HPV Vaccination in Immunosuppressed Patients with Established Skin Warts and Non-Melanoma Skin Cancer: A Single-Institutional Cohort Study
Abstract
:1. Introduction
2. Methods
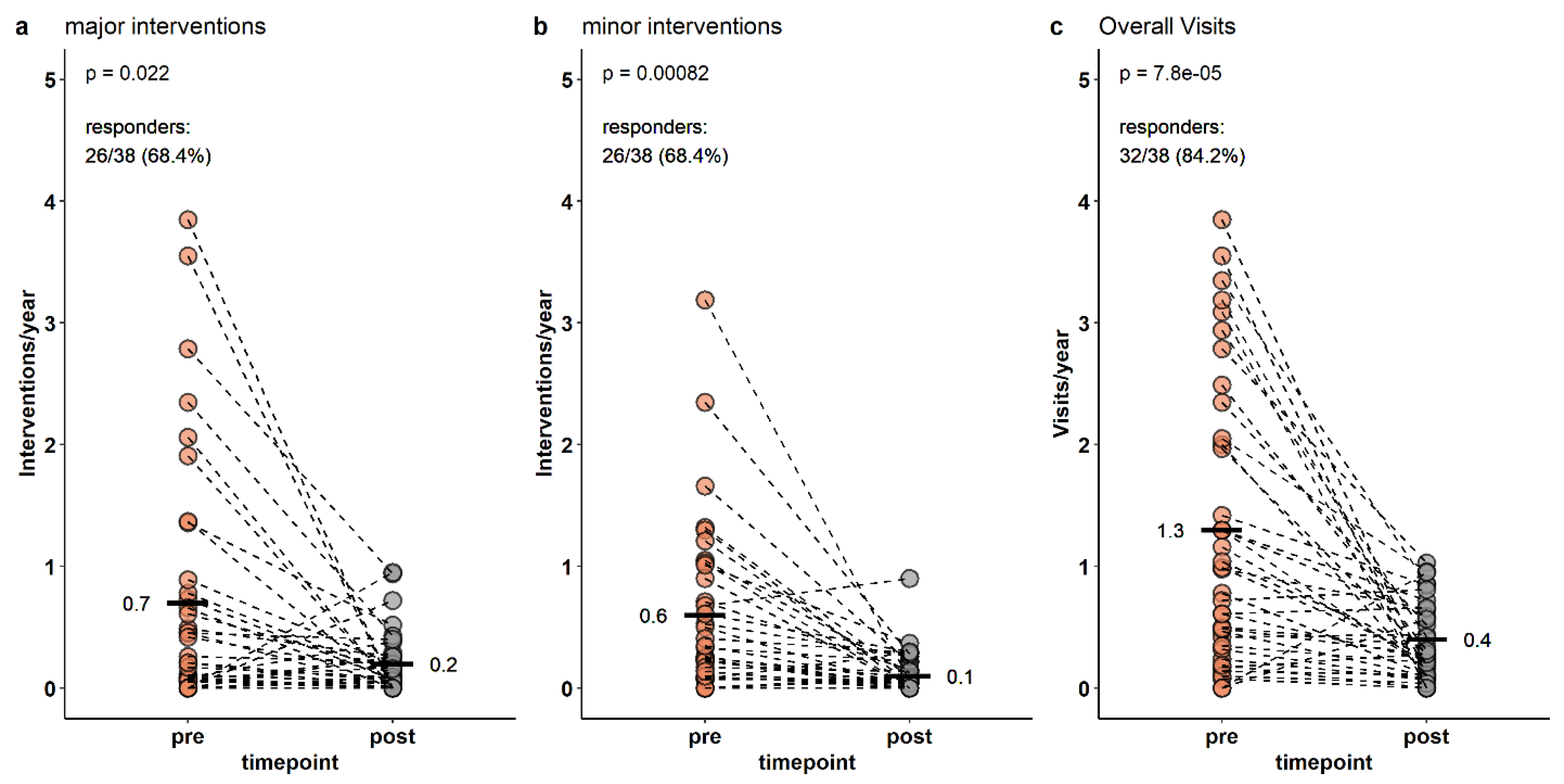
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madeleine, M.M.; Patel, N.S.; Plasmeijer, E.I.; Engels, E.; Bavinck, J.B.; Toland, A.; Green, A. Epidemiology of keratinocyte carcinomas after organ transplantation. Br. J. Dermatol. 2017, 177, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Euvrard, S.; Kanitakis, J.; Claudy, A. Skin cancers after organ transplantation. N. Engl. J. Med. 2003, 348, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Euvrard, S.; Chardonnet, Y.; Pouteil-Noble, C.; Kanitakis, J.; Chignol, M.C.; Thivolet, J.; Touraine, J.L. Association of skin malignancies with various and multiple carcinogenic and noncarcinogenic human papillomaviruses in renal transplant recipients. Cancer 1993, 72, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Nindl, I.; Rösl, F. Molecular concepts of virus infections causing skin cancer in organ transplant recipients. Am. J. Transplant. 2008, 8, 2199–2204. [Google Scholar] [CrossRef]
- de Jong-Tieben, L.M.; Berkhout, R.J.; ter Schegget, J.; Vermeer, B.J.; de Fijter, J.W.; Bruijn, J.A.; Westendorp, R.G.J.; Bavinck, J.N.B. The prevalence of human papillomavirus DNA in benign keratotic skin lesions of renal transplant recipients with and without a history of skin cancer is equally high: A clinical study to assess risk factors for keratotic skin lesions and skin cancer. Transplantation 2000, 69, 44–49. [Google Scholar] [CrossRef]
- Harwood, C.A.; Surentheran, T.; McGregor, J.M.; Spink, P.J.; Leigh, I.M.; Breuer, J.; Proby, C.M. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J. Med. Virol. 2000, 61, 289–297. [Google Scholar] [CrossRef]
- Wang, J.; Aldabagh, B.; Yu, J.; Arron, S.T. Role of human papillomavirus in cutaneous squamous cell carcinoma: A meta-analysis. J. Am. Acad. Dermatol. 2014, 70, 621–629. [Google Scholar] [CrossRef]
- de Villiers, E.M.; Fauquet, C.; Broker, T.R.; Bernard, H.-U.; zur Hausen, H. Classification of papillomaviruses. Virology 2004, 324, 17–27. [Google Scholar] [CrossRef]
- Reusser, N.M.; Downing, C.; Guidry, J.; Tyring, S.K. HPV Carcinomas in Immunocompromised Patients. J. Clin. Med. 2015, 4, 260–281. [Google Scholar] [CrossRef]
- Gupta, R.; Rady, P.L.; Doan, H.Q.; Tyring, S.K. Development of a β-HPV vaccine: Updates on an emerging frontier of skin cancer prevention. J. Clin. Virol. 2020, 126, 104348. [Google Scholar] [CrossRef]
- CDC. Human Papillomavirus. In Epidemiology and Prevention of Vaccine-Preventable Diseases Pink Book; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019. [Google Scholar]
- Nichols, A.; Nahm, W.; Rabinovitz, H.; Ioannides, T. Keratinocyte Carcinomas in Immunocompromised Patients Are Reduced After Administration of the Nonavalent Human Papillomavirus Vaccine. J. Drugs Dermatol. 2022, 21, 526–528. [Google Scholar] [CrossRef]
- Nichols, A.J.; Gonzalez, A.; Clark, E.S.; Khan, W.N.; Rosen, A.C.; Guzman, W.; Rabinovitz, H.; Badiavas, E.V.; Kirsner, R.S.; Ioannides, T. Combined Systemic and Intratumoral Administration of Human Papillomavirus Vaccine to Treat Multiple Cutaneous Basaloid Squamous Cell Carcinomas. JAMA Dermatol. 2018, 154, 927–930. [Google Scholar] [CrossRef]
- Bossart, S.; Imstepf, V.; Hunger, R.E.; Seyed Jafari, S.M. Nonavalent Human Papillomavirus Vaccination as a Treatment for Skin Warts in Immunosuppressed Adults: A Case Series. Acta Derm. Venereol. 2020, 100, adv00078. [Google Scholar] [CrossRef] [PubMed]
- Garbuglia, A.R.; Lapa, D.; Sias, C.; Khan, W.N.; Rosen, A.C.; Guzman, W.; Rabinovitz, H.; Badiavas, E.V.; Kirsner, R.S.; Ioannides, T. The Use of Both Therapeutic and Prophylactic Vaccines in the Therapy of Papillomavirus Disease. Front. Immunol. 2020, 11, 188. [Google Scholar] [CrossRef] [PubMed]
- Wenande, E.; Bech-Thomsen, N.; Togsverd-Bo, K.; Haedersdal, M. Off-Label 9-Valent Human Papillomavirus Vaccination for Actinic Keratosis: A Case Series. Case Rep. Dermatol. 2021, 13, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, S.; Negbenebor, N.; Sadoughifar, R.; Ahmad, S.; Kroumpouzos, G. Global impact on dermatology practice due to the COVID-19 pandemic. Clin. Dermatol. 2021, 39, 479–487. [Google Scholar] [CrossRef]
- Elek, P.; Fadgyas-Freyler, P.; Váradi, B.; Mayer, B.; Zemplényi, A.; Csanádi, M. Effects of lower screening activity during the COVID-19 pandemic on breast cancer patient pathways: Evidence from the age cut-off of organized screening. Health Policy 2022, 126, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Jepson, C.; Xie, D.; Roy, J.A.; Shou, H.; Hsu, J.Y.; Anderson, A.H.; Landis, J.R.; He, J.; Feldman, H.I.; et al. Statistical methods for recurrent event analysis in cohort studies of CKD. Clin. J. Am. Soc. Nephrol. 2017, 12, 2066–2073. [Google Scholar] [CrossRef]
- Faust, H.; Toft, L.; Sehr, P.; Müller, M.; Bonde, J.; Forslund, O.; Østergaard, L.; Tolstrup, M.; Dillner, J. Human Papillomavirus neutralizing and cross-reactive antibodies induced in HIV-positive subjects after vaccination with quadrivalent and bivalent HPV vaccines. Vaccine 2016, 34, 1559–1565. [Google Scholar] [CrossRef]
- Hama, N.; Ohtsuka, T.; Yamazaki, S. Detection of mucosal human papilloma virus DNA in bowenoid papulosis, Bowen’s disease and squamous cell carcinoma of the skin. J. Dermatol. 2006, 33, 331–337. [Google Scholar] [CrossRef]
- Iftner, A.; Klug, S.J.; Garbe, C.; Blum, A.; Stancu, A.; Wilczynski, S.P.; Iftner, T. The prevalence of human papillomavirus genotypes in nonmelanoma skin cancers of nonimmunosuppressed individuals identifies high-risk genital types as possible risk factors. Cancer Res. 2003, 63, 7515–7519. [Google Scholar] [PubMed]
- Ben Ayed, I.; Tounsi, H.; Jaballah, A.; Ardhaoui, M.; Maaloul, A.; Lassili, T.; Mezghani, N.; Abdelhak, S.; Boubaker, S. Mucosal human papillomavirus detection and TP53 immunohistochemical expression in non-melanoma skin cancer in Tunisian patients. J. Cutan. Pathol. 2019, 46, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Hufbauer, M.; Lazić, D.; Akgül, B.; Brandsma, J.; Pfister, H.; Weissenborn, S. Enhanced human papillomavirus type 8 oncogene expression levels are crucial for skin tumorigenesis in transgenic mice. Virology 2010, 403, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Walsh, S.B.; Verney, Z.M.; Kopelovich, L.; Elmets, C.A.; Athar, M. Procarcinogenic effects of cyclosporine A are mediated through the activation of TAK1/TAB1 signaling pathway. Biochem. Biophys. Res. Commun. 2011, 408, 363–368. [Google Scholar] [CrossRef]
- Strickley, J.D.; Messerschmidt, J.L.; Awad, M.E.; Li, T.; Hasegawa, T.; Ha, D.T.; Nabeta, H.W.; Bevins, P.A.; Ngo, K.H.; Asgari, M.M.; et al. Immunity to commensal papillomaviruses protects against skin cancer. Nature 2019, 575, 519–522. [Google Scholar] [CrossRef]
| Patient Characteristics | |
|---|---|
| N = 38 | |
| Age (years) | 62 (50, 73) |
| Sex (male) | 28 (76%) |
| Previous NMSC | 21 (55%) |
| previous cSCC | 15 (39%) |
| Only Wart lesions | 17 (45%) |
| Underlying disease | |
| TPL | 25 (66%) |
| other cancer | 4 (11%) |
| autoimmune | 2 (5%) |
| recalcitrant warts | 7 (18%) |
| number of IS | |
| 0 | 8 (21%) |
| 1 | 7 (18%) |
| 2 | 10 (26%) |
| 3 | 13 (34%) |
| localisation | |
| solitary | 16 (42%) |
| multiple | 22 (58%) |
| Variable | Overall | Before | During | After |
|---|---|---|---|---|
| Visits | 1040 [25.5 (IQR: 14–38)] | 425 [10 (IQR: 4–16)] | 318 [6 (IQR: 3–12)] | 297 [5 (IQR: 2.25–12.75)] |
| Overall interventions | 915 [21 (IQR: 10–35)] | 394 [8 (IQR: 3–15.75)] | 285 [4.5 (IQR: 2.25–11)] | 236 [4.5 (IQR: 1–10.75)] |
| Minor interventions | 422 [8.5 (IQR: 2.25–15)] | 203 [3.5 (IQR: 1–7)] | 102 [1.5 (IQR: 0–3.75)] | 117 [1 (IQR: 0–3.75)] |
| Major interventions | 493 [9.5 (IQR: 3.25–19.25)] | 191 [2 (IQR: 0.25–6.75)] | 183 [1.5 (IQR: 0–6)] | 119 [2 (IQR: 0–5.75)] |
| follow up time | 100 [2.7 (IQR: 1.868–3.235)] | 37 [0.96 (IQR: 0.712–1.165)] | 25 [0.585 (IQR: 0.55–0.608)] | 38 [0.765 (IQR: 0.35–1.71)] |
| Crude Model | Intermediate Model | Full Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR 1 | 95% CI 2 | p-Value | HR 1 | 95% CI 2 | p-Value | HR 1 | 95% CI 2 | p-Value | |
| Gardasil | 0.27 | 0.14, 0.51 | <0.001 | 0.21 | 0.11, 0.41 | <0.001 | 0.2 | 0.10, 0.41 | <0.001 |
| Gender [male] | 0.5 | 0.28, 0.88 | 0.017 | 0.5 | 0.28, 0.88 | 0.017 | |||
| Age at vaccionation (per year) | 0.99 | 0.98, 1.00 | 0.13 | 0.99 | 0.98, 1.00 | 0.13 | |||
| Indication [TPL] | 0.6 | 0.31, 1.19 | 0.15 | 0.6 | 0.31, 1.16 | 0.13 | |||
| Skin lesion [SCC Tumor] | 3.69 | 2.31, 5.89 | <0.001 | 3.68 | 2.31, 5.86 | <0.001 | |||
| national COVID Cases [per 1000] | 1.01 | 0.99, 1.03 | 0.5 | ||||||
| Responder Major Interventions | Responder Minor Interventions | Responder Overall Interventions | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OR 1 | 95% CI 2 | p-Value | OR 1 | 95% CI 2 | p-Value | OR 1 | 95% CI 2 | p-Value | |
| high intervention burden | 1.53 | 1.08, 2.18 | 0.024 | 0.76 | 0.54, 1.08 | 0.14 | 1.19 | 0.94, 1.53 | 0.2 |
| age at vacc (years) | 1 | 1.0, 1.01 | 0.4 | 1 | 0.99, 1.01 | >0.9 | 1 | 0.99, 1.00 | 0.7 |
| no of IS (per 1) | 0.95 | 0.75, 1.22 | 0.7 | 1.17 | 0.93, 1.48 | 0.2 | 1 | 0.84, 1.19 | >0.9 |
| indication (TPL) | 1.37 | 0.76, 2.47 | 0.3 | 0.7 | 0.40, 1.23 | 0.2 | 1.23 | 0.81, 1.86 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bossart, S.; Daneluzzi, C.; Moor, M.B.; Hirzel, C.; Heidemeyer, K.; Seyed Jafari, S.M.; Hunger, R.E.; Sidler, D. HPV Vaccination in Immunosuppressed Patients with Established Skin Warts and Non-Melanoma Skin Cancer: A Single-Institutional Cohort Study. Vaccines 2023, 11, 1490. https://doi.org/10.3390/vaccines11091490
Bossart S, Daneluzzi C, Moor MB, Hirzel C, Heidemeyer K, Seyed Jafari SM, Hunger RE, Sidler D. HPV Vaccination in Immunosuppressed Patients with Established Skin Warts and Non-Melanoma Skin Cancer: A Single-Institutional Cohort Study. Vaccines. 2023; 11(9):1490. https://doi.org/10.3390/vaccines11091490
Chicago/Turabian StyleBossart, Simon, Cloé Daneluzzi, Matthias B. Moor, Cédric Hirzel, Kristine Heidemeyer, S. Morteza Seyed Jafari, Robert E. Hunger, and Daniel Sidler. 2023. "HPV Vaccination in Immunosuppressed Patients with Established Skin Warts and Non-Melanoma Skin Cancer: A Single-Institutional Cohort Study" Vaccines 11, no. 9: 1490. https://doi.org/10.3390/vaccines11091490
APA StyleBossart, S., Daneluzzi, C., Moor, M. B., Hirzel, C., Heidemeyer, K., Seyed Jafari, S. M., Hunger, R. E., & Sidler, D. (2023). HPV Vaccination in Immunosuppressed Patients with Established Skin Warts and Non-Melanoma Skin Cancer: A Single-Institutional Cohort Study. Vaccines, 11(9), 1490. https://doi.org/10.3390/vaccines11091490






