Abstract
Background: Assessing the real-world safety of preventive products against respiratory syncytial virus (RSV) in pregnant women holds significant public health implications, especially as vaccination programs become more widespread. This generic protocol describes a post-authorisation safety study (PASS) to evaluate the safety of RSV vaccination in pregnant women using a target trial emulation framework. Methods: This generic protocol, adapted from an ongoing PASS, is designed using the target trial emulation framework to evaluate the safety of an RSV vaccine in pregnant women. Emulating target trial conditions have the ability to minimise confounding and bias. In this pragmatic real-world observational study, RSV-vaccinated pregnant women are matched (1:N) with unexposed women based on gestational age, calendar time, maternal age, immunocompromised status, and high-risk pregnancy. Key adverse outcomes include preterm birth, stillbirth, hypertensive disorders of pregnancy, Guillain-Barré Syndrome (GBS), low birth weight (LBW), and small for gestational age (SGA). Future studies may add additional outcomes per vaccine risk profile and Global Alignment of Immunization safety Assessment (GAIA) recommendations. Distinguishing outcomes measured during pregnancy from those assessed at or after birth is crucial for analysis and interpretation. Conclusions: This protocol offers a structured approach to evaluating the safety of RSV vaccines in pregnant women. It aims to guide researchers in designing studies and should be adapted to specific settings and data availability.
1. Introduction
Respiratory syncytial virus (RSV) is a major cause of lower respiratory tract infections (LRTIs) in young children. In 2019 alone, RSV was responsible for 33 million LRTIs globally, leading to 3.6 million hospitalisations and around 100,000 deaths [1]. Notably, about half of the global burden of RSV falls upon infants under the age of 6 months, with a significant number occurring during the neonatal period [1]. Given the substantial impact of RSV, introducing an RSV vaccine for maternal immunisation during pregnancy may offer promise in reducing the burden of RSV-associated illness in infants [2]. The mechanism underlying maternal immunisation involves transferring antibodies across the placenta to protect the infant during its vulnerable neonatal period and beyond [3].
The development of RSV vaccines is progressing swiftly, with multiple candidate vaccines advancing through phases 2 and 3 of development [4]. In 2023, the first maternal RSV vaccine was approved by the European Medicines Agency (EMA), the Food and Drug Administration (FDA) and the Medicines and Healthcare products Regulatory Agency (MHRA) [5,6,7]. These approvals in pregnant women in the EU, US and UK are supported by evidence from a global randomized, double-blinded, and placebo-controlled trial (MATISSE). This trial assessed the vaccine’s efficacy, safety, and immunogenicity against RSV-related medically attended LRTI in infants born to vaccinated healthy women during weeks 24 to 36 of gestation. The study enrolled 7358 pregnant women, receiving either the RSV vaccine or placebo. At the time of analysis, 5654 infants (79%) completed 6-month follow-up after birth, with follow-up ongoing for a total of 24 months [8].
Immunocompromised pregnant women and those with high-risk pregnancies were excluded from the trial [8]. As a result, there is a need for further post-authorisation safety studies (PASS) to understand the vaccine’s safety profile in these populations. Additionally, Guillain-Barré Syndrome (GBS), a rare neurological disorder, has been reported as an adverse event in the clinical trial of the approved vaccine in older adults [9]. Although GBS has not been reported in clinical trials in pregnant women, we identify it as a safety event of interest.
In the MATISSE trial, there was no evidence of a difference in the percentage of preterm births between the vaccine and placebo groups overall. However, analyses stratified by income level revealed a statistically significant difference in upper-middle-income countries [10]. Of note, another trial of a candidate maternal RSV vaccine of a similar type stopped early due to a higher risk of preterm birth in the vaccine group [11]. As the first vaccine to be approved for maternal indication and given this potential uncertainty about preterm birth, the regulatory agencies each approved the vaccine to be used at different gestational ages [12]. The EMA decided that the vaccine could be administered beginning at 24 weeks of gestation. The FDA approved vaccination of pregnant women at 32 through 36 weeks of gestation, while the MHRA has recommended that the vaccine may be given in the third trimester of pregnancy (weeks 28 to 36 of gestation) [13].
In light of the importance of addressing gaps in safety data, a protocol for a PASS to assess the safety of this RSV vaccine in pregnant women and their offspring in real-world settings was developed as an additional pharmacovigilance activity outlined in the EU and UK-approved risk management plans and as a post-marketing requirement to the FDA [14,15]. This manuscript provides a generic framework of the PASS protocol to assist researchers in the preparation of their own protocols during the post-marketing phase in the coming years.
2. Objectives
The primary objective of a PASS on maternal RSV immunisation study is to estimate the occurrence of adverse maternal, pregnancy and birth outcomes in women who receive the vaccine during pregnancy (and their offspring), compared with a matched comparator group of pregnant women who do not receive the RSV vaccine during pregnancy (and their offspring). The secondary objectives may include assessing the occurrence of these adverse outcomes in immunocompromised or high-risk pregnant women who receive the RSV vaccine compared to those who do not, as these groups were not studied in clinical trials. Due to variations in timing of authorised administration, another objective may be to estimate the occurrence of adverse outcomes by gestational week of vaccination to better understand possible effect modification by week of gestation.
3. Methods
3.1. Study Design
We recommend an observational retrospective comparative cohort study of pregnant women who receive the RSV vaccine compared to an unexposed pregnant comparator group (or an active comparator group when there is one). To assess whether the RSV vaccine increases the risk of any adverse outcome in pregnancy or offspring, we propose designing the study to emulate a hypothetical pragmatic randomised trial (Table 1) [16]. This trial would assess the safety of administering one dose of the RSV vaccine between 24 and 36 weeks of gestation in pregnant women compared to pregnant women who do not receive an RSV vaccine (Figure 1A). As we cannot truly randomise participants, we need to emulate this feature by carefully addressing confounding biases through various techniques such as matching and careful assessment of inclusion criteria. These techniques also have the potential to address both selection bias and immortal time bias. Time zero, designated as day 0, marks the point at which the eligibility criteria are fulfilled, and the RSV vaccine is administered to the pregnant woman.

Table 1.
A summary of the protocol of the MATISSE trial and emulation of this target trial to estimate the effect of RSV vaccination during pregnancy.
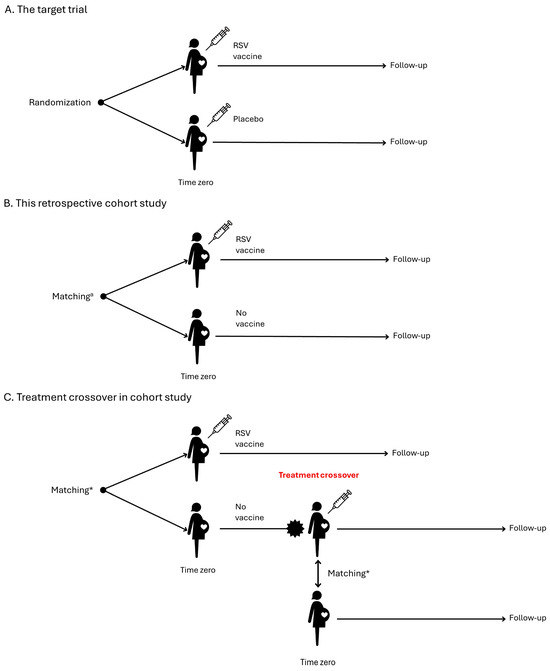
Figure 1.
Study design. * Matching should be on gestational age (same week of gestation), calendar time (same week) and maternal age. Additional characteristics may be added.
At Time zero, exposed participants should be matched to unexposed pregnant participants eligible to receive the vaccine (1:N ratio) based on (Figure 1B):
- Gestational age (same week of gestation) to account for timing of vaccination in pregnancy and the risk of preterm birth
- Calendar time (same week) because of RSV seasonality and probability of exposure
- Maternal age (year of birth) because of the association with adverse pregnancy outcomes
- Immunocompromised status because of the association with adverse maternal outcomes
- High-risk pregnancy because of the association with adverse pregnancy outcomes
- Additional characteristics may be added that would confound the association.
A primary analytical concern is the gestational age at Time zero, which directly impacts the risk of preterm birth. Therefore, it is imperative to ensure a robust match on gestational age, and in fact, we recommend emulating a series of separate target trials for different gestational ages at vaccination, i.e., 24, 25, 26, …, or 36 weeks to study effect modification [17]. Follow-up of the exposed and unexposed (or active comparator) pregnant women should start on day 1, and end at the earliest of N months after birth depending on the outcome being measured, maternal death, disenrollment or migration, end of data availability in the data source, treatment crossover, or occurrence of a given outcome. Treatment crossover, or protocol violation, occurs when previously unexposed pregnant women in the matched comparator cohort receive vaccination with an RSV vaccine during pregnancy. To uphold statistical power, unexposed pregnant women within the matched comparator cohort ought to be censored upon treatment violation, while exposed pregnant women should remain uncensored (see Section 3.10). When unvaccinated pregnant women receive an RSV vaccine, they become eligible for inclusion in the vaccinated cohort (Figure 1C). Follow-up of offspring to assess birth outcomes will depend on the risk window for measuring the outcome and will end at the earliest of N months of age, neonatal death, disenrollment or migration, end of data availability in the data source, or occurrence of a given outcome.
3.2. Study Setting
To have sufficient power, we recommend using real-world secondary data derived from electronic health records or administrative claims data that can be linked on an individual level to birth registries and outcomes in offspring (mother-child linkage). The specific study setting will be defined by the availability and nature of the data source(s) utilised and will depend on the country or region covered by the data source. To assess whether data sources are fit for purpose, we stress the importance of ascertainment the accuracy of estimating the start and end of pregnancy, the ability to detect ongoing pregnancies, the capacity to identify exposure to RSV vaccines including timing of vaccine dose, the feasibility of linking mother and child, the capability to measure outcomes and confounders of interest, and the lag time for data availability for each study component. We recommend the use of quality indicators and external benchmarks to assess whether a data source is fit for purpose [18,19]. Key aspects of this evaluation include:
- Population coverage: The data must cover a representative sample of the population under study. This includes demographics such as age, sex, ethnicity, and geographic location.
- Data granularity: The data must be granular enough to capture individual-level information on healthcare encounters, including diagnoses, procedures, prescriptions, and outcomes.
- Longitudinal data: Longitudinal data, tracking individuals over time, is essential for understanding disease progression, treatment patterns, and outcomes.
- Data quality: High-quality data is crucial for reliable analysis. This involves accurate information recording, standardised coding systems, and measures to address missing or erroneous data.
- Data linkage: The ability to link data across different sources (e.g., primary care, hospitals, pharmacies, birth registers) enables a comprehensive view of healthcare utilisation and outcomes.
- Privacy and ethical considerations: Data sources must adhere to strict privacy regulations and ethical guidelines to protect patient confidentiality and ensure informed consent.
- Timeliness: Timely updates ensure that researchers can access the most current information, allowing for real-time or near-real-time analyses.
3.3. Study Period
The first maternal vaccine was launched on 23 August 2023 [4]. In a hypothetical scenario focused on a study of its safety, we propose commencing data collection retrospectively from 24 August 2022. This means that gathering data starting from the day following approval, allowing for a one-year look-back period. This period is essential for capturing prior medical history, including diseases, comorbidities, and medication use, which are critical factors to consider in the study’s analysis and interpretation. Data collection would continue until the last data availability for each data access provider based on the calendar date of the last data extraction. Given the anticipated need for a significant number of participants, we expect a follow-up period of at least 5 years, with the latest data extraction planned for 2029. However, we recommend regular progress reports, with reporting triggered by the number of exposures.
3.4. Study Population
The study population comprises all pregnant women who receive an RSV vaccine during pregnancy from the earliest indicated timing of gestation for which the vaccine is licensed and a matched comparator group of pregnant women who did not receive the RSV vaccine or received another RSV vaccine. Identification of pregnancies may vary across data sources which is very important since most data sources that link to birth registries only detect pregnancies when they end. Validated data source-specific algorithms or the ConcePTION pregnancy algorithm could be employed to determine the start and end dates of ongoing pregnancies. The ConcePTION algorithm was created to identify ongoing pregnancies using diagnosis and procedure data related to pregnancy [20]. The identification of ongoing pregnancies is important to avoid selection bias towards pregnancies that end prematurely. In instances where pregnancy data are only available when the pregnancy has ended, a minimum of 10 months of administrative follow-up from the last menstrual period (LMP) in the data source, unless they died, is recommended for all pregnancy and birth outcomes. This period covers the gestational period plus an additional month to identify offspring outcomes at birth [17]. It is important to anticipate that women may have more than one pregnancy, and those subsequent pregnancies could be included to increase power.
3.5. Recommended In- and Exclusion Criteria
Participants are eligible if they:
- have an identified start of pregnancy date.
- are enrolled in the healthcare system for a least 12 months prior to Time zero (date of vaccination).
- have at least one day of follow-up after Time zero for maternal outcomes and at least 10 months of follow-up after the LMP for birth and pregnancy outcomes.
Participants are excluded if they:
- are exposed to an RSV vaccine prior to the current pregnancy or before or after the gestational week for which the vaccine is licensed (vaccination in a prior pregnancy is permitted).
- cannot be linked in the data source to their child (mother-child linkage).
3.6. Exposure
The exposure of interest is RSV vaccination during pregnancy. The vaccine data could be obtained from pharmacy dispensing records, general practice records, immunisation registers, vaccination records, medical records or other databanks. We will consider a single administration of one dose between 24–36 weeks of gestation. An woman is considered exposed from the date of vaccination until the end of pregnancy for all pregnancy and birth outcomes.
3.7. Outcomes
The proposed key outcomes of interest, along with their respective outcome-specific exposure windows, are described in Table 2 and encompass preterm birth, time between vaccination and birth, stillbirth, hypertensive disorders of pregnancy, Guillain-Barré Syndrome (GBS), low birth weight (LBW), and small for gestational age (SGA). The outcome time between vaccination and birth holds more statistical power than preterm birth, as preterm birth is a categorical outcome while the time between vaccination and birth is continuous [21,22]. Distinguishing between outcomes measured during pregnancy and those assessed at or after birth is crucial, as it affects statistical analysis and interpretation.

Table 2.
Outcomes of interest.
Additional outcomes for maternal immunisation may be added based on the risk profile of the vaccine [30]. The clinical definitions for outcomes of interest should be based on either the Global Alignment on Immunization Safety Assessment (GAIA), Brighton Collaboration, or the official World Health Organization (WHO) definitions [31,32]. While these definitions can be adjusted, utilising these official, globally recognised definitions will enhance harmonisation and study comparability. The outcomes should be ascertained using algorithms based on codes for diagnoses, procedures, medical products and information from each specific databank. We recommend working with each data source to understand how information can be retrieved and how outcomes and other variables are coded.
3.8. Covariates
Crucial covariates of interest include matching variables: gestational age, calendar time, maternal age, immunocompromised status and high-risk pregnancy, for which definitions are provided in Table 3. Similar to outcomes, we recommend covariates to be identified using diagnoses, procedures, medical products and information from each specific databank. Additionally, variables associated with the outcomes and exposure of interest should be included [33,34,35,36,37,38]. An example is presented in Supplementary Table S1. Maternal vaccinations such as influenza, SARS-CoV-2, Tetanus, Diphtheria and Pertussis (Tdap) given prior to RSV vaccination should also be recorded, as well as RSV monoclonal antibodies and RSV vaccination of the offspring.

Table 3.
Covariates of interest.
3.9. Study Size
All women meeting eligibility criteria during the study observation period should be included in the study. It is currently unknown how many women will receive RSV vaccination during pregnancy, as the National Immunisation Technical Advisory Groups need to determine whether this will be included in routine maternal immunisation programs in the country or region where the study is conducted. Minimal detectable risks can be calculated based on the assumption that every vaccinated pregnant individual could be compared with N unvaccinated pregnant individual(s) (1:N ratio). The desired alpha level is 5%, and risk ratio (RR) values of 1.5, 2.0, 2.5, and 4.0 may be considered (Supplementary Table S2). For pregnancy and birth outcomes, the required number of exposed women to achieve a power of 80% under different RR values may assume prevalence rates in the unexposed group ranging from 0.2% to 6.0%. To detect an RR of 1.5 with 95% confidence interval (CI) for preterm birth, approximately 1200 pregnancies ending in live birth would need to be included in a data source. For rare outcomes during pregnancy such as GBS, we calculated the power obtainable assuming a maximum sample size of 30,000 exposed pregnant women, with an incidence rate of 1/100,000 person-years in the comparator group [39].
3.10. Data Analysis
Detailed methodology for summary and statistical analyses should be documented in a statistical analysis plan and be drafted prior to any analyses. Any major modifications to the prespecified analyses should be clearly reported. Changes should be annotated and amended when they are major.
3.10.1. Baseline Characteristics
Baseline characteristics for both maternal RSV-exposed and pregnant comparator cohorts should be reported as means, standard deviations, medians and other quartiles for continuous variables, and counts and proportions for categorical variables. Any missing baseline characteristics and the duration of the look-back period should be explicitly described. To assess the comparability of matched cohorts, a standardised difference between the index and comparator cohort can be computed for each baseline characteristic. In cases where categorical variables have more than two levels, an overall standardised difference should be calculated.
3.10.2. Measures of Effect
Primary analyses should estimate counts, proportions, and risk or rate ratios with 95% CI for events occurring and measurable during pregnancy, and counts, proportions, and prevalence ratios with 95% CI for outcomes measurable at birth. Comparison of outcomes by exposure status should be conducted using multivariable generalised linear models. These analyses should control for potential confounding by matching. Balance of covariates should be analysed using standardised mean differences (SMDs), some characteristics and risk factors for the exposure or outcomes may remain unbalanced, which should be analysed using plots of SMDs. To address this, propensity score methods can be employed and propensity scores can be adjusted or additionally matched on if necessary [40]. To obtain more power for the analysis of preterm birth, we recommend that time from vaccination to birth should be analysed separately [21,22].
The primary analyses may face-selective censoring due to treatment crossover in the comparator cohort, necessitating the use of inverse probability censoring weights. To maintain statistical power, unexposed pregnant women in the matched comparator cohort should be censored upon treatment violation, while exposed pregnant women should not be censored. Weights can be used to correct for the bias introduced by such censoring, ensuring more accurate and reliable results. Additionally, a sensitivity analysis can be conducted to explore the effect of censoring the matched pair if treatment crossover happens in the previously unexposed pregnant woman. Another sensitivity analysis may be carried out to explore the effect of exclusion of medically indicated iatrogenic births.
For subgroup analyses, secondary analyses can be conducted among immunocompromised or high-risk pregnancies and stratified by weeks of gestation at the time of vaccination.
3.10.3. Meta-Analysis
In case multiple data sources are utilised, we recommend employing a common analytics tool, such as R studio, to conduct the analyses separately within each data source consistently. Subsequently, utilising the main estimates obtained from each data source, appropriate random-effects meta-analytic methods should be applied to derive a combined effect estimate [41,42]. To assess potential heterogeneity across data sources, it is advisable to visually inspect and check using forest plots, displaying the study site estimates and a pooled estimate with 95% CIs. In situations where there are zero events, we suggest pooling incidence rates, as they can be zero and still yield valid standard errors.
3.11. Quality Control
We want to highlight that it is important to conduct quality control procedures to ensure the existence and accuracy of records. Quality control can be conducted using the INSIGHT level 1–3 data quality checks [43]. Briefly, level 1 verifies data completeness, level 2 ensures data consistency and level 3 checks for study variables and whether data are fit for purpose.
3.12. Protection of Human Subjects
Ethical considerations are paramount in any research involving human subjects. In this generic protocol for a retrospective database study, we describe data that exist in a deidentified/pseudonymised structured format without including personal patient information, thus eliminating the need for informed consent from participants. However, in the event of conducting a new study, it is essential to adhere to ethical guidelines and obtain approval and consent to safeguard the rights and well-being of participants if needed. There must be prospective approval from relevant Institutional Review Boards or Ethics Committees for the study protocol and amendments. Furthermore, we highly recommend conducting the study according to the guidelines for Good Pharmacoepidemiology Practice and the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP) guide on Methodological Standards in Pharmacoepidemiology [44,45]. Additionally, the study protocol should be registered before data collection begins and adhere to transparency and scientific independence principles outlined by the ENCePP Code of Conduct [46].
4. Discussion
This generic protocol has been developed to assess the safety of RSV vaccines in pregnant women and their offspring in real-world settings utilising secondary sources of data. It presents the emulation of a target trial, assessing the safety of administering a single dose of an RSV vaccine to pregnant women between 24–36 weeks of gestation compared to pregnant women who do not receive RSV vaccination during pregnancy [8]. The emulation of target trial conditions will help to reduce bias due to censoring, immortal time, competing events and confounding, improve the interpretability of estimands and highlight any remaining challenges with the data [17]. However, despite efforts to minimise bias and improve interpretability, limitations persist in target trial emulation using observational data. These may include challenges in accurately capturing all relevant confounders. Data sources that routinely collect data often provide comprehensive information on treatments and outcomes but may lack sufficient detail on clinical factors that require adjustment [47]. Fitness for purpose of data can be assessed in feasibility studies [18,19].
The study will use code lists or observations in birth registers to identify the covariates and outcomes of interest. An important consideration of using code lists is the potential for outcome misclassification, which can introduce bias and impact the interpretation of findings. The code lists could be sourced from Vaccine Monitoring Collaboration for Europe (VAC4EU) (https://vac4eu.org/), as these have undergone thorough review by medical professionals. Only codes labelled as “narrow” are included to mitigate the false-positive rate. For preterm birth, validation may be needed since preterm birth can either be spontaneous or medically indicated (iatrogenic), whereas our focus is only on spontaneous preterm birth. Codes and outcome definitions may be scrutinised in Safety Platform for Emergency Vaccines companion guides. Validation of outcomes, using Brighton or WHO definitions, may be conducted when resources are available.
In addition to outcome misclassification, it is also crucial to address exposure misclassification. Since RSV vaccination has only recently received approval for maternal immunisation, and many countries’ National Immunisation Technical Advisory Groups have yet to decide on its inclusion in routine immunisation schedules, it can be challenging to determine whether current data sources accurately capture RSV vaccination. This challenge is reflected by the current situation in Europe, where other maternal immunisation practices vary widely across countries, and some prefer to provide monoclonal antibodies or vaccines to neonates [2,48] Therefore, before commencing data collection, it is imperative to liaise with relevant public health organisations to understand if and how RSV vaccination will be integrated into routine immunisation programs for maternal immunisation and in which settings it is provided. Subsequently, it is essential to verify if the data source adequately captures this information and compare it with national statistics to ensure comprehensive coverage and prevent exposure misclassification in the comparator group.
It is important to note that a non-exposed comparator group may be chosen because presently there is only one approved maternal vaccine for preventing RSV infection in offspring. In the event that other RSV vaccines receive approval, it is crucial to consider their inclusion in the study design. This necessitates either incorporating all different RSV vaccines in the exposed group or excluding women specifically vaccinated with other RSV vaccines from the unexposed group.
Because of safety concerns, the primary outcome measure under examination is preterm birth, considering the varying recommendations by EMA, FDA and MHRA regarding the optimal gestational age for vaccination [5,6,7,12,13]. Notably, concerns over preterm births have led to the suspension of a phase 3 trial for another candidate RSV prefusion subunit vaccine [10]. However, a significant challenge in analysing preterm birth in observational studies lies in its direct relationship with gestational age at the time of vaccination. For instance, administering the vaccine at 35 weeks of gestation cannot result in an preterm birth of less than 35 weeks. Therefore, we emphasise the critical importance of matching participants based on gestational age at the time of vaccination. In fact, participants will be enrolled in a sequence of weekly trials based on their gestational age at the time of vaccination. Additionally, we have added the outcome time between vaccination and birth. This outcome holds more statistical power than preterm birth, as preterm birth is a categorical outcome while time between vaccination and birth is continuous. Also, this approach allows us to examine the median time between vaccination and preterm births, which is crucial for identifying specific underlying mechanisms. For instance, it can reveal whether preterm infants are born days, weeks, or months after vaccination. Moreover, this outcome is independent of the accuracy of gestational age assessment [21,22]. Other outcomes can be chosen, and we refer to the GAIA initiative, which defined more than 20 outcomes relevant to maternal immunisation [31].
5. Conclusions
This generic protocol is designed to offer guidance for researchers and health authorities in evaluating the safety of RSV vaccination during pregnancy. A target trial emulation approach of observational data can be selected to minimise bias and improve interpretability. We envision this protocol serving as a valuable tool, fostering alignment of research efforts and aiding in the monitoring of the safety of RSV vaccination in pregnant women and their offspring.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines13030272/s1, Table S1: Risk factors for the outcomes of interest; Table S2: Power and sample size estimates for Guillain-Barré Syndrome and pregnancy and birth outcomes.
Author Contributions
Conceptualization, O.d.B., L.N., J.C., O.R., H.-W.U., F.A., S.S., H.R., K.B., C.d.L. and M.S.; methodology, L.N., J.C., O.R. and H.-W.U.; writing—original draft preparation, O.d.B.; writing—review and editing, O.d.B., L.N., J.C., O.R., H.-W.U., F.A., S.S., H.R., K.B., C.d.L. and M.S.; visualization, O.d.B.; supervision, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was sponsored by Pfizer, Inc.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to express our gratitude to the PROMISE Consortium for their valuable contributions to the peer-review of the deliverable on which this manuscript is based, in particular: Lydie Marcelon, Anders Peter Hviid, Se Li, Rolf Kramer, Claire Thorne, Carlo Giaquinto, Veena Kumar, Jim Janimak, Louis Bont, Harish Nair, and Jenny Hendricks. The PROMISE Consortium has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under Grant Agreement 101034339. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA.
Conflicts of Interest
S.S., H.R. and C.D. are employees of Pfizer, Inc and have received Pfizer stock. M.S. leads a department that conducts studies on RSV vaccines for the European Medicines Agency (EMA) and Pfizer. She has not received personal fees or other personal benefits; however University Medical Center Utrecht (UMCU) has received major funding (>EUR 100,000). K.B. is the local principal investigator of a phase 3 clinical trial on maternal RSV vaccination funded by Pfizer. O.d.B., L.N., J.C., O.R., H.-W.U., and F.A. have no conflict of interest to declare.
References
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; A Madhi, S.; Omer, S.B.; Simões, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047. [Google Scholar] [CrossRef] [PubMed]
- Mazur, N.I.; Terstappen, J.; Baral, R.; Bardají, A.; Beutels, P.; Buchholz, U.J.; Cohen, C.; Crowe, J.E.; Cutland, C.L.; Eckert, L.; et al. Respiratory syncytial virus prevention within reach: The vaccine and monoclonal antibody landscape. Lancet Infect. Dis. 2023, 23, e2. [Google Scholar] [CrossRef]
- Omer, S.B. Maternal immunization. N. Engl. J. Med. 2017, 376, 1256. [Google Scholar] [CrossRef]
- PATH. RSV Vaccine and mAb Snapshot. Available online: https://www.path.org/our-impact/resources/rsv-vaccine-and-mab-snapshot/ (accessed on 20 April 2024).
- Pfizer Inc. European Commission Approves Pfizer’s ABRYSVOTM to Help Protect Infants Through Maternal Immunization and Older Adults from RSV. Available online: https://www.pfizer.com/news/press-release/press-release-detail/european-commission-approves-pfizers-abrysvotm-help-protect (accessed on 4 April 2024).
- U.S. Food & Drugs Administration. FDA Approves First Vaccine for Pregnant Individuals to Prevent RSV in Infants. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-vaccine-pregnant-individuals-prevent-rsv-infants (accessed on 4 April 2024).
- PMLiVE. Pfizer’s RSV Vaccine Granted MHRA Approval to Protect Infants and Older Adults. Available online: https://pmlive.com/pharma_news/pfizers_rsv_vaccine_granted_mhra_approval_to_protect_infants_and_older_adults_1504343/ (accessed on 4 April 2024).
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.; Pahud, B.A.; Llapur, C.; Baker, J.; Marc, G.P.; Radley, D.; Shittu, E.; et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N. Engl. J. Med. 2023, 388, 1451. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Marc, G.P.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N. Engl. J. Med. 2023, 388, 1465. [Google Scholar] [CrossRef] [PubMed]
- Pfizer Inc. Respiratory Syncytial Virus Bivalent Stabilized Prefusion F Subunit Vaccine (RSVpreF)—VRBPAC Briefing Document. Available online: https://www.fda.gov/media/165625/download (accessed on 20 April 2024).
- Dieussaert, I.; Kim, J.H.; Luik, S.; Seidl, C.; Pu, W.; Stegmann, J.; Swamy, G.; Webster, P.; Dormitzer, P. RSV prefusion F protein-based maternal vaccine—Preterm birth and other outcomes. N. Engl. J. Med. 2024, 390, 1009. [Google Scholar] [CrossRef]
- Willemsen, J.E.; Borghans, J.A.M.; Bont, L.J.; Drylewicz, J. Disagreement FDA and EMA on RSV maternal vaccination: Possible consequence for global mortality. Pediatr. Infect. Dis. J. 2024, 43, e1. [Google Scholar] [CrossRef]
- Boytchev, H. FDA advisers back Pfizer’s maternal RSV vaccine after voicing safety concerns. BMJ 2023, 381, 1187. [Google Scholar] [CrossRef]
- Pfizer Inc. Abrysvo: EPAR—Risk Management Plan. Available online: https://www.ema.europa.eu/en/documents/rmp-summary/abrysvo-epar-risk-management-plan_en.pdf (accessed on 4 April 2024).
- U.S. Food & Drug Administration. Summary Basis for Regulatory Action—ABRYSVO. Available online: https://www.fda.gov/media/172126/download?attachment (accessed on 4 April 2024).
- Hernán, M.A.; Robins, J.M. Using big data to emulate a target trial when a randomized trial is not available. Am. J. Epidemiol. 2016, 183, 758. [Google Scholar] [CrossRef]
- Hernández-Díaz, S.; Huybrechts, K.F.; Chiu, Y.-H.; Yland, J.J.; Bateman, B.T.; Hernán, M.A. Emulating a target trial of interventions initiated during pregnancy with healthcare databases: The example of COVID-19 vaccination. Epidemiology 2023, 34, 238. [Google Scholar] [CrossRef]
- Gatto, N.M.; Campbell, U.B.; Rubinstein, E.; Jaksa, A.; Mattox, P.; Mo, J.; Reynolds, R.F. The structured process to identify fit-for-purpose data: A data feasibility assessment framework. Clin. Pharmacol. Ther. 2022, 111, 122. [Google Scholar] [CrossRef]
- Willame, C.; Baril, L.; van den Bosch, J.; Ferreira, G.L.C.; Williams, R.; Rosillon, D.; Cohet, C. Importance of feasibility assessments before implementing non-interventional pharmacoepidemiologic studies of vaccines: Lessons learned and recommendations for future studies. Pharmacoepidemiol. Drug Saf. 2016, 25, 1397. [Google Scholar] [CrossRef] [PubMed]
- GitHub—ARS-toscana/ConcePTIONAlgorithmPregnancies: Repository of the Script of the ConcePTION Algorithm for Pregnancies. Available online: https://github.com/ARS-toscana/ConcePTIONAlgorithmPregnancies (accessed on 5 April 2024).
- Boytchev, H. Maternal RSV vaccine: Further analysis is urged on preterm births. BMJ 2023, 381, 10. [Google Scholar] [CrossRef] [PubMed]
- Science Media Centre (SMC). Expert Reaction to BMJ Investigation Suggesting Monitoring of the RSV Vaccine from Pfizer. Available online: https://www.sciencemediacentre.org/expert-reaction-to-bmj-investigation-suggesting-monitoring-of-the-rsv-vaccine-from-pfizer/ (accessed on 18 July 2024).
- Quinn, J.-A.; Munoz, F.M.; Gonik, B.; Frau, L.; Cutland, C.; Mallett-Moore, T.; Kissou, A.; Wittke, F.; Das, M.; Nunes, T.; et al. Preterm birth: Case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine 2016, 34, 6047. [Google Scholar]
- Da Silva, F.T.; Gonik, B.; McMillan, M.; Keech, C.; Dellicour, S.; Bhange, S.; Tila, M.; Harper, D.M.; Woods, C.; Kawai, A.T.; et al. Stillbirth: Case definition and guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2016, 34, 6057. [Google Scholar] [CrossRef] [PubMed]
- Magee, L.A.; Brown, M.A.; Hall, D.R.; Gupte, S.; Hennessy, A.; Karumanchi, S.A.; Kenny, L.C.; McCarthy, F.; Myers, J.; Poon, L.C.; et al. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022, 27, 148–169. [Google Scholar]
- Rouse, C.E.; Eckert, L.O.; Wylie, B.J.; Lyell, D.J.; Jeyabalan, A.; Kochhar, S.; McElrath, T.F. Hypertensive disorders of pregnancy: Case definitions & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2016, 34, 6069. [Google Scholar]
- Sejvar, J.J.; Kohl, K.S.; Gidudu, J.; Amato, A.; Bakshi, N.; Baxter, R.; Burwen, D.R.; Cornblath, D.R.; Cleerbout, J.; Edwards, K.M.; et al. Guillain-Barré syndrome and Fisher syndrome: Case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2011, 29, 599. [Google Scholar] [CrossRef]
- Cutland, C.L.; Lackritz, E.M.; Mallett-Moore, T.; Bardají, A.; Chandrasekaran, R.; Lahariya, C.; Nisar, M.I.; Tapia, M.D.; Pathirana, J.; Kochhar, S.; et al. Low birth weight: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2017, 35, 6492. [Google Scholar]
- Schlaudecker, E.P.; Munoz, F.M.; Bardají, A.; Boghossian, N.S.; Khalil, A.; Mousa, H.; Nesin, M.; Nisar, M.I.; Pool, V.; Spiegel, H.M.; et al. Small for gestational age: Case definition & guidelines for data collection, analysis, and presentation of maternal immunisation safety data. Vaccine 2017, 35, 6518. [Google Scholar]
- Hviid, A.; Faksova, K.; Marcelon, L.; Neveu, D. D2.4 Report on Identification of Adverse Events for Safety Evaluations of RSV Vaccination and Monoclonal Antibodies. Available online: https://www.ed.ac.uk/sites/default/files/atoms/files/promise_d2.4-adverse-events-for-safety-evaluations_v1.0.pdf (accessed on 20 April 2024).
- Bonhoeffer, J.; Kochhar, S.; Hirschfeld, S.; Heath, P.T.; Jones, C.E.; Bauwens, J.; Honrado, Á.; Heininger, U.; Muñoz, F.M.; Eckert, L.; et al. Global alignment of immunization safety assessment in pregnancy—The GAIA project. Vaccine 2016, 34, 5993. [Google Scholar] [CrossRef]
- Kohl, K.S.; Bonhoeffer, J.; Braun, M.M.; Chen, R.T.; Duclos, P.; Heijbel, H.; Heininger, U.; Loupi, E.; Marcy, S.M.; The Brighton Collaboration. The Brighton Collaboration: Creating a Global Standard for Case Definitions (and Guidelines) for Adverse Events Following Immunization. Available online: https://europepmc.org/article/MED/21249832/NBK20499#free-full-text (accessed on 4 April 2024).
- NIH. What Are the Risk Factors for Preterm Labor and Birth? 2023. Available online: https://www.nichd.nih.gov/health/topics/preterm/conditioninfo/who_risk (accessed on 8 April 2024).
- NIH. What Are the Risk Factors for Stillbirth? 2023. Available online: https://www.nichd.nih.gov/health/topics/stillbirth/topicinfo/risk (accessed on 8 April 2024).
- NIH. Who Is at Risk of Preeclampsia? Available online: https://www.nichd.nih.gov/health/topics/preeclampsia/conditioninfo/risk (accessed on 8 April 2024).
- WHO. Guillain–Barré Syndrome. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/guillain-barr%C3%A9-syndrome (accessed on 8 April 2024).
- Valero De Bernabé, J.; Soriano, T.; Albaladejo, R.; Juarranz, M.; Calle, M.E.; Martínez, D.; Domínguez-Rojas, V. Risk factors for low birth weight: A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 116, 3–15. [Google Scholar] [CrossRef]
- MSD Manual. Small-for-Gestational-Age (SGA) Newborns. 2024. Available online: https://www.msdmanuals.com/home/children-s-health-issues/general-problems-in-newborns/small-for-gestational-age-sga-newborn (accessed on 8 April 2024).
- Rothman, K.J. Episheet. Available online: http://krothman.hostbyet2.com/Episheet.xls (accessed on 14 May 2024).
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.D.; Higgins, J.P.T.; Deeks, J.J. Interpretation of random effects meta-analyses. BMJ 2011, 342, d549. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, V.; Navarro, C.L.A.; Riera-Arnau, J.; Elbers, R.J.H.J.; Alsina, E.; Dodd, C.; Sturkenboom, M.C.J.M. INSIGHT: A Tool for Fit-for-Purpose Evaluation and Quality Assessment of Observational Data Sources for Real World Evidence on Medicine and Vaccine Safety. medRxiv 2023. [Google Scholar] [CrossRef]
- The European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP). Guide on Methodological Standards in Pharmacoepidemiology (Revision 11). Available online: https://encepp.europa.eu/encepp-toolkit/methodological-guide_en?prefLang=nl#related-documents (accessed on 14 May 2024).
- Public Policy Committee; International Society of Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practice (GPP). Pharmacoepidemiol. Drug Saf. 2016, 25, 2. [Google Scholar] [CrossRef]
- Gini, R.; Fournie, X.; Dolk, H.; Kurz, X.; Verpillat, P.; Simondon, F.; Strassmann, V.; Apostolidis, K.; Goedecke, T. The ENCePP code of conduct: A best practise for scientific independence and transparency in noninterventional postauthorisation studies. Pharmacoepidemiol. Drug Saf. 2019, 28, 422. [Google Scholar] [CrossRef]
- Hernán, M.A.; Wang, W.; Leaf, D.E. Target trial emulation: A framework for causal inference from observational data. JAMA 2022, 328, 2446. [Google Scholar] [CrossRef]
- Maltezou, H.C.; Effraimidou, E.; Cassimos, D.C.; Medic, S.; Topalidou, M.; Konstantinidis, T.; Theodoridou, M.; Rodolakis, A. Vaccination programs for pregnant women in Europe, 2021. Vaccine 2021, 39, 6137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).