Abstract
The aim of this study was to evaluate the usefulness of serum interleukin (IL)-37 and IL-18 as disease activity markers of adult-onset Still’s disease (AOSD) and to compare their related clinical features. Forty-five patients with a set of high and subsequent low disease activity status of AOSD were enrolled. Modified Pouchot (mPouchot) score and serologic disease activity markers including levels of IL-37 and IL-18 were compared between high and low disease activity status. The relationships between disease activity parameters and differences in levels of cytokines according to each disease manifestation were evaluated in high disease activity status. mPouchot score and all disease activity markers including IL-37 and IL-18 significantly declined after treatment. Though both cytokines positively correlated with mPouchot score, the two did not correlate with each other in high disease activity status. IL-18 positively correlated with ferritin, AST, and LDH while IL-37 correlated better with CRP. The expression level of IL-37 was related to leukocytosis while IL-18 was related to pleuritis, pneumonitis, abnormal LFT, and hyperferritinemia. In addition, patients in the IL-18 dominant group presented with higher LDH levels and required a higher mean corticosteroid dose. In conclusion, IL-37 and IL-18 are disease activity markers reflecting different aspects of AOSD that can complement each other.
1. Introduction
Adult-onset Still’s disease (AOSD) is a rare systemic auto-inflammatory disorder characterized by four cardinal symptoms of spiking fever, arthralgia or arthritis, evanescent salmon-colored rash, and leukocytosis with neutrophil predominance [1]. The diagnosis of AOSD is still challenging owing to its heterogeneous clinical manifestations and few disease-specific markers [2]. Likewise, it is difficult to assess disease activity of AOSD. Pouchot et al. described a systemic disease activity score comprised of 12 main signs and symptoms of AOSD; this scoring system was later modified by Rau et al. [3,4]. Several biomarkers were proposed as potential disease activity parameters of AOSD including C-reactive protein (CRP), ferritin, and interleukin (IL)-18 [1,2,5]. However, no reliable disease activity parameter exists yet [6]. Given the potential polycyclic evolution of AOSD, it is necessary to identify biomarkers for accurate assessment of disease activity to improve AOSD management [1].
Although the pathogenesis of AOSD is unclear, many proinflammatory cytokines, such as IL-1β, IL-6, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and IL-18 seem to be involved; levels of these cytokines positively correlated with disease activity [6]. Among these cytokines, IL-18 is the most promising biomarker of AOSD as its serum level is particularly high in AOSD compared to other inflammatory diseases such as rheumatoid arthritis (RA), ankylosing spondylitis (AS), polymyalgia rheumatica, and sepsis [1,2,7,8]. IL-18 has not only been suggested as a potential biomarker for differential diagnosis of AOSD, but also for evaluation of disease activity [1,2]. However, its use in routine investigations has not been widely accepted as evidence of its reliability is insufficient [1,6,9].
IL-37 is a newly discovered member of the IL-1 cytokine family and has been identified as an inhibitor of immune responses in contrast to IL-18, another member of the IL-1 family [10]. Studies have shown elevated expression of IL-37 and its positive correlations with the disease activity parameters in patients with autoimmune diseases such as RA, systemic lupus erythematosus, AS, and systemic juvenile idiopathic arthritis [11,12,13,14,15]. In addition, a recent study identified increased expression of IL-37 and its positive correlation with disease activity markers in patients with AOSD [16]. However, our knowledge about the role of IL-37 in the pathogenesis of AOSD and its clinical applicability as a biomarker of the disease is very limited. Moreover, no study has compared IL-37 and IL-18 as disease activity parameters in patients with AOSD.
In this study, we evaluated how serum levels of IL-37 and IL-18 reflect the activity of AOSD using paired serum samples of high and low disease activity status in a cohort of patients with AOSD.
2. Materials and Methods
2.1. Study Population and the Evaluation Period
Sixty patients with AOSD were enrolled in this study, all of whom met the criteria of Yamaguchi et al. Four major criteria proposed by Yamaguchi include fever, arthralgia or arthritis, non-pruritic salmon-colored rash, and leukocytosis with granulocyte predominance. Minor criteria include sore throat, lymphadenopathy, hepatomegaly and/or splenomegaly, abnormal liver function, and negative test results for antinuclear antibody and rheumatoid factor. At least five criteria, including two major criteria and no exclusion criteria (infection, malignancy, and other rheumatic diseases), are required for the diagnosis of AOSD (Supplementary Table S1) [17]. Those with concurrent infection, malignancies, or other rheumatic diseases were not eligible. The medical records of patients were reviewed thoroughly regarding AOSD activity status assessed by the modified Pouchot (mPouchot) score proposed by Rau et al. [3,4]. The total score that ranges from 0 to 12 is calculated by assigning one point to each of these items: the presence of fever, evanescent rash, sore throat, arthritis, myalgia, pleuritis, pericarditis, pneumonitis, lymphadenopathy, hepatomegaly or abnormal liver function tests, white blood cell count (WBC) > 15,000/mm3, and serum ferritin > 3000 ng/mL. Consistent with previous studies, a high disease activity status of AOSD was defined as mPouchot score ≥ 4 [4,8]. Low disease activity status of AOSD was defined arbitrarily as mPouchot score ≤ 2 [18]. A set of high and subsequent low disease activity statuses that fall within a 12-month period were determined in each patient. For comparison of clinical parameters between high and low disease activity status of AOSD, patients without a record of definite high disease activity status or those with a high and low disease activity status interval longer than 12 months were excluded from the study.
A retrospective analysis of AOSD patients who prospectively visited a single university hospital for periodic examination or admission between August 2016 and April 2017 was performed. This analysis was an evaluation of clinical information from the initial diagnosis of AOSD until the time of investigation, April 2017. The study protocol was approved by the Institutional Review Board of Hanyang University Hospital, and informed consent was received from all participants (IRB no. HYUH 2016-06-007).
2.2. Laboratory Studies
Serial serum samples were collected from all study patients at one- to three-month intervals depending on the clinical situation. In addition to routine clinical laboratory studies including complete blood cell count and liver function tests, disease activity parameters such as ferritin, interleukin (IL)-18, and IL-37 levels were measured. Serum samples were obtained in the morning after overnight fasting, and care was taken to avoid hemolysis. The serum ferritin level was analyzed using the electrochemiluminescence method (Cobas e 601, Roche Diagnostics, Mannheim, Germany). Erythrocyte sedimentation rate (ESR) level was measured using the Westergren method (Starrsed, R & R mechatronic, Zwaag, The Netherlands). The AU5822 automated clinical chemistry analyzer (Beckman Coulter, Brea, CA, USA) was used for the measurement of levels of C-reactive protein (CRP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT), lactate dehydrogenase (LDH) using the immunoturbidimetric method (CRP), UV method (AST and ALT), and lactate to pyruvate method (LDH).
For measurement of cytokines, samples were centrifuged at 2500 rpm for 15 min at 5 °C within 30 min of collection at room temperature and maintained at −70 °C until use. Serum IL-18 (Medical and Biological Laboratories, Nagoya, Japan) and IL-37 (Elabscience Biotechnology Inc., Huston, TX, USA) were measured using commercial enzyme-linked immunosorbent assay kits, following the manufacturers’ instructions. If the absorbance of a sample for the IL-18 measurement exceeded 2500 pg/mL, the sample was diluted 20–200-fold. And, if the sample for the IL-37 measurement exceeded 1000 pg/mL, it was diluted 10-fold. The researcher who read the ELISA measurements was blinded to the patient information [2].
2.3. Statistical Analysis
Data are expressed according to the properties of the variables. The mPouchot score, clinical parameters included in the mPouchot score, and laboratory parameters were compared between high and low disease activity status using the McNemar test, Exact McNemar test, or Wilcoxon signed-rank test, as appropriate.
Clinical characteristics and laboratory parameters were analyzed in high disease activity statuses of AOSD. The relationships among the disease activity parameters were evaluated using Spearman’s correlation coefficients. The patients were divided into two groups, the IL-37 dominant group (patients with IL-37 ≥ median and IL-18 < median) and the IL-18 dominant group (patients with IL-18 ≥ median and IL-37 < median); clinical characteristics and laboratory parameters were compared using Wilcoxon rank-sum test and Fisher’s exact test, as appropriate. Serum levels of IL-37 and IL-18 were compared according to the presence or absence of each disease manifestation of AOSD by the Wilcoxon rank-sum test.
All statistical analyses were performed using SAS software version 9.4 (SAS, Cary, NC, USA) and R version 3.5.3. (The R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Among the 60 patients recruited for this study, 45 were included. Nine patients did not show definite high disease activity defined as mPouchot score ≥ 4, and six patients did not cycle between high and low disease activity status within a 12-month period; these 15 were excluded from the study.
3.1. Demographic and Clinical Characteristics
The demographic and clinical characteristics of patients with AOSD included in this study are shown in Table 1. The overall mean age of the patients was 47.1 (±14.0) years, with a mean disease duration of 59.5 (±48.2) months; female patients were more prevalent (86.7%). Retrospective analyses of the medical records of all patients revealed that the most common AOSD onset symptom was a combined pattern of systemic and articular diseases (68.9%). A total of 57.8% of patients showed two or more systemic disease flare-ups by April 2017, the end of the observation period.

Table 1.
Demographic and clinical characteristics of patients with adult-onset Still’s disease a.
The mean interval between high disease activity status and subsequent low disease activity status was 6.0 (±2.7) months. During this period, 17.8% of patients underwent steroid pulse therapy, and the most frequently used immune-modulating medications were methotrexate (77.8%), cyclosporin A (40.0%), and leflunomide (22.2%). Approximately one-third of patients (33.3%) had a re-flare of AOSD within one year after a low disease activity status, with a mean duration until re-flare of 4.3 (±4.8) months. More than one-half of patients (54.3%) had a re-flare within two years after achieving low disease activity status.
3.2. Paired Comparison of Clinical Features and Laboratory Parameters between High and Low Disease Activity Status
As shown in Table 2, the mPouchot score and all the laboratory parameters investigated, including IL-37 and IL-18, were significantly lower in the subsequent low disease activity status compared to the high disease activity status. The most common disease manifestations of AOSD in high disease activity status were fever (86.7%), skin rash (82.2%), arthritis (73.3%), myalgia (68.9%), and sore throat (66.7%). The least frequent clinical findings were pleuritis (13.3%) and pericarditis (4.4%), which failed to show a significant difference between high and low disease activity states.

Table 2.
Paired comparison of clinical characteristics between high and low disease activity status in patients with adult-onset Still’s disease a.
3.3. Correlations between Disease Activity Parameters in High Disease Activity Status
As shown in Table 3, IL-37 and IL-18 showed different patterns of correlations with other serologic disease activity markers in high disease activity status of AOSD. Unlike IL-18, which correlated better with serum levels of ferritin, LDH, and AST, IL-37 correlated better with CRP. IL-37 and IL-18 showed comparable correlations with systemic disease activity score (mPouchot score) (Spearman’s rho = 0.355 and 0.399, respectively, p < 0.05). However, no association was established between IL-37 and IL-18.

Table 3.
Correlations between disease activity parameters in high disease activity status of adult-onset Still’s disease.
3.4. IL-37 and IL-18 in High Disease Activity Status of AOSD
As shown in Figure 1, serum IL-37 and IL-18 levels were diffusely scattered at high disease activity status of AOSD, showing no linear correlation. Clinical characteristics were compared between two groups, each with 10 patients, with contrasting serum concentration levels of IL-37 and IL-18 (Table 4, Supplementary Table S2). There were no significant differences between the IL-37 dominant group and the IL-18 dominant group in demographics, AOSD disease course, incidence of disease flares, use of immune-modulating drugs at high disease activity status, and re-flare rate after low disease activity status. However, the IL-18 dominant group showed higher LDH levels at high disease activity status, and these patients required a higher mean steroid dose for achieving low disease activity status (p = 0.01 and 0.02, respectively). However, there was no difference in mPouchot score between the two groups in high disease activity status.
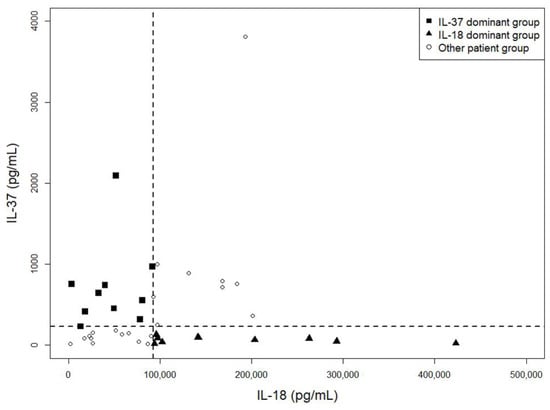
Figure 1.
Scatter plot of IL-37 and IL-18 levels at a high disease activity status of adult-onset Still’s disease. Serum IL-37 and IL-18 levels were diffusely scattered at a high disease activity status of AOSD, showing no linear correlation. Each symbol represents an individual patient and dotted lines represent the median values of IL-37 and IL-18. AOSD, adult-onset Still’s disease; IL, interleukin.

Table 4.
Comparison of disease activity parameters and medications used between the IL-37 and IL-18 dominant groups in patients with adult-onset Still’s disease a.
As shown in Table 5, IL-37 and IL-18 serum levels showed different patterns of increase according to the presence of each disease manifestation of AOSD at high disease activity status. Serum IL-18 levels were higher in patients with pleuritis, pneumonitis, abnormal liver function test, and hyperferritinemia (p < 0.05). In contrast, serum IL-37 level was only higher in patients with leukocytosis (p < 0.01). There was no concurrent increase in serum levels of IL-37 and IL-18 related to the presence of any clinical domain of AOSD.

Table 5.
Comparison of serum IL-37 and IL-18 levels according to disease manifestations at high disease activity status of patients with adult-onset Still’s disease.
4. Discussion
AOSD is a systemic auto-inflammatory disorder that presents with heterogeneous clinical manifestations. Its pathogenesis is largely unknown, and no single efficient disease activity marker for this disease has been identified [1,2]. Regarding its heterogeneity in the clinical presentation, we might need multi-modal approaches for investigating pathogenic mechanisms and assessing disease activity. In this study, we demonstrated that IL-37 level reflects disease activity of AOSD, as does the known cytokine biomarker IL-18, but in different ways [1].
IL-37 and IL-18 comprise the IL-18 subfamily of the IL-1 family as both bind to the IL-18 receptor [19,20]. IL-18 plays a key role in the polarized type 1 innate and adaptive responses that can possibly extend to the mediation of autoimmune diseases [21]. In contrast, IL-37 is known to be negatively involved in the development and pathogenesis of autoimmune diseases [16,22,23]. The expression level of IL-37 is low in healthy human tissues but is stimulated in severe inflammatory conditions to inhibit excessive immune response [22,24]. Proinflammatory stimuli including cytokines such as IL-1β or engagement of various Toll-like receptors activate the production of IL-37 [10,25]. Though both IL-18 and IL-37 bind to the α chain of the IL-18 receptor (IL-18Rα) on the cell surface, the IL-37 combined complex transduces anti-inflammatory signals while the IL-18 combined complex initiates a strong proinflammatory process that produces IFN-γ [10,26,27]. IL-18 induces the production of IFN-γ, IL-17A, and TNF-α, which play an important role in the disease manifestations of AOSD [28]. IL-6 is also elevated and known to be related to some clinical features of AOSD such as fever, arthritis, and increased production of acute-phase proteins [29,30]. Circulating IL-18 Binding Protein (IL-18BP) provides the main regulatory mechanism on IL-18 mediated inflammation by sequestrating IL-18 and prevents its binding to the receptor [10]. IL-18BP also binds to IL-37 and hence high level of IL-18BP could reduce the anti-inflammatory activity of IL-37. In addition, IL-37 can translocate into the nucleus and regulate gene expression of proinflammatory cytokines such as TNF-α, IL-1α, and IL-6 [10]. Previous studies conducted in murine models revealed the improvement of clinical features related to inflammation such as fatigue, arthritis, insulin resistance, and gouty arthritis after treatment with recombinant IL-37 [31,32,33,34]. Gout is another auto-inflammatory disease that shares the common pathophysiology of IL-1 mediated inflammation with AOSD. Joosten, et al. previously identified rare genetic variants of IL-37 in gout patients by sequencing all coding bases of IL-37 [34]. These results all indicate the potential mechanistic role of IL-37 in the pathogenesis of AOSD.
Previous studies supported the usefulness of IL-18 in AOSD differential diagnosis, disease activity evaluation, subset prediction (systemic or chronic articular subtypes), and severity assessment [1,2,5,7,8,18]. However, there is scarce information about the role of IL-37 in AOSD [16]. Chi et al. recently reported that the serum level of IL-37 was higher in patients with AOSD compared to healthy subjects, and a significant difference in the serum level of IL-37 was observed between patients with active and inactive disease status [16]. The serum level of IL-37 was even higher in patients with inactive disease compared to healthy subjects. In addition, their results showed a significant decrease in serum level of IL-37 after treatment in 10 patients with serial work-up data [16]. Though previous studies suggested both IL-37 and IL-18 as estimators of disease activity in patients with AOSD, controversies exist as to whether IL-18 could serve as a marker for disease remission and follow-up [1]. We noted four studies that demonstrated changes in serum level of IL-18 between high and subsequent low disease activity status after treatment [2,18,35,36]. Two studies from the same institution showed no significant decrease in serum IL-18 level after treatment in 16 and 17 patients with AOSD, respectively [18,35]. However, they did not apply specific criteria for a high disease activity status of AOSD. Contrary to their results, our previous study indicated that serum IL-18 declined significantly in 18 patients with AOSD who responded to therapy but not in non-responders [2]. In addition, the serum IL-18 level was higher in patients with low disease activity compared to those in remission, unlike other serologic disease activity markers. Therefore, we previously suggested IL-18 as an efficient marker for remission and follow-up in patients with AOSD [2]. In the current study, the serum IL-18 level decreased significantly in patients who achieved low disease activity status along with other disease activity markers including leukocytes, ESR, CRP, AST, ALT, ferritin, and IL-37 (Table 2). Our results are also compatible with those of another report of 11 patients with AOSD [36]. The differing results regarding change of serum IL-18 level could be due to different criteria for high and low disease activity status or differences in baseline characteristics of study populations, such as disease subset type. Previous studies have demonstrated that serum IL-18 level tends to be higher in patients presenting with systemic subset compared to chronic articular subtype [1,37]. After applying clear set criteria for both high and low disease activity status, we noted that both IL-37 and IL-18 could be used as disease activity evaluators even for follow-up of AOSD activity.
We evaluated IL-37 and IL-18 in relation to other disease activity parameters and various clinical features in high disease activity status of AOSD to compare their roles in the activity of this disease. In addition to there being no correlation between IL-18 and IL-37 serum levels, the differences in their patterns of positive correlations with other serologic markers suggest their different pathogenic roles in disease activity (Table 3). In addition, there were different patterns in the increase of both serum cytokines regarding the presence of each clinical manifestation of AOSD (Table 5). IL-37 seems to be more related to non-specific markers of inflammation such as CRP and leukocytes [1]. In contrast, IL-18 seems to be more related to pleuritis, pneumonitis, and serologic markers associated with liver injury such as AST, ALT, ferritin, and LDH [7,30,38,39,40]. We also demonstrated that some patients with AOSD could be grouped as those with the dominant increase in the serum level of IL-37 or IL-18 (Figure 1). Though patients in both groups showed no difference in mPouchot score, those in the IL-18 dominant group presented with higher serum level of LDH at high disease activity status. In addition, they required a higher mean corticosteroid dose to achieve low disease activity status with no difference in the cumulative dosage of corticosteroid (Table 4 and Supplementary Table S2). LDH performs a prominent role in the active metabolism of the overall body and unusual LDH isoform level in serum serves as a significant biomarker of different diseases [41]. In a case report, Motoo et al. demonstrated that the increased serum LDH during an AOSD flare-up was mainly of liver origin (LDH isoenzymes 4 and 5) [40]. Macrophage-derived IL-18 plays a fundamental role in hepatocyte apoptosis via activation of the Fas/Fas ligand pathway [39,42,43]. Priori et al. demonstrated that the serum IL-18 level is markedly increased in patients with AOSD-related hepatitis. In addition, they histologically revealed that IL-18 was highly expressed by activated CD68+ liver macrophages and Kupffer cells in a patient with AOSD [38]. Therefore, it is possible that IL-18 related hypermetabolic changes or hepatic injury could have led to a higher intensity of therapy in IL-18 dominant group patients in the current study. It has been known that hyperferritinemia in AOSD patients is primarily due to increased secretion in the liver and spleen in patients with AOSD [7,30]. CRP is produced only by hepatocytes, predominantly induced by IL-6, in response to inflammation [44]. However, its increase in serum could be dampened by severe liver dysfunction [45]. In contrast, the increase in serum level of IL-18 or ferritin could reflect the status of liver injury [1,46,47,48]. Patients with AOSD present with heterogeneous clinical features of multi-systemic involvement [29]. Likewise, not all patients in this study presented with an abnormal liver function test or serum ferritin level higher than 3000 ng/mL in high disease activity status (Table 2). Therefore, other serologic markers could be better markers of disease activity compared to IL-18 or ferritin in a substantial portion of patients with AOSD. For a better evaluation of disease activity in patients with AOSD, we might need additional useful biomarkers or a multi-biomarker disease activity index that properly reflects a patient status such as what has developed for patients with RA [49].
In all relevant previous studies regarding correlations between serum level of IL-18 and other serologic markers in patients with active AOSD, serum IL-18 level positively correlated with serum ferritin level and systemic disease scores of AOSD, alike our study results [7,8,18,35,38,50]. However, its correlations with serum ESR or CRP have shown inconsistent results, probably due to the different characteristics of patients studied [7,8,18,35,38]. Only two studies conducted by Priori et al. showed significant positive correlations between serum IL-18 level and non-specific inflammatory markers. However, unlike ours, their analyses included patients in inactive disease status of AOSD [8,38]. According to a previous study of IL-37 in patients with AOSD conducted by Chi et al., serum IL-37 level positively correlated with non-specific disease activity markers including leukocytes and CRP but not with liver enzymes. However, unlike our study results, serum IL-37 level correlated well with IL-18 level in 62 patients with AOSD [16]. In addition, more diverse clinical features (fever, sore throat, rash, lymphadenopathy, splenomegaly, myalgia, and arthralgia) were significantly related to higher levels of IL-37. We noted that a third of their study population (21 patients) were inactive AOSD patients with a mean mPouchot score of 0.2 ± 0.5 indicating almost no disease-related symptoms. Therefore, the difference between the two groups could have been more easily reflected in their analysis results owing to the conflicting disease activities among patients [51]. With very limited information especially regarding the role of IL-37 in AOSD, we are unaware whether how differently both IL-37 and IL-18 change in their serum concentrations depending on the disease activity of AOSD [22]. Therefore, to examine the correlation between both cytokines in patients with AOSD, it would be better to control the disease activity of AOSD in the analysis to get more reliable results.
The current study and previous relevant study findings, overall, indicate that both serum levels of IL-37 and IL-18 can be good markers of disease activity in patients with AOSD. We add new finding by demonstrating different patterns of increase in serum levels of both cytokines and related clinical features in the high disease activity status of AOSD. Measuring both serum levels of IL-37 and IL-18 could complement each other to better understand this heterogeneous, multi-systemic disease. Our study has several limitations. First, the sample size of this study was small owing to the rarity of the disease. However, our study was the largest comparing paired serum samples of both high and low disease activity statuses of AOSD. Second, our evaluation did not include other potential mechanistic cytokines of AOSD, such as IL-1β, IL-6, or TNF-α [1]. Future studies with larger sample sizes that evaluate various serologic cytokine profiles are needed. Third, there could be the concern that the use of immune-modulating drugs at high disease activity status could have affected patient cytokine profile to some extent. However, we noted that there were no significant differences in the frequencies of immune-modulating drug use between compared groups of Table 4 and Table 5. Fourth, as our study was a retrospective observational study, patient visit interval or therapeutic protocol was not constant among patients. Study patients were treated by a single rheumatologist for the purpose of controlling disease activity with optimal medical therapies available. The patient visit interval was determined by clinical situation and varied from two weeks to three months during the observation period. Last, there was no consensus in defining high or low disease activity status of AOSD or in defining re-flare of disease after achieving low disease activity status. Therefore, we referred to previous studies that applied similar definitions [4,8,18]. However, we applied the operational definition of disease re-flare as an increase in mPouchot score, increase in dosage, or addition of immune-modulating drugs including glucocorticoids.
5. Conclusions
We identified that serum levels of IL-37 and IL-18 reflect different clinical features of AOSD. Both could be utilized as disease activity markers that can complement each other.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/10/5/910/s1, Table S1: Yamaguchi criteria for the diagnosis of adult-onset Still’s disease. Table S2: Comparison of clinical characteristics between the IL-37 and IL-18 dominant groups in patients with adult-onset Still’s disease.
Author Contributions
Conceptualization, D.H.Y. and S.W.N.; methodology, D.H.Y. and S.W.N.; software, S.W.N. and D.H.Y.; validation, S.W.N. and D.H.Y.; formal analysis, J.H.L. and S.W.N.; investigation, S.W.N. and S.K.; resources, D.H.Y. and S.K.; data curation, S.W.N. and S.K.; writing—original draft preparation, S.W.N.; writing—review and editing, D.H.Y. and S.W.N.; visualization, S.W.N. and J.H.L.; supervision, D.H.Y.; project administration, D.H.Y.; funding acquisition, D.H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Hanyang University Hospital (IRB no. HYUH 2016-06-007. Approved: June, 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the ethical issues.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mitrovic, S.; Fautrel, B. New markers for adult-onset still’s disease. Jt. Bone Spine 2018, 85, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Kim, J.J.; Lee, J.S.; Park, W.; Kim, T.H.; Jun, J.B.; Yoo, D.H. Interleukin-18 as an efficient marker for remission and follow-up in patients with inactive adult-onset still’s disease. Scand. J. Rheumatol. 2014, 43, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Pouchot, J.; Sampalis, J.S.; Beaudet, F.; Carette, S.; Decary, F.; Salusinsky-Sternbach, M.; Hill, R.O.; Gutkowski, A.; Harth, M.; Myhal, D.; et al. Adult still’s disease: Manifestations, disease course, and outcome in 62 patients. Medicine 1991, 70, 118–136. [Google Scholar] [CrossRef]
- Rau, M.; Schiller, M.; Krienke, S.; Heyder, P.; Lorenz, H.; Blank, N. Clinical manifestations but not cytokine profiles differentiate adult-onset still’s disease and sepsis. J. Rheumatol. 2010, 37, 2369–2376. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Terajima, H.; Harigai, M.; Hara, M.; Kamatani, N. Interleukin-18 as a novel diagnostic marker and indicator of disease severity in adult-onset still’s disease. Arthritis Rheum. 2001, 44, 1716–1717. [Google Scholar] [CrossRef]
- Feist, E.; Mitrovic, S.; Fautrel, B. Mechanisms, biomarkers and targets for adult-onset still’s disease. Nat. Rev. Rheumatol. 2018, 14, 603–618. [Google Scholar] [CrossRef]
- Kawashima, M.; Yamamura, M.; Taniai, M.; Yamauchi, H.; Tanimoto, T.; Kurimoto, M.; Miyawaki, S.; Amano, T.; Takeuchi, T.; Makino, H. Levels of interleukin-18 and its binding inhibitors in the blood circulation of patients with adult-onset still’s disease. Arthritis Rheum. 2001, 44, 550–560. [Google Scholar] [CrossRef]
- Priori, R.; Colafrancesco, S.; Alessandri, C.; Minniti, A.; Perricone, C.; Iaiani, G.; Palazzo, D.; Valesini, G. Interleukin 18: A biomarker for differential diagnosis between adult-onset still’s disease and sepsis. J. Rheumatol. 2014, 41, 1118–1123. [Google Scholar] [CrossRef]
- Shimizu, T.; Kikuchi-Taura, A.; Tsuji, S.; Matsushita, M.; Ohshima, S.; Saeki, Y. Up-regulation of cd64 expression on monocytes in patients with active adult-onset still disease: A possible biomarker of disease activity. J. Clin. Rheumatol. 2018. [Google Scholar] [CrossRef]
- Cavalli, G.; Dinarello, C.A. Suppression of inflammation and acquired immunity by il-37. Immunol. Rev. 2018, 281, 179–190. [Google Scholar] [CrossRef]
- Zhao, P.W.; Jiang, W.G.; Wang, L.; Jiang, Z.Y.; Shan, Y.X.; Jiang, Y.F. Plasma levels of il-37 and correlation with tnf-alpha, il-17a, and disease activity during dmard treatment of rheumatoid arthritis. PLoS ONE 2014, 9, e95346. [Google Scholar] [CrossRef]
- Ye, L.; Ji, L.; Wen, Z.; Zhou, Y.; Hu, D.; Li, Y.; Yu, T.; Chen, B.; Zhang, J.; Ding, L.; et al. Il-37 inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells of patients with systemic lupus erythematosus: Its correlation with disease activity. J. Transl. Med. 2014, 12, 69. [Google Scholar] [CrossRef]
- Song, L.; Qiu, F.; Fan, Y.; Ding, F.; Liu, H.; Shu, Q.; Liu, W.; Li, X. Glucocorticoid regulates interleukin-37 in systemic lupus erythematosus. J. Clin. Immunol. 2013, 33, 111–117. [Google Scholar] [CrossRef]
- Chen, B.; Huang, K.; Ye, L.; Li, Y.; Zhang, J.; Zhang, J.; Fan, X.; Liu, X.; Li, L.; Sun, J.; et al. Interleukin-37 is increased in ankylosing spondylitis patients and associated with disease activity. J. Transl. Med. 2015, 13, 36. [Google Scholar] [CrossRef]
- Feng, M.; Kang, M.; He, F.; Xiao, Z.; Liu, Z.; Yao, H.; Wu, J. Plasma interleukin-37 is increased and inhibits the production of inflammatory cytokines in peripheral blood mononuclear cells in systemic juvenile idiopathic arthritis patients. J. Transl. Med. 2018, 16, 277. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Liu, D.; Sun, Y.; Hu, Q.; Liu, H.; Cheng, X.; Ye, J.; Shi, H.; Yin, Y.; Liu, M.; et al. Interleukin-37 is increased in adult-onset still’s disease and associated with disease activity. Arthritis Res. Ther. 2018, 20, 54. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Ohta, A.; Tsunematsu, T.; Kasukawa, R.; Mizushima, Y.; Kashiwagi, H.; Kashiwazaki, S.; Tanimoto, K.; Matsumoto, Y.; Ota, T.; et al. Preliminary criteria for classification of adult still’s disease. J. Rheumatol. 1992, 19, 424–430. [Google Scholar] [PubMed]
- Kim, H.A.; An, J.M.; Nam, J.Y.; Jeon, J.Y.; Suh, C.H. Serum s100a8/a9, but not follistatin-like protein 1 and interleukin 18, may be a useful biomarker of disease activity in adult-onset still’s disease. J. Rheumatol. 2012, 39, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Netea, M.G. New insights in the immunobiology of il-1 family members. Front. Immunol. 2013, 4, 167. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Risser, P.; Mao, W.; Baldwin, D.T.; Zhong, A.W.; Filvaroff, E.; Yansura, D.; Lewis, L.; Eigenbrot, C.; Henzel, W.J.; et al. Il-1h, an interleukin 1-related protein that binds il-18 receptor/il-1rrp. Cytokine 2001, 13, 1–7. [Google Scholar] [CrossRef]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef]
- Jia, H.; Liu, J.; Han, B. Reviews of interleukin-37: Functions, receptors, and roles in diseases. BioMed Res. Int. 2018, 2018, 3058640. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Jiang, B.; Deng, J.; Du, J.; Xiong, W.; Guan, Y.; Wen, Z.; Huang, K.; Huang, Z. Il-37 alleviates rheumatoid arthritis by suppressing il-17 and il-17-triggering cytokine production and limiting th17 cell proliferation. J. Immunol. 2015, 194, 5110–5119. [Google Scholar] [CrossRef]
- Bufler, P.; Gamboni-Robertson, F.; Azam, T.; Kim, S.H.; Dinarello, C.A. Interleukin-1 homologues il-1f7b and il-18 contain functional mrna instability elements within the coding region responsive to lipopolysaccharide. Biochem. J. 2004, 381, 503–510. [Google Scholar] [CrossRef]
- Nold, M.F.; Nold-Petry, C.A.; Zepp, J.A.; Palmer, B.E.; Bufler, P.; Dinarello, C.A. Il-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 2010, 11, 1014–1022. [Google Scholar] [CrossRef]
- Tsutsumi, N.; Kimura, T.; Arita, K.; Ariyoshi, M.; Ohnishi, H.; Yamamoto, T.; Zuo, X.; Maenaka, K.; Park, E.Y.; Kondo, N.; et al. The structural basis for receptor recognition of human interleukin-18. Nat. Commun. 2014, 5, 5340. [Google Scholar] [CrossRef]
- Nold-Petry, C.A.; Lo, C.Y.; Rudloff, I.; Elgass, K.D.; Li, S.; Gantier, M.P.; Lotz-Havla, A.S.; Gersting, S.W.; Cho, S.X.; Lao, J.C.; et al. Il-37 requires the receptors il-18ralpha and il-1r8 (sigirr) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat. Immunol. 2015, 16, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Kadavath, S.; Efthimiou, P. Adult-onset still’s disease-pathogenesis, clinical manifestations, and new treatment options. Ann. Med. 2015, 47, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, R.; Ruscitti, P.; Shoenfeld, Y. A comprehensive review on adult onset still’s disease. J. Autoimmun. 2018, 93, 24–36. [Google Scholar] [CrossRef]
- Sabnis, G.R.; Gokhale, Y.A.; Kulkarni, U.P. Tocilizumab in refractory adult-onset still’s disease with aseptic meningitis--efficacy of interleukin-6 blockade and review of the literature. Semin. Arthritis Rheum. 2011, 40, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Justice, J.N.; Boyle, K.E.; D’Alessandro, A.; Eisenmesser, E.Z.; Herrera, J.J.; Hansen, K.C.; Nemkov, T.; Stienstra, R.; Garlanda, C.; et al. Interleukin 37 reverses the metabolic cost of inflammation, increases oxidative respiration, and improves exercise tolerance. Proc. Natl. Acad. Sci. USA 2017, 114, 2313–2318. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, G.; Koenders, M.; Kalabokis, V.; Kim, J.; Tan, A.C.; Garlanda, C.; Mantovani, A.; Dagna, L.; Joosten, L.A.; Dinarello, C.A. Treating experimental arthritis with the innate immune inhibitor interleukin-37 reduces joint and systemic inflammation. Rheumatology 2016, 55, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Ballak, D.B.; Li, S.; Cavalli, G.; Stahl, J.L.; Tengesdal, I.W.; van Diepen, J.A.; Kluck, V.; Swartzwelter, B.; Azam, T.; Tack, C.J.; et al. Interleukin-37 treatment of mice with metabolic syndrome improves insulin sensitivity and reduces pro-inflammatory cytokine production in adipose tissue. J. Biol. Chem. 2018, 293, 14224–14236. [Google Scholar] [CrossRef] [PubMed]
- Kluck, V.; van Deuren, R.C.; Cavalli, G.; Shaukat, A.; Arts, P.; Cleophas, M.C.; Crisan, T.O.; Tausche, A.K.; Riches, P.; Dalbeth, N.; et al. Rare genetic variants in interleukin-37 link this anti-inflammatory cytokine to the pathogenesis and treatment of gout. Ann. Rheum. Dis. 2020, 79, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Suh, C.H.; Lee, Y.M.; Suh, Y.J.; Lee, S.K.; Kim, S.S.; Nahm, D.H.; Park, H.S. Serum cytokine profiles in patients with adult onset still’s disease. J. Rheumatol. 2003, 30, 2422–2427. [Google Scholar]
- Kudela, H.; Drynda, S.; Lux, A.; Horneff, G.; Kekow, J. Comparative study of interleukin-18 (il-18) serum levels in adult onset still’s disease (aosd) and systemic onset juvenile idiopathic arthritis (sjia) and its use as a biomarker for diagnosis and evaluation of disease activity. BMC Rheumatol. 2019, 3, 4. [Google Scholar] [CrossRef]
- Inoue, N.; Shimizu, M.; Tsunoda, S.; Kawano, M.; Matsumura, M.; Yachie, A. Cytokine profile in adult-onset still’s disease: Comparison with systemic juvenile idiopathic arthritis. Clin. Immunol. 2016, 169, 8–13. [Google Scholar] [CrossRef]
- Priori, R.; Barone, F.; Alessandri, C.; Colafrancesco, S.; McInnes, I.B.; Pitzalis, C.; Valesini, G.; Bombardieri, M. Markedly increased il-18 liver expression in adult-onset still’s disease-related hepatitis. Rheumatology 2011, 50, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Finotto, S.; Siebler, J.; Hausding, M.; Schipp, M.; Wirtz, S.; Klein, S.; Protschka, M.; Doganci, A.; Lehr, H.A.; Trautwein, C.; et al. Severe hepatic injury in interleukin 18 (il-18) transgenic mice: A key role for il-18 in regulating hepatocyte apoptosis in vivo. Gut 2004, 53, 392–400. [Google Scholar] [CrossRef]
- Motoo, Y.; Ohta, H.; Okai, T.; Sawabu, N. Adult-onset still’s disease: Hepatic involvement and various serum markers relating to the disease activity. Jpn. J. Med. 1991, 30, 247–250. [Google Scholar] [CrossRef][Green Version]
- Khan, A.A.; Allemailem, K.S.; Alhumaydhi, F.A.; Gowder, S.J.T.; Rahmani, A.H. The biochemical and clinical perspectives of lactate dehydrogenase: An enzyme of active metabolism. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Matsui, K.; Okamura, H.; Nakanishi, K. Pathophysiological roles of interleukin-18 in inflammatory liver diseases. Immunol. Rev. 2000, 174, 192–209. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Kayagaki, N.; Kuida, K.; Nakano, H.; Hayashi, N.; Takeda, K.; Matsui, K.; Kashiwamura, S.; Hada, T.; Akira, S.; et al. Caspase-1-independent, fas/fas ligand-mediated il-18 secretion from macrophages causes acute liver injury in mice. Immunity 1999, 11, 359–367. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: A critical update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Pieri, G.; Agarwal, B.; Burroughs, A.K. C-reactive protein and bacterial infection in cirrhosis. Ann. Gastroenterol. 2014, 27, 113–120. [Google Scholar]
- Lim, K.B.; Schiano, T.D. Still disease and the liver-an underappreciated association. Gastroenterol. Hepatol. 2011, 7, 844–846. [Google Scholar]
- Slaats, J.; Ten Oever, J.; van de Veerdonk, F.L.; Netea, M.G. Il-1beta/il-6/crp and il-18/ferritin: Distinct inflammatory programs in infections. PLoS Pathog. 2016, 12, e1005973. [Google Scholar] [CrossRef]
- Kowdley, K.V.; Belt, P.; Wilson, L.A.; Yeh, M.M.; Neuschwander-Tetri, B.A.; Chalasani, N.; Sanyal, A.J.; Nelson, J.E.; Nash Clinical Research Network. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2012, 55, 77–85. [Google Scholar] [CrossRef]
- Hirata, S.; Tanaka, Y. Assessment of disease activity in rheumatoid arthritis by multi-biomarker disease activity (mbda) score. Nihon Rinsho Meneki Gakkai Kaishi 2016, 39, 37–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, D.Y.; Lan, J.L.; Lin, F.J.; Hsieh, T.Y. Proinflammatory cytokine profiles in sera and pathological tissues of patients with active untreated adult onset still’s disease. J. Rheumatol. 2004, 31, 2189–2198. [Google Scholar]
- Whitley, E.; Ball, J. Statistics review 6: Nonparametric methods. Crit Care 2002, 6, 509–513. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).