Interleukin 34 Serves as a Novel Molecular Adjuvant against Nocardia Seriolae Infection in Largemouth Bass (Micropterus Salmoides)
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Fish, Bacterial Strains, Plasmids, Reagents, and Growth Conditions
2.3. Sequence and Phylogenetic Analysis
2.4. Plasmid Construction and Cloning of pcIL-34 and pcHrp1
2.5. In vitro Expression Analysis of pcIL-34 and pcHrp1
2.6. Vaccination and Sampling
2.7. Challenge
2.8. Detection of pcIL-34 and pcHrp1 in the Muscle of Immnunized Fish by PCR
2.9. In Vivo Transcription Analysis of pcIL-34 and pcHrp1 by RT-PCR
2.10. In vivo Expression Analysis of pcIL-34 and pcHrp1 by Western Blotting
2.11. Western Blot Analysis
2.12. Serum Lysozyme Activity
2.13. Enzyme-Linked Immunosorbent Assay (ELISA)
2.14. qRT-PCR Analysis of the Expression of Immune-Related Genes
2.15. Statistical Analysis
3. Results
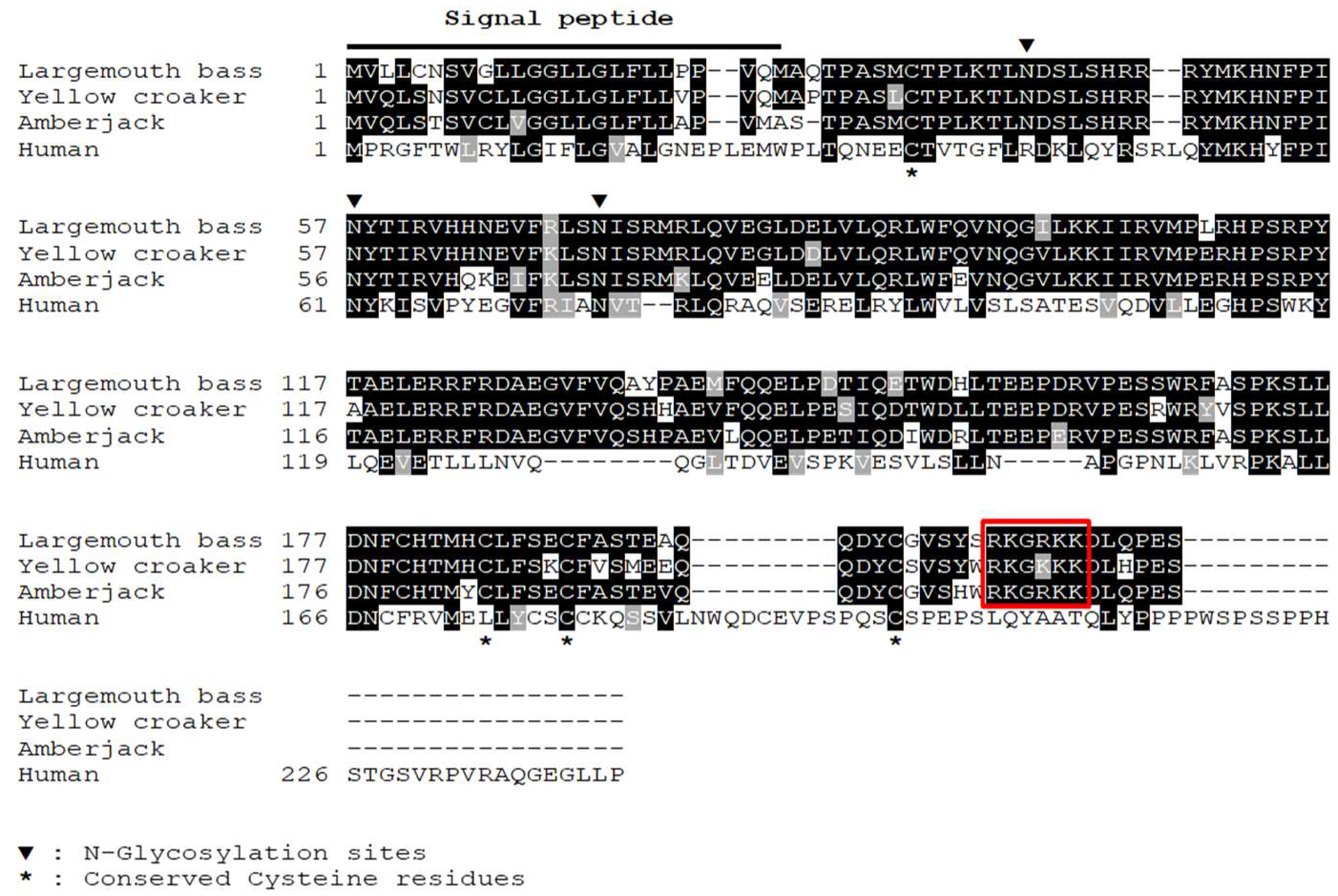
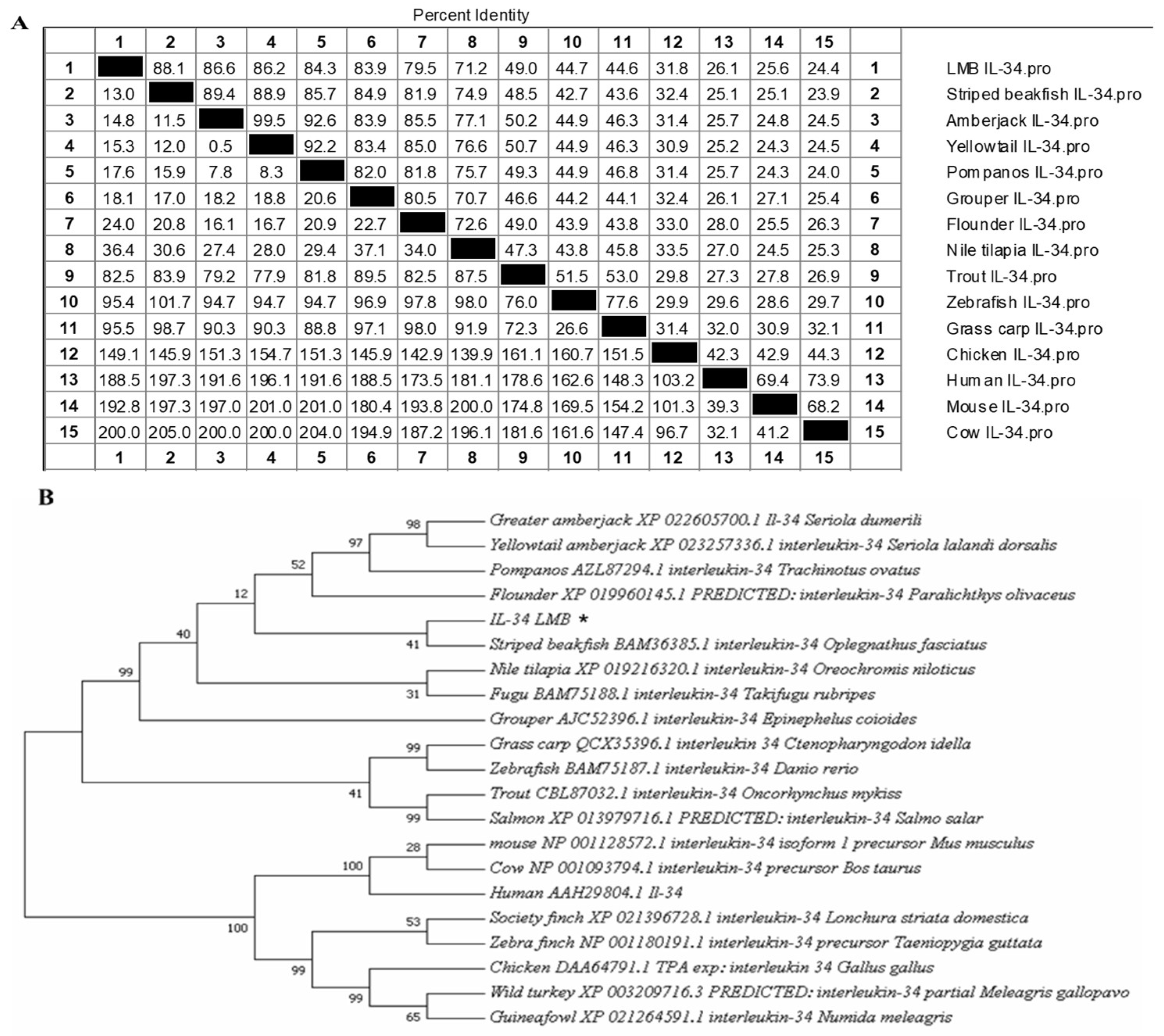
3.1. Sequence Analysis of IL-34
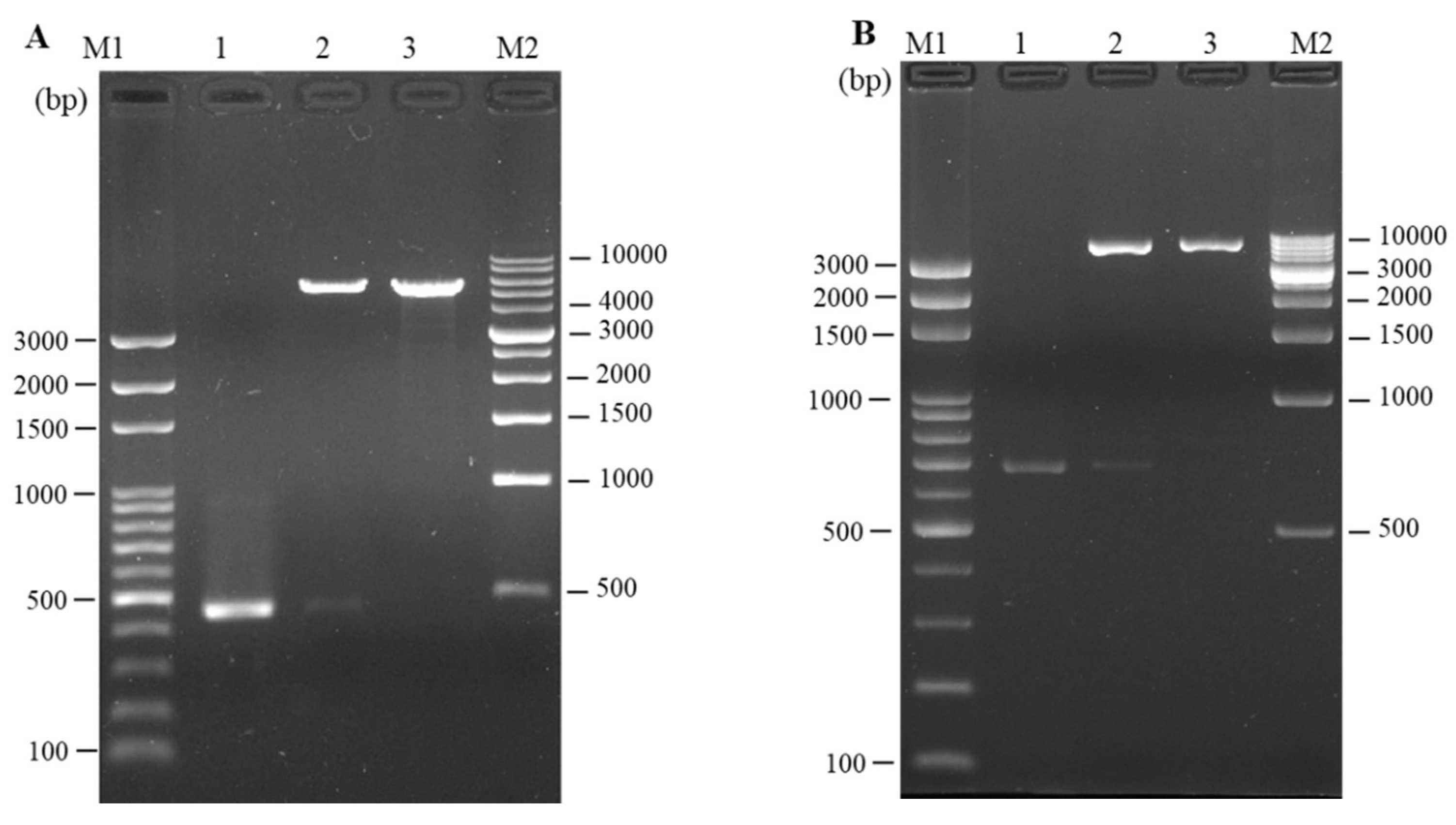
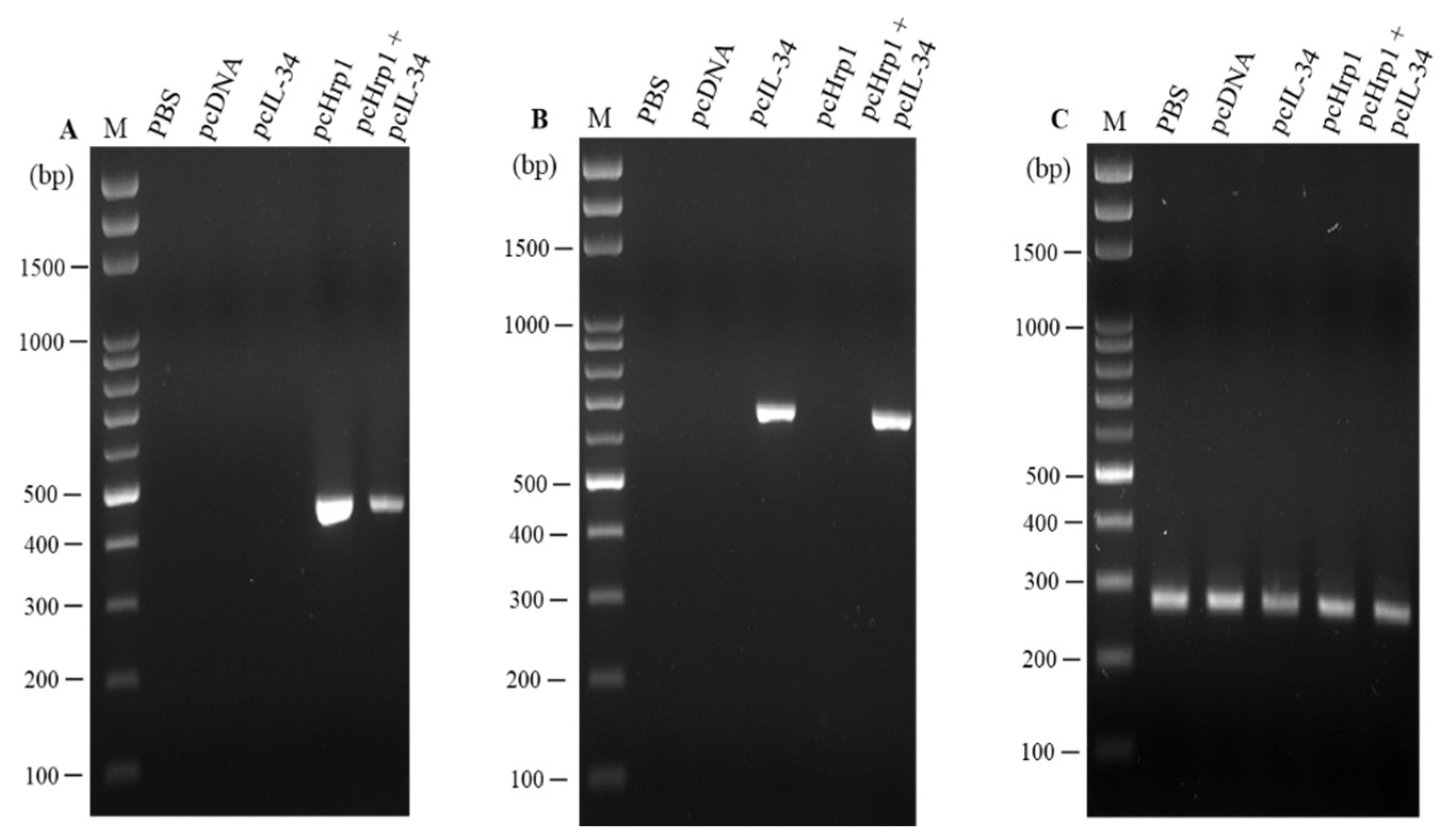
3.2. Cloning and In Vitro Expression of Plasmid pcIL-34 and pcHrp1
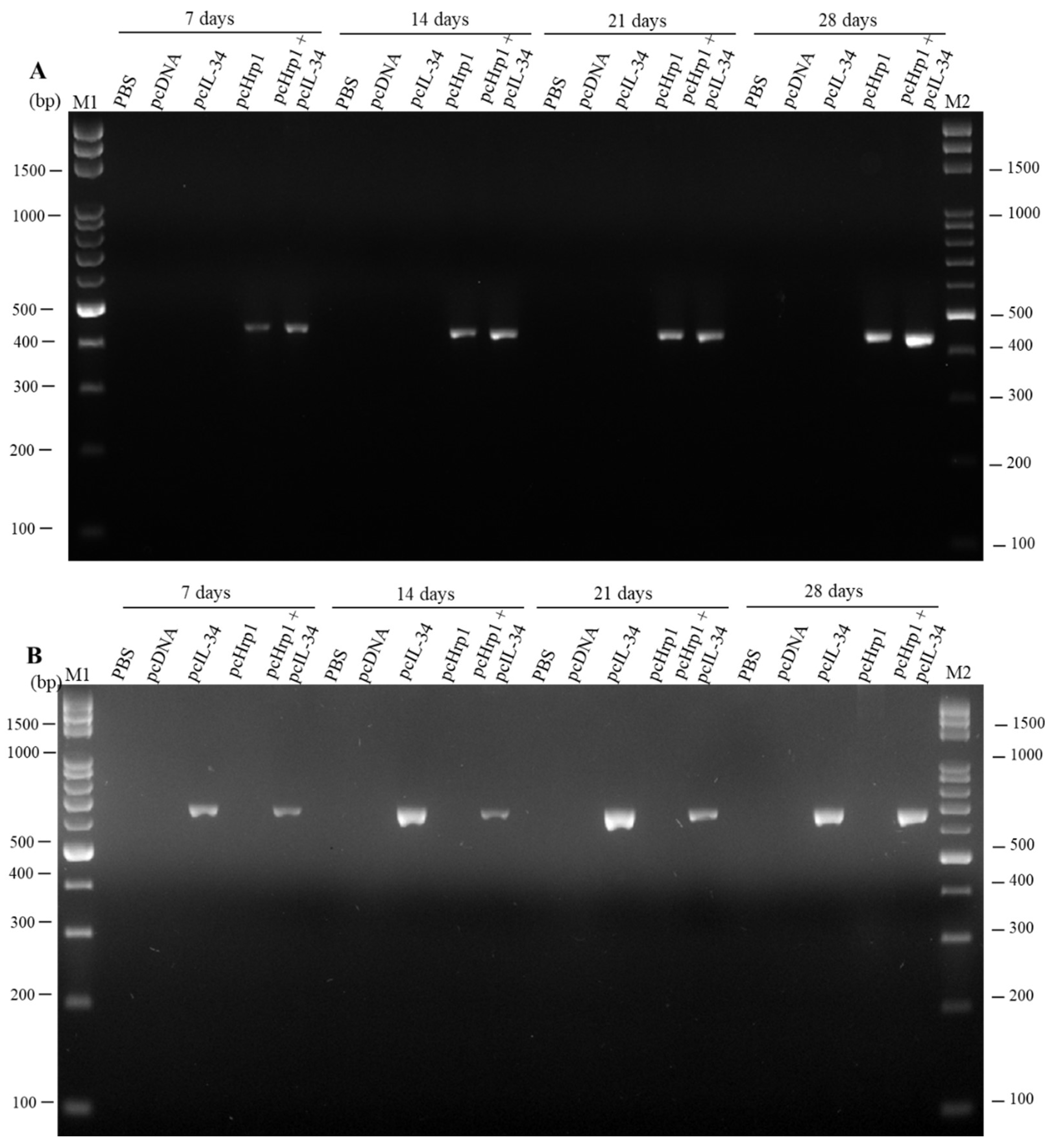
3.3. Detection of pcIL-34 and pcHrp1 in the Muscle of Immnunized Fish by PCR
3.4. In vivo Transcription Analysis of pcIL-34 and pcHrp1 by RT-PCR
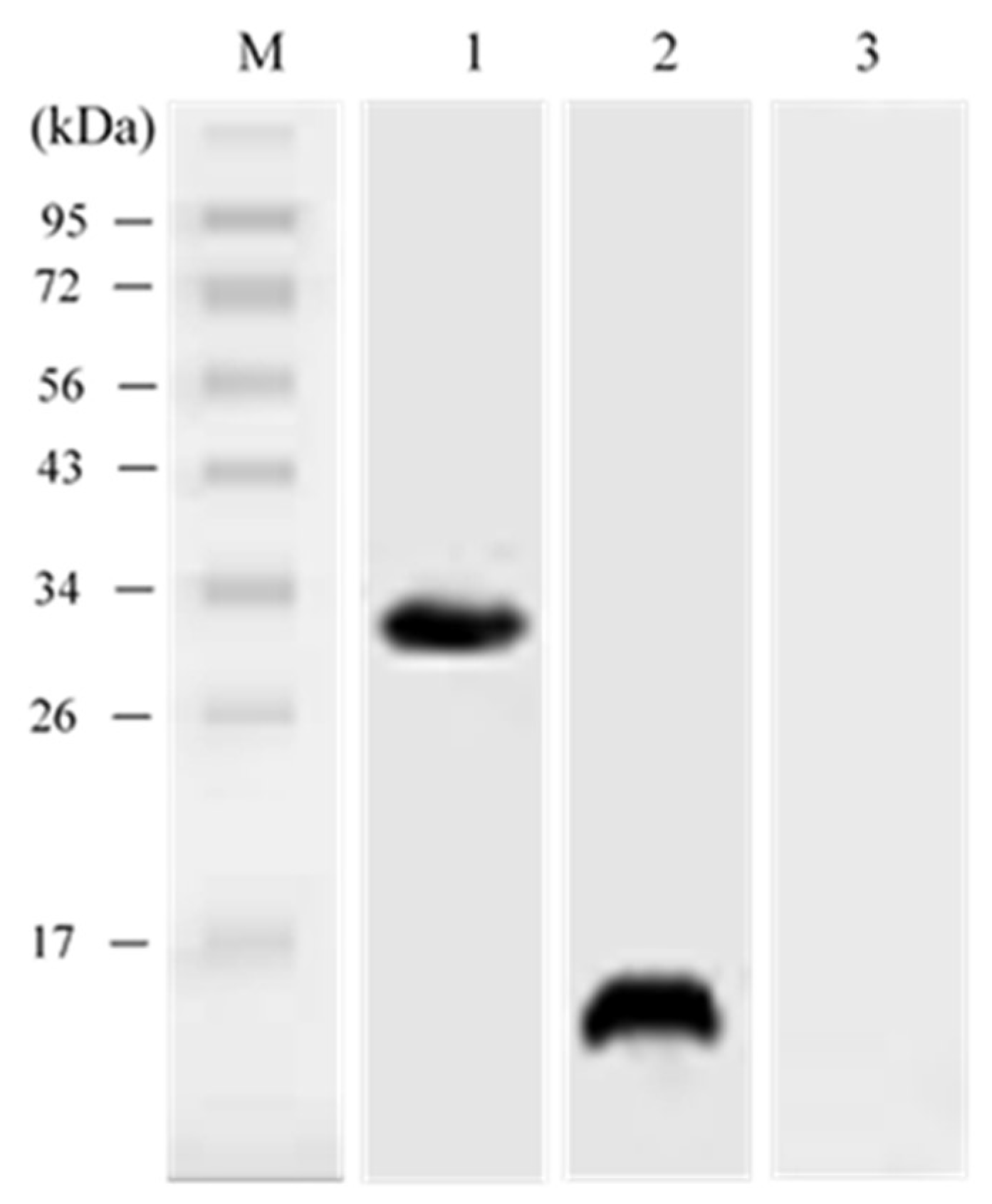
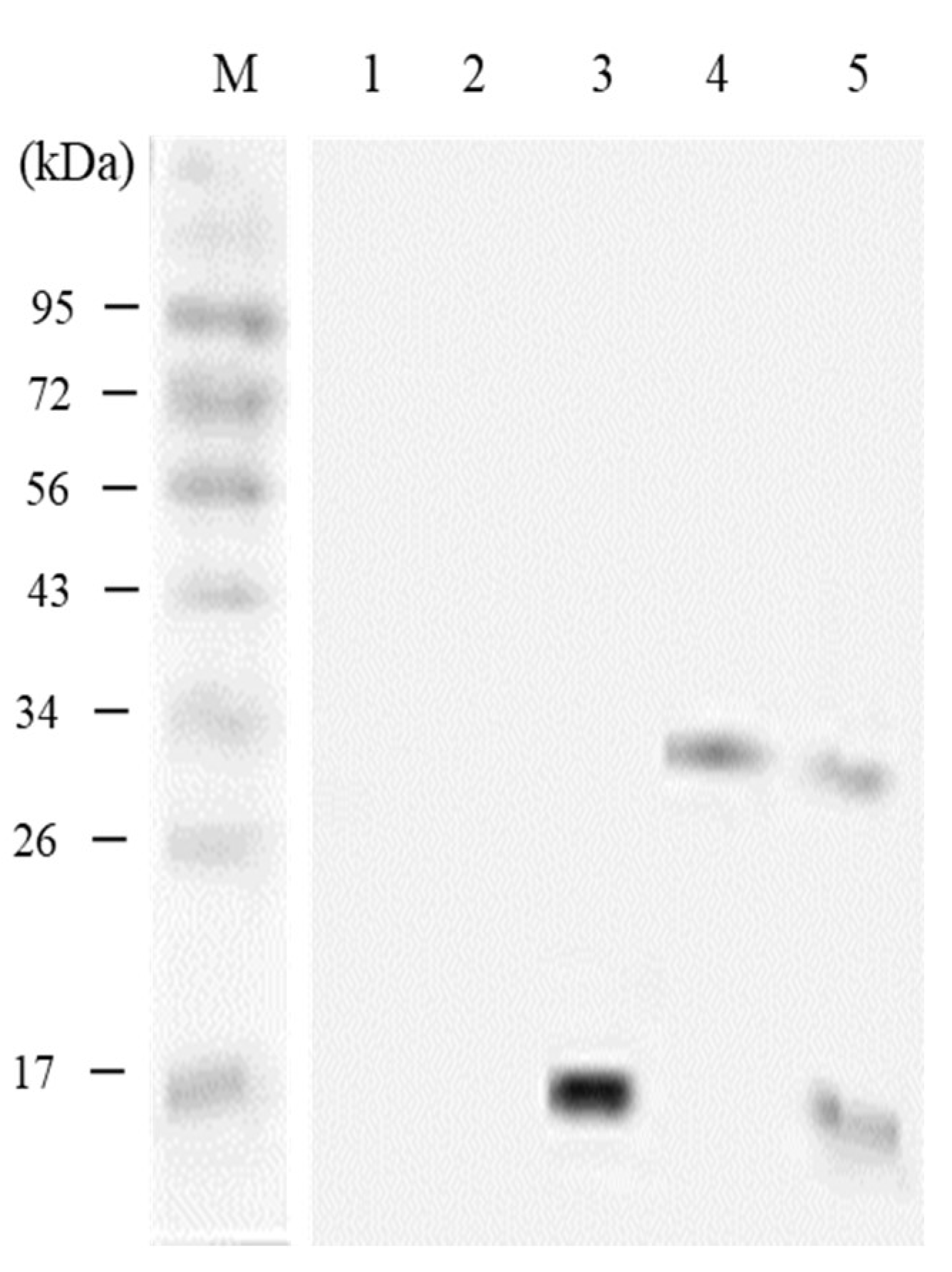
3.5. In vivo Expression Analysis of pcIL-34 and pcHrp1 by Western Blotting
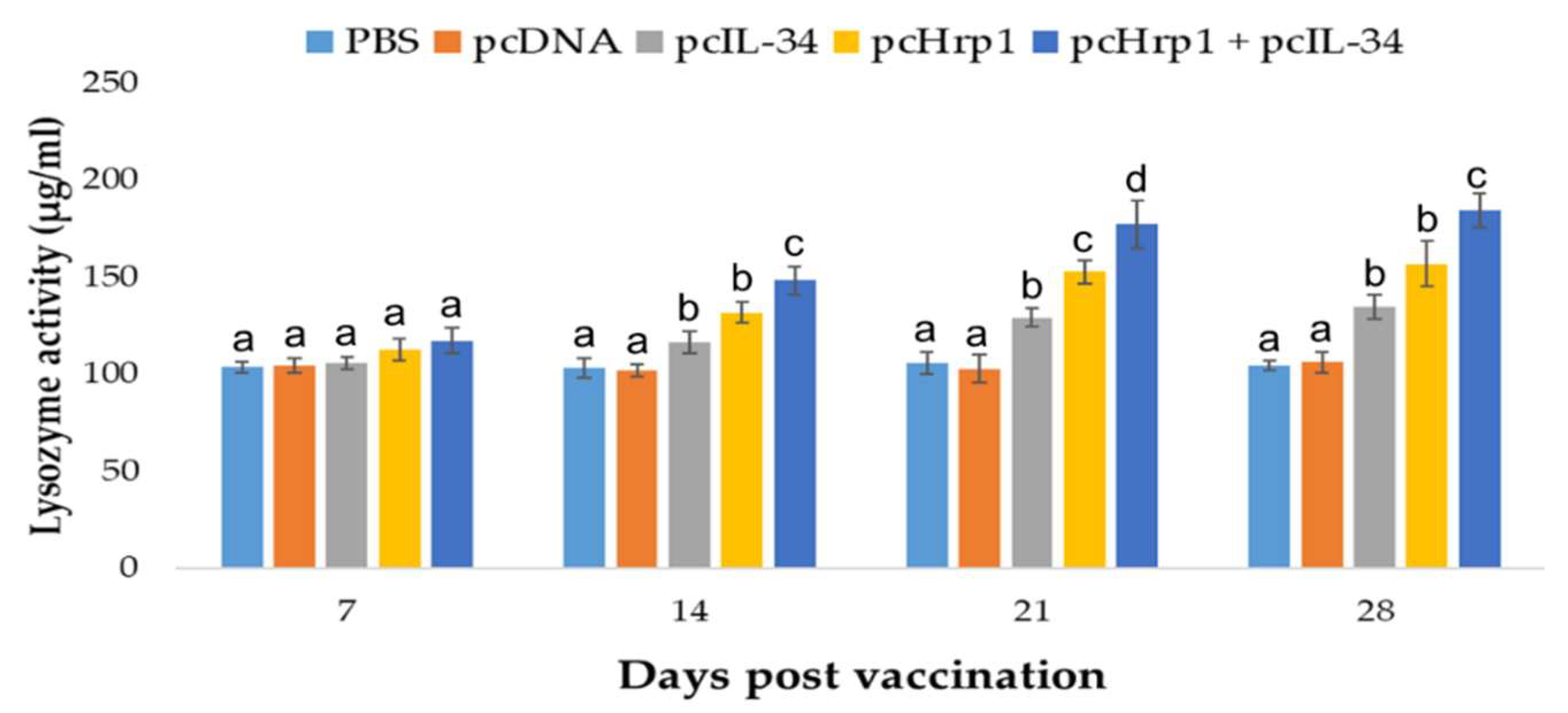
3.6. Serum Lysozyme Activity
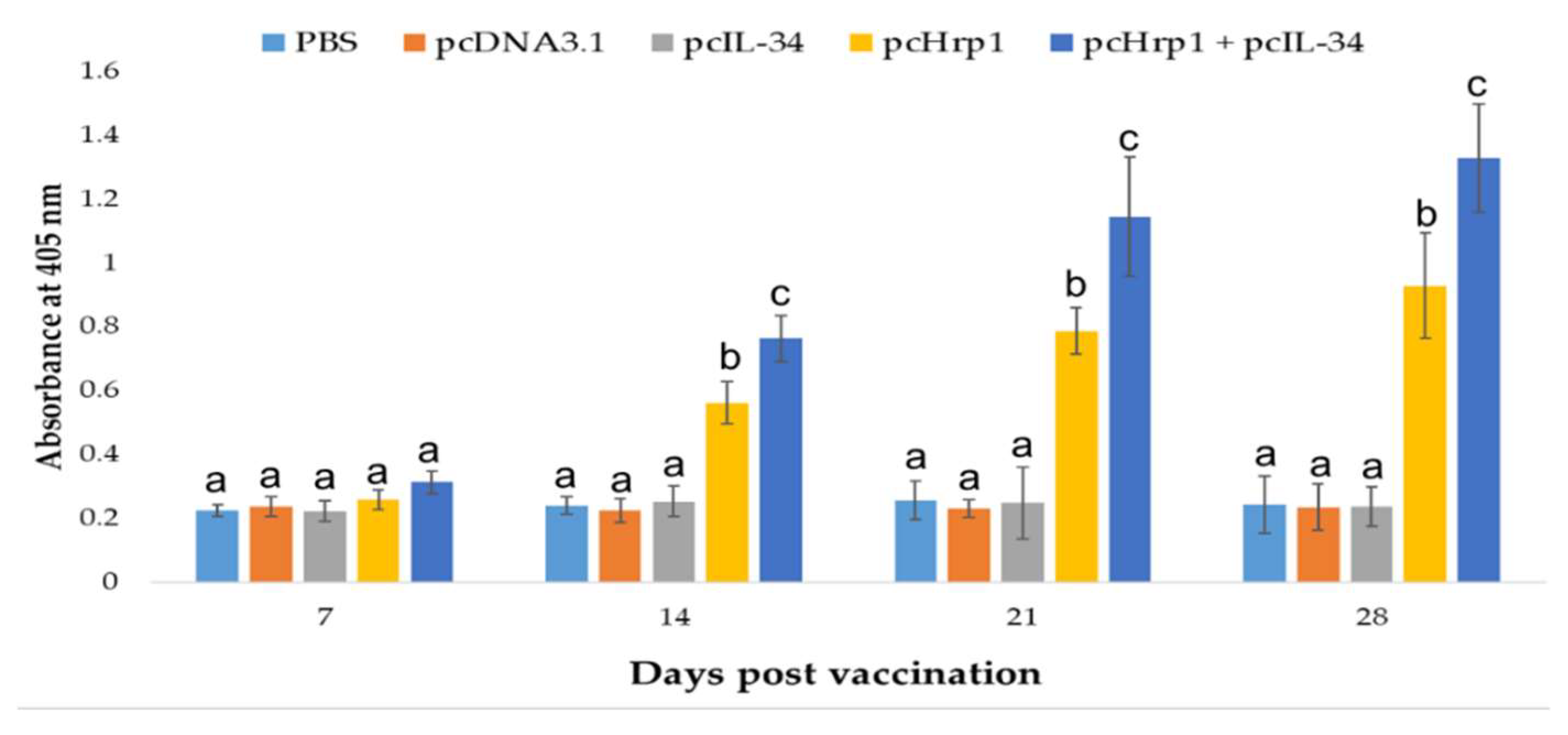
3.7. Specific Serum Antibody Production
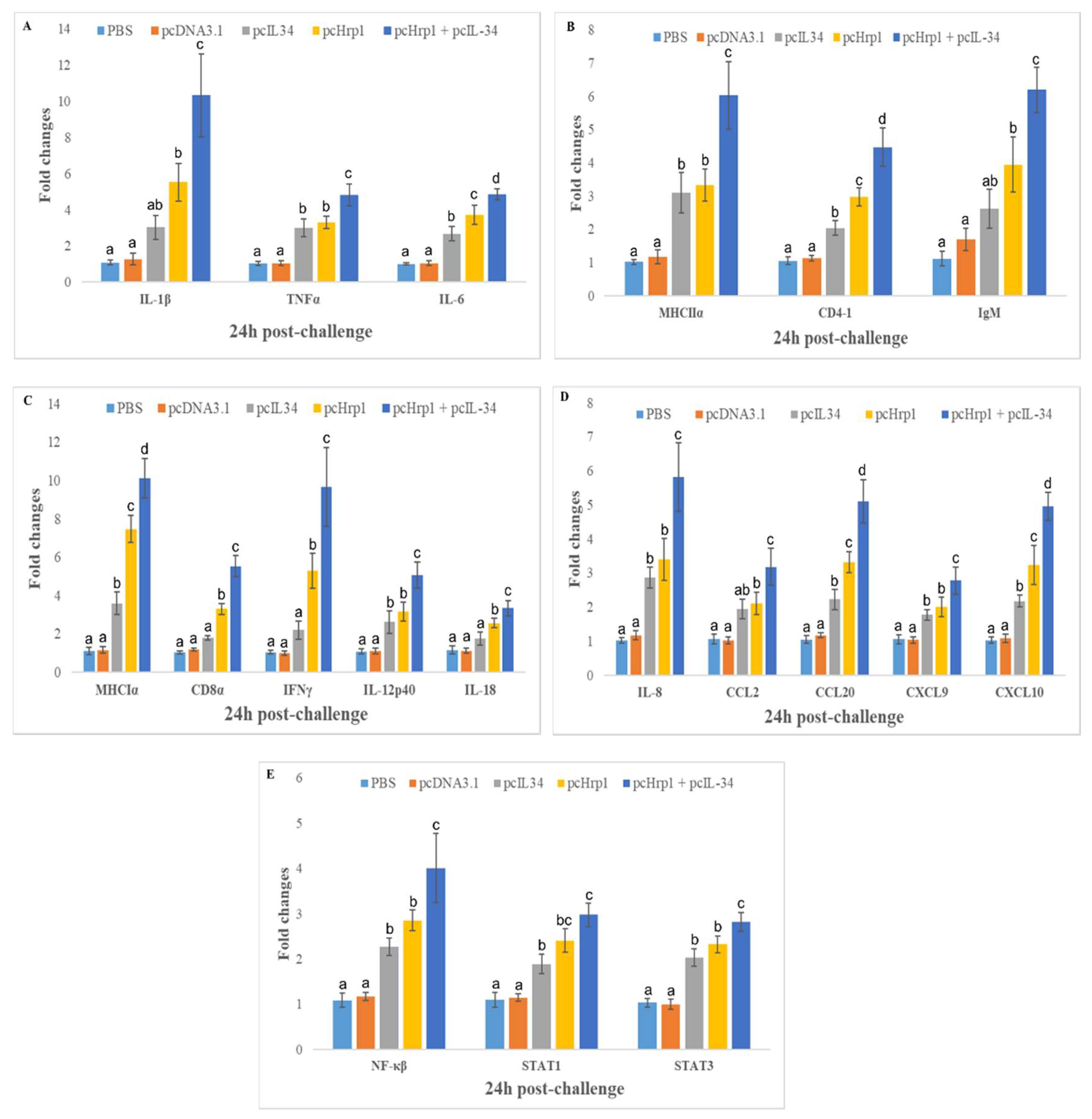
3.8. Expression of Immune-Related Genes
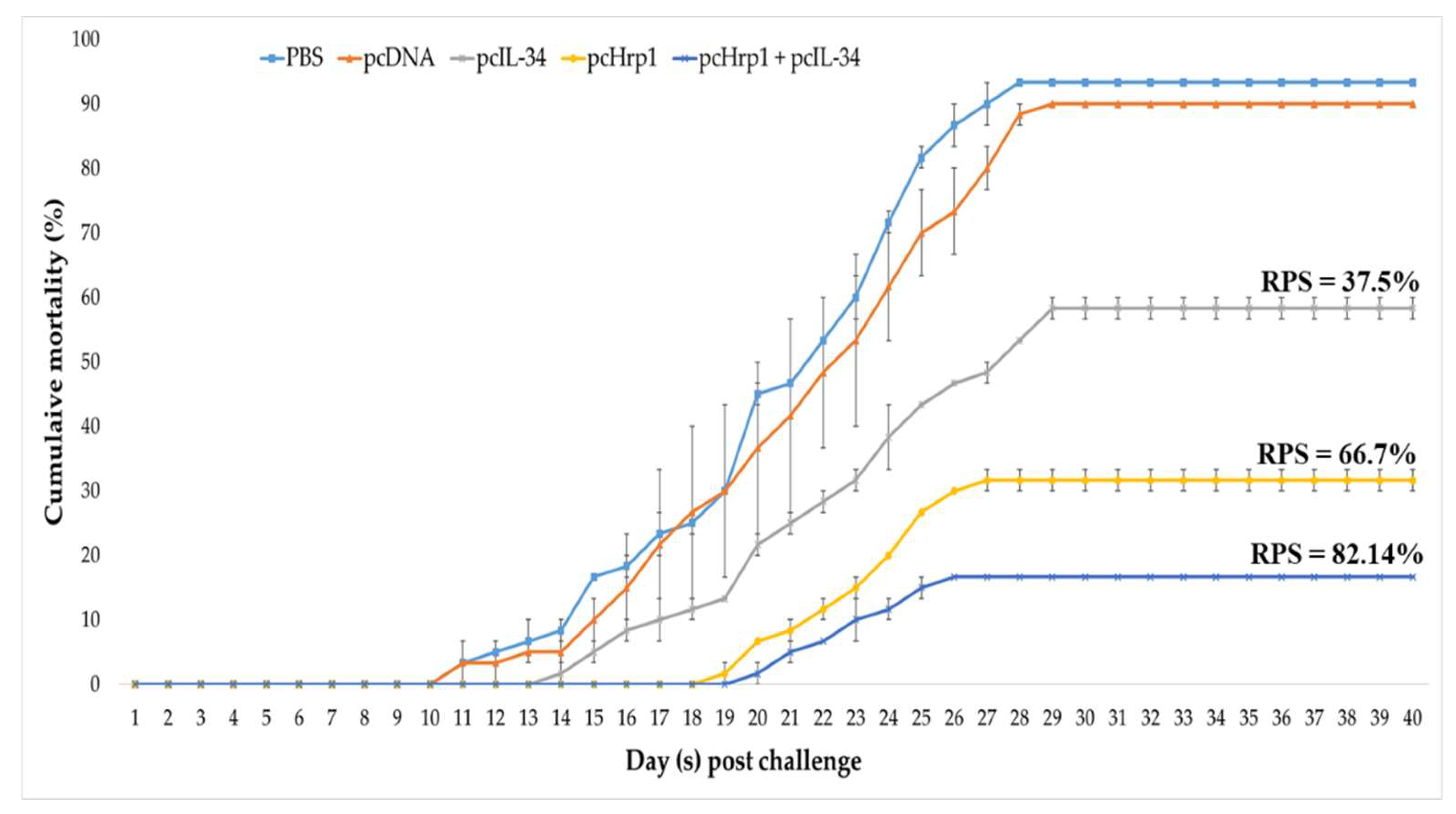
3.9. Immuno-Protective Efficacy against N. seriolae Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kariya, T.; Kubota, S.; Nakamura, Y.; Kira, K. Nocardia infection in cultured yellowtails (Seriola quinqueradiata and S. purpursacens) I. Bacteriological study. Fish Pathol. 1968, 3, 16–23. [Google Scholar] [CrossRef]
- Chen, S.C.; Lee, J.L.; Lai, C.C.; Gu, Y.W.; Wang, C.T.; Chang, H.Y. Nocardiosis in sea bass, Lateolabrax japonicus, in Taiwan. J. Fish Dis. 2000, 23, 299–307. [Google Scholar] [CrossRef]
- Huang, S.L.; Lai, K.C.; Su, S.C.; Shei, M.C.; Chen, S.N. Isolation and characterization of the pathogenic bacterium, Nocardia seriolae, from female broodstock of striped mullet (Mugil cephalus). J. Fish. Res. 2004, 12, 61–69. [Google Scholar]
- Labrie, L.; Ng, J.; Tan, Z.; Komar, C.; Ho, E.; Grisez, L. Nocardial infections in fish: An emerging problem in both freshwater and marine aquaculture systems in Asia. In Diseases in Asian Aquaculture VI; Reantaso, M.G.B., Mohan, V., Crumlish, M., Subasinghe, R.P., Eds.; Fish Health Section, Asian Fisheries Society: Manila, Philippines, 2008; pp. 297–312. [Google Scholar]
- Vu-Khac, H.; Duong, V.Q.B.; Chen, S.C.; Pham, T.H.; Nguyen, T.T.G.; Trinh, T.T.H. Isolation and genetic characterization of Nocardia seriolae from snubnose pompano Trachinotus blochii in Vietnam. Dis. Aquat. Org. 2016, 120, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Shimahara, Y.; Huang, Y.F.; Tsai, M.A.; Wang, P.C.; Yoshida, T.; Lee, J.L.; Chen, S.C. Genotypic and phenotypic analysis of fish pathogen, Nocardia seriolae, isolated in Taiwan. Aquaculture 2009, 294, 165–171. [Google Scholar] [CrossRef]
- Silveira, M.M.; Oliveira, L.; Schuch, R.A.; McBride, A.J.A.; Dellagostin, O.A.; Hartwig, D.D. DNA vaccines against leptospirosis: A literature review. Vaccine 2017, 35, 5559–5567. [Google Scholar] [CrossRef] [PubMed]
- McNeel, D.G.; Becker, J.T.; Johnson, L.E.; Olson, B.M. DNA Vaccines for Prostate Cancer. Curr. Cancer Ther. Rev. 2012, 8, 254–263. [Google Scholar] [CrossRef]
- Liu, C.; Hu, X.; Cao, Z.; Sun, Y.; Chen, X.; Zhang, Z. Construction and characterization of a DNA vaccine encoding the SagH against Streptococcus iniae. Fish Shellfish Immunol. 2019, 89, 71–75. [Google Scholar] [CrossRef]
- Xu, H.; Xing, J.; Tang, X.; Sheng, X.; Zhan, W. Intramuscular administration of a DNA vaccine encoding OmpK antigen induces humoral and cellular immune responses in flounder (Paralichthys olivaceus) and improves protection against Vibrio anguillarum. Fish Shellfish Immunol. 2019, 86, 618–626. [Google Scholar] [CrossRef]
- Xing, J.; Xu, H.; Tang, X.; Sheng, X.; Zhan, W. A DNA Vaccine Encoding the VAA Gene of Vibrio anguillarum Induces a Protective Immune Response in Flounder. Front. Immunol. 2019, 10, 499. [Google Scholar] [CrossRef]
- Jiao, X.D.; Zhang, M.; Hu, Y.; Sun, L. Construction and evaluation of DNA vaccines encoding Edwardsiella tarda antigens. Vaccine 2009, 27, 5195–5202. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.S.; Sun, L. Comparative study of the immune effect of an Edwardsiella tarda antigen in two forms: Subunit vaccine vs DNA vaccine. Vaccine 2011, 29, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, J.; Zhang, H.; Liu, Q.; Xiao, J.; Zhang, Y. Design and evaluation of an Edwardsiella tarda DNA vaccine co-encoding antigenic and adjuvant peptide. Fish Shellfish Immunol. 2016, 59, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Kato, G.; Kato, K.; Jirapongpairoj, W.; Kondo, H.; Hirono, I. Development of DNA vaccines against Nocardia seriolae infection in fish. Fish Pathol. 2014, 49, 165–172. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.; Hou, S.; Fu, W.; Cai, J.; Xia, L.; Lu, Y. Comparison of protective efficacy between two DNA vaccines encoding DnaK and GroEL against fish nocardiosis. Fish Shellfish Immunol. 2019, 95, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Salonius, K.; Simard, N.; Harland, R.; Ulmer, J.B. The road to licensure of a DNA vaccine. Curr. Opin. Investig. Drugs 2007, 8, 635–641. [Google Scholar] [PubMed]
- Dalmo, R.A. DNA vaccines for fish: Review and perspectives on correlates of protection. J. Fish Dis. 2018, 41, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ingolotti, M.; Kawalekar, O.; Shedlock, D.J.; Muthumani, K.; Weiner, D.B. DNA vaccines for targeting bacterial infections. Expert Rev. Vaccines 2010, 9, 747–763. [Google Scholar] [CrossRef]
- Reynes-Cerpa, S.; Maisey, K.; Reyes-López, F.; Toro-Ascuy, D.; Sandino, A.M.; Imarai, M. Fish cytokines and immune response. New Adv. Contrib. Fish Biol. 2012, 3–57. [Google Scholar] [CrossRef]
- Tafalla, C.; Bøgwald, J.; Dalmo, R.A. Adjuvants and immunostimulants in fish vaccines: Current knowledge and future perspectives. Fish Shellfish Immunol. 2013, 35, 1740–1750. [Google Scholar] [CrossRef]
- Guo, M.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. The Immune Adjuvant Effects of Flounder (Paralichthys olivaceus) Interleukin-6 on E. tarda Subunit Vaccine OmpV. Int. J. Mol. Sci. 2017, 18, 1445. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Long, B.; Wang, K.; Wang, J.; He, Y.; Wang, X.; Yang, Q.; Liu, T.; Chen, D.; Geng, Y.; et al. Interleukin-8 holds promise to serve as a molecular adjuvant in DNA vaccination model against Streptococcus iniae infection in fish. Oncotarget 2016, 7, 83938–83950. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, Q.; Xu, L.; Li, S.; Wang, D.; Zhao, J.; Liu, H.; Feng, J.; Lu, T. Effects of different cytokines on immune responses of rainbow trout in a virus DNA vaccination model. Oncotarget 2017, 8, 112222–112235. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Tang, X.; Sheng, X.; Xing, J.; Zhan, W. The effects of IL-1β, IL-8, G-CSF and TNF-α as molecular adjuvant on the immune response to an E. tarda subunit vaccine in flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2018, 77, 374–384. [Google Scholar] [CrossRef]
- Xu, H.; Xing, J.; Tang, X.; Sheng, X.; Zhan, W. Generation and functional evaluation of a DNA vaccine co-expressing Vibrio anguillarum VAA protein and flounder interleukin-2. Fish Shellfish Immunol. 2019, 93, 1018–1027. [Google Scholar] [CrossRef]
- Wang, E.; Liu, T.; Wu, J.; Wang, K.; Chen, D.; Geng, Y.; Huang, X.; Ouang, P.; Lai, W.; Ai, X. Molecular characterization, phylogenetic analysis and adjuvant effect of channel catfish interleukin-1βs against Streptococcus iniae. Fish Shellfish Immunol. 2019, 87, 155–165. [Google Scholar] [CrossRef]
- Matsumoto, M.; Araki, K.; Hayashi, K.; Takeuchi, Y.; Shiozaki, K.; Suetake, H.; Yamamoto, A. Adjuvant effect of recombinant interleukin-12 in the Nocardiosis formalin-killed vaccine of the amberjack Seriola dumerili. Fish Shellfish Immunol. 2017, 67, 263–269. [Google Scholar] [CrossRef]
- Xu, H.; Xing, J.; Tang, X.; Sheng, X.; Zhan, W. The effects of CCL3, CCL4, CCL19 and CCL21 as molecular adjuvants on the immune response to VAA DNA vaccine in flounder (Paralichthys olivaceus). Dev. Comp. Immunol. 2019, 103, 103492. [Google Scholar] [CrossRef]
- Kumari, R.; Kole, S.; Soman, P.; Rathore, G.; Triphathi, G.; Makesh, M.; Rajendran, K.V.; Bedekar, M.K. Bicistronic DNA vaccine against Edwardsiella tarda infection in Labeo rohita: Construction and comparative evaluation of its protective efficacy against monocistronic DNA vaccine. Aquaculture 2018, 485, 201–209. [Google Scholar] [CrossRef]
- Ge, Y.; Huang, M.; Yao, Y.M. Immunomodulation of interleukin-34 and its potential significance as a disease Biomarker and Therapeutic target. Int. J. Biol. Sci. 2019, 15, 1835–1845. [Google Scholar] [CrossRef]
- Wang, T.; Kono, T.; Monte, M.; Kuse, H.; Costa, M.; Korenaga, H.; Maehr, T.; Husain, M.; Sakai, M.; Secombes, C.J. Identification of IL-34 in teleost fish: Differential expression of rainbow trout IL-34, MCSF1 and MCSF2, ligands of the MCSF receptor. Mol. Immunol. 2013, 53, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.Q.; Li, Y.W.; Zhou, L.; Li, A.X.; Luo, X.C.; Dan, X.M. Grouper (Epinephelus coioides) IL-34/MCSF2 and MCSFR1/MCSFR2 were involved in mononuclear phagocytes activation against Cryptocaryon irritans infection. Fish Shellfish Immunol. 2015, 67, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, L.; Wu, C.; Lou, B. Molecular characterization and expression analysis of large yellow croaker (Larimichthys crocea) interleukin-12A, 16 and 34 after poly I:C and Vibrio anguillarum challenge. Fish Shellfish Immunol. 2018, 74, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Jiang, X.; Gao, J.; Li, X.; Xu, J.; Wang, J.; Gao, Q.; Zou, J. Functional characterisation of interleukin 34 in grass carp Ctenopharyngodon idella. Fish Shellfish Immunol. 2019, 92, 91–100. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Suzuki, S. A PCR method to detect Nocardia seriolae in fish samples. Fish Pathol. 2003, 38, 93–97. [Google Scholar] [CrossRef]
- Rombel, I.T.; Sykes, K.F.; Rayner, S.; Johnston, S.A. ORF-FINDER: A vector for high-throughput gene identification. Gene 2002, 282, 33–41. [Google Scholar] [CrossRef]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [CrossRef]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; Heijne von, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Pitti, T.; Chen, C.T.; Lin, H.N.; Choong, W.K.; Hsu, W.L.; Sung, T.Y. N-GlyDE: A two-stage N-linked glycosylation site prediction incorporating gapped dipeptides and pattern-based encoding. Sci. Rep. 2019, 9, 15975. [Google Scholar] [CrossRef]
- Ye, J.; McGinnis, S.; Madden, T.L. BLAST: Improvements for better sequence analysis. Nucleic Acids Res. 2006, 34, W6–W9. [Google Scholar] [CrossRef]
- Kozak, M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. Biol. Chem. 1991, 266, 19867–19870. [Google Scholar]
- Hoang, H.H.; Wang, P.C.; Chen, S.C. The protective efficacy of recombinant hypoxic response protein 1 of Nocardia seriolae in largemouth bass (Micropterus salmoides). Vaccine 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Nguyen, H.T.; Wang, P.C.; Chen, S.C. Identification and expression analysis of two pro-inflammatory cytokines, TNF-alpha and IL-8, in cobia (Rachycentron canadum L.) in response to Streptococcus dysgalactiae infection. Fish Shellfish Immunol. 2017, 67, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Byadgi, O.; Chen, C.W.; Wang, P.C.; Tsai, M.A.; Chen, S.C. De Novo Transcriptome Analysis of Differential Functional Gene Expression in Largemouth Bass (Micropterus salmoides) after Challenge with Nocardia seriolae. Int. J. Mol. Sci. 2016, 17, 1315. [Google Scholar] [CrossRef]
- Panzarin, V.; Toffan, A.; Abbadi, M.; Buratin, A.; Mancin, M.; Braaen, S.; Olsen, C.M.; Bargelloni, L.; Rimstad, E.; Cattoli, G. Molecular Basis for Antigenic Diversity of Genus Betanodavirus. PLoS ONE 2016, 11, e0158814. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, S.; Pang, H.; Jian, J.; Wu, Z. Immunogenicity and efficacy of DNA vaccine encoding antigenic AcfA via addition of the molecular adjuvant Myd88 against Vibrio alginolyticus in Epinephelus coioides. Fish Shellfish Immunol. 2017, 66, 71–77. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Liu, R.; Jia, J. Identification and evaluation of an outer membrane protein OmpU from a pathogenic Vibrio harveyi isolate as vaccine candidate in turbot (Scophthalmus maximus). Lett. Appl. Microbiol. 2011, 53, 22–29. [Google Scholar] [CrossRef]
- Xu, L.; Zhao, J.; Liu, M.; Ren, G.; Jian, F.; Yin, J.; Feng, J.; Liu, H.; Lu, T. Bivalent DNA vaccine induces significant immune responses against infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus in rainbow trout. Sci. Rep. 2017, 7, 5700. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Nguyen, T.T.T.; Chen, Y.C.; Vu-Khac, H.; Wang, P.C.; Chen, S.C. Enhanced immune responses and effectiveness of refined outer membrane protein vaccines against Vibrio harveyi in orange-spotted grouper (Epinephelus coioides). J. Fish Dis. 2018, 41, 1349–1358. [Google Scholar] [CrossRef]
- Ellis, A.E. Lysozyme Assays. InTech. Fish Immunol. 1990, 1, 101–103. [Google Scholar]
- Martyniuk, C.J.; Doperalski, N.J.; Prucha, M.S.; Zhang, J.L.; Kroll, K.J.; Conrow, R.; Barber, D.S.; Denslow, N.D. High contaminant loads in Lake Apopka’s riparian wetland disrupt gene networks involved in reproduction and immune function in largemouth bass. Comp. Biochem. Physiol. D Genom. Proteom. 2016, 19, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Baghdadi, M.; Umeyama, Y.; Hama, N.; Kobayashi, T.; Han, N.; Wada, H.; Seino, K.I. Interleukin-34, a comprehensive review. J. Leukoc. Biol. 2018, 104, 931–951. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Leo, C.; Chen, X.; Wong, B.R.; Williams, L.T.; Lin, H.; He, X. The mechanism of shared but distinct CSF-1R signaling by the non-homologous cytokines IL-34 and CSF-1. Biochim. Biophys. Acta 2012, 1824, 938–945. [Google Scholar] [CrossRef]
- Hølvold, L.B.; Myhr, A.I.; Dalmo, R.A. Strategies and hurdles using DNA vaccines to fish. Vet. Res. 2014, 45, 21. [Google Scholar] [CrossRef]
- Roupie, V.; Romano, M.; Zhang, L.; Korf, H.; Lin, M.Y.; Franken, K.L.M.C. Immunogenicity of eight dormancy regulon-encoded proteins of Mycobacterium tuberculosis in DNA-vaccinated and tuberculosis-infected mice. Infect. Immun. 2007, 75, 941–949. [Google Scholar] [CrossRef]
- Bashir, N.; Kounsar, F.; Mukhopadhyay, S.; Hasnain, S.E. Mycobacterium tuberculosis conserved hypothetical protein rRv2626c modulates macrophage effector functions. Immunology 2010, 130, 34–45. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, M.; Chaudhry, A.; Sharma, S. Rv2626c and Rv2032 activate TH1 response and downregulate regulatory T cells in peripheral blood mononuclear cells of tuberculosis patients. Comp. Immunol. Microbiol. Infect. Dis. 2019, 62, 46–53. [Google Scholar] [CrossRef]
- Gjessing, M.C.; Falk, K.; Weli, S.C.; Koppang, E.O.; Kvellestad, A. A sequential study of incomplete Freund’s adjuvant-induced peritonitis in Atlantic cod. Fish Shellfish Immunol. 2012, 32, 141–150. [Google Scholar] [CrossRef]
- Veenstra, K.A.; Wang, T.; Alnabulsi, A.; Douglas, A.; Russell, K.S.; Tubbs, L.; Arous, J.B.; Secombes, C.J. Analysis of adipose tissue immune gene expression after vaccination of rainbow trout with adjuvanted bacterins reveals an association with side effects. Mol. Immunol. 2017, 88, 89–98. [Google Scholar] [CrossRef]
- Xu, W.; Jiao, C.; Bao, P.; Liu, Q.; Wang, P.; Zhang, R.; Liu, X.; Zhang, Y. Efficacy of MontanideTM ISA 763 A VG as aquatic adjuvant administrated with an inactivated Vibrio harveyi vaccine in turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 2019, 84, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jin, P.; Liu, Q.; Wang, Q.; Zhang, Y.; Liu, X. A CpG-riched plasmids as vaccine adjuvant reduce antigen dose of an inactivated Vibrio anguillarum vaccine in turbot (Scophthalamus maximus L.). Fish Shellfish Immunol. 2020, 98, 312–317. [Google Scholar] [CrossRef]
- Zou, J.; Carrington, A.; Collet, B.; Dijkstra, J.M.; Yoshiura, Y.; Bols, N.; Secombes, C. Identification and bioactivities of IFN-γ in rainbow trout Oncorhynchus mykiss: The first Th1-type cytokine characterized functionally in fish. J. Immunol. 2005, 175, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Tonheim, T.C.; Bøgwald, J.; Dalmo, R.A. What happens to the DNA vaccine in fish? A review of current knowledge. Fish Shellfish Immunol. 2008, 25, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Z.; Wang, W.; Hou, S.; Cai, J.; Xia, L.; Lu, Y. Development of DNA vaccines encoding ribosomal proteins (RplL and RpsA) against Nocardia seriolae infection in fish. Fish Shellfish Immunol. 2020, 96, 201–212. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, Y.H.; Liu, C.S.; Sun, L. Construction and comparative study of monovalent and multivalent DNA vaccines against Streptococcus iniae. Fish Shellfish Immunol. 2012, 33, 1303–1310. [Google Scholar] [CrossRef]
- Lee, L.Y.Y.; Izzard, L.; Hurt, A.C. A Review of DNA Vaccines Against Influenza. Front. Immunol. 2018, 9, 1568. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights to the function of lysozyme. PLoS Pathog. 2017, 21, e1006512. [Google Scholar] [CrossRef]
- Brott, A.S.; Clarke, A.J. Peptidoglycan O-Acetylation as a Virulence Factor: Its Effect on Lysozyme in the Innate Immune System. Antibiotics (Basel) 2019, 8, 94. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Tang, X.; Liu, F.; Sheng, X.; Xing, J.; Zhan, W. Recombinant NADP-dependent isocitrate dehydrogenase of Edwardsiella tarda induces both Th1 and Th2 type immune responses and evokes protective efficacy against edwardsiellosis. Vaccine 2018, 36, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Prados-Rosales, R.; Carreño, L.; Cheng, T.; Blanc, C.; Weinrick, B.; Malek, A.; Lowary, T.; Baena, A.; Joe, M.; Bai, Y.; et al. Enhanced control of Mycobacterium tuberculosis extrapulmonary dissermination in mice by an arabinomannan-protein conjugate vaccine. PLoS Pathog. 2017, 13, e1006250. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.E.; Burtnick, M.N.; Stokes, M.G.; Whelan, A.O.; Williamson, E.D.; Atkins, T.P.; Prior, J.L.; Brett, P.J. Burkholderia pseudomallei capsular polysaccharide conjugates provide protection against acute melioidosis. Infect. Immun. 2014, 82, 3206–3213. [Google Scholar] [CrossRef] [PubMed]
- Rawool, D.B.; Bitsaktsis, C.; Li, Y.; Gosselin, D.R.; Lin, Y.; Kurkure, N.V.; Metzger, D.W.; Gosselin, E.J. Utilization of Fc receptors as a mucosal vaccine strategy against an intracellular bacterium, Francisella tularensis. J. Immunol. 2008, 180, 5548–5557. [Google Scholar] [CrossRef]
- Singh, A.K.; Kingston, J.J.; Murali, H.S.; Batra, H.V. A recombinant bivalent fusion protein rVE confers active and passive protection against Yersinia enterocolitica infection in mice. Vaccine 2014, 32, 1233–1239. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef]
- Ismail, N.; Olano, J.P.; Feng, H.M.; Walker, D.H. Current status of immune mechanism of killing of intracellular microorganisms. FEMS Microbiol. Lett. 2002, 207, 111–120. [Google Scholar] [CrossRef]
- Yung, S.C.; Murphy, P.M. Antimicrobial chemokines. Front. Immunol. 2012, 3, 276. [Google Scholar] [CrossRef]
- Gomes, R.N.; Teixeira-Cunha, M.G.; Figueiredo, R.T.; Almeida, P.E.; Alves, S.C.; Bozza, P.T.; Bozza, F.A.; Bozza, M.T.; Zimmerman, G.A.; Castro-Faria-Neto, H.C. Bacterial clearance in septic mice is modulated by MCP-1/CCL2 and nitric oxide. Shock 2013, 39, 63–69. [Google Scholar] [CrossRef]
- Depaolo, R.W.; Lathan, R.; Rollins, B.J.; Karpus, W.J. The chemokine CCL2 is required for control of murine gastric Salmonella enterica infection. Infect. Immun. 2005, 73, 6514–6522. [Google Scholar] [CrossRef]
- Collin, M.; Linge, H.M.; Bjartell, A.; Giwercman, A.; Malm, J.; Egesten, A. Constitutive expression of the antibacterial CXC chemokine GCP-2/CXCL6 by epithelial cells of male reproductive tract. J. Reprod. Immunol. 2008, 79, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Valdivia-Silva, J.; Medina-Tayamo, J.; Gracia-Zepeda, E.A. Chemokine-derived peptides: Novel antimicrobial and antineoplastic agents. Int. J. Mol. Sci. 2015, 16, 12958–12985. [Google Scholar] [CrossRef] [PubMed]
- Söbirk, S.K.; Mörgelin, M.; Egesten, A.; Bates, P.; Shannon, O.; Collin, M. Human chemokines as antimicrobial peptides with direct parasiticidal effect on Leishmania mexicana in vitro. PLoS ONE 2013, 8, e58129. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, L.J.; Nie, C.Q.; Hansen, D.S. The role of chemokines in severe malaria: More than meets the eye. Parasitology 2014, 141, 602–613. [Google Scholar] [CrossRef]
- Domingo-Gonzalez, R.; Prince, O.; Cooper, A.; Khader, S.A. Cytokines and Chemokines in Mycobacterium tuberculosis Infection. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Zhou, R.P.; Wu, X.S.; Xie, Y.Y.; Dai, B.B.; Hu, W.; Ge, J.F.; Chen, F.H. Functions of interleukin-34 and its emerging association with rheumatoid arthritis. Immunology 2016, 149, 362–373. [Google Scholar] [CrossRef]
- Truong, A.D.; Hong, Y.; Lee, J.; Lee, K.; Kil, D.Y.; Lillehoj, H.S.; Hong, Y.H. Interleukin-34 Regulates Th1 and Th17 Cytokine Production by Activating Multiple Signaling Pathways through CSF-1R in Chicken Cell Lines. Int. J. Mol. Sci. 2018, 19, 1665. [Google Scholar] [CrossRef]
- Lin, X.; Luo, H.; Yan, X.; Song, Z.; Gao, X.; Xia, Y.; Zhang, L.; Yin, Y.; Cao, J. Interleukin-34 ameliorates survival and bacterial clearance in polymicrobial sepsis. Crit. Care Med. 2018, 46, e584–e590. [Google Scholar] [CrossRef]
- Mikalsen, A.B.; Torgersen, J.; Aleström, P.; Hellemann, A.L.; Koppang, E.O.; Rimstad, E. Protection of atlantic salmon Salmo salar against infectious pancreatic necrosis after DNA vaccination. Dis. Aquat. Organ. 2004, 60, 11–20. [Google Scholar] [CrossRef]
- Kanellos, T.; Sylvester, I.D.; D’Mello, F.; Howard, C.R.; Mackie, A.; Dixon, P.F.; Chang, K.C.; Ramstad, A.; Midtlyng, P.J.; Russel, P.H. DNA Vaccination Can Protect Cyprinus Carpio Against Spring Viraemia of Carp Virus. Vaccine 2006, 24, 4927–4933. [Google Scholar] [CrossRef]
- Caipang, C.M.; Takano, T.; Hirono, I.; Aoki, T. Genetic Vaccines Protect Red Seabream, Pagrus Major, Upon Challenge With Red Seabream Iridovirus (RSIV). Fish Shellfish Immunol. 2006, 21, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Pasnik, D.J.; Smith, S.A. Immune and histopathologic responses of DNA-vaccinated hybrid striped bass Morone Saxatilis X M. Chrysops after acute Mycobacterium marinum infection. Dis. Aquat. Organ. 2006, 73, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tan, W.; Wang, W.; Hou, S.; Chen, G.; Xia, L.; Lu, Y. Identification of common antigens of three pathogenic Nocardia species and development of DNA vaccine against fish nocardiosis. Fish Shellfish Immunol. 2019, 95, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Munang’andu, H.M.; Evensen, Ø. Correlates of protective immunity of fish vaccines. Fish Shellfish Immunol. 2019, 85, 132–140. [Google Scholar] [CrossRef]
- Cai, S.H.; Huang, Y.C.; Lu, Y.S.; Wu, Z.H.; Wang, B.; Tang, J.F.; Jian, J.C. Expression and immunogenicity analysis of accessory colonization factor A from Vibrio alginolyticus strain HY9901. Fish Shellfish Immunol. 2013, 43, 454–462. [Google Scholar] [CrossRef]
- Xing, J.; Xu, H.; Wang, Y.; Tang, X.; Sheng, X.; Zhan, W. Protective efficacy of six immunogenic recombinant proteins of Vibrio anguillarum and evaluation them as vaccine candidate for flounder (Paralichthys olivaceus). Microb. Pathog. 2017, 107, 155–163. [Google Scholar] [CrossRef]
- Xing, J.; Xu, H.; Wang, Y.; Tang, X.; Sheng, X.; Zhan, W. Identification of immunogenic proteins and evaluation of four recombinant proteins as potential vaccine antigens from Vibrio anguillarum in flounder (Paralichthys olivaceus). Vaccine 2017, 35, 3196–3203. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Primer Sequence (5′ → 3′) | Application | References |
|---|---|---|---|
| Hrp1-F | TTGCTAGCGCCACCATGGCCACGGCACG (NheI) | Plasmid construction, PCR, and RT-PCR | This study |
| Hrp1-R | GTAAGCTTCTAATGATGATGATGATGATGTGTCCAAGGCGCGCA (HindIII) | Plasmid construction | |
| Hrp1-R2 | CTATGTCCA AGGCGCGCAGACC | PCR and RT-PCR | |
| IL-34-F | CGAAGCTTGCCACCATGGTTCTACTGTGCA (HindIII) | Plasmid construction, PCR, and RT-PCR | |
| IL-34-R | GAATTCTCAATGATGATGATGATGATGGCTTTCGGGCTGTAA (EcoRI) | Plasmid construction | |
| IL-34-R2 | TCAGCTTTCGGGCTGTAAGTCTTTC | PCR and RT-PCR | |
| RPS40 | F: CAGAAATGGCACGATAAGCA | qRT-PCR | Martyniuk et al., 2016 |
| R: GACCTTTACGCCCAAATCC | |||
| β-actin | F: CCACCACAGCCGAGAGGGAA | qRT-PCR | Omkar et al, 2016 |
| R: TCATGGTGGATGGGGCCAGG | |||
| IL-1β | F: TTGCCATAGAGAGGTTTA | qRT-PCR | |
| R: ACACTATATGCTCTTCCA | |||
| IL-12p40 | F: TCTTCCATCCTTGTGGTCTTCC | qRT-PCR | |
| R: CAGTTCCAGGTCAAAGTGGTC | |||
| TNFα | F: CTAGTGAAGAACCAGATTGT | qRT-PCR | |
| R: AGGAGACTCTGAACGATG | |||
| IL-8 | F: GAGCCATTTTTCCTGGTGACT | qRT-PCR | |
| R: TCCTCATTGGTGCTGAAAGATC | |||
| NF-κB | F: AGGATGACTGAAGCTCCGTT | qRT-PCR | |
| R: GGACACGAGGAGGATCGGAGT | |||
| IFNγ | F: TGCAGGCTCTCAAACACATC | qRT-PCR | Hoang et al, 2020 |
| R: TGTTTTCGGTCAGTGTGCTC | |||
| MHCI-α | F: GTGGTTCAACGTCAACATCG | qRT-PCR | |
| R: ACCCAGACTTGTTCGGTGTC | |||
| MHCII-α | F: GAGGACCTTGCTGTCATTGG | qRT-PCR | |
| R: GCGTACCAAACCTCTTCACC | |||
| CD4-1 | F: GCTCCAGCGGGGAATAATTT | qRT-PCR | |
| R: GCCAGGCAAGCTCAAAGTTA | |||
| CD8-α | F: GGAAGGGGATCCTGTTGACA | qRT-PCR | |
| R: CCAGCACTCGAAACCAGATG | |||
| IgM | F: CTGGACCAGTCTCCCTCTGA | qRT-PCR | |
| R: CGAGGTACTGAGTGCTGCTG | |||
| STAT3 | F: CCACCCAAAGAACGTGAACT | qRT-PCR | |
| R: TCAATGGTCAGGCCTCTCTT | |||
| CCL20 | F: ACAACCACGGAAAACTGCCG | qRT-PCR | |
| R: TCCTCACCCACTCATCCTTC | |||
| STAT1 | F: TAAAACTCCGGTTCCTGGTG | qRT-PCR | This study |
| R: CCGTTTGACTCCTCCATGTT | |||
| IL-18 | F: TTGATGGCAAGAAGATGGTGG | qRT-PCR | |
| R: AAGCCTTGTGTGCAGTTTCCT | |||
| IL-6 | F: GGAACCCTGAACAGGTAACG | qRT-PCR | |
| R: TGTGCGGTCATCTTTCTGTGG | |||
| CCL2 | F: GCGAGTGGTCAGCTACATCA | qRT-PCR | |
| R: GATGAGCTCCTTCACCCAAG | |||
| CXCL9 | F: GGAAGATGTTTGTGTCCACAG | qRT-PCR | |
| R: GGCGTTTTGGGTAGACTGTG | |||
| CXCL10 | F: GAATCGGGACAGCAGTGTCT | qRT-PCR | |
| R: CAGTTGCTGGGTAGATCTGGA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, H.H.; Wang, P.-C.; Chen, S.-C. Interleukin 34 Serves as a Novel Molecular Adjuvant against Nocardia Seriolae Infection in Largemouth Bass (Micropterus Salmoides). Vaccines 2020, 8, 151. https://doi.org/10.3390/vaccines8020151
Hoang HH, Wang P-C, Chen S-C. Interleukin 34 Serves as a Novel Molecular Adjuvant against Nocardia Seriolae Infection in Largemouth Bass (Micropterus Salmoides). Vaccines. 2020; 8(2):151. https://doi.org/10.3390/vaccines8020151
Chicago/Turabian StyleHoang, Huy Hoa, Pei-Chi Wang, and Shih-Chu Chen. 2020. "Interleukin 34 Serves as a Novel Molecular Adjuvant against Nocardia Seriolae Infection in Largemouth Bass (Micropterus Salmoides)" Vaccines 8, no. 2: 151. https://doi.org/10.3390/vaccines8020151
APA StyleHoang, H. H., Wang, P.-C., & Chen, S.-C. (2020). Interleukin 34 Serves as a Novel Molecular Adjuvant against Nocardia Seriolae Infection in Largemouth Bass (Micropterus Salmoides). Vaccines, 8(2), 151. https://doi.org/10.3390/vaccines8020151





