Abstract
Background: Toxoplasma gondii (T. gondii) is a significant opportunistic zoonotic protozoan, presenting a substantial risk to human health and livestock. Consequently, the development of an effective vaccine against toxoplasmosis is imperative. This study focuses on the GRA12 protein as a target for developing a recombinant protein vaccine, with its efficacy evaluated through immunization trials in cats. Methods: We expressed recombinant GRA12 protein in E. coli and immunized cats with the purified antigen. The cats were categorized into four groups: G1 (PBS control), G2 (ISA 201 adjuvant alone), G3 (rGRA12 vaccine), and G4 (rGRA12 combined with ISA 201 adjuvant). All cats underwent subcutaneous immunizations on days 0, 14, and 28. Subsequently, serum levels of IgG (including IgG1 and IgG2a subclasses) and cytokines (IFN-γ, IL-2, TNF-α, IL-4, IL-10) were measured by enzyme-linked immunosorbent assay (ELISA). Two weeks after the third immunization (42 DPI), each cat was intraperitoneally infected with 1 × 106 T. gondii RH tachyzoites. Oocyst shedding, survival duration, and T. gondii burden were monitored to assess vaccine-induced immunity. Results: The results indicate that immunization with recombinant rGRA12 protein significantly elevated IgG, IgG1, and IgG2a antibody levels in cats. G4 displayed elevated IgG levels post-immunization compared to G1 and G2, with an IgG1/IgG2a ratio > 1, indicating a mixed Th1/Th2 immune response. G4 also showed significantly increased IFN-γ, IL-2, TNF-α, and IL-4 levels compared to G1 (p < 0.05), while IL-10 remained unchanged. After T. gondii infection, total oocyst counts were 4.61 × 106 (G1), 4.49 × 106 (G2), 3.58 × 106 (G3), and 2.59 × 106 (G4), with G3/G4 showing 20.1–27.9% reduction relative to G1 (p < 0.05). Survival analysis revealed that groups G3 and G4 exhibited significantly longer median survival times (38 and 60 days, respectively; G4 with no mortality) compared to G1 and G2 (19 and 26 days, respectively). Additionally, parasite burdens in the brain, heart, lungs, liver, and spleen were significantly reduced in G3/G4 compared to G1/G2 (p < 0.01). Conclusions: In summary, the recombinant GRA12 vaccine significantly enhanced host survival and reduced parasite burden, demonstrating its potential as an effective toxoplasmosis vaccine candidate. These findings provide valuable data for future toxoplasmosis vaccine development.
1. Introduction
T. gondii, an obligatory intracellular protozoan, exhibits a pronounced tropism for nucleated cells in virtually all endothermic vertebrate taxa, including human [1]. Humans and animals are commonly infected by eating undercooked meat containing tissue cysts or by consuming food or water contaminated with sporulated oocysts from feline feces [2]. While infections often remain asymptomatic in individuals with healthy immune systems, immunocompromised people are at much higher risk for serious conditions like encephalitis, myocarditis, and pneumonia [3,4]. If a pregnant woman acquires an acute infection, the parasite can cross the placenta and result in serious pregnancy outcomes, such as miscarriage, intrauterine fetal death, premature birth, or congenital defects [5]. In livestock, T. gondii also causes major reproductive losses, particularly abortions in economically significant species like swine and sheep [6,7].
Felids, particularly domestic cats, serve as the definitive hosts for T. gondii and are the only animals capable of shedding environmentally resistant oocysts. A single cat can release up to 55 million oocysts per day during the acute stage of infection [8,9]. Global economic growth and urbanization have contributed to rising domestic cat populations, amplifying zoonotic transmission risks. Meta-analysis of studies from 1967 to 2017 revealed a global T. gondii seroprevalence of 35% in domestic cats and 59% in wild felids [10]. In certain regions of China, such as Jilin and Shandong, pet owners had T. gondii infection rates of 18.10% and 18.04%, respectively, significantly higher than the general population’s rate of 7.88% [11,12]. Similarly, a serological study in western Thailand found that dairy cows exposed to pets were four times more likely to test positive for T. gondii than cows without such exposure [13,14]. These findings highlight the urgent need to develop safe and effective vaccines targeting feline hosts to help control the spread of toxoplasmosis.
T. gondii contains three specialized apical secretory organelles—micronemes, rhoptries, and dense granules—that play key roles during host cell invasion. As the parasite’s tachyzoites form invades, microneme proteins (MICs) are secreted from the apical end to facilitate recognition and attachment to the host cell membrane. Rhoptry proteins (ROPs) then act in coordination with MICs to assist in host cell entry and the formation of a protective parasitophorous vacuole (PV). Following this, dense granule proteins (GRAs) are secreted to modify the PV environment, allowing for nutrient acquisition that supports parasite replication [15].
Among the GRAs, GRA12 behaves similarly to GRA2 and GRA6 by being released into the PV shortly after invasion. It first localizes to the posterior invaginated pocket to assemble the nanotubular network, eventually spreading throughout the vacuolar space, where it is present in both soluble and membrane-bound forms [16]. GRA12 has 53 predicted post-translational modification sites, a transmembrane domain, and multiple predicted B-cell and T-cell epitopes. Immunological analyses suggest that GRA12 is strongly immunogenic and non-allergenic, making it a promising vaccine target [17]. Notably, deletion of the GRA12 gene severely reduces chronic-stage cyst development in vivo, although it does not affect parasite growth or cyst differentiation in vitro [18]. Overall, this study aims to demonstrates the immunogenicity and protective potential of the recombinant GRA12 (rGRA12) subunit vaccine, including its capacity to reduce T.gondii transmission in felids and mitigate host damage, providing a scientific basis for further Toxoplasma vaccine development.
2. Materials and Methods
2.1. Ethics Statement
The animal experiments were approved by the Laboratory Animal Ethics Committee of South China Agricultural University (Ethics Number: 2024F069), and the experimental animals were raised at the Laboratory Animal Center of South China Agricultural University.
2.2. Cats
To maximize animal welfare while maintaining scientific validity, we implemented the 3Rs principle (Replacement, Reduction, and Refinement) throughout our experimental design. This included using the minimum number of animals required for statistically significant results, optimizing protocols to minimize discomfort. All the care procedures were approved by the Animal Ethics Committee of South China Agricultural University (no.SCAU2022f208). Twelve 4-month-old British Shorthair cats (2–3 kg) were enrolled in this study. The animals were previously monitored for 2 months prior to the beginning of the experiment. The cats were randomly allocated to individual cages and provided ad libitum access to commercially available cat food and fresh drinking water. Serum was collected from all cats one week pre-experiment to verify naive status. Modified agglutination testing (MAT) and sucrose flotation techniques were employed to rule out prior T. gondii infection.
2.3. Parasite Propagation and Harvest
Tachyzoites of the T. gondii RH strain were obtained from the Laboratory of Parasitology at South China Agricultural University. These tachyzoites were used to infect human foreskin fibroblast (HFF) cells maintained in medium supplemented with 2% serum. Once 80% of cells exhibited cytopathic effects, they were detached using a cell scraper. The resulting suspension was homogenized by passing it 20–25 times through a 27-gauge needle. The homogenate was then subjected to two centrifugation steps: first at 1500× g for 5 min at 4 °C to remove cellular debris, and then at 3000× g for 7 min at 4 °C to collect the tachyzoites. The final pellet was resuspended in sterile phosphate-buffered saline (PBS), and concentration of tachyzoites was quantified using hemocytometry [19].
2.4. Construction of Plasmids, and Expression and Purification of rGRA12
The protocol for obtaining rGRA12 was conducted following the method described by Wang [20]. The DNA sequence of the T. gondii GRA12 gene was obtained from the GenBank database (accession number: FJ011096.1). Genomic DNA was isolated from T. gondii RH strain tachyzoites and used as a template to amplify the GRA12 gene via standard PCR. Based on its sequence, the predicted molecular weight of GRA12 antigen was 47.8 kDa. The 1311 bp GRA12 fragment was amplified using primers GRA12F (5′-TGAGCTCATCATGAGGGCGATCGTGGCATCGACG-3′, SacI site underlined) and GRA12R (5′-CAAGCTTGTTGTGTTTGCTGCCTGCAGAGCCGCG-3′, HindIII site underlined), and then cloned into the pET-28a vector via SacI/HindIII restriction sites. The recombinant plasmids were verified by SacI/HindIII double digestion and sequencing of the gel-purified fragments. Sequence-confirmed positive clones were subsequently transformed into E. coli BL21 for protein expression. E. coli BL21(DE3) harboring pET-28a-GRA12 was inoculated from a 5 mL starter culture into 500 mL of kanamycin-supplemented LB medium. The culture was grown at 37 °C with 170 rpm shaking to OD600 0.4–0.5, at which point protein expression was induced with 0.1% IPTG. Following 4 h of continued shaking under identical conditions, the bacterial cells were harvested for subsequent protein purification. The purified recombinant GRA12 protein (47.8 kDa) was subsequently used for feline immunization.
2.5. Grouping, Immunization, and Challenge Infection of Cats
The GRA12 protein was heterologously expressed and purified in our laboratory. A total of twelve 6-month-old female domestic cats with blue coats were randomly divided into four groups (three cats per group): G1 (PBS control), G2 (ISA 201 adjuvant alone), G3 (rGRA12 vaccine), and G4 (rGRA12 combined with ISA 201 adjuvant). Subcutaneous immunizations were administered three times at two-week intervals. Table 1 and Figure 1 provides a clear overview of the immunization timeline. Blood samples collected on days 0, 14, 28, and 42, and serum was isolated for the evaluation of antibody titers and cytokine concentrations.

Table 1.
Immunization and challenge of cats.
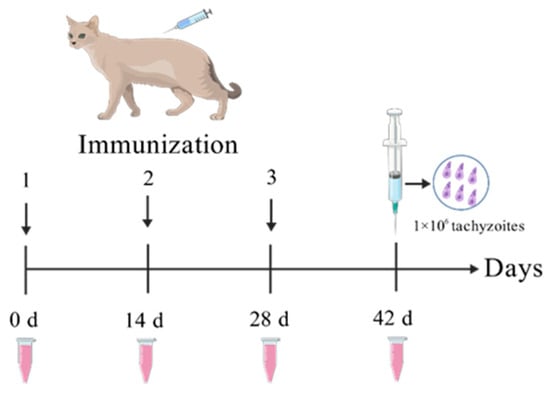
Figure 1.
rGRA12 immunization procedure diagram.
All cats were challenged on day 42 with 1 × 106 tachyzoites of the T. gondii RH strain. Survival was monitored daily for 60 days, with group-specific survival times recorded.
2.6. Enzyme-Linked Immunosorbent Assays for IgG, IgG1, and IgG2a
Serum samples were collected from all groups on days 0, 14, 28, and 42 following immunization. The rGRA12 antigen was prepared at a concentration of 10 μg/mL in a carbonate–bicarbonate coating buffer (50 mM, pH 9.6). A 96-well plate was coated with 100 μL of this antigen solution and incubated overnight at 4 °C. The plate was then washed three times with PBST (PBS containing 0.05% Tween-20), and nonspecific sites were blocked using 100 μL of 1% BSA per well at 37 °C for 1 h. Serum samples were then diluted 1:400 in PBS, and 100 μL of each diluted sample was added to the wells. After incubation at 37 °C for 1 h and another three washes with PBST, 100 μL of horseradish peroxidase (HRP)-conjugated secondary antibody (diluted 1:2000 in PBS) was added and incubated for an additional hour at 37 °C. The plate was then washed five times with PBST, and 100 μL of TMB substrate was added to initiate color development. After a 10 min incubation in the dark, the reaction was stopped by adding 50 μL of 2 M sulfuric acid. Absorbance at 450 nm was measured within 15 min using a microplate reader. All tests were performed in triplicate. Total IgG levels were measured on days 0, 14, 28, and 42, while IgG1 and IgG2a subclass levels were specifically assessed on day 42.
2.7. Cytokine Assays
To detect cytokines on day 42 post-immunization, blood was collected from vein. The levels of IL-2, IL-4, IL-10, IFN-γ, and TNF-α were examined using commercial ELISA kits, according to the manufacturer’s instructions (Shanghai Meilian Biotechnology Co., Ltd., Shanghai, China). Data from three independent experiments were analyzed, including three replicates per serum. All procedures were performed according to the manufacturer’s detailed protocols.
2.8. Quantitative Detection Process of Cat Oocysts
Fresh fecal samples were homogenized in distilled water (1:10) within 24 h of collection. One gram of the homogenized material was mixed with 10 mL of saturated sucrose solution, vortexed for 30 s, and filtered through a 100 μm mesh. The filtrate was centrifuged at 1200× g for 10 min at 4 °C. The upper layer of the sucrose solution was gently aspirated using a Pasteur pipette. For samples that tested positive for oocysts under microscopy, a secondary purification step was conducted: 9 mL of the supernatant was diluted with 40 mL of deionized water and centrifuged again under the same conditions. The supernatant was discarded, and the pellet was resuspended in 1 mL of distilled water. The final suspension was loaded onto a modified McMaster slide, allowed to settle for 3 min, and examined under a light microscope to count the oocysts. Oocyst counts were used to calculate the number of oocysts per gram of feces, which served as an estimate of total oocyst excretion. Group-wise daily average oocyst shedding was statistically analyzed, and kinetic shedding curves were constructed. Protective Fraction (PF) was calculated using the following formula: PF = (P2 − P1)/P2, where P2 was the mean count in the control group and P1 was the mean count in the immunized group.
2.9. Quantitative Analysis of Parasitic Burden in Tissues
Experimental procedures were performed as follows: Primers targeted the T. gondii B1 gene (F: 5′-TCCTTCGTCCGTCGTAAT-3′; R: 5′-TTCTTCAGCCGTCTTGTG-3) [21]. Genomic DNA extracted from harvested T. gondii tachyzoites was used as a template to amplify the B1 gene by PCR. The purified target DNA fragment was ligated into the pMD18-T vector to construct the recombinant plasmid, which was then transformed into DH5α competent cells. Following sequence verification, positive clones were selected for overnight culture in LB medium supplemented with 100 μg/mL ampicillin (37 °C, 220 rpm). DNA concentration and purity were determined by UV spectrophotometry, with A260/A280 ratios between 1.8 and 2.0 considered acceptable. The plasmid was serially diluted to generate RT-qPCR standards (109 to 104 copies/μL). For RT-qPCR, 20 μL reactions comprised 10 μL of 2× SYBR Green qPCR Mix, 0.5 μL of each primer (10 μM), 1.0 μL of template DNA, and 8 μL of RNase free water. Thermal cycling conditions were as follows: initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 31 s, and 72 °C for 30 s. To quantify tissue parasite burden, brain, heart, lung, liver, and spleen tissues were collected immediately after euthanasia for pathological and molecular analyses.
2.10. Statistical Analysis
Statistical analyses were conducted using IBM SPSS Statistics 26.0 with a hierarchical approach (International Business Machines Corporation, New York City, NY, USA): one-way ANOVA followed by Tukey’s post hoc test was applied for multi-group comparisons, while unpaired Student’s t-tests evaluated differences between two groups. Statistical significance was defined as * p < 0.05, ** p < 0.01, and *** p < 0.001.
3. Results
3.1. IgG Antibody Detection
Serum IgG concentrations in four feline cohorts (G1–G4) were quantified by ELISA pre- and post-immunization (Figure 2). Total IgG titers increased progressively after multiple vaccinations, reaching peak concentrations at day 42. G3 and G4 exhibited significantly higher IgG levels than G1 (p < 0.01), beginning on day 14 post-immunization, with G4 maintaining significantly higher levels than G3 at days 28 and 42 (p < 0.01). However, no statistically significant difference was observed between the G1 and G2 groups (p > 0.05).
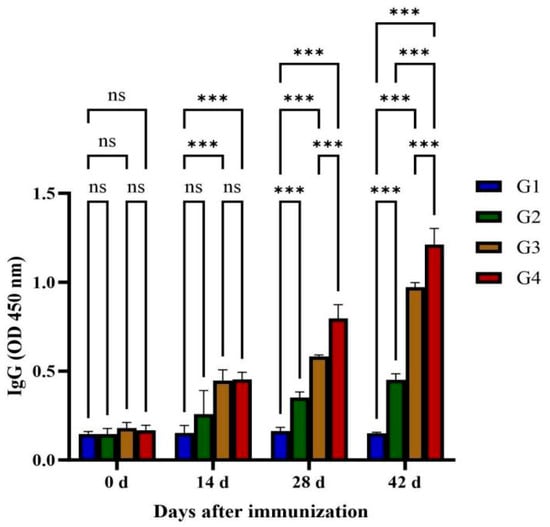
Figure 2.
Quantitative detections of total specific anti-GRA12 IgG antibodies in cat sera. Groups were defined as follows: G1: PBS group, G2: ISA 201 group, G3: rGRA12 group, and G4: rGRA12 + ISA 201 group. ns p > 0.05; *** p < 0.001.
3.2. IgG Antibody Isotype Determination
Serum IgG isotype profiles (IgG1/IgG2a) were characterized to explore potential Th1/Th2 immune polarization. As shown in Figure 3, G1 and G2 showed no statistically significant difference in IgG1 and IgG2a levels at 42 days post-immunization (p > 0.05). Vaccination with rGRA12 protein (G3/G4) demonstrated concurrent upregulation of both IgG subclasses (p < 0.05), displaying IgG2a predominance over IgG1 (ratio > 1). This isotypic polarization suggests coordinated Th1/Th2 immune activation following antigenic challenge.
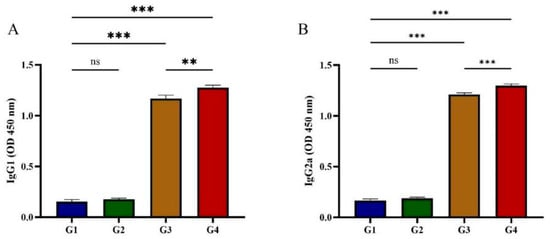
Figure 3.
Determination of IgG isotypes in post-immunization cat serum. (A) Post-immunization IgG1 changes in feline serum. (B) Post-immunization IgG2a changes in feline serum. Groups were defined as follows: G1: PBS group, G2: ISA 201 group, G3: rGRA12 group, and G4: rGRA12 + ISA 201 group. ns p > 0.05; ** p < 0.01; *** p < 0.001.
3.3. Cytokine Production Assay
Serum concentrations of Th1-type cytokines (IFN-γ, IL-2, TNF-α) and Th2-type cytokines (IL-4, IL-10) were quantified following rGRA12 protein administration (Figure 4). By day 42 post-immunization, both G3 and G4 demonstrated significantly elevated IFN-γ, IL-2, and TNF-α levels compared to G1 and G2 (p < 0.01) (Figure 4A–C). After immunization with rGRA12 protein, IL-4 levels in G3 remained stable (p > 0.05), whereas G4 exhibited a pronounced increase (p < 0.01) (Figure 4D). No significant differences in IL-10 concentrations were observed in either G3 or G4 (p > 0.05) (Figure 4E). Compared to G3, G4 displayed statistically significant upregulation of IFN-γ, IL-2, TNF-α, and IL-4, while IL-10 levels remained comparable between the two groups (p > 0.05).
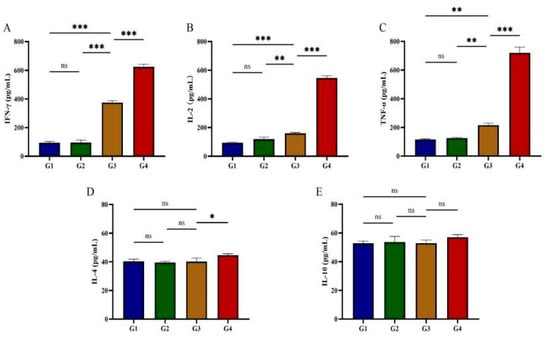
Figure 4.
Cytokine production in post-immunization cat serum. (A) Post-immunization IFN-γ changes in feline serum. (B) Post-immunization IL-2 changes in feline serum. (C) Post-immunization TNF-α changes in feline serum. (D) Post-immunization IL-4 changes in feline serum. (E) Post-immunization IL-10 changes in feline serum. Groups were defined as follows: G1: PBS group, G2: ISA 201 group, G3: rGRA12 group, and G4: rGRA12 + ISA 201 group. ns p > 0.05; * p < 0.05; ** p < 0.01; *** p < 0.001.
3.4. Feline Oocyst Volume Statistics
On day 42 post-vaccination, cats were intraperitoneally challenged with 1 × 106 RH T. gondii tachyzoites. Fecal samples were collected daily from day 1 to day 20 post-infection for oocyst shedding quantification. All infected cats excreted T. gondii oocysts; however, total oocyst shedding in G3 and G4 was significantly reduced compared to G1 and G2 (Figure 5B). Shedding dynamic analysis showed distinct patterns: G1 initiated oocyst shedding on day 3 post-infection, persisting for 12 days with a total output of 4.61 × 106 oocysts; G2 commenced shedding on day 3, producing 4.49 × 106 oocysts over 12 days; G3 delayed shedding onset to day 4, with 3.58 × 106 oocysts shed during a 10-day period; G4 exhibited the most delayed onset (day 4) and shortest shedding duration (9 days), yielding 2.59 × 106 oocysts (Figure 5A). Compared to G1, total oocyst shedding in G2, G3, and G4 decreased by 2.62%, 20.10%, and 27.88%, respectively. rGRA12 vaccination significantly suppressed oocyst excretion (p < 0.05) and reduced potential environmental transmission risks. Detailed statistical summaries of oocyst shedding parameters are provided in Table 2.
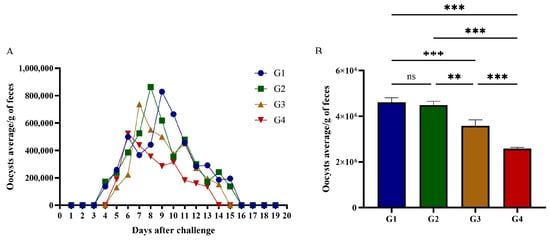
Figure 5.
Oocyst shedding in cats challenged with tachyzoites of T. gondii. (A) Excretion of T. gondii oocysts in cats immunized with rGRA12 protein. (B) The total number of T. gondii oocysts excreted by each group of cats. Groups were defined as follows: G1: PBS group, G2: ISA 201 group, G3: rGRA12 group, and G4: rGRA12 + ISA 201 group. ns p > 0.05; ** p< 0.01; *** p < 0.001.

Table 2.
Assessment of oocyst shedding in post-immunization cats.
3.5. Protective Efficacy of Recombinant Protein Vaccination in Cats
Immunization efficacy was evaluated by survival analysis in T. gondii-infected cats. At 42 days post-immunization, all groups were challenged with RH. As shown in Figure 6, the vaccinated groups (G3 and G4) demonstrated significantly higher survival rates compared to both G1 and G2 groups. Importantly, within the vaccinated groups, G4 exhibited superior survival compared to G3. Median survival times were 20 days for G1, 30 days for G2, and 38 days for G3, while G4 achieved 100% survival, with no mortality observed. rGRA12 vaccination, when formulated with ISA 201 adjuvant, enhanced protection against T. gondii infection.
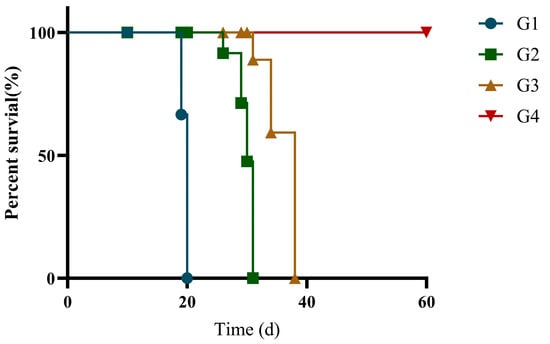
Figure 6.
Survival rate of the immunized cats. Groups were defined as follows: G1: PBS group, G2: ISA 201 group, G3: rGRA12 group, and G4: rGRA12 + ISA 201 group. Log-rank test demonstrated an overall significant difference in survival rate between all groups of mice (χ2 = 68.21, df = 3, p < 0.0001).
3.6. Quantification of Parasite Burden in Various Organs of Cats
Absolute RT-qPCR was used to quantify parasite loads in multiple organs (brain, heart, lung, liver, spleen) across all groups. Compared to G1 and G2, cats immunized with rGRA12 alone (G3) displayed significantly reduced T. gondii loads across all tested organs (p < 0.01) (Figure 7). Notably, G4 demonstrated enhanced protection compared to G3, with statistically lower parasite burdens in every organ analyzed (p < 0.05).
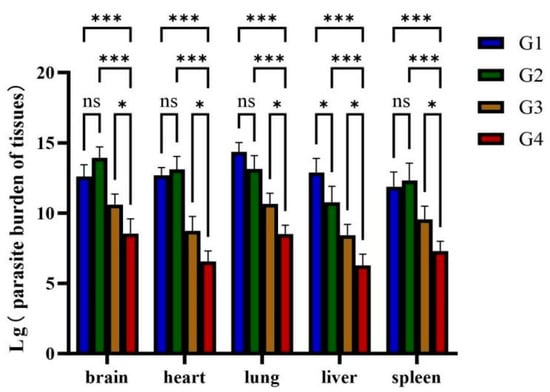
Figure 7.
Parasite burden in various organs of cats. Groups were defined as follows: G1: PBS group, G2: ISA 201 group, G3: rGRA12 group, and G4: rGRA12 + ISA 201 group. ns p > 0.05; * p < 0.05; *** p < 0.001.
4. Discussion
In China, the reported 15.3% seroprevalence of T. gondii in edible livestock, with regional and temporal variations, highlights the widespread environmental contamination by feline-derived oocysts and its potential impact on food safety [22]. Given the significant health threats posed by T. gondii to immunocompromised populations and its considerable economic impact on livestock industries, there is an urgent demand for effective vaccines against toxoplasmosis [23]. Among the existing vaccine strategies, recombinant protein-based vaccines have emerged as safe and potent alternatives to traditional vaccine approaches. These subunit vaccines serve as leading candidates for preventing both acute and chronic T. gondii infections, and show great promise for protective use [24].
Research shows that anti-T. gondii IgG plays a vital role in preventing parasite adhesion to host cells and works alongside macrophages to clear the parasite [25]. In this study, cats immunized with rGRA12 exhibited a gradual increase in T. gondii-specific IgG levels following each immunization, reaching their highest point following the third dose. The vaccinated groups (G3 and G4) had significantly greater IgG titers than the control groups (G1 and G2) (p < 0.05). Among the vaccinated groups, G4 demonstrated significantly higher anti-T. gondii IgG levels than G3, indicating that the ISA 201 adjuvant enhanced the immunogenicity of rGRA12. Subclass analysis revealed significant increases in both IgG1 and IgG2a in G4, with IgG2a levels surpassing those of IgG1. This pattern suggests that rGRA12 immunization induced a mixed Th1/Th2 response in cats, with a Th1-predominant bias. Previous studies have shown that IgG2a supports Th1-driven adaptive immunity by activating T helper cell responses, whereas IgG1 promotes the differentiation of naive Th cells toward a Th2 phenotype [26].
After rGRA12 immunization, cats exhibited elevated serum concentrations of the pro-inflammatory cytokines IFN-γ and IL-2. IFN-γ, primarily produced by NK cells, CD4+ T cells, and CD8+ T cells, plays a central role in defending against T. gondii by inducing antimicrobial mechanisms such as nitric oxide (NO) and reactive oxygen species (ROS), as well as altering host cell metabolism to inhibit parasite replication [27,28,29,30]. The dynamic equilibrium between Th1 and Th2 responses plays a pivotal role in modulating macrophage effector functions during T. gondii infection. Th1 cells activate macrophages through two principal mechanisms: (1) secretion of pro-inflammatory cytokines (IFN-γ, TNF-α, IL-2) and (2) promotion of IgG2a antibody production. Conversely, Th2 cells stimulate anti-inflammatory cytokine secretion (IL-4, IL-5, IL-6, IL-10) and IgG1 production, resulting in the suppression of macrophage functions. Notably, low levels of IL-4 and IL-10 were detected in this study, suggesting partial Th2 activation. IL-4 antagonizes IFN-γ activity in murine models, enhancing IgG1 synthesis and FcR2 expression on macrophages while suppressing IgG2a [31,32,33,34]. Additionally, IL-4 subverts host defense against T. gondii by inhibiting Th1 immunity—an immunosuppressive effect that may predispose one to pregnancy complications [35].
In this study, subcutaneous administration with rGRA12 resulted in oocyst shedding reductions of 20.10% in Group G3 and 27.88% in Group G4. In contrast, Garcia reported a 90.8% reduction in oocyst shedding in cats immunized intranasally with crude T. gondii antigens, while Zulpo observed an 86.7% reduction following intranasal administration of recombinant ROP2 (rROP2) protein. These comparisons highlight the significant impact that the route of immunization can have on the effectiveness of oocyst suppression [36,37].
As T. gondii oocyst formation primarily occurs in the intestinal epithelial cells of felines [38], intranasal vaccination may offer distinct advantages due to the favorable mucosal environment—featuring neutral pH, minimal enzymatic activity, and a large surface area—which helps preserve antigen stability and reduce required dosages. Additionally, intranasal immunization stimulates the common mucosal immune system, inducing dual mucosal and systemic immune responses that enhance intestinal secretory IgA (sIgA) production [39,40]. While sIgA is well-studied in humans, experimental findings also demonstrate its ability to inhibit T. gondii invasion of intestinal cells and limit parasite replication both in vitro and in vivo [41,42], indicating conserved protective functions across mammalian species.
The addition of adjuvants represents a critical strategy for enhancing vaccine immunogenicity while maintaining safety. ISA 201, an oil-based adjuvant from the Montanide™ ISA series, offers ready-to-use convenience, low viscosity, and excellent injectability, while demonstrating an absence of pyrogenic reactions, granuloma formation, or cyst induction [43]. Zulpo immunized cats with crude T. gondii rhoptry proteins adjuvanted with Quil-A and observed a reduction in oocyst shedding rates from 98.4% to 53.0%. Furthermore, immunization with the recombinant ROP2 protein plus Quil-A adjuvant resulted in an 86.7% decrease in oocyst shedding [37,44]. These findings suggest that, beyond antigen optimization, the strategic selection of adjuvants is critical for enhancing vaccine immunogenicity and promoting the production of immunologically active molecules, ultimately eliciting more robust and durable protective immunity.
The delayed mortality observed in cats vaccinated with rGRA12 may indicate the establishment of a regulated Th1/Th2 immune response. While Th1-related cytokines such as IFN-γ are essential for parasite control, excessive Th1 activity has been associated with early death during toxoplasmosis. In contrast, Th2 cytokines like IL-4 and IL-10 help moderate this response, reducing the risk of acute-phase mortality by dampening excessive inflammation [45,46]. This immunological balance likely facilitates effective parasite elimination while minimizing tissue damage. Consistent with this interpretation, analysis of tissue samples showed significantly lower T. gondii burdens in the vaccinated groups (G3 and G4) compared to controls (G1) (p < 0.05), confirming the systemic protective effect of rGRA12 immunization.
Studies have demonstrated that the peak period of oocyst shedding in cats infected with T. gondii occurs between 5 and 8 days post-infection, with oocyst shedding spanning 7 to 20 days [44,47]. Therefore, in this study, fecal oocyst shedding was monitored in all experimental cat groups for 20 days following infection with RH strain tachyzoites. After immunization with rGRA12 protein, G4 stopped shedding oocysts by day 16, showing an earlier cessation and a shorter shedding duration compared to other groups. However, as oocyst shedding persisted in all groups, potential public health risks remain. We did not examine possible differences in oocyst infectivity between treatment groups.
While the observed differences in oocyst shedding counts between experimental groups were close on a logarithmic scale, we rigorously verified our quantification through repeated measurements. After systematically excluding potential technical artifacts, we hypothesize that although the reduction in oocyst shedding post-immunization is not statistically significant on a logarithmic scale, immunization may still exert subtle effects by potentially damaging oocyst structure, attenuating infectivity, impairing sporulation efficiency, or compromising sporozoite viability.
This study demonstrated that rGRA12 protein reduced parasite burden in cats; however, it did not assess histopathological changes in feline tissues to evaluate potential mitigation of T. gondii-induced organ damage. The current study employed a relatively small sample size (n = 3), which may compromise statistical power (reducing the ability to detect true effects, resulting in unstable findings) and generalizability (limiting the coverage of diverse population characteristics and variations). Furthermore, although T. gondii oocyst development primarily occurs in the feline small intestine, this investigation measured only systemic IgG and its subclasses, without examining mucosal IgA responses. Moreover, the immunization strategy employed in this study was relatively restricted. In contrast to subcutaneous administration, intranasal vaccination has been shown to elicit both mucosal and systemic immune responses, significantly enhancing intestinal secretory IgA (sIgA) production, which may confer superior efficacy in reducing oocyst shedding. This study utilized only a single T. gondii strain genotype for immune protection analysis. Whether the rGRA12 protein can demonstrate comparable protective effects against T. gondii strains of different genotypes remains to be determined.
5. Conclusions
Subcutaneous immunization with the rGRA12 protein formulated with ISA 201 adjuvant elicited a mixed immune response skewed toward Th1 in cats, prolonging survival and decreasing T. gondii loads in critical organs such as the liver, lungs, and brain. These results demonstrate the vaccine candidate’s dual protective capacity: it can effectively limit parasite replication while enhancing host immune defense. Future investigations should explore combinatorial approaches incorporating multiple T. gondii antigens with highly immunogenic epitopes, while also evaluating diverse adjuvant formulations and alternative immunization routes to optimize protective immunity. Additionally, future research should specifically examine morphological and infectivity changes in oocysts post-immunization, as well as investigate whether vaccination can suppress secondary oocyst shedding in cats upon re-infection. This approach may also be adapted for kittens, thereby reducing public health risks.
Author Contributions
Conceptualization, H.Y. and Y.Z.; Methodology, X.-X.Z., Z.Y. (Ziguo Yuan), H.Y. and Y.Z.; Software, J.Y., L.N., Y.S., Z.Y. (Zipeng Yang), L.Y., H.R., W.L. and Y.M.; Validation, J.Y., L.N. and Y.S.; Formal analysis, J.Y., L.N., Y.S., Z.Y. (Zipeng Yang), L.Y., H.R., W.L. and Y.M.; Investigation, J.Y., L.N. and Y.S.; Writing—original draft, J.Y.; Writing—review and editing, Z.Y. (Ziguo Yuan) and H.Y.; Visualization, X.-X.Z., Z.Y. (Ziguo Yuan) and H.Y.; Supervision, H.Y. and Y.Z.; Project administration, Z.Y. (Ziguo Yuan) and Y.Z.; Funding acquisition, H.Y. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Natural Science Foundation of Guangdong Province (2023A1515011795, 2025A1515012622) and the National Natural Science Foundation of China (31972707).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of South China Agricultural University under Contract no. SCAU2022f208 (22 August 2022) and all animal experiments were performed in strict accordance with the Animal Ethics Procedures and Guidelines of the People’s Republic of China.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dubey, J.P. The history of Toxoplasma gondii—The first 100 years. J. Eukaryot. Microbiol. 2008, 55, 467–475. [Google Scholar] [CrossRef]
- Dunay, I.R.; Gajurel, K.; Dhakal, R.; Liesenfeld, O.; Montoya, J.G. Treatment of Toxoplasmosis: Historical Perspective, Animal Models, and Current Clinical Practice. Clin. Microbiol. Rev. 2018, 31, e00057-17. [Google Scholar] [CrossRef]
- Hunter, C.A.; Remington, J.S. Immunopathogenesis of toxoplasmic encephalitis. J. Infect. Dis. 1994, 170, 1057–1067. [Google Scholar] [CrossRef]
- Eza, D.E.; Lucas, S.B. Fulminant toxoplasmosis causing fatal pneumonitis and myocarditis. HIV Med. 2006, 7, 415–420. [Google Scholar] [CrossRef]
- Jones, J.L.; Lopez, A.; Wilson, M.; Schulkin, J.; Gibbs, R. Congenital toxoplasmosis: A review. Obstet. Gynecol. Surv. 2001, 56, 296–305. [Google Scholar] [CrossRef]
- Pan, M.; Lyu, C.; Zhao, J.; Shen, B. Sixty Years (1957–2017) of Research on Toxoplasmosis in China-An Overview. Front. Microbiol. 2017, 8, 1825. [Google Scholar] [CrossRef]
- Ortega-Mora, L.M.; Gottstein, B.; Conraths, F.J.; Buxton, D. Protozoal Abortion in Farm Ruminants: Guidelines for Diagnosis and Control; CABI: Wallingford, UK, 2007. [Google Scholar]
- Torrey, E.F.; Yolken, R.H. Toxoplasma oocysts as a public health problem. Trends Parasitol. 2013, 29, 380–384. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Montazeri, M.; Mikaeili Galeh, T.; Moosazadeh, M.; Sarvi, S.; Dodangeh, S.; Javidnia, J.; Sharif, M.; Daryani, A. The global serological prevalence of Toxoplasma gondii in felids during the last five decades (1967–2017): A systematic review and meta-analysis. Parasit. Vectors 2020, 13, 82. [Google Scholar] [CrossRef]
- Li, X.T.; Wang, L.; Ding, Y.; Sun, W.W. Toxoplasma gondii infection in pet cats and their owners in northeastern China: An important public health concern. BMC Vet. Res. 2022, 18, 9. [Google Scholar] [CrossRef]
- Coordinating Office of the National Survey on the Important Human Parasitic Diseases. A national survey on current status of the important parasitic diseases in human population. Chin. J. Parasitol. Parasit. Dis. 2005, 23, 332–340. [Google Scholar]
- Jung, B.K.; Song, H.; Lee, S.E.; Kim, M.J.; Cho, J.; Shin, E.H.; Chai, J.-Y. Seroprevalence and Risk Factors of Toxoplasma gondii Infection among Cat Sitters in Korea. Korean J. Parasitol. 2017, 55, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Arunvipas, P.; Jittapalapong, S.; Inpankaew, T.; Pinyopanuwat, N.; Chimnoi, W.; Maruyama, S. Seroprevalence and risk factors influenced transmission of Toxoplasma gondii in dogs and cats in dairy farms in Western Thailand. Afr. J. Agric. Res. 2013, 8, 591–595. [Google Scholar]
- Nam, H.W. GRA proteins of Toxoplasma gondii: Maintenance of host-parasite interactions across the parasitophorous vacuolar membrane. Korean J. Parasitol. 2009, 47, S29–S37. [Google Scholar] [CrossRef]
- Michelin, A.; Bittame, A.; Bordat, Y.; Travier, L.; Mercier, C.; Dubremetz, J.F.; Lebrun, M. GRA12, a Toxoplasma dense granule protein associated with the intravacuolar membranous nanotubular network. Int. J. Parasitol. 2009, 39, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, A.D.; Dalimi, A.; Ghaffarifar, F.; Pirestani, M. Antigenic properties of dense granule antigen 12 protein using bioinformatics tools in order to improve vaccine design against Toxoplasma gondii. Clin. Exp. Vaccine Res. 2020, 9, 81–96. [Google Scholar] [CrossRef]
- Fox, B.A.; Guevara, R.B.; Rommereim, L.M.; Falla, A.; Bellini, V.; Pètre, G.; Rak, C.; Cantillana, V.; Dubremetz, J.-F.; Cesbron-Delauw, M.-F.; et al. Toxoplasma gondii Parasitophorous Vacuole Membrane-Associated Dense Granule Proteins Orchestrate Chronic Infection and GRA12 Underpins Resistance to Host Gamma Interferon. mBio 2019, 10, e00589-19. [Google Scholar] [CrossRef]
- Liang, X.H. Biological Roles of De Novo Synthesis of Saturated Fatty Acids and Phosphatidylcholine in T. gondii. Ph.D. Thesis, Huazhong Agricultural University, Hangzhou, China, 2022. [Google Scholar]
- Wang, Y.H. Prokaryotic Expression and Immunogenicity Study of Toxoplasma Gondii Dense Granule Protein GRA12. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2017. [Google Scholar]
- Zhu, X.; Yang, T.; Yang, G.; Zhao, Y.; Yao, F. Establishment and application of SYBR Green I fluorescent quantitative PCR for detection of Toxoplasma gondii. J. Pathog. Biol. 2007, 428–432. [Google Scholar]
- Yang, Z.; Yuan, H.; Nie, L.; Wen, Q.; Li, H.; Yang, L.; Song, Y.; Luo, X.; Zhang, X.; Yuan, Z. Deciphering the epidemiological dynamics: Toxoplasma gondii seroprevalence in mainland China’s food animals, 2010–2023. Front. Cell Infect. Microbiol. 2024, 14, 1381537. [Google Scholar] [CrossRef]
- Pappas, G.; Roussos, N.; Falagas, M.E. Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int. J. Parasitol. 2009, 39, 1385–1394. [Google Scholar] [CrossRef]
- Şahar, E.A.; Can, H.; İz, S.G.; Döşkaya, A.D.; Kalantari-Dehaghi, M.; Deveci, R.; Gürüz, A.Y.; Döşkaya, M. Development of a hexavalent recombinant protein vaccine adjuvanted with Montanide ISA 50 V and determination of its protective efficacy against acute toxoplasmosis. BMC Infect. Dis. 2020, 20, 493. [Google Scholar] [CrossRef]
- Dupont, C.D.; Christian, D.A.; Hunter, C.A. Immune response and immunopathology during toxoplasmosis. Semin. Immunopathol. 2012, 34, 793–813. [Google Scholar] [CrossRef]
- Finkelman, F.D.; Holmes, J.; Katona, I.M.; Urban, J.F., Jr.; Beckmann, M.P.; Park, L.S.; Schooley, K.A.; Coffman, R.L.; Mosmann, T.R.; Paul, W.E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 1990, 8, 303–333. [Google Scholar] [CrossRef]
- Mahmoudzadeh, S.; Nozad Charoudeh, H.; Marques, C.S.; Bahadory, S.; Ahmadpour, E. The role of IL-12 in stimulating NK cells against Toxoplasma gondii infection: A mini-review. Parasitol. Res. 2021, 120, 2303–2309. [Google Scholar] [CrossRef]
- Saraav, I.; Cervantes-Barragan, L.; Olias, P.; Fu, Y.; Wang, Q.; Wang, L.; Wang, Y.; Mack, M.; Baldridge, M.T.; Stappenbeck, T.; et al. Chronic Toxoplasma gondii infection enhances susceptibility to colitis. Proc. Natl. Acad. Sci. USA 2021, 118, e2106730118. [Google Scholar] [CrossRef]
- Bhadra, R.; Gigley, J.P.; Weiss, L.M.; Khan, I.A. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc. Natl. Acad. Sci. USA 2011, 108, 9196–9201. [Google Scholar] [CrossRef]
- Yarovinsky, F. Innate immunity to Toxoplasma gondii infection. Nat. Rev. Immunol. 2014, 14, 109–121. [Google Scholar] [CrossRef]
- Snapper, C.M.; Paul, W.E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 1987, 236, 944–947. [Google Scholar] [CrossRef]
- Alexander, J.; Jebbari, H.; Bluethmann, H.; Brombacher, F.; Roberts, C.W. The role of IL-4 in adult acquired and congenital toxoplasmosis. Int. J. Parasitol. 1998, 28, 113–120. [Google Scholar] [CrossRef]
- Bessieres, M.H.; Swierczynski, B.; Cassaing, S.; Miedouge, M.; Olle, P.; Seguela, J.P.; Pipy, B. Role of IFN-gamma, TNF-alpha, IL4 and IL10 in the regulation of experimental Toxoplasma gondii infection. J. Eukaryot. Microbiol. 1997, 44, 87s. [Google Scholar] [CrossRef]
- Perussia, B.; Dayton, E.T.; Lazarus, R.; Fanning, V.; Trinchieri, G. Immune interferon induces the receptor for monomeric IgG1 on human monocytic and myeloid cells. J. Exp. Med. 1983, 158, 1092–1113. [Google Scholar] [CrossRef]
- Petersen, E.; Nielsen, H.V.; Christiansen, L.; Spenter, J. Immunization with E. coli produced recombinant T. gondii SAG1 with alum as adjuvant protect mice against lethal infection with Toxoplasma gondii. Vaccine 1998, 16, 1283–1289. [Google Scholar] [CrossRef]
- Garcia, J.L.; Navarro, I.T.; Biazzono, L.; Freire, R.L.; da Silva Guimarães Junior, J.; Cryssafidis, A.L.; Bugni, F.M.; da Cunha, I.A.L.; Hamada, F.N.; Dias, R.C.F. Protective activity against oocyst shedding in cats vaccinated with crude rhoptry proteins of the Toxoplasma gondii by the intranasal route. Vet. Parasitol. 2007, 145, 197–206. [Google Scholar] [CrossRef]
- Zulpo, D.L.; Igarashi, M.; Sammi, A.S.; Santos, J.R.; Sasse, J.P.; da Cunha, I.A.L.; Taroda, A.; de Barros, L.D.; de Almeida, J.C.; Jenkins, M.C.; et al. rROP2 de Toxoplasma gondii como potencial vacina contra a eliminação de oocistos em gatos domésticos. Rev. Bras. Parasitol. Veterinária 2017, 26, 67–73. [Google Scholar] [CrossRef]
- Pavot, V.; Rochereau, N.; Genin, C.; Verrier, B.; Paul, S. New insights in mucosal vaccine development. Vaccine 2012, 30, 142–154. [Google Scholar] [CrossRef]
- Rose, M.A.; Zielen, S.; Baumann, U. Mucosal immunity and nasal influenza vaccination. Expert Rev. Vaccines 2012, 11, 595–607. [Google Scholar] [CrossRef]
- Mack, D.G.; McLeod, R. Human Toxoplasma gondii-specific secretory immunoglobulin A reduces T. gondii infection of enterocytes in vitro. J. Clin. Investig. 1992, 90, 2585–2592. [Google Scholar] [CrossRef][Green Version]
- Bonenfant, C.; Dimier-Poisson, I.; Velge-Roussel, F.; Buzoni-Gatel, D.; Del Giudice, G.; Rappuoli, R.; Bout, D. Intranasal immunization with SAG1 and nontoxic mutant heat-labile enterotoxins protects mice against Toxoplasma gondii. Infect. Immun. 2001, 69, 1605–1612. [Google Scholar] [CrossRef]
- Dimier-Poisson, I.; Aline, F.; Bout, D.; Mévélec, M.N. Induction of protective immunity against toxoplasmosis in mice by immunization with Toxoplasma gondii RNA. Vaccine 2006, 24, 1705–1709. [Google Scholar] [CrossRef]
- Iyer, A.V.; Ghosh, S.; Singh, S.N.; Deshmukh, R.A. Evaluation of three ‘ready to formulate’ oil adjuvants for foot-and-mouth disease vaccine production. Vaccine 2000, 19, 1097–1105. [Google Scholar] [CrossRef]
- Zulpo, D.L.; Headley, S.A.; Biazzono, L.; da Cunha, I.A.; Igarashi, M.; de Barros, L.D.; Taroda, A.; Cardim, S.T.; Bogado, A.L.G.; Navarro, I.T.; et al. Oocyst shedding in cats vaccinated by the nasal and rectal routes with crude rhoptry proteins of Toxoplasma gondii. Exp. Parasitol. 2012, 131, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.W.; Ferguson, D.J.; Jebbari, H.; Satoskar, A.; Bluethmann, H.; Alexander, J. Different roles for interleukin-4 during the course of Toxoplasma gondii infection. Infect. Immun. 1996, 64, 897–904. [Google Scholar] [CrossRef]
- Neyer, L.E.; Grunig, G.; Fort, M.; Remington, J.S.; Rennick, D.; Hunter, C.A. Role of interleukin-10 in regulation of T-cell-dependent and T-cell-independent mechanisms of resistance to Toxoplasma gondii. Infect. Immun. 1997, 65, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Frenkel, J.K. Cyst-induced toxoplasmosis in cats. J. Protozool. 1972, 19, 155–177. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).