Abstract
Hematopoietic cell transplantation (HCT) and chimeric antigen receptor (CAR)-T cell patients are immunocompromised, remain at high risk following SARS-CoV-2 infection, and are less likely than immunocompetent individuals to respond to vaccination. As part of the safety lead-in portion of a phase 2 clinical trial in patients post HCT/CAR-T for hematological malignancies (HM), we tested the immunogenicity of the synthetic modified vaccinia Ankara-based COVID-19 vaccine COH04S1 co-expressing spike (S) and nucleocapsid (N) antigens. Thirteen patients were vaccinated 3–12 months post HCT/CAR-T with two to four doses of COH04S1. SARS-CoV-2 antigen-specific humoral and cellular immune responses, including neutralizing antibodies to ancestral virus and variants of concern (VOC), were measured up to six months post vaccination and compared to immune responses in historical cohorts of naïve healthy volunteers (HV) vaccinated with COH04S1 and naïve healthcare workers (HCW) vaccinated with the FDA-approved mRNA vaccine Comirnaty® (Pfizer, New York, NY, USA). After one or two COH04S1 vaccine doses, HCT/CAR-T recipients showed a significant increase in S- and N-specific binding antibody titers and neutralizing antibodies with potent activity against SARS-CoV-2 ancestral virus and VOC, including the highly immune evasive Omicron XBB.1.5 variant. Furthermore, vaccination with COH04S1 resulted in a significant increase in S- and N-specific T cells, predominantly CD4+ T lymphocytes. Elevated S- and N-specific immune responses continued to persist at six months post vaccination. Furthermore, both humoral and cellular immune responses in COH04S1-vaccinated HCT/CAR-T patients were superior or comparable to those measured in COH04S1-vaccinated HV or Comirnaty®-vaccinated HCW. These results demonstrate robust stimulation of SARS-CoV-2 S- and N-specific immune responses including cross-reactive neutralizing antibodies by COH04S1 in HM patients post HCT/CAR-T, supporting further testing of COH04S1 in immunocompromised populations.
1. Introduction
Hematopoietic cell transplantation (HCT) and chimeric antigen receptor (CAR)-T cell cancer patients are at increased risk of hospitalization and death following SARS-CoV-2 infection [1,2,3]. The use of COVID-19 vaccines in this population has resulted in better outcomes, but vaccine response rates remain below those measured in healthy volunteers (HV) [4,5,6,7,8]. This is due to suppressed immunity post cell therapy and is further worsened by transplantation-related morbidities [9].
Two doses of FDA-approved mRNA vaccines have been shown to be poorly immunogenic in HCT/CAR-T patients and often require subsequent doses to improve the response rate [4,10,11,12,13]. For this reason, current guidelines recommend three doses as a primary series followed by one or more booster doses with updated bivalent mRNA vaccines [14,15]. Major risk factors for low vaccine responses have been shown to include vaccination less than 12 months from transplant, low lymphocyte count, and use of immunosuppressive drugs [5,9,16,17].
We are conducting a phase 2 clinical trial to test the safety and immunogenicity of dual-antigen COVID-19 vaccine COH04S1, a fully synthetic modified vaccinia Ankara (sMVA) vector co-expressing SARS-CoV-2 Wuhan-Hu-1-based spike (S) and nucleocapsid (N) antigens [18,19]. In a phase 1 clinical trial in HV, COH04S1 has been shown to be safe and to induce robust antigen-specific humoral and cellular immune responses [20,21]. Additionally, COH04S1 demonstrated protection in small and large animal models against SARS-CoV-2 ancestral virus and several variants of concern (VOC), including Omicron subvariants [19,22,23].
In this study, we demonstrate robust stimulation of S- and N-specific humoral and cellular immune responses in HCT/CAR-T patients vaccinated with COH04S1 within one year post cell therapy. This includes neutralizing antibodies (NAb) with potent reactivity against SARS-CoV-2 ancestral virus and several variants of concern (VOC), including the Beta, Delta, Omicron BA.1, and the highly immune evasive Omicron XBB.1.5 variants. In addition, humoral and cellular responses elicited by COH04S1 in HCT/CAR-T patients surpass or are comparable to responses measured in HV vaccinated with COH04S1 as well as healthcare workers (HCW) vaccinated with the FDA-approved mRNA vaccine Comirnaty® (BNT162b2, Pfizer, New York, NY, USA). These results support further clinical testing of COH04S1 in individuals with weakened immune systems.
2. Materials and Methods
2.1. Study Design
The randomized, multi-center, observer-blind study of COH04S1 versus Comirnaty® in patients post HCT/CAR-T for hematological malignancies (HM) was approved by an external institutional review board (IRB) and is registered (NCT04977024). The open-label safety lead-in portion, conducted at City of Hope, enrolled between September 2021 and September 2022 and included 13 patients. After giving informed consent, patients were vaccinated with two to four doses of COH04S1 at 2.5 × 108 plaque forming units (pfu) diluted in PBS/7.5% lactose. Immunological assessment was carried out at baseline and at days 28, 56, 120, and 180. For patients receiving additional vaccine doses at days 56 and 140, subsequent blood draws were taken at days 84, 140, and 180 (Figure S1). Immunological results for patients at all timepoints following a reported positive COVID-19 test (4/13 patients), or after boosting with FDA-approved SARS-CoV-2 vaccines (3/13 patients) were not included in the immunological analysis (Table S1 and Figures S2–S4). Historical cohorts of naïve HV vaccinated with COH04S1 [20] (NCT04639466, 2.5 × 108 pfu, two doses) and naïve HCW vaccinated with two doses of Comirnaty® [21] (City of Hope IRB#20720) were used as comparator. HV and HCW were enrolled between December 2020 and May 2021.
2.2. Binding IgG
To measure S-, receptor binding domain (RBD)-, and N-specific binding IgG, a quantitative ELISA calibrated with the WHO international standard serum was used as described previously [21]. Briefly, 1 μg/mL S, RBD, and N proteins (Sino Biological 40589-V08B1, 40592-V08H, 40588-V08B) in PBS were used to coat 96-well plates. After blocking in sample buffer (0.5% casein/154 mM NaCl/10 mM Tris-HCl/0.1% Tween-20 [pH 7.6]/8% Normal goat serum), 6-fold serial dilutions of serum in sample buffer were added to the plates. Plates were incubated for 2 h at 37 °C, after which anti-human IgG HRP secondary antibody (1:3000, BioRad, Hercules, CA, USA, 204005) in sample buffer was added for 1 h. WHO International reference panel (NIBSC, 20/268) assigned values were used to create a standard curve. For each sample, the first absorbance value to fall within the standard curve range was used to calculate the IgG titer and expressed as BAU/mL.
2.3. Neutralizing Antibodies
SARS-CoV-2-specific NAb against ancestral and variant viruses were measured by microneutralization assay using SARS-CoV-2 pseudovirus (PsV) [21]. Lentiviral-based PsV were produced using the pALD system (Aldevron, Fargo, ND, USA) and Spike expression plasmids with C-terminal 19-amino acid deletion (custom order, cloned into pTwist-CMV-BetaGlobin vector, Twist Biosciences, South San Francisco, CA, USA). Serial dilutions of serum starting from 1:20 were prepared in triplicate, mixed with PsV and added to poly-L-lysin-coated 96-well plates. Plates were incubated overnight at 4 °C after which 10,000 HEK293T-ACE2 cells [24] were added to each well in the presence of 3 μg/mL polybrene and incubated at 37 °C for 48 h. Luminescence was quantified using SpectraMax L (Molecular Devices, San Jose, CA, USA) after adding Luciferase Assay Reagent (Promega E1483).
2.4. ELISPOT
IFNγ/IL-4 secretion was evaluated on thawed PBMCs using the human IFNγ/IL-4 FluoroSpot FLEX kit (Mabtech, Nacka Strand, Sweden, X-01A16B) following manufacturer instructions. Briefly, 150,000 cells/well in CTL-test serum-free media (Immunospot CTLT-010, Immunospot, Shaker Heights, OH, USA) were added to duplicate wells and stimulated with S, N, and membrane (M) peptide pools (15-mers, 11 aa overlap, >70% purity; GenScript and in-house synthesis). The S peptide library was divided into 4 sub-pools spanning the S1 and S2 domains. Each peptide pool (2 μg/mL) and αCD28 (0.1 μg/mL, Mabtech) was added to the cells, and plates were incubated for 48 h at 37 °C. Primary and secondary antibodies were added according to the manufacturer’s protocol. Fluorescent spot forming units (SFU) were acquired using CTL S6 Fluorocore (Immunospot).
2.5. Activation-Induced Marker (AIM) Assay
S- and N-specific T-cell phenotype analysis was carried out using an AIM assay [25]. Briefly, 106 PBMCs were stimulated for 24 h with 1 µg/mL of either S-15mer megapool [25] or N peptide pool, in RPMI media with 5% human serum (BioIVT). DMSO (equimolar amount) and PHA (20 µg/mL) were used as negative and positive controls, respectively. Cells were stained at room temperature with live/dead, anti-CD19, anti-CD14, anti-CD3, anti-CD4, anti-CD8, anti-OX40, anti-CD137, anti-CD69, anti-CCR7, and anti-CD45RA (Table S5). After washing, cells were resuspended in PBS and acquired on the Attune NxT cytometer (Thermo-Fisher, Waltham, MA, USA). Data were analyzed with the FlowJo X software (v10.8). Gating strategy is shown in Figure S9.
2.6. Statistics
GraphPad Prism (v9.4.1) was used to calculate statistical power. Student’s t distribution with N–1 degrees of freedom was applied on log10-transformed responses to compare baseline values to post-vaccination timepoints. The two-tailed Mann–Whitney test was used to compare the immunogenicity of COH04S1 and Comirnaty®.
3. Results
A phase 2 randomized clinical trial investigating the safety and immunogenicity of COH04S1 compared to the FDA-approved mRNA vaccine Comirnaty® in allogeneic (allo) and autologous (auto) HCT recipients and CAR-T cell therapy patients is ongoing at City of Hope (NCT04977024). The FDA-required open-label safety lead-in segment was designed with six patients in each treatment group (auto-HCT, allo-HCT, and CAR-T in a 3 + 3 design). Six auto-HCT, six allo-HCT, and one CAR-T patient were enrolled and received two doses of COH04S1 on days 0 and 28. Due to changes in the CDC vaccination guidelines, a protocol amendment was subsequently submitted to allow for a third and fourth COH04S1 vaccine dose at days 56 and 140, respectively. Five out of thirteen patients received a third dose, and four of these received an additional fourth dose (Figure S1). Of the thirteen patients, nine (69%) received the first dose 3–6 months post therapy, and four (31%) received the first dose 6–12 months post therapy. Pre-treatment diagnosis was heterogeneous. Concomitant immunosuppressive or immunotherapeutic treatments were allowed, except for high dose corticosteroids (>0.5 mg/kg/day). Most patients reported receiving FDA-approved COVID-19 vaccines and/or monoclonal antibodies before HCT/CAR-T. Three patients were boosted with an FDA-approved COVID-19 vaccine after receiving COH04S1, and four COVID-19 asymptomatic/mild-symptomatic cases were reported during the trial. Those patients were subsequently removed from the immune analysis. Complete patient characteristics are presented in Table 1 and Table S1.

Table 1.
Total and grouped patient characteristics.
Pre-existing S- and RBD-specific binding antibodies were measured at baseline in most subjects. Vaccination with one and two doses of COH04S1 resulted in a significant increase in S- and RBD-specific IgG (0.0004 < p < 0.013), and elevated S- and RBD-specific IgG responses were maintained for up to six months (Figure 1A and Figure S2, and Table S2). N-specific IgG were low to absent at baseline and were significantly elevated after two COH04S1 vaccine doses (d56 p = 0.0159), after which they remained relatively stable up to day 180. Using a pseudoviral-based neutralization assay, elevated NAb responses against the Wuhan-Hu-1 strain, and the Beta and Delta variants were measured at baseline in most patients (NT50 GM 37, 31, and 43, respectively), while Omicron BA.1 and XBB.1.5 variant-specific NAb responses were low to absent in most patients (NT50 GM 10). A significant increase in SARS-CoV-2-specific NAb titers against all strains was observed after one dose (d28 0.008 < p < 0.0079), and these NAb titers further increased after the second dose (d56 0.0013 < p < 0.0023) (Figure 1B and Figure S3, and Table S2). In patients who received two doses and those receiving additional COH04S1 vaccine doses, NAb titers remained elevated for at least six months post vaccination. Importantly, cross-NAb responses against the highly NAb-evasive XBB.1.5 Omicron subvariant were measured in most patients after one and two doses (7/13 and 8/12, respectively), and in all patients after three or more COH04S1 doses (Figure 1B and Figure S4).
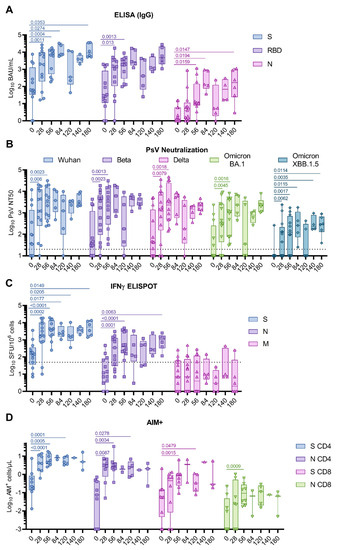
Figure 1.
COH04S1-elicited humoral and T cell immunity in cancer patients post hematopoietic cell transplantation and cellular therapy. Patients (n = 13) were vaccinated two to four times with COH04S1, and blood samples were evaluated for SARS-CoV-2-specific humoral and T cell immunity at the indicated timepoints. (A) Binding antibodies. Binding antibody titers to spike (S, circles), receptor-binding domain (RBD, squares), and nucleocapsid (N, triangles) antigens were measured with quantitative ELISA. (B) NAb responses. 50% neutralizing antibody titers (NT50) against ancestral SARS-CoV-2 (Wuhan-Hu-1, circles), Beta (squares), Delta (up-pointing triangles), and Omicron BA.1 (down-pointing triangles) and XBB.1.5 (diamonds) variants were measured using a pseudovirus (PsV) assay. Dotted lines represent the lower limit of detection. (C) IFNγ T cells. IFNγ T cells were quantified via ELISPOT following stimulation of PBMCs with S (circles), N (squares), and membrane (M, triangles) peptide libraries. Shown are the IFNγ spot forming units (SFU) measured in 106 PBMCs. Dotted line represents the arbitrary threshold for a positive response (50 SFU/106 PBMCs). (D) Activation-Induced Markers (AIM+) T cells. CD4+ and CD8+ AIM+ T cells per μL of blood were quantified via cytofluorimetry in PBMCs stimulated with S (CD4+ and CD8+ AIM+ T cells indicated as circles and up-pointing triangles, respectively) and N (CD4+ and CD8+ AIM+ T cells indicated as squares and down-pointing triangles, respectively) peptide libraries. Data are presented as box plots extending from 25th to 75th percentile, with lines indicating medians, and whiskers going from minimum to maximum values. Student’s t test on log10-transformed data was applied to compare baseline to post-vaccine geometric mean fold rise (GMFR) values. p values ≤ 0.05 are indicated. Where not indicated, p > 0.05.
S-specific IFNγ T cell responses at baseline were above the arbitrary threshold of positivity for most patients. In contrast, N- and M-specific T cell responses measured at baseline were low to absent in most patients. Vaccination with one or two doses of COH04S1 significantly increased S- and N-specific IFNγ T cell responses compared to baseline (0.0001 ≤ p < 0.0002), while it had no impact on M-specific T cell levels (Figure 1C and Figure S5, and Table S3). Significantly elevated S- and N-specific IFNγ T cells were measured up to six months post vaccination (d180 p = 0.0149 and 0.0063. Figure 1C). Although S- and N-specific IL-4 T cells significantly increased after COH04S1 vaccination (0.0068 < p < 0.04), they remained at low levels throughout the study, indicating a substantial Th1-biased cellular immune response (Figure S6). Interestingly, after vaccination with COH04S1, the only CAR-T cell patient of the study mounted a robust, albeit temporary, T cell response to S and N antigens despite the limited increase in SARS-CoV-2-specific humoral responses and the evident lack of response to a subsequent booster vaccination with Spikevax® (mRNA-1273, Moderna, Cambridge, MA, USA) (Figure S7). A similar increase in SARS-CoV-2-specific NAb, and S- and N-specific IFNγ T cell responses was measured in auto- and allo-transplant recipients, and in patients that underwent HCT/CAR-T 3 to 6 and 6 to 12 months prior to COH04S1 vaccination (Figure S8), indicative of an immune response to vaccination with COH04S1 that is independent from the type and time of transplant.
In a subset of samples, analysis of activation-induced markers (AIM) was performed with flow cytometry to assess broader antigen-specific T cell responses. Consistent with the measured IFNγ-specific T cell responses by ELISPOT (Figure 1C), a significant increase in S- and N-specific CD4+ T cells was observed after one and two COH04S1 vaccine doses (0.0001 ≤ p < 0.0034) (Figure 1D and Figure S9–S10, and Table S3). S- and N-specific CD8+ T cells significantly increased compared to baseline after two vaccine doses (p = 0.0015 and 0.0009). Further analysis of naïve/memory subtypes after two vaccine doses revealed a predominance of S- and N-specific CD4+ T cells with effector memory (>70%) and central memory (>20%) phenotype, while S- and N-specific CD8+ T cells were predominantly effector memory (>30% effector memory and >30% terminally differentiated effectors) (Figure S10).
To better estimate the magnitude of the vaccine-elicited immune responses, S- and N-specific responses elicited in COH04S1-vaccinated HCT/CAR-T patients were compared to those elicited in naïve HV vaccinated with COH04S1 [20] and to those elicited by Comirnaty® in naïve HCW [21] (Table S4). Baseline S-specific humoral responses measured prior to vaccination were significantly higher in COH04S1-vaccinated HCT/CAR-T patients than in COH04S1- or Comirnaty®-vaccinated naïve volunteers (0.0001 < p < 0.0157. Figure 2A). S-specific IgG titers remained significantly elevated in COH04S1-vaccinated HCT/CAR-T patients compared to COH04S1-vaccinated HV or Comirnaty®-vaccinated HCW at both one and six months post vaccination (0.0001 < p < 0.0033. Figure 2A). Baseline N-specific humoral responses were low to absent in all three vaccine cohorts and similar between COH04S1-vaccinated HCT/CAR-T patients and COH04S1- or Comirnaty-vaccinated naïve volunteers (Figure 2B). Vaccination with COH04S1 induced comparable N-specific IgG titers in HCT/CAR-T patients and HV, and resulted in significantly elevated N-specific IgG titers compared to those measured in Comirnaty®-vaccinated HCW (0.0001 < p < 0.0008. Figure 2B).
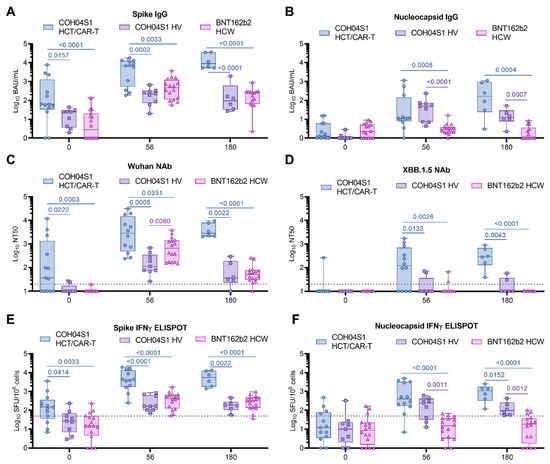
Figure 2.
Immunogenicity of COH04S1 in HCT/CAR-T patients and COH04S1 or Comirnaty® in healthy adults. SARS-CoV-2 binding antibodies (IgG) to SARS-CoV-2 spike (S, (A)), nucleocapsid (N, (B)), neutralizing antibody titers to SARS-CoV-2 Wuhan-Hu-1 ancestral strain (C), and XBB.1.5 variant (D), and IFNγ T cells specific for SARS-CoV-2 S (E) and N (F) were measured in samples of COH04S1-vaccinated HCT/CAR-T patients (circles) at baseline (n = 13), day 56 (n = 12), and 180 (n = 6) post vaccination, and compared to responses measured in healthy volunteers (HV, squares, d0-d56 n = 9, d180 n = 6) vaccinated with COH04S1 or healthcare workers (HCW, triangles, n = 17) vaccinated with the FDA-approved mRNA vaccine Comirnaty® at the same timepoints. Dotted lines in (C,D) represent the lower limit of detection. Dotted lines in (E,F) represent the arbitrary threshold of positivity (50 spots/106 cells). At the day 180 timepoint, 4/6 HCT/CAR-T patients had received three/four COH04S1 doses. Data are presented as box plots extending from 25th to 75th percentile, with lines indicating medians, and whiskers going from minimum to maximum values. Values were compared using the two-tailed Mann–Whitney test. p values ≤ 0.05 are indicated. Where not indicated, p > 0.05.
COH04S1-vaccinated HCT/CAR-T patients showed elevated Wuhan-specific NAb responses at baseline compared to COH04S1- or Comirnaty®-vaccinated naïve volunteers, while NAb titers against Omicron XBB.1.5 at baseline were below the limit of detection in all subjects in the three vaccine cohorts with the exception of one HCT patient (Figure 2C,D). After two vaccine doses, at one and at six months post vaccination, NAb titers measured in COH04S1-vaccinated patients were significantly higher than those measured in COH04S1-vaccinated HV or Comirnaty®-vaccinated HCW for all tested SARS-CoV-2 variants, including Omicron XBB.1.5 (0.0001 ≤ p < 0.0331. Figure 2C,D and Figure S11). Strikingly, while XBB.1.5-specific NAb titers were below the detection limit in most COH04S1- and Comirnaty®-vaccinated HV and HCW, COH04S1 vaccination of HCT/CAR-T patients resulted in Omicron XBB.1.5 median NT50 titers above 102. At peak response, SARS-CoV-2-specific NAb titers against all variants, except XBB.1.5, were consistently higher in Comirnaty®-vaccinated HCW than in COH04S1-vaccinated HV. In contrast, at six months post vaccination, NAb titers against all SARS-CoV-2 variants were similar between Comirnaty®-vaccinated HCW and COH04S1-vaccinated HV, albeit significantly lower than in COH04S1-vaccinated HCT/CAR-T patients (Figure S11).
Similar to the observed S-specific antibody and NAb responses, baseline S-specific T cells were significantly higher in COH04S1 HCT/CAR-T patients than in healthy adults vaccinated with COH04S1 or Comirnaty®, and they remained significantly higher after two vaccine doses, at both one and six months post vaccination (0.0001 < p < 0.0414. Figure 2E). No difference in baseline N-specific T cells was measured across cohorts, with most subjects showing IFNγ N-specific T cell levels below the threshold of positivity (Figure 2F). COH04S1 vaccination in both HCT/CAR-T patients and HV resulted in significantly increased N-specific T cells compared to Comirnaty®-vaccinated HCW at both one month and six months post vaccination (0.0001 ≤ p < 0.0012), with overall highest N-specific responses measured in COH04S1-vaccinated HCT/CAR-T patients (Figure 2F). M-specific T cells measured in both COH04S1 and Comirnaty® vaccinees were uniformly very low throughout the time course (Figure S11).
4. Discussion
Numerous studies have reported on the poor response rate and durability of the immune response to mRNA vaccination in HCT/CAR-T patients, which is further complicated in patients vaccinated less than one year post therapy [5,26]. Here we demonstrate that a heterogeneous group of HCT/CAR-T patients vaccinated with two or more doses of dual-antigen sMVA-vectored COH04S1 vaccine less than one year post treatment can mount robust humoral and cellular responses to S and N antigens, including NAb, with potent cross-reactivity against the SARS-CoV-2 ancestral virus and several VOC. In addition, NAb and T cell responses elicited in COH04S1-vaccinated HCT/CAR-T patients significantly exceed those measured in a cohort of COH04S1-vaccinated HV or HCW vaccinated with the FDA-approved mRNA vaccine Comirnaty®.
The dual antigen design differentiates COH04S1 from FDA-approved COVID-19 vaccines that exclusively utilize S as an immunogen. While S-elicited NAb are considered the principal immune correlate of protection, S and in particular its RBD are known as mutational hotspots allowing SARS-CoV-2 to evolve into new variants with the ability to escape protective NAb responses [27]. In contrast, the N protein is considered less susceptible than the S protein to evasion by humoral and cellular immune responses [27]. N-specific antibodies have been shown to contribute to NK cell activation [28] and to enhance control of SARS-CoV-2 through NK-mediated antibody-dependent cellular cytotoxicity (ADCC) [29,30]. Importantly, there is strong evidence in animal and human studies that N-specific T cells play a major role in protection from severe disease [31,32,33]. Therefore, use of a dual antigen vaccine based on S and N should be encouraged, especially in immunocompromised individuals with limited ability to mount robust humoral and cellular responses through vaccination.
While the emergence of Omicron may have resulted in a more favorable outcome in patients undergoing HCT/CAR-T [34], the more recent surges of further evolved Omicron subvariants BQ.1.1 and XBB have de facto eliminated the possibility of benefiting from monoclonal antibody treatments such as Evusheld® (tixagevimab/cilgavimab), which were extensively used in this patient population as a complement to vaccines and antivirals. Additionally, compared to previous variants, the appearance of Omicron has resulted in significantly increased breakthrough infections in boosted HCT/CAR-T patients [35]. There were four breakthrough cases of COVID-19 in our cohort (two in allo-HCT and two in auto-HCT patients), occurring during a time spanning consecutive Omicron waves, and it is encouraging that all these cases were asymptomatic/mild-symptomatic and did not require hospitalization.
While the auto-HCT patients had documented pre-transplant vaccination with FDA-approved vaccines, donor vaccination record for most allo-HCT patients was unavailable. However, it is likely that the majority of allo-HCT recipients were transplanted with a non-naïve donor graft, given that these transplants took place at a time when vaccination rates in the US and Europe reached up to 78% [36]. Multiple studies have shown that donor natural or vaccine-induced immunity can be transferred to the recipient and boosted via vaccination [37,38,39]. How COH04S1 compares to Comirnaty® in stimulating SARS-CoV-2-specific immunity in this heavily treated, likely non-naïve population is being evaluated in the randomized blinded portion of the trial.
Vaccine-elicited preexisting immunity to S in the graft likely contributed to the stimulation of the increased S-specific humoral and cellular immune responses observed in COH04S1-vaccinated HCT, especially when compared to healthy naïve adults vaccinated with COH04S1 or Comirnaty®. This indicates that COH04S1 can effectively restimulate donor-derived S-specific memory B and T cells most likely elicited through mRNA vaccination. In contrast, N-specific immunity at baseline was low to absent in most patients, suggesting that N-specific responses occurred most likely de novo and were solely due to post-transplant response to COH04S1 vaccination. Consistent with other studies on HCT patients, we observed more than 90% of the circulating specific CD4+ T cells displaying a memory phenotype after two COH04S1 vaccine doses [40]. This is important considering the role of vaccination-elicited memory T cell responses for protection against emerging SARS-CoV-2 VOC [41].
Most patients received the COH04S1 vaccine 3–6 months post therapy, at a time when the T cell compartment is not fully reconstituted [42] and a T cell response to SARS-CoV-2 vaccination is often not mounted [43]. The SARS-CoV-2-specific T cell responses in these vaccinated patients using the sMVA-based COH04S1 vaccine is consistent with our previous observations of elevated CMV-specific cellular responses in HCT recipients vaccinated post HCT with an MVA-based Triplex cytomegalovirus (CMV) vaccine [44]. These prior findings and our current observations with COH04S1 indicate that MVA-based vaccines are highly immunogenic in this patient population at early timepoints post transplant. These observations are noteworthy, as the FDA has chosen the configuration of the updated mRNA vaccine for the fall 2023 rollout. The fact that COH04S1 provides strong recognition of Omicron subvariants without updating, and the knowledge that it causes potent stimulation of immunity that exceeds what we documented in the HV cohort, portends continued efficacy as Omicron evolves. Since the T cell response to S is less susceptible to antibody resistance mutations, these patients might be equally or better served by receiving a booster of COH04S1 rather than updated mRNA boosters.
5. Conclusions
Despite the small and heterogeneous population evaluated in this study, the remarkably robust S- and N-specific humoral and cellular responses observed in COH04S1-vaccinated HCT/CAR-T patients are encouraging. These observations warrant further studies with COH04S1 in other immunocompromised patient populations that are known to respond poorly to vaccination with approved mRNA vaccines.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11091492/s1, Table S1: Patient characteristics and treatments. Table S2: Humoral response geometric mean and geometric mean fold increase by study day. Table S3: Cellular response geometric mean and geometric mean fold increase by study day. Table S4: Comparison of COH04S1 HCT/CAR-T patient population with healthy adults vaccinated with COH04S1 and Comirnaty®. Table S5: Flow Cytometry Reagents. Figure S1: Patients’ vaccination and sampling schedule. Figure S2: COH04S1 vaccine-elicited binding IgG in cancer patients post hematopoietic cell transplantation and cellular therapy. Figure S3: COH04S1 vaccine-elicited SARS-CoV-2-specific neutralizing antibodies (NAb) in cancer patients post hematopoietic cell transplantation and cellular therapy. Figure S4: Comparison of humoral and cellular responses elicited by 2 or 3/4 COH04S1 immunizations. Figure S5: COH04S1 vaccine-elicited cellular immunity in cancer patients post hematopoietic cell transplantation and cellular therapy. Figure S6: COH04S1-mediated cellular immunity in cancer patients post hematopoietic cell transplantation and cellular therapy. Figure S7: SARS-CoV-2-specific humoral and cellular immunity in CAR-T cell therapy patient COH206 vaccinated with two doses of COH04S1. Figure S8: Differential immunogenicity of two doses of COH04S1 at early and late timepoints post transplant and in auto-HCT and allo-HCT patients. Figure S9: Gating strategy for the evaluation of S- and N-specific T cells. Figure S10: AIM assay and memory phenotype. Figure S11: Immunogenicity of COH04S1 in HCT/CAR-T patients and COH04S1 or Comirnaty® in healthy adults.
Author Contributions
Study conceptualization: D.J.D. and S.T. Study design: S.T., S.S.D. and D.J.D. Immunological analysis: F.C., S.O.-F., C.L.R., J.L., M.L., K.F., J.M.-O., M.-A.G., F.W. and D.J.D. Sample processing: Q.Z., S.O.-F., K.F. and J.M.-O. Clinical PI: S.S.D. Patients oversight: S.S.D., B.B., H.A., A.S. (Amandeep Salhotra), A.S. (Anthony Stein), N.N., M.R., L.N., M.M.A.M., J.D. (Jana Dickter), D.D.N., A.P. (Alfredo Puing), S.J.F., R.A.T., J.A.Z. and R.N. Study data collection and participant oversight: J.D. (Jennifer Drake), P.F. and C.S. Statistical analysis: W.R. and F.C. Immunological analysis support: T.K., A.G. and A.S. (Alessandro Sette). Laboratory support: A.P. (Angela Patterson) and S.D. Manuscript writing: F.C., F.W., S.O.-F. and D.J.D. COH04S1 conceptualization and development: F.W., F.C. and D.J.D. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by the Carol Moss Foundation, donors Julie and Roger Baskes, Judd Malkin and Michael Zweig.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by Advarra (Columbia, MD, USA) Institutional Review Board (protocol 21163, approved on 27 April 2021). This study was conducted at City of Hope Medical Center between September 2021 and September 2022. Clinical Trials Registration: NCT04977024.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and/or its Supplementary Materials.
Acknowledgments
The authors would like to thank all the patients who volunteered in the study and all the investigators and study site personnel who assisted in the clinical trial completion. We would like to thank Melinda Ji for her involvement. We acknowledge and thank Christoph Pittius and Yuriy Shostak (Research Business Development, City of Hope) for excellent project management.
Conflicts of Interest
While unknown whether publication of this report will aid in receiving grants and contracts, it is possible that this publication will be of benefit to City of Hope (COH). COH had no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript. D.J.D. and F.W. are co-inventors on a patent application covering the design and construction of the synthetic MVA platform (PCT/US2021/016247). D.J.D., F.W., and F.C. are co-inventors on a patent application covering the development of a COVID-19 vaccine (PCT/US2021/032821). D.J.D. is a consultant for GeoVax. A.S. is a consultant for AstraZeneca Pharmaceuticals, Calyptus Pharmaceuticals, Inc, Darwin Health, EmerVax, EUROIMMUN, F. Hoffman-La Roche Ltd., Fortress Biotech, Gilead Sciences, Gritstone Oncology, Guggenheim Securities, Moderna, Pfizer, RiverVest Venture Partners, and Turnstone Biologics. LJI has filed for patent protection for various aspects of T cell epitope and vaccine design work. A.G. is a consultant for Pfizer. All other authors declare no competing interests. GeoVax Labs Inc. has taken a worldwide exclusive license for COH04S1 under the name of GEO-CM04S1.
References
- Spanjaart, A.M.; Ljungman, P.; de La Camara, R.; Tridello, G.; Ortiz-Maldonado, V.; Urbano-Ispizua, A.; Barba, P.; Kwon, M.; Caballero, D.; Sesques, P.; et al. Poor outcome of patients with COVID-19 after CAR T-cell therapy for B-cell malignancies: Results of a multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) Infectious Diseases Working Party and the European Hematology Association (EHA) Lymphoma Group. Leukemia 2021, 35, 3585–3588. [Google Scholar] [CrossRef]
- Varma, A.; Kosuri, S.; Ustun, C.; Ibrahim, U.; Moreira, J.; Bishop, M.R.; Nathan, S.; Mehta, J.; Moncayo, D.; Heng, J.; et al. COVID-19 infection in hematopoietic cell transplantation: Age, time from transplant and steroids matter. Leukemia 2020, 34, 2809–2812. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bhatt, N.S.; St Martin, A.; Abid, M.B.; Bloomquist, J.; Chemaly, R.F.; Dandoy, C.; Gauthier, J.; Gowda, L.; Perales, M.A.; et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: An observational cohort study. Lancet Haematol. 2021, 8, e185–e193. [Google Scholar] [CrossRef]
- Maillard, A.; Redjoul, R.; Klemencie, M.; Labussiere Wallet, H.; Le Bourgeois, A.; D’Aveni, M.; Huynh, A.; Berceanu, A.; Marchand, T.; Chantepie, S.; et al. Antibody response after 2 and 3 doses of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic cell transplant recipients. Blood 2022, 139, 134–137. [Google Scholar] [CrossRef]
- Ni, B.; Yanis, A.; Dee, K.; Chappell, J.D.; Dulek, D.E.; Kassim, A.A.; Kitko, C.L.; Thomas, L.D.; Halasa, N. SARS-CoV-2 vaccine safety and immunogenicity in patients with hematologic malignancies, transplantation, and cellular therapies. Blood Rev. 2022, 56, 100984. [Google Scholar] [CrossRef]
- Lindemann, M.; Klisanin, V.; Thummler, L.; Fisenkci, N.; Tsachakis-Muck, N.; Ditschkowski, M.; Schwarzkopf, S.; Klump, H.; Reinhardt, H.C.; Horn, P.A.; et al. Humoral and Cellular Vaccination Responses against SARS-CoV-2 in Hematopoietic Stem Cell Transplant Recipients. Vaccines 2021, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, F.; Papanicolaou, G.; Dadwal, S.; Pergam, S.A.; Wingard, J.R.; Boghdadly, Z.E.; Abidi, M.Z.; Waghmare, A.; Shahid, Z.; Michaels, L.; et al. Frequently Asked Questions on Coronavirus Disease 2019 Vaccination for Hematopoietic Cell Transplantation and Chimeric Antigen Receptor T-Cell Recipients from the American Society for Transplantation and Cellular Therapy and the American Society of Hematology. Transpl. Cell Ther. 2023, 29, 10–18. [Google Scholar] [CrossRef]
- Barnes, E.; Goodyear, C.S.; Willicombe, M.; Gaskell, C.; Siebert, S.; de Silva, T., I; Murray, S.M.; Rea, D.; Snowden, J.A.; Carroll, M.; et al. SARS-CoV-2-specific immune responses and clinical outcomes after COVID-19 vaccination in patients with immune-suppressive disease. Nat. Med. 2023, 29, 1760–1774. [Google Scholar] [CrossRef] [PubMed]
- Bordat, J.; Maury, S.; Leclerc, M. Allogeneic hematopoietic stem cell transplantation in the COVID-19 era. Front. Immunol. 2023, 14, 1100468. [Google Scholar] [CrossRef]
- Abid, M.B.; Rubin, M.; Ledeboer, N.; Szabo, A.; Longo, W.; Mohan, M.; Shah, N.N.; Fenske, T.S.; Abedin, S.; Runaas, L.; et al. Efficacy of a third SARS-CoV-2 mRNA vaccine dose among hematopoietic cell transplantation, CAR T cell, and BiTE recipients. Cancer Cell 2022, 40, 340–342. [Google Scholar] [CrossRef]
- Henig, I.; Isenberg, J.; Yehudai-Ofir, D.; Leiba, R.; Ringelstein-Harlev, S.; Ram, R.; Avni, B.; Amit, O.; Grisariu, S.; Azoulay, T.; et al. Third BNT162b2 mRNA SARS-CoV-2 Vaccine Dose Significantly Enhances Immunogenicity in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation. Vaccines 2023, 11, 775. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Bergman, P.; Blennow, O.; Hansson, L.; Mielke, S.; Nowak, P.; Soderdahl, G.; Osterborg, A.; Smith, C.I.E.; Vesterbacka, J.; et al. Real-world assessment of immunogenicity in immunocompromised individuals following SARS-CoV-2 mRNA vaccination: A one-year follow-up of the prospective clinical trial COVAXID. EBioMedicine 2023, 94, 104700. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.R.; Sekine, T.; Trubach, D.; Niessl, J.; Chen, P.; Bergman, P.; Blennow, O.; Hansson, L.; Mielke, S.; Nowak, P.; et al. Additive effects of booster mRNA vaccination and SARS-CoV-2 Omicron infection on T cell immunity across immunocompromised states. Sci. Transl. Med. 2023, 15, eadg9452. [Google Scholar] [CrossRef]
- Solera, J.T.; Ierullo, M.; Arbol, B.G.; Mavandadnejad, F.; Kurtesi, A.; Qi, F.; Hu, Q.; Gingras, A.C.; Ferreira, V.H.; Humar, A.; et al. Bivalent COVID-19 mRNA vaccine against omicron subvariants in immunocompromised patients. Lancet Infect. Dis. 2023, 23, E266–E267. [Google Scholar] [CrossRef] [PubMed]
- Bankova, A.K.; Pasin, C.; Huang, A.; Cicin-Sain, C.; Epp, S.; Audige, A.; Mueller, N.J.; Nilsson, J.; Vilinovszki, O.; Nair, G.; et al. Antibody response to a third SARS-CoV-2 vaccine dose in recipients of an allogeneic haematopoietic cell transplantation. Br. J. Haematol. 2023, 201, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Majcherek, M.; Matkowska-Kocjan, A.; Szymczak, D.; Karasek, M.; Szeremet, A.; Kiraga, A.; Milanowska, A.; Kuznik, E.; Kujawa, K.; Wrobel, T.; et al. Two Doses of BNT162b2 mRNA Vaccine in Patients after Hematopoietic Stem Cell Transplantation: Humoral Response and Serological Conversion Predictors. Cancers 2022, 14, 325. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.; Ihorst, G.; Bartsch, I.; Zeiser, R.; Wasch, R.; Bertz, H.; Finke, J.; Huzly, D.; Wehr, C. Cellular and Humoral SARS-CoV-2 Vaccination Responses in 192 Adult Recipients of Allogeneic Hematopoietic Cell Transplantation. Vaccines 2022, 10, 1782. [Google Scholar] [CrossRef]
- Chiuppesi, F.; Salazar, M.D.; Contreras, H.; Nguyen, V.H.; Martinez, J.; Park, Y.; Nguyen, J.; Kha, M.; Iniguez, A.; Zhou, Q.; et al. Development of a multi-antigenic SARS-CoV-2 vaccine candidate using a synthetic poxvirus platform. Nat. Commun. 2020, 11, 6121. [Google Scholar] [CrossRef] [PubMed]
- Chiuppesi, F.; Nguyen, V.H.; Park, Y.; Contreras, H.; Karpinski, V.; Faircloth, K.; Nguyen, J.; Kha, M.; Johnson, D.; Martinez, J.; et al. Synthetic multiantigen MVA vaccine COH04S1 protects against SARS-CoV-2 in Syrian hamsters and non-human primates. npj Vaccines 2022, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Chiuppesi, F.; Zaia, J.A.; Frankel, P.H.; Stan, R.; Drake, J.; Williams, B.; Acosta, A.M.; Francis, K.; Taplitz, R.A.; Dickter, J.K.; et al. Safety and immunogenicity of a synthetic multiantigen modified vaccinia virus Ankara-based COVID-19 vaccine (COH04S1): An open-label and randomised, phase 1 trial. Lancet Microbe 2022, 3, E252–E264. [Google Scholar] [CrossRef]
- Chiuppesi, F.; Zaia, J.A.; Faircloth, K.; Johnson, D.; Ly, M.; Karpinski, V.; La Rosa, C.; Drake, J.; Marcia, J.; Acosta, A.M.; et al. Vaccine-induced spike- and nucleocapsid-specific cellular responses maintain potent cross-reactivity to SARS-CoV-2 Delta and Omicron variants. iScience 2022, 25, 104745. [Google Scholar] [CrossRef]
- Wussow, F.; Kha, M.; Faircloth, K.; Nguyen, V.H.; Iniguez, A.; Martinez, J.; Park, Y.; Nguyen, J.; Kar, S.; Andersen, H.; et al. COH04S1 and Beta Sequence Modified Vaccine Protect Hamsters From SARS-CoV-2 Variants. iScience 2022, 25, 104457. [Google Scholar] [CrossRef]
- Wussow, F.; Kha, M.; Kim, T.; Ly, M.; Yll-Pico, M.; Kar, S.; Lewis, M.G.; Chiuppesi, F.; Diamond, D.J. Synthetic multiantigen MVA vaccine COH04S1 and variant-specific derivatives protect Syrian hamsters from SARS-CoV-2 Omicron subvariants. npj Vaccines 2023, 8, 41. [Google Scholar] [CrossRef]
- Crawford, K.H.D.; Eguia, R.; Dingens, A.S.; Loes, A.N.; Malone, K.D.; Wolf, C.R.; Chu, H.Y.; Tortorici, M.A.; Veesler, D.; Murphy, M.; et al. Protocol and Reagents for Pseudotyping Lentiviral Particles with SARS-CoV-2 Spike Protein for Neutralization Assays. Viruses 2020, 12, 513. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e1415. [Google Scholar] [CrossRef] [PubMed]
- Chaekal, O.K.; Gomez-Arteaga, A.; Chen, Z.; Soave, R.; Shore, T.; Mayer, S.; Phillips, A.; Hsu, J.M.; Drelick, A.; Kodiyanplakkal, R.P.L.; et al. Predictors of Covid-19 Vaccination Response after In-Vivo T-Cell-Depleted Stem Cell Transplantation. Transpl. Cell Ther. 2022, 28, 618.e1–618.e10. [Google Scholar] [CrossRef]
- Carabelli, A.M.; Peacock, T.P.; Thorne, L.G.; Harvey, W.T.; Hughes, J.; Consortium, C.-G.U.; Peacock, S.J.; Barclay, W.S.; de Silva, T.I.; Towers, G.J.; et al. SARS-CoV-2 variant biology: Immune escape, transmission and fitness. Nat. Rev. Microbiol. 2023, 21, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Fielding, C.A.; Sabberwal, P.; Williamson, J.C.; Greenwood, E.J.D.; Crozier, T.W.M.; Zelek, W.; Seow, J.; Graham, C.; Huettner, I.; Edgeworth, J.D.; et al. SARS-CoV-2 host-shutoff impacts innate NK cell functions, but antibody-dependent NK activity is strongly activated through non-spike antibodies. eLife 2022, 11, e74489. [Google Scholar] [CrossRef] [PubMed]
- Dangi, T.; Sanchez, S.; Class, J.; Richner, M.; Visvabharathy, L.; Chung, Y.R.; Bentley, K.; Stanton, R.J.; Koralnik, I.J.; Richner, J.M.; et al. Improved control of SARS-CoV-2 by treatment with a nucleocapsid-specific monoclonal antibody. J. Clin. Investig. 2022, 132. [Google Scholar] [CrossRef]
- Lopez-Munoz, A.D.; Kosik, I.; Holly, J.; Yewdell, J.W. Cell surface SARS-CoV-2 nucleocapsid protein modulates innate and adaptive immunity. Sci. Adv. 2022, 8, eabp9770. [Google Scholar] [CrossRef]
- Matchett, W.E.; Joag, V.; Stolley, J.M.; Shepherd, F.K.; Quarnstrom, C.F.; Mickelson, C.K.; Wijeyesinghe, S.; Soerens, A.G.; Becker, S.; Thiede, J.M.; et al. Cutting Edge: Nucleocapsid Vaccine Elicits Spike-Independent SARS-CoV-2 Protective Immunity. J. Immunol. 2021, 207, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Hajnik, R.L.; Plante, J.A.; Liang, Y.; Alameh, M.G.; Tang, J.; Bonam, S.R.; Zhong, C.; Adam, A.; Scharton, D.; Rafael, G.H.; et al. Dual spike and nucleocapsid mRNA vaccination confer protection against SARS-CoV-2 Omicron and Delta variants in preclinical models. Sci. Transl. Med. 2022, 14, eabq1945. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Felce, S.L.; Dong, D.; Penkava, F.; Mentzer, A.J.; Yao, X.; Liu, G.; Yin, Z.; Chen, J.L.; Lu, Y.; et al. An immunodominant NP(105–113)-B*07:02 cytotoxic T cell response controls viral replication and is associated with less severe COVID-19 disease. Nat. Immunol. 2022, 23, 50–61. [Google Scholar] [CrossRef]
- Tan, J.Y.; Wee, L.E.; Tan, Y.H.; Conceicao, E.P.; Lim, F.W.I.; Chen, Y.; Than, H.; Quek, J.K.S.; Nagarajan, C.; Goh, Y.T.; et al. Favorable outcomes of COVID-19 in vaccinated hematopoietic stem cell transplant recipients: A single-center experience. Transpl. Infect. Dis. 2023, 25, e14024. [Google Scholar] [CrossRef]
- Pinana, J.L.; Martino, R.; Vazquez, L.; Lopez-Corral, L.; Perez, A.; Chorao, P.; Avendano-Pita, A.; Pascual, M.J.; Sanchez-Salinas, A.; Sanz-Linares, G.; et al. SARS-CoV-2-reactive antibody waning, booster effect and breakthrough SARS-CoV-2 infection in hematopoietic stem cell transplant and cell therapy recipients at one year after vaccination. Bone Marrow Transpl. 2023, 58, 567–580. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID Data Tracker. 2023. Available online: https://covid.cdc.gov/covid-data-tracker (accessed on 1 June 2023).
- Leclerc, M.; Redjoul, R.; Le Bouter, A.; Beckerich, F.; Robin, C.; Parinet, V.; Pautas, C.; Menouche, D.; Bouledroua, S.; Roy, L.; et al. Impact of donor vaccination on recipient response to early SARS-CoV-2 mRNA vaccination after allogeneic HSCT. Lancet Haematol. 2022, 9, e318–e321. [Google Scholar] [CrossRef]
- La Rosa, C.; Chiuppesi, F.; Park, Y.; Zhou, Q.; Yang, D.; Gendzekhadze, K.; Ly, M.; Li, J.; Kaltcheva, T.; Ortega Francisco, S.; et al. Functional SARS-CoV-2-specific T cells of donor origin in allogeneic stem cell transplant recipients of a T-cell-replete infusion: A prospective observational study. Front. Immunol. 2023, 14, 1114131. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, C.; Aldoss, I.; Park, Y.; Yang, D.; Zhou, Q.; Gendzekhadze, K.; Kaltcheva, T.; Rida, W.; Dempsey, S.; Arslan, S.; et al. Hematopoietic stem cell donor vaccination with cytomegalovirus triplex augments frequencies of functional and durable cytomegalovirus-specific T cells in the recipient: A novel strategy to limit antiviral prophylaxis. Am. J. Hematol. 2023, 98, 588–597. [Google Scholar] [CrossRef]
- Harrington, P.; Doores, K.J.; Saha, C.; Saunders, J.; Child, F.; Dillon, R.; Saglam, S.; Raj, K.; McLornan, D.; Avenoso, D.; et al. Repeated vaccination against SARS-CoV-2 elicits robust polyfunctional T cell response in allogeneic stem cell transplantation recipients. Cancer Cell 2021, 39, 1448–1449. [Google Scholar] [CrossRef]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, P847–P859. [Google Scholar] [CrossRef]
- Velardi, E.; Tsai, J.J.; van den Brink, M.R.M. T cell regeneration after immunological injury. Nat. Rev. Immunol. 2021, 21, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Clemenceau, B.; Guillaume, T.; Coste-Burel, M.; Peterlin, P.; Garnier, A.; Le Bourgeois, A.; Jullien, M.; Ollier, J.; Grain, A.; Bene, M.C.; et al. SARS-CoV-2 T-Cell Responses in Allogeneic Hematopoietic Stem Cell Recipients following Two Doses of BNT162b2 mRNA Vaccine. Vaccines 2022, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Aldoss, I.; La Rosa, C.; Baden, L.R.; Longmate, J.; Ariza-Heredia, E.J.; Rida, W.N.; Lingaraju, C.R.; Zhou, Q.; Martinez, J.; Kaltcheva, T.; et al. Poxvirus Vectored Cytomegalovirus Vaccine to Prevent Cytomegalovirus Viremia in Transplant Recipients: A Phase 2, Randomized Clinical Trial. Ann. Intern. Med. 2020, 172, 306–316. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).