Abstract
(1) Background: Vestibular migraine (VM) and Meniere’s disease (MD) share multiple features in terms of clinical presentations and auditory-vestibular dysfunctions, e.g., vertigo, hearing loss, and headache. Therefore, differentiation between VM and MD is of great significance. (2) Methods: We retrospectively analyzed the medical records of 110 patients with VM and 110 patients with MD. We at first established a regression equation by using logistic regression analysis. Furthermore, sensitivity, specificity, accuracy, positive predicted value (PV), and negative PV of screened parameters were assessed and intuitively displayed by receiver operating characteristic curve (ROC curve). Then, two visualization tools, i.e., nomograph and applet, were established for convenience of clinicians. Furthermore, other patients with VM or MD were recruited to validate the power of the equation by ROC curve and the Gruppo Italiano per la Valutazione degli Interventi in Terapia Intensiva (GiViTI) calibration belt. (3) Results: The clinical manifestations and auditory-vestibular functions could help differentiate VM from MD, including attack frequency (X5), phonophobia (X13), electrocochleogram (ECochG) (X18), head-shaking test (HST) (X23), ocular vestibular evoked myogenic potential (o-VEMP) (X27), and horizontal gain of vestibular autorotation test (VAT) (X30). On the basis of statistically significant parameters screened by Chi-square test and multivariable double logistic regression analysis, we established a regression equation: P = 1/[1 + e−(−2.269 × X5 − 2.395 × X13 + 2.141 × X18 + 3.949 × X23 + 2.798 × X27 − 4.275 × X30(1) − 5.811 × X30(2) + 0.873)] (P, predictive value; e, natural logarithm). Nomographs and applets were used to visualize our result. After validation, the prediction model showed good discriminative power and calibrating power. (4) Conclusions: Our study suggested that a diagnostic algorithm based on available clinical features and an auditory-vestibular function regression equation is clinically effective and feasible as a differentiating tool and could improve the differential diagnosis between VM and MD.
1. Introduction
Meniere’s disease (MD) is a peripheral vestibular disorder that causes episodic vertigo and fluctuating hearing loss, tinnitus, and aural fullness. The prevalence of MD roughly stands somewhere between 34–190 per 100,000 [1]. The establishment of vestibular migraine (VM) diagnosis entails the presence of vertigo attacks associated with migrainous symptoms, such as headache and photophobia, among others [2]. VM represents one of the most common vestibular disorders and afflicts up to 1% of the general population [3] and 11% of patients visiting dizziness clinics [4]. Inevitably, some patients have episodes of vertigo that have the features of both diseases [5]. Though different mechanisms have been proposed, distinguishing between these two conditions remains challenging [6]. It has been well accepted that some patients might suffer from both diseases [7]. It has been reported that 30% of patients with vestibular migraine have no headache, and 51% of patients with Meniere’s disease experience migraine [8]. The vestibular symptoms of VM tend to be indistinguishable from those of MD. Moreover, it should be pointed out that the treatment approaches of the two conditions are different, and the non-targeted treatment without clear differential diagnosis might lead to even more unfavorable prognosis. Therefore, identifying measures or markers that differentiate VM from MD is an urgent task for both researchers and clinicians.
The current diagnostic criteria for MD and VM developed by the Classification Committee of the Bárány Society are mainly based on subjective complaints but lack objective measures [1,2]. To date, a few studies have employed some objective tests for the differential diagnosis of MD and VM. Multiple studies have found that loss of VOR, as detected by the caloric test, is more common and severe in MD than in VM; vHIT can provide additional information, and the two tests can be used in tandem. Vestibular evoked myogenic potentials (VEMPs) have been proposed as a marker for the differential diagnosis between MD and VM [9]. What is more, EH is a common pathology shared by both MD and VM, as revealed by gadolinium-contrasted MRI, and MD patients present EH much more frequently than their VM counterparts [10,11]. Two studies based on the same VM diagnostic criteria made an explicit comparison between MD and VM with regards to physical examination results [7,12]. Both studies found that abnormal headshake nystagmus (HSN) and abnormal vibration-induced nystagmus (VIN) are more frequent in the MD population. Murofushi et al. [5] suggested the term VM/MD overlapping syndrome to describe the cases where it was not possible to distinguish between MD and VM. Separate identification of VM and MD is the premise for diagnosing the presence of comorbidity. In addition, the application of artificial intelligence may lead to development of novel strategies for differential diagnosis of vertigo and dizziness [13,14]. Patients with MD and patients with VM are treated differently, and therefore, more accurate differential diagnosis of these two disorders should help to avoid mismanagement. Until now, no ascertained indicators are available that can be used for confirming or refuting the diagnosis of VM or MD.
Up to now, there exist no simple strategies for differentiating VM from MD. Misdiagnosis of VM or MD leads to erroneous management in the subsequent treatment of these two conditions. Hence, in this study, we used more comprehensive clinical features and auxiliary examination findings to establish a predictive model to facilitate the screening and the diagnosis of VM and MD for clinicians and medical care providers.
2. Materials and Methods
2.1. Data Collection and Processing
A total of 110 cases of VM and 110 cases of MD were retrospectively analyzed from the Department of Otorhinolaryngology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China, from February 2020 to February 2022.
Inclusion criteria: Diagnostic criteria of VM and probable VM were jointly formulated; the Committee for Classification of Vestibular Disorders of the Barany Society and the International Headache Society (IHS) developed a consensus document with diagnostic criteria that was included in the appendix of the new international classification of headache disorders (ICHD)-3 beta version [2]. The diagnostic criteria for MD cover two categories—definite MD and probable MD—and the criteria were jointly formulated by the Classification Committee of the Barany Society and some other scientific societies [1]. All patient records were reviewed by two specialists to decide if a patient met the diagnostic criteria of the definite or probable MD or VM for inclusion in the study.
Exclusion criteria: (1) having bilateral MD or family history and autoimmune disease history; (2) having reported overlapping symptoms of MD and VM or if satisfying the diagnostic criteria for both disorders based on their history; (3) having further vestibular or neurological disorders (e.g., benign paroxysmal positional vertigo, vestibular paroxysmia, inner and outer labyrinthine fistula, vestibular neuritis, vestibular schwannoma, cerebellar ataxia, extrapyramidal motor disorders, dementia, multiple sclerosis, stroke) or middle ear disease (e.g., cholesteatoma, otosclerosis, chronic otitis media, tympanic effusion); (4) having a history of ear surgery, brain surgery, or concussion; (5) having metabolic diseases or mental illnesses; (6) pregnant women and age < 18 years.
Then, we prospectively recruited 28 patients with MD and 28 patients with VM in the same center at different periods as validation groups, whose inclusion and exclusion criteria were mentioned above. Informed consent was obtained from all participants before their enrollment.
All the data pertaining to these patients were collected, the medical records of these patients were reviewed, and information regarding their demography, clinical manifestations, and auxiliary examinations was retrieved. The data were entered into EpiData 3.1 (The Epidata Association, Odense, Denmark) statistical software, which was used for database design and data entry [15]. Clinical variables included descriptions of vertigo/dizziness, illness duration, visual motion, nausea and vomiting, hearing loss and other otologic symptoms (e.g., tinnitus), headache or migraine, and personal and family neurotologic histories. Audiometric-vestibular assessments included pure tone audiometry (PTA), otoacoustic emission (OAE), electrocochleogram (ECochG), head-shaking test (HST), videonystagmography (VNG), caloric tests, vestibular evoked myogenic potentials (VEMPs), video-head impulse test (v-HIT), vestibular autorotation test (VAT), tests of sensory integration and balance, such as the sensory organization test (SOT). Somatic Self-rating Scale (SSS), Generalized Anxiety Disorder (GAD-7), and The Patient Health Questionnaire-9 (PHQ-9) were used for the assessment of patient self-reported dizziness-related handicap, anxiety, and depression, respectively. Patients were removed from the research if 2/3 of their examination items of the overall data were missing or unavailable [16,17]. Subsequently, the data were systematically imported into a Microsoft Excel chart. Each parameter or feature was assigned a value for subsequent logistic regression. The methodology and parameters of the tests are shown in Table 1.

Table 1.
Methodology and parameters of the tests.
This study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China (NO. 20210873). All procedures performed in the studies involving human participants were in strict accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
2.2. Statistical Analysis
Data were entered into EpiData 3.1 database and then analyzed using SPSS 23.0 (IBM, Armonk, NY, USA). Univariate Chi-square test (UCST) was applied to determine statistically significant variables that might be important in the differentiation of VM from MD. p < 0.05 was considered statistically significant. Then, statistically significant parameters were subjected to multivariable double logistic regression analysis and a regression equation (mathematical model) was established. Confidence intervals and ORs of the significant parameters were calculated.
Each significant parameter, separately or in combination with other parameters, was further analyzed for sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy in the diagnosis of VM or MD. Discriminative power of the regression model was verified by ROC curves. Then, visualization tools, i.e., nomographs and applets, were established through R software and Excel, respectively. ROC curve (C-index) and GiViTI calibration belt were used to estimate the discriminative power and calibrating ability of the prediction model in both the model construction group and validation group through R software (R, A Language and Environment for Statistical Computing, R Core Team, R Foundation for Statistical Computing, Vienna, Austria. 2021, URL http://www.R-project.org, R version 4.0.5, accessed on 9 May 2021).
3. Results
3.1. Demographics, Past History, and Auxiliary Examination Findings of VM and MD
A database involving a total of 220 patients was established and consisted of several parts (i.e., baseline data, clinical manifestations, auxiliary examination results). Demographics, clinical features, and auxiliary examination findings of VM and MD patients were recorded in all patients, and a value was assigned to each variable. Features in VM and MD were analyzed and screened to search for differentiating ones by using the univariate Chi-square test. We found that illness duration (p = 0.005), attack frequency (p = 0.000), hearing impairment (p = 0.043), aural fullness (p = 0.002), headache (p = 0.000), photophobia (p = 0.000), phonophobia (p = 0.000), PTA (p = 0.000), glycerin test result (p = 0.000), ECochG (p = 0.000), HST (p = 0.000), caloric test (p = 0.000), o-VEMP (p = 0.000), horizontal gain (p = 0.000), and horizontal phase (p = 0.000) were significant indicators in distinguishing between VM and MD (Table 2).

Table 2.
Differentiation of vestibular migraine (VM) and Meniere’s disease (MD).
3.2. Variables That Could Differentiate VM and MD
Multivariable double logistic regression analysis of the clinical and auxiliary examination parameters demonstrated that attack frequency, phonophobia, ECochG, HST, o-VEMP, and VAT (horizontal gain) could help differentiate between VM and MD (Table 3). In order to evaluate the sensitivity and specificity of selected parameters in the differentiation between VM and MD, sensitivity, specificity, accuracy, positive PV, and negative PV were tabulated for various parameters (Table 4).

Table 3.
Multivariate logistic regression analysis of clinical and auditory-vestibular function features in patients with VM and MD.

Table 4.
Sensitivity, specificity, accuracy, positive PV, and negative PV tabulated for significant parameters.
3.3. Predictive Variable Models for Differentiation of VM from MD
On the basis of statistically significant parameters screened by the Chi-square test and further multivariable double logistic regression analysis, we established regression equations. Regression equation P = 1/[1 + e−(−2.269 × X5 − 2.395 × X13 + 2.141 × X18 + 3.949 × X23 + 2.798 × X27 − 4.275 × X30(1) − 5.811 × X30(2) + 0.873)] (where P is predictive value and e is natural logarithm) was established to predict the differential diagnosis between VM and MD. As shown in the flow, if p ≥ 0.314, the predictable diagnosis is MD; if p < 0.314, the predictable diagnosis is VM. (Figure 1). The diagnosis point or threshold (predictive boundary value) 0.314 was obtained by the ROC curve. The area under the curve (AUC) of ROC curves of attack frequency, phonophobia, HST, ECochG, o-VEMP, and horizontal gai, were all greater than 0.5 (Figure 2).
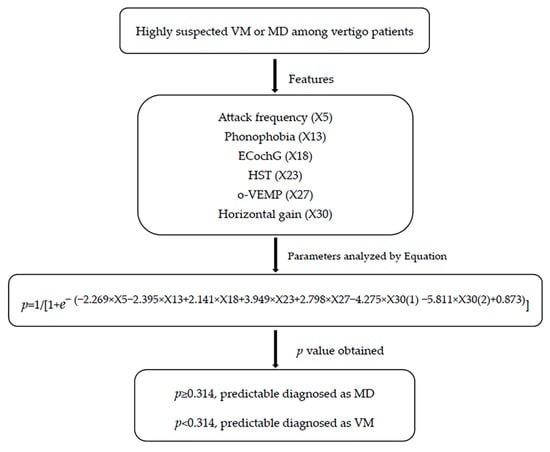
Figure 1.
Differential diagnostic flow between VM and MD based on clinical and auditory-vestibular function features. EcochG, electrocochleogram; HST, head shake test; o-VEMP, Ocular Vestibular Evoked Myogenic Potential; VM, vestibular migraine; MD, Meniere’s disease.
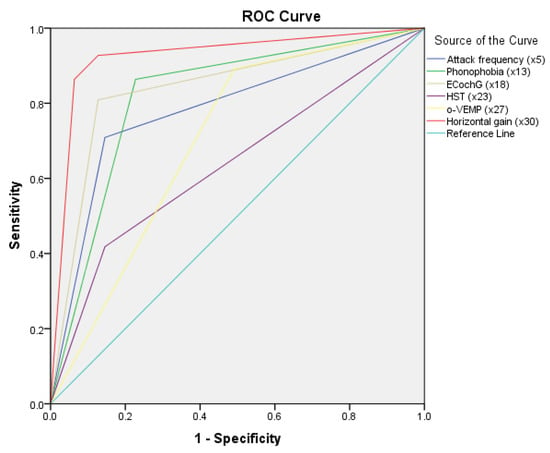
Figure 2.
Receiver operating characteristic (ROC) curves of logistic model and specificity variables. EcochG, electrocochleogram; HST, head shake test; o-VEMP, Ocular Vestibular Evoked Myogenic Potential; VM, vestibular migraine; MD, Meniere’s disease.
The following method was used for the regression equation: (1) collect patients’ clinical and auditory-vestibular function features, including attack frequency, phonophobia, HST, ECochG, o-VEMP, and horizontal gain of VAT. (2) If attack frequency is less than 3 times in one month, X5 = 0. If attack frequency is greater than 3 times in one month, X5 = 1. If patients have no phonophobia, X13 = 0. If patients have phonophobia, X13 = 1. If the results of HST, ECochG, and o-VEMP are normal, the value of X18, X23, and X27 is 0; if abnormal, the value is 1. If the horizontal gain of VAT is normal, the value of X30(1) is 1, and X30(2) is 0. If the horizontal gain of VAT is subnormal, the value of X30(1) is 0, and X30(2) is 0. If the horizontal gain of VAT is paranormal, the value of X30(1) is 0, and X30(2) is 1. Then, calculate the p value. (3) As shown in the flow, if p ≥ 0.314, the predictable diagnosis is MD; if p < 0.314, the predictable diagnosis is VM.
The area under the curve (AUC) of ROC curves of attack frequency, phonophobia, HST, ECochG, o-VEMP, and horizontal gain were all greater than 0.5.
3.4. Nomograph and Applet, as Two Visualization Tools
On the basis of the results of the logistic regression, we determined the predictive factors for diagnosis between VM and MD. To facilitate the differentiation between VM and MD, we screened out six characteristic variables and then constructed a nomographic risk prediction model (Figure 3). In addition, we inputted the value of each single risk factor of the patients through the formula module of Excel software (Supplementary Applet) and then calculated the specific value of diagnosis by means of a regression equation. The model tools made it easy for clinicians to differentiate VM and MD.
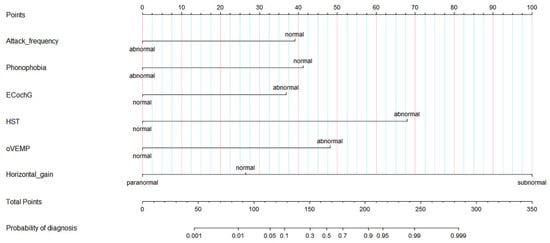
Figure 3.
Diagnostic nomogram estimated by clinical and auditory-vestibular function features for patients with VM and MD. EcochG, electrocochleogram; HST, head shake test; o-VEMP, Ocular Vestibular Evoked Myogenic Potential; VM, vestibular migraine; MD, Meniere’s disease.
The following method was used for the nomogram: (1) the patient-related values were input on the diagnostic variable axes. (2) An intersection line was drawn perpendicular to the score axis from the marked points of each variable axis to obtain the corresponding scores of each variable. (3) Finally, all scores were added, the corresponding points on the total score axis were determined, and a vertical line was drawn perpendicular to the probability axis from the six points on the total score axis. The value obtained from the intersection is the probability of differential diagnosis between VM and MD.
3.5. Internal Validation of the Prediction Model
The development and validation of the predictive model was according to the requirements of the TRIPOD (Transparent Reporting of a multi-variable prediction model for Individual Prognosis or Diagnosis) Statement [17], an international guideline specifically designed for diagnosis or prognosis. The prediction model needs the verification of samples in different periods, regions, and centers to truly reflect the real prediction efficiency [16]. Therefore, we prospectively collected the clinical data of new patients as the validation population of the center in different periods, verified the model, and evaluated its clinical application value. A total of 28 patients with VM and 28 patients with MD were prospectively recruited as the validation set. Moreover, there was no significant difference in clinical features between the model and the validation group (p > 0.05). The internal validation was performed by evaluating the performance of the model with respect to its discriminative and calibrating ability.
3.6. Discriminative Power
The area under the receiver operating characteristic curve (AUROC) is commonly used in clinical practice to quantify the predictive model discrimination power. In the two groups, ROC curves were plotted. In model group, AUROC was 0.982 (95% CI: 0.968~0.996), and the cutoff value was 31.4% (Figure 4). In the validation group, AUROC was 0.880 (95% CI: 0.789~0.970).
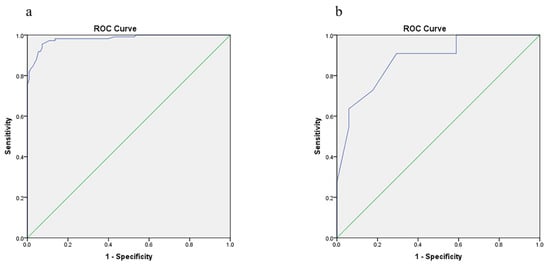
Figure 4.
AUROC curve used to estimate the discriminative power the prediction model. (a) Model construction group; (b) validation group. AUROC, Area Under Receiver Operating Characteristic.
3.7. Calibrating Ability
The calibration ability refers to the consistency between the predicted probability and the actual probability. The 80% CI and 95% CI of the GiViTI calibration belt did not cover the 45° diagonal bisector line, suggesting the model has good discriminative power. The Hosmer-Lemeshow test was conducted for the prediction model in the model group, with x2 = 4.562 and p = 0.727. In the validation group, x2 = 5.534 and p = 0.372. The Hosmer-Lemeshow test yielded a p > 0.05 of the prediction model in the two groups, indicating that the difference was not statistically significant (Figure 5).
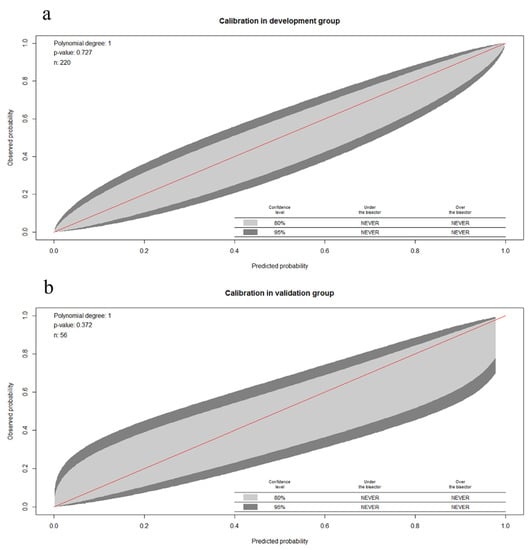
Figure 5.
GiViTI calibration belt used to estimate the calibrating ability of the prediction model. (a) Model construction group; (b) validation group. GiViTI, Gruppo Italiano per la Valutazione degli Interventi in Terapia Intensiva.
The 80% CI and 95% CI of the GiViTI calibration belt did not cover the 45° diagonal bisector line. The Hosmer-Lemeshow test was conducted for the prediction model in the model group, with x2 = 4.562 and p = 0.727. In the validation group, x2 = 5.534 and p = 0.372.
4. Discussion
In this study, by examining demographic data, clinical history, and auxiliary examination results in a large cohort containing 110 MD patients and 110 VM patients, we identified features that could help distinguish between MD and VM. First and foremost, we found a profile of differentiating (predictive) factors, including attack frequency (X5), phonophobia (X13), ECochG (X18), HST (X23), o-VEMP(X27), and VAT (horizontal gain) (X30). Secondly, we, for the first time, proposed a mathematical model (a regression equation) to help differentiate between VM and MD, i.e., P = 1/[1 + e−(−2.269 × X5 − 2.395 × X13 + 2.141 × X18 + 3.949 × X23 + 2.798 × X27 − 4.275 × X30(1) − 5.811 × X30(2) + 0.873)] (where P is the predictive value and e is the natural logarithm). Finally, we established two visualization tools, i.e., a nomograph and an applet, to facilitate the clinical application of the model. In addition, the internal validation was conducted by evaluating the performance of the model with respect to its discriminative and calibrating abilities, and the results suggested that the diagnostic algorithm could achieve better diagnostic efficiency and could improve the differential diagnosis between VM and MD in clinical practice.
4.1. Clinical Symptoms
Previous studies showed that older age at illness onset and male sex favored MD, whereas younger age at illness onset and female sex favored VM [1,5,7,18], which was similar in our study. Some major symptoms of MD, such as fluctuating hearing loss, tinnitus, and aural fullness, may be found in VM patients. In addition, we found that the attack frequency in VM was about 4–8 times in one month, and MD about 2–3 times in one month. Nonetheless, in VM, hearing loss is unlikely to progress to a more severe condition [19]. Similarly, migraine headache, photophobia, and even migraine auras are common during Meniere attacks [20]. Although 51% of MD patients have migraine [21], 52.5% of headaches reportedly occurred concomitantly with vestibular symptoms, a rate similar to the rate found in our study (46.7%) [22]. Moreover, headaches of VM patients tend to be more severe and intermittent and last for about 11.16 days per month [22]. Hearing loss is more frequent in MD than that in VM: 81.8% and 46.7%, respectively. Similarly, Lopez-Escamez et al. found that 77.3% of MD patients, 26.3% of VM patients, and 15.4% pVM patients had hearing loss during attacks [18]. Comparison of accompanying symptoms between MD and VM revealed that tinnitus, aural fullness, and hearing loss were less common in patients with VM than in their MD counterparts. The overlapping pathologies of VM and MD might be ascribed to the inflammation of the trigeminal nerve vessel system, which impairs inner ear function. The VM damage predominantly affects the vestibular region, causing neuritis and vasoconstriction expansion [23]. According to its proposed mechanism, symptoms of VM could be linked to a generally “hyperexcitable” brain [24]. However, the MD principally involves a “fragile” inner ear [25].
Photo- and phonophobia were more frequent in VM patients than in MD patients. Our study found that 77.3% of VM patients and 13.6% of MD patients had phonophobia, playing an important role in the model. The sensitivity, specificity, and accuracy of the phonophobia as predictive factors in the model were 86.4%, 77.3%, and 81.8%, respectively. Beh SC et al. reported that phonophobia was as high as 90.1% in VM [26], close to the rate in our study. Ghavami et al. showed that 51% of MD patients had phonophobia [21]. Phonophobia might be an independent symptom of MD, which was found in 84% of MD patients and was unrelated to the presence of migraine [27]; this finding is inconsistent with the results of our study. Phonophobia might be attributed to lowered hearing threshold, which results from higher brainstem auditory neurol sensitivity [28]. This mechanism may explain our finding that phonophobia was infrequent in MD patients.
In addition, in our study, we found anxiety and depression were slightly more common among patients with VM than MD; however, there was no statistically significant difference between two groups. The patients’ treatment is often unsatisfactory because of the close interactions between vestibular, psychiatric, and neurological disorders. Therefore, clinicians need to consider psychological issues when treating VM or MD patients and realize their different presentations to provide appropriate treatment according to the nature of the disease.
4.2. Auditory Function Results
Of our MD patients, 70.9% and 80.9% yielded positive results with the glycerin test and EchochG, respectively. The higher positive rate might be ascribed to the fact that our hospital is a large general hospital, and most of the patients had serious MD. In general, the results of this test are more likely to be negative at the very early and very late stages of MD [29]. A systematic review and meta-analysis of the role of EChochG in MD diagnosis indicated that the diagnostic specificity was 83.8% [30]. The specificity of EChochG in our cohort was 87.3%, which was comparable to the aforementioned results. The glycerin test and ECochG are two traditional tests that indirectly detect the endolymphatic hydrops (EH). Although EH is a common pathology shared by MD and VM, as exhibited by gadolinium-contrasted MRI, MD patients had a substantially higher rate of EH than VM patients [11,31]. The glycerin test and EChochG are believed to be the most convenient, widely used, and specific tests for the diagnosis MD. Although contrast-enhanced MRI can visualize EH with high sensibility and specificity, some of its limitations restrict its extensive application, since it is costly, time-consuming, and not readily available in all hospitals.
4.3. Vestibular Function Findings
The head-shaking test (HST) is induced by oscillating the head at high frequency in the horizontal plane. It is used for both peripheral and central vestibular disorders. It is important to diagnose vestibular dysfunction; we routinely perform the horizontal HST [32]. The abnormal rate in MD was about 39.8%, and that in VM was about 14.5%, much lower than previously reported: 71% in MD, and 50% in VM [12]. This may be attributed to patients usually visiting their doctor in interval episodes. In addition, the positivity rate of HSN can decrease with the development of vestibular compensation. VAT horizonal gain decreased in 86.4% of MD patients and increased in 87.3% of VM patients. VAT horizonal gain was the most important factor or contributor in our predictive model, the sensitivity and specificity of which were 92.7% and 87.3%, respectively. VAT is a testing technique that examines the head and eye movements at high frequencies (2 to 6 Hz). VAT can provide supplementary information to other tests for VOR assessment. The pathways responsible for the VOR are very complex and principally consist of vestibular ganglia, vestibular nuclei, and oculomotor nuclei. The peripheral damage renders the primary reflex pathway incomplete, thereby down-regulating VOR and reducing the gain. If the central vestibule is structurally abnormal, the inhibitory action of the vestibular nuclei will be weakened, leading to VOR hyperfunction and gain increase. In addition, Mert Cemal Gökgöz [33] confirmed that horizontal phase values were sensitive markers for discriminating decompensated MD from compensated MD. The vertical high-frequency VOR plays an important role in visual stabilization during daily activities, such as ambulation. A high vertical phase value, in the range of 4 to 5 Hz, was associated with presence of migraine.
We found that the o-VEMP was abnormal in 25.5% of VM patients and 74.7% of MD patients. Similarly, Taylor et al. [34] and Salviz et al. [35] reported a significantly higher AR (asymmetry ratio) of ACS (air-conducted sound) cVEMP amplitudes in patients with unilateral MD (46% and 29%) as compared to those with VM (16% in both studies). VEMPs are short-latency manifestations of vestibuloocular and vestibulocollic reflexes that originate from the utricle and saccule. Thus, VEMPs have mostly been applied to the diagnosis of disorders involving the peripheral vestibular system. The high AR may be attributed to the distended saccular membrane in contact with the stapes footplate or probably to a progressive loss of vestibular hair cells and primary vestibular neurons. Moreover, Baier and Dieterich [36] found no difference in ACS cVEMPs between VM and MD. Histopathological analyses in MD have shown that, compared to the utricle, the saccule is affected more often by ELH. Furthermore, the trigeminovascular system branches into the labyrinthine arteries of both labyrinths, and the distribution might result in symmetrical involvement of both labyrinth organs in VM.
In our cohort, the caloric test yielded abnormal results in 54.5% of MD patients and 30.9% of VM patients; pathological vHIT occurred in 63.6% of MD patients and 58.7% of VM patients. Both vHIT and the caloric test can evaluate the VOR of the horizontal semicircular canal. The semicircular canal paralysis was detected by the caloric test more frequently and was more severe in MD patients than their VM counterparts; no significant difference in vHIT was found between them [37]. Blödow and colleagues compared the results of the caloric test and vHIT in 53 patients with VM and MD and found that vHIT was more sensitive for the diagnosis of peripheral hypofunction. Blödow considered that the reason for the inconsistent results was that the caloric test detects the low frequency of the semicircular canal, while vHIT detects the high frequency of the semicircular canal. The vestibular victims of MD may be frequency dependent [38]. In addition, McGarvie reported that due to endolymphatic hydrops, MD patients’ membranous semicircular canal expands. When stimulated by the caloric test, the endolymph forms local circulation in the expanded membranous semicircular canal, which reduces the flow of endolymph on both sides of crista ampullaris and reduces the deviation of crista ampullaris. During vHIT, the enlarged membranous semicircular canal did not affect the internal lymph flow caused by head flick, so the result was normal [39].
Differentiation between MD and VM faces various challenges, even if relevant diagnostic criteria are available. MD is now diagnosed on the basis of the consensus proposed by the Bárány Society [1]. In terms of VM, the Bárány Society and the International Headache Society have established a consensus document, with diagnostic criteria, which was included in the appendix of the new International Classification of Headache Disorders (ICHD)-3 beta version, which means that VM can be deemed as an emerging entity needing further research [2]. The constantly revised diagnostic guideline and multidisciplinary international expert workshops [40] have contributed greatly to the assessment of MD and VM and their treatment.
Our study has some limitations. First, our study was conducted in a single tertiary care general hospital and caution should be exercised when extrapolating the model to other hospitals with different resources and patient sources. A multicenter and prospective study with additional clinicians can enhance the robustness of the model. Another limitation is that this study failed to cover more factors, such as the temporal relation between headache and vestibular symptoms and biological markers [41], among others. In addition, another issue left unaddressed was the endotyping of the MD subjects [42,43,44,45]. The patients were not sorted in terms of interictal phase and acute stage of VM and MD. Statistically, we performed internal validation on the lesser dataset, which partially addressed the validation issue; however, this is not as robust as validation of the predictive models using external or cross validation. These are problems we will address in our future studies.
5. Conclusions
In this study, we for the first time worked out a predictive model for distinguishing between MD and VM. Attack frequency, phonophobia, EChochG, HST, o-VEMP, and VAT (horizontal gain) may form a battery to differentiate the two conditions. The predictive model had high sensitivity and specificity, good discriminative power, and good calibrating ability in both model and validation groups. The use of nomographs in combination with applets also facilitated the doctor–patient communication, and the intuitive display improves the diagnostic efficiency. Our study paves the way for future commodity studies of VM and MD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11164745/s1, Supplementary Applet: Differential diagnosis applet of VM and MD. Use method of applet: (1) firstly, assign values to each influence factor, enter 0, 1, or 2 according to the change of predictor; (2) then after pressing enter automatically get forecast value and the result.
Author Contributions
S.Z. and W.K. designed the research and directed its implication. D.L. drafted the manuscript. E.T., J.W., Z.G., L.Z. and J.C. contributed to the manuscript’s modifications. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81873701) and the National Natural Science Foundation of China (No. 82171152).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China (No. 20210873).
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lopez-Escamez, J.A.; Carey, J.; Chung, W.H.; Goebel, J.A.; Magnusson, M.; Mandalà, M.; Newman-Toker, D.E.; Strupp, M.; Suzuki, M.; Trabalzini, F.; et al. Diagnostic criteria for Menière’s disease. J. Vestib. Res. 2015, 25, 1–7. [Google Scholar] [CrossRef]
- Lempert, T.; Olesen, J.; Furman, J.; Waterston, J.; Seemungal, B.; Carey, J.; Bisdorff, A.; Versino, M.; Evers, S.; Newman-Toker, D. Vestibular migraine: Diagnostic criteria. J. Vestib. Res. Equilib. Orientat. 2012, 22, 167–172. [Google Scholar] [CrossRef]
- Neuhauser, H.K.; Radtke, A.; von Brevern, M.; Feldmann, M.; Lezius, F.; Ziese, T.; Lempert, T. Migrainous vertigo: Prevalence and impact on quality of life. Neurology 2006, 67, 1028–1033. [Google Scholar] [CrossRef]
- Hochman, M.S. The interrelations of migraine, vertigo, and migrainous vertigo. Neurology 2001, 57, 1522. [Google Scholar] [CrossRef]
- Murofushi, T.; Tsubota, M.; Kitao, K.; Yoshimura, E. Simultaneous Presentation of Definite Vestibular Migraine and Definite Ménière’s Disease: Overlapping Syndrome of Two Diseases. Front. Neurol. 2018, 9, 749. [Google Scholar] [CrossRef]
- Pyykkö, I.; Pyykkö, N.; Manchaiah, V. Vestibular drop attacks in Ménière’s disease and its association with migraine. Eur. Arch. Otorhinolaryngol. 2020, 277, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Neff, B.A.; Staab, J.P.; Eggers, S.D.; Carlson, M.L.; Schmitt, W.R.; Van Abel, K.M.; Worthington, D.K.; Beatty, C.W.; Driscoll, C.L.; Shepard, N.T. Auditory and vestibular symptoms and chronic subjective dizziness in patients with Ménière’s disease, vestibular migraine, and Ménière’s disease with concomitant vestibular migraine. Otol. Neurotol. 2012, 33, 1235–1244. [Google Scholar] [CrossRef]
- Moshtaghi, O.; Sahyouni, R.; Lin, H.W.; Ghavami, Y.; Djalilian, H.R. A Historical Recount: Discovering Menière’s Disease and Its Association With Migraine Headaches. Otol. Neurotol. 2016, 37, 1199–1203. [Google Scholar] [CrossRef]
- Dlugaiczyk, J.; Habs, M.; Dieterich, M. Vestibular evoked myogenic potentials in vestibular migraine and Menière’s disease: cVEMPs make the difference. J. Neurol. 2020, 267, 169–180. [Google Scholar] [CrossRef]
- Gürkov, R.; Kantner, C.; Strupp, M.; Flatz, W.; Krause, E.; Ertl-Wagner, B. Endolymphatic hydrops in patients with vestibular migraine and auditory symptoms. Eur. Arch. Oto-Rhino-Laryngol. 2014, 271, 2661–2667. [Google Scholar] [CrossRef]
- Oh, S.Y.; Dieterich, M.; Lee, B.N.; Boegle, R.; Kirsch, V. Endolymphatic Hydrops in Patients with Vestibular Migraine and Concurrent Meniere’s Disease. Front. Neurol. 2021, 12, 594481. [Google Scholar] [CrossRef]
- Shin, J.E.; Kim, C.H.; Park, H.J. Vestibular abnormality in patients with Meniere’s disease and migrainous vertigo. Acta Oto-Laryngol. 2013, 133, 154–158. [Google Scholar] [CrossRef]
- Groezinger, M.; Huppert, D.; Strobl, R.; Grill, E. Development and validation of a classification algorithm to diagnose and differentiate spontaneous episodic vertigo syndromes: Results from the DizzyReg patient registry. J. Neurol. 2020, 267, 160–167. [Google Scholar] [CrossRef]
- Kabade, V.; Hooda, R.; Raj, C.; Awan, Z.; Young, A.S.; Welgampola, M.S.; Prasad, M. Machine Learning Techniques for Differential Diagnosis of Vertigo and Dizziness: A Review. Sensors 2021, 21, 7565. [Google Scholar] [CrossRef] [PubMed]
- Lauritsen, J.M.; Bruus, M. EpiData Entry. A Comprehensive Tool for Validated Entry and Documentation of Data; The EpiData Association: Odense, Denmark, 2008. [Google Scholar]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): The TRIPOD Statement. Br. J. Surg. 2015, 102, 148–158. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Wolff, R.F.; Riley, R.D.; Whiting, P.F.; Westwood, M.; Collins, G.S.; Reitsma, J.B.; Kleijnen, J.; Mallett, S. PROBAST: A Tool to Assess Risk of Bias and Applicability of Prediction Model Studies: Explanation and Elaboration. Ann. Intern. Med. 2019, 170, W1–W33. [Google Scholar] [CrossRef]
- Lopez-Escamez, J.A.; Dlugaiczyk, J.; Jacobs, J.; Lempert, T.; Teggi, R.; von Brevern, M.; Bisdorff, A. Accompanying Symptoms Overlap during Attacks in Menière’s Disease and Vestibular Migraine. Front. Neurol. 2014, 5, 265. [Google Scholar] [CrossRef] [PubMed]
- Radtke, A.; Neuhauser, H.; von Brevern, M.; Hottenrott, T.; Lempert, T. Vestibular migraine-validity of clinical diagnostic criteria. Cephalalgia 2011, 31, 906–913. [Google Scholar] [CrossRef]
- Brantberg, K.; Baloh, R.W. Similarity of vertigo attacks due to Meniere’s disease and benign recurrent vertigo, both with and without migraine. Acta Oto-Laryngol. 2011, 131, 722–727. [Google Scholar] [CrossRef]
- Ghavami, Y.; Mahboubi, H.; Yau, A.Y.; Maducdoc, M.; Djalilian, H.R. Migraine features in patients with Meniere’s disease. Laryngoscope 2016, 126, 163–168. [Google Scholar] [CrossRef]
- Vuralli, D.; Yildirim, F.; Akcali, D.T.; Ilhan, M.N.; Goksu, N.; Bolay, H. Visual and Postural Motion-Evoked Dizziness Symptoms Are Predominant in Vestibular Migraine Patients. Pain Med. 2018, 19, 178–183. [Google Scholar] [CrossRef]
- Huang, T.C.; Wang, S.J.; Kheradmand, A. Vestibular migraine: An update on current understanding and future directions. Cephalalgia 2020, 40, 107–121. [Google Scholar] [CrossRef]
- Aurora, S.K.; Wilkinson, F. The brain is hyperexcitable in migraine. Cephalalgia 2007, 27, 1442–1453. [Google Scholar] [CrossRef]
- Vass, Z.; Shore, S.E.; Nuttall, A.L.; Miller, J.M. Direct evidence of trigeminal innervation of the cochlear blood vessels. Neuroscience 1998, 84, 559–567. [Google Scholar] [CrossRef]
- Beh, S.C.; Masrour, S.; Smith, S.V.; Friedman, D.I. The Spectrum of Vestibular Migraine: Clinical Features, Triggers, and Examination Findings. Headache 2019, 59, 727–740. [Google Scholar] [CrossRef]
- Saberi, A.; Nemati, S.; Amlashi, T.T.; Tohidi, S.; Bakhshi, F. Phonophobia and migraine features in patients with definite meniere’s disease: Pentad or triad/tetrad? Acta Oto-Laryngol. 2020, 140, 548–552. [Google Scholar] [CrossRef]
- Kalita, J.; Misra, U.K.; Bansal, R. Phonophobia and brainstem excitability in migraine. Eur. J. Neurosci. 2021, 53, 1988–1997. [Google Scholar] [CrossRef]
- Levine, S.; Margolis, R.H.; Daly, K.A. Use of electrocochleography in the diagnosis of Meniere’s disease. Laryngoscope 1998, 108, 993–1000. [Google Scholar] [CrossRef]
- Ayub, A.; Qi, L.; Nunez, D.A. A systematic review and meta-analysis of extratympanic electrocochleography in Ménière’s disease diagnosis. Int. J. Audiol. 2019, 58, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Yoshida, T.; Suga, K.; Kato, M.; Otake, H.; Kato, K.; Teranishi, M.; Sone, M.; Sugiura, S.; Kuno, K.; et al. Endolymphatic space size in patients with vestibular migraine and Ménière’s disease. J. Neurol. 2014, 261, 2079–2084. [Google Scholar] [CrossRef]
- Yang, T.H.; Lee, J.H.; Oh, S.Y.; Kang, J.J.; Kim, J.S.; Dieterich, M. Clinical implications of head-shaking nystagmus in central and peripheral vestibular disorders: Is perverted head-shaking nystagmus specific for central vestibular pathology? Eur. J. Neurol. 2020, 27, 1296–1303. [Google Scholar] [CrossRef]
- Gökgöz, M.C.; Satar, B.; Hıdır, Y.; Ceyhan, A.; Çoban, V.K. Recognizing Decompensated Meniere’s Disease Using High Frequency Rotational Test. J. Int. Adv. Otol. 2020, 16, 165–170. [Google Scholar] [CrossRef]
- Taylor, R.L.; Zagami, A.S.; Gibson, W.P.; Black, D.A.; Watson, S.R.; Halmagyi, M.G.; Welgampola, M.S. Vestibular evoked myogenic potentials to sound and vibration: Characteristics in vestibular migraine that enable separation from Meniere’s disease. Cephalalgia 2012, 32, 213–225. [Google Scholar] [CrossRef]
- Salviz, M.; Yuce, T.; Acar, H.; Taylan, I.; Yuceant, G.A.; Karatas, A. Diagnostic value of vestibular-evoked myogenic potentials in Ménière’s disease and vestibular migraine. J. Vestib. Res. Equilib. Orientat. 2016, 25, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Baier, B.; Dieterich, M. Vestibular-evoked myogenic potentials in “vestibular migraine” and Menière’s disease: A sign of an electrophysiological link? Ann. N. Y. Acad. Sci. 2009, 1164, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Blödow, A.; Heinze, M.; Bloching, M.B.; von Brevern, M.; Radtke, A.; Lempert, T. Caloric stimulation and video-head impulse testing in Ménière’s disease and vestibular migraine. Acta Oto-Laryngol. 2014, 134, 1239–1244. [Google Scholar] [CrossRef]
- Yilmaz, M.S.; Egilmez, O.K.; Kara, A.; Guven, M.; Demir, D.; Genc Elden, S. Comparison of the results of caloric and video head impulse tests in patients with Meniere’s disease and vestibular migraine. Eur. Arch. Oto-Rhino-Laryngol. 2021, 278, 1829–1834. [Google Scholar] [CrossRef]
- McGarvie, L.A.; Curthoys, I.S.; MacDougall, H.G.; Halmagyi, G.M. What does the head impulse test versus caloric dissociation reveal about vestibular dysfunction in Ménière’s disease? Ann. N. Y. Acad. Sci. 2015, 1343, 58–62. [Google Scholar] [CrossRef]
- Mallampalli, M.P.; Rizk, H.G.; Kheradmand, A.; Beh, S.C.; Abouzari, M.; Bassett, A.M.; Buskirk, J.; Ceriani, C.E.J.; Crowson, M.G.; Djalilian, H.; et al. Care Gaps and Recommendations in Vestibular Migraine: An Expert Panel Summit. Front. Neurol. 2022, 12, 812678. [Google Scholar] [CrossRef]
- Flook, M.; Frejo, L.; Gallego-Martinez, A.; Martin-Sanz, E.; Rossi-Izquierdo, M.; Amor-Dorado, J.C.; Soto-Varela, A.; Santos-Perez, S.; Batuecas-Caletrio, A.; Espinosa-Sanchez, J.M.; et al. Differential Proinflammatory Signature in Vestibular Migraine and Meniere Disease. Front. Immunol. 2019, 10, 1229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, Z.; Tian, E.; Liu, D.; Wang, J.; Kong, W. Meniere disease subtyping: The direction of diagnosis and treatment in the future. Expert Rev. Neurother. 2022, 22, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Eckhard, A.H.; Zhu, M.; O’Malley, J.T.; Williams, G.H.; Loffing, J.; Rauch, S.D.; Nadol, J.B., Jr.; Liberman, M.C.; Adams, J.C. Inner ear pathologies impair sodium-regulated ion transport in Meniere’s disease. Acta Neuropathol. 2019, 137, 343–357. [Google Scholar] [CrossRef]
- Bächinger, D.; Brühlmann, C.; Honegger, T.; Michalopoulou, E.; Monge Naldi, A.; Wettstein, V.G.; Muff, S.; Schuknecht, B.; Eckhard, A.H. Endotype-Phenotype Patterns in Meniere’s Disease Based on Gadolinium-Enhanced MRI of the Vestibular Aqueduct. Front. Neurol. 2019, 10, 303. [Google Scholar] [CrossRef]
- Bächinger, D.; Luu, N.N.; Kempfle, J.S.; Barber, S.; Zürrer, D.; Lee, D.J.; Curtin, H.D.; Rauch, S.D.; Nadol, J.B., Jr.; Adams, J.C.; et al. Vestibular Aqueduct Morphology Correlates with Endolymphatic Sac Pathologies in Menière’s Disease-A Correlative Histology and Computed Tomography Study. Otol. Neurotol. 2019, 40, e548–e555. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).