Abstract
Background: Cases of autoimmune bullous dermatosis (AIBD) have been reported following COVID-19 vaccination. Objective: We aimed to provide an overview of clinical characteristics, treatments, and outcomes of AIBDs following COVID-19 vaccination. Methods: We conducted a systematic review and searched the Embase, Cochrane Library, and Medline databases from their inception to 27 March 2024. We included all studies reporting ≥ 1 patient who developed new-onset AIBD or experienced flare of AIBD following at least one dose of any COVID-19 vaccine. Results: We included 98 studies with 229 patients in the new-onset group and 216 in the flare group. Among the new-onset cases, bullous pemphigoid (BP) was the most frequently reported subtype. Notably, mRNA vaccines were commonly associated with the development of AIBD. Regarding the flare group, pemphigus was the most frequently reported subtype, with the mRNA vaccines being the predominant vaccine type. The onset of AIBD ranged from 1 to 123 days post-vaccination, with most patients displaying favorable outcomes and showing improvement or resolution from 1 week to 8 months after treatment initiation. Conclusions: Both new-onset AIBD and exacerbation of pre-existing AIBD may occur following COVID-19 vaccination. Healthcare practitioners should be alert, and post-vaccination monitoring may be essential.
1. Introduction
To mitigate the transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1,2,3], various vaccines have been rapidly developed, including mRNA vaccines (BioNTech/Pfizer (Comirnaty; BNT162b2) and Moderna (Spikevax; mRNA-1273)), viral-vectored vaccines (AstraZeneca (Covishield; AZD1222/ChAdOx1) and Johnson & Johnson (COVID-19 Vaccine Janssen; Ad26.COV2.S/JNJ-78436735)), and inactivated vaccines (Sinopharm (BBIBP-CorV) and Sinovac (CoronaVac)) [4,5,6]. With the introduction of global mass vaccination, reports of post-vaccination cutaneous adverse events have emerged, including injection site reactions, urticaria, and morbilliform eruptions [7,8,9,10,11]. Furthermore, cases of autoimmune bullous dermatosis (AIBD) have been documented [12,13,14,15].
AIBD is characterized by the presence of autoantibodies targeting specific adhesion molecules, such as desmoglein, BP180, or BP230, within the skin or mucosae [16]. Clinical manifestations of AIBD range from localized vesiculobullous eruption to widespread potentially life-threatening skin detachment [17]. Following COVID-19 vaccination, various subtypes of AIBD have been reported, including diseases with intraepidermal detachment, such as pemphigus vulgaris (PV), pemphigus foliaceus (PF), pemphigus erythematosus (PE), pemphigus vegetans (PVeg), as well as diseases with subepidermal detachment, such as bullous pemphigoid (BP), mucous membrane pemphigoid (MMP), and linear IgA bullous dermatosis (LABD) [6,18,19]. The potential association between COVID-19 vaccination and AIBD requires further investigation, and a comprehensive review of this topic is needed. Given the increasing number of COVID-19 vaccine administrations, we conducted a systematic review to provide an overview of the clinical characteristics, treatment, and outcomes of AIBDs following COVID-19 vaccination.
2. Methods
This systematic review was registered with PROSPERO (CRD42023390478), and it was performed in accordance with the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [20,21,22]. Comprehensive searches were performed in the Embase, Cochrane Library, and Medline databases from their inception to 27 March 2024 using relevant terms, including ‘COVID-19’, ‘vaccine’, ‘autoimmune bullous dermatosis’, ‘vesiculobullous skin diseases’, ‘pemphigus’, ‘pemphigus vulgaris’, ‘pemphigus foliaceus’, ‘pemphigus erythematosus’, ‘bullous pemphigoid’, ‘mucous membrane pemphigoid’, and ‘linear IgA bullous dermatosis’. These terms were applied as free text, medical subject headings (MeSH in PubMed and Emtree in Embase), and abbreviations in the literature search. Boolean operators were used to combine keywords, and a primary search strategy was developed without language or publication data limitations (Table S1). Additionally, the reference lists of all identified articles were screened to identify further relevant studies.
We included studies reporting at least one patient who developed new-onset AIBD or experienced an exacerbation of AIBD following administration of at least one dose of any COVID-19 vaccine. Exacerbation was defined as the presence of increased body surface area involvement, the presence of vesiculobullous lesions or skin erythema, subjective worsening reported by the patient, worsening described in physical examination findings, or clinician assessment or plan indicating exacerbation, rebound, or worsening of AIBD compared to previous examination. Review articles, conference abstracts, and in vitro or animal model studies were excluded. Two experienced authors (Wu and Wang) independently conducted the literature search, data extraction, and quality assessments. Any discrepancies between the reviewers were resolved by a third author (Huang). The quality of case reports and series was assessed using the appraisal tool developed by Murad et al. [23], while observational studies were evaluated using the National Institute of Health quality assessment tool (Tables S2 and S3) [24].
Data extraction was performed independently by two authors (Wu and Wang) and included the following information from the included studies: author, year of publication, country, demographic information of patients (age and sex), blister sites, COVID-19 vaccination details (vaccine type and dose), onset time, classification of cases as new-onsets or exacerbations, AIBD subtype, other potential triggers, pathology examinations (Hematoxylin and Eosin stains and immunofluorescence study), enzyme-linked immunoassay (ELISA) results (such as BP180, BP 230, desmoglein [dsg] 1, and desmoglein 3), prior and post-exacerbation treatments, outcomes, and reactions to subsequent COVID-19 vaccination. The patient groups were further categorized based on the occurrence of new AIBD onset or exacerbation of AIBD, and all patients were classified according to AIBD subtypes.
3. Results
3.1. Literature Search
As shown in Figure 1, 333 studies were identified after searching three major databases and performing a manual search of the reference lists of identified studies. We excluded 91 studies as duplicates, and 75 studies were excluded for being unrelated to the study question after assessing the title or abstract. The full texts of the remaining 167 studies were reviewed, and 98 studies were identified as meeting the inclusion criteria for qualitative synthesis. A total of 74 studies reporting new-onset AIBD, 15 studies reporting exacerbation of AIBD, and 9 studies reporting both new onset and exacerbation of AIBD were included in this study (Table 1 and Table 2). The quality assessments of case reports and series consistently received scores ranging from five to seven according to the methodology proposed by Murad et al. [23]. For observational studies, all of the assessments were rated as ‘fair’ using the National Institute of Health quality assessment tool [24].
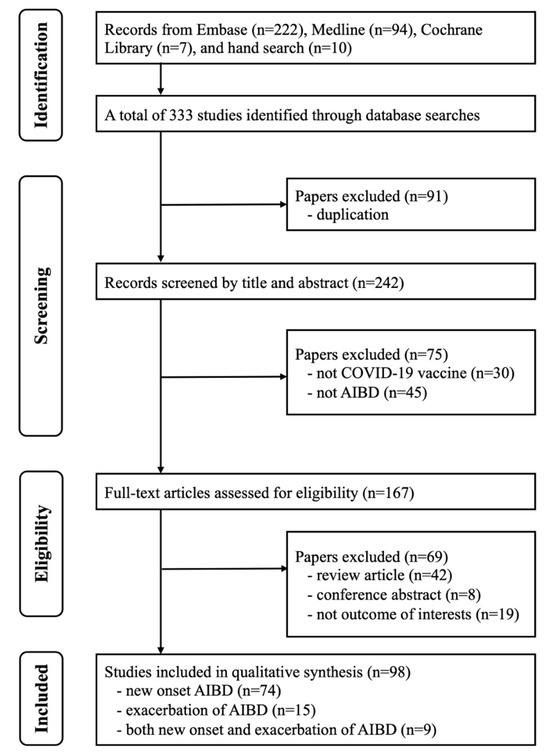
Figure 1.
PRISMA flowchart of the selection of studies.

Table 1.
Characteristics of the included studies reporting new onset of autoimmune bullous dermatosis.

Table 2.
Characteristics of the included studies reporting exacerbation of autoimmune bullous dermatosis.
3.2. Patient Characteristics
Detailed patient information is presented in Table 1 and Table 2. The characteristics of the included studies are summarized in Table 3. The new-onset group comprised 229 patients, mostly from America, with ages ranging from 11 to 97 years. Although most studies did not report patients’ sex, a slight male predominance was noted among those that did. The most frequently encountered diagnosis in the group was BP in 174 patients, followed by PV in 23 and PF in 16.

Table 3.
Summary of characteristics of the included studies.
The flare group included 216 patients, with ages ranging from 20 to 88 years, who primarily had pemphigus (specific subtype unspecified). Most patients were from Asia (44%) and America (41%). Similarly to the new-onset group, most studies did not provide information on patients’ sex, but a slight male predominance existed among those that did.
3.3. Vaccine Type, Vaccine Dose, and Time to AIBD Onset Following Vaccination
In the new-onset group, 55% of patients received the BioNTech/Pfizer vaccine, followed by the Moderna vaccine (16%) and the Oxford-AstraZeneca vaccine (13%). However, it is noteworthy that the vaccine type was not reported for a large number of patients. Most cases of new-onset AIBD occurred after the second (39%) or first vaccine dose (34%), while 15% of AIBD patients experienced onset following both doses. The onset times varied widely, ranging from 1 to 123 days after vaccination.
In the flare group, most patients were administered the BioNTech/Pfizer vaccine (56%), followed by the Sinovac vaccine (18%) and the Moderna vaccine (16%). Flares were most frequently reported after the third vaccine dose (63%), followed by the first dose (24%) and the second dose (10%). The onset of AIBD symptoms ranged from 1 day to 92 days following vaccination.
3.4. Other Potential Non-Vaccine Triggers
In the new-onset group, most studies did not provide information on other potential non-vaccine triggers. However, some BP patients had pre-existing neurological or psychiatric disorders, such as dementia, depression, or Alzheimer’s disease, which are known to be associated with the development of BP [28,114,115]. Additionally, dipeptidyl peptidase 4 (DPP-4) inhibitors, a well-established risk factor for BP [116], were used by some patients [34,44,45,48]. In the majority of cases, patients denied any new medication use.
In the flare group, the information regarding other potential triggers was unavailable in most studies. Nevertheless, two patients had a history of COVID-19 infection prior to receiving the COVID-19 vaccines, and subsequently experienced a BP eruption [99,103].
3.5. The Assessment of Naranjo Scores for New-Onset AIBD or AIBD Flares
To evaluate the potential causal relationship between COVID-19 vaccination and AIBD development, we applied the Naranjo scores to all cases (Tables S4 and S5) [117]. In the new-onset group, 87% of cases were categorized as ‘possible’, and 13% as ‘probable’. In the flare group, 92% of cases were classified as ‘possible’, and 8% as ‘probable’. Notably, all cases deemed ‘probable’ in causality had experienced a disease flare following both doses of COVID-19 vaccines, contributing to the overall score for these cases [6,13,25,37,38,40,41,42,49,50,53,54,68,71,75,86,91].
3.6. Treatment and Outcomes for New-Onset AIBD or AIBD Flares
In the new onset group, BP patients with limited involvement were treated with topical corticosteroids, while those with more extensive involvements received a variety of systemic immunomodulators, including corticosteroids, doxycycline, nicotinamide, methotrexate, azathioprine, cyclosporine, mycophenolate mofetil, cyclophosphamide, dapsone, colchicine, or hydroxychloroquine [5,6,14,15,28,31,32,35,38,39,42,43,44,45,49,51,53,56,70,71,72,73,75,77,82,86,87,92,93]. DPP-4 inhibitors were suspended in patients using these medications [34,44,45,48]. Intravenous immunoglobulin G (IVIG) was administered in selected cases, and biologics, such as dupilumab and omalizumab, were utilized [26,34,49,55,56]. Rituximab was introduced in three cases, leading to significant improvement [51,56,63]. Most patients with pemphigus were managed with systemic corticosteroids and immunomodulators, with rituximab administered in 29% of cases [51,63,74,75,78,79,81,89]. In one case of PVeg, intralesional injections of onabotulinum toxin, corticosteroids, and mycophenolate mofetil were used, resulting in resolution after 6 months [87]. The majority of patients demonstrated improvement (56%) or resolution (35%) after treatment, with resolution times ranging from 1 week to 8 months. One case of BP showed improvement after prednisolone treatment, but the patient died due to pulmonary embolism one month after discharge [29]. Disease flare after both vaccine doses was observed in 69% of reported cases, but most studies lacked data on subsequent vaccinations.
In the flare group, the predominant treatment approach involved topical or systemic corticosteroids supplemented by immunomodulators, such as doxycycline, nicotinamide, methotrexate, azathioprine, or mycophenolate mofetil, in refractory cases [6,18,30,105,106]. Additional corticosteroid therapy was used in most patients experiencing a flare of AIBD, with further immunosuppressants utilized for treatment-resistant cases [14,18,32,106]. Rituximab was administered in six cases, resulting in four cases experiencing disease improvement; one case died 15 days after the administration of COVID-19 vaccination due to sepsis, and one case had ongoing treatment and no final outcome was reported [51,99,105,108,109,110]. The majority of cases showed improvement (65%) or resolution (22%) after treatment, with resolution times ranging from 1 to 10 weeks. Only three of the reported cases (20%) experienced a similar flare following their initial COVID-19 vaccination and exhibited disease exacerbation after the second dose [6,18,30,105].
4. Discussion
In this systematic review, we have compiled all available reports of new-onset AIBD or AIBD flares following COVID-19 vaccination. Our analysis included 98 studies, encompassing 229 patients in the new-onset group and 216 patients in the flare group. Among the new-onset cases, BP was the most frequently reported subtype, while pemphigus was the most commonly reported subtype in the flare group. As we know, clinical relapse is commonly seen in pemphigus, with a relapse rate as high as 82% [118]. The chronic and relapsing features of pemphigus may contribute to the larger number of flare cases relative to BP. Notably, both new onset and exacerbation of AIBDs were frequently observed following the administration of mRNA vaccines. However, we should recognize that mRNA vaccines were the most frequently administered vaccine worldwide. Onset time varied widely among both new-onset and flare groups, ranging from 1 to 123 days. Most patients achieved favorable outcomes, with improvement or resolution occurring within 1 week to 8 months after treatment initiation.
The potential association between vaccination and AIBD has been investigated in the previous research [119]. Various vaccines, including influenza, tetanus and diphtheria, hepatitis B, herpes zoster, and quadrivalent human papillomavirus, have been reported to be associated with AIBD development [120]. With the substantial increase in COVID-19 vaccinations, the link between newly developed vaccines and AIBD has been reexamined. The theory of molecular mimicry between specific basement membrane proteins and the spike protein of SARS-CoV-2 has been proposed as a potential cause [121]. Additionally, mRNA vaccines are suggested to activate pro-inflammatory pathways by interacting with toll-like receptors, potentially leading to increased production of interleukin (IL) -4, IL-17, interferon-γ, and tumor necrosis factor-α cytokines [71,79,122]. Because autoreactive T cells and the dysregulation of T helper (Th)1 and Th2 responses play a crucial role in both pemphigus and pemphigoid [123], the vaccine trigger and cytokine modulation may promote an imbalance between Th2 responses against cutaneous antigens, fostering the generation of autoreactive B cells and contributing to AIBD development [122]. Vaccine-induced inflammation may also disrupt the basement membrane, leading to the production of anti-basement membrane antibodies [121]. Furthermore, human leukocyte antigen (HLA) molecules, including alleles HLA-DQB1*0503 and HLA-DRB1*0402 in pemphigus, as well as HLA-DQB1*0301 in pemphigoid, may represent key predisposing factors for drug-induced AIBDs [124]. However, none of the included cases underwent HLA examinations, necessitating further investigations.
On the contrary, Birabaharan et al. conducted a cohort study involving over 1.5 million individuals who received mRNA COVID-19 vaccinations, which revealed no difference in the risk of new-onset BP within a 6-month period between vaccinated patients and those who remained unvaccinated [33]. Another investigation by Kasperkiewicz et al. demonstrated that circulating anti-SARS-CoV-2 antibodies did not cross-react with the main AIBD autoantigens, including dsg 1, dsg 3, envoplakin, BP180, BP230, and type VII collagen [125]. This perspective is consistent with the findings of previous systematic reviews, which posited that the hypothesized causal relationship is likely to be a relatively rare occurrence [126,127]. In our study, we not only included a substantially larger sample size compared to previous studies, but we also employed the Naranjo score to investigate causality. Patients with severe or extensive AIBD are usually advised against re-exposure to the same vaccine. However, in our study, 23 patients who experienced new onset or exacerbation of AIBD were re-exposed to the same vaccine, leading to recurrence. This implicates COVID-19 vaccines as the likely causative agents, supported by the high Naranjo rating score of 7. Our research provides evidence suggesting a potential association between COVID-19 vaccination and the development of AIBD to some extent, as indicated by the short onset interval and the absence of other triggers in most cases. These findings are in accordance with the previous literature, underscoring that mRNA vaccines were the most commonly reported vaccine type in both new onset and exacerbation of AIBD cases, followed by inactivated and viral-vectored vaccines [127].
It is worth noting that some studies reported potential non-vaccine triggers, such as neurological or psychiatric disorders, use of DPP-4 inhibitor, polypharmacy, or a history of COVID-19 infection [28,34,43,44,46,49,50,92,99]. The etiology and pathogenesis of AIBD remain largely elusive. However, the occurrence of exacerbation of AIBD has been reported in association with specific triggering factors, including medications, physical stimuli, infections, and organ transplantations [128]. We outlined these cases and assigned lower scores on the Naranjo score, which consequently decreased the overall rating. Only 13% of the new-onset AIBD patients and 8% of AIBD flare cases were rated as probable according to the Naranjo score. Nevertheless, it is essential to acknowledge that most studies did not report such triggers, limiting the calculation of the Naranjo score. Given that the existing data predominantly consist of anecdotal, single-case reports with a low level of evidence, real-world, population-based studies are warranted to elucidate a definitive link between COVID-19 vaccinations and risk of AIBD. However, this should not dissuade the current vaccination recommendations for patients with AIBD, given the favorable risk–benefit ratio.
Our study has certain limitations. Firstly, most of the included studies were case reports, case series, and retrospective observational studies from database collections. Some studies lacked comprehensive documentation of patients’ clinical conditions, while others were deficient in critical information, including vaccine dosage, additional triggers, laboratory findings, treatment modalities, and disease outcomes. Secondly, not all studies presented results of skin biopsies, immunofluorescence studies, or ELISA tests, thereby raising questions about the accuracy of disease diagnoses in some cases. Thirdly, essential parameters for assessing disease severity in AIBD patients, such as the bullous pemphigoid disease area index (BPDAI), the pemphigus area and activity score (PAAS), and the percentage of body surface area affected, were not reported among all studies. These parameters are pivotal for evaluating disease severity before vaccination, after vaccination, and following treatment. Fourthly, only a limited number of cases provided information regarding whether patients received subsequent vaccine doses, and the duration of follow-up was relatively short. In our analysis, most patients in the new-onset and flare groups showed improvement or resolution. However, given the chronic and relapsing nature of AIBD, future long-term follow-up studies are imperative to establish a stronger evidence base, and ongoing monitoring is essential for these patients [129].
5. Conclusions
In conclusion, both new-onset AIBD and exacerbation of pre-existing AIBD may occur following COVID-19 vaccination. Healthcare practitioners should raise concerns for AIBD when administering COVID-19 vaccines, and post-vaccination monitoring may be essential. Current evidence continues to favor COVID-19 vaccination in individuals with AIBD, owing to its significant protective benefits against SARS-CoV-2. More studies are imperative to elucidate the underlying mechanisms of the association between COVID-19 vaccines and the development of AIBD.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines12050465/s1, Table S1: Search strategy; Table S2: Quality assessment of case reports; Table S3: Quality assessment of observational cohort and cross-sectional studies; Table S4: The assessment of Naranjo score for cases of new onset autoimmune bullous dermatosis; Table S5: The assessment of Naranjo score for cases of exacerbation of autoimmune bullous dermatosis.
Author Contributions
Conceptualization: C.-C.C.; Data curation: C.-Y.W. and C.-C.C.; Methodology: P.-C.W., I.-H.H. and C.-C.C.; Investigation: I.-H.H. and C.-Y.W.; Analysis and software: P.-C.W.; Writing—original draft preparation: P.-C.W. and I.-H.H.; Writing—review and editing: C.-C.C. and C.-Y.W.; Visualization: P.-C.W. and C.-Y.W.; Supervision: C.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were generated in support of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Chi, C.C. Aiming at a bright future. Dermatol. Sin. 2022, 40, 1–2. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 25 April 2023).
- McMahon, D.E.; Kovarik, C.L.; Damsky, W.; Rosenbach, M.; Lipoff, J.B.; Tyagi, A.; Chamberlin, G.; Fathy, R.; Nazarian, R.M.; Desai, S.R.; et al. Clinical and pathologic correlation of cutaneous COVID-19 vaccine reactions including V-REPP: A registry-based study. J. Am. Acad. Dermatol. 2022, 86, 113–121. [Google Scholar] [CrossRef]
- Agharbi, F.Z.; Eljazouly, M.; Basri, G.; Faik, M.; Benkirane, A.; Albouzidi, A.; Chiheb, S. Bullous pemphigoid induced by the AstraZeneca COVID-19 vaccine. Ann. Dermatol. Venereol. 2022, 149, 56–57. [Google Scholar] [CrossRef]
- Akoglu, G. Pemphigus vulgaris after SARS-CoV-2 vaccination: A case with new-onset and two cases with severe aggravation. Dermatol. Ther. 2022, 35, e15396. [Google Scholar] [CrossRef]
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad. Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef]
- Freeman, E.E.; Sun, Q.; McMahon, D.E.; Singh, R.; Fathy, R.; Tyagi, A.; Blumenthal, K.; Hruza, G.J.; French, L.E.; Fox, L.P. Skin reactions to COVID-19 vaccines: An American Academy of Dermatology/International League of Dermatological Societies registry update on reaction location and COVID vaccine type. J. Am. Acad. Dermatol. 2022, 86, e165–e167. [Google Scholar] [CrossRef]
- Hung, W.-K.; Chi, C.-C.; Wang, S.-H. AZ arm: Delayed cutaneous reaction to ChAdOx1 nCoV-19 (AZD1222) vaccine. Dermatol. Sin. 2022, 40, 52–53. [Google Scholar] [CrossRef]
- Lin, P.T.; Chi, C.C. Erythrodermic psoriasis following ChAdOx1 nCOV-19 vaccination: A case report. Dermatol. Sin. 2022, 40, 62–63. [Google Scholar] [CrossRef]
- Grieco, T.; Maddalena, P.; Sernicola, A.; Muharremi, R.; Basili, S.; Alvaro, D.; Cangemi, R.; Rossi, A.; Pellacani, G. Cutaneous adverse reactions after COVID-19 vaccines in a cohort of 2740 Italian subjects: An observational study. Dermatol. Ther. 2021, 34, e15153. [Google Scholar] [CrossRef]
- Hsieh, T.S.; Chen, J.S.; Tsai, T.F. Dyshidrotic bullous pemphigoid developing after Moderna mRNA-1273 vaccination. Dermatol. Sin. 2023, 41, 52–53. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Y.; Tang, H.; Fan, M.; Wang, W.; Ding, Y.; Shen, S.; Zhou, W.; Zhang, Y.; Wang, Z. Bullous Pemphigoid After Vaccination With the Inactivated Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine: Two Cases in China. Wound. Manag. Prev. 2022, 68, 22–25. [Google Scholar] [CrossRef]
- Afacan, E.; Edek, Y.C.; Ilter, N.; Gulekon, A. Can COVID-19 vaccines cause or exacerbate bullous pemphigoid? A report of seven cases from one center. Int. J. Dermatol. 2022, 61, 626–627. [Google Scholar] [CrossRef]
- Hung, W.K.; Chi, C.C. Incident bullous pemphigoid in a psoriatic patient following mRNA-1273 SARS-CoV-2 vaccination. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e407–e409. [Google Scholar] [CrossRef]
- Witte, M.; Zillikens, D.; Schmidt, E. Diagnosis of Autoimmune Blistering Diseases. Front. Med. 2018, 5, 296. [Google Scholar] [CrossRef]
- Holtsche, M.M.; Boch, K.; Schmidt, E. Autoimmune bullous dermatoses. J. Dtsch. Dermatol. Ges. 2023, 21, 405–412. [Google Scholar] [CrossRef]
- Damiani, G.; Pacifico, A.; Pelloni, F.; Iorizzo, M. The first dose of COVID-19 vaccine may trigger pemphigus and bullous pemphigoid flares: Is the second dose therefore contraindicated? J. Eur. Acad. Dermatol. Venereol. 2021, 35, e645–e647. [Google Scholar] [CrossRef]
- Coto-Segura, P.; Fernandez-Prada, M.; Mir-Bonafe, M.; Garcia-Garcia, B.; Gonzalez-Iglesias, I.; Alonso-Penanes, P.; Gonzalez-Guerrero, M.; Gutierrez-Palacios, A.; Miranda-Martinez, E.; Martinon-Torres, F. Vesiculobullous skin reactions induced by COVID-19 mRNA vaccine: Report of four cases and review of the literature. Clin. Exp. Dermatol. 2022, 47, 141–143. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kuo, L.T.; Shao, S.H.; Chi, C.C. Ten essential steps for performing a systematic review: A quick tutorial. Dermatol. Sin. 2022, 40, 204–206. [Google Scholar] [CrossRef]
- Shao, S.C.; Kuo, L.T.; Huang, Y.T.; Lai, P.C.; Chi, C.C. Using Grading of Recommendations Assessment, Development, and Evaluation (GRADE) to rate the certainty of evidence of study outcomes from systematic reviews: A quick tutorial. Dermatol. Sin. 2023, 41, 3–7. [Google Scholar] [CrossRef]
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef]
- National Institutes of Health. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 16 January 2023).
- Khalid, M.; Lipka, O.; Becker, C. Moderna COVID-19 vaccine induced skin rash. Vis. J. Emerg. Med. 2021, 25, 101108. [Google Scholar] [CrossRef]
- Nakamura, K.; Kosano, M.; Sakai, Y.; Saito, N.; Takazawa, Y.; Omodaka, T.; Kiniwa, Y.; Okuyama, R. Case of bullous pemphigoid following coronavirus disease 2019 vaccination. J. Dermatol. 2021, 48, e606–e607. [Google Scholar] [CrossRef]
- Perez-Lopez, I.; Moyano-Bueno, D.; Ruiz-Villaverde, R. Bullous pemphigoid and COVID-19 vaccine. Med. Clin. 2021, 157, e333–e334. [Google Scholar] [CrossRef]
- Tomayko, M.M.; Damsky, W.; Fathy, R.; McMahon, D.E.; Turner, N.; Valentin, M.N.; Rallis, T.; Aivaz, O.; Fox, L.P.; Freeman, E.E. Subepidermal blistering eruptions, including bullous pemphigoid, following COVID-19 vaccination. J. Allergy Clin. Immunol. 2021, 148, 750–751. [Google Scholar] [CrossRef]
- Alshammari, F.; Abuzied, Y.; Korairi, A.; Alajlan, M.; Alzomia, M.; AlSheef, M. Bullous pemphigoid after second dose of mRNA- (Pfizer-BioNTech) COVID-19 vaccine: A case report. Ann. Med. Surg. 2022, 75, 103420. [Google Scholar] [CrossRef]
- Avallone, G.; Cavallo, F.; Astrua, C.; Caldarola, G.; Conforti, C.; De Simone, C.; di Meo, N.; di Stefani, A.; Genovese, G.; Maronese, C.A.; et al. Cutaneous adverse reactions following SARS-CoV-2 vaccine booster dose: A real-life multicentre experience. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e876–e879. [Google Scholar] [CrossRef]
- Bailly-Caille, B.; Jouen, F.; Dompmartin, A.; Morice, C. A case report of anti-P200 pemphigoid following COVID-19 vaccination. JAAD Case Rep. 2022, 23, 83–86. [Google Scholar] [CrossRef]
- Bardazzi, F.; Carpanese, M.A.; Abbenante, D.; Filippi, F.; Sacchelli, L.; Loi, C. New-onset bullous pemphigoid and flare of pre-existing bullous pemphigoid after the third dose of the COVID-19 vaccine. Dermatol. Ther. 2022, 35, e15555. [Google Scholar] [CrossRef]
- Birabaharan, M.; Kaelber, D.C.; Orme, C.M.; Paravar, T.; Karris, M.Y. Evaluating risk of bullous pemphigoid after mRNA COVID-19 vaccination. Br. J. Dermatol. 2022, 187, 271–273. [Google Scholar] [CrossRef]
- Bostan, E.; Yel, B.; Akdogan, N.; Gokoz, O. New-onset bullous pemphigoid after inactivated COVID-19 vaccine: Synergistic effect of the COVID-19 vaccine and vildagliptin. Dermatol. Ther. 2022, 35, e15241. [Google Scholar] [CrossRef]
- Daines, B.; Madigan, L.M.; Vitale, P.A.; Khalighi, M.; Innes, M. A new eruption of bullous pemphigoid following mRNA COVID-19 vaccination. Dermatol. Online J. 2022, 28, 11. [Google Scholar] [CrossRef]
- Darrigade, A.S.; Oules, B.; Sohier, P.; Jullie, M.L.; Moguelet, P.; Barbaud, A.; Soria, A.; Vignier, N.; Lebrun-Vignes, B.; Sanchez-Pena, P.; et al. Sweet-like syndrome and multiple COVID arm syndrome following COVID-19 vaccines: ‘specific’ patterns in a series of 192 patients. Br. J. Dermatol. 2022, 187, 615–617. [Google Scholar] [CrossRef]
- Dell’Antonia, M.; Anedda, S.; Usai, F.; Atzori, L.; Ferreli, C. Bullous pemphigoid triggered by COVID-19 vaccine: Rapid resolution with corticosteroid therapy. Dermatol. Ther. 2022, 35, e15208. [Google Scholar] [CrossRef]
- Desai, A.D.; Shah, R.; Haroon, A.; Wassef, C. Bullous Pemphigoid Following the Moderna mRNA-1273 Vaccine. Cureus 2022, 14, e24126. [Google Scholar] [CrossRef]
- Fu, P.A.; Chen, C.W.; Hsu, Y.T.; Wei, K.C.; Lin, P.C.; Chen, T.Y. A case of acquired hemophilia A and bullous pemphigoid following SARS-CoV-2 mRNA vaccination. J. Formos. Med. Assoc. 2022, 121, 1872–1876. [Google Scholar] [CrossRef]
- Gambichler, T.; Hamdani, N.; Budde, H.; Sieme, M.; Skrygan, M.; Scholl, L.; Dickel, H.; Behle, B.; Ganjuur, N.; Scheel, C.; et al. Bullous pemphigoid after SARS-CoV-2 vaccination: Spike-protein-directed immunofluorescence confocal microscopy and T-cell-receptor studies. Br. J. Dermatol. 2022, 186, 728–731. [Google Scholar] [CrossRef]
- Hali, F., Sr.; Araqi, L., Jr.; Marnissi, F.; Meftah, A.; Chiheb, S. Autoimmune Bullous Dermatosis Following COVID-19 Vaccination: A Series of Five Cases. Cureus 2022, 14, e23127. [Google Scholar] [CrossRef]
- Larson, V.; Seidenberg, R.; Caplan, A.; Brinster, N.K.; Meehan, S.A.; Kim, R.H. Clinical and histopathological spectrum of delayed adverse cutaneous reactions following COVID-19 vaccination. J. Cutan. Pathol. 2022, 49, 34–41. [Google Scholar] [CrossRef]
- Maronese, C.A.; Caproni, M.; Moltrasio, C.; Genovese, G.; Vezzoli, P.; Sena, P.; Previtali, G.; Cozzani, E.; Gasparini, G.; Parodi, A.; et al. Bullous Pemphigoid Associated With COVID-19 Vaccines: An Italian Multicentre Study. Front. Med. 2022, 9, 841506. [Google Scholar] [CrossRef]
- Maronese, C.A.; Di Zenzo, G.; Genovese, G.; Barei, F.; Monestier, A.; Pira, A.; Moltrasio, C.; Marzano, A.V. Reply to “New-onset bullous pemphigoid after inactivated COVID-19 vaccine: Synergistic effect of the COVID-19 vaccine and vildagliptin”. Dermatol. Ther. 2022, 35, e15496. [Google Scholar] [CrossRef]
- Nakahara, Y.; Yamane, M.; Sunada, M.; Aoyama, Y. SARS-CoV-2 vaccine-triggered conversion from systemic lupus erythematosus (SLE) to bullous SLE and dipeptidyl peptidase 4 inhibitors-associated bullous pemphigoid. J. Dermatol. 2022, 50, 162–165. [Google Scholar] [CrossRef]
- Nida, S.S.; Tobon, G.J.; Wilson, M.; Chauhan, K. A patient develops bullous rash after receiving the second dose of COVID-19 vaccine. Cureus 2022, 14, e29786. [Google Scholar] [CrossRef]
- Pauluzzi, M.; Stinco, G.; Errichetti, E. Bullous pemphigoid in a young male after COVID-19 mRNA vaccine: A report and brief literature review. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e257–e259. [Google Scholar] [CrossRef]
- Russo, R.; Gasparini, G.; Cozzani, E.; D’Agostino, F.; Parodi, A. Absolving COVID-19 Vaccination of Autoimmune Bullous Disease Onset. Front. Immunol. 2022, 13, 834316. [Google Scholar] [CrossRef]
- Savoldy, M.A.; Tadicherla, T.; Moureiden, Z.; Ayoubi, N.; Baldwin, B.T. The Successful Treatment of COVID-19-Induced Bullous Pemphigoid With Dupilumab. Cureus 2022, 14, e30541. [Google Scholar] [CrossRef]
- Schmidt, V.; Blum, R.; Mohrenschlager, M. Biphasic bullous pemphigoid starting after first dose and boosted by second dose of mRNA-1273 vaccine in an 84-year-old female with polymorbidity and polypharmacy. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e88–e90. [Google Scholar] [CrossRef]
- Shakoei, S.; Kalantari, Y.; Nasimi, M.; Tootoonchi, N.; Ansari, M.S.; Razavi, Z.; Etesami, I. Cutaneous manifestations following COVID-19 vaccination: A report of 25 cases. Dermatol. Ther. 2022, 35, e15651. [Google Scholar] [CrossRef]
- Shanshal, M. Dyshidrosiform Bullous Pemphigoid Triggered by COVID-19 Vaccination. Cureus 2022, 14, e26383. [Google Scholar] [CrossRef]
- Wan, V.; Chen, D.; Shiau, C.J.; Jung, G.W. Association between COVID-19 vaccination and bullous pemphigoid—A case series and literature review. SAGE Open Med. Case Rep. 2022, 10, 2050313X221131868. [Google Scholar] [CrossRef]
- Young, J.; Mercieca, L.; Ceci, M.; Pisani, D.; Betts, A.; Boffa, M.J. A case of bullous pemphigoid after the SARS-CoV-2 mRNA vaccine. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e13–e16. [Google Scholar] [CrossRef]
- Zhang, Y.; Lang, X.; Guo, S.; He, H.; Cui, H. Bullous pemphigoid after inactivated COVID-19 vaccination: Case report. Dermatol. Ther. 2022, 35, e15595. [Google Scholar] [CrossRef]
- Baffa, M.E.; Maglie, R.; Montefusco, F.; Pipito, C.; Senatore, S.; Antiga, E. Severe bullous pemphigoid following COVID-19 vaccination resistant to rituximab and successfully treated with dupilumab. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e135–e137. [Google Scholar] [CrossRef]
- Cowan, T.L.; Huang, C.; Murrell, D.F. Autoimmune blistering skin diseases triggered by COVID-19 vaccinations: An Australian case series. Front. Med. 2023, 9, 1117176. [Google Scholar] [CrossRef]
- Dawoud, N.M.; Aslam, H.; Alshehri, M.A.; Dawoud, M.M. COVID-19 Vaccine-Triggered Bullous Pemphigoid: Two New Cases from Saudi Arabia. Indian J. Dermatol. 2023, 68, 590. [Google Scholar] [PubMed]
- Mulianto, N.; Hashfi, A.F. Bullous pemphigoid associated with COVID-19 vaccine in child: A case report. J. Pak. Assoc. Dermatol. 2023, 33, 730–735. [Google Scholar]
- Sun, L.; Brazão, C.; Mancha, D.; Soares-de-Almeida, L.; Filipe, P. Reply to: ‘Severe bullous pemphigoid following COVID-19 vaccination resistant to rituximab and successfully treated with dupilumab’ by Baffa et al. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e578–e580. [Google Scholar] [CrossRef] [PubMed]
- Oguz Topal, I.; Tokmak, A.; Kurmuş, G.I.; Kalkan, G.; Demirseren, D.D.; Tosun, M.; Emre, S.; Özkök Akbulut, T.; Kaya Özden, H.; Koska, M.; et al. Skin manifestations following anti-COVID-19 vaccination: A multicentricstudy from Turkey. J. Cosmet. Dermatol. 2023, 22, 354–363. [Google Scholar] [CrossRef]
- Üstün, P.; Satılmış, A.; Kılıç, İ.İ.; Adışen, E. COVID-19 Vaccine Induced Bullous Pemphigoid: Case Report and Review of the Literature. J. Turk. Acad. Dermatol. 2023, 17, 27–30. [Google Scholar] [CrossRef]
- Diab, R.; Rakhshan, A.; Salarinejad, S.; Pourani, M.R.; Ansar, P.; Abdollahimajd, F. Clinicopathological characteristics of cutaneous complications following COVID-19 vaccination: A case series. J. Cosmet. Dermatol. 2024, 23, 725–730. [Google Scholar] [CrossRef]
- Yamamoto, S.; Koga, H.; Tsutsumi, M.; Ishii, N.; Nakama, T. Bullous pemphigoid associated with prodromal-phase by repeated COVID-19 vaccinations. J. Dermatol. 2024, 51, e6–e7. [Google Scholar] [CrossRef]
- Mustin, D.E.; Huffaker, T.B.; Feldman, R.J. New-Onset Pemphigoid Gestationis Following COVID-19 Vaccination. Cutis 2023, 111, E2–E4. [Google Scholar] [CrossRef]
- Rungraungrayabkul, D.; Rattanasiriphan, N.; Juengsomjit, R. Mucous Membrane Pemphigoid Following the Administration of COVID-19 Vaccine. Head Neck Pathol. 2023, 17, 587–588. [Google Scholar] [CrossRef]
- Calabria, E.; Antonelli, A.; Lavecchia, A.; Giudice, A. Oral mucous membrane pemphigoid after SARS-CoV-2 vaccination. Oral Diseases 2024, 30, 782–783. [Google Scholar] [CrossRef]
- Hali, F.; Kerouach, A.; Alatawna, H.; Chiheb, S.; Lakhdar, H. Linear IgA bullous dermatosis following Oxford AstraZeneca COVID-19 vaccine. Clin. Exp. Dermatol. 2022, 47, 611–613. [Google Scholar] [CrossRef]
- Han, J.; Russo, G.; Stratman, S.; Psomadakis, C.E.; Rigo, R.; Owji, S.; Luu, Y.; Mubasher, A.; Gonzalez, B.R.; Ungar, J.; et al. Toxic epidermal necrolysis-like linear IgA bullous dermatosis after third Moderna COVID-19 vaccine in the setting of oral terbinafine. JAAD Case Rep. 2022, 24, 101–104. [Google Scholar] [CrossRef]
- Nahm, W.J.; Juarez, M.; Wu, J.; Kim, R.H. Eosinophil-rich linear IgA bullous dermatosis induced by mRNA COVID-19 booster vaccine. J. Cutan. Pathol. 2023, 50, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Solimani, F.; Mansour, Y.; Didona, D.; Dilling, A.; Ghoreschi, K.; Meier, K. Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e649–e651. [Google Scholar] [CrossRef] [PubMed]
- Agharbi, F.Z.; Basri, G.; Chiheb, S. Pemphigus vulgaris following second dose of mRNA-(Pfizer-BioNTech) COVID-19 vaccine. Dermatol. Ther. 2022, 35, e15769. [Google Scholar] [CrossRef] [PubMed]
- Aryanian, Z.; Balighi, K.; Azizpour, A.; Kamyab Hesari, K.; Hatami, P. Coexistence of Pemphigus Vulgaris and Lichen Planus following COVID-19 Vaccination. Case Rep. Dermatol. Med. 2022, 2022, 2324212. [Google Scholar] [CrossRef] [PubMed]
- Calabria, E.; Canfora, F.; Mascolo, M.; Varricchio, S.; Mignogna, M.D.; Adamo, D. Autoimmune mucocutaneous blistering diseases after SARS-CoV-2 vaccination: A Case report of Pemphigus Vulgaris and a literature review. Pathol. Res. Pract. 2022, 232, 153834. [Google Scholar] [CrossRef]
- Corra, A.; Barei, F.; Genovese, G.; Zussino, M.; Spigariolo, C.B.; Mariotti, E.B.; Quintarelli, L.; Verdelli, A.; Caproni, M.; Marzano, A.V. Five cases of new-onset pemphigus following vaccinations against coronavirus disease 2019. J. Dermatol. 2022, 50, 229–233. [Google Scholar] [CrossRef]
- Das, P.; Arora, S.; Singh, G.K.; Bellad, P.; Rahman, R.; Bahuguna, A.; Sapra, D.; Shrivastav, R.; Gupta, A. A study of COVID-19 vaccine (Covishield) induced dermatological adverse effects from India. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e402–e404. [Google Scholar] [CrossRef] [PubMed]
- Hatami, P.; Balighi, K.; Nicknam Asl, H.; Aryanian, Z. COVID vaccination in patients under treatment with rituximab: A presentation of two cases from Iran and a review of the current knowledge with a specific focus on pemphigus. Dermatol. Ther. 2022, 35, e15216. [Google Scholar] [CrossRef]
- Knechtl, G.V.; Seyed Jafari, S.M.; Berger, T.; Rammlmair, A.; Feldmeyer, L.; Borradori, L. Development of pemphigus vulgaris following mRNA SARS-CoV-19 BNT162b2 vaccination in an 89-year-old patient. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e251–e253. [Google Scholar] [CrossRef]
- Koutlas, I.G.; Camara, R.; Argyris, P.P.; Davis, M.D.P.; Miller, D.D. Development of pemphigus vulgaris after the second dose of the mRNA-1273 SARS-CoV-2 vaccine. Oral Dis. 2022, 28 (Suppl. S2), 2612–2613. [Google Scholar] [CrossRef] [PubMed]
- Norimatsu, Y.; Yoshizaki, A.; Yamada, T.; Akiyama, Y.; Toyama, S.; Sato, S. Pemphigus vulgaris with advanced hypopharyngeal and gastric cancer following SARS-CoV-2 vaccination. J Dermatol 2022, 50, e74–e75. [Google Scholar] [CrossRef]
- Saffarian, Z.; Samii, R.; Ghanadan, A.; Vahidnezhad, H. De novo severe pemphigus vulgaris following SARS-CoV-2 vaccination with BBIBP-CorV. Dermatol. Ther. 2022, 35, e15448. [Google Scholar] [CrossRef]
- Singh, A.; Bharadwaj, S.J.; Chirayath, A.G.; Ganguly, S. Development of severe pemphigus vulgaris following ChAdOx1 nCoV-19 vaccination and review of literature. J. Cosmet. Dermatol. 2022, 21, 2311–2314. [Google Scholar] [CrossRef]
- Thongprasom, K.; Pengpis, N.; Phattarataratip, E.; Samaranayake, L. Oral pemphigus after COVID-19 vaccination. Oral. Dis. 2022, 28 (Suppl. S2), 2597–2598. [Google Scholar] [CrossRef]
- Hui, H.Z.; Wang, Y.J.; Cheng, J.R.; Mao, H.; Guo, H.X.; Diao, Q.C.; Shi, B.J. Rituximab for COVID-19 Vaccine-Associated Pemphigus Vulgaris. Am. J. Ther. 2023, 30, E544–E546. [Google Scholar] [CrossRef]
- Khalayli, N.; Omar, A.; Kudsi, M. Pemphigus vulgaris after the second dose of COVID-19 vaccination: A case report. J. Med. Case Rep. 2023, 17, 322. [Google Scholar] [CrossRef] [PubMed]
- Alami, S.; Benzekri, L.; Senouci, K.; Meziane, M. Pemphigus foliaceus triggered after inactivated SARS-CoV-2 vaccine: Coincidence or causal link? Dermatol. Ther. 2022, 35, e15775. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Young, P.A.; So, J.Y.; Pol-Rodriguez, M.; Rieger, K.E.; Lewis, M.A.; Winge, M.C.G.; Bae, G.H. New-onset pemphigus vegetans and pemphigus foliaceus after SARS-CoV-2 vaccination: A report of 2 cases. JAAD Case Rep. 2022, 27, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Lua, A.C.Y.; Ong, F.L.L.; Choo, K.J.L.; Yeo, Y.W.; Oh, C.C. An unusual presentation of pemphigus foliaceus following COVID-19 vaccination. Australas. J. Dermatol. 2022, 63, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Pourani, M.; Bidari-Zerehpoosh, F.; Ayatollahi, A.; Robati, R.M. New onset of pemphigus foliaceus following BBIBP COVID-19 vaccine. Dermatol. Ther. 2022, 35, e15816. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Nogueira, M.; Figueiras, O.; Coelho, A.; Cunha Velho, G.; Raposo, I. Pemphigus foliaceous after mRNA COVID-19 vaccine. Eur. J. Dermatol. 2022, 32, 428–429. [Google Scholar] [CrossRef]
- Rouatbi, J.; Aounallah, A.; Lahouel, M.; Sriha, B.; Belajouza, C.; Denguezli, M. Two cases with new onset of pemphigus foliaceus after SARS-CoV-2 vaccination. Dermatol. Ther. 2022, 35, e15827. [Google Scholar] [CrossRef]
- Yildirici, S.; Yayli, S.; Demirkesen, C.; Vural, S. New onset of pemphigus foliaceus following BNT162b2 vaccine. Dermatol. Ther. 2022, 35, e15381. [Google Scholar] [CrossRef]
- Almasi-Nasrabadi, M.; Ayyalaraju, R.S.; Sharma, A.; Elsheikh, S.; Ayob, S. New onset pemphigus foliaceus following AstraZeneca COVID-19 vaccination. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1–e3. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.N.; Nguyen, T.T.P.; Vu, T.T.P.; Nguyen, H.T. Pemphigus Foliaceus after COVID-19 Vaccination: A Report of Two Cases. Case Rep. Dermatol. Med. 2023, 2023, 1218388. [Google Scholar] [CrossRef] [PubMed]
- Weschawalit, S.; Pongcharoen, P.; Suthiwartnarueput, W.; Srivilaithon, W.; Daorattanachai, K.; Jongrak, P.; Chakkavittumrong, P. Cutaneous Adverse Events After COVID-19 Vaccination. Clin. Cosmet. Investig. Dermatol. 2023, 16, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, F.; Lamberti, A.; Cota, C.; Rubegni, P.; Cinotti, E. Reply to ‘development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2’ by Solimani F et al. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e976–e978. [Google Scholar] [CrossRef]
- Lansang, R.P.; Amdemichael, E.; Sajic, D. IgA pemphigus following COVID-19 vaccination: A case report. SAGE Open Med. Case Rep. 2023, 11, 2050313X231181022. [Google Scholar] [CrossRef]
- Kianfar, N.; Dasdar, S.; Salehi Farid, A.; Balighi, K.; Mahmoudi, H.; Daneshpazhooh, M. Exacerbation of Autoimmune Bullous Diseases After Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination: Is There Any Association? Front. Med. 2022, 9, 957169. [Google Scholar] [CrossRef]
- Happaerts, M.; Vanassche, T. Acquired hemophilia following COVID-19 vaccination: Case report and review of literature. Res. Pract. Thromb. Haemost. 2022, 6, e12785. [Google Scholar] [CrossRef]
- Juay, L.; Chandran, N.S. Three cases of vesiculobullous non-IgE-mediated cutaneous reactions to tozinameran (Pfizer-BioNTech COVID-19 vaccine). J. Eur. Acad. Dermatol. Venereol. 2021, 35, e855–e857. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Ruggiero, A.; Battista, T.; Fabbrocini, G.; Megna, M. Bullous pemphigoid and COVID-19 vaccination: Management and treatment reply to ‘Bullous pemphigoid in a young male after COVID-19 mRNA vaccine: A report and brief literature review’ by Pauluzzi et al. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e35–e36. [Google Scholar] [CrossRef]
- Massip, E.; Marcant, P.; Font, G.; Faiz, S.; Duvert-Lehembre, S.; Alcaraz, I.; Vermersch-Langlin, A.; Veron, M.; Macaire, C.; Faure, K.; et al. Cutaneous manifestations following COVID-19 vaccination: A multicentric descriptive cohort. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e253–e255. [Google Scholar] [CrossRef]
- Rasner, C.J.; Schultz, B.; Bohjanen, K.; Pearson, D.R. Autoimmune bullous disorder flares following severe acute respiratory syndrome coronavirus 2 vaccination: A case series. J. Med. Case Rep. 2023, 17, 408. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, S.; Matsuzaki, Y.; Yao, S.; Sagara, C.; Akasaka, E.; Koga, H.; Ishii, N.; Hashimoto, T.; Sawamura, D. Case report: A case of epidermolysis bullosa acquisita with IgG and IgM anti-basement membrane zone antibodies relapsed after COVID-19 mRNA vaccination. Front. Med. 2023, 10, 1093827. [Google Scholar] [CrossRef] [PubMed]
- Avallone, G.; Giordano, S.; Astrua, C.; Merli, M.; Senetta, R.; Conforti, C.; Ribero, S.; Marzano, A.V.; Quaglino, P. Reply to ‘The first dose of COVID-19 vaccine may trigger pemphigus and bullous pemphigoid flares: Is the second dose therefore contraindicated?’ by Damiani G et al. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e433–e435. [Google Scholar] [CrossRef] [PubMed]
- Martora, F.; Fabbrocini, G.; Nappa, P.; Megna, M. Reply to ‘Development of severe pemphigus vulgaris following SARS-CoV-2 vaccination with BNT162b2’ by Solimani et al. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e750–e751. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.K.; Darji, K.; Chaudhry, S.B. Severe flare of pemphigus vulgaris after first dose of COVID-19 vaccine. JAAD Case Rep. 2022, 22, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.A.; Saleh, N.A. Pemphigus vulgaris relapse during the coronavirus disease pandemic. Dermatol. Ther. 2022, 35, e15354. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Ma, S.H.; Wang, L.H.; Chang, Y.T.; Wu, C.Y. Pemphigus aggravation following Pfizer-BioNTech vaccination: A case report and review of literature. Int. J. Rheum. Dis. 2023, 26, 1187–1190. [Google Scholar] [CrossRef]
- Ligrone, L.; Lembo, S.; Cillo, F.; Spennato, S.; Fabbrocini, G.; Raimondo, A. A severe relapse of pemphigus vulgaris after SARS-CoV-2 vaccination. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e1369–e1371. [Google Scholar] [CrossRef]
- Al Salmi, A.; Al Khamisani, M.; Al Shibli, A.; Al Maqbali, S. Adverse cutaneous reactions reported post COVID-19 vaccination in Al Buraimi governorate, Sultanate of Oman. Dermatol. Ther. 2022, 35, e15820. [Google Scholar] [CrossRef]
- Ozgen, Z.; Aksoy, H.; Akin Cakici, O.; Koku Aksu, A.E.; Erdem, O.; Kara Polat, A.; Gurel, M.S. COVID-19 severity and SARS-CoV-2 vaccine safety in pemphigus patients. Dermatol. Ther. 2022, 35, e15417. [Google Scholar] [CrossRef]
- Kasperkiewicz, M.; Strong, R.; Yale, M.; Dunn, P.; Woodley, D.T. Safety of the COVID-19 vaccine booster in patients with immunobullous diseases: A cross-sectional study of the International Pemphigus and Pemphigoid Foundation. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e9–e10. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.H.; Wu, P.C.; Liu, C.W.; Huang, Y.C. Association between bullous pemphigoid and psychiatric disorders: A systematic review and meta-analysis. J. Dtsch. Dermatol. Ges. 2022, 20, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, K.; Chi, C.C.; Vincent, A.; Groves, R.W.; Venning, V.; Wojnarowska, F. The association of bullous pemphigoid with cerebrovascular disease and dementia: A case-control study. Arch. Dermatol. 2010, 146, 1251–1254. [Google Scholar] [CrossRef]
- Liu, S.D.; Chen, W.T.; Chi, C.C. Association between medication use and bullous pemphigoid: A systematic review and meta-analysis. JAMA Dermatol. 2020, 156, 891–900. [Google Scholar] [CrossRef]
- Murayama, H.; Sakuma, M.; Takahashi, Y.; Morimoto, T. Improving the assessment of adverse drug reactions using the Naranjo Algorithm in daily practice: The Japan Adverse Drug Events Study. Pharmacol. Res. Perspect. 2018, 6, e00373. [Google Scholar] [CrossRef]
- Ujiie, I.; Ujiie, H.; Iwata, H.; Shimizu, H. Clinical and immunological features of pemphigus relapse. Br. J. Dermatol. 2019, 180, 1498–1505. [Google Scholar] [CrossRef]
- Vadala, M.; Poddighe, D.; Laurino, C.; Palmieri, B. Vaccination and autoimmune diseases: Is prevention of adverse health effects on the horizon? EPMA J. 2017, 8, 295–311. [Google Scholar] [CrossRef]
- Kasperkiewicz, M.; Woodley, D.T. Association between vaccination and autoimmune bullous diseases: A systematic review. J. Am. Acad. Dermatol. 2022, 86, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Aashish; Rai, A.; Khatri, G.; Priya; Hasan, M.M. Bullous pemphigoid following COVID-19 vaccine: An autoimmune disorder. Ann. Med. Surg. 2022, 80, 104266. [Google Scholar] [CrossRef] [PubMed]
- Cozzani, E.; Gasparini, G.; Russo, R.; Parodi, A. May bullous pemphigoid be worsened by COVID-19 vaccine? Front. Med. 2022, 9, 931872. [Google Scholar] [CrossRef]
- Hertl, M.; Eming, R.; Veldman, C. T cell control in autoimmune bullous skin disorders. J. Clin. Investig. 2006, 116, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Sernicola, A.; Mazzetto, R.; Tartaglia, J.; Ciolfi, C.; Miceli, P.; Alaibac, M. Role of Human Leukocyte Antigen Class II in Antibody-Mediated Skin Disorders. Medicina 2023, 59, 1950. [Google Scholar] [CrossRef] [PubMed]
- Kasperkiewicz, M.; Bednarek, M.; Tukaj, S. Case Report: Circulating Anti-SARS-CoV-2 Antibodies Do Not Cross-React With Pemphigus or Pemphigoid Autoantigens. Front. Med. 2021, 8, 807711. [Google Scholar] [CrossRef]
- Kasperkiewicz, M. Association between COVID-19 vaccination and autoimmune bullous diseases: A random coincidence or rare event. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e665–e666. [Google Scholar] [CrossRef] [PubMed]
- Kasperkiewicz, M.; Woodley, D.T. Association between vaccination and immunobullous disorders: A brief, updated systematic review with focus on COVID-19. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e498–e500. [Google Scholar] [CrossRef] [PubMed]
- Moro, F.; Fania, L.; Sinagra, J.L.M.; Salemme, A.; Di Zenzo, G. Bullous pemphigoid: Trigger and predisposing factors. Biomolecules 2020, 10, 1432. [Google Scholar] [CrossRef]
- Chen, Y.N.; Chi, C.C. Levels of evidence and study designs: A brief introduction to dermato-epidemiologic research methodology. Dermatol. Sin. 2023, 41, 199–205. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).