Complications of the Percutaneous Mitral Valve Edge-To-Edge Repair: Role of Transesophageal Echocardiography
Abstract
:1. Introduction
2. Pericardial Effusion/Tamponade
3. Thromboembolic Events
4. Device-Related Complications
4.1. Functional Device Failure
4.1.1. Persistent Mitral Regurgitation
4.1.2. Mitral Stenosis
4.2. Structural Device Failure
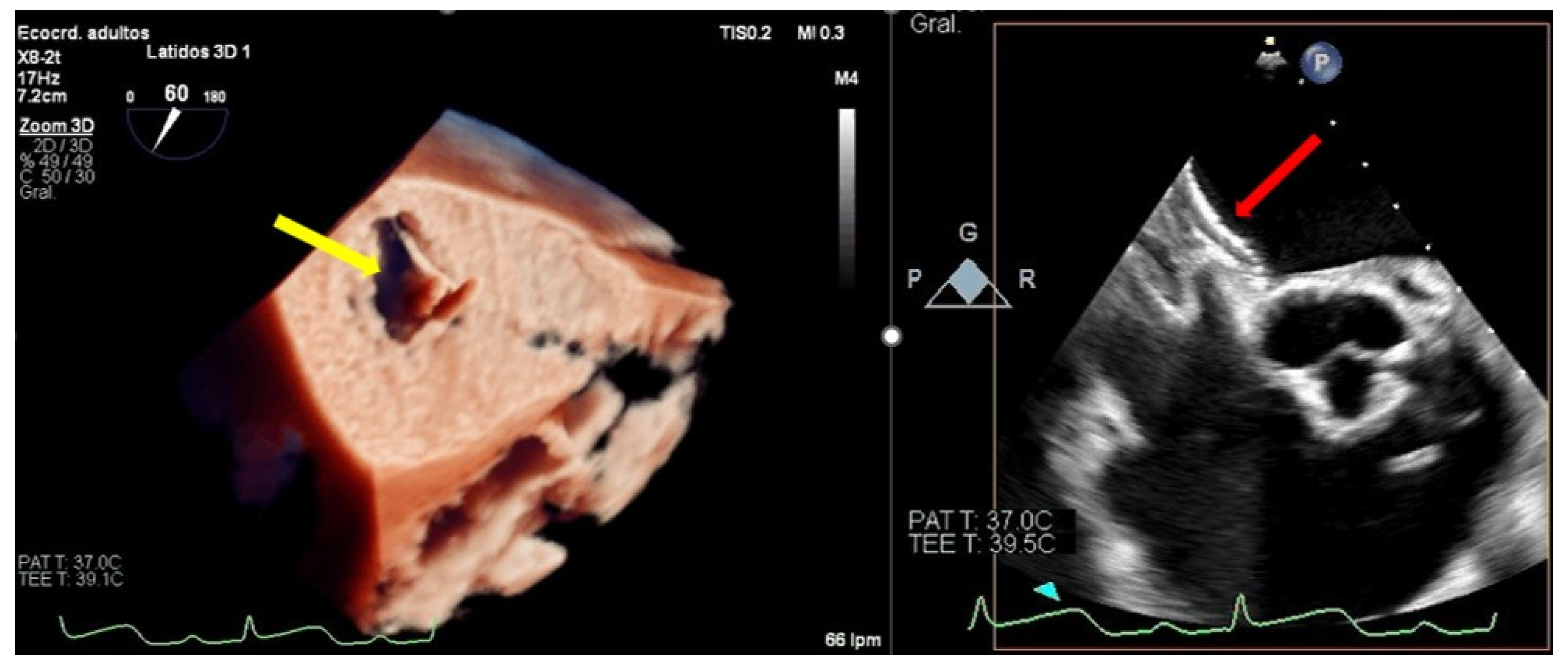
4.2.1. Single Leaflet Device Attachment
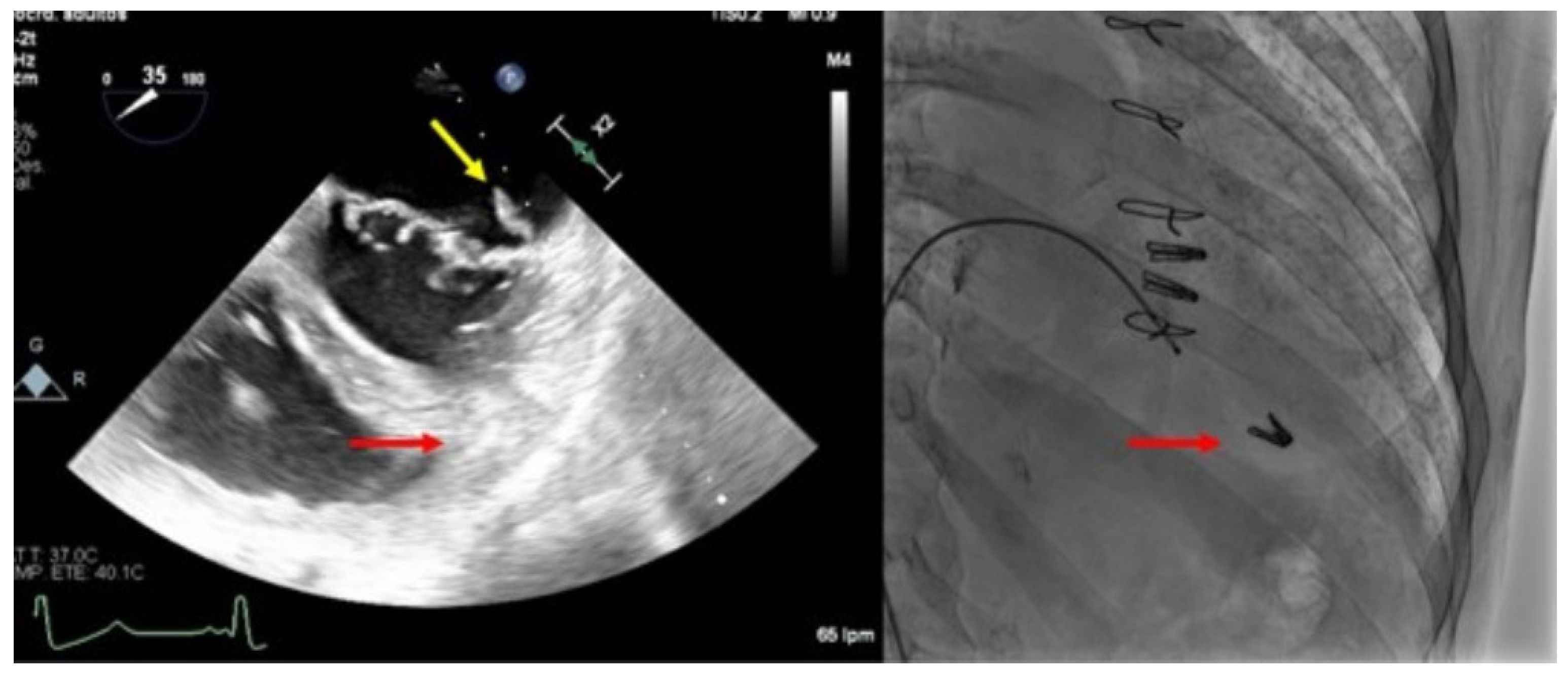
4.2.2. Clip Embolization
5. Post-TEER Persistent Atrial Septal Defects
6. Complications Due to Transesophageal Probe Monitoring
7. Access Site Complications
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schnitzler, K.; Hell, M.; Geyer, M.; Kreidel, F.; Münzel, T.; von Bardeleben, R.S. Complications Following MitraClip Implantation. Curr. Cardiol. Rep. 2021, 23, 131. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, L.; Ielasi, A.; Rensing, B.J.W.M.; Eefting, F.D.; Timmers, L.; Latib, A.; Swaans, M.J. Complications Following Percutaneous Mitral Valve Repair. Front. Cardiovasc. Med. 2019, 6, 146. [Google Scholar] [CrossRef] [PubMed]
- Agricola, E.; Meucci, F.; Ancona, F.; Pardo Sanz, A.; Zamorano, J.L. Echocardiographic guidance in transcatheter structural cardiac interventions. EuroIntervention 2022, 17, 1205–1226. [Google Scholar] [CrossRef] [PubMed]
- Altiok, E.; Becker, M.; Hamada, S.; Reith, S.; Marx, N.; Hoffmann, R. Optimized guidance of percutaneous edge-to edge repair of the mitral valve using real-time 3-D transesophageal echocardiography. Clin. Res. Cardiol. 2011, 100, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, N.C.; Beigel, R.; Ho, S.Y.; Nietlispach, F.; Cheng, R.; Agricola, E.; Siegel, R.J. Imaging for Mitral Interventions: Methods and Efficacy. JACC Cardiovasc. Imaging 2018, 11, 872–901. [Google Scholar] [CrossRef] [PubMed]
- Praz, F.; Braun, D.; Unterhuber, M.; Spirito, A.; Orban, M.; Brugger, N.; Brinkmann, I.; Spring, K.; Moschovitis, A.; Nabauer, M.; et al. Edge-to-Edge Mitral Valve Repair With Extended Clip Arms: Early Experience From a Multicenter Observational Study. JACC Cardiovasc. Interv. 2019, 12, 1356–1365. [Google Scholar] [CrossRef]
- Eggebrecht, H.; Schelle, S.; Puls, M.; Plicht, B.; Von Bardeleben, R.S.; Butter, C.; May, A.E.; Lubos, E.; Boekstegers, P.; Ouarrak, T.; et al. Risk and outcomes of complications during and after MitraClip implantation: Experience in 828 patients from the German TRAnscatheter mitral valve interventions (TRAMI) registry. Catheter. Cardiovasc. Interv. 2015, 86, 728–735. [Google Scholar] [CrossRef]
- Von Bardeleben, R.S.; Hobohm, L.; Kreidel, F.; Ostad, M.A.; Schulz, E.; Konstantinides, S.; Lankeit, M.; Feldman, T.; Münzel, T.; Keller, K. Incidence and in-hospital safety outcomes of patients undergoing percutaneous mitral valve edge-to-edge repair using MitraClip: Five-year German national patient sample including 13,575 implants. EuroIntervention 2019, 14, 1725–1732. [Google Scholar] [CrossRef]
- Maisano, F.; Franzen, O.; Baldus, S.; Schäfer, U.; Hausleiter, J.; Butter, C.; Ussia, G.P.; Sievert, H.; Richardt, G.; Widder, J.D.; et al. Percutaneous Mitral Valve Interventions in the Real World: Early and 1-Year Results from the ACCESS-EU, A Prospective, Multicenter, Nonrandomized Post-Approval Study of the MitraClip Therapy in Europe. J. Am. Coll. Cardiol. 2013, 62, 1052–1061. [Google Scholar] [CrossRef]
- Elbadawi, A.; Elgendy, I.Y.; Mohamed, A.H.; Almahmoud, M.F.; Omer, M.; Abuzaid, A.; Mahmoud, K.; Ogunbayo, G.O.; Denktas, A.; Paniagua, D.; et al. Temporal Trends and Outcomes of Transcatheter Mitral Valve Repair and Surgical Mitral Valve Intervention. Cardiovasc. Revascularization Med. 2020, 21, 1560–1566. [Google Scholar] [CrossRef]
- Orban, M.; Braun, D.; Sonne, C.; Orban, M.; Thaler, R.; Grebmer, C.; Lesevic, H.; Schömig, A.; Mehilli, J.; Massberg, S.; et al. Dangerous liaison: Successful percutaneous edge-to-edge mitral valve repair in patients with end-stage systolic heart failure can cause left ventricular thrombus formation. EuroIntervention 2014, 10, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Bilge, M.; Saatci Yaşar, A.; Ali, S.; Alemdar, R. Left atrial spontaneous echo contrast and thrombus formation at septal puncture during percutaneous mitral valve repair with the MitraClip system of severe mitral regurgitation: A report of two cases. Anadolu. Kardiyol. Derg. 2014, 14, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Khalique, O.K.; Hahn, R.T. Percutaneous Mitral Valve Repair: Multi-Modality Cardiac Imaging for Patient Selection and Intra-Procedural Guidance. Front. Cardiovasc. Med. 2019, 6, 142. [Google Scholar] [CrossRef] [PubMed]
- Sorajja, P.; Vemulapalli, S.; Feldman, T.; Mack, M.; Holmes, D.R.; Stebbins, A.; Kar, S.; Thourani, V.; Ailawadi, G. Outcomes With Transcatheter Mitral Valve Repair in the United States: An STS/ACC TVT Registry Report. J. Am. Coll. Cardiol. 2017, 70, 2315–2327. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Asch, F.M.; Bruce, C.; Gillam, L.D.; Grayburn, P.A.; Hahn, R.T.; Inglessis, I.; Islam, A.M.; Lerakis, S.; Little, S.H.; et al. Guidelines for the Evaluation of Valvular Regurgitation After Percutaneous Valve Repair or Replacement: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2019, 32, 431–475. [Google Scholar] [CrossRef]
- Altiok, E.; Hamada, S.; Brehmer, K.; Kuhr, K.; Reith, S.; Becker, M.; Schröder, J.; Almalla, M.; Lehmacher, W.; Marx, N.; et al. Analysis of Procedural Effects of Percutaneous Edge-to-Edge Mitral Valve Repair by 2D and 3D Echocardiography. Circ. Cardiovasc. Imaging 2012, 5, 748–755. [Google Scholar] [CrossRef]
- Avenatti, E.; Mackensen, G.B.; El-Tallawi, K.C.; Reisman, M.; Gruye, L.; Barker, C.M.; Little, S.H. Diagnostic Value of 3-Dimensional Vena Contracta Area for the Quantification of Residual Mitral Regurgitation After MitraClip Procedure. JACC Cardiovasc. Interv. 2019, 12, 582–591. [Google Scholar] [CrossRef]
- Koell, B.; Ludwig, S.; Weimann, J.; Waldschmidt, L.; Hildebrandt, A.; Schofer, N.; Schirmer, J.; Westermann, D.; Reichenspurner, H.; Blankenberg, S.; et al. Long-Term Outcomes of Patients With Elevated Mitral Valve Pressure Gradient After Mitral Valve Edge-to-Edge Repair. JACC Cardiovasc. Interv. 2022, 15, 922–934. [Google Scholar] [CrossRef]
- Neuss, M.; Schau, T.; Isotani, A.; Pilz, M.; Schöpp, M.; Butter, C. Elevated Mitral Valve Pressure Gradient After MitraClip Implantation Deteriorates Long-Term Outcome in Patients With Severe Mitral Regurgitation and Severe Heart Failure. JACC Cardiovasc. Interv. 2017, 10, 931–939. [Google Scholar] [CrossRef]
- Li, C.H.; Arzamendi, D.; Carreras, F. Role of Imaging Techniques in Percutaneous Treatment of Mitral Regurgitation. Rev. Esp. Cardiol. 2016, 69, 421–436. [Google Scholar] [CrossRef]
- Kreidel, F.; Frerker, C.; Schlüter, M.; Alessandrini, H.; Thielsen, T.; Geidel, S.; Schäfer, U.; Kuck, K.-H. Repeat MitraClip Therapy for Significant Recurrent Mitral Regurgitation in High Surgical Risk Patients: Impact of Loss of Leaflet Insertion. JACC Cardiovasc. Interv. 2015, 8, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Ikenaga, H.; Makar, M.; Rader, F.; Siegel, R.J.; Kar, S.; Makkar, R.R.; Shiota, T. Mechanisms of mitral regurgitation after percutaneous mitral valve repair with the MitraClip. Eur. Hear. J. Cardiovasc. Imaging 2019, 21, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, M.; Besler, C.; Lurz, P.; Noack, T. Bail-out edge-to-edge mitral repair for an acute single leaflet device attachment: A case report. Eur. Heart J. Case Rep. 2021, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Lisko, J.C.; Greenbaum, A.B.; Guyton, R.A.; Kamioka, N.; Grubb, K.J.; Gleason, P.T.; Byku, I.; Condado, J.F.; Jadue, A.; Paone, G.; et al. Electrosurgical Detachment of MitraClips From the Anterior Mitral Leaflet Prior to Transcatheter Mitral Valve Implantation. JACC Cardiovasc. Interv. 2020, 13, 2361–2370. [Google Scholar] [CrossRef] [PubMed]
- Schueler, R.; Öztürk, C.; Wedekind, J.A.; Werner, N.; Stöckigt, F.; Mellert, F.; Nickenig, G.; Hammerstingl, C. Persistence of iatrogenic atrial septal defect after interventional mitral valve repair with the MitraClip system: A note of caution. JACC Cardiovasc. Interv. 2015, 8, 450–459. [Google Scholar] [CrossRef]
- Paukovitsch, M.; Schneider, L.M.; Reichart, C.; Nita, N.; Rottbauer, W.; Keßler, M.; Markovic, S. Prevalence of iatrogenic atrial septal defects (iASD) after mitral valve (MV) transcatheter edge-to-edge repair (TEER) in the long-term follow-up. Open Heart 2021, 8, e001732. [Google Scholar] [CrossRef]
- Rodríguez Nieto, J.; Piserra López-Fernández de Heredia, A.; Perea Armijo, J. What 3D transillumination provides: Thrombus or tear? Rev. Esp. Cardiol. 2021, 74, 263. [Google Scholar] [CrossRef]
- Freitas-Ferraz, A.B.; Rodés-Cabau, J.; Vega, L.J.; Beaudoin, J.; O’Connor, K.; Turgeon, P.Y.; Paradis, J.-M.; Ferreira-Neto, A.; Asmarats, L.; Champagne, J.; et al. Transesophageal echocardiography complications associated with interventional cardiology procedures. Am. Heart J. 2019, 221, 19–28. [Google Scholar] [CrossRef]
- Bhatty, S.; Cooke, R.; Shetty, R.; Jovin, I. Femoral vascular access-site complications in the cardiac catheterization laboratory: Diagnosis and management. Interv. Cardiol. 2011, 3, 503–514. [Google Scholar] [CrossRef]
- Steppich, B.; Stegmüller, F.; Rumpf, P.M.; Pache, J.; Sonne, C.; Lesevic, H.; Braun, D.; Hausleiter, J.; Kasel, A.M.; Ott, I. Vascular complications after percutaneous mitral valve repair and venous access closure using suture or closure device. J. Interv. Cardiol. 2017, 31, 223–229. [Google Scholar] [CrossRef]
- McGee, D.C.; Gould, M.K. Preventing complications of central venous catheterization. N. Engl. J. Med. 2003, 348, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Ramirez, R.; Steinhaus, D.A.; Yousuf, O.K.; Giocondo, M.J.; Ramza, B.M.; Wimmer, A.P.; Gupta, S.K. Comparative outcomes of vascular access closure methods following atrial fibrillation/flutter catheter ablation: Insights from VAscular Closure for Cardiac Ablation Registry. J. Interv. Card. Electrophysiol. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
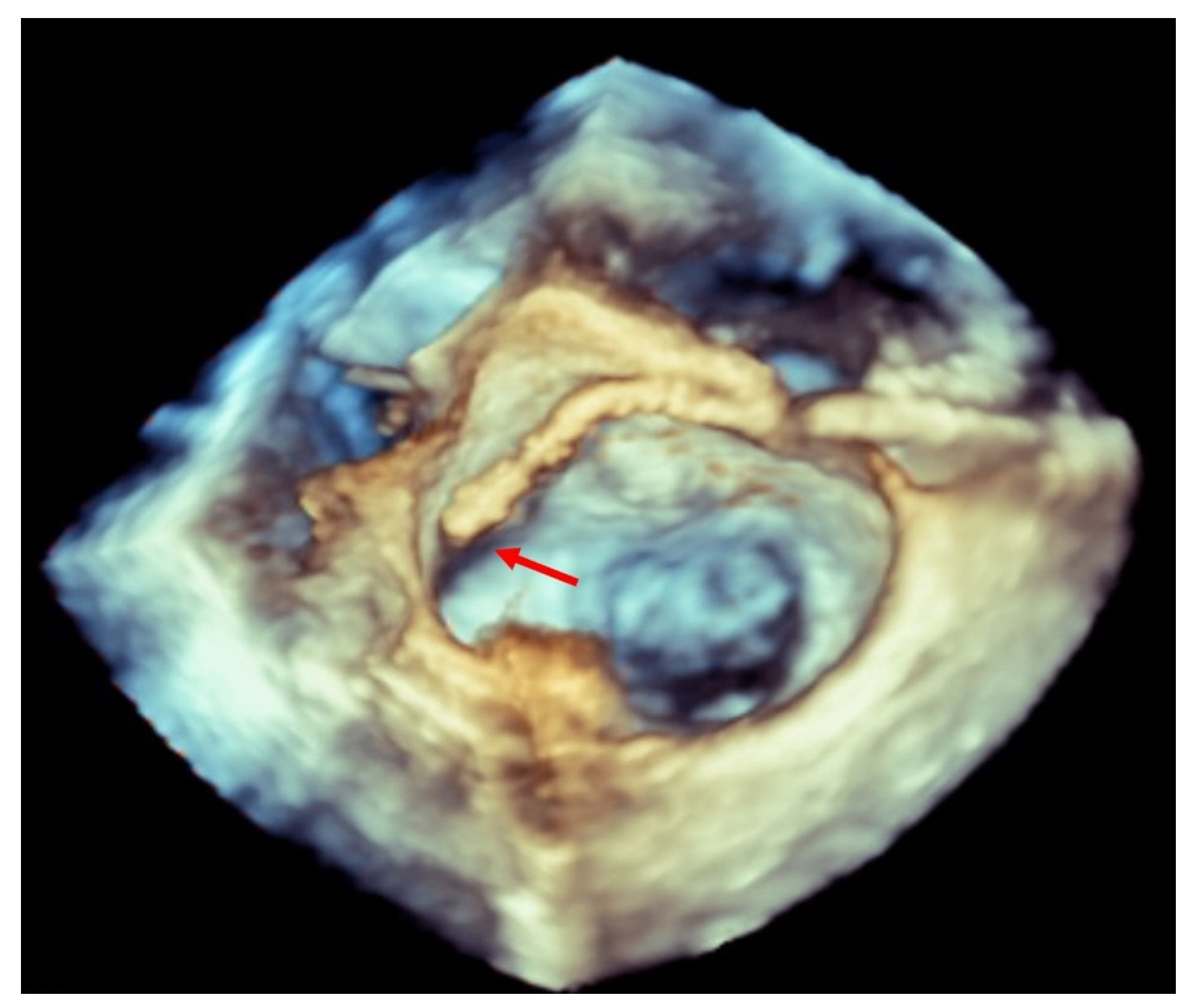
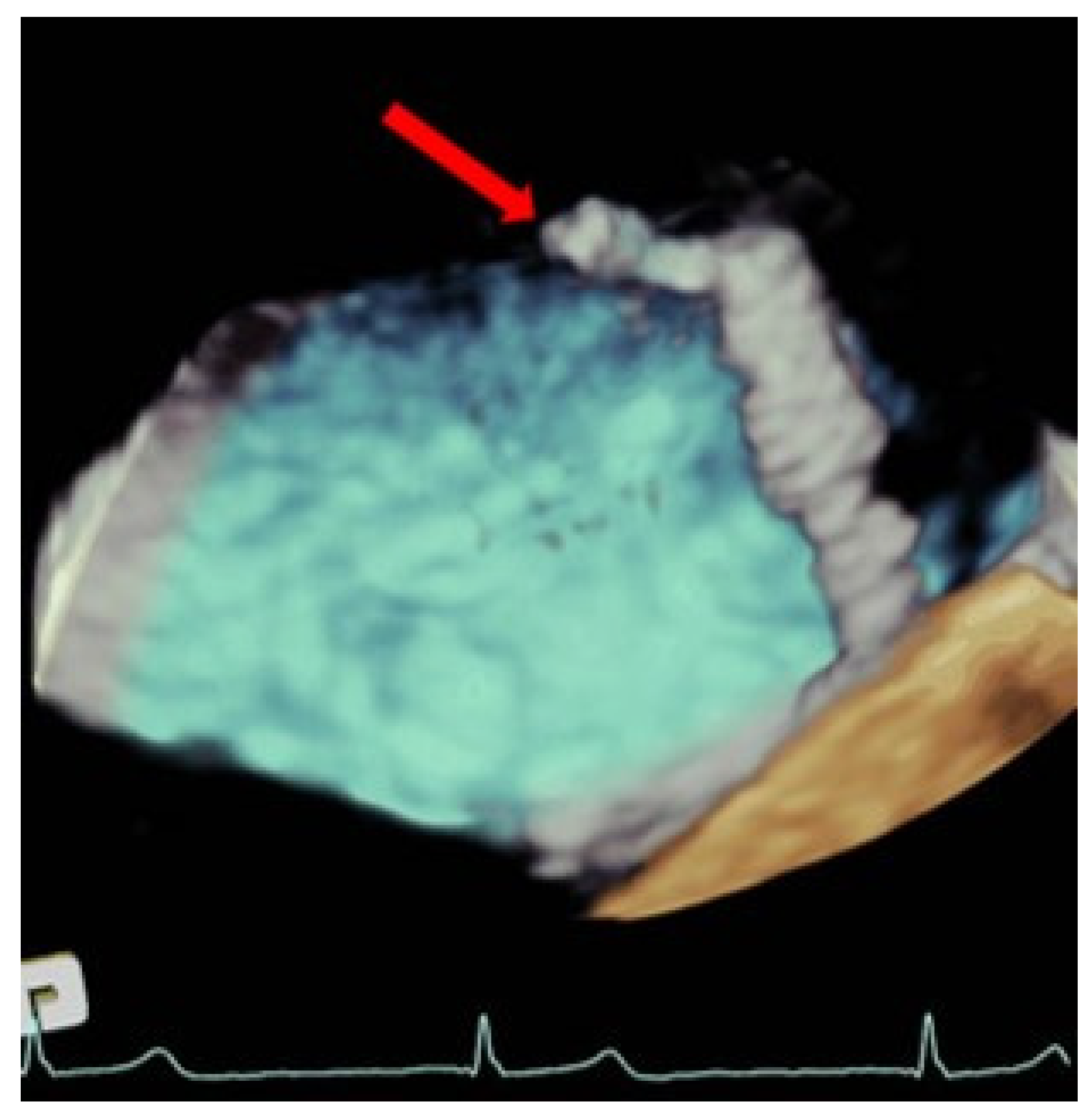

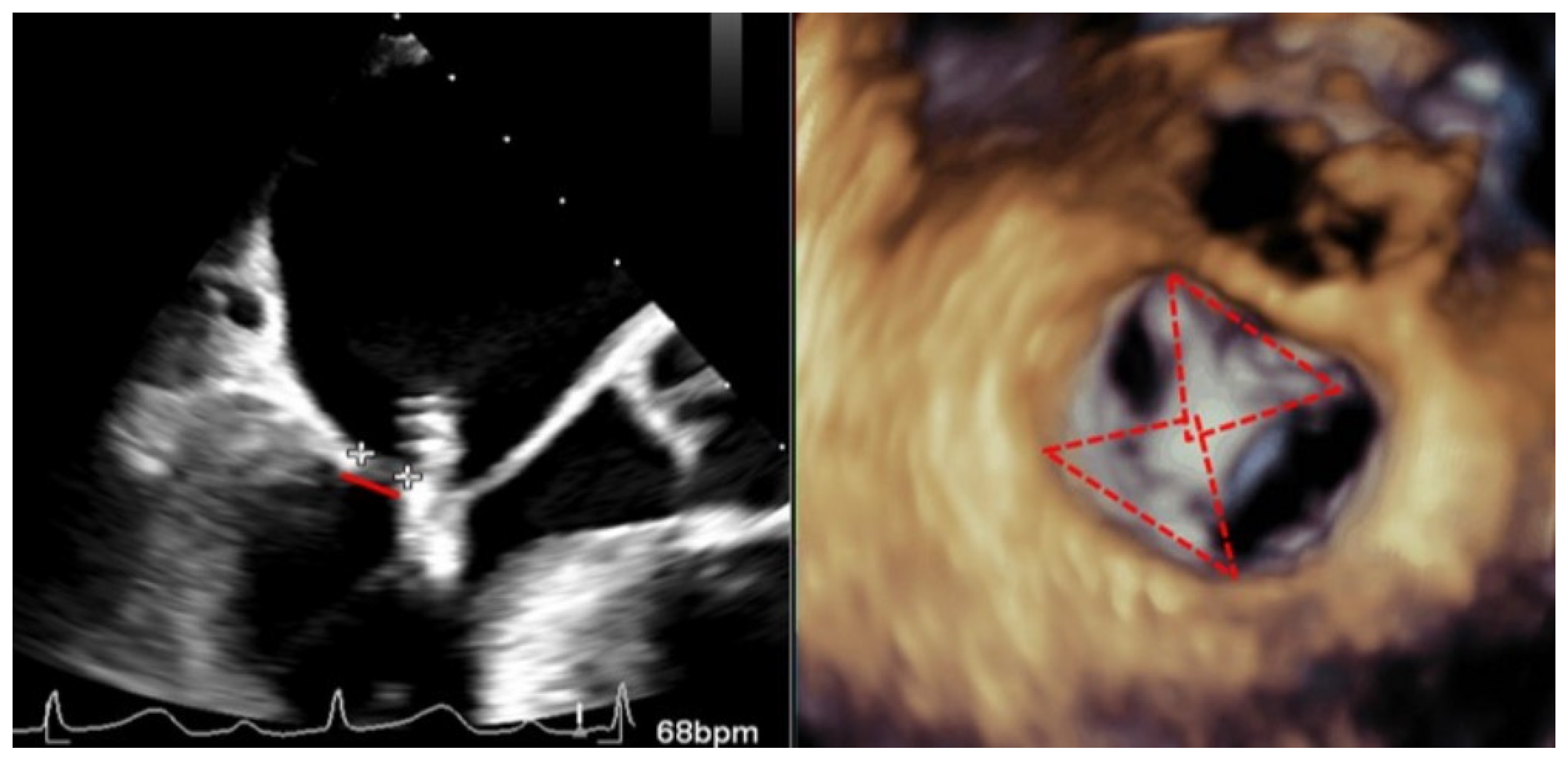
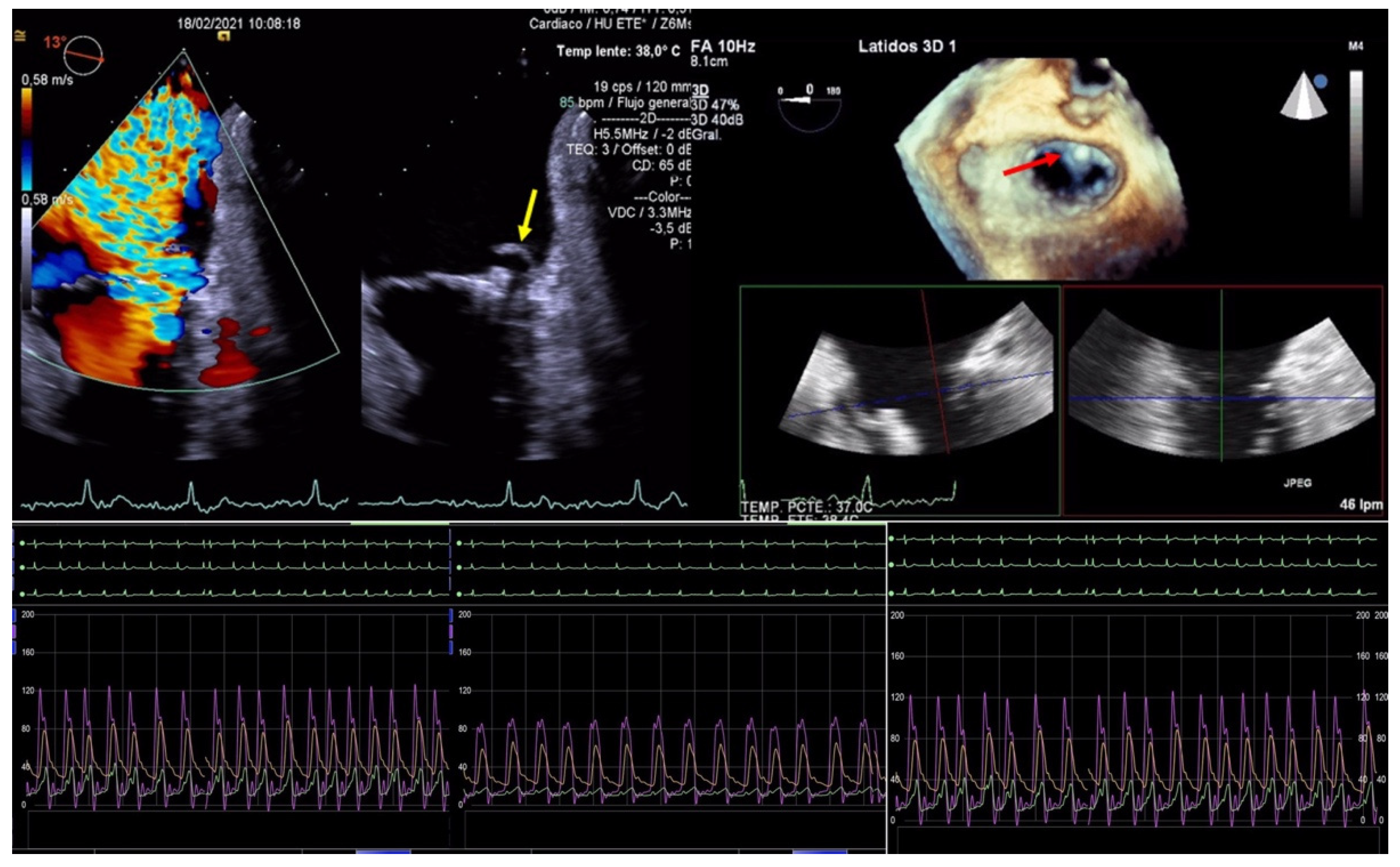
| EV-I | EV | EV-II | TCVT | GRASP | ACCESS-EU | TRAMI | TVT | COAPT | MITRA FR | Mitra Expand | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of study | Trial | Trial | Trial | Obs | Obs | Obs | Obs | Obs | Trial | Trial | Obs |
| Year of publication | 2005 | 2009 | 2011 | 2014 | 2013 | 2013 | 2015 | 2017 | 2018 | 2018 | 2019 |
| Devices generation | 1st | 1st | 1st | 1st | 1st | 1st | 1st | 1st | 1st 2nd | 1st 2nd | 3rd |
| Number of patients | 27 | 107 | 279 | 628 | 117 | 567 | 828 | 2952 | 302 | 144 | 107 |
| Complications | |||||||||||
| Related to the procedure | |||||||||||
| In-hospital death | 0% | 0.9% | 1.0% | 2.9% | 0.9% | 3.4% | 2.2% | 2.7% | ND | ND | 0.9% |
| Pericardial tamponade | 0% | 2.8% | 1.6% | 1.1% | 0% | 1.1% | 1.9% | 1% | ND | 1.4% | 0% |
| Thromboembolic events a | 0% | 0.9% | 1.0% | 0.2% | 0.9% | 1.1% | 0.9% | 0.5% | 0.7% | 1.4% | 0% |
| Acute renal failure | 0% | 0% | <1.0% | 0% | 0% | 4.8% | 0.7% | ND | ND | ND | 1% |
| Major bleeding | 3% | 3.7% | ND | 1.1% | ND | ND | 7.4% | 3.9% | ND | 3.5% | 1% |
| Major vascular complications | 0% | ND | 1.0% | 0.7% | ND | ND | 1.4% | 1.1% | ND | ND | ND |
| Related to the clip implantation | |||||||||||
| Single-leaflet device attachment | 0% | 2.8% | 5.0% | ND | ND | 4.8% | 2% | 1.5% | ND | ND | 4% |
| Clip embolization | 0% | 0% | 0.0% | 0.7% | ND | 0% | 0% | 0.1% | ND | ND | 0% |
| Early partial leaflet detachment b | 11% | 9% | 0.0% | ND | ND | 0.2% | 2% | ND | ND | ND | 0% |
| Thrombus formation on clip | 0% | ND | ND | ND | ND | ND | 0.1% | ND | ND | ND | 0% |
| Isolated leaflet damage | 0% | ND | ND | ND | ND | ND | ND | ND | ND | ND | 2% |
| Relevant mitral stenosis | 0% | ND | 0.0% | ND | ND | ND | 0.5% | ND | ND | ND | ND |
| Conversion to open surgery | 0% | 1.8% | 0.0% | 0% | 0% | 0% | 0% | 0.7% | ND | 0% | 4% |
| No procedural success c | 3% | 26% | 23% | 4.6% | 0% | 9% | 3.4% | 8.2% | 2% | 4.2% | 7% |
| Cardiac surgery during the first 30 days | 3% | 0.9% | ND | 0% | 0% | ND | 0.9% | ND | ND | 0% | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, G.; Mesa, D.; Ojeda, S.; de Lezo, J.S.; Gonzalez-Manzanares, R.; Dueñas, G.; Pan, M. Complications of the Percutaneous Mitral Valve Edge-To-Edge Repair: Role of Transesophageal Echocardiography. J. Clin. Med. 2022, 11, 4747. https://doi.org/10.3390/jcm11164747
Flores G, Mesa D, Ojeda S, de Lezo JS, Gonzalez-Manzanares R, Dueñas G, Pan M. Complications of the Percutaneous Mitral Valve Edge-To-Edge Repair: Role of Transesophageal Echocardiography. Journal of Clinical Medicine. 2022; 11(16):4747. https://doi.org/10.3390/jcm11164747
Chicago/Turabian StyleFlores, Guisela, Dolores Mesa, Soledad Ojeda, Javier Suárez de Lezo, Rafael Gonzalez-Manzanares, Guillermo Dueñas, and Manuel Pan. 2022. "Complications of the Percutaneous Mitral Valve Edge-To-Edge Repair: Role of Transesophageal Echocardiography" Journal of Clinical Medicine 11, no. 16: 4747. https://doi.org/10.3390/jcm11164747
APA StyleFlores, G., Mesa, D., Ojeda, S., de Lezo, J. S., Gonzalez-Manzanares, R., Dueñas, G., & Pan, M. (2022). Complications of the Percutaneous Mitral Valve Edge-To-Edge Repair: Role of Transesophageal Echocardiography. Journal of Clinical Medicine, 11(16), 4747. https://doi.org/10.3390/jcm11164747






