishima, F.; Ishida, K.; Hamada, S.; Kume, K.; Kikuta, K.; Hirota, M.; et al. The usefulness of endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of pancreatic neuroendocrine tumors based on the World Health Organization classification. Scand. J. Gastroenterol. 2014, 49, 1367–1374. [Google Scholar] [CrossRef]
- Paiella, S.; Landoni, L.; Rota, R.; Valenti, M.; Elio, G.; Crinò, S.F.; Manfrin, E.; Parisi, A.; Cingarlini, S.; D’Onofrio, M.; et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis and grading of pancreatic neuroendocrine tumors: A retrospective analysis of 110 cases. Endoscopy 2020, 52, 988–994. [Google Scholar] [CrossRef] [PubMed]
- Crinò, S.F.; Ammendola, S.; Meneghetti, A.; Bernardoni, L.; Conti Bellocchi, M.C.; Gabbrielli, A.; Landoni, L.; Paiella, S.; Pin, F.; Parisi, A.; et al. Comparison between EUS-guided fine-needle aspiration cytology and EUS-guided fine-needle biopsy histology for the evaluation of pancreatic neuroendocrine tumors. Pancreatology 2021, 21, 443–450. [Google Scholar] [CrossRef]
- Rimbaş, M.; Crino, S.F.; Gasbarrini, A.; Costamagna, G.; Scarpa, A.; Larghi, A. EUS-guided fine-needle tissue acquisition for solid pancreatic lesions: Finally moving from fine-needle aspiration to fine-needle biopsy? Endosc. Ultrasound 2018, 7, 137–140. [Google Scholar] [CrossRef]
- Eusebi, L.H.; Thorburn, D.; Toumpanakis, C.; Frazzoni, L.; Johnson, G.; Vessal, S.; Luong, T.V.; Caplin, M.; Pereira, S.P. Endoscopic ultrasound-guided fine-needle aspiration vs fine-needle biopsy for the diagnosis of pancreatic neuroendocrine tumors. Endosc. Int. Open 2019, 7, E1393–E1399. [Google Scholar] [CrossRef]
- Di Leo, M.; Poliani, L.; Rahal, D.; Auriemma, F.; Anderloni, A.; Ridolfi, C.; Spaggiari, P.; Capretti, G.; Di Tommaso, L.; Preatoni, P.; et al. Pancreatic Neuroendocrine Tumours: The Role of Endoscopic Ultrasound Biopsy in Diagnosis and Grading Based on the WHO 2017 Classification. Dig. Dis. 2019, 37, 325–333. [Google Scholar] [CrossRef]
- Leeds, J.S.; Nayar, M.K.; Bekkali, N.L.H.; Wilson, C.H.; Johnson, S.J.; Haugk, B.; Darne, A.; Oppong, K.W. Endoscopic ultrasound-guided fine-needle biopsy is superior to fine-needle aspiration in assessing pancreatic neuroendocrine tumors. Endosc. Int. Open 2019, 7, E1281–E1287. [Google Scholar] [CrossRef]
- Melita, G.; Pallio, S.; Tortora, A.; Crinò, S.F.; Macrì, A.; Dionigi, G. Diagnostic and Interventional Role of Endoscopic Ultrasonography for the Management of Pancreatic Neuroendocrine Neoplasms. J. Clin. Med. 2021, 10, 2638. [Google Scholar] [CrossRef]
- Bernstein, J.; Ustun, B.; Alomari, A.; Bao, F.; Aslanian, H.R.; Siddiqui, U.; Chhieng, D.; Cai, G. Performance of endoscopic ultrasound-guided fine needle aspiration in diagnosing pancreatic neuroendocrine tumors. CytoJournal 2013, 10, 10. [Google Scholar] [CrossRef]
- Boutsen, L.; Jouret-Mourin, A.; Borbath, I.; van Maanen, A.; Weynand, B. Accuracy of Pancreatic Neuroendocrine Tumour Grading by Endoscopic Ultrasound-Guided Fine Needle Aspiration: Analysis of a Large Cohort and Perspectives for Improvement. Neuroendocrinology 2018, 106, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Heidsma, C.M.; Tsilimigras, D.I.; Rocha, F.; Abbott, D.E.; Fields, R.; Smith, P.M.; Poultsides, G.A.; Cho, C.; van Eijck, C.; van Dijkum, E.N.; et al. Clinical relevance of performing endoscopic ultrasound-guided fine-needle biopsy for pancreatic neuroendocrine tumors less than 2 cm. J. Surg. Oncol. 2020, 122, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Gratian, L.; Pura, J.; Dinan, M.; Roman, S.; Reed, S.; Sosa, J.A. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann. Surg. Oncol. 2014, 21, 3515–3521. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, S.M.; In, H.; Winchester, D.J.; Talamonti, M.S.; Baker, M.S. Surgical resection provides an overall survival benefit for patients with small pancreatic neuroendocrine tumors. J. Gastrointest. Surg. 2015, 19, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, V.; Haglund, C.; Seppänen, H. Outcomes of resected nonfunctional pancreatic neuroendocrine tumors: Do size and symptoms matter? Surgery 2015, 158, 1556–1563. [Google Scholar] [CrossRef]
- Hofland, J.; Falconi, M.; Christ, E.; Castaño, J.P.; Faggiano, A.; Lamarca, A.; Perren, A.; Petrucci, S.; Prasad, V.; Ruszniewski, P.; et al. European Neuroendocrine Tumor Society 2023 guidance paper for functioning pancreatic neuroendocrine tumour syndromes. J. Neuroendocrinol. 2023, 35, e13318. [Google Scholar] [CrossRef]
- Delle Fave, G.; O’Toole, D.; Sundin, A.; Taal, B.; Ferolla, P.; Ramage, J.K.; Ferone, D.; Ito, T.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Gastroduodenal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 119–124. [Google Scholar] [CrossRef]
- Ramage, J.K.; De Herder, W.W.; Delle Fave, G.; Ferolla, P.; Ferone, D.; Ito, T.; Ruszniewski, P.; Sundin, A.; Weber, W.; Zheng-Pei, Z.; et al. ENETS Consensus Guidelines Update for Colorectal Neuroendocrine Neoplasms. Neuroendocrinology 2016, 103, 139–143. [Google Scholar] [CrossRef]
- Delconte, G.; Cavalcoli, F.; Magarotto, A.; Centonze, G.; Bezzio, C.; Cattaneo, L.; Rausa, E.; Kelly, M.E.; Bonitta, G.; Milione, M.; et al. Does ProCore Fine-Needle Biopsy Really Improve the Clinical Outcome of Endoscopic Ultrasound-Guided Sampling of Pancreatic Masses? Dig. Dis. 2022, 40, 78–84. [Google Scholar] [CrossRef]
- Sugawara, T.; Rodriguez Franco, S.; Kirsch, M.J.; Colborn, K.L.; Ishida, J.; Grandi, S.; Al-Musawi, M.H.; Gleisner, A.; Del Chiaro, M.; Schulick, R.D. Evaluation of Survival Following Surgical Resection for Small Nonfunctional Pancreatic Neuroendocrine Tumors. JAMA Netw. Open 2023, 6, e234096. [Google Scholar] [CrossRef]
- Ito, T.; Masui, T.; Komoto, I.; Doi, R.; Osamura, R.Y.; Sakurai, A.; Ikeda, M.; Takano, K.; Igarashi, H.; Shimatsu, A.; et al. JNETS clinical practice guidelines for gastroenteropancreatic neuroendocrine neoplasms: Diagnosis, treatment, and follow-up: A synopsis. J. Gastroenterol. 2021, 56, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Sadot, E.; Reidy-Lagunes, D.L.; Tang, L.H.; Do, R.K.; Gonen, M.; D’Angelica, M.I.; DeMatteo, R.P.; Kingham, T.P.; Groot Koerkamp, B.; Untch, B.R.; et al. Observation versus Resection for Small Asymptomatic Pancreatic Neuroendocrine Tumors: A Matched Case-Control Study. Ann. Surg. Oncol. 2016, 23, 1361–1370. [Google Scholar] [CrossRef]
- Massironi, S.; Rossi, R.E.; Zilli, A.; Casazza, G.; Ciafardini, C.; Conte, D. A wait-and-watch approach to small pancreatic neuroendocrine tumors: Prognosis and survival. Oncotarget 2016, 7, 18978–18983. [Google Scholar] [CrossRef] [PubMed]
- Partelli, S.; Ramage, J.K.; Massironi, S.; Zerbi, A.; Kim, H.B.; Niccoli, P.; Panzuto, F.; Landoni, L.; Tomazic, A.; Ibrahim, T.; et al. Management of Asymptomatic Sporadic Nonfunctioning Pancreatic Neuroendocrine Neoplasms (ASPEN) ≤ 2 cm: Study Protocol for a Prospective Observational Study. Front. Med. 2020, 7, 598438. [Google Scholar] [CrossRef] [PubMed]
- Partelli, S.; Massironi, S.; Zerbi, A.; Niccoli, P.; Kwon, W.; Landoni, L.; Panzuto, F.; Tomazic, A.; Bongiovanni, A.; Kaltsas, G.; et al. Management of asymptomatic sporadic non-functioning pancreatic neuroendocrine neoplasms no larger than 2 cm: Interim analysis of prospective ASPEN trial. Br. J. Surg. 2022, 109, 1186–1190. [Google Scholar] [CrossRef]
- Paik, W.H.; Lee, H.S.; Lee, K.J.; Jang, S.I.; Lee, W.J.; Hwang, J.H.; Cho, C.M.; Park, C.H.; Han, J.; Woo, S.M.; et al. Malignant potential of small pancreatic neuroendocrine neoplasm and its risk factors: A multicenter nationwide study. Pancreatology 2021, 21, 208–214. [Google Scholar] [CrossRef]
- Morse, B.; Al-Toubah, T.; Montilla-Soler, J. Anatomic and functional imaging of neuroendocrine tumors. Curr. Treat. Options Oncol. 2020, 21, 75. [Google Scholar] [CrossRef]
- Zilli, A.; Arcidiacono, P.G.; Conte, D.; Massironi, S. Clinical impact of endoscopic ultrasonography on the management of neuroendocrine tumors: Lights and shadows. Dig. Liver Dis. Off. J. Ital. Soc. Gastroenterol. Ital. Assoc. Study Liver 2018, 50, 6–14. [Google Scholar] [CrossRef]
- Rossi, R.E.; Elvevi, A.; Gallo, C.; Palermo, A.; Invernizzi, P.; Massironi, S. Endoscopic techniques for diagnosis and treatment of gastro-entero-pancreatic neuroendocrine neoplasms: Where we are. World J. Gastroenterol. 2022, 28, 3258–3273. [Google Scholar] [CrossRef]
- Lee, D.W.; Kim, M.K.; Kim, H.G. Diagnosis of Pancreatic Neuroendocrine Tumors. Clin. Endosc. 2017, 50, 537–545. [Google Scholar] [CrossRef]
- Rust, E.; Hubele, F.; Marzano, E.; Goichot, B.; Pessaux, P.; Kurtz, J.E.; Imperiale, A. Nuclear medicine imaging of gastro-entero-pancreatic neuroendocrine tumors. The key role of cellular differentiation and tumor grade: From theory to clinical practice. Cancer Imaging 2012, 12, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, I.; Henze, M.; Engelbrecht, S.; Eisenhut, M.; Runz, A.; Schäfer, M.; Schilling, T.; Haufe, S.; Herrmann, T.; Haberkorn, U. Comparison of 68Ga-DOTATOC PET and 111In-DTPAOC (Octreoscan) SPECT in patients with neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Van Binnebeek, S.; Vanbilloen, B.; Baete, K.; Terwinghe, C.; Koole, M.; Mottaghy, F.M.; Clement, P.M.; Mortelmans, L.; Bogaerts, K.; Haustermans, K.; et al. Comparison of diagnostic accuracy of (111)In-pentetreotide SPECT and (68)Ga-DOTATOC PET/CT: A lesion-by-lesion analysis in patients with metastatic neuroendocrine tumours. Eur. Radiol. 2016, 26, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Castaldi, P.; Rindi, G.; Giordano, A.; Rufini, V. Diagnostic performance of Gallium-68 somatostatin receptor PET and PET/CT in patients with thoracic and gastroenteropancreatic neuroendocrine tumours: A meta-analysis. Endocrine 2012, 42, 80–87. [Google Scholar] [CrossRef]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601. [Google Scholar] [CrossRef]
- Binderup, T.; Knigge, U.; Loft, A.; Federspiel, B.; Kjaer, A. 18F-fluorodeoxyglucose positron emission tomography predicts survival of patients with neuroendocrine tumors. Clin. Cancer Res. 2010, 16, 978–985. [Google Scholar] [CrossRef]
- Muffatti, F.; Partelli, S.; Cirocchi, R.; Andreasi, V.; Mapelli, P.; Picchio, M.; Gianolli, L.; Falconi, M. Combined 68Ga-DOTA-peptides and 18F-FDG PET in the diagnostic work-up of neuroendocrine neoplasms (NEN). Clin. Transl. Imaging 2019, 7, 181–188. [Google Scholar] [CrossRef]
- Mapelli, P.; Tam, H.H.; Sharma, R.; Aboagye, E.O.; Al-Nahhas, A. Frequency and significance of physiological versus pathological uptake of 68Ga-DOTATATE in the pancreas: Validation with morphological imaging. Nucl. Med. Commun. 2014, 35, 613–619. [Google Scholar] [CrossRef]
- Mapelli, P.; Partelli, S.; Salgarello, M.; Doraku, J.; Muffatti, F.; Schiavo Lena, M.; Pasetto, S.; Bezzi, C.; Bettinardi, V.; Andreasi, V.; et al. Dual Tracer 68Ga-DOTATOC and 18F-FDG PET Improve Preoperative Evaluation of Aggressiveness in Resectable Pancreatic Neuroendocrine Neoplasms. Diagnostics 2021, 11, 192. [Google Scholar] [CrossRef]
- Paiella, S.; Landoni, L.; Tebaldi, S.; Zuffante, M.; Salgarello, M.; Cingarlini, S.; D’Onofrio, M.; Parisi, A.; Deiro, G.; Manfrin, E.; et al. Dual-Tracer (68Ga-DOTATOC and 18F-FDG-)-PET/CT Scan and G1-G2 Nonfunctioning Pancreatic Neuroendocrine Tumors: A Single-Center Retrospective Evaluation of 124 Nonmetastatic Resected Cases. Neuroendocrinology 2022, 112, 143–152. [Google Scholar] [CrossRef]
- Leon, A.; Torta, M.; Dittadi, R.; degli Uberti, E.; Ambrosio, M.R.; Delle Fave, G.; De Braud, F.; Tomassetti, P.; Gion, M.; Dogliotti, L. Comparison between two methods in the determination of circulating chromogranin A in neuroendocrine tumors (NETs): Results of a prospective multicenter observational study. Int. J. Biol. Markers 2005, 20, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Welin, S.; Stridsberg, M.; Cunningham, J.; Granberg, D.; Skogseid, B.; Oberg, K.; Eriksson, B.; Janson, E.T. Elevated plasma chromogranin A is the first indication of recurrence in radically operated midgut carcinoid tumors. Neuroendocrinology 2009, 89, 302–307. [Google Scholar] [CrossRef]
- Sansone, A.; Lauretta, R.; Vottari, S.; Chiefari, A.; Barnabei, A.; Romanelli, F.; Appetecchia, M. Specific and Non-Specific Biomarkers in Neuroendocrine Gastroenteropancreatic Tumors. Cancers 2019, 11, 1113. [Google Scholar] [CrossRef]
- Oberg, K.; Couvelard, A.; Delle Fave, G.; Gross, D.; Grossman, A.; Jensen, R.T.; Pape, U.F.; Perren, A.; Rindi, G.; Ruszniewski, P.; et al. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Biochemical Markers. Neuroendocrinology 2017, 105, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Y.; Gong, Y.F.; Zhuang, H.K.; Zhou, Z.X.; Huang, S.Z.; Zou, Y.P.; Huang, B.W.; Sun, Z.H.; Zhang, C.Z.; Tang, T.Q.; et al. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J. Gastroenterol. 2020, 26, 2305–2322. [Google Scholar] [CrossRef]
- Kulke, M.H.; Anthony, L.B.; Bushnell, D.L.; de Herder, W.W.; Goldsmith, S.J.; Klimstra, D.S.; Marx, S.J.; Pasieka, J.L.; Pommier, R.F.; Yao, J.C.; et al. NANETS treatment guidelines: Well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 2010, 39, 735–752. [Google Scholar] [CrossRef]
- Knigge, U.; Hansen, C.P. Surgery for GEP-NETs. Best Pract. Res. Clin. Gastroenterol. 2012, 26, 819–831. [Google Scholar] [CrossRef]
- Massironi, S.; Conte, D.; Rossi, R.E. Somatostatin analogues in functioning gastroenteropancreatic neuroendocrine tumours: Literature review, clinical recommendations and schedules. Scand. J. Gastroenterol. 2016, 51, 513–523. [Google Scholar] [CrossRef]
- Pavel, M.; O’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Caplin, M.E.; Pavel, M.; Ruszniewski, P. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 1556–1557. [Google Scholar] [CrossRef] [PubMed]
- Jann, H.; Denecke, T.; Koch, M.; Pape, U.F.; Wiedenmann, B.; Pavel, M. Impact of octreotide long-acting release on tumour growth control as a first-line treatment in neuroendocrine tumours of pancreatic origin. Neuroendocrinology 2013, 98, 137–143. [Google Scholar] [CrossRef]
- Wolin, E.M.; Jarzab, B.; Eriksson, B.; Walter, T.; Toumpanakis, C.; Morse, M.A.; Tomassetti, P.; Weber, M.M.; Fogelman, D.R.; Ramage, J.; et al. Phase III study of pasireotide long-acting release in patients with metastatic neuroendocrine tumors and carcinoid symptoms refractory to available somatostatin analogues. Drug Des. Dev. Ther. 2015, 9, 5075–5086. [Google Scholar] [CrossRef]
- Feldman, J.M.; Jones, R.S. Carcinoid syndrome from gastrointestinal carcinoids without liver metastasis. Ann. Surg. 1982, 196, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Zavras, N.; Schizas, D.; Machairas, N.; Damaskou, V.; Economopoulos, N.; Machairas, A. Carcinoid syndrome from a carcinoid tumor of the pancreas without liver metastases: A case report and literature review. Oncol. Lett. 2017, 13, 2373–2376. [Google Scholar] [CrossRef]
- Famerée, L.; Van Lier, C.; Borbath, I.; Yildiz, H.; Lemaire, J.; Baeck, M. Misleading clinical presentation of carcinoid syndrome. Acta Gastro-Enterol. Belg. 2021, 84, 501–503. [Google Scholar] [CrossRef]
- Rossi, R.E.; Ciafardini, C.; Sciola, V.; Conte, D.; Massironi, S. Chromogranin A in the Follow-up of Gastroenteropancreatic Neuroendocrine Neoplasms: Is It Really Game Over? A Systematic Review and Meta-analysis. Pancreas 2018, 47, 1249–1255. [Google Scholar] [CrossRef]
- Zandee, W.T.; van Adrichem, R.C.; Kamp, K.; Feelders, R.A.; van Velthuysen, M.F.; de Herder, W.W. Incidence and prognostic value of serotonin secretion in pancreatic neuroendocrine tumours. Clin. Endocrinol. 2017, 87, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Depoilly, T.; Leroux, R.; Andrade, D.; Nicolle, R.; Dioguardi Burgio, M.; Marinoni, I.; Dokmak, S.; Ruszniewski, P.; Hentic, O.; Paradis, V.; et al. Immunophenotypic and molecular characterization of pancreatic neuroendocrine tumors producing serotonin. Modern pathology 2022, 35, 1713–1722. [Google Scholar] [CrossRef]
- Haq, A.U.; Yook, C.R.; Hiremath, V.; Kasimis, B.S. Carcinoid syndrome in the absence of liver metastasis: A case report and review of literature. Med. Pediatr. Oncol. 1992, 20, 221–223. [Google Scholar] [CrossRef]
- Oberg, K.; Janson, E.T.; Eriksson, B. Tumour markers in neuroendocrine tumours. Ital. J. Gastroenterol. Hepatol. 1999, 31 (Suppl. S2), S160–S162. [Google Scholar] [PubMed]
- Eriksson, B.; Oberg, K.; Stridsberg, M. Tumor markers in neuroendocrine tumors. Digestion 2000, 62 (Suppl. S1), 33–38. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).