Impact of an Oral Hygiene Intervention in People with and without Dementia on Oral Health Parameters—Results from the Oral Health, Bite Force, and Dementia (OrBiD) Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Study Intervention
2.3. Measurements
2.4. Statistical Analysis
2.5. Ethical Consideration
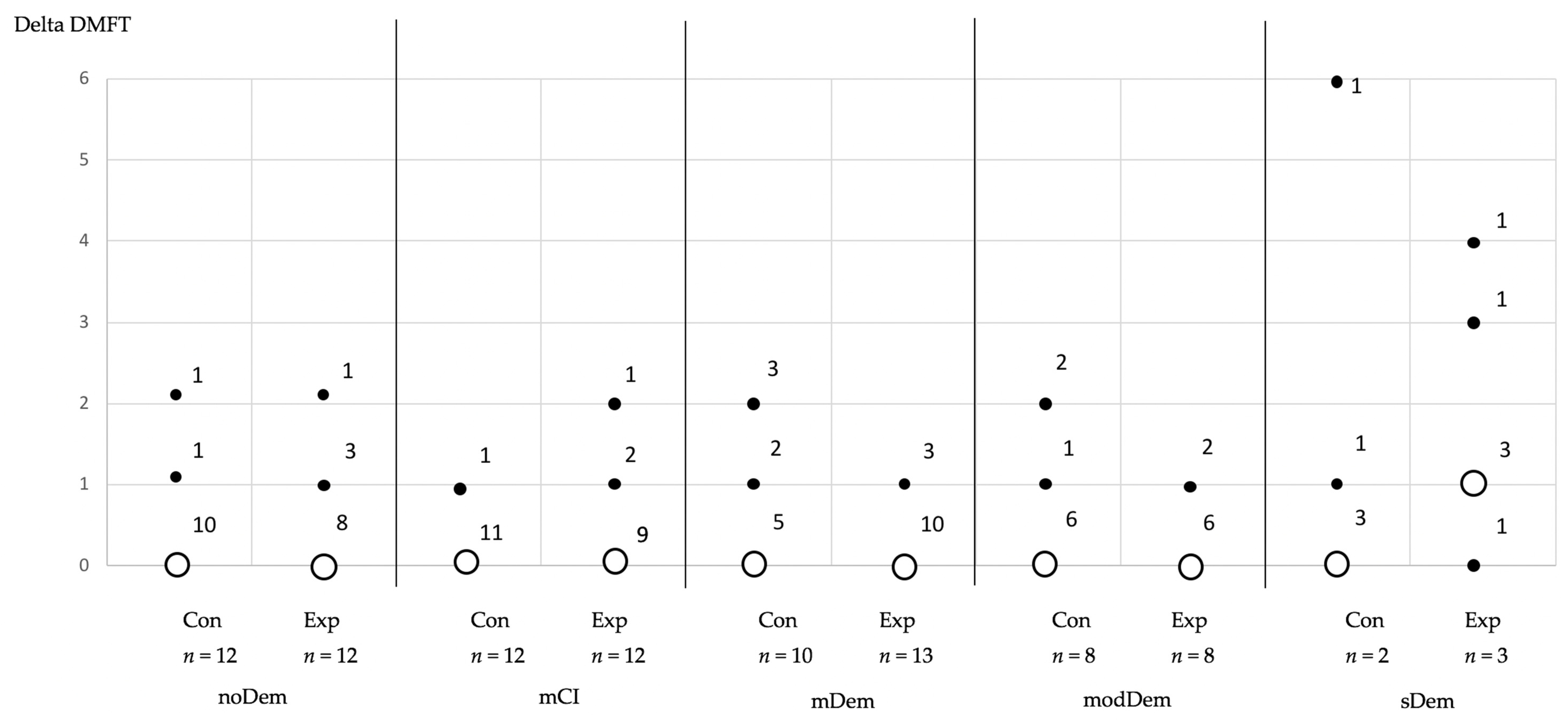
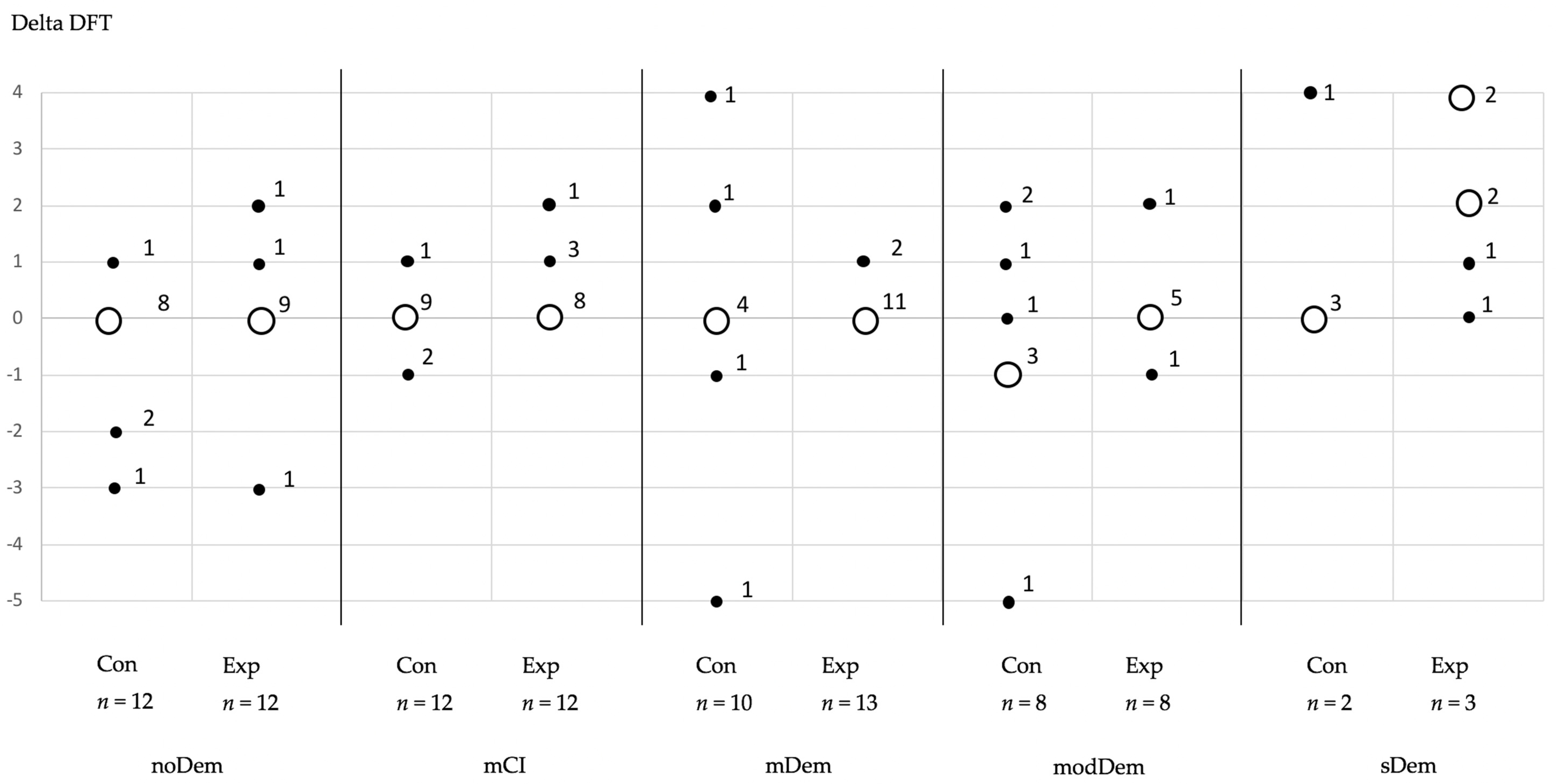
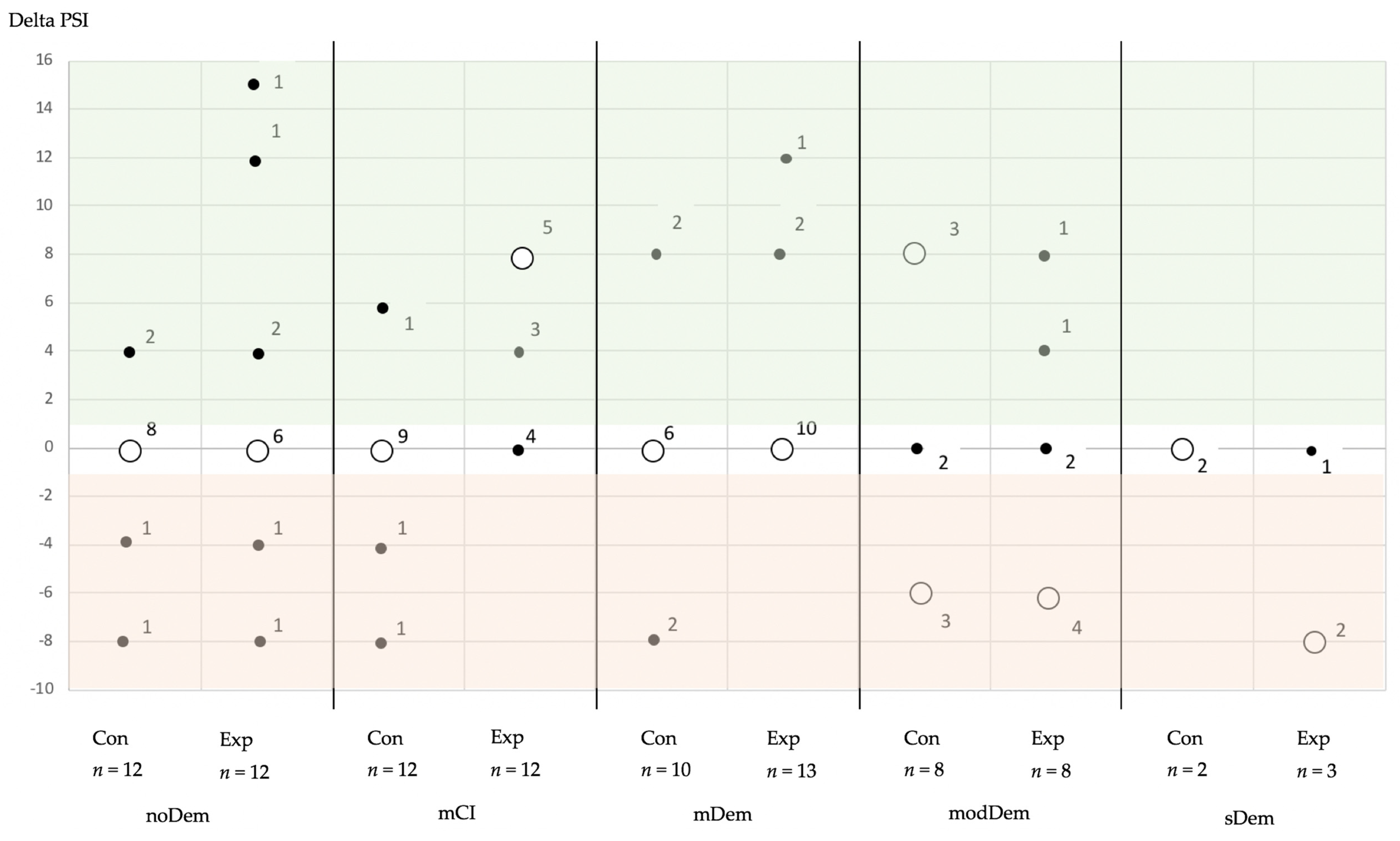
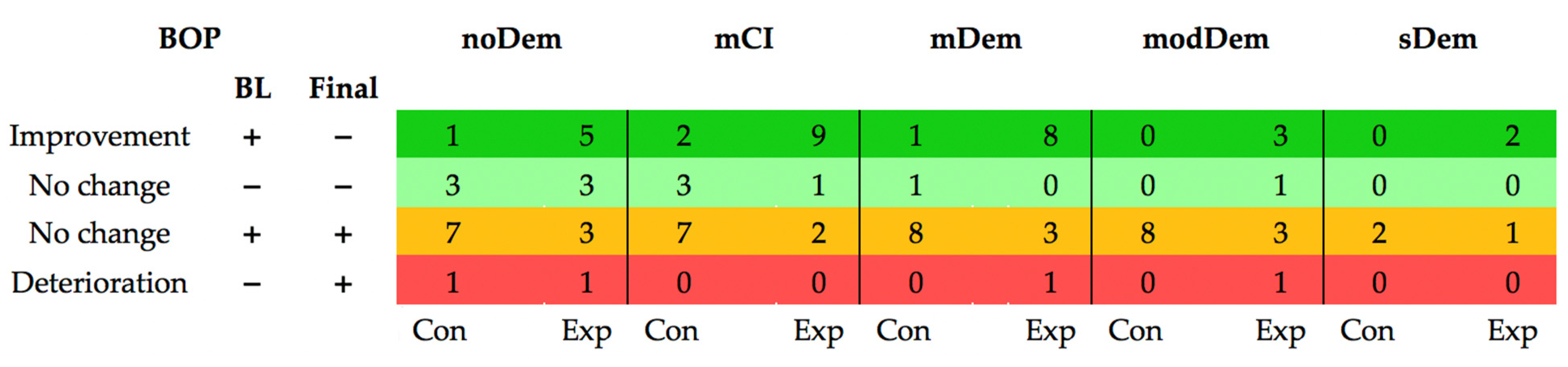
3. Results
Longitudinal Alterations of the Outcome Parameters
4. Discussion
4.1. Limitations of the Study
4.2. Discussion of the Results
4.3. Future Perspective
- Ensuring that the dentist is called upon in cases of reduced mobility and cases of incipient vulnerability, by implementing mobile dental concepts;
- Referral of patients with an initial diagnosis of dementia by the physician to the dentist, enabling the early admission of older vulnerable patients for dental care and treatment including prevention;
- Educational programs for relatives on the topic of oral health in old age—as well as its relevance in dementia;
- Strengthening of interdisciplinary cooperation with medical doctors, LTCF, nutritionists, etc., and the establishment of multidisciplinary health care teams [47];
- A combination of comprehensive theoretical and practical training of nurses (according to the concept of “teach the teacher”) and relatives, thus enabling a reduction in psychological barriers to oral care;
- Reduction in barriers to the implementation of training concepts in the long-term care sector and an increase in financial support for the implementation of support measures, both from the state and from other cost units (e.g., health insurance companies);
- Improvement of evaluation programs, which are used in the care sector to estimate the need for care, to enhance dental problem detection [18];
- Prompt admission of patients when a need for treatment is diagnosed, to avoid anesthesia;
- Minimum 6-monthly dental recalls and the professional cleaning of teeth and dentures [47];
- Improvement of individual oral hygiene at home by setting up individual oral care programs (e.g., the use of toothpaste with a high fluoride content, etc.).
5. Conclusions
6. Clinical Relevance
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista. Geschätzte Prävalenz von Demenz in Ausgewählten OECD-Ländern je 1.000 Einwohner in den Jahren 2021 und 2050. 2022. Available online: https://de.statista.com/statistik/daten/studie/182591/umfrage/demenz-praevalenz-in-europa/ (accessed on 7 February 2022).
- DESTASTIS. Pflegestatistik 2015–Pflege im Rahmen der Pflegeversicherung–Deutschlandergebnisse; Statistisches Bundesamt: Wiesbaden, Germany, 2017.
- Nitschke, I. Zur Mundgesundheit von Pflegebedürftigen und Menschen mit Behinderung in Deutschland–Eine Systematische Übersicht (Review) auf der Grundlage aktueller Einzelstudien (2000–2012); IDZ-Information: Köln, Germany, 2012; p. 3. Available online: https://www.idz.institute/publikationen/idz-information/zur-mundgesundheit-von-pflegebeduerftigen-und-menschen-mit-behinderungen-in-deutschland.html (accessed on 6 February 2022).
- Nitschke, I.; Hahnel, S.; Jockusch, J. Health-Related Social and Ethical Considerations towards the Utilization of Dental Medical Services by Seniors: Influencing and Protective Factors, Vulnerability, Resilience and Sense of Coherence. Int. J. Environ. Res. Public Health 2021, 18, 2048. [Google Scholar] [CrossRef]
- Chalmers, J.; Pearson, A. Oral hygiene care for residents with dementia: A literature review. J. Adv. Nurs. 2005, 52, 410–419. [Google Scholar] [CrossRef]
- Delwel, S.; Binnekade, T.T.; Perez, R.S.; Hertogh, C.M.; Scherder, E.J.; Lobbezoo, F. Oral health and orofacial pain in older people with dementia: A systematic review with focus on dental hard tissues. Clin. Oral. Investig. 2017, 21, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Dolan, T.A.; Atchison, K.A. Implications of access, utilization and need for oral care by the non-institutionalized and institutionalized elderly in the dental delivery system. J. Dent. Educ. 1993, 57, 876–887. [Google Scholar] [CrossRef]
- Jones, J.A.; Lavallee, N.; Alman, J.; Sinclair, C.; Garcia, R.I. Caries incidence in patients with dementia. Gerodontology 1993, 10, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ship, J.A. Oral health of patients with Alzheimer’s disease. J. Am. Dent. Assoc. 1992, 123, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Rejnefelt, I.; Andersson, P.; Renvert, S. Oral health status in individuals with dementia living in special facilities. Int. J. Dent. Hyg. 2006, 4, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.G.; Wekstein, D.R. Providing dental care for patients diagnosed with Alzheimer’s disease. Dent. Clin. N. Am. 1997, 41, 915–943. [Google Scholar] [PubMed]
- Farias, I.P.S.E.; Sousa, S.A.; Almeida, L.F.D.; Santiago, B.M.; Pereira, A.C.; Cavalcanti, Y.W. Does non-institutionalized elders have a better oral health status compared to institutionalized ones? A systematic review and meta-analysis. Cienc. Saude Colet. 2020, 25, 2177–2192. [Google Scholar] [CrossRef] [PubMed]
- Jockusch, J.; Hopfenmüller, W.; Nitschke, I. Influence of cognitive impairment and dementia on oral health and the utilization of dental services: Findings of the Oral Health, Bite force and Dementia Study (OrBiD). BMC Oral Health 2021, 21, 399. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, I.; Stillhart, A.; Kunze, J. Utilization of dental services in old age. Swiss Dent. J. 2015, 125, 433–447. [Google Scholar] [PubMed]
- Adams, R. Qualified nurses lack adequate knowledge related to oral health, resulting in inadequate oral care of patients on medical wards. J. Adv. Nurs. 1996, 24, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Logan, H.L.; Ettinger, R.; McLeran, H.; Casko, R.; Dal Secco, D. Common misconceptions about oral health in the older adult: Nursing practices. Spec. Care Dentist. 1991, 11, 243–247. [Google Scholar] [CrossRef]
- Nitschke, I.; Majdani, M.; Sobotta, B.A.; Reiber, T.; Hopfenmüller, W. Dental care of frail older people and those caring for them. J. Clin. Nurs. 2010, 19, 1882–1890. [Google Scholar] [CrossRef]
- Jockusch, J.; Hopfenmüller, W.; Sobotta, B.A.J.; Nitschke, I. Interrater reliability and concurrent validity of oral/dental items in the resident assessment instrument minimum data set 2.0. Gerodontology 2021, 38, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, I.; Ramm, C.; Schrock, A. Mundgesundheit bei Demenz: Ergebnisse einer telefonischen Beratungsstelle [Oral health in dementia: Results of a telephone helpline]. Z Gerontol. Geriatr. 2015, 48, 550–556. (In German) [Google Scholar] [CrossRef] [PubMed]
- Jockusch, J.; Hopfenmüller, W.; Ettinger, R.; Nitschke, I. Outpatient, dental care of adult vulnerable patients under general anaesthesia-a retrospective evaluation of need for treatment and dental follow-up care. Clin. Oral Investig. 2020, 25, 2407–2417. [Google Scholar] [CrossRef]
- Bassim, C.W.; Gibson, G.; Ward, T.; Paphides, B.M.; Denucci, D.J. Modification of the risk of mortality from pneumonia with oral hygiene care. J. Am. Geriatr. Soc. 2008, 56, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Manchery, N.; Subbiah, G.K.; Nagappan, N.; Premnath, P. Are oral health education for carers effective in the oral hygiene management of elderly with dementia? A systematic review. Dent. Res. J. 2020, 17, 1–9. [Google Scholar] [CrossRef]
- Albrecht, M.; Kupfer, R.; Reissmann, D.R.; Mühlhauser, I.; Köpke, S. Oral health educational interventions for nursing home staff and residents. Cochrane Database Syst. Rev. 2016, 9, CD010535. [Google Scholar] [CrossRef]
- Jockusch, J.; Hopfenmüller, W.; Nitschke, I. Chewing function and related parameters as a function of the degree of dementia: Is there a link between the brain and the mouth? J. Oral Rehabil. 2021, 48, 1160–1172. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Rubenstein, L.Z.; Harker, J.O.; Salvà, A.; Guigoz, Y.; Vellas, B. Screening for undernutrition in geriatric practice: Developing the short-form mini-nutritional assessment (MNA-SF). J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M366–M372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitschke, I.; Kunze, J.; Hopfenmüller, W.; Reiber, T. Die zahnmedizinische funktionelle Kapazität–ein Instrument in der Gerostomatologie. Quintessenz 2012, 63, 207–210. [Google Scholar]
- WHO. Oral Health Surveys: Basic Methods; World Health Organization: Geneva, Switzerland, 2013.
- Nitschke, I.; Micheelis, W. Krankheits-und Versorgungsprävalenzen bei Älteren Senioren mit Pflegebedarf. Fünfte Dtsch. Mundgesundheitsstudie 2016, 39, 557. [Google Scholar]
- Charles, C.; Charles, A. Periodontal screening and recording. J. Calif. Dent. Assoc. 1994, 22, 43–46. [Google Scholar] [PubMed]
- Greene, J.G.; Vermillion, J.R. The simplified oral hygiene index. J. Am. Dent. Assoc. 1964, 68, 7–13. [Google Scholar] [CrossRef]
- Teare, M.D.; Dimairo, M.; Shephard, N.; Hayman, A.; Whitehead, A.; Walters, S.J. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: A simulation study. Trials 2014, 15, 264. [Google Scholar] [CrossRef] [PubMed]
- Malekmahmoodi, M.; Shamsi, M.; Roozbahani, N.; Moradzadeh, R. A randomized controlled trial of an educational intervention to promote oral and dental health of patients with type 2 diabetes mellitus. BMC Public Health 2020, 20, 287. [Google Scholar] [CrossRef] [Green Version]
- IBM SPSS Statistics for Windows, Version 23; IBM Corp: Armonk, NY, USA; Chicago, IL, USA, 2014.
- Jablonski, R.A.; Kolanowski, A.M.; Azuero, A.; Winstead, V.; Jones-Townsend, C.; Geisinger, M.L. Randomised clinical trial: Efficacy of strategies to provide oral hygiene activities to nursing home residents with dementia who resist mouth care. Gerodontology 2018, 35, 365–375. [Google Scholar] [CrossRef]
- Aagaard, K.; Meléndez-Torres, G.J.; Overgaard, C. Improving oral health in nursing home residents: A process evaluation of a shared oral care intervention. J. Clin. Nurs. 2020, 29, 3392–3402. [Google Scholar] [CrossRef] [PubMed]
- Perneczky, R.; Wagenpfeil, S.; Komossa, K.; Grimmer, T.; Diehl, J.; Kurz, A. Mapping scores onto stages: Mini-mental state examination and clinical dementia rating. Am. J. Geriatr. Psychiatry 2006, 14, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Hopcraft, M.S.; Morgan, M.V.; Satur, J.G.; Wright, F.A. Utilizing dental hygienists to undertake dental examination and referral in residential aged care facilities. Community Dent. Oral Epidemiol. 2011, 39, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Schimmel, M.; Riesen, M.; Ilgner, A.; Wicht, M.J.; Warncke, M.; Ellwood, R.P.; Nitschke, I.; Müller, F.; Noack, M.J. High-fluoride toothpaste: A multicenter randomized controlled trial in adults. Community Dent. Oral Epidemiol. 2014, 42, 333–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmerman, S.; Sloane, P.D.; Ward, K.; Wretman, C.J.; Stearns, S.C.; Poole, P.; Preisser, J.S. Effectiveness of a Mouth Care Program Provided by Nursing Home Staff vs Standard Care on Reducing Pneumonia Incidence: A Cluster Randomized Trial. JAMA Netw. Open 2020, 3, e204321. [Google Scholar] [CrossRef]
- Pearson, A.; Chalmers, J. Oral hygiene care for adults with dementia in residential aged care facilities. JBI Libr. Syst. Rev. 2004, 2, 1–89. [Google Scholar] [CrossRef] [PubMed]
- Nihtilä, A.; Tuuliainen, E.; Komulainen, K.; Autonen-Honkonen, K.; Nykänen, I.; Hartikainen, S.; Ahonen, R.; Tiihonen, M.; Suominen, A.L. Preventive oral health intervention among old home care clients. Age Ageing 2017, 46, 846–851. [Google Scholar] [CrossRef] [Green Version]
- Locker, D.; Matear, D.; Stephens, M.; Jokovic, A. Oral health-related quality of life of a population of medically compromised elderly people. Community Dent. Health 2002, 19, 90–97. [Google Scholar]
- Canadian Dental Association. Optimal Health for Frail Older Adults: Best Practices along the Continuum of Care; Canadian Dental Association: Ottawa, ON, Canada, 2009. [Google Scholar]
- Chalmers, J.M. Minimal intervention dentistry: Part 1. Strategies for addressing the new caries challenge in older patients. J. Can. Dent. Assoc. 2006, 72, 427–433. [Google Scholar]
- Nicol, R.; Petrina Sweeney, M.; McHugh, S.; Bagg, J. Effectiveness of health care worker training on the oral health of elderly residents of nursing homes. Community Dent. Oral Epidemiol. 2005, 33, 115–124. [Google Scholar] [CrossRef]
- Rozas, N.S.; Sadowsky, J.M.; Jeter, C.B. Strategies to improve dental health in elderly patients with cognitive impairment: A systematic review. J. Am. Dent. Assoc. 2017, 148, 236–245.e3. [Google Scholar] [CrossRef]
| Item | noDem | mCI | mDem | modDem | sDem | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Con (n = 12) | Exp (n = 12) | Con (n = 12) | Exp (n = 12) | Con (n = 10) | Exp (n = 13) | Con (n = 9) | Exp (n = 8) | Con (n = 5) | Exp (n = 6) | |||||||||||
| Sex (female) (n) | 7 | 6 | 8 | 8 | 6 | 12 | 6 | 6 | 4 | 5 | ||||||||||
| Age (Median (Range)) | 74.5 (63–83) | 76 (62–92) | 78 (65–94) | 83 (61–95) | 85.5 (65–95) | 82 (71–93) | 91 (76–99) | 86 (61–98) | 78 (67–94) | 90.5 (86–93) | ||||||||||
| MMSE (Median (Range)) | 29 (28–30) | 29 (28–30) | 26.5 (25–27) | 27 (25–27) | 21.5 (18–24) | 21 (18–24) | 13 (10–17) | 12.5 (10–17) | 5 (1–9) | 3.5 (0–9) | ||||||||||
| Living situation (n) | ||||||||||||||||||||
| Community-dwelling | 9 | 12 | 7 | 7 | 2 | 2 | 0 | 0 | 0 | 0 | ||||||||||
| LTCF | 3 | 0 | 5 | 5 | 8 | 11 | 9 | 8 | 5 | 6 | ||||||||||
| MNA (n) | BL | Final | BL | Final | BL | Final | BL | Final | BL | Final | BL | Final | BL | Final | BL | Final | BL | Final | BL | Final |
| n = 12 | n = 12 | n = 12 | n = 12 | n = 12 | n = 12 | n = 12 | n = 12 | n = 10 | n = 10 | n = 13 | n = 13 | n = 7 | n = 8 | n = 8 | n = 6 | n = 5 | n = 5 | n = 5 | n = 6 | |
| Normal (24–30) | 10 | 10 | 10 | 11 | 8 | 9 | 7 | 10 | 5 | 6 | 4 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| At risk (17–23.5) | 1 | 2 | 2 | 1 | 4 | 3 | 5 | 2 | 5 | 3 | 7 | 9 | 5 | 4 | 5 | 5 | 3 | 3 | 2 | 2 |
| Malnourished (<17) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 2 | 4 | 2 | 1 | 2 | 2 | 3 | 4 |
| Oral functional capacity (n) | BL n = 12 | Final n = 12 | BL n = 12 | Final n = 12 | BL n = 12 | Final n = 12 | BL n = 12 | Final n = 12 | BL n = 10 | Final n = 10 | BL n = 13 | Final n = 13 | BL n = 9 | Final n = 9 | BL n = 8 | Final n = 8 | BL n = 5 | Final n = 5 | BL n = 6 | Final n = 6 |
| Therapeutic capability (n) | ||||||||||||||||||||
| Normal | 10 | 10 | 9 | 9 | 6 | 5 | 6 | 5 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Slightly reduced | 2 | 2 | 3 | 3 | 6 | 6 | 6 | 7 | 5 | 2 | 2 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| Greatly reduced | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 4 | 7 | 10 | 8 | 8 | 7 | 6 | 6 | 2 | 1 | 1 | 0 |
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 1 | 2 | 1 | 2 | 3 | 4 | 4 | 6 |
| Oral hygiene ability (n) | ||||||||||||||||||||
| Normal | 8 | 9 | 8 | 9 | 3 | 3 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Slightly reduced | 4 | 3 | 4 | 3 | 8 | 8 | 7 | 8 | 3 | 2 | 3 | 4 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Greatly reduced | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 2 | 6 | 5 | 7 | 6 | 8 | 5 | 5 | 5 | 2 | 1 | 1 | 0 |
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 3 | 3 | 1 | 4 | 2 | 3 | 3 | 4 | 5 | 6 |
| Self-responsibility (n) | ||||||||||||||||||||
| Normal | 11 | 10 | 12 | 12 | 9 | 9 | 7 | 6 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Reduced | 1 | 2 | 0 | 0 | 3 | 2 | 5 | 6 | 5 | 1 | 5 | 6 | 4 | 2 | 3 | 0 | 0 | 0 | 0 | 0 |
| None | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 7 | 8 | 7 | 5 | 7 | 5 | 8 | 5 | 5 | 6 | 6 |
| Resilience capacity level (n) | ||||||||||||||||||||
| Normal | 8 | 8 | 6 | 8 | 3 | 2 | 2 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Slightly reduced | 4 | 4 | 6 | 4 | 7 | 9 | 6 | 6 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Greatly reduced | 0 | 0 | 0 | 0 | 2 | 0 | 4 | 4 | 5 | 1 | 5 | 6 | 4 | 2 | 3 | 0 | 0 | 0 | 0 | 0 |
| None | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 7 | 8 | 7 | 5 | 7 | 5 | 8 | 5 | 5 | 6 | 6 |
| Item | noDem | mCI | mDem | modDem | sDem | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Con (n = 12) | Exp (n = 12) | Con (n = 12) | Exp (n = 12) | Con (n = 10) | Exp (n = 13) | Con (n = 9) | Exp (n = 8) | Con (n = 5) | Exp (n = 6) | ||
| DMFT (related to 32 teeth) | |||||||||||
| BL | Median (Range) Mean ± SD | 29.5 (21–32) 28.5 ± 3.7 | 28 (18–32) 27.3 ± 3.8 | 28.5 (20–32) 28.4 ± 3.3 | 26 (17–32) 25.3 ± 4.4 | 27 (23–31) 26.7 ± 2.7 | 29 (20–32) 27.2 ± 3.6 | 30 (19–32) 28 ± 4.1 | 25.5 (20–32) 26.8 ± 4.5 | 18 (14–27) 19.4 ± 5.5 | 25.5 (17–31) 24.5 ± 4.7 |
| Final | Median (Range) Mean ± SD | 30 (21–32) 28.8 ± 3.6 | 28 (18–32) 27.7 ± 3.8 | 29 (20–32) 28.5 ± 3.3 | 26 (17–32) 25.6 ± 4.3 | 28 (23–32) 27.5 ± 2.9 | 29 (21–30) 27.4 ± 3.5 | 30 (19–32) 28.6 ± 4.4 | 26 (21–32) 27 ± 4.2 | 21 (14–27) 20.8 ± 5.1 | 26 (21–32) 26.2 ± 4 |
| Δ DMFT (related to 32 teeth) | |||||||||||
| Median (Range) Mean ± SD | 0 (0–2) 0.3 ± 0.6 | 0 (0–2) 0.4 ± 0.7 | 0 (0–1) 0.1 ± 0.3 | 0 (0–2) 0.3 ± 0.7 | 0 (0–1) 0.2 ± 0.4 | 0.5 (0–2) 0.8 ± 0.9 | 0 (0–2) 0.6 ± 0.9 | 0 (0–1) 0.3 ± 0.5 | 0 (0–6) 1.4 ± 2.6 | 1 (0–4) 1.7 ± 1.5 | |
| DT | |||||||||||
| BL | Median (Range) | 0 (0–1) | 0 (0–1) | 0 (0–6) | 0 (0–2) | 0.5 (0–8) | 0 (0–4) | 1 (0–7) | 0.5 (0–3) | 1 (0–6) | 0 (0–14) |
| Final | Median (Range) | 0 (0–3) | 0 (0–0) | 0 (0–6) | 0 (0–2) | 1.5 (0–12) | 0 (0–4) | 1 (0–8) | 0.5 (0–2) | 1 (0–6) | 5 (0–19) |
| MT | |||||||||||
| BL | Median (Range) | 21.5 (8–29) | 17.5 (4–24) | 16 (4–30) | 12 (4–28) | 13.5 (3–23) | 10 (4–27) | 13 (4–25) | 12 (5–21) | 7 (0–22) | 8 (6–13) |
| Final | Median (Range) | 21.5 (9–30) | 18.5 (4–24) | 16 (4–30) | 11.5 (4–28) | 14 (3–25) | 10 (4–27) | 11 (5–26) | 12 (5–22) | 9 (0–22) | 7.5 (6–10) |
| FT | |||||||||||
| BL | Median (Range) | 6 (3–18) | 10 (5–21) | 10 (2–20) | 12 (0–22) | 13.5 (3–19) | 16 (2–24) | 10 (4–22) | 13 (6–24) | 8 (1–17) | 16.5 (3–20) |
| Final | Median (Range) | 6 (1–16) | 10 (5–23) | 10 (2–21) | 13.5 (2–22) | 12 (3–19) | 16 (1–24) | 9 (0–25) | 13 (6–23) | 12 (1–17) | 14 (3–20) |
| DFT | |||||||||||
| BL | Median (Range) | 6 (3–18) | 10 (5–21) | 13 (2–21) | 12.5 (1–22) | 14 (4–20) | 16 (2–24) | 13 (5–26) | 13.5 (7–24) | 8 (5–18) | 17.5 (8–20) |
| Final | Median (Range) | 6 (2–16) | 10 (5–23) | 12.5 (2–22) | 13.5 (2–22) | 14.5 (3–20) | 16 (3–24) | 13 (6–25) | 14 (8–24) | 12 (5–18) | 19.5 (12–22) |
| Δ DFT (related to 32 teeth) | |||||||||||
| Median (Range) Mean ± SD | 0 (−4–1) −0.6 ± 1.4 | 0 (−3–2) 0 ± 1.1 | 0 (−1–1) −0.8 ± 0.5 | 0 (0–2) 0.4 ± 0.7 | 0 (−5–4) 0.2 ± 2.9 | 0 (0–1) 0.2 ± 0.4 | 0 (−5–2) −0.2 ± 2.2 | 0 (−1–1) 0.1 ± 0.6 | 0 (0–4) 0.8 ± 1.8 | 2 (0–4) 2.2 ± 1.6 | |
| Degree of Dementia | Subgroup | PSI Code—Final | Total | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1–2 | 3 | 4 | |||||
| noDem MMSE 28–30 | Con | PSI Code—Baseline | 1–2 | 2 | 1 | 0 | 3 | |
| 3 | 2 | 5 | 1 | 8 | ||||
| 4 | 0 | 0 | 1 | 1 | ||||
| Total | 4 | 6 | 2 | 12 | ||||
| Exp | PSI Code—Baseline | 1–2 | 0 | 1 | 1 | 0 | 2 | |
| 3 | 0 | 2 | 3 | 1 | 6 | |||
| 4 | 1 | 1 | 0 | 2 | 4 | |||
| Total | 1 | 4 | 4 | 3 | 12 | |||
| mCI MMSE 25–27 | Con | PSI Code—Baseline | 1–2 | 2 | 1 | 0 | 3 | |
| 3 | 1 | 5 | 1 | 7 | ||||
| 4 | 0 | 0 | 2 | 2 | ||||
| Total | 3 | 6 | 3 | 12 | ||||
| Exp | PSI Code—Baseline | 3 | 3 | 2 | 0 | 5 | ||
| 4 | 0 | 5 | 2 | 7 | ||||
| Total | 3 | 7 | 2 | 12 | ||||
| mDem MMSE 18–24 | Con | PSI Code—Baseline | 3 | 3 | 2 | 5 | ||
| 4 | 2 | 3 | 5 | |||||
| Total | 5 | 5 | 10 | |||||
| Exp | PSI Code—Baseline | 3 | 0 | 6 | 0 | 6 | ||
| 4 | 1 | 2 | 4 | 7 | ||||
| Total | 1 | 8 | 4 | 13 | ||||
| modDem MMSE 10–17 | Con | PSI Code—Baseline | 3 | 1 | 3 | 4 | ||
| 4 | 3 | 1 | 4 | |||||
| Total | 4 | 4 | 8 | |||||
| Exp | PSI Code—Baseline | 3 | 1 | 2 | 4 | 7 | ||
| 4 | 0 | 1 | 0 | 1 | ||||
| Total | 1 | 3 | 4 | 8 | ||||
| sDem MMSE < 10 | Con | PSI Code—Baseline | 4 | 2 | 2 | |||
| Total | 2 | 2 | ||||||
| Exp | PSI Code—Baseline | 3 | 2 | 2 | ||||
| 4 | 1 | 1 | ||||||
| Total | 3 | 3 | ||||||
| Item | noDem | mCI | mDem | modDem | sDem | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Con (n = 12) | Exp (n = 12) | Con (n = 12) | Exp (n = 12) | Con (n = 10) | Exp (n = 13) | Con (n = 9) | Exp (n = 8) | Con (n = 5) | Exp (n = 6) | ||
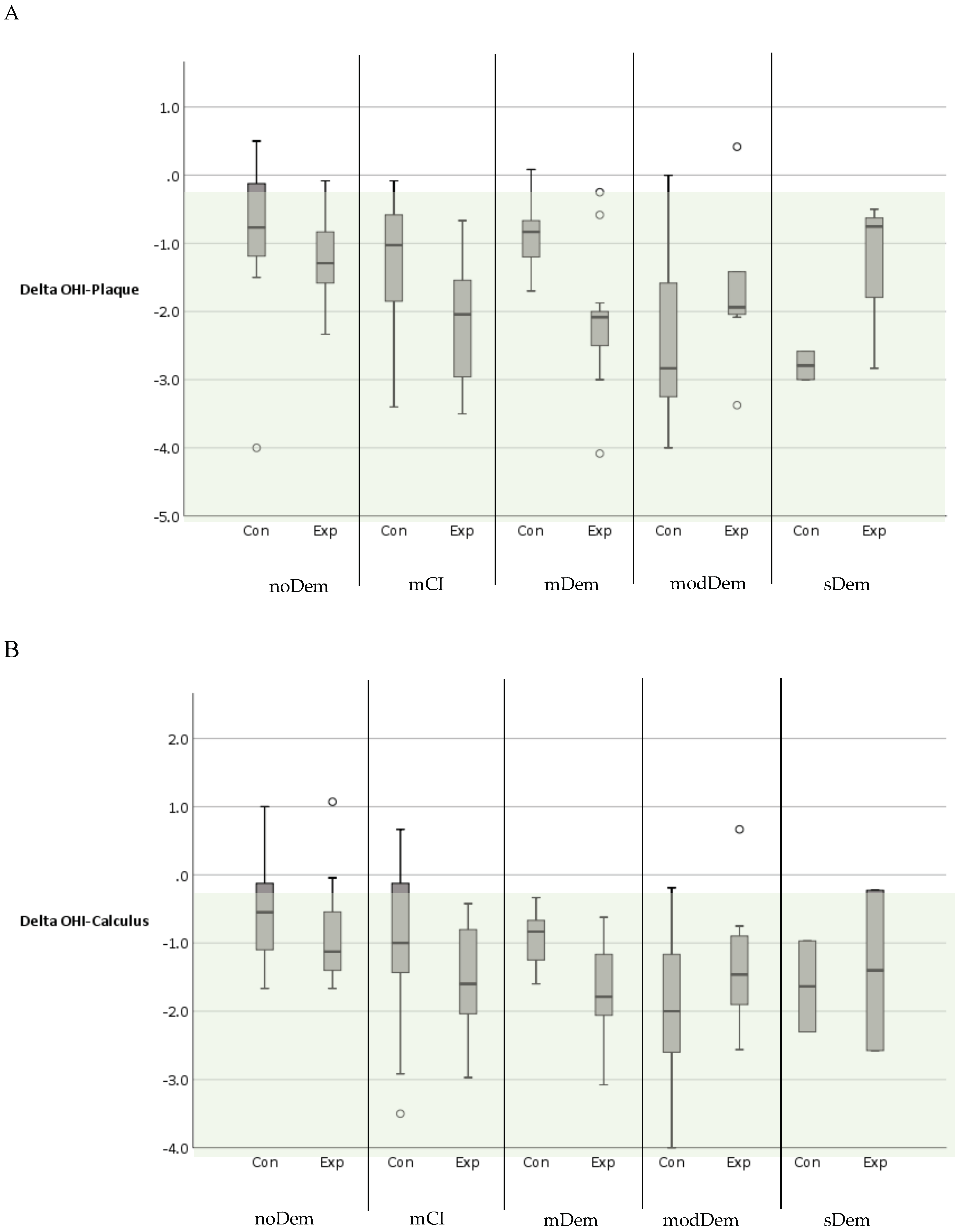
| OHI-Plaque (DI) | |||||||||||
| BL | n = 12 | n = 12 | n = 12 | n = 12 | n = 10 | n = 13 | n = 9 | n = 8 | n = 3 | n = 5 | |
| Median (Range) | 2 (0.7–5) | 1.9 (1–2.5) | 2.4 (0.8–4.5) | 2.8 (1.3–4.2) | 3.2 (1.8–3.8) | 3.7 (2–5.5) | 5 (2.4–6) | 3.8 (2–4.8) | 4.5 (2.5–5.3) | 3.3 (3–4.8) | |
| Final | n = 12 | n = 12 | n = 12 | n = 12 | n = 9 | n = 13 | n = 9 | n = 8 | n = 2 | n = 3 | |
| Median (Range) | 1.1 (0.5–2) | 0.3 (0–1.9) | 1.3 (0.3–2) | 0.5 (0.1–1.4) | 2.2 (0.3–3) | 2 (0–3) | 2.1 (1.3–2.4) | 2 (1.2–2.6) | 2.1 (1.5–2.8) | 2.4 (0.5–3) | |
| Delta | n = 12 | n = 12 | n = 12 | n = 12 | n = 9 | n = 13 | n = 9 | n = 8 | n = 2 | n = 3 | |
| Median | −0.8 | −1.3 | −1.0 | −2.0 | −0.8 | −2.1 | −2.8 | −1.9 | −2.8 | −0.8 | |
| Range | −4–0.5 | −2.3 to −0.1 | −3.4 to −0.1 | −3.5 to −0.7 | −1.7–0.1 | −4.1 to −0.2 | −4–0 | −3.4–0.4 | −3 to −2.6 | −2.8 to −0.5 | |
| OHI-Calculus (CI) | |||||||||||
| BL | n = 12 | n = 12 | n = 12 | n = 12 | n = 10 | n = 13 | n = 9 | n = 8 | n = 3 | n = 4 | |
| Median (Range) | 1.5 (0–3) | 1.6 (0.5–2.7) | 2 (0–4.2) | 1.9 (0.7–3.3) | 2.8 (1.3–3.7) | 3.2 (1.2–4.5) | 4 (2.2–6) | 2.7 (1.3–3.8) | 3.3 (2.3–3.7) | 3.3 (2.7–4) | |
| Final | n = 12 | n = 12 | n = 12 | n = 12 | n = 9 | n = 13 | n = 9 | n = 8 | n = 2 | n = 3 | |
| Median (Range) | 1.0 (0–1.8) | 0.4 (0–1.6) | 0.9 (0.5–1.7) | 0.4 (0–0.9) | 1.8 (0.3–3) | 1.6 (0–3) | 2 (1.1–2.2) | 1.4 (0.4–2.5) | 1.9 (1.4–2.4) | 2.2 (0.9–3.3) | |
| Delta | n = 12 | n = 12 | n = 12 | n = 12 | n = 9 | n = 13 | n = 9 | n = 8 | n = 2 | n = 2 | |
| Median | −0.6 | −1.1 | −1 | −1.6 | −0.8 | −1.8 | −2 | −1.5 | −1.6 | −1.4 | |
| Range | −1.7–1 | −1.7–1.1 | −3.5–0.7 | −3 to −0.4 | −1.6 to −0.3 | −3.1 to −0.6 | −4 to −0.2 | −2.6–0.7 | −2.3 to −1.0 | −2.6 to −0.2 | |
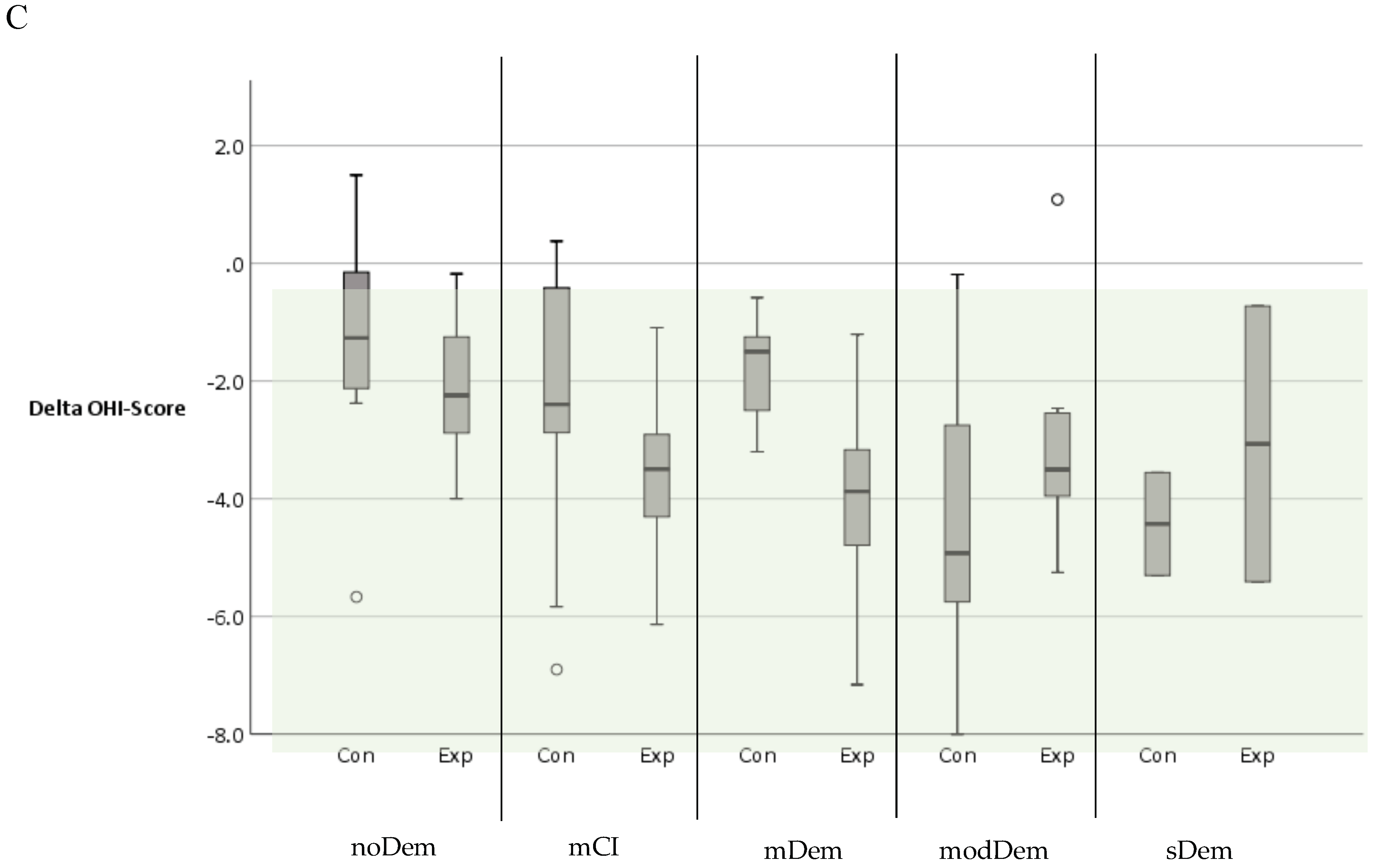
| OHI-Score | |||||||||||
| BL | n = 12 | n = 12 | n = 12 | n = 12 | n = 10 | n = 13 | n = 9 | n = 8 | n = 3 | n = 4 | |
| Median (Range) | 3.5 (1–7.7) | 3.3 (1.8–4.7) | 4.7 (1.7–8.7) | 4.8 (2.7–7.5) | 5.9 (3.2–7.4) | 6.7 (3.2–9.5) | 8.9 (4.7–12) | 7 (3.3–7.8) | 8.2 (4.8–8.7) | 6.5 (6–8.8) | |
| Final | n = 12 | n = 12 | n = 12 | n = 12 | n = 9 | n = 13 | n = 9 | n = 8 | n = 2 | n = 3 | |
| Median (Range) | 2.1 (0.8–3.8) | 0.7 (0–3.3) | 2.2 (0.8–3.3) | 1.1 (0.3–2.3) | 4.1 (0.7–6) | 3.1 (0–6) | 4 (2.4–4.5) | 3.6 (1.8–5.1) | 4 (2.9–5.1) | 4.6 (0.6–6.3) | |
| Delta | n = 12 | n = 12 | n = 12 | n = 12 | n = 9 | n = 13 | n = 9 | n = 8 | n = 2 | n = 2 | |
| Median | −1.3 | −2.2 | −2.4 | −3.5 | −1.5 | −3.9 | −4.9 | −3.5 | −4.4 | −3.1 | |
| Range | −5.7–1.5 | −4 to −0.2 | −6.9–0.4 | −6.1 to −1.1 | −3.2 to −0.6 | −7.2 to −1.2 | −8 to −0.2 | −5.2–1.1 | −5.3 to −3.6 | −5.4 to −0.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jockusch, J.; Nitschke, S.; Hopfenmüller, W.; Schierz, O.; Hahnel, S.; Nitschke, I. Impact of an Oral Hygiene Intervention in People with and without Dementia on Oral Health Parameters—Results from the Oral Health, Bite Force, and Dementia (OrBiD) Pilot Study. J. Clin. Med. 2022, 11, 1356. https://doi.org/10.3390/jcm11051356
Jockusch J, Nitschke S, Hopfenmüller W, Schierz O, Hahnel S, Nitschke I. Impact of an Oral Hygiene Intervention in People with and without Dementia on Oral Health Parameters—Results from the Oral Health, Bite Force, and Dementia (OrBiD) Pilot Study. Journal of Clinical Medicine. 2022; 11(5):1356. https://doi.org/10.3390/jcm11051356
Chicago/Turabian StyleJockusch, Julia, Siri Nitschke, Werner Hopfenmüller, Oliver Schierz, Sebastian Hahnel, and Ina Nitschke. 2022. "Impact of an Oral Hygiene Intervention in People with and without Dementia on Oral Health Parameters—Results from the Oral Health, Bite Force, and Dementia (OrBiD) Pilot Study" Journal of Clinical Medicine 11, no. 5: 1356. https://doi.org/10.3390/jcm11051356
APA StyleJockusch, J., Nitschke, S., Hopfenmüller, W., Schierz, O., Hahnel, S., & Nitschke, I. (2022). Impact of an Oral Hygiene Intervention in People with and without Dementia on Oral Health Parameters—Results from the Oral Health, Bite Force, and Dementia (OrBiD) Pilot Study. Journal of Clinical Medicine, 11(5), 1356. https://doi.org/10.3390/jcm11051356






