RAndomized Clinical Trial Of NAfamostat Mesylate, A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Aims
2.2. Treatments
2.3. Statistical Analysis
2.3.1. Main Analysis
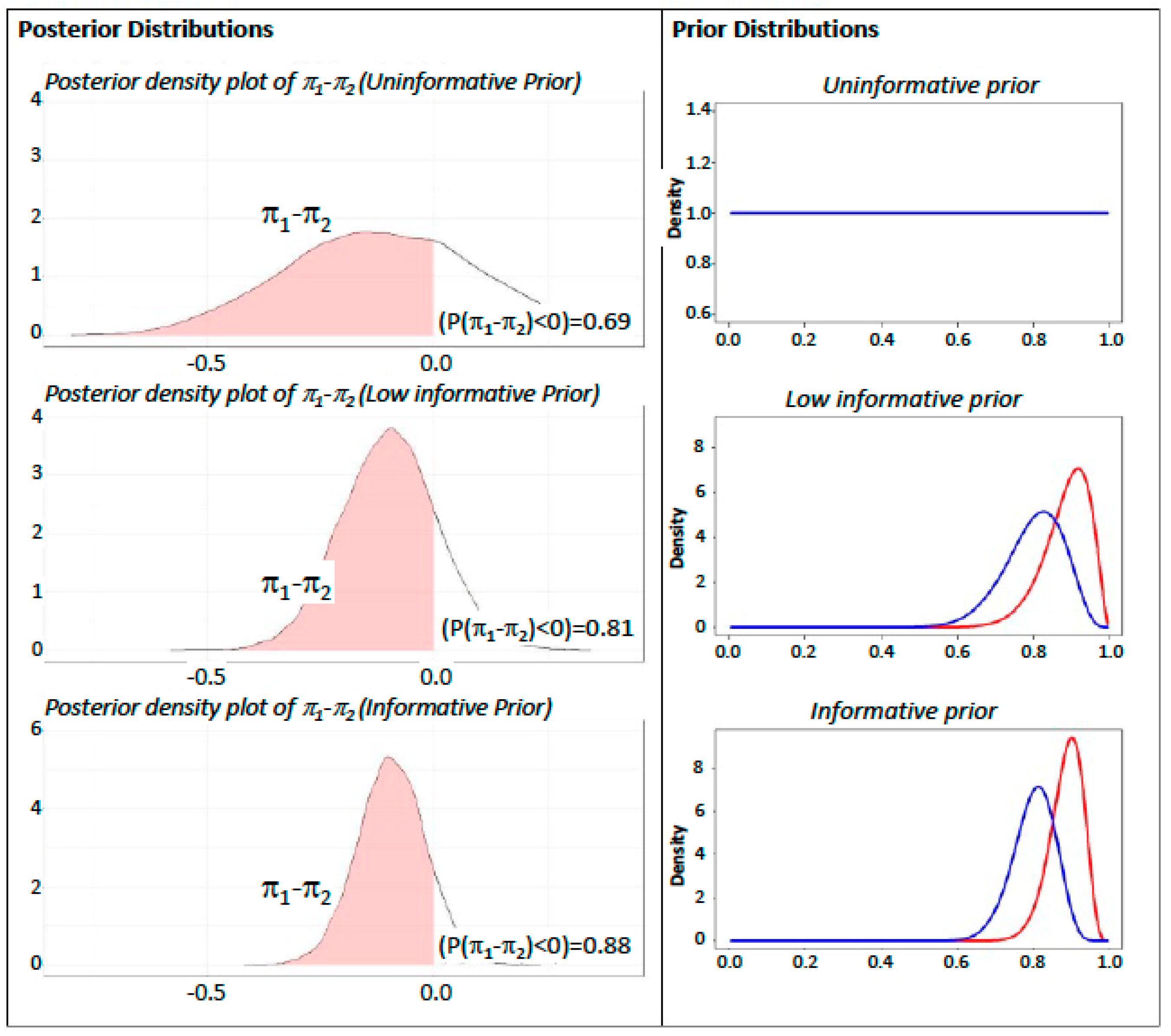
2.3.2. Outcome Evaluation
- A first resampling of the response rate from , which is the posterior distribution for the treatment group.
- A second resampling of from .
- A posterior distribution, for the parameter related to the difference in proportions, has been obtained by calculating from the previously resampled distributions [20].
2.3.3. Sensitivity Analysis
- Power Prior without discounting (Informative). A for the treatment arm and a for the control arm were derived considering the number of successes reported in the literature in a similar research framework [24].
- Power Prior 50% discounting (Low Informative). The second scenario provides the same informative Beta as previously indicated, also applying a 50% down weight on parameters, to control the effect of prior information on final inference as indicated in the literature [25].
- Power Prior 100% discounting (Uninformative). A prior in both treatments arms has been considered.The inference results have been analyzed evaluating the hypothesis that .
3. Results
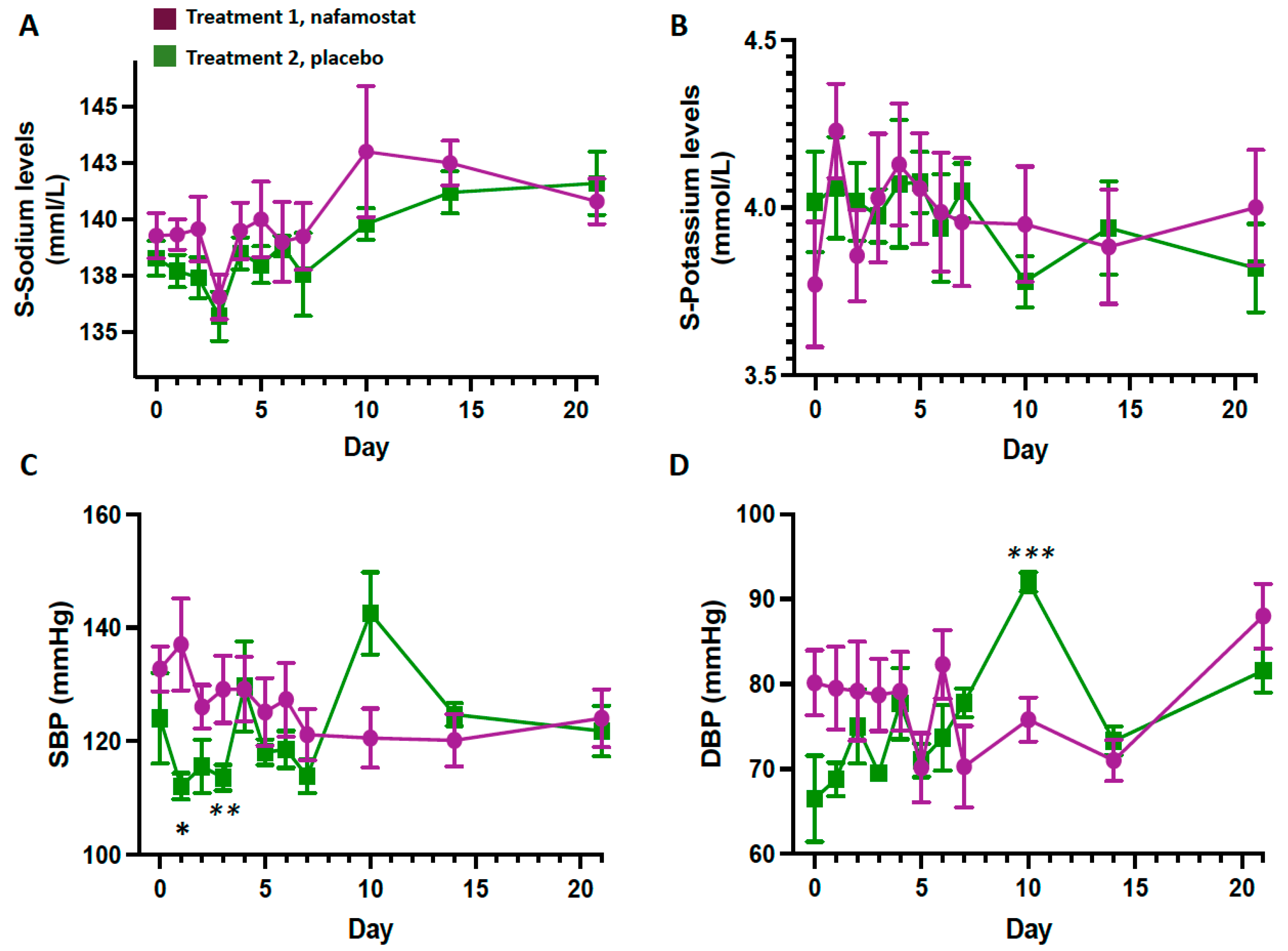
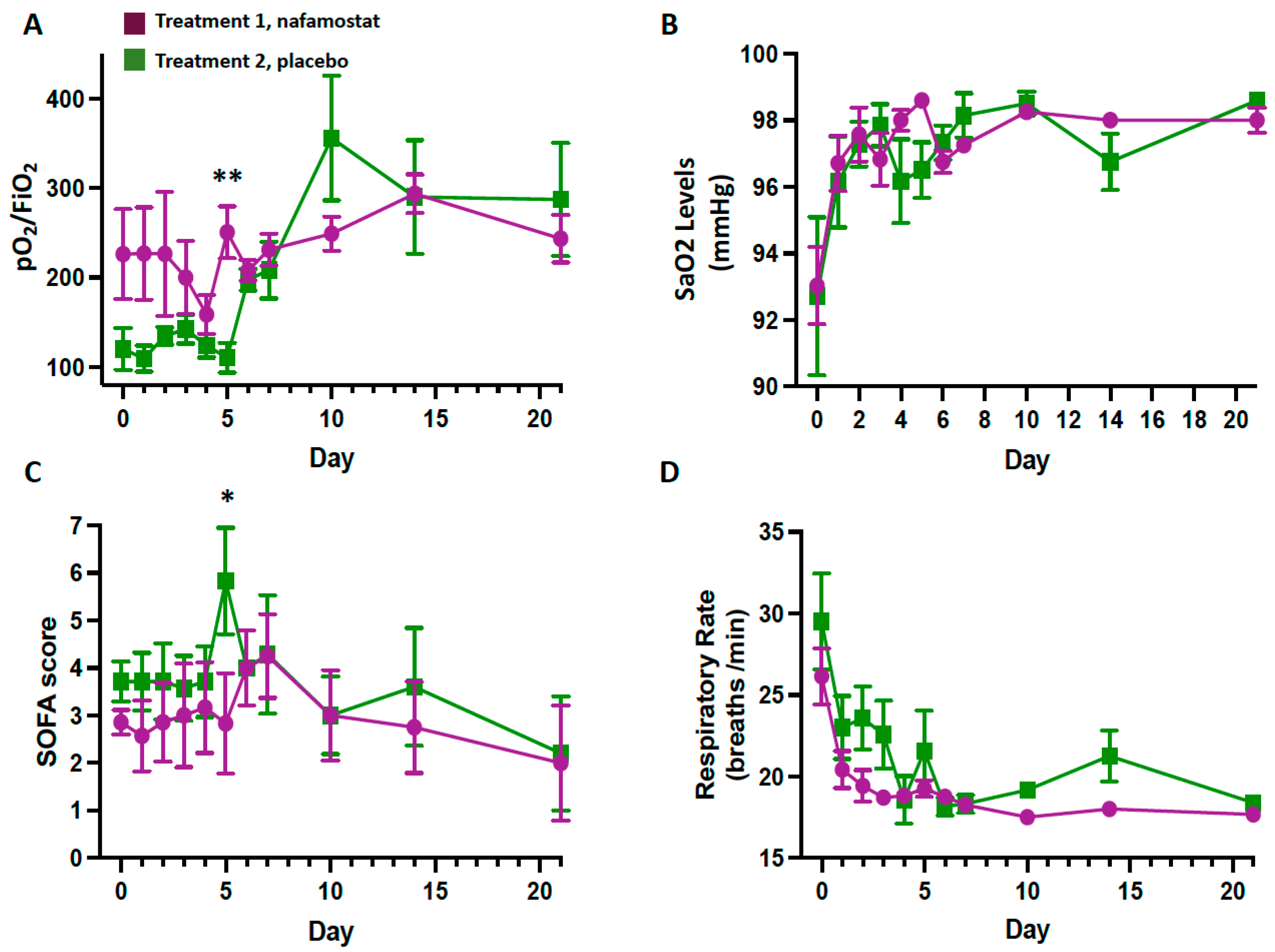
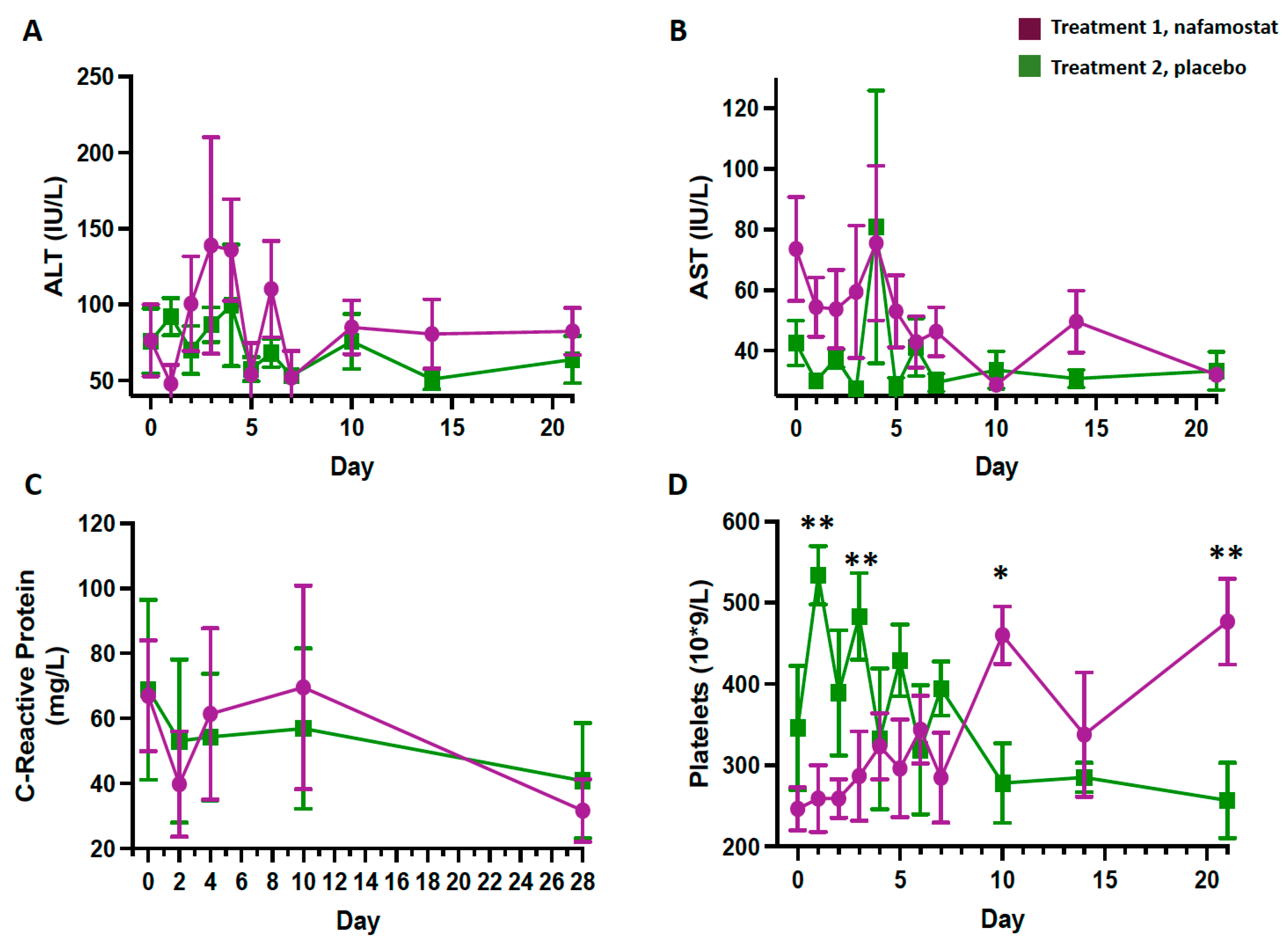
3.1. Safety Outcomes
3.2. Outcomes
4. Discussion
Limitations of the Study and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isgrò, M.A.; Trillò, G.; Russo, L.; Tornesello, A.L.; Buonaguro, L.; Tornesello, M.L.; Miscio, L.; Normanno, N.; Bianchi, A.A.M.; Buonaguro, F.M.; et al. Bimodal antibody-titer decline following BNT162b2 mRNA anti-SARS-CoV-2 vaccination in healthcare workers of the INT—IRCCS “Fondazione Pascale” Cancer Center (Naples, Italy). Infect. Agents Cancer 2022, 17, 40. [Google Scholar] [CrossRef]
- Yang, H.; Xie, Y.; Li, C. Understanding the mechanisms for COVID-19 vaccine’s protection against infection and severe disease. Expert. Rev. Vaccines 2023, 22, 186–192. [Google Scholar] [CrossRef]
- Chuenkitmongkol, S.; Solante, R.; Burhan, E.; Chariyalertsak, S.; Chiu, N.-C.; Do-Van, D.; Masliyana, H.; Kao-Pin, H.; Sasisopin, K.; Prasad, S.K. Expert review on global real-world vaccine effectiveness against SARS-CoV-2. Expert. Rev. Vaccines 2022, 21, 1255–1268. [Google Scholar] [CrossRef]
- Botton, J.; Jabagi, M.J.; Bertrand, M.; Baricault, B.; Drouin, J.; le Vu, S.; Weill, A.; Farrington, P.; Zureik, M.; Dray-Spira, R. Risk for Myocardial Infarction, Stroke, and Pulmonary Embolism Following COVID-19 Vaccines in Adults Younger Than 75 Years in France. Ann. Intern. Med. 2022, 175, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Prats-Uribe, A.; Feng, Q.; Wang, Y.; Gill, D.; Paredes, R.; Prieto-Alhambra, D. Clinical and Genetic Risk Factors for Acute Incident Venous Thromboembolism in Ambulatory Patients with COVID-19. JAMA Intern. Med. 2022, 182, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Tobias, S.; Georg, H.; Nai-Huei, W.; Andreas, N.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Voigtlaender, M.; Edler, C.; Gerling, M.; Schädler, J.; Ondruschka, B.; Schröder, A.S.; Sperhake, J.; Ehrhardt, S.; Wang, L.; Haddad, M.; et al. Thromboembolic events in deceased patients with proven SARS-CoV-2 infection: Frequency, characteristics and risk factors. Thromb. Res. 2022, 218, 171–176. [Google Scholar] [CrossRef]
- Knight, R.; Walker, V.; Ip, S.; Cooper, J.A.; Bolton, T.; Keene, S.; Denholm, R.; Akbari, A.; Abbasizanjani, H.; Torabi, F.; et al. Association of COVID-19 with Major Arterial and Venous Thrombotic Diseases: A Population-Wide Cohort Study of 48 Million Adults in England and Wales. Circulation 2022, 146, 892–906. [Google Scholar] [CrossRef]
- Eslamifar, Z.; Behzadifard, M.; Soleimani, M.; Behzadifard, S. Coagulation abnormalities in SARS-CoV-2 infection: Overexpression tissue factor. Thromb. J. 2020, 18, 38. [Google Scholar] [CrossRef]
- Yamamoto, M.; Matsuyama, S.; Li, X.; Takeda, M.; Kawaguchi, Y.; Inoue, J.-I.; Matsuda, Z. Identification of Nafamostat as a Potent Inhibitor of Middle East Respiratory Syndrome Coronavirus S Protein-Mediated Membrane Fusion Using the Split-Protein-Based Cell-Cell Fusion Assay. Antimicrob. Agents Chemother. 2016, 60, 6532–6539. [Google Scholar] [CrossRef]
- Maruyama, Y.; Yoshida, H.; Uchino, S.; Yokoyama, K.; Yamamoto, H.; Takinami, M.; Hosoya, T. Nafamostat mesilate as an anticoagulant during continuous veno-venous hemodialysis: A three-year retrospective cohort study. Int. J. Artif. Organs 2011, 34, 571–576. [Google Scholar] [CrossRef]
- Choi, J.-Y.; Kang, Y.-J.; Jang, H.M.; Jung, H.-Y.; Cho, J.-H.; Park, S.-H.; Kim, Y.-L.; Kim, C.-D. Nafamostat Mesilate as an Anticoagulant during Continuous Renal Replacement Therapy in Patients with High Bleeding Risk: A Randomized Clinical Trial. Medicine 2015, 94, e2392. [Google Scholar] [CrossRef]
- Yu, G.; Li, S.; Wan, R.; Wang, X.; Hu, G. Nafamostat mesilate for prevention of post-ERCP pancreatitis: A meta-analysis of prospective, randomized, controlled trials. Pancreas 2015, 44, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Minakata, D.; Fujiwara, S.-I.; Ikeda, T.; Kawaguchi, S.-I.; Toda, Y.; Ito, S.; Ochi, S.-I.; Nagayama, T.; Mashima, K.; Umino, K.; et al. Comparison of gabexate mesilate and nafamostat mesilate for disseminated intravascular coagulation associated with hematological malignancies. Int. J. Hematol. 2019, 109, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Kastenhuber, E.R.; Mercadante, M.; Nilsson-Payant, B.; Johnson, J.L.; Jaimes, J.A.; Muecksch, F.; Weisblum, Y.; Bram, Y.; Chandar, V.; Whittaker, G.R.; et al. Coagulation factors directly cleave SARS-CoV-2 spike and enhance viral entry. Elife 2022, 11, e77444. [Google Scholar] [CrossRef]
- Aoyama, T.; Ino, Y.; Ozeki, M.; Oda, M.; Sato, T.; Koshiyama, Y.; Suzuki, S.; Fujita, M. Pharmacological studies of FUT-175, nafamstat mesilate. I. Inhibition of protease activity in in vitro and in vivo experiments. Jpn. J. Pharmacol. 1984, 35, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Uchiba, M.; Okajima, K.; Abe, H.; Okabe, H.; Takatsuki, K. Effect of nafamostat mesilate, a synthetic protease inhibitor, on tissue factor-factor VIIa complex activity. Thromb. Res. 1994, 74, 155–161. [Google Scholar] [CrossRef]
- Albert, J. (Ed.) Bayesian Computation with R; Springer: New York, NY, USA, 2009; pp. 19–37, 87–115. [Google Scholar]
- Kawasaki, Y.; Shimokawa, A.; Miyaoka, E. Comparison of Three Calculation Methods for a Bayesian Inference of P(π1 > π2). J. Mod. Appl. Stat. Methods 2013, 12, 256–268. [Google Scholar] [CrossRef]
- Barry, J. Doing Bayesian Data Analysis: A Tutorial with R and BUGS. Eur. J. Psychol. 2011, 7, 778–779. [Google Scholar] [CrossRef]
- Lunn, D.; Spiegelhalter, D.; Thomas, A.; Best, N. The BUGS project: Evolution, critique and future directions. Stat. Med. 2009, 28, 3049–3067. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing. 2015. Available online: https://www.r-project.org/ (accessed on 2 August 2020).
- Chen, M.-H.; Ibrahim, J.G. Power prior distributions for regression models. Stat. Sci. 2000, 15, 46–60. [Google Scholar] [CrossRef]
- Zhuravel, S.V.; Khmelnitskiy, O.K.; Burlaka, O.O.; Gritsan, A.I.; Goloshchekin, B.M.; Kim, S.; Hong, K.Y. Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: A randomised Phase II clinical trial. E Clin. Med. 2021, 41, 101169. [Google Scholar] [CrossRef]
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Dunson, D.B.; Vehtari, A.; Rubin, D.B. (Eds.) Bayesian Data Analysis. In Bayesian Data Analysis; Chapman and Hall/CRC: New York, NY, USA, 2013. [Google Scholar]
- Ota, S.; Hara, Y.; Kanoh, S.; Shinoda, M.; Kawano, S.; Fujikura, Y.; Kawana, A.; Shinkai, M. Acute eosinophilic pneumonia caused by camostat mesilate: The first case report. Respir. Med. Case Rep. 2016, 19, 21–23. [Google Scholar] [CrossRef]
- Iwashita, K.; Kitamura, K.; Narikiyo, T.; Adachi, M.; Shiraishi, N.; Miyoshi, T.; Nagano, J.; Tuyen, D.G.; Nonoguchi, H.; Tomita, K. Inhibition of prostasin secretion by serine protease inhibitors in the kidney. J. Am. Soc. Nephrol. 2003, 14, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Nimishakavi, S.; Raymond, W.W.; Gruenert, D.C.; Caughey, G.H. Divergent Inhibitor Susceptibility among Airway Lumen-Accessible Tryptic Proteases. PLoS ONE 2015, 10, e0141169. [Google Scholar] [CrossRef]
- Muto, S.; Imai, M.; Asano, Y. Mechanisms of hyperkalemia caused by nafamostat mesilate. Gen. Pharmacol. 1995, 26, 1627–1632. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Sanga, V.; Barton, M. Potential harmful effects of discontinuing ACE-inhibitors and ARBs in COVID-19 patients. eLife 2020, 9, e57278. [Google Scholar] [CrossRef]
- Quinn, T.M.; Gaughan, E.E.; Bruce, A.; Antonelli, J.; O’Connor, R.; Li, F.; McNamara, S.; Koch, O.; MacKintosh, C.; Dockrell, D.; et al. Randomised controlled trial of intravenous nafamostat mesylate in COVID pneumonitis: Phase 1b/2a experimental study to investigate safety, Pharmacokinetics and Pharmacodynamics. EBio Med. 2022, 76, 103856. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S. Sun Pharma Laboratories Limited, Mumbai, India. Unpublished work. 2020. [Google Scholar]
- Kim, D. Korea Cancer Center Hospital, Seoul, Republic of Korea. Unpublished work. 2020. [Google Scholar]
- Soma, T.; Fujii, K.; Yoshifuji, A.; Maruki, T.; Itoh, K.; Taniyama, D.; Kikuchi, T.; Hasegawa, N.; Nakamura, M. Nafamostat Mesylate Monotherapy in Patients with Moderate COVID-19: A Single-Center, Retrospective Study. Jpn. J. Infect. Dis. 2022, 75, 484–489. [Google Scholar] [CrossRef]
- Inokuchi, R.; Kuno, T.; Komiyama, J.; Uda, K.; Miyamoto, Y.; Taniguchi, Y.; Abe, T.; Ishimaru, M.; Adomi, M.; Tamiya, N.; et al. Association between Nafamostat Mesylate and In-Hospital Mortality in Patients with Coronavirus Disease 2019: A Multicenter Observational Study. J. Clin. Med. 2021, 11, 446. [Google Scholar] [CrossRef]
- Hoffmann, M.; Schroeder, S.; Kleine-Weber, H.; Müller, M.A.; Drosten, C.; Pöhlmann, S. Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrob. Agents Chemother. 2020, 64, e00754-20. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.-G.; Zhang, M.; Yu, D.; Shao, J.-P.; Chen, Y.-C.; Liu, X.-Q. A method for quantifying the unstable and highly polar drug nafamostat mesilate in human plasma with optimized solid-phase extraction and ESI-MS detection: More accurate evaluation for pharmacokinetic study. Anal. Bioanal. Chem. 2008, 391, 1063–1071. [Google Scholar] [CrossRef]
- Yamaori, S.; Fujiyama, N.; Kushihara, M.; Funahashi, T.; Kimura, T.; Yamamoto, I.; Sone, T.; Isobe, M.; Ohshima, T.; Matsumura, K.; et al. Involvement of Human Blood Arylesterases and Liver Microsomal Carboxylesterases in Nafamostat Hydrolysis. Drug Metab. Pharmacokinet. 2006, 21, 147–155. [Google Scholar] [CrossRef]
- Broglio, K. Randomization in clinical trials: Permuted blocks and stratification. JAMA 2018, 319, 2223–2224. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; Mcmurray, J.V.; Pfeffer, M.A.; Solomon, S.D. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with Covid-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhu, L.; Cai, J.; Lei, F.; Qin, J.J.; Xie, J.; Liu, Y.M.; Zhao, Y.C.; Huang, X.; Lin, L.; et al. Association of Inpatient Use of Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers with Mortality Among Patients with Hypertension Hospitalized With COVID-19. Circ. Res. 2020, 126, 1671–1681. [Google Scholar] [CrossRef]
- World Health Organization. WHO R&D Blueprint Informal Consultation on Prioritization of Candidate Therapeutic Agents for Use in Novel Coronavirus 2019 Infection; World Health Organization: Geneva, Switzerland, 2020.
- Lesaffre, E. The Randomized Controlled Trial: Methodological Perspectives. In Understanding Evidence-Based Rheumatology; Springer: Berlin/Heidelberg, Germany, 2014; pp. 159–178. [Google Scholar]
- Lachin, J.M.; Foulkes, M.A. Evaluation of Sample Size and Power for Analyses of Survival with Allowance for Nonuniform Patient Entry, Losses to Follow-Up, Noncompliance, and Stratification. Biometrics 1986, 42, 507–519. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- R_Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: 2020. Available online: https://www.r-project.org/ (accessed on 2 August 2020).
- Keaven Anderson, K. gsDesign: Group Sequential Design. 30-1, Package Version; 2016. Available online: https://cranr-project.org/package=gsdesign (accessed on 2 August 2020).
| Variable | Group 1 Nafamostat (n = 7) | Group 2 Placebo (n = 7) | All Patients (n = 14) |
|---|---|---|---|
| Male sex, n (%) | 6 (85%) | 4 (57%) | 10 (71) |
| Age, years | 64 [46–68] | 58 [58–77] | 62 [57–74] |
| Body temperature, °C | 37.5 (36.2–38.5) | 37.7 (36.9–39.0) | 37.5 [36.8–38.7] |
| SBP, mmHg | 133 [130–140] | 139 [118–154] | 135 [127–143] |
| DBP, mmHg | 80 [70–92] | 84 [63–91] | 82 [69–91] |
| Heart rate/min | 88 [72–95] | 96 [81–103] | 90 [80–102] |
| Respiratory rate/min | 28 [23–30] | 29 [23–33] | 28 [23–30] |
| Seven-category ordinal scale, score | 4 [4–5] | 5 [5–5] | 5 [4–5] |
| SOFA score, Units | 3 [2–3] | 4 [3–5] | 3 [2–4] |
| C-reactive protein, mg/L | 59 [29–118] | 40 [12–150] | 49 [13–121] |
| D-dimer, μg/L | 189 [150–259] | 286 [156–657] | 218 [154–513] |
| S-Creatinine, μmol/L | 70 [64–87] | 62 [53–104] | 68 [54–87] |
| eGFR, mL/min/1.73 m2 | 92 [80–106] | 98 [68–104] | 94 [80–103] |
| Fibrinogen, g/L | 4.90 [4.50–6.80] | 4.30 [3.60–4.80] | 4.65 [4.27–5.20] |
| PT, s | 98 [96–101] | 98 [87–108] | 98 [87–106] |
| INR | 1.07 [1.04–1.11] | 1.08 [1.04–1.14] | 1.08 [1.04–1.12] |
| Platelets, 109/L | 237 [202–304] | 268 [132–355] | 251 [185–316] |
| SatO2, % | 93 [90–94] | 93 [85–99] | 93 [90–97] |
| pO2/FiO2 ratio | 161 [110–379] | 98 [73–162] | 121 [97–231] |
| Procalcitonin, µg/L | 0.08 [0.05–0.14] | 0.25 [0.04–0.93] | 0.08 [0.04–0.65] |
| PSI, Units | 84 [56–96] | 98 [93–107] | 94 [75–100] |
| CURB-65, Units | 1 [0–2] | 2 [1–2] | 1 [1–2] |
| S-Ferritin, µg/L | 1884 [554–4635] | 1358 [943–1721] | 1358 [886–2507] |
| S-K+, mmol/L | 4.0 [3.4–4.1] | 4.3 [3.9–4.4] | 4.0 [3.7–4.3] |
| S-Na+, mmol/L | 138 [137–141] | 139 [137–140] | 138 [137–141] |
| Variable | Group 1 Nafamostat (n = 7) | Group 2 Placebo (n = 7) | p |
|---|---|---|---|
| (A) Outcomes at day 7 | |||
| Body temperature, °C | 36.2 [36.0–38.5] | 36.1 [36.0–36.8] | 0.527 |
| SBP, mmHg | 115 [104–120] | 130 [109–150] | 1.000 |
| DBP, mmHg | 70 [66–72] | 80 [65–89] | 0.476 |
| Heart rate, bpm | 79 [58–95] | 71 [60–81] | 0.476 |
| Respiratory rate/min | 18 [18–18] | 18 [16–20] | 1.000 |
| Seven-category ordinal scale, score | 3 [1–6] | 6 [4–6] | 0.295 |
| SOFA score, Units | 4 [1–7] | 5 [1–7] | 0.927 |
| C-reactive protein, mg/L | 33 [12–144] | 48 [2.9–120] | 0.876 |
| D-dimer, µg/L | 163 [152–248] | 626 [300–4279] | 0.012 |
| S-Creatinine, μmol/L | 106 [92–136] | 66 [49–94] | 0.109 |
| eGFR ml/min/1.73 m2 | 59 [45–89] | 95 [63–109] | 0.257 |
| S-Fibrinogen, g/L | 6.00 [4.00–7.25] | 3.70 [2.80–6.10] | 0.164 |
| PT, s | 93 [79–104] | 89 [76–103] | 0.648 |
| INR | 1.11 [1.04–1.18] | 1.10 [1.06–1.20] | 1.000 |
| Platelets, 109/L | 495 [308–625] | 259 [228–270] | 0.048 |
| SatO2, % | 97 [97–98] | 98 [97–100] | 0.230 |
| pO2/FiO2 | 233 [165–294] | 172 [156–257] | 0.648 |
| S-Procalcitonin, µg/L | 0.08 [0.04–0.33] | 0.06 [0.04–0.21] | 1.000 |
| PSI, Units | 83 [50–113] | 97 [58–114] | 0.648 |
| CURB-65, Units | 2 [1–2] | 1 [1–3] | 0.527 |
| S-Ferritin, µg/L | 1003 [287–2409] | 894 [607–1319] | 1.000 |
| S-K+, mmol/L | 4.0 [3.6–4.2] | 4.0 [3.7–4.4] | 1.000 |
| S-Na+, mmol/L | 139 [135–143] | 138 [136–139] | 0.648 |
| (B) Outcomes at day 14 | |||
| Body temperature, °C | 36.1 [36.0–36.7] | 36.0 [36.0–37.1] | 0.610 |
| SBP, mmHg | 115 [102–123] | 135 [121–171] | 1.000 |
| DBP, mmHg | 78 [71–83] | 92 [87–95] | 0.476 |
| Heart rate, bpm | 80 [67–80] | 83 [66–111] | 0.476 |
| Respiratory rate/min | 18 [16–19] | 18 [18–27] | 0.171 |
| Seven-category ordinal scale, score | 1 [1–4] | 4 [1–6] | 0.432 |
| SOFA score, Units | 1 [1–6]] | 2 [1–7] | 1.000 |
| C-reactive protein, mg/L | 10 [3–10] | 10 [10–108] | 1.000 |
| D-dimer, µg/L | 274 [161–453] | 421 [192–777] | 0.063 |
| S-Creatinine, μmol/L | 92 [89–92] | 65 [49–70] | 0.016 |
| eGFR ml/min/1.73 m2 | 83 [76–89] | 102 [93–105] | 0.029 |
| S-Fibrinogen, g/L | 5.1 [3.6–7.7] | 4.7 [3.1–5.9] | 0.413 |
| PT, sec | 87 [67–101] | 104 [90–107] | 0.556 |
| INR | 1.12 [1.02–1.23] | 1.04 [1.03–1.12] | 1.000 |
| Platelets, 109/L | 425 [267–490] | 206 [166–425] | 0.413 |
| SatO2, % | 98 [97–98] | 98 [93–98] | 0.762 |
| pO2/FiO2 | 314 [208–357] | 219 [108–542] | 0.476 |
| S-Procalcitonin, µg/L | 0.04 [0.04–0.11] | 0.04 [0.04–2.30] | 1.000 |
| PSI, Units | 73 [52–82] | 108 [63–160] | 0.762 |
| CURB-65, Units | 1 [1–2] | 2 [1–2] | 0.730 |
| S-Ferritin, µg/L | 736 [62–736] | 404 [281–404] | 1.000 |
| S-K+, mmol/L | 3.9 [3.8–4.3] | 3.7 [3.6–4.0] | 0.905 |
| S-Na+, mmol/L | 142 [140–145] | 143 [139–143] | 1.000 |
| Control (n = 7) | Treatment (n = 7) | ||
|---|---|---|---|
| 29% (2) | 43% (3) | ||
| Predictive Posterior Estimates (95% CI) | |||
| Informative | 5 (3; 7) | 6 (3; 7) | −0.09 (−0.24; 0.05) |
| Low Informative | 5 (2; 7) | 6 (3; 7) | −0.1 (−0.31; 0.11) |
| Uninformative | 2 (0; 6) | 3 (0; 6) | −0.11 (−0.52; 0.32) |
| Authors, Journal | ID, Registration Date, Acronym (If Available) | Country | Trial Design | Primary Endpoint | Patients (n) | Dose | Outcome/Side Effects |
|---|---|---|---|---|---|---|---|
| Rossi G.P. et al. Submitted [30] | NCT04352400 Posted April 2020 RACONA | Italy | Prospective randomized, double-blind, placebo-controlled | Clinical efficacy and safety | Target number: 256 Enrolled: 15 | 0.10 mg/kg/h i.v. for 7 days | No adverse events, including no hyperkalemia |
| Quinn T.M. et al. eBioMedicine 2022 [31] | NCT04473053 Posted 16 July 2020 DEFINE | Scotland | Prospective, open label, controlled, multicenter | Safety and tolerability | In-hospital patients (21 vs. 21 SoC) | 0.2 mg/Kg/h i.v. for 7 days | No serious adverse events. Hyperkalemia (14.3% of patients) and higher plasma creatinine levels (mean difference 10.57 umol/L, 95% CI 2.43–18.92). |
| Unpublished No available results [32] | CTRI/2020/06/026220 Posted July 2020 | India | Prospective, randomized open-label, controlled | Proportion of patients with clinical improvement at day 14 | In-hospital patients | 0.1 mg/kg/h i.v. for 10 days | N.A. |
| Zhuravel S.V. et al. EClinical Medicine 2021 [24] | NCT04623021 Posted 10 November 2020 | Russia | Prospective, randomized open-label (nafamostat vs. SOC), multicenter | Time to clinical improvement | In-hospital patients * (53 vs. 51 vs. SOC) | 0.2 mg/kg/h for 10 days or discharge | No overall difference in primary endpoint. In high-risk patients (NEWS > 7) nafamostat was superior to SOC (11 vs. 14 days; RR, 2.89; 95% CI, 1.17 to 7.14; p = 0.012). No serious adverse events. Phlebitis (13% vs. 3%) |
| Unpublished No available results [33] | NCT04628143 Posted 13 November 2020 | South Korea | Prospective, open-label, controlled | Time to clinical improvement | In-hospital patients vs. SOC (total 31) | N.A. | N.A. |
| Soma T. et al. Jpn J Infect Dis 2022 [34] | N.A. | Japan | Retrospective single centre | Clinical deterioration | Moderate-risk patients ** (31 vs. 33 conservative supportive treatment) | 0.06–0.2 mg/Kg/h i.v. for 5 days | No differences in deterioration or death Hyperkalemia, i.e., >5 mmol/L, (21% vs. unknown) No need additional treatment after discontinuation |
| Inokuchi R. et al. J Clin Med 2022 [35] | N.A. | Japan | Retrospective observational | Mortality | In-hospital patients (121 vs. 15859) | N.A. | No difference in mortality. No side effects reported. |
| ID Registration | Country | Trial Design | Primary Outcome Measure | Patients (Condition, Dose, Target Number and Control) |
|---|---|---|---|---|
| NCT05555641 27 September 2022 | Wuhan, Hubei, China, | Randomized Parallel Assignment | Incidence of severe bleeding | ECMO anticoagulated critically ill patients Dose and target number N.A. Unfractionated heparin |
| NCT04390594 15 May 2020 | Senegal | Randomized Parallel Assignment | SARS-CoV-2 viral load level at day 7 | In-hospital patients Dose: 0.1–0.2 mg/kg/h Target number: total 186 SOC |
| NCT04483960 23 July 2020 ASCOT ADAPT | Melbourne | Randomized Factorial assignment Open label | Death from any cause or requirement of new intensive respiratory support or vasopressor/inotropic support | In-hospital patients Dose: 0.2mg/kg/hour Target number: N.A. Enoxaparin, dalteparin or tinzaparin |
| NCT04871646 4 May 2021 | Republic of Korea | Double-blind Randomized Parallel Assignment Multicenter | Time to recovery | In-hospital patients Dose: N.A. Target number: N.A. SOC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seccia, T.M.; Shagjaa, T.; Morpurgo, M.; Caroccia, B.; Sanga, V.; Faoro, S.; Venturini, F.; Iadicicco, G.; Lococo, S.; Mazzitelli, M.; et al. RAndomized Clinical Trial Of NAfamostat Mesylate, A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia. J. Clin. Med. 2023, 12, 6618. https://doi.org/10.3390/jcm12206618
Seccia TM, Shagjaa T, Morpurgo M, Caroccia B, Sanga V, Faoro S, Venturini F, Iadicicco G, Lococo S, Mazzitelli M, et al. RAndomized Clinical Trial Of NAfamostat Mesylate, A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia. Journal of Clinical Medicine. 2023; 12(20):6618. https://doi.org/10.3390/jcm12206618
Chicago/Turabian StyleSeccia, Teresa Maria, Tungalagtamir Shagjaa, Margherita Morpurgo, Brasilina Caroccia, Viola Sanga, Sonia Faoro, Francesca Venturini, Girolama Iadicicco, Sara Lococo, Maria Mazzitelli, and et al. 2023. "RAndomized Clinical Trial Of NAfamostat Mesylate, A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia" Journal of Clinical Medicine 12, no. 20: 6618. https://doi.org/10.3390/jcm12206618
APA StyleSeccia, T. M., Shagjaa, T., Morpurgo, M., Caroccia, B., Sanga, V., Faoro, S., Venturini, F., Iadicicco, G., Lococo, S., Mazzitelli, M., Farnia, F., Fioretto, P., Kobayashi, Y., Gregori, D., & Rossi, G. P. (2023). RAndomized Clinical Trial Of NAfamostat Mesylate, A Potent Transmembrane Protease Serine 2 (TMPRSS2) Inhibitor, in Patients with COVID-19 Pneumonia. Journal of Clinical Medicine, 12(20), 6618. https://doi.org/10.3390/jcm12206618








