Proper Selection Does Make the Difference: A Propensity-Matched Analysis of Percutaneous and Surgical Cut-Down Transfemoral TAVR
Abstract
1. Background
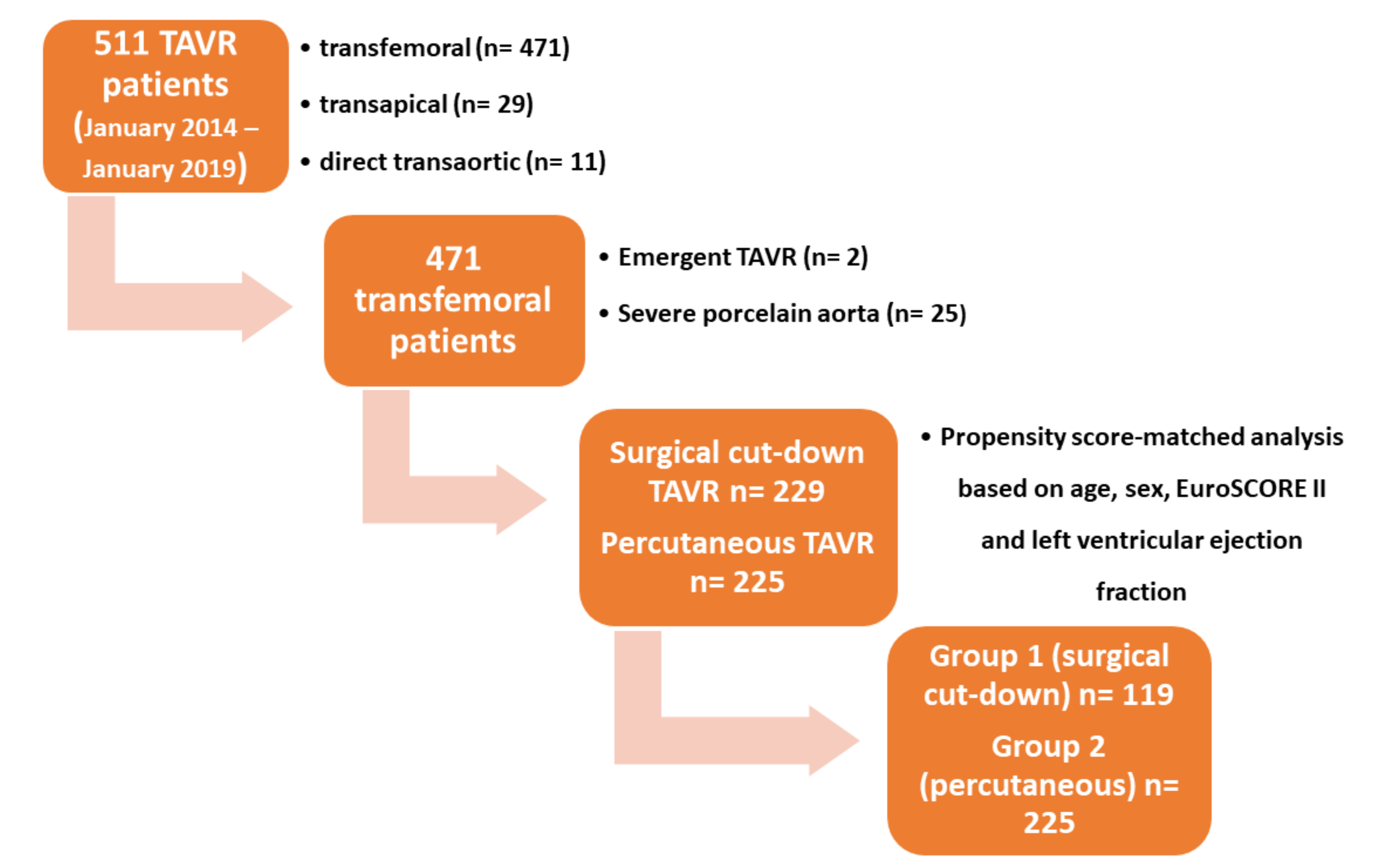
2. Methods
2.1. Patients
2.2. Ethical Committee
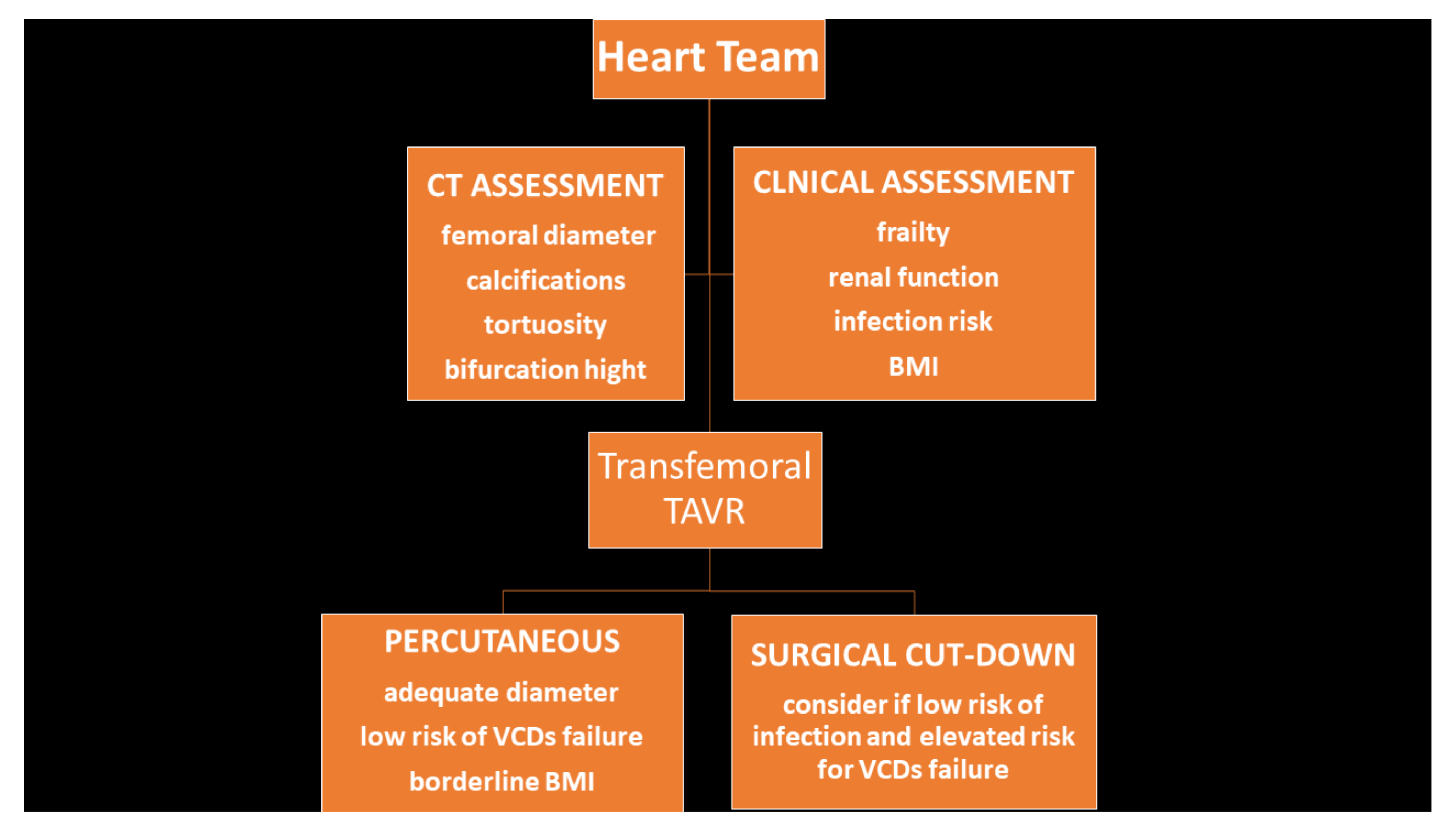
2.3. Surgical Cut-Down Technique
2.4. Percutaneous Access Technique
2.5. Outcomes and Follow-Up
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. In-Hospital Outcomes
3.3. Clinical Outcome at Follow-Up
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Ates, I.; Cilingiroglu, M. Percutaneous access versus surgical cut down for TAVR: Where do we go from here? Catheter Cardiovasc. Interv. 2018, 91, 1363–1364. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Webb, J.G.; Svensson, L.G.; Kodali, S.K.; Satler, L.F.; Fearon, W.F.; Davidson, C.J.; Eisenhauer, A.C.; Makkar, R.R.; Bergman, G.W.; et al. Vascular complications after transcatheter aortic valve replacement: Insights from the PARTNER (Placement of AoRTic TraNscathetER Valve) trial. J. Am. Coll. Cardiol. 2012, 60, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Briasoulis, A.; Holmes, A.A.; Takagi, H.; Slovut, D.P. Percutaneous versus surgical cut-down access in transfemoral transcatheter aortic valve replacement: A meta-analysis. J. Card. Surg. 2016, 31, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Drafts, B.C.; Choi, C.H.; Sangal, K.; Cammarata, M.W.; Applegate, R.J.; Gandhi, S.K.; Kincaid, E.H.; Kon, N.; Zhao, D.X. Comparison of outcomes with surgical cut-down versus percutaneous transfemoral transcatheter aortic valve replacement: TAVR transfemoral access comparisons between surgical cut-down and percutaneous approach. Catheter Cardiovasc. Interv. 2018, 91, 1354–1362. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Lefèvre, T.; Chevalier, B.; Hovasse, T.; Romano, M.; Garot, P.; Mylotte, D.; Uribe, J.; Farge, A.; Donzeau-Gouge, P.; et al. Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc. Interv. 2011, 4, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Lung, B.; Lancellotti, P.; Lanac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Kappetein, A.P.; Head, S.J.; Généreux, P.; Piazza, N.; van Mieghem, N.M.; Blackstone, E.H.; Brott, T.G.; Cohen, D.J.; Cutlip, D.E.; van Es, G.A.; et al. Valve Academic Research Consortium (VARC)-2. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The Valve Academic Research Consortium-2 consensus document (VARC-2). Eur. J. Cardiothorac. Surg. 2012, 42, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Van Mieghem, N.M.; Nuis, R.J.; Piazza, N.; Apostolos, T.; Ligthart, J.; Schultz, C.; de Jaegere, P.P.; Serruys, P.W. Vascular complications with transcatheter aortic valve implantation using the 18 Fr Medtronic CoreValve System: The Rotterdam experience. EuroIntervention 2010, 5, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Ducrocq, G.; Francis, F.; Serfaty, J.M.; Himbert, D.; Maury, J.M.; Pasi, N.; Marouene, S.; Provenchère, S.; Iung, B.; Castier, Y.; et al. Vascular complications of transfemoral aortic valve implantation with the Edwards SAPIEN prosthesis: Incidence and impact on outcome. EuroIntervention 2010, 5, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Toggweiler, S.; Gurvitch, R.; Leipsic, J.; Wood, D.A.; Willson, A.B.; Binder, R.K.; Cheung, A.; Ye, J.; Webb, J.G. Percutaneous aortic valve replacement: Vascular outcomes with a fully percutaneous procedure. J. Am. Coll. Cardiol. 2012, 59, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Jilaihawi, H.; Kashif, M.; Takahashi, N.; Chakravarty, T.; Pokhrel, H.; Patel, J.; Forrester, J.S.; Nakamura, M.; Cheng, W.; et al. Transfemoral access assessment for transcatheter aortic valve replacement: Evidence-based application of computed tomography over invasive angiography. Circ. Cardiovasc. Imaging 2014, 8, e001995. [Google Scholar] [CrossRef] [PubMed]
- Vincent, F.; Spillemaeker, H.; Kyheng, M.; Belin-Vincent, C.; Delhaye, C.; Piérache, A.; Denimal, T.; Verdier, B.; Debry, N.; Moussa, M. Ultrasound Guidance to Reduce Vascular and Bleeding Complications of Percutaneous Transfemoral Transcatheter Aortic Valve Replacement: A Propensity Score-Matched Comparison. J. Am. Heart Assoc. 2020, 9, e014916. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, B.; Graham, M.M.; Afilalo, J.; Hassan, A.; Arora, R.C. Frailty as a risk predictor in cardiac surgery: Beyond the eyeball test. J. Thorac. Cardiovasc. Surg. 2019, 157, 1905–1909. [Google Scholar] [CrossRef] [PubMed]
- Baekke, P.S.; Jørgensen, T.H.; Søndergaard, L. Impact of early hospital discharge on clinical outcomes after transcatheter aortic valve implantation. Catheter Cardiovasc. Interv. 2020. [Google Scholar] [CrossRef] [PubMed]
- Torsello, G.B.; Kasprzak, B.; Klenk, E.; Tessarek, J.; Osada, N.; Torsello, G.F. Endovascular suture versus cutdown for endovascular aneurysm repair: A prospective randomized pilot study. J. Vasc. Surg. 2003, 38, 78–82. [Google Scholar] [CrossRef]
- Goldsweig, A.M.; Tak, H.J.; Chen, L.W.; Aronow, H.D.; Shah, B.; Kolte, D.; Desai, N.R.; Szerlip, M.; Velagapudi, P.; Abbott, J.D. Relative Costs of Surgical and Transcatheter Aortic Valve Replacement and Medical Therapy. Circ. Cardiovasc. Interv. 2020, 13, e008681. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Chakravarty, T.; Jilaihawi, H.; Doctor, N.; Dohad, S.; Fontana, G.; Cheng, W.; Makkar, R.R. Complete percutaneous approach for arterial access in transfemoral transcatheter aortic valve replacement: A comparison with surgical cut-down and closure. Catheter Cardiovasc. Interv. 2014, 84, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Holper, E.M.; Kim, R.J.; Mack, M.; Brown, D.; Brinkman, W.; Herbert, M.; Stewart, W.; Vance, K.; Bowers, B.; Dewey, T. Randomized trial of surgical cutdown versus percutaneous access in transfemoral TAVR. Catheter Cardiovasc. Interv. 2014, 83, 457–464. [Google Scholar] [CrossRef] [PubMed]


| Unmatched | |||
|---|---|---|---|
| Variables | Surgical Cut-Down | Percutaneous | p-Value |
| Total Population (n) | 219 | 225 | |
| Age, Median (q1–q3) | 81 (77–85) | 83 (79–86) | <0.01 |
| Male, n (%) | 105 (47.9) | 130 (57.8) | 0.04 |
| Female, n (%) | 114 (52.1) | 95 (42.2) | |
| BMI, Median (q1–q3), kg/m2 | 25.1 (22.2–28.7) | 25.1 (23.4–28.0) | 0.37 |
| Hypertension, n (%) | 166 (76.2) | 180 (80.0) | 0.36 |
| Diabetes, n (%) | 55 (25.2) | 52 (23.1) | 0.66 |
| COPD | 13 (6) | 11 (5) | 0.33 |
| Peripheral Vascular Disease, n (%) | 82 (37.6) | 55 (24.4) | <0.01 |
| Creatinin, Median (q1–q3), mg/dL | 1.00 (0.81–1.26) | 1.02 (0.81–1.29) | 0.35 |
| Hb, Mean (SD), (g/dL) | 12.4 (1.7) | 12.6 (1.6) | 0.34 |
| EuroSCORE II log, Median (q1–q3) | 4.13 (2.52–6.75) | 3.77 (2.33–5.22) | 0.21 |
| Atrial Fibrillation, n (%) | 36 (16.7) | 34 (15.3) | 0.70 |
| Mean Aortic Gradient, Median (q1–q3), mmHg | 46 (39–55) | 43 (35–52) | 0.02 |
| EF, Median (q1–q3) | 61 (53–67) | 59 (50–66) | 0.05 |
| PAPs, median (q1–q3), mmHg | 35 (31–42) | 35 (31–42) | 0.22 |
| Unmatched | |||
|---|---|---|---|
| Variables | Group 1 Surgical Cut-Down | Group 2 Percutaneous | p-Value |
| Total Population (n) | 119 | 225 | |
| Age, Median (q1–q3) | 83 (78–85) | 83 (79–86) | 0.45 |
| Male, n (%) | 67 (56.3) | 130 (57.8) | 0.79 |
| Female, n (%) | 52 (43.7) | 95 (42.2) | |
| BMI, Median (q1–q3), kg/m2 | 24.7 (22.3–27.5) | 25.1 (23.4–28.0) | 0.05 |
| Hypertension, n (%) | 89 (74.8) | 180 (80.0) | 0.27 |
| Diabetes, n (%) | 27 (22.7) | 52 (23.1) | 0.93 |
| COPD | 7 (6) | 11 (5) | 0.21 |
| Peripheral Vascular Disease, n (%) | 37 (31.1) | 55 (24.4) | 0.19 |
| Creatinin, Median (q1–q3), mg/dL | 1.00 (0.82–1.32) | 1.02 (0.81–1.29) | 0.81 |
| Hb, Mean (SD), (g/dL) | 12.5 (1.6) | 12.6 (1.6) | 0.57 |
| EuroSCORE II log, Median (q1–q3) | 4.09 (2.61–7.29) | 3.77 (2.33–5.22) | 0.22 |
| Atrial Fibrillation, n (%) | 22 (18.6) | 34 (15.3) | 0.44 |
| Mean Aortic Gradient, Median (q1–q3), mmHg | 45 (38–54) | 43 (35–52) | 0.30 |
| EF, Median (q1–q3) | 61 (48–67) | 59 (50–66) | 0.35 |
| PAPs, Median (q1–q3), mmHg | 35 (31–42) | 35 (31–42) | 0.37 |
| Matched Populations | |||
|---|---|---|---|
| Variables | Group 1 | Group 2 | p-Value |
| History of Coronaropathy, n (%) | 45 (37.8) | 92 (40.9) | 0.58 |
| Previous CABG o PCI, n (%) | 19 (16.0) | 50 (22.2) | 0.17 |
| Previous Cardiac Surgery (%) | 31 (26.1) | 39 (17.3) | 0.07 |
| Previous SAVR, n (%) | 8 (7.0) | 0 (0.0) | <0.01 |
| Severe Peripheral Vascular Disease, n (%) | 37 (31.1) | 55 (24.4) | 0.19 |
| EF, Median (q1–q3) | 61 (48–67) | 59 (50–66) | 0.35 |
| Variable | Group 1 | Group 2 | p-Value |
|---|---|---|---|
| Type of Anesthesia | |||
| Deep Sedation, n (%) | 119 (100.0) | 224 (99.5) | 1.0 |
| Local Anesthesia + Mild Sedation, n (%) | 0 (0.0) | 1 (0.5) | |
| Type of TAV | |||
| Self-Expanding TAV, n (%) | 5 (4.2) | 12 (5.3) | 0.80 |
| Balloon-Expandable TAV, n (%) | 114 (95.8) | 213 (94.7) | |
| TAV’s Diameter | |||
| 20 mm, n (%) | 3 (2.5) | 1 (0.5) | 0.17 |
| 23 mm, n (%) | 52 (43.7) | 87 (38.7) | |
| 26 mm, n (%) | 47 (39.5) | 109 (48.4) | |
| 29 mm, n (%) | 17 (14.3) | 28 (12.4) | |
| Femoral Sheaths | |||
| 14F eSheath n (%) | 100 (84) | 191 (84.9) | 0.35 |
| 14F EnVeo R InLine Sheath n (%) | 5 (4.2) | 12 (5.3) | |
| 16F eSheath n (%) | 14 (11.8) | 22 (9.8) |
| Outcome | Group 1 | Group 2 | p-Value |
|---|---|---|---|
| Intraprocedural Death, n (%) | 1 (0.8) | 5 (2.2) | 0.67 |
| Cardiac Arrest, n (%) | 1 (0.8) | 4 (1.8) | 0.66 |
| Cardiovascular Mortality, n (%) | 1 (0.8) | 4 (1.8) | 0.66 |
| More than Mild PVL, n (%) | 7 (6.0) | 19 (8.7) | 0.09 |
| Device Embolization, n (%) | 0 (0) | 0 (0) | 0.00 |
| Need for CPB/ECMO, n (%) | 1 (0.8) | 1 (0.4) | 1.00 |
| Conversion to Sternotomy, n (%) | 1 (0.8) | 1 (0.4) | 1.00 |
| Device Success, n (%) | 116 (98.3) | 219 (97.3) | 0.72 |
| Minor Vascular Complications, n (%) | 6 (5.1) | 10 (4.5) | 0.97 |
| Major Vascular Complications, n (%) | 3 (2.6) | 6 (2.7) | 1.00 |
| Coronary Occlusion, n (%) | 0 (0) | 1 (0.4) | 1.00 |
| New Onset AF, n (%) | 6 (5.1) | 13 (5.8) | 0.79 |
| AMI, n (%) | 1 (0.8) | 0 (0) | 0.34 |
| Minor Neurological Events, n (%) | 1 (0.85) | 2 (0.9) | 0.48 |
| Major Neurological Events, n (%) | 0 (0.0) | 5 (2.2) | 0.38 |
| Major Bleedings, n (%) | 9 (7.7) | 22 (9.9) | 0.78 |
| Minor Bleedings, n (%) | 3 (2.6) | 3 (1.3) | 0.44 |
| PPI, n (%) | 3 (5.6) | 12 (5.4) | 0.28 |
| Temporary Postoperative CVVH, n (%) | 1 (0.8) | 2 (0.9) | 1.00 |
| Outcomes | Group 1 | Group 2 | p-Value |
|---|---|---|---|
| Length of Stay, Median (q1–q3), days | 7 (5–9) | 5 (4–7) | <0.01 |
| Discharged Home, n (%) | 18 (15.5) | 194 (88.2) | <0.01 |
| Rehabilitation, n (%) | 98 (84.5) | 26 (11.8) | <0.01 |
| Follow-Up Mortality, n (%) | 37 (31.6) | 48 (21.8) | 0.05 |
| Follow-Up Cardiovascular Mortality al, n (%) | 11 (9.4) | 22 (10.1) | 0.85 |
| Median Follow-Up (q1–q3), Days | 949 (624–1434) | 1039 (703–1553) | 0.27 |
| NYHA 1 at Follow-Up, n (%) | 94 (83.9) | 184 (86.4) | 0.83 |
| NYHA 2 at Follow-Up, n (%) | 16 (14.3) | 26 (12.2) | |
| NYHA 3 at Follow-Up, n (%) | 2 (1.8) | 3 (1.4) | |
| Cardiovascular Rehospitalization, n (%) | 7 (6.3) | 22 (10.3) | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gennari, M.; Rigoni, M.; Mastroiacovo, G.; Trabattoni, P.; Roberto, M.; Bartorelli, A.L.; Fabbiocchi, F.; Tamborini, G.; Muratori, M.; Fusini, L.; et al. Proper Selection Does Make the Difference: A Propensity-Matched Analysis of Percutaneous and Surgical Cut-Down Transfemoral TAVR. J. Clin. Med. 2021, 10, 909. https://doi.org/10.3390/jcm10050909
Gennari M, Rigoni M, Mastroiacovo G, Trabattoni P, Roberto M, Bartorelli AL, Fabbiocchi F, Tamborini G, Muratori M, Fusini L, et al. Proper Selection Does Make the Difference: A Propensity-Matched Analysis of Percutaneous and Surgical Cut-Down Transfemoral TAVR. Journal of Clinical Medicine. 2021; 10(5):909. https://doi.org/10.3390/jcm10050909
Chicago/Turabian StyleGennari, Marco, Marta Rigoni, Giorgio Mastroiacovo, Piero Trabattoni, Maurizio Roberto, Antonio L. Bartorelli, Franco Fabbiocchi, Gloria Tamborini, Manuela Muratori, Laura Fusini, and et al. 2021. "Proper Selection Does Make the Difference: A Propensity-Matched Analysis of Percutaneous and Surgical Cut-Down Transfemoral TAVR" Journal of Clinical Medicine 10, no. 5: 909. https://doi.org/10.3390/jcm10050909
APA StyleGennari, M., Rigoni, M., Mastroiacovo, G., Trabattoni, P., Roberto, M., Bartorelli, A. L., Fabbiocchi, F., Tamborini, G., Muratori, M., Fusini, L., Pepi, M., Muti, P., Polvani, G., & Agrifoglio, M. (2021). Proper Selection Does Make the Difference: A Propensity-Matched Analysis of Percutaneous and Surgical Cut-Down Transfemoral TAVR. Journal of Clinical Medicine, 10(5), 909. https://doi.org/10.3390/jcm10050909









