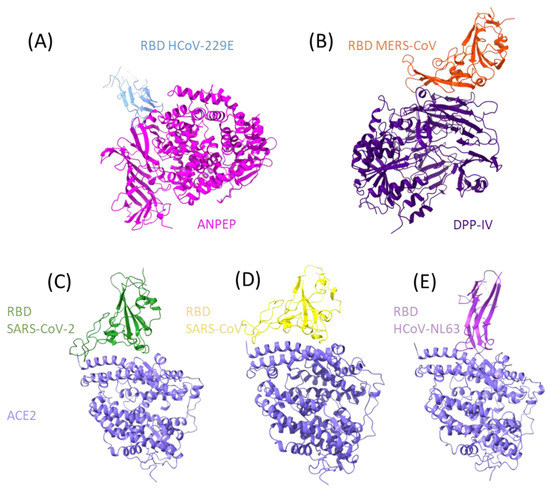
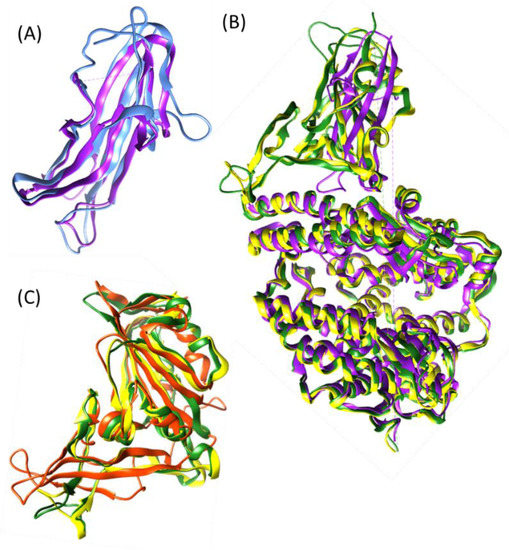
Abstract
Coronaviruses interact with protein or carbohydrate receptors through their spike proteins to infect cells. Even if the known protein receptors for these viruses have no evolutionary relationships, they do share ontological commonalities that the virus might leverage to exacerbate the pathophysiology. ANPEP/CD13, DPP IV/CD26, and ACE2 are the three protein receptors that are known to be exploited by several human coronaviruses. These receptors are moonlighting enzymes involved in several physiological processes such as digestion, metabolism, and blood pressure regulation; moreover, the three proteins are expressed in kidney, intestine, endothelium, and other tissues/cell types. Here, we spot the commonalities between the three enzymes, the physiological functions of the enzymes are outlined, and how blocking either enzyme results in systemic deregulations and multi-organ failures via viral infection or therapeutic interventions is addressed. It can be difficult to pinpoint any coronavirus as the target when creating a medication to fight them, due to the multiple processes that receptors are linked to and their extensive expression.
1. Introduction
The designation Coronaviridae refers to a family of viruses that all display protein homotrimers across the whole surface of their viral membrane, giving them a crown-like appearance. The coronaviruses are encapsulated positive-stranded RNA viruses [1], and the family contains four distinct genera (alpha, beta, gamma, and delta coronavirus) all of which encode nearly all functionally identical proteins [2]. Known human coronaviruses belong specifically to the genera Alphacoronavirus or Betacoronavirus, whose common ancestor infected bats [2]. Table 1 depicts the seven distinct coronaviruses that can infect humans: HCoV- 229E, HCoV- NL63, HCoV- OC43, HCoV- HKU1, SARS-CoV, MERS-CoV, and SARS-CoV-2. The latter three have been the main causes of epidemics in recent years [1,3,4].

Table 1.
Human coronaviruses and their most probable ancestor host. Seven human coronaviruses have emerged from zoonotic transmission events.
The structural glycoprotein known as the spike protein (S) enables the host receptor identification and entry into the cell [12]. The S proteins of coronaviruses must be cleaved to activate the fusion peptide and infect the cell [13]. S proteins have two functional subdomains that allow membrane fusion. The host recognition involves the subdomain 1 (S1), which has a C-terminal domain (CTD) and an N-terminal domain (NTD). The receptor binding domain (RBD) may be in the NTD or the CTD, depending on the virus. The fusion machinery, which consists of the fusion peptide and the heptad repeats required for the fusion, is present in the second subdomain (S2). The S2 sequence is far more conserved among the four genera than the S1 sequence, as the virus must adapt to the receptors of the hosts [14]. The adaptability of the coronaviruses has led to their evolutionary success because they respond rapidly to selective pressures [15,16] and can jump to another species with highly identical receptors [14,17].
Coronaviruses are known to primarily use a host receptor, which can be either a protein or a carbohydrate [17]. It is hypothesized that an ancient coronavirus acquired a host galectin sequence that resulted in the ability to bind carbohydrates, so binding to carbohydrates is a more recent feature [18]. Even though each coronavirus has a primary host receptor, it has been shown that several S proteins can bind to other components of the cell membrane [19,20,21]. Even more, the S protein’s glycosylation can also interact with the lectins of the host [20]. The reported protein receptors employed by mammalian coronaviruses are the murine carcinoembryonic antigen-related cell adhesion molecule 1a (mCEACAM 1a), aminopeptidase N (ANPEP also CD13), dipeptidyl protease IV (DPP- IV also CD26), and angiotensin converting enzyme 2 (ACE2) [17]. Since the first one is receptor for the murine hepatitis virus (MHV) and it does not infect humans, herein, we will discuss the other three enzymes solely.
4. Role of Proteases in Physiological Processes
4.1. Role of the Proteases in Digestion
Digestion is the conjunction of the sequential process of breaking down food, so that its nutrients can be taken up and absorbed. The digestive system is essential for hormone secretion; it provides the body with amino acids, lipids, carbohydrates, and micronutrients that regulate the immune system and interact with the microbiome, all of which have a variety of effects on the organism. As a result, the digestive system is essential for preserving the homeostasis of the body. Specialized epithelial cells along the gastrointestinal tract express enzymes that catabolize macronutrients and protein transporters to absorb them. Proteins from food are hydrolyzed into oligopeptides and amino acids through the gastrointestinal tract and then absorbed in the small intestine. Small intestinal epithelial cells express the digestive enzymes ACE2, ANPEP, and DPP-IV, which are crucial for the last stages of digestion.
ACE2 has been described as a key regulator of dietary amino acid homeostasis, antimicrobial peptide expression, maturation of the local immune system, and interaction with the microbiome [61]. Hashimoto and collaborators write that Ace2 knockout mice have an increased propensity to develop severe colitis and showed that this inflammatory condition was associated with malnutrition. As ACE2 is necessary for the expression of the neutral amino acid transporter B0AT1 (SL6A19 gene), the mice were unable to absorb neutral amino acids from their diet, and consequently serum levels of valine, threonine, tyrosine and tryptophan were reduced [67]. Evidence shows that ACE2 is necessary not only for its functions as a digestive enzyme but also for amino acid absorption, aiding homeostasis [61]; this is also true for ANPEP, which co-localizes with B0AT1 on the luminal membrane of the small intestine in rats [68].
4.2. Role of the Proteases in Angiogenesis
Angiogenesis is the processes by which new blood vessels develop from existing ones, to supply nutrients and oxygen to all tissues. In mice with arthritis, soluble ANPEP (sANPEP) induced endothelial cell migration and monocyte mobilization [69]. In response to hypoxia, ANPEP and other angiogenic factors are upregulated [70]. ANPEP suppression by interference RNA (RNAi) resulted in the inhibition of capillary tube formation of HUVEC on Matrigel [71]. Similarly, Rinkevich et al. 2015 reported that inhibition of DPP-IV decreases skin-scar formation during wound healing. This occurs because DPP-IV cleaves the angiogenic factor, the high mobility group box 1 protein (HMGB1), at its N-terminal region, which induces endothelial cell migration and capillary-like structure formation, as well as vascular network formation [72]. Moreover, another substrate, the stromal-cell-derived factor alpha (SDF-1a) induced mobilization of bone-marrow-derived stem cells and the expression of the angiogenic vascular endothelial growth factor (VEGF) [73]. The role of ACE2 during angiogenesis lies in the product Ang (1-7), which promotes angiogenesis and cell proliferation via the receptor Mas [74]; besides this, the activation of this receptor in the brain also decreases oxidative stress, neuroinflammation, improves cerebral blood flow and therefore neuronal survival [75], and tissue repair [76,77,78], but also tumor growth [74,79]. That is, that the participation of these enzymes in angiogenesis lies in the signaling generated by their substrates.
4.3. Role of the Proteases in the RAAS System
The RAAS is a regulator of the osmolarity of the whole organism: it affects blood ions concentration, blood volume, pressure, and vascular wall resistance; part of the system promotes sodium reabsorption and fluid retention, which increases blood pressure, while the other promotes fluid excretion [60]. The kidneys, lungs, suprarenal glands, the entire vasculature, and the brain have important roles in the system. Although RAAS affects the entire body, there are local RAASs that might primarily affect a specific organ [80]. By sensing blood pressure in the vasculature, this mechanism promotes an increase in blood flow. Under physiological conditions, the RAAS peptides are produced in a dynamic and balanced manner, and any alteration could lead to an acute event of poor blood pressure regulation or a chronic cardiometabolic disorder; in fact, RAAS connects the cardiovascular, renal, and the metabolism systems.
Figure 3 can help to understand the global RAAS. The juxtaglomerular cells in the kidneys cleave pro-renin in response to a drop in blood pressure and sodium in blood. Following this, renin cleaves angiotensinogen to produce angiotensin I, which in turn is transformed to angiotensin II (Ang II) by the angiotensin converting enzyme (ACE). The two receptors for Ang II, type I (AT1R), and type II receptors (AT2R) cause a variety of effects depending on tissue and receptor type. For example, vasoconstriction is induced via AT1R, which increases sodium reabsorption in the kidney and stimulates the production of aldosterone in the adrenal cortex; in the brain, they promote water intake and retention via the hypothalamus–pituitary gland axis, which releases the antidiuretic hormone [75,81]. The overall consequence is an increase in vasculature tone. AT2R is associated to wound healing and tissue repair, inhibits ACE, and enhances the activity of angiotensin receptor blockers (ARB) [82].
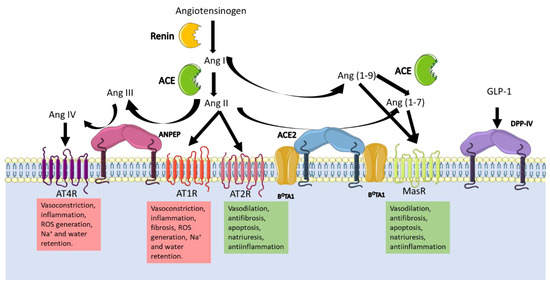
Figure 3.
Schematic representation of the peptide flux involved in the RAAS highlighting the participation of the viral receptors ANPEP, DPP-IV, and ACE2. When hypotension is detected, the Angiotensinogen produced in the liver, is catalyzed by the enzyme renin synthetized in the juxtaglomerular cells in the kidney. The product Ang I is either cleaved by the ACE to produce Ang II, which activates any of the Ang II receptors (AT1R and AT2R). Ang I and Ang II can be also cleaved by ACE2 to produce Ang (1-9) and Ang (1-7), respectively. The additional cleavage of ACE to Ang (1-9) produces Ang (1-7) which finally activates the MasR. Additionally, Ang II can be further catalyzed twice by ANPEP producing Ang IV, which activates the AT4R. The effects every Ang II Receptor has, counteracts others so the accurate balance is necessary in order to control blood pressure, osmolarity and inflammation. Ang II is also necessary for aldosterone secretion, which reabsorbs sodium in the kidney, and the antidiuretic hormone release which retains water. The overall effect of the system is the increase of blood pressure. The blockage of the enzymes either with coronaviruses or with other molecules, alters the homeostasis of blood pressure and osmolarity.
Ang II is regulated by multiple proteases that cleave it to produce different vasoactive peptides that cause a range of effects. The protease (discussed here) implicated in the RAAS with the most evidence in the literature is ACE2, because it hydrolyses angiotensin I (Ang I) producing Ang (1-9), and Ang II producing Ang (1–7) and angiotensin A; subsequent hydrolysis of Ang (1-9) by ACE produces the second metabolite of ACE2. As mentioned above, vasoactive peptides induce vasodilation or vasoconstriction depending on the receptor stimulated. The receptor Mas (MasR), specific for Ang (1-7), promotes anti-inflammatory, antifibrotic, and vasodilatory properties [60]. Different polymorphisms of the Ace2 gene are known to be associated with RAAS pathologies at different levels [62].
Additionally, aminopeptidases play crucial functions in blood-pressure regulation [81]. In the brain, the inhibition of ANPEP activity in the intracerebroventricular space increases arterial blood pressure [29], as its substrate, Ang III, accumulates, causing blood pressure to rise. In addition, the inhibition of ANPEP increases the release of vasopressin, the antidiuretic hormone that retains water, thus contributing to increased vessel tone [81]. In the renal tubule, ANPEP, via Ang IV, decreases basolateral Na+/K+ ATPase, thereby increasing urinary Na+ excretion and decreasing transcellular Na+ transport in the renal artery. Therefore, ANPEP promotes natriuresis by increasing Ang IV and reducing the Ang III concentration.
The precise molecular interactions between DDP-IV and RAAS are still unknown; what is clear is that DDP- IV coimmunoprecipitates with the sodium/hydrogen exchanger-3 in the proximal tubules of the nephrons, so its inhibition reduces the expression of the exchanger and thus the sodium uptake [46]. Besides this, renal accumulation of Ang II induces both glomerular and proximal tubule injury by reducing the expression of megalin, which promotes the reabsorption of filtered albumin and other low-molecular-weight proteins. Nonetheless, Ang II stimulates the activity of DPP-IV, which, together with ANPEP, supports the reabsorption of oligopeptides through its catalytic activity [83].
4.4. Role of the Proteases in Metabolism
Metabolic dysregulations, such as obesity and diabetes, cause other complications, such as cardiovascular disease and chronic kidney disease. The role of DPP-IV in metabolism is to degrade the glucagon-like peptide-1 and -2 (GLP1/GLP-2) and glucose-dependent insulinotropic peptide (GIP), which leads to insulin secretion [46]. DPP-IV is upregulated in patients with type 2 diabetes mellitus; this increases insulin resistance and confers cardiovascular risk [46]. Treatment that inhibits DPP-IV activity improves glucose tolerance, hypertension, and has positive consequences in different tissues such as the heart, vasculature, adipose tissue, and kidney [46,72]. Furthermore, obese and insulin-resistant patients have elevated levels of sDPP-IV, which contributes to inflammation by activating T cells via CD45 contacts and activating antigen-presenting cells via interactions with the mannose-6-phosphate receptor and adenosine deaminase [84,85].
The role of ACE2 in glucose metabolism is still uncertain and it is thought to act as a compensatory mechanism to reduce the effects of the Ang II/AT1R axis [86]. Elevated Ang II concentrations can lead to hyperglycemia, dyslipidemia, impaired vascular function, and inflammation. ACE2 deficiency increased vascular inflammation and atherosclerosis due to the Ang II/AT1R axis, whereas stimulation of the ACE2-Ang-(1-7)/Mas axis was reported to reduce obesity [84].
Increased plasma ACE2 concentration is associated with an increased risk of heart failure incidence, myocardial infarction, stroke, and diabetes [87]. Furthermore, patients with impaired fasting glucose, impaired glucose tolerance, or diabetes had high urinary ACE2 levels as well as high fasting blood glucose and triglyceride, elevated high-sensitivity C-reactive protein, high serum creatinine and urinary albumin-to-creatinine ratio, and elevated systolic blood pressure [88,89].
4.5. Role of the Proteases in the Respiratory System
Under physiological conditions, the viral receptors are expressed in the epithelial cells of the respiratory airways. ACE2 is co-expressed with TMPRRS2 and cathepsin L in type II pneumocytes, endothelial cells, and the nasal epithelial cells [90]. They must control blood pressure as part of their role as enzymes in the tissue. ACE2 processes Ang II into Ang (1-7), and other vasopeptides that contribute to maintaining the blood pressure in the capillaries surrounding the lungs and upper airways. ANPEP is present in epithelial cells and alveolar macrophages and it could aggravate lung injury by enhancing the generation of reactive oxygen species and NF-kB activation during inflammation [91]. DPP-IV, which is expressed in epithelial cells and T lymphocytes, exhibits comparable behavior. The three enzymes process peptides from the mucosa but have an important role in immunomodulating the tissue [92].
5. Pathogenesis of the Viruses
Human coronaviruses infect the organism through cells expressing any of their principal receptors and co-receptors that will process the viral S protein. They enter the body either through the respiratory airways or the gut, primarily affecting the lungs and gastrointestinal tract, but multi-organ failure is imminent. For instance, HCoV-229E, HCoV-NL63, HCoV-OC43, and HCoV-HKU1 cause common colds by infecting superior airways while SARS-CoV, MERS-CoV, and SARS-CoV-2 can also affect class II pneumocytes from the lungs. Coronaviruses have a tissue tropism towards the receptors and the proteases needed for S protein processing [13]. Once the virus has entered the body, it interacts with the matching host receptor using its S protein, and several host proteases fully activate it to allow the membrane fusion [93]. The host’s proteases prime the S protein by cleaving the S1 from S2, allowing the fusion of viral and host membranes. The various proteases cleave at various points during the replicative cycle; the transmembrane serine protease (TMPRSS 2), elastase, plasmin, and trypsin catalyze at the membrane and extracellular levels; the cathepsin-L in endosomes and the furin-like convertases cleave the newly synthesized S protein at the Golgi apparatus [13]. The replicative cycle begins upon the fusing of either the cell membrane or the endosome membrane [94].
There are no reports indicating symptoms other than fever, throat pain, dry cough, tiredness, headache, runny nose, and diarrhea for the coronaviruses that cause common cold. However, the pandemic of coronaviruses (SARS-CoV-2, SARS- CoV and MERS-CoV) could cause more complicated symptoms. Due to their entrance path and the presence of the receptors, the most notable and primary pathologies are located in the respiratory airways, yet the gut may suffer tissue damage as well as other organs [95]. Acute myocardial injury, myocarditis, and other cardiopathies, hyperglycemia, diabetic ketoacidosis, gastrointestinal abnormalities, elevated aminotransferases and bilirubin, neurologic distortions, thromboembolic events, and renal injury are some of the complications associated with severe COVID-19 [95].
The pathology of the coronavirus infection starts with tissue damage and the loss of function of the infected cell. The immune response and pathophysiology of the common cold coronaviruses remains unclear, but certainly the response is known for pandemic coronaviruses. Coronaviruses infection provokes an immunological signature where proinflammatory cytokines are released (IFN, IL-6, IL-8, IP-10, TNF-α, MCP-1, IL-1β, IL-17, etc.), the accumulation of neutrophils and macrophages in the infected tissue, and lymphopenia of both T and B cells [92]. The infection causes inflammasome NLRP3 activation, which ends in an uncontrolled death driven by pyroptosis and the release of IL-1β [96]. It has been reported that SARS-CoV and MERS-CoV are both detected by the innate receptor toll-like receptor 7 (TLR-7), which triggers the synthesis of pro-inflammatory cytokines, such as type I IFN, IL-6, TNF-α, and the IFN inducible genes [97,98], these communicate to neighbor cells to activate an antiviral state. SARS-CoV-2 is also susceptible to type I IFN [99], in fact we now know that the timing for its synthesis could drive the course of the diseases. A single cell RNA analysis revealed that type I IFN response is not activated in severe patients [100], and another report showed that severe patients had low serum levels of type I and III IFN and high levels of other pro-inflammatory cytokines [101] as well as the viral titers [102]. It remains to be discovered how the immediate response of these patients came about, because it was reported that the delay of IFN cascade caused a rapid viral replication enhancing lung damage and a robust pro-inflammatory cytokine response in a mouse model [103].
The consequence of the proinflammatory cytokine production is the recruitment of immunological cells who can combat and repair the tissue. Nevertheless, this may cause edema, impeding lung function and causing poor gas exchange. Liquid in the pulmonary tissue causes cell death, which in turn causes loss of function and increases inflammation. Beside this, it is likely that the capillaries at the lungs worsen the scenario, either by endothelial cell infection or by capillary wall disruption [104]. The latter causes the activation of the coagulation cascades, increasing the possibility of micro thrombi deposition in the pulmonary network, activating systemic inflammatory mediators, and enhancing other coagulopathies [61]. In the case of SARS-CoV-2, the RAAS dysregulates because the binding of the virus to the ACE2 receptors enhances vascular permeability and the disruption of intercellular junctions. All these contributes to lung inflammation, cell death, and fibrosis.
The catalytic sites of the aforementioned enzymes are not blocked when they interact with the corresponding coronavirus S protein; however, any coronavirus receptor is downregulated because the virus is internalized with it through endocytosis [105]. In fact, DPP-IV and ANPEP are found in membrane domains associated with caveolae [106]. As consequence the product–substrate balance could be altered locally or systemically. Coronaviruses using DPP-IV or ANPEP may have an unbalanced pro-inflammatory response because of their participation in modulating the immune system and cytokine processing. These enzymes are also known to interact with extracellular matrix (ECM) proteins, promote cell migration, adhesion, and tissue remodeling; however, the precise immunological response and the balance of vasoactive peptides must be thoroughly investigated. SARS-CoV-2 pandemics, in contrast to past coronavirus outbreaks, prompted a significant effort to advance understanding of the virus and the physiological reactions of affected organisms. According to studies, SARS-CoV-2 and SARS-CoV entry cause membrane ACE2 downregulation, which puts Ang II and Ang (1-7) out of balance, leading to an increase in inflammation, angiogenesis, pro-oxidation, and pro-fibrotic responses [84]. Protease dysregulation causes change in the concentrations of substrates such as Ang II, apelin-13, dynorphin-13, and products such as Ang (1-7), Ang (1-9), apelin-12, dynorphin-12 [107].
The viruses can infect endothelial cells, causing the exposure of prothrombotic molecules, platelet adhesion, activation of coagulation cascades by the release of plasminogen activator inhibitor 1 (PAI-1), von Willebrand factor (vWF) antigen, soluble thrombomodulin, and tissue factor pathway inhibitor (TFPI), as well as the proangiogenic factors VEGF, hypoxia-inducible factor 1a (HIF-1a), IL-6, and TNF receptor super family 1A and 12 [108]. The vascular damage makes systemic angiopathy and thromboembolism probable, which can lead to multi-organ failure and death [109]. Similarly, myocardium failure is caused by pericytes expressing ACE2 [61]. However, the inhibition of ACE2 catalytic activity could lead to acute myocardial injury associated with SARS-CoV-2 due to Ang II accumulation [62].
7. Conclusions
Metabolic disorders, hypertension, and cardiovascular and renal diseases are known to be risk factors for developing severe COVID-19 disease [165], but they are also co-morbidities for all coronavirus diseases. The coronavirus receptors (ACE2, DPP-IV, and ANPEP) are interconnected in physiological processes that impact locally and, more significantly, systemically. Any of these receptors may be involved in chronic conditions that affect vital aspects of homeostasis, such as metabolism, blood pressure, and vascular integrity. ACE2, DPP-IV, and ANPEP proteases are also widely expressed, with a significant role in endothelial cells, the network that connects all tissues and organs. Additionally, several polymorphisms of the proteases, affect the susceptibility and course of coronavirus diseases. Each of these elements has effects on systemic physiology; thus, coronavirus infections can cause multiple organ failure, making it difficult to develop treatments or bullets to block them all.
Proposed and applied treatments against SARS-CoV-2 has opened a debate regarding the expression of ACE2. On one hand, enhanced ACE2 expression might facilitate the infection and replication of the virus but would favor Ang 1-7 accumulation, and as consequence vasodilation and anti-inflammatory responses. In the other hand, limiting the expression of ACE2 provokes Ang II accumulation, and therefore vasoconstriction and inflammation via AT1R signaling, fibrosis, and tissue injuries. ANPEP and DPP-IV are also involved in processing peptides of the RAAS and metabolism. Therefore, the accurate expression and enzymatic activity of the protein receptors is essential for the maintenance of the homeostasis of the organism. Any alteration could provoke systemic complications, including organ failure. Because of this, diseases that employ these receptors generally create difficulties, especially if they are new viruses that the immune system is not familiar with. The most important issue is how to combat the viral spread and develop prophylactic and treatments against the agents. This represents an important challenge because viral receptors-based drugs represent a double-edged sword against coronavirus infection.
Although several approaches have been tried, it is obvious that medicine must be more individually tailored to treat each case according to its receptor expression levels and metabolism. Treatment of coronavirus infections with drugs that inhibit the receptor’s catalytic function may result in complex unintended side outcomes. Other treatments that involve modifying the RAAS system have been assessed in clinical trial but findings are inconclusive. The soluble receptors systems may be an attractive alternative for hijacking the virus before it infects the cell. Ongoing research in this field should continue because it is still in experimental phases. Meanwhile, it seems that preventive vaccinations and mAbs against viral proteins can possibly block viral entry without affecting the enzymes’ activity, and a healthy balance of metabolites remains the most effective treatment for coronavirus infections without presenting significant adverse events. Hopefully, the research in soluble molecules systems creates a drug-design platform to solve future outbreaks.
Author Contributions
Conceptualization, G.I.L.-C.; writing—original draft preparation, G.I.L.-C.; writing—review and editing, M.P.-P., M.M.H.-A., H.F.V. and M.V.J.; project administration, G.I.L.-C.; funding acquisition, M.V.J. All authors have read and agreed to the published version of the manuscript.
Funding
M.V.J. was financially supported by Dirección General de Asuntos del Personal Académico (DGAPA)- PAPIIT-IN200823, Universidad Nacional Autónoma de México,. M.P.-P. is a postdoctoral researcher fellow with CVU 694877 from Consejo Nacional de Ciencia y Tecnología (CONACYT) at Universidad Nacional Autónoma de México (UNAM). G.I.L.-C. is a postdoctoral researcher fellow from DGAPA-PAPIIT IV201220, UNAM.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was generated.
Acknowledgments
We acknowledge Laura Díaz-Álvarez and Erandi Pérez-Figueroa for helpful revisions and comments. Graphical abstract and Figure 3 were drawn using pictures from Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/, accessed on 27 November 2022). Molecular graphics and analyses (Figure 1 and Figure 2) performed with UCSF ChimeraX (https://www.rbvi.ucsf.edu/chimerax, accessed on 27 November 2022), developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from National Institutes of Health R01-GM129325 and the Office of Cyber Infrastructure and Computational Biology, National Institute of Allergy and Infectious Diseases [126].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Woo, P.C.Y.; Lau, S.K.P.; Lam, C.S.F.; Lau, C.C.Y.; Tsang, A.K.L.; Lau, J.H.N.; Bai, R.; Teng, J.L.L.; Tsang, C.C.C.; Wang, M.; et al. Discovery of Seven Novel Mammalian and Avian Coronaviruses in the Genus Deltacoronavirus Supports Bat Coronaviruses as the Gene Source of Alphacoronavirus and Betacoronavirus and Avian Coronaviruses as the Gene Source of Gammacoronavirus and Deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ding, Y.; Du, J.; Fan, Y. 2020 update on human coronaviruses: One health, one world. Med. Nov. Technol. Devices 2020, 8, 100043. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Corman, V.M.; Baldwin, H.J.; Tateno, A.F.; Zerbinati, R.M.; Annan, A.; Owusu, M.; Nkrumah, E.E.; Maganga, G.D.; Oppong, S.; Adu-Sarkodie, Y.; et al. Evidence for an Ancestral Association of Human Coronavirus 229E with Bats. J. Virol. 2015, 89, 11858–11870. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Shi, M.; Chommanard, C.; Queen, K.; Zhang, J.; Markotter, W.; Kuzmin, I.V.; Holmes, E.C.; Tong, S. Surveillance of Bat Coronaviruses in Kenya Identifies Relatives of Human Coronaviruses NL63 and 229E and Their Recombination History. J. Virol. 2017, 91, e01953-16. [Google Scholar] [CrossRef] [PubMed]
- Kapikian, A.Z. The coronaviruses. Dev. Biol. Stand 1975, 28, 42–64. [Google Scholar] [PubMed]
- Corman, V.M.; Muth, D.; Niemeyer, D.; Drosten, C. Hosts and Sources of Endemic Human Coronaviruses. Adv. Virus Res. 2018, 100, 163–188. [Google Scholar] [CrossRef]
- van der Hoek, L. Human coronaviruses: What do they cause? Antivir. Ther. 2007, 12, 651–658. [Google Scholar] [CrossRef]
- Corman, V.M.; Eckerle, I.; Bleicker, T.; Zaki, A.; Landt, O.; Eschbach-Bludau, M.; van Boheemen, S.; Gopal, R.; Ballhause, M.; Bestebroer, T.M.; et al. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Eurosurveillance 2012, 17, 20285. [Google Scholar] [CrossRef]
- Singh, D.; Yi, S.V. On the origin and evolution of SARS-CoV-2. Exp. Mol. Med. 2021, 53, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Li, F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu. Rev. Virol. 2016, 3, 237–261. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.K.; Whittaker, G.R. Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015, 202, 120–134. [Google Scholar] [CrossRef]
- López-Cortés, G.I.; Palacios-Pérez, M.; Zamudio, G.S.; Veledíaz, H.F.; Ortega, E.; José, M.V. Neutral evolution test of the spike protein of SARS-CoV-2 and its implications in the binding to ACE2. Sci. Rep. 2021, 11, 18847. [Google Scholar] [CrossRef] [PubMed]
- López-Cortés, G.I.; Palacios-Pérez, M.; Veledíaz, H.F.; Hernández-Aguilar, M.; López-Hernández, G.R.; Zamudio, G.S.; José, M.V. The Spike Protein of SARS-CoV-2 Is Adapting Because of Selective Pressures. Vaccines 2022, 10, 864. [Google Scholar] [CrossRef]
- Jaimes, J.A.; André, N.M.; Chappie, J.S.; Millet, J.K.; Whittaker, G.R. Phylogenetic Analysis and Structural Modeling of SARS-CoV-2 Spike Protein Reveals an Evolutionary Distinct and Proteolytically Sensitive Activation Loop. J. Mol. Biol. 2020, 432, 3309–3325. [Google Scholar] [CrossRef]
- Millet, J.K.; Jaimes, A.J.; Whittaker, G.R. Molecular diversity of coronavirus host cell entry receptors. FEMS Microbiol. Rev. 2020, 45, fuaa057. [Google Scholar] [CrossRef]
- Peng, G.; Sun, D.; Rajashankar, K.R.; Qian, Z.; Holmes, K.V.; Li, F. Crystal structure of mouse coronavirus receptor-binding domain complexed with its murine receptor. Proc. Natl. Acad. Sci. USA 2011, 108, 10696–10701. [Google Scholar] [CrossRef]
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020, 526, 135–140. [Google Scholar] [CrossRef]
- Hoffmann, D.; Mereiter, S.; Jin Oh, Y.; Monteil, V.; Elder, E.; Zhu, R.; Canena, D.; Hain, L.; Laurent, E.; Grünwald-Gruber, C.; et al. Identification of lectin receptors for conserved SARS-CoV-2 glycosylation sites. bioRxiv 2021. [Google Scholar] [CrossRef]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Antón-Plágaro, C.; Shoemark, D.K.; Simón-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Mina-Osorio, P. The moonlighting enzyme CD13: Old and new functions to target. Trends Mol. Med. 2008, 14, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovac, J.; Čupić, B.; Breljak, D.; Zekušić, M.; Boranić, M. Expression of CD13/aminopeptidase N and CD10/neutral endopeptidase on cultured human keratinocytes. Immunol. Lett. 2004, 91, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Look, A.T.; A Ashmun, R.; Shapiro, L.H.; Peiper, S.C. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J. Clin. Investig. 1989, 83, 1299–1307. [Google Scholar] [CrossRef]
- Griffin, J.D.; Ritz, J.; Nadler, L.M.; Schlossman, S.F. Expression of myeloid differentiation antigens on normal and malignant myeloid cells. J. Clin. Investig. 1981, 68, 932–941. [Google Scholar] [CrossRef]
- López-Cortés, G.I.; Díaz-Alvarez, L.; Ortega, E. Leukocyte Membrane Enzymes Play the Cell Adhesion Game. Front. Immunol. 2021, 12, 742292. [Google Scholar] [CrossRef]
- Chen, L.; Lin, Y.-L.; Peng, G.; Li, F. Structural basis for multifunctional roles of mammalian aminopeptidase N. Proc. Natl. Acad. Sci. USA 2012, 109, 17966–17971. [Google Scholar] [CrossRef]
- Tresnan, D.B.; Levis, R.; Holmes, K.V. Feline aminopeptidase N serves as a receptor for feline, canine, porcine, and human coronaviruses in serogroup I. J. Virol. 1996, 70, 8669. [Google Scholar] [CrossRef]
- Danziger, R.S. Aminopeptidase N in arterial hypertension. Heart Fail. Rev. 2008, 13, 293–298. [Google Scholar] [CrossRef]
- Lu, C.; Amin, M.A.; Fox, D.A. CD13/Aminopeptidase N Is a Potential Therapeutic Target for Inflammatory Disorders. J. Immunol. 2020, 204, 3–11. [Google Scholar] [CrossRef]
- Luan, Y.; Xu, W. The Structure and Main Functions of Aminopeptidase N. Curr. Med. Chem. 2007, 14, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Garay-Canales, C.A.; Díaz-Alvarez, L.; Lopez-Cortes, G.I. Novel immunotherapy strategies involving matrix metalloproteinase (MMP) family. In Immunotherapy in Resistant Cancer: From the Lab Bench Work to Its Clinical Perspectives; Morales-Montor, J., Segovia-Mendoza, M., Eds.; Academic Press Inc.: Cambridge, MA, USA, 2020; Volume 2, pp. 227–247. [Google Scholar]
- Gabrilovac, J.; Breljak, D.; Čupić, B. Regulation of aminopeptidase N (EC 3.4.11.2; APN.; CD13) on the HL-60 cell line by TGF-β1. Int. Immunopharmacol. 2008, 8, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Santos, N.; Langner, J.; Herrmann, M.; Riemann, D. Aminopeptidase N/CD13 is directly linked to signal transduction pathways in monocytes. Eur. PMC 2000, 201, 22–32. [Google Scholar] [CrossRef]
- Subramani, J.; Ghosh, M.; Rahman, M.M.; Caromile, L.A.; Gerber, C.; Rezaul, K.; Han, D.K.; Shapiro, L.H. Tyrosine Phosphorylation of CD13 Regulates Inflammatory Cell–Cell Adhesion and Monocyte Trafficking. J. Immunol. 2013, 191, 3905–3912. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Gerber, C.; Rahman, M.M.; Vernier, K.M.; Pereira, F.E.; Subramani, J.; Caromile, L.A.; Shapiro, L.H. Molecular mechanisms regulating CD13-mediated adhesion. Immunology 2014, 142, 636–647. [Google Scholar] [CrossRef]
- Licona-Limón, I.; Garay-Canales, C.A.; Muñoz-Paleta, O.; Ortega, E. CD13 mediates phagocytosis in human monocytic cells. J. Leukoc. Biol. 2015, 98, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Mina-Osorio, P.; Winnicka, B.; O’Conor, C.; Grant, C.L.; Vogel, L.K.; Rodriguez-Pinto, D.; Holmes, K.V.; Ortega, E.; Shapiro, L.H. CD13 is a novel mediator of monocytic/endothelial cell adhesion. J. Leukoc. Biol. 2008, 84, 448–459. [Google Scholar] [CrossRef]
- Mina-Osorio, P.; Shapiro, L.H.; Ortega, E. CD13 in cell adhesion: Aminopeptidase N (CD13) mediates homotypic aggregation of monocytic cells. J. Leukoc. Biol. 2006, 79, 719–730. [Google Scholar] [CrossRef]
- Pérez-Figueroa, E.; Álvarez-Carrasco, P.; Ortega, E. Crosslinking of membrane CD13 in human neutrophils mediates phagocytosis and production of reactive oxygen species, neutrophil extracellular traps and proinflammatory cytokines. Front. Immunol. 2022, 13, 6681. [Google Scholar] [CrossRef]
- Mendoza-Coronel, E.; Ortega, E. Macrophage Polarization Modulates FcγR- and CD13-Mediated Phagocytosis and Reactive Oxygen Species Production, Independently of Receptor Membrane Expression. Front. Immunol. 2017, 8, 303. [Google Scholar] [CrossRef]
- Cheng, H.C.; Abdel-Ghany, M.; Pauli, B.U. A Novel Consensus Motif in Fibronectin Mediates Dipeptidyl Peptidase IV Adhesion and Metastasis. J. Biol. Chem. 2003, 278, 24600–24607. [Google Scholar] [CrossRef] [PubMed]
- Klemann, C.; Wagner, L.; Stephan, M.; von Hörsten, S. Cut to the chase: A review of CD26/dipeptidyl peptidase-4’s (DPP4) entanglement in the immune system. Clin. Exp. Immunol. 2016, 185, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Iwata, B.S.; Morimoto, C. CD26/Dipeptidyl Peptidase IV in Context. J. Exp. Med. 1999, 190, 301–305. [Google Scholar] [CrossRef]
- Engel, M.; Hoffmann, T.; Wagner, L.; Wermann, M.; Heiser, U.; Kiefersauer, R.; Huber, R.; Bode, W.; Demuth, H.-U.; Brandstetter, H. The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism. Proc. Natl. Acad. Sci. USA 2003, 100, 5063–5068. [Google Scholar] [CrossRef]
- Nistala, R.; Savin, V. Diabetes, hypertension, and chronic kidney disease progression: Role of DPP4. Am. J. Physiol. Physiol. 2017, 312, F661–F670. [Google Scholar] [CrossRef]
- Raha, A.A.; Chakraborty, S.; Henderson, J.; Mukaetova-Ladinska, E.; Zaman, S.; Trowsdale, J.; Raha-Chowdhury, R. Investigation of CD26, a potential SARS-CoV-2 receptor, as a biomarker of age and pathology. Biosci. Rep. 2020, 40, 20203092. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.A.; McCaughan, G.W.; Baker, E.; Sutherland, G.R. Genomic organization, exact localization, and tissue expression of the human CD26 (dipeptidyl peptidase IV) gene. Immunogenetics 1994, 40, 331–338. [Google Scholar] [CrossRef]
- Salgado, F.J.; Vela, E.; Martın, M.; Franco, R.; Nogueira, M.; Cordero, O.J. Mechanisms of CD26/dipeptidyl peptidase IV cytokine-dependent regulation on human activated lymphocytes. Cytokine 2000, 12, 1136–1141. [Google Scholar] [CrossRef]
- Fan, H.; Tansi, F.L.; Weihofen, W.A.; Böttcher, C.; Hu, J.; Martinez, J.; Saenger, W.; Reutter, W. Molecular mechanism and structural basis of interactions of dipeptidyl peptidase IV with adenosine deaminase and human immunodeficiency virus type-1 transcription transactivator. Eur. J. Cell Biol. 2012, 91, 265–273. [Google Scholar] [CrossRef]
- Morimoto, C.; Schlossman, S.F. The structure and function of CD26 in the T-cell immune response. Immunol. Rev. 1998, 161, 55–70. [Google Scholar] [CrossRef]
- Kameoka, J.; Tanaka, T.; Nojima, Y.; Schlossman, S.F.; Morimoto, C. Direct Association of Adenosine Deaminase with a T Cell Activation Antigen, CD26. Science 1993, 261, 466–469. [Google Scholar] [CrossRef]
- Lambeir, A.-M.; Durinx, C.; Scharpé, S.; De Meester, I. Dipeptidyl-Peptidase IV from Bench to Bedside: An Update on Structural Properties, Functions, and Clinical Aspects of the Enzyme DPP IV. Crit. Rev. Clin. Lab. Sci. 2003, 40, 209–294. [Google Scholar] [CrossRef]
- A Havre, P.; Dang, L.H.; Ohnuma, K.; Iwata, S.; Morimoto, C.; Dang, N.H. CD26 Expression on T-Anaplastic Large Cell Lymphoma (ALCL) Line Karpas 299 is associated with increased expression of Versican and MT1-MMP and enhanced adhesion. BMC Cancer 2013, 13, 517. [Google Scholar] [CrossRef] [PubMed]
- Gine, S.; Mariño, M.; Mallol, J.; Canela, E.I.; Morimoto, C.; Callebaut, C.; Hovanessian, A.; Casadó, V.; Lluis, C.; Franco, R. Regulation of epithelial and lymphocyte cell adhesion by adenosine deaminase-CD26 interaction. Biochem. J. 2002, 361, 203–209. [Google Scholar] [CrossRef]
- Samavati, L.; Uhal, B.D. ACE2, Much More Than Just a Receptor for SARS-COV-2. Front. Cell. Infect. Microbiol. 2020, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Zhang, Q.; Gefter, J.; Sneddon, W.B.; Mamonova, T.; Friedman, P.A. ACE2 interaction with cytoplasmic PDZ protein enhances SARS-CoV-2 invasion. iScience 2021, 24, 102770. [Google Scholar] [CrossRef]
- Kliche, J.; Kuss, H.; Ali, M.; Ivarsson, Y. Cytoplasmic short linear motifs in ACE2 and integrin β3 link SARS-CoV-2 host cell receptors to mediators of endocytosis and autophagy. Sci. Signal 2021, 14, 1117. [Google Scholar] [CrossRef]
- Hrenak, J.; Simko, F. Renin–angiotensin system: An important player in the pathogenesis of acute respiratory distress syndrome. Int. J. Mol. Sci. 2020, 21, 8038. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.L.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef]
- Brandi, M.L. Are sex hormones promising candidates to explain sex disparities in the COVID-19 pandemic? Rev. Endocr. Metab. Disord. 2022, 23, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Purkayastha, A.; Sen, C.; Garcia Jr, G.; Langerman, J.; Shia, D.W.; Meneses, L.K.; Vijayaraj, P.; Durra, A.; Koloff, C.R.; Freund, D.R.; et al. Direct Exposure to SARS-CoV-2 and Cigarette Smoke Increases Infection Severity and Alters the Stem Cell-Derived Airway Repair Response. Cell Stem Cell 2020, 27, 869–875.e4. [Google Scholar] [CrossRef]
- Simõese Silva, A.C.; Silveira, K.D.; Ferreira, A.J.; Teixeira, M.M. ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br. J. Pharmacol. 2013, 169, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shine, M.; Pyle, A.M.; Zhang, Y. US-align: Universal structure alignments of proteins, nucleic acids, and macromolecular complexes. Nat. Methods 2022, 19, 1109–1115. [Google Scholar] [CrossRef]
- Zhang, Y.; Skolnick, J. TM-align: A protein structure alignment algorithm based on the TM-score. Nucleic Acids Res. 2005, 33, 2302–2309. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef]
- Jando, J.; Camargo, S.; Herzog, B.; Verrey, F. Expression and regulation of the neutral amino acid transporter B0AT1 in rat small intestine. PLoS ONE 2017, 12, e0184845. [Google Scholar] [CrossRef]
- Du, Y.; Lu, C.; Morgan, R.L.; Stinson, W.A.; Campbell, P.L.; Cealey, E.; Fu, W.; Lepore, N.J.; Hervoso, J.L.; Cui, H.; et al. Angiogenic and Arthritogenic Properties of the Soluble Form of CD13. J. Immunol. 2019, 203, 360–369. [Google Scholar] [CrossRef]
- Bhagwat, S.V.; Lahdenranta, J.; Giordano, R.; Arap, W.; Pasqualini, R.; Shapiro, L.H. CD13/APN is activated by angiogenic signals and is essential for capillary tube formation. Blood J. Am. Soc. Hematol. 2001, 97, 652–659. [Google Scholar] [CrossRef]
- Fukasawa, K.; Fujii, H.; Saitoh, Y.; Koizumi, K.; Aozuka, Y.; Sekine, K.; Yamada, M.; Saiki, I.; Nishikawa, K. Aminopeptidase N (APN/CD13) is selectively expressed in vascular endothelial cells and plays multiple roles in angiogenesis. Cancer Lett. 2006, 243, 135–143. [Google Scholar] [CrossRef]
- Marchetti, C.; Di Carlo, A.; Facchiano, F.; Senatore, C.; De Cristofaro, R.; Luzi, A.; Federici, M.; Romani, M.; Napolitano, M.; Capogrossi, M.C.; et al. High mobility group box 1 is a novel substrate of dipeptidyl peptidase-IV. Diabetologia 2011, 55, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Fish, J.E.; White, M.D.; Yu, S.; Smyth, J.W.; Shaw, R.M.; DiMaio, J.M.; Srivastava, D. Stromal Cell-Derived Factor-1 Alpha is Cardioprotective After Myocardial Infarction. Circulation 2008, 117, 2224. [Google Scholar] [CrossRef] [PubMed]
- Hinsley, E.E.; de Oliveira, C.E.; Hunt, S.; Coletta, R.D.; Lambert, D.W. Angiotensin 1-7 inhibits angiotensin II-stimulated head and neck cancer progression. Eur. J. Oral Sci. 2017, 125, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Kangussu, L.M.; Marzano, L.A.; Souza, C.F.; Dantas, C.C.; Miranda, A.S.; Simoes e Silva, A.C. The Renin-Angiotensin System and the Cerebrovascular Diseases: Experimental and Clinical Evidence. Protein Pept. Lett. 2019, 27, 463–475. [Google Scholar] [CrossRef]
- Meng, Y.; Yu, C.H.; Li, W.; Li, T.; Luo, W.; Huang, S.; Wu, P.S.; Cai, S.X.; Li, X. Angiotensin-converting enzyme 2/angiotensin-(1-7)/mas axis protects against lung fibrosis by inhibiting the MAPK/NF-κB pathway. Am. J. Respir. Cell Mol. Biol. 2014, 50, 723–736. [Google Scholar] [CrossRef]
- Passos-Silva, D.G.; Verano-Braga, T.; Santos, R.A.S. Angiotensin-(1–7): Beyond the cardio-renal actions. Clin. Sci. 2012, 124, 443–456. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, T.; Chen, Y.; Sun, Y. Angiotensin 1-7 Promotes Cardiac Angiogenesis Following Infarction. Curr. Vasc. Pharmacol. 2015, 13, 37–42. [Google Scholar] [CrossRef]
- Yu, C.; Tang, W.; Wang, Y.; Shen, Q.; Wang, B.; Cai, C.; Meng, X.; Zou, F. Downregulation of ACE2/Ang-(1-7)/Mas axis promotes breast cancer metastasis by enhancing store-operated calcium entry. Cancer Lett. 2016, 376, 268–277. [Google Scholar] [CrossRef]
- Yang, T.; Xu, C. Physiology and Pathophysiology of the Intrarenal Renin-Angiotensin System: An Update. J. Am. Soc. Nephrol. 2017, 28, 1040–1049. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Goto, Y.; Maruyama, M.; Hattori, A. Biochemical and enzymatic properties of the M1 family of aminopeptidases involved in the regulation of blood pressure. Heart Fail. Rev. 2007, 13, 285–291. [Google Scholar] [CrossRef]
- Chaudhary, M. Anti-Hypertensive Potential and Epigenetics of Angiotensin II type 2 Receptor (AT2R). Curr. Hypertens. Rev. 2021, 17, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Aroor, A.; Zuberek, M.; Duta, C.; Meuth, A.; Sowers, J.R.; Whaley-Connell, A.; Nistala, R. Angiotensin II Stimulation of DPP4 Activity Regulates Megalin in the Proximal Tubules. Int. J. Mol. Sci. 2016, 17, 780. [Google Scholar] [CrossRef]
- Valencia, I.; Peiró, C.; Lorenzo, Ó.; Sánchez-Ferrer, C.F.; Eckel, J.; Romacho, T. DPP4 and ACE2 in Diabetes and COVID-19: Therapeutic Targets for Cardiovascular Complications? Front. Pharmacol. 2020, 11, 1161. [Google Scholar] [CrossRef] [PubMed]
- Ikushima, H.; Munakata, Y.; Ishii, T.; Iwata, S.; Terashima, M.; Tanaka, H.; Schlossman, S.F.; Morimoto, C. Internalization of CD26 by mannose 6-phosphate/insulin-like growth factor II receptor contributes to T cell activation. Proc. Natl. Acad. Sci. USA 2000, 97, 8439–8444. [Google Scholar] [CrossRef]
- Bindom, S.M.; Lazartigues, E. The sweeter side of ACE2: Physiological evidence for a role in diabetes. Mol. Cell. Endocrinol. 2009, 302, 193–202. [Google Scholar] [CrossRef]
- Narula, S.; Yusuf, S.; Chong, M.; Ramasundarahettige, C.; Rangarajan, S.; Bangdiwala, I.S.; van Eikels, M.; Leineweber, K.; Wu, A.; Pigeyre, M.; et al. Plasma ACE2 and risk of death or cardiometabolic diseases: A case-cohort analysis. Lancet 2020, 396, 968–976. [Google Scholar] [CrossRef]
- Park, S.E.; Kim, W.J.; Park, S.W.; Park, J.W.; Lee, N.; Park, C.Y.; Youn, B.S. High urinary ACE2 concentrations are associated with severity of glucose intolerance and microalbuminuria. Eur. J. Endocrinol. Eur. Fed. Endocr. Soc. 2013, 168, 203–210. [Google Scholar] [CrossRef]
- Cherney, D.Z.; Xiao, F.; Zimpelmann, J.; Har, R.L.; Lai, V.; Scholey, J.W.; Reich, H.N.; Burns, K.D. Urinary ACE2 in healthy adults and patients with uncomplicated type 1 diabetes. Can. J. Physiol. Pharmacol. 2014, 92, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.P.; Ho, J.C.; Cheung, M.C.; Ng, K.C.; Ching, R.H.; Lai, K.L.; Kam, T.T.; Gu, H.; Sit, K.Y.; Hsin, M.K.; et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 2022, 603, 715–720. [Google Scholar] [CrossRef]
- Gong, T.; Zhang, X.; Peng, Z.; Ye, Y.; Liu, R.; Yang, Y.; Chen, Z.; Zhang, Z.; Hu, H.; Yin, S.; et al. Macrophage-derived exosomal aminopeptidase N aggravates sepsis-induced acute lung injury by regulating necroptosis of lung epithelial cell. Commun. Biol. 2022, 5, 543. [Google Scholar] [CrossRef]
- Devarakonda, C.K.V.; Meredith, E.; Ghosh, M.; Shapiro, L.H. Coronavirus Receptors as Immune Modulators. J. Immunol. 2021, 206, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M. Proteolytic activation of SARS-CoV-2 spike protein. Microbiol. Immunol. 2021, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Channappanavar, R.; Kanneganti, T.-D. Coronaviruses: Innate Immunity, Inflammasome Activation, Inflammatory Cell Death, and Cytokines. Trends Immunol. 2020, 41, 1083–1099. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fehr, A.R.; Zheng, J.; Wohlford-Lenane, C.; Abrahante, J.E.; Mack, M.; Sompallae, R.; McCray, P.B.; Meyerholz, D.K.; Perlman, S. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J. Clin. Investig. 2019, 129, 3625–3639. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Züst, R.; Weber, F.; Spiegel, M.; Lang, K.S.; Akira, S.; Thiel, V.; Ludewig, B. Control of coronavirus infection through plasmacytoid dendritic-cell–derived type I interferon. Blood 2006, 109, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.K.; Firmal, P.; Alam, A.; Ganguly, D.; Chattopadhyay, S. Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past. Front Immunol 2020, 11, 1949. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.A.P.M.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tsang, O.T.-Y.; et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 2020, 369, 1210–1220. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, S.; Jeong, H.W.; Ahn, J.Y.; Choi, S.J.; Lee, H.; Choi, B.; Nam, S.K.; Sa, M.; Kwon, J.-S.; et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci. Immunol. 2020, 5, 1554. [Google Scholar] [CrossRef]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Péré, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Al-Maiahy, T.J.; Alexiou, A.; Mukerjee, N.; Batiha, G.E.-S. An insight into the placental growth factor (PlGf)/angii axis in Covid-19: A detrimental intersection. Biotechnol. Genet. Eng. Rev. 2022, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Hansen, G.H.; NIELS-Christiansen, L.L.; Thorsen, E.; Immerdal, L.; Santos, A.N.; Kehlen, A.; Langner, J.; Danielsen, E.M. Caveolae/lipid rafts in fibroblast-like synoviocytes: Ectopeptidase-rich membrane microdomains. Biochem. J. 2001, 354, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, M.E.; Hemmati, R.; Tashakor, A.; Homaei, A.; Yousefzadeh, M.; Hemati, K.; Hosseinkhani, S. Induced dysregulation of ACE2 by SARS-CoV-2 plays a key role in COVID-19 severity. Biomed. Pharmacother. 2021, 137, 11363. [Google Scholar] [CrossRef] [PubMed]
- Norooznezhad, A.H.; Mansouri, K. Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID-19). Microvasc. Res. 2021, 137, 104188. [Google Scholar] [CrossRef] [PubMed]
- Pelle, M.C.; Zaffina, I.; Lucà, S.; Forte, V.; Trapanese, V.; Melina, M.; Giofrè, F.; Arturi, F. Endothelial Dysfunction in COVID-19: Potential Mechanisms and Possible Therapeutic Options. Life 2022, 12, 1605. [Google Scholar] [CrossRef]
- Brand, J.M.V.D.; Smits, S.L.; Haagmans, B.L. Pathogenesis of Middle East respiratory syndrome coronavirus. J. Pathol. 2014, 235, 175–184. [Google Scholar] [CrossRef]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19). Diabetes Metab. Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef]
- Frydrych, L.M.; Bian, G.; O’Lone, D.E.; Ward, P.A.; Delano, M.J. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J. Leukoc. Biol. 2018, 104, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef]
- Booth, C.M. Clinical Features and Short-term Outcomes of 144 Patients With SARS in the Greater Toronto Area. JAMA 2003, 289, 2801–2809. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.J.; Santos, R.A.; Bradford, C.N.; Mecca, A.P.; Sumners, C.; Katovich, M.J.; Raizada, M.K. Therapeutic Implications of the Vasoprotective Axis of the Renin-Angiotensin System in Cardiovascular Diseases. Hypertension 2010, 55, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.C.J.L.; Krieger, J.E.; Pereira, A.C. Renin^|^ndash;Angiotensin System, Hypertension, and Chronic Kidney Disease: Pharmacogenetic Implications. J. Pharmacol. Sci. 2012, 120, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Frühbeck, G.; Catalán, V.; Valentí, V.; Moncada, R.; Gómez-Ambrosi, J.; Becerril, S.; Silva, C.; Portincasa, P.; Escalada, J.; Rodríguez, A. FNDC4 and FNDC5 reduce SARS-CoV-2 entry points and spike glycoprotein S1-induced pyroptosis, apoptosis, and necroptosis in human adipocytes. Cell Mol. Immunol. 2021, 18, 2457–2459. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Colón, G.J.; Ratnasiri, K.; Chen, H.; Jiang, S.; Zanley, E.; Rustagi, A.; Verma, R.; Chen, H.; Andrews, J.R.; Mertz, K.D.; et al. SARS-CoV-2 infection drives an inflammatory response in human adipose tissue through infection of adipocytes and macrophages. Sci. Transl. Med. 2022, 14, eabm9151. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef]
- Kassir, R. Risk of COVID-19 for patients with obesity. Obes. Rev. 2020, 21, e13034. [Google Scholar] [CrossRef]
- Boni, M.F.; Lemey, P.; Jiang, X.; Lam, T.T.Y.; Perry, B.W.; Castoe, T.A.; Rambaut, A.; Robertson, D.L. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat. Microbiol. 2020, 5, 1408–1417. [Google Scholar] [CrossRef]
- Ryan, P.M.D.; Caplice, N.M. Is Adipose Tissue a Reservoir for Viral Spread, Immune Activation, and Cytokine Amplification in Coronavirus Disease 2019? Obesity (Silver Spring) 2020, 28, 1191–1194. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Ayajiki, K.; Nishio, Y.; Sugaya, T.; Kashiwagi, A.; Okamura, T. Evidence for a Causal Role of the Renin-Angiotensin System in Vascular Dysfunction Associated With Insulin Resistance. Hypertension 2004, 43, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Negrel, R.; Sharma, A.M. Physiology and Pathophysiology of the Adipose Tissue Renin-Angiotensin System. Hypertension 2000, 35, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Rathod, M.A.; Rogers, P.M.; Vangipuram, S.D.; McAllister, E.J.; Dhurandhar, N.V. Adipogenic Cascade Can Be Induced Without Adipogenic Media by a Human Adenovirus. Obesity 2009, 17, 657–664. [Google Scholar] [CrossRef]
- Iyer, A.; Fairlie, D.P.; Prins, J.B.; Hammock, B.D.; Brown, L. Inflammatory lipid mediators in adipocyte function and obesity. Nat. Rev. Endocrinol. 2010, 6, 71–82. [Google Scholar] [CrossRef]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Lee, I.T.; Cheng, R.; Nakayama, T.; Jiang, S.; Demeter, J.; Bevacqua, R.J.; Chang, C.A.; et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021, 33, 1565–1576.e5. [Google Scholar] [CrossRef]
- Tang, X.; Uhl, S.; Zhang, T.; Xue, D.; Li, B.; Vandana, J.J.; Acklin, J.A.; Bonnycastle, L.L.; Narisu, N.; Erdos, M.R.; et al. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab. 2021, 33, 1577–1591.e7. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Green, W.D.; Beck, M.A.; Algaith, T.; Herbst, C.H.; Alsukait, R.F.; Alluhidan, M.; Alazemi, N.; Shekar, M. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes. Rev. 2020, 21, e13128. [Google Scholar] [CrossRef]
- Ana, H.D.A.; de Medeiros, A.F.; Medeiros, I.; de Lima, V.C.; Luz, A.B.; Maciel, B.L.; Passos, T.S. Tamarind ( Tamarindus indica L.) Seed a Candidate Protein Source with Potential for Combating SARS-CoV-2 Infection in Obesity. Drug Target Insights 2021, 15, 5–12. [Google Scholar]
- Jia, H.; Neptune, E.; Cui, H. Targeting ACE2 for COVID-19 Therapy: Opportunities and Challenges. Am. J. Respir. Cell Mol. Biol. 2021, 64, 416–425. [Google Scholar] [CrossRef]
- Asai, A.; Konno, M.; Ozaki, M.; Otsuka, C.; Vecchione, A.; Arai, T.; Kitagawa, T.; Ofusa, K.; Yabumoto, M.; Hirotsu, T.; et al. COVID-19 Drug Discovery Using Intensive Approaches. Int. J. Mol. Sci. 2020, 21, 2839. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Vardeny, O.; Michel, T.; McMurray, J.J.; Pfeffer, M.A.; Solomon, S.D. Renin–Angiotensin–Aldosterone System Inhibitors in Patients with COVID-19. N. Engl. J. Med. 2020, 382, 1653–1659. [Google Scholar] [CrossRef]
- Nojomi, M.; Yassin, Z.; Keyvani, H.; Makiani, M.J.; Roham, M.; Laali, A.; Dehghan, N.; Navaei, M.; Ranjbar, M. Effect of Arbidol (Umifenovir) on COVID-19: A randomized controlled trial. BMC Infect. Dis. 2020, 20, 954. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.C.; Zhang, H.X.; Zhang, Z.; Rinkiko, S.; Cui, Y.M.; Zhu, Y.Z. The Two-Way Switch Role of ACE2 in the Treatment of Novel Coronavirus Pneumonia and Underlying Comorbidities. Molecules 2021, 26, 142. [Google Scholar] [CrossRef]
- El Ouafi, Z.; Rhalem, W.; Habib, N.; Azami, A.I.; Sehli, S.; Al Idrissi, N.; Bakkali, F.; Abderrazak, R.; Merzouki, M.; Allali, I.; et al. Molecular Modeling Targeting the ACE2 Receptor with Cannabis sativa’s Active Ingredients for Antiviral Drug Discovery against SARS-CoV-2 Infections. Bioinform. Biol. Insights 2022, 16, 11779322221145380. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, S.; Lockey, R.F.; Kolliputi, N. Soluble ACE2 as a potential therapy for COVID-19. Am. J. Physiol. Physiol. 2021, 320, C279–C281. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Benthin, C.; Zeno, B.; Albertson, T.E.; Boyd, J.; Christie, J.; Hall, R.; Poirier, G.; Ronco, J.J.; Tidswell, M.; et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care 2017, 21, 234. [Google Scholar] [CrossRef]
- Murça, T.M.; Moraes, P.L.; Capuruço, C.A.; Santos, S.H.; Melo, M.B.; Santos, R.A.; Shenoy, V.; Katovich, M.J.; Raizada, M.K.; Ferreira, A.J. Oral administration of an angiotensin-converting enzyme 2 activator ameliorates diabetes-induced cardiac dysfunction. Regul. Pept. 2012, 177, 107–115. [Google Scholar] [CrossRef]
- Iwanaga, N.; Cooper, L.; Rong, L.; Maness, N.J.; Beddingfield, B.; Qin, Z.; Crabtree, J.; Tripp, R.A.; Yang, H.; Blair, R.; et al. ACE2-IgG1 fusions with improved in vitro and in vivo activity against SARS-CoV-2. iScience 2022, 25, 103670. [Google Scholar] [CrossRef]
- de Ligt, M.; Hesselink, M.K.; Jorgensen, J.; Hoebers, N.; Blaak, E.E.; Goossens, G.H. Resveratrol supplementation reduces ACE2 expression in human adipose tissue. Adipocyte 2021, 10, 408–411. [Google Scholar] [CrossRef]
- Jardine, M.J.; Kotwal, S.S.; Bassi, A.; Hockham, C.; Jones, M.; Wilcox, A.; Pollock, C.; Burrell, L.M.; McGree, J.; Rathore, V.; et al. Angiotensin receptor blockers for the treatment of covid-19: Pragmatic, adaptive, multicentre, phase 3, randomised controlled trial. BMJ 2022, 379, e072175. [Google Scholar] [CrossRef]
- Kotfis, K.; Lechowicz, K.; Drożdżal, S.; Niedźwiedzka-Rystwej, P.; Wojdacz, T.K.; Grywalska, E.; Biernawska, J.; Wiśniewska, M. COVID-19—The potential beneficial therapeutic effects of spironolactone during SARS-CoV-2 infection. Pharmaceuticals 2021, 14, 71. [Google Scholar] [CrossRef]
- Saeedi Saravi, S.S.; Beer, J.H. Apelin-potential therapy for COVID-19? J. Mol. Cell Cardiol. 2020, 145, 84–87. [Google Scholar] [CrossRef]
- Valdez-Cruz, N.A.; Garcia-Hernandez, E.; Espitia, C.; Cobos-Marín, L.; Altamirano, C.; Bando-Campos, C.G.; Cofas-Vargas, L.F.; Coronado-Aceves, E.W.; Gonzalez-Hernandez, R.A.; Hernandez-Peralta, P.; et al. Integrative overview of antibodies against SARS-CoV-2 and their possible applications in COVID-19 prophylaxis and treatment. Microb. Cell Fact. 2021, 20, 88. [Google Scholar] [CrossRef]
- Cascella, M.; Rajnik, M.; Dulebohn, S.C.; di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19); StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Berry, J.D.; Hay, K.; Rini, J.M.; Yu, M.; Wang, L.; Plummer, F.A.; Corbett, C.R.; Andonov, A. Neutralizing epitopes of the SARS-CoV S-protein cluster independent of repertoire, antigen structure or mAb technology. MAbs 2010, 2, 53–66. [Google Scholar] [CrossRef]
- Du, L.; Yang, Y.; Zhou, Y.; Lu, L.; Li, F.; Jiang, S. MERS-CoV spike protein: A key target for antivirals. Expert Opin. Ther. Targets 2017, 21, 131–143. [Google Scholar] [CrossRef]
- Wang, P.; Casner, R.G.; Nair, M.S.; Yu, J.; Guo, Y.; Wang, M.; Chan, J.F.W.; Cerutti, G.; Iketani, S.; Liu, L.; et al. A monoclonal antibody that neutralizes SARS-CoV-2 variants, SARS-CoV, and other sarbecoviruses. Emerg. Microbes Infect. 2022, 11, 147–157. [Google Scholar] [CrossRef]
- Akbar, R.; Bashour, H.; Rawat, P.; Robert, P.A.; Smorodina, E.; Cotet, T.-S.; Flem-Karlsen, K.; Frank, R.; Mehta, B.B.; Vu, M.H.; et al. Progress and challenges for the machine learning-based design of fit-for-purpose monoclonal antibodies. Mabs 2022, 14. [Google Scholar] [CrossRef]
- Pang, J.; Liu, M.; Ling, W.; Jin, T. Friend or foe? ACE2 inhibitors and GLP-1R agonists in COVID-19 treatment. Obes. Med. 2021, 22, 100312. [Google Scholar] [CrossRef]
- Haber, P.K.; Ye, M.; Wysocki, J.; Maier, C.; Haque, S.K.; Batlle, D. Angiotensin-converting enzyme 2-independent action of presumed angiotensin-converting enzyme 2 activators: Studies in vivo, ex vivo, and in vitro. Hypertension 2014, 63, 774–782. [Google Scholar] [CrossRef]
- Shapira, T.; Monreal, I.A.; Dion, S.P.; Buchholz, D.W.; Imbiakha, B.; Olmstead, A.D.; Jager, M.; Désilets, A.; Gao, G.; Martins, M.; et al. A TMPRSS2 inhibitor acts as a pan-SARS-CoV-2 prophylactic and therapeutic. Nature 2022, 605, 340–348. [Google Scholar] [CrossRef]
- Fraser, B.J.; Beldar, S.; Seitova, A.; Hutchinson, A.; Mannar, D.; Li, Y.; Kwon, D.; Tan, R.; Wilson, R.P.; Leopold, K.; et al. Structure and activity of human TMPRSS2 protease implicated in SARS-CoV-2 activation. Nat. Chem. Biol. 2022, 18, 963–971. [Google Scholar] [CrossRef]
- Howell, R.; Clarke, M.A.; Reuschl, A.K.; Chen, T.; Abbott-Imboden, S.; Singer, M.; Lowe, D.M.; Bennett, C.L.; Chain, B.; Jolly, C.; et al. Executable network of SARS-CoV-2-host interaction predicts drug combination treatments. Npj Digit. Med. 2022, 5, 18. [Google Scholar] [CrossRef]
- Leach, D.A.; Mohr, A.; Giotis, E.S.; Cil, E.; Isac, A.M.; Yates, L.L.; Barclay, W.S.; Zwacka, R.M.; Bevan, C.L.; Brooke, G.N. The antiandrogen enzalutamide downregulates TMPRSS2 and reduces cellular entry of SARS-CoV-2 in human lung cells. Nat. Commun. 2021, 12, 4068. [Google Scholar] [CrossRef]
- Chen, Y.; Lear, T.B.; Evankovich, J.W.; Larsen, M.B.; Lin, B.; Alfaras, I.; Kennerdell, J.R.; Salminen, L.; Camarco, D.P.; Lockwood, K.C.; et al. A high-throughput screen for TMPRSS2 expression identifies FDA-approved compounds that can limit SARS-CoV-2 entry. Nat. Commun. 2021, 12, 3907. [Google Scholar] [CrossRef]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef]
- Zhao, M.M.; Zhu, Y.; Zhang, L.; Zhong, G.; Tai, L.; Liu, S.; Yin, G.; Lu, J.; He, Q.; Li, M.J.; et al. Novel cleavage sites identified in SARS-CoV-2 spike protein reveal mechanism for cathepsin L-facilitated viral infection and treatment strategies. Cell Discov. 2022, 8, 53. [Google Scholar] [CrossRef]
- Zhao, M.M.; Yang, W.L.; Yang, F.Y.; Zhang, L.; Huang, W.J.; Hou, W.; Fan, C.F.; Jin, R.H.; Feng, Y.M.; Wang, Y.C.; et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Target. Ther. 2021, 6, 134. [Google Scholar] [CrossRef]
- Zadeh, N.M.; Asl, N.S.M.; Forouharnejad, K.; Ghadimi, K.; Parsa, S.; Mohammadi, S.; Omidi, A. Mechanism and adverse effects of COVID-19 drugs: A basic review. Int. J. Physiol. Pathophysiol. Pharmacol. 2021, 13, 102. [Google Scholar]
- Forni, D.; Cagliani, R.; Clerici, M.; Sironi, M. Molecular Evolution of Human Coronavirus Genomes. Trends Microbiol. 2016, 25, 35–48. [Google Scholar] [CrossRef]
- Heilmann, E.; Costacurta, F.; Moghadasi, S.A.; Ye, C.; Pavan, M.; Bassani, D.; Volland, A.; Ascher, C.; Weiss, A.K.H.; Bante, D.; et al. SARS-CoV-2 3CL pro mutations selected in a VSV-based system confer resistance to nirmatrelvir, ensitrelvir, and GC376. Sci. Transl. Med. 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Silveira, M.A.D.; De Jong, D.; Berretta, A.A.; dos Santos Galvão, E.B.; Ribeiro, J.C.; Cerqueira-Silva, T.; Amorim, T.C.; da Conceicao, L.F.M.R.; Gomes, M.M.D.; Teixeira, M.B.; et al. Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial. Biomed. Pharmacother. 2021, 138, 111526. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Fernandez, J.; Ochoa, J.; Ojeda, M.L.; Nogales, F.; Carreras, O.; Díaz-Castro, J. Inflammation and oxidative stress, the links between obesity and COVID-19: A narrative review. J. Physiol. Biochem. 2022, 78, 581–591. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).