Rickettsia Vaccine Candidate pVAX1-OmpB24 Stimulates TCD4+INF-γ+ and TCD8+INF-γ+ Lymphocytes in Autologous Co-Culture of Human Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
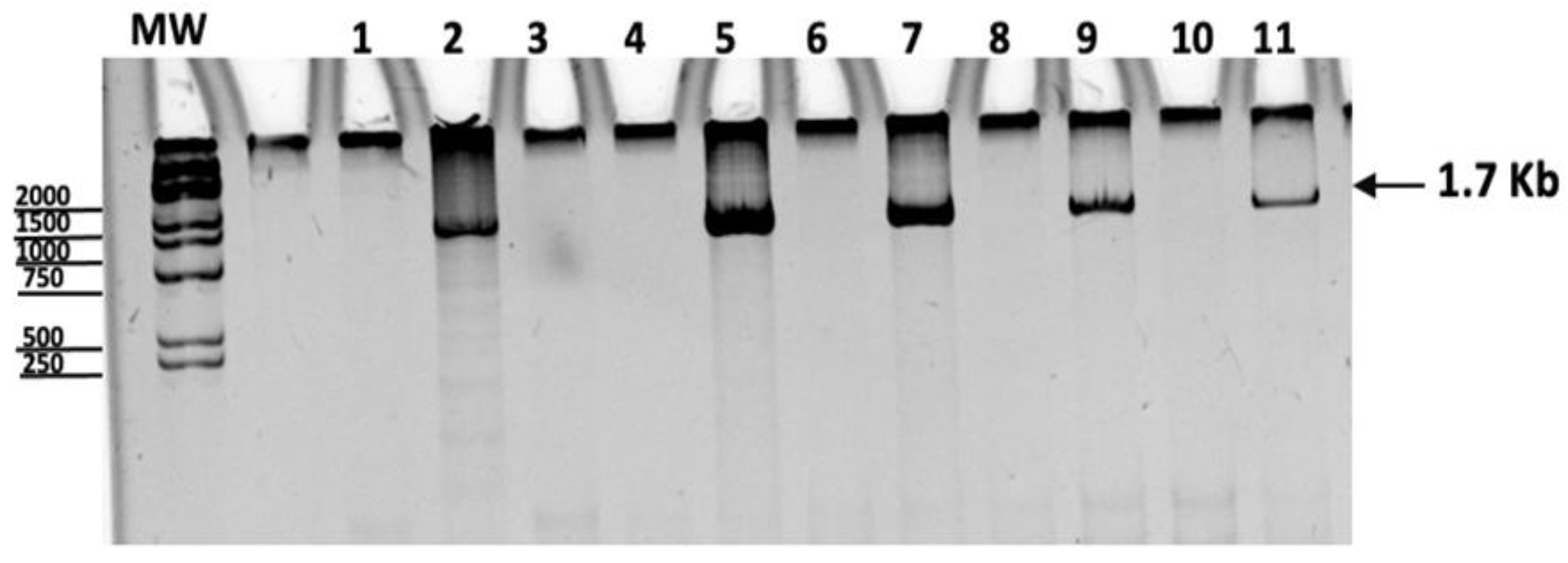
2.2. Molecular Identification of Rickettsia in Patients
2.3. Isolation of Lymphocytes and Monocytes from Peripheral Blood
2.4. Differentiation and Transfection of Monocyte-Derived Macrophages
2.5. Autologous Culture of Lymphocytes with Transfected Macrophages
2.6. Flow Cytometric Analysis
3. Results
3.1. Study Population
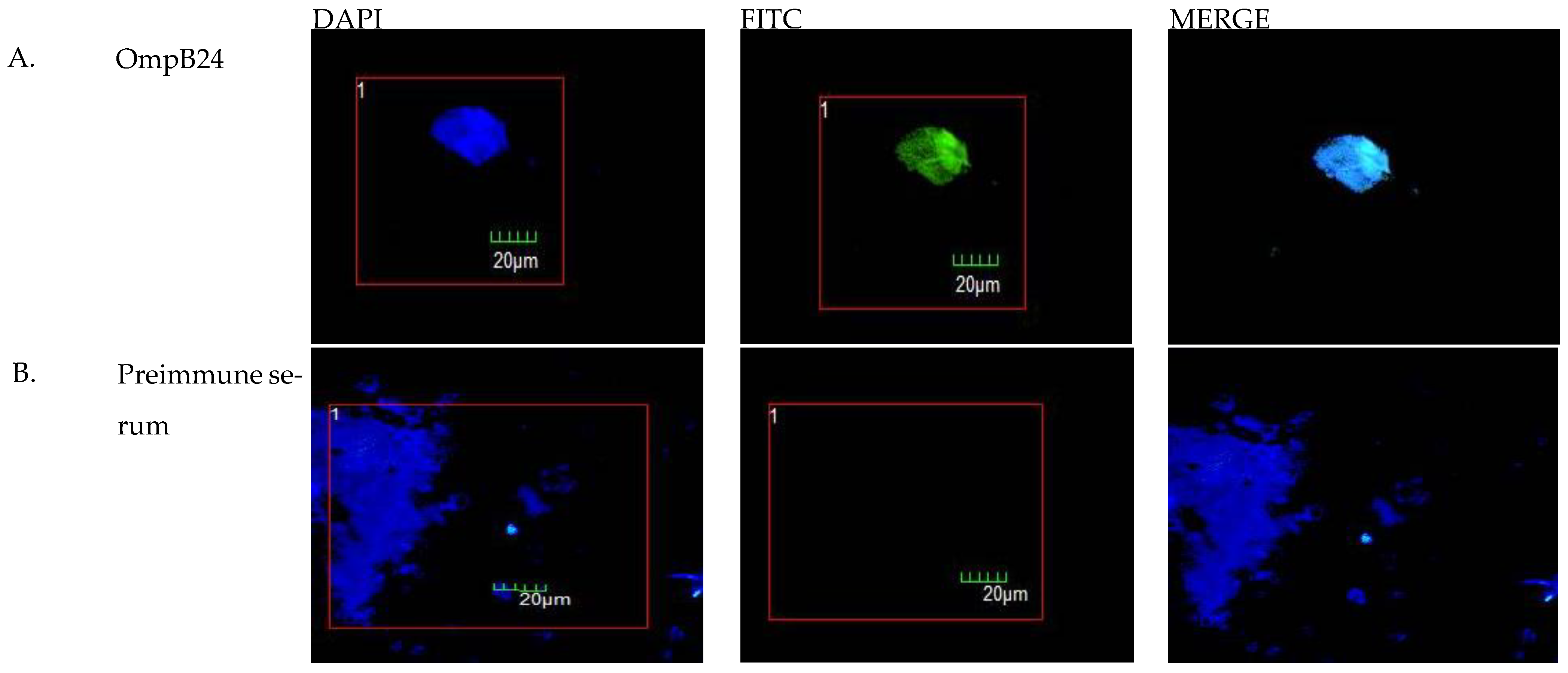
3.2. Transfection of Macrophages with pVAX1-OmpB24
3.3. Flow Cytometric Analysis
TCD4+INF-γ+ and TCD8+INF-γ+ Lymphocytes Induced by Human Macrophages Transfected with pVAX1-OmpB24
3.4. PD1 Expression in TCD4+ and TCD8+ Lymphocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osterloh, A. The Neglected Challenge: Vaccination against Rickettsiae. PLoS Negl. Trop. Dis. 2020, 14, e0008704. [Google Scholar] [CrossRef]
- Moreira, J.; Bressan, C.S.; Brasil, P.; Siqueira, A.M. Epidemiology of Acute Febrile Illness in Latin America. Clin. Microbiol. Infect. 2018, 24, 827–835. [Google Scholar] [CrossRef]
- Blanton, L.S.; Wilson, N.M.; Quade, B.R.; Walker, D.H. Susceptibility of Rickettsia Rickettsii to Tigecycline in a Cell Culture Assay and Animal Model for Rocky Mountain Spotted Fever. Am. J. Trop. Med. Hyg. 2019, 101, 1091–1095. [Google Scholar] [CrossRef]
- Helgren, T.R.; Chen, C.; Wangtrakuldee, P.; Edwards, T.E.; Staker, B.L.; Abendroth, J.; Sankaran, B.; Housley, N.A.; Myler, P.J.; Audia, J.P.; et al. Rickettsia Prowazekii Methionine Aminopeptidase as a Promising Target for the Development of Antibacterial Agents. Bioorg. Med. Chem. 2017, 25, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Qi, Y.; Xiong, X.; Jiao, J.; Duan, C.; Wen, B. Rickettsia Rickettsii Outer Membrane Protein YbgF Induces Protective Immunity in C3H/HeN Mice. Hum. Vaccines Immunother. 2015, 11, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Xiong, X.; Qi, Y.; Jiao, J.; Duan, C.; Wen, B. Surface Protein Adr2 of Rickettsia Rickettsii Induced Protective Immunity against Rocky Mountain Spotted Fever in C3H/HeN Mice. Vaccine 2014, 32, 2027–2033. [Google Scholar] [CrossRef]
- Riley, S.P.; Cardwell, M.M.; Chan, Y.G.Y.; Pruneau, L.; Del Piero, F.; Martinez, J.J. Failure of a Heterologous Recombinant Sca5/OmpB Protein-Based Vaccine to Elicit Effective Protective Immunity against Rickettsia Rickettsii Infections in C3H/HeN Mice. Pathog. Dis. 2015, 73, ftv101. [Google Scholar] [CrossRef]
- Giordano, M. Patologia, parassitologia ed igiene del paesi caldi. J. Am. Med. Assoc. 1951, 145, 358–359. [Google Scholar] [CrossRef]
- Cox, H.R. Cultivation of Rickettsiae of the Rocky Mountain Spotted Fever, Typhus and Q Fever Groups in the Embryonic Tissues of Developing Chicks. Science 1941, 94, 399–403. [Google Scholar] [CrossRef]
- Kenyon, R.H.; Pedersen, C.E. Preparation of Rocky Mountain Spotted Fever Vaccine Suitable for Human Immunization. J. Clin. Microbiol. 1975, 1, 500–503. [Google Scholar] [CrossRef]
- Clements, M.L.; Wisseman, C.L.; Woodward, T.E.; Fiset, P.; Dumler, J.S.; McNamee, W.; Black, R.E.; Rooney, J.; Hughes, T.P.; Levine, M.M. Reactogenicity, Immunogenicity, and Efficacy of a Chick Embryo Cell-Derived Vaccine for Rocky Mountain Spotted Fever. J. Infect. Dis. 1983, 148, 922–930. [Google Scholar] [CrossRef] [PubMed]
- DuPont, H.L.; Hornick, R.B.; Dawkins, A.T.; Heiner, G.G.; Fabrikant, I.B.; Wisseman, C.L.; Woodward, T.E. Rocky Mountain Spotted Fever: A Comparative Study of the Active Immunity Induced by Inactivated and Viable Pathogenic Rickettsia Rickettsii. J. Infect. Dis. 1973, 128, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Crocquet-Valdes, P.A.; Díaz-Montero, C.M.; Feng, H.M.; Li, H.; Barrett, A.D.; Walker, D.H. Immunization with a Portion of Rickettsial Outer Membrane Protein A Stimulates Protective Immunity against Spotted Fever Rickettsiosis. Vaccine 2001, 20, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xiong, X.; Jiao, J.; Yang, X.; Jiang, Y.; Wen, B.; Gong, W. Th1 Epitope Peptides Induce Protective Immunity against Rickettsia Rickettsii Infection in C3H/HeN Mice. Vaccine 2017, 35, 7204–7212. [Google Scholar] [CrossRef] [PubMed]
- Moderzynski, K.; Heine, L.; Rauch, J.; Papp, S.; Kuehl, S.; Richardt, U.; Fleischer, B.; Osterloh, A. Cytotoxic Effector Functions of T Cells Are Not Required for Protective Immunity against Fatal Rickettsia Typhi Infection in a Murine Model of Infection: Role of TH1 and TH17 Cytokines in Protection and Pathology. PLoS Negl. Trop. Dis. 2017, 11, e0005404. [Google Scholar] [CrossRef]
- Meng, Y.; Xiong, X.; Qi, Y.; Duan, C.; Gong, W.; Jiao, J.; Wen, B. Protective Immunity against Rickettsia Heilongjiangensis in a C3H/HeN Mouse Model Mediated by Outer Membrane Protein B-Pulsed Dendritic Cells. Sci. China Life Sci. 2015, 58, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Dzul-Rosado, K.; Balam-Romero, J.; Valencia-Pacheco, G.; Lugo-Caballero, C.; Arias-León, J.; Peniche-Lara, G.; Zavala-Castro, J. Immunogenicity of OmpA and OmpB Antigens from Rickettsia Rickettsii on Mononuclear Cells from Rickettsia Positive Mexican Patients. J. Vector Borne Dis. 2017, 54, 317. [Google Scholar] [CrossRef]
- Blair, P.J.; Jiang, J.; Schoeler, G.B.; Moron, C.; Anaya, E.; Cespedes, M.; Cruz, C.; Felices, V.; Guevara, C.; Mendoza, L.; et al. Characterization of Spotted Fever Group Rickettsiae in Flea and Tick Specimens from Northern Peru. J. Clin. Microbiol. 2004, 42, 4961–4967. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Jang, W.-J.; Kim, J.-H.; Ryu, J.-S.; Lee, S.-H.; Park, K.-H.; Paik, H.-S.; Koh, Y.-S.; Choi, M.-S.; Kim, I.-S. Spotted Fever Group and Typhus Group Rickettsioses in Humans, South Korea. Emerg. Infect. Dis. 2005, 11, 237–244. [Google Scholar] [CrossRef]
- Zavala-Castro, J.E.; Zavala-Velázquez, J.E.; Walker, D.H.; Arcila, E.E.R.; Laviada-Molina, H.; Olano, J.P.; Ruiz-Sosa, J.A.; Small, M.A.; Dzul-Rosado, K.R. Fatal Human Infection with Rickettsia Rickettsii, Yucatán, Mexico. Emerg. Infect. Dis. 2006, 12, 672–674. [Google Scholar] [CrossRef]
- Zavala-Velazquez, J.E.; Ruiz-Sosa, J.; Vado-Solis, I.; Billings, A.N.; Walker, D.H. Serologic Study of the Prevalence of Rickettsiosis in Yucatán: Evidence for a Prevalent Spotted Fever Group Rickettsiosis. Am. J. Trop. Med. Hyg. 1999, 61, 405–408. [Google Scholar] [CrossRef]
- Álvarez de Haro, N. Cultivos Celulares Para el Desarrollo de Vacunas DNA Frente a Virus de Peces Utilizando el Modelo de Trucha Arco Iris/Rhabdovirus de la Septicemia Hemorrágica Vírica (VHSV). Ph.D. Thesis, Universidad de León, León, Spain, 2015. [Google Scholar] [CrossRef]
- Li, Z.; Díaz-Montero, C.M.; Valbuena, G.; Yu, X.-J.; Olano, J.P.; Feng, H.-M.; Walker, D.H. Identification of CD8 T-Lymphocyte Epitopes in OmpB of Rickettsia Conorii. Infect. Immun. 2003, 71, 3920–3926. [Google Scholar] [CrossRef] [PubMed]
- Dolskiy, A.A.; Grishchenko, I.V.; Yudkin, D.V. Cell Cultures for Virology: Usability, Advantages, and Prospects. Int. J. Mol. Sci. 2020, 21, 7978. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K.; et al. A More Efficient Method to Generate Integration-Free Human IPS Cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.; Bisceglia, H.; Girerd-Chambaz, Y.; Burdin, N.; Chaux, P. HLA-DR and HLA-DP Restricted Epitopes from Human Cytomegalovirus Glycoprotein B Recognized by CD4+ T-Cell Clones from Chronically Infected Individuals. J. Clin. Immunol. 2012, 32, 1305. [Google Scholar] [CrossRef]
- Dokka, S.; Toledo, D.; Shi, X.; Ye, J.; Rojanasakul, Y. High-Efficiency Gene Transfection of Macrophages by Lipoplexes. Int. J. Pharm. 2000, 206, 97–104. [Google Scholar] [CrossRef]
- Osorio, J.S.; Bionaz, M. Plasmid Transfection in Bovine Cells: Optimization Using a Realtime Monitoring of Green Fluorescent Protein and Effect on Gene Reporter Assay. Gene 2017, 626, 200–208. [Google Scholar] [CrossRef]
- Duarte, F.B.; Brígido, M.d.M.; Melo, E.d.O.; Báo, S.N.; Martins, C.F. Strategies for Transfection of Bovine Mesenchymal Stem Cells with PBC1-Anti-CD3 Vector. Anim. Biotechnol. 2022, 33, 1014–1024. [Google Scholar] [CrossRef]
- Mbawuike, I.N.; Zhang, Y.; Wang, Y.; Song, L. Cationic Liposome-Mediated Enhanced Generation of Human HLA-Restricted RSV-Specific CD8+ CTL+. J. Clin. Immunol. 2002, 22, 164–175. [Google Scholar] [CrossRef]
- Schwendener, R.A. Liposomes as Vaccine Delivery Systems: A Review of the Recent Advances. Ther. Adv. Vaccines 2014, 2, 159–182. [Google Scholar] [CrossRef]
- U’Ren, L.; Kedl, R.; Dow, S. Vaccination with Liposome--DNA Complexes Elicits Enhanced Antitumor Immunity. Cancer Gene Ther. 2006, 13, 1033–1044. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.H.; Popov, V.L.; Feng, H.M. Establishment of a Novel Endothelial Target Mouse Model of a Typhus Group Rickettsiosis: Evidence for Critical Roles for Gamma Interferon and CD8 T Lymphocytes. Lab. Investig. J. Tech. Methods Pathol. 2000, 80, 1361–1372. [Google Scholar] [CrossRef]
- Gruver, A.; Hudson, L.; Sempowski, G. Immunosenescence of Ageing. J. Pathol. 2007, 211, 144–156. [Google Scholar] [CrossRef]
- Zavala, J.; Ruiz, A.; Zavala, J. Las Rickettsias Del Grupo de Las Fiebres Manchadas: Respuesta Inmune y Sus Proteínas Inmunodominantes. Rev. Médica Chile 2004, 132, 381–387. [Google Scholar] [CrossRef]
- Mansueto, P.; Vitale, G.; Cascio, A.; Seidita, A.; Pepe, I.; Carroccio, A.; di Rosa, S.; Rini, G.B.; Cillari, E.; Walker, D.H. New Insight into Immunity and Immunopathology of Rickettsial Diseases. Clin. Dev. Immunol. 2011, 2012, e967852. [Google Scholar] [CrossRef] [PubMed]
- Secretaría de Salud Guía de Práctica Clínica SS-595-13 “Prevención, Diagnóstico y Tratamiento de la Fiebre Manchada por Rickettsia Rickettsii en Población Pediátrica y Adulta en el Primer y Segundo Nivel de Atención”. Centro Nacional de Excelencia Tecnologica en Salud. Mexico: Secretaria de Salud. 2013. Available online: https://www.actuamed.com.mx/informacion-medica/prevencion-diagnostico-y-tratamiento-de-fiebre-manchada-por-rickettsia-rickettsii (accessed on 14 December 2022).
- Ávila-Aguirre, L.M.; Martínez-Díaz, H.C.; Betancourt-Ruiz, P.; Hidalgo, M. 12. Historia de la rickettsiosis en Colombia. Fondo Editor. Biogénesis 2020, 1, 218–239. [Google Scholar]
- Sampedro-Núñez, M.; Serrano-Somavilla, A.; Adrados, M.; Cameselle-Teijeiro, J.M.; Blanco-Carrera, C.; Cabezas-Agricola, J.M.; Martínez-Hernández, R.; Martín-Pérez, E.; Muñoz de Nova, J.L.; Díaz, J.Á.; et al. Analysis of Expression of the PD-1/PD-L1 Immune Checkpoint System and Its Prognostic Impact in Gastroenteropancreatic Neuroendocrine Tumors. Sci. Rep. 2018, 8, 17812. [Google Scholar] [CrossRef] [PubMed]
- Wykes, M.N.; Lewin, S.R. Immune Checkpoint Blockade in Infectious Diseases. Nat. Rev. Immunol. 2018, 18, 91–104. [Google Scholar] [CrossRef]
- Jurado, J.O.; Alvarez, I.B.; Pasquinelli, V.; Martínez, G.J.; Quiroga, M.F.; Abbate, E.; Musella, R.M.; Chuluyan, H.E.; García, V.E. Programmed Death (PD)-1:PD-Ligand 1/PD-Ligand 2 Pathway Inhibits T Cell Effector Functions during Human Tuberculosis. J. Immunol. 2008, 181, 116–125. [Google Scholar] [CrossRef]
- Shen, L.; Gao, Y.; Liu, Y.; Zhang, B.; Liu, Q.; Wu, J.; Fan, L.; Ou, Q.; Zhang, W.; Shao, L. PD-1/PD-L Pathway Inhibits M.Tb-Specific CD4+ T-Cell Functions and Phagocytosis of Macrophages in Active Tuberculosis. Sci. Rep. 2016, 6, 38362. [Google Scholar] [CrossRef]
- Chang, T.T.; Kuchroo, V.K.; Sharpe, A.H. Role of the B7-CD28/CTLA-4 Pathway in Autoimmune Disease. Curr. Dir. Autoimmun. 2002, 5, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.L.; Cotterell, S.E.; Gorak, P.M.; Engwerda, C.R.; Kaye, P.M. Blockade of CTLA-4 Enhances Host Resistance to the Intracellular Pathogen, Leishmania Donovani. J. Immunol. Baltim. 1998, 161, 4153–4160. [Google Scholar] [CrossRef]
- Gazi, M.; Caro-Gomez, E.; Goez, Y.; Cespedes, M.A.; Hidalgo, M.; Correa, P.; Valbuena, G. Discovery of a Protective Rickettsia Prowazekii Antigen Recognized by CD8+ T Cells, RP884, Using an In Vivo Screening Platform. PLoS ONE 2013, 8, e76253. [Google Scholar] [CrossRef]
- Nair, S.; Archer, G.E.; Tedder, T.F. Isolation and Generation of Human Dendritic Cells. Curr. Protoc. Immunol. 2012, 99, 7–32. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.B.; Sureda, M.; Rebollo, J. Células dendríticas I: Aspectos básicos de su biología y funciones. Inmunología 2012, 31, 21–30. [Google Scholar] [CrossRef]
- Elgueta, R.; Benson, M.J.; de Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular Mechanism and Function of CD40/CD40L Engagement in the Immune System. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef] [PubMed]
- la Mora, J.D.-D.; Licona-Enríquez, J.D.; Leyva-Gastélum, M.; Mora, D.D.-D.L.; Rascón-Alcantar, A.; Álvarez-Hernández, G. A Fatal Case Series of Rocky Mountain Spotted Fever in Sonora, México. Biomédica 2018, 38, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.; Pillai, S. Inmunología Celular y Molecular; Octava; Elsevier: Barcelona, Spain, 2015; ISBN 978-84-9022-909-5. [Google Scholar]
- Vasilevko, V.; Ghochikyan, A.; Holterman, M.J.; Agadjanyan, M.G. CD80 (B7-1) and CD86 (B7-2) Are Functionally Equivalent in the Initiation and Maintenance of CD4+ T-Cell Proliferation after Activation with Suboptimal Doses of PHA. DNA Cell Biol. 2002, 21, 137–149. [Google Scholar] [CrossRef]
- Galletti, M.F.B.M.; Fujita, A.; Rosa, R.D.; Martins, L.A.; Soares, H.S.; Labruna, M.B.; Daffre, S.; Fogaça, A.C. Virulence Genes of Rickettsia Rickettsii Are Differentially Modulated by Either Temperature Upshift or Blood-Feeding in Tick Midgut and Salivary Glands. Parasit. Vectors 2016, 9, 331. [Google Scholar] [CrossRef]
- Hoan, N.X.; Khuyen, N.; Giang, D.P.; Binh, M.T.; Toan, N.L.; Anh, D.T.; Trung, N.T.; Bang, M.H.; Meyer, C.G.; Velavan, T.P.; et al. Vitamin D Receptor ApaI Polymorphism Associated with Progression of Liver Disease in Vietnamese Patients Chronically Infected with Hepatitis B Virus. BMC Med. Genet. 2019, 20, 201. [Google Scholar] [CrossRef]
- Niebylski, M.L.; Peacock, M.G.; Schwan, T.G. Lethal Effect of Rickettsia Rickettsii on Its Tick Vector (Dermacentor andersoni). Appl. Environ. Microbiol. 1999, 65, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, H.; Li, Z.; Liu, Y.; Gao, J.; Zeng, X.; Gou, C.; Zhu, X.; Guo, X.; Pan, L.; et al. Association of Taq I T/C and Fok I C/T polymorphisms of vitamin D receptor gene with outcome of hepatitis B virus infection. Zhonghua Yi Xue Za Zhi 2006, 86, 1952–1956. [Google Scholar] [PubMed]
| Age | Gender | State | Symptom Onset Date | Clinical Signs | |||||
|---|---|---|---|---|---|---|---|---|---|
| Species | Fever | Exanthema | Headache | Arthralgias/Myalgias | Exposure | ||||
| 26 | F | Baja California | 24/11/16 | R. rickettsii | + | - | + | + | Tick |
| 29 | M | Baja California | 31/08/16 | R. rickettsii | + | - | + | + | - |
| 54 | M | Baja California | 18/08/16 | R. rickettsii | + | - | + | + | Tick |
| 25 | M | Baja California | - | R. rickettsii | + | + | + | + | Tick |
| 51 | F | Baja California | 21/03/17 | R. rickettsii | + | + | + | + | - |
| 33 | F | Yucatan | 03/10/15 | R. typhi | + | - | + | + | Fleas |
| 31 | F | Yucatan | 09/11/16 | R. typhi | + | + | + | - | |
| 39 | M | Yucatan | 14/10/16 | R. typhi | + | - | + | + | Fleas and Ticks |
| 28 | M | Yucatan | - | R. typhi | + | - | - | - | Tick |
| 32 | M | Yucatan | - | R. typhi | + | - | - | - | - |
| 72 | M | Yucatan | 01/08/13 | R. felis | + | + | - | + | Tick |
| 43 | M | Yucatan | 01/04/13 | R. felis | + | - | + | + | Tick |
| 18 | F | Yucatan | 01/05/06 | R. felis | + | - | - | - | Tick |
| 16 | F | Yucatan | 01/11/13 | R. felis | + | + | - | - | Tick |
| Macrophage Markers (%) | No Stimulation | Stimulation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Concavalina A | pVax | OmpB-24 | |||||||
| Healthy Donors | Patient | Healthy Donors | Patient | Healthy Donors | Patient | Healthy Donors | Patient | ||
| R. rickettsii | CD14+CD40+ | 25.30 | 32.00 a | 52.80 | 11.92 | 15.20 | 17.70 a | 9.97 | 12.50 a |
| CD14+CD80+ | 32.00 | 23.90 | 39.40 | 18.40 | 27.80 | 48.10 a | 37.30 | 22.40 | |
| CD14+HLA-I | 97.80 | 97.00 | 95.40 | 97.00 a | 99.20 | 94.10 | 98.10 | 85.50 | |
| CD14+HLA-II | 46.10 | 34.30 | 79.20 | 21.90 | 37.90 | 28.10 | 37.30 | 26.60 | |
| R. typhi | CD14+CD40+ | 32.70 | 22.10 | 15.40 | 32.20 a | 32.90 | 20.50 | 39.00 | 18.30 |
| CD14+CD80+ | 16.70 | 16.30 | 11.50 | 21.00 a | 23.00 | 19.10 | 22.80 | 9.91 | |
| CD14+HLA-I | 99.20 | 91.80 | 93.10 | 96.40 a | 93.70 | 92.20 | 97.00 | 97.30 a | |
| CD14+HLA-II | 48.00 | 25.20 | 27.70 | 36.10 a | 40.10 | 28.30 | 53.20 | 33.50 | |
| R. felis | CD14+CD40+ | 10.40 | 6.70 | 23.70 | 4.70 | 18.90 | 5.20 | 18.50 | 7.30 |
| CD14+CD80+ | 16.60 | 3.20 | 27.70 | 9.20 | 30.40 | 9.20 | 25.90 | 9.40 | |
| CD14+HLA-I | 98.90 | 96.30 | 97.00 | 96.80 | 97.40 | 97.70 a | 97.20 | 97.70 a | |
| CD14+HLA-II | 28.90 | 66.80 a | 33.00 | 62.10 a | 44.60 | 92.50 a | 49.20 | 94.60 a | |
| (MFI) | No Stimulation | Stimulation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Concanavalin A | pVax | OmpB-24 | |||||||
| Healthy Donors | Patient | Healthy Donors | Patient | Healthy Donors | Patient | Healthy Donors | Patient | ||
| R. rickettsii | CD14+CD40+ | 4662 | 2514 | 4791 | 2975 | 4008 | 3525 | 4537 | 2871 |
| CD14+CD80+ | 20,569 | 2476 | 29,801 | 2619 | 41,331 | 2275 | 29,801 | 3332 | |
| CD14+HLA-I | 51,054 | 53,824 a | 62,133 | 65,078 a | 58,748 | 55,552 | 38,630 | 42,342 a | |
| CD14+HLA-II | 10,110 | 8117 | 5039 | 2000 | 6000 | 12,000 a | 10,000 | 8000 | |
| R. typhi | CD14+CD40+ | 1270 | 5136 a | 2115 | 3991 a | 1633 | 4924 a | 5191 | 5439 a |
| CD14+CD80+ | 3132 | 2771 | 1839 | 4316 a | 2400 | 4352 a | 1946 | 4325 a | |
| CD14+HLA-I | 80,092 | 55,417 | 34,830 | 65,897 a | 77,776 | 76,401 | 82,276 | 73,816 | |
| CD14+HLA-II | 7697 | 9795 a | 3325 | 10,248 a | 6972 | 11,197 a | 8010 | 14,788 a | |
| R. felis | CD14+CD40+ | 5125 | 189,210 a | 2799 | 465,000 a | 6531 | 340,706 a | 147,873 | 321,110 a |
| CD14+CD80+ | 2223 | 47,918 a | 2270 | 49,923 a | 2332 | 95,271 a | 2917 | 125,643 a | |
| CD14+HLA-I | 47,429 | 75,097 a | 51,132 | 65,897 a | 62,588 | 165,240 a | 58,606 | 74,065 a | |
| CD14+HLA-II | 9156 | 72,806 a | 7646 | 78,536 a | 12,698 | 76,929 a | 15,171 | 78,807 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzul-Rosado, K.; Donis-Maturano, L.; Arias-León, J.; Machado-Contreras, J.; Valencia-Pacheco, G.; Panti-Balam, C.; Balam-Romero, J.; Ku-González, A.; Peniche-Lara, G.; Mosqueda, J.; et al. Rickettsia Vaccine Candidate pVAX1-OmpB24 Stimulates TCD4+INF-γ+ and TCD8+INF-γ+ Lymphocytes in Autologous Co-Culture of Human Cells. Vaccines 2023, 11, 173. https://doi.org/10.3390/vaccines11010173
Dzul-Rosado K, Donis-Maturano L, Arias-León J, Machado-Contreras J, Valencia-Pacheco G, Panti-Balam C, Balam-Romero J, Ku-González A, Peniche-Lara G, Mosqueda J, et al. Rickettsia Vaccine Candidate pVAX1-OmpB24 Stimulates TCD4+INF-γ+ and TCD8+INF-γ+ Lymphocytes in Autologous Co-Culture of Human Cells. Vaccines. 2023; 11(1):173. https://doi.org/10.3390/vaccines11010173
Chicago/Turabian StyleDzul-Rosado, Karla, Luis Donis-Maturano, Juan Arias-León, Jesús Machado-Contreras, Guillermo Valencia-Pacheco, Candi Panti-Balam, Javier Balam-Romero, Angela Ku-González, Gaspar Peniche-Lara, Juan Mosqueda, and et al. 2023. "Rickettsia Vaccine Candidate pVAX1-OmpB24 Stimulates TCD4+INF-γ+ and TCD8+INF-γ+ Lymphocytes in Autologous Co-Culture of Human Cells" Vaccines 11, no. 1: 173. https://doi.org/10.3390/vaccines11010173
APA StyleDzul-Rosado, K., Donis-Maturano, L., Arias-León, J., Machado-Contreras, J., Valencia-Pacheco, G., Panti-Balam, C., Balam-Romero, J., Ku-González, A., Peniche-Lara, G., Mosqueda, J., Zazueta, O. E., Lugo-Caballero, C., & Puerto-Manzano, F. (2023). Rickettsia Vaccine Candidate pVAX1-OmpB24 Stimulates TCD4+INF-γ+ and TCD8+INF-γ+ Lymphocytes in Autologous Co-Culture of Human Cells. Vaccines, 11(1), 173. https://doi.org/10.3390/vaccines11010173








