The Effectiveness of Paracetamol to Reduce the Post-Vaccination SARS-CoV-2 Adverse Effects in an Italian Vaccination Center
Abstract
:1. Introduction
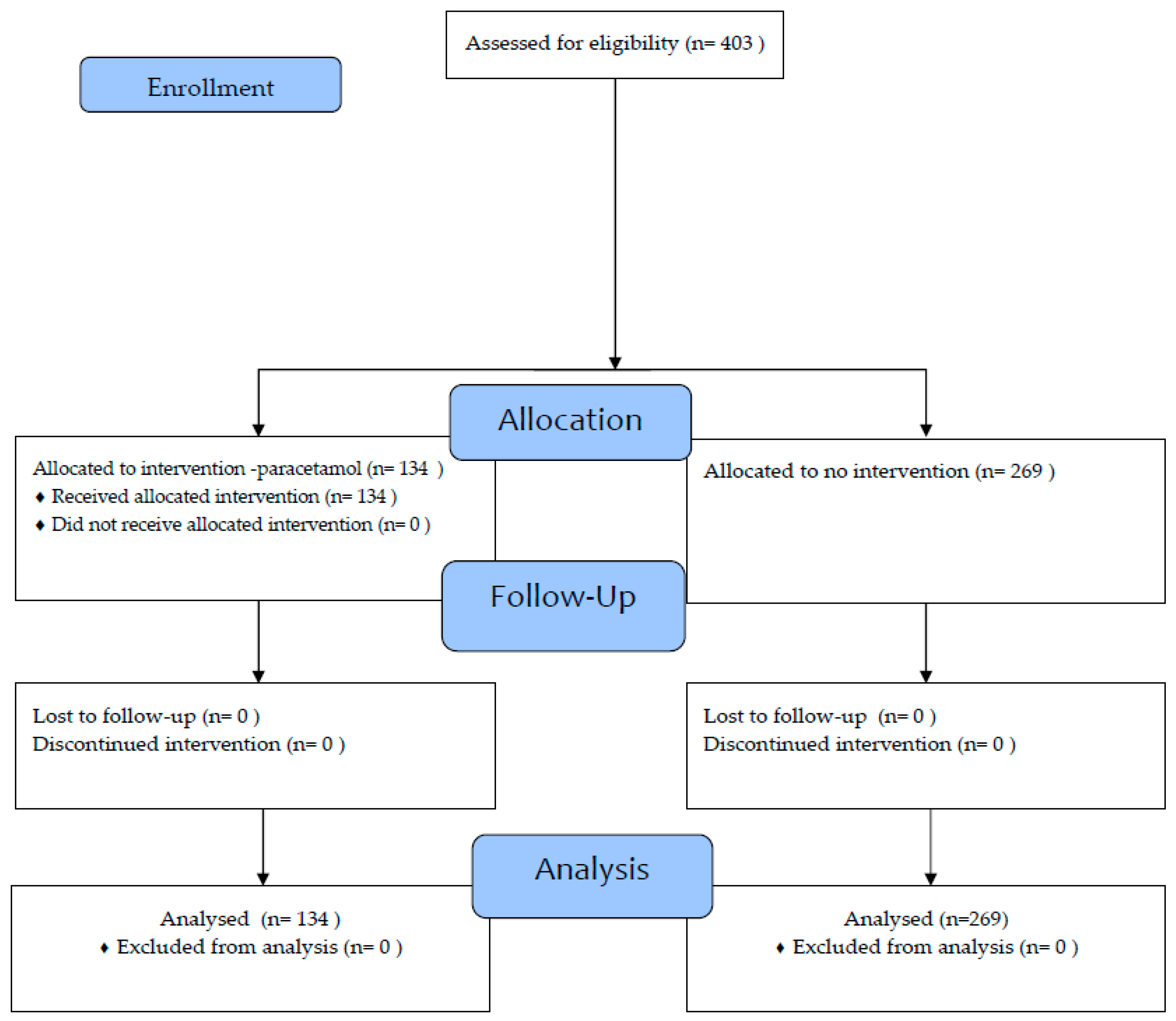
2. Materials and Methods
2.1. Study Design and Setting
2.2. Statistical Analysis
3. Results
3.1. First Administration
3.2. Second Administration
3.3. Multivariate Analyses
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- CDC COVID Data Tracker. Centers for Disease Control and Prevention. Available online: https://covid.cdc.gov/coviddata-tracker (accessed on 1 August 2023).
- EpiCentro COVID-19 Integrated Surveillance: Key National Data. Available online: https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-integrated-surveillance-data (accessed on 1 August 2023).
- Lopez Bernal, J.; Andrews, N.; Gower, C.; Gallagher, E.; Simmons, R.; Thelwall, S.; Stowe, J.; Tessier, E.; Groves, N.; Dabrera, G.; et al. Effectiveness of COVID-19 Vaccines against the B.1.617.2 (Delta) Variant. N. Engl. J. Med. 2021, 385, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine Side-Effects and SARS-CoV-2 Infection after Vaccination in Users of the COVID Symptom Study App in the UK: A Prospective Observational Study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Kazama, I. Targeting Lymphocyte Kv1.3-Channels to Suppress Cytokine Storm in Severe COVID-19: Can It Be a Novel Therapeutic Strategy? Drug Discov. Ther. 2020, 14, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Kazama, I.; Senzaki, M. Does Immunosuppressive Property of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) Reduce COVID-19 Vaccine-Induced Systemic Side Effects? Drug Discov. Ther. 2021, 15, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Aoki, F.Y.; Yassi, A.; Cheang, M.; Murdzak, C.; Hammond, G.W.; Seklà, L.H.; Wright, B. Effects of Acetaminophen on Adverse Effects of Influenza Vaccination in Health Care Workers. CMAJ Can. Med. Assoc. J. J. Assoc. Medicale Can. 1993, 149, 1425–1430. [Google Scholar]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and Immunogenicity of the ChAdOx1 NCoV-19 Vaccine against SARS-CoV-2: A Preliminary Report of a Phase 1/2, Single-Blind, Randomised Controlled Trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Choi, S.-H.; Chung, J.-W.; Hwang, M.-H.; Kim, M.-C. Systemic Adverse Events and Use of Antipyretics Predict the Neutralizing Antibody Positivity Early after the First Dose of ChAdOx1 Coronavirus Disease Vaccine. J. Clin. Med. 2021, 10, 2844. [Google Scholar] [CrossRef] [PubMed]
- Koufoglou, E.; Kourlaba, G.; Michos, A. Effect of Prophylactic Administration of Antipyretics on the Immune Response to Pneumococcal Conjugate Vaccines in Children: A Systematic Review. Pneumonia 2021, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Prymula, R.; Siegrist, C.-A.; Chlibek, R.; Zemlickova, H.; Vackova, M.; Smetana, J.; Lommel, P.; Kaliskova, E.; Borys, D.; Schuerman, L. Effect of Prophylactic Paracetamol Administration at Time of Vaccination on Febrile Reactions and Antibody Responses in Children: Two Open-Label, Randomised Controlled Trials. Lancet 2009, 374, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Doedée, A.M.C.M.; Boland, G.J.; Pennings, J.L.A.; de Klerk, A.; Berbers, G.A.M.; van der Klis, F.R.M.; de Melker, H.E.; van Loveren, H.; Janssen, R. Effects of Prophylactic and Therapeutic Paracetamol Treatment during Vaccination on Hepatitis B Antibody Levels in Adults: Two Open-Label, Randomized Controlled Trials. PLoS ONE 2014, 9, e98175. [Google Scholar] [CrossRef] [PubMed]
- Graham, G.G.; Scott, K.F.; Day, R.O. Tolerability of Paracetamol. Drug Saf. 2005, 28, 227–240. [Google Scholar] [CrossRef] [PubMed]
| Variables | n (%) or Median (Range) |
|---|---|
| Gender | |
| Female | 214 (53.1%) |
| Male | 189 (46.9%) |
| Age (years) | 49.7 (18.5–103) |
| Role | |
| Nurses | 107 (26.6%) |
| Physicians | 60 (14.9%) |
| Other healthcare professionals | 22 (5.5%) |
| Administratives | 53 (13.2%) |
| Other | 76 (19.9%) |
| Health Care Assistant | 41 (13.2%) |
| Physiotherapists | 44 (10.9%) |
| Symptoms | Paracetamol Yes n (%) n Tot = 134 | Paracetamol NO n (%) n Tot = 269 | p |
|---|---|---|---|
| Fever | |||
| No | 122 (91%) | 244 (90.7%) | 0.912 |
| Yes | 12 (9.0%) | 25 (9.3%) | |
| Headache | |||
| No | 111 (82.8%) | 218 (81%) | 0.661 |
| Yes | 23 (17.2%) | 51 (19%) | |
| Localized pain | |||
| No | 59 (44.0%) | 124 (46.1%) | 0.695 |
| Yes | 75 (56.0%) | 145 (53.9%) | |
| Asthenia | |||
| No | 117 (87.3%) | 10 (76.6%) | 0.011 |
| Yes | 17 (12.7%) | 63 (23.4%) | |
| Number of Symptoms | 1 (0–4) | 1 (0.4) | 0.314 |
| Symptoms | Paracetamol Yes n (%) n Tot = 134 | Paracetamol NO n (%) n Tot = 269 | p |
|---|---|---|---|
| Fever | |||
| No | 138 (85.2%) | 183 (80.3%) | 0.209 |
| Yes | 24 (14.8%) | 45 (19.7%) | |
| Headache | |||
| No | 128 (79.5%) | 0.209 | 0.400 |
| Yes | 33 (20.5%) | 55 (24.1%) | |
| Localized pain | |||
| No | 68 (42.0%) | 87 (38.2%) | 0.448 |
| Yes | 94 (58.0%) | 141(61.8%) | |
| Asthenia | |||
| No | 127 (78.4%) | 119 (52.2%) | 0.001 |
| Yes | 35 (21.6%) | 109 (47.8%) | |
| Number of Symptoms | 1 (0–4) | 2 (0–4) | 0.001 |
| Outcome Variables | Paracetamol 1 OR (95% CI) | Paracetamol 2 OR (95% CI) |
|---|---|---|
| Fever | 0.845 (0.55–1.31) | 0.72 (0.42–1.24) |
| Headache | 0.92 (0.64–1.33) | 0.86 (0.52–1.41) |
| Localized pain | 1.09 (0.80–1.47) | 0.92 (0.60–1.40) |
| Asthenia | 0.42 (0.29–0.60) | 0.30 (0.18–0.48) |
| Time 1 β (p) | Time 2 β (p) | |
|---|---|---|
| Paracetamol | −0.026 (0.596) | −0.146 (0.003) |
| R2 of the model | 0.059 | 0.105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, E.; Glavasc, A.; Morandini, B.; Grassi, M.C.; La Torre, G. The Effectiveness of Paracetamol to Reduce the Post-Vaccination SARS-CoV-2 Adverse Effects in an Italian Vaccination Center. Vaccines 2023, 11, 1493. https://doi.org/10.3390/vaccines11091493
Ricci E, Glavasc A, Morandini B, Grassi MC, La Torre G. The Effectiveness of Paracetamol to Reduce the Post-Vaccination SARS-CoV-2 Adverse Effects in an Italian Vaccination Center. Vaccines. 2023; 11(9):1493. https://doi.org/10.3390/vaccines11091493
Chicago/Turabian StyleRicci, Eleonora, Anamaria Glavasc, Barbara Morandini, Maria Caterina Grassi, and Giuseppe La Torre. 2023. "The Effectiveness of Paracetamol to Reduce the Post-Vaccination SARS-CoV-2 Adverse Effects in an Italian Vaccination Center" Vaccines 11, no. 9: 1493. https://doi.org/10.3390/vaccines11091493
APA StyleRicci, E., Glavasc, A., Morandini, B., Grassi, M. C., & La Torre, G. (2023). The Effectiveness of Paracetamol to Reduce the Post-Vaccination SARS-CoV-2 Adverse Effects in an Italian Vaccination Center. Vaccines, 11(9), 1493. https://doi.org/10.3390/vaccines11091493







