A Systematic Review of Reported Cases of Immune Thrombocytopenia after COVID-19 Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection of Studies
2.2. Data Extraction
3. Results
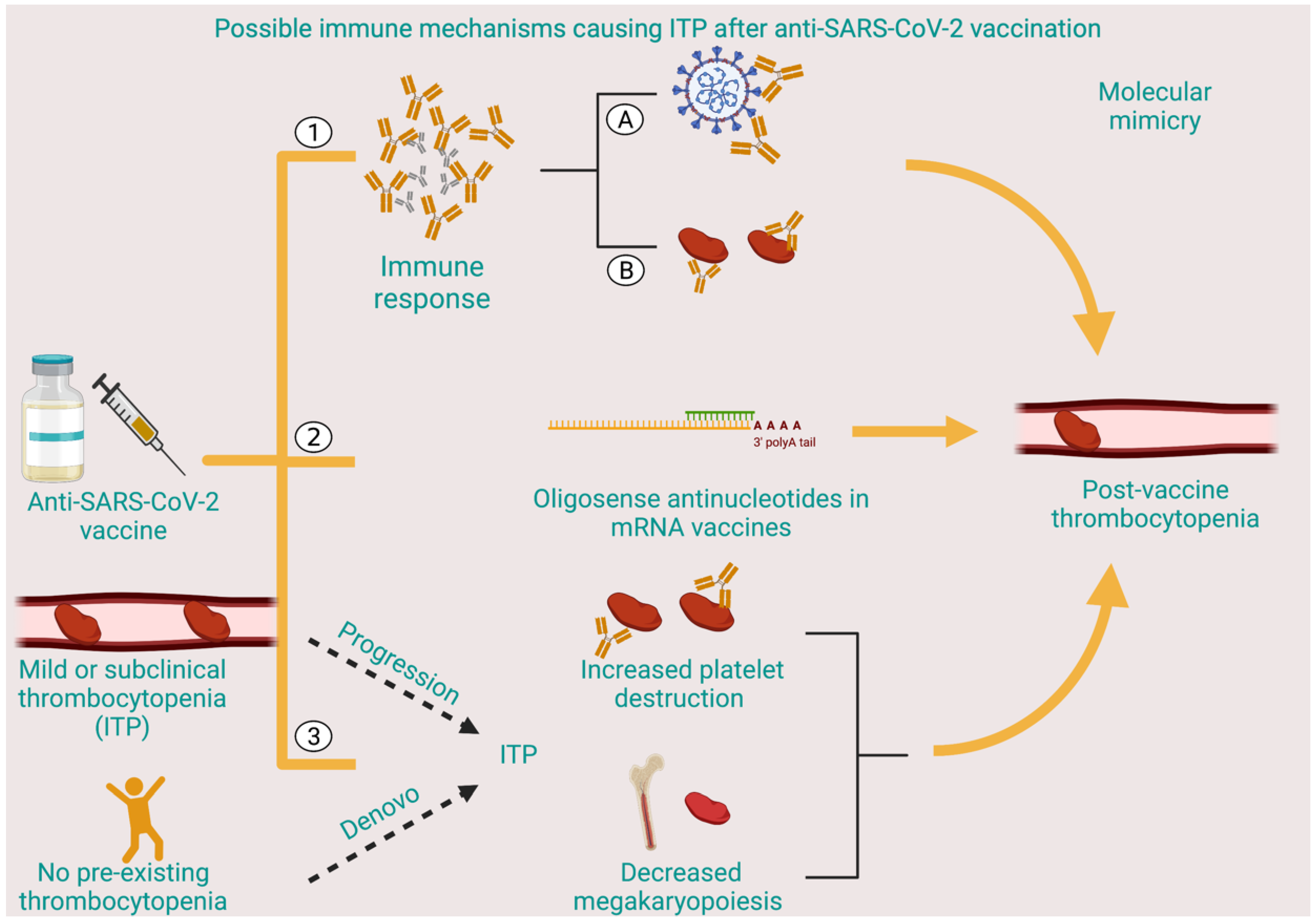
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Onisâi, M.; Vlădăreanu, A.-M.; Spînu, A.; Găman, M.; Bumbea, H. Idiopathic thrombocytopenic purpura (ITP)—New era for an old disease. Rom. J. Intern. Med. 2019, 57, 273–283. [Google Scholar] [CrossRef]
- Lambert, M.P.; Gernsheimer, T.B. Clinical updates in adult immune thrombocytopenia. Blood 2017, 129, 2829–2835. [Google Scholar] [CrossRef]
- Cooper, N.; Ghanima, W. Immune Thrombocytopenia. N. Engl. J. Med. 2019, 381, 945–955. [Google Scholar] [CrossRef]
- Shah, S.R.A.; Dolkar, S.; Mathew, J.; Vishnu, P. COVID-19 vaccination associated severe immune thrombocytopenia. Exp. Hematol. Oncol. 2021, 10, 42. [Google Scholar] [CrossRef]
- Moulis, G.; Sommet, A.; Sailler, L.; Lapeyre-Mestre, M.; Montastruc, J.-L.; the French Association of Regional; The French Association of Regional Pharmacovigilance Centers. Drug-induced immune thrombocytopenia: A descriptive survey in the French PharmacoVigilance database. Platelets 2011, 23, 490–494. [Google Scholar] [CrossRef]
- Lee, E.J.; Cines, D.B.; Gernsheimer, T.; Kessler, C.; Michel, M.; Tarantino, M.D.; Semple, J.W.; Arnold, D.M.; Godeau, B.; Lambert, M.P.; et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am. J. Hematol. 2021, 96, 534–537. [Google Scholar] [CrossRef]
- Long, B.; Bridwell, R.; Gottlieb, M. Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines. Am. J. Emerg. Med. 2021, 49, 58–61. [Google Scholar] [CrossRef]
- Saluja, P.; Gautam, N.; Yadala, S.; Venkata, A.N. Thrombotic thrombocytopenic purpura (TTP) after COVID-19 vaccination: A systematic review of reported cases. Thromb. Res. 2022, 214, 115–121. [Google Scholar] [CrossRef]
- Banerjee, S.; Sandhu, M.; Tonzi, E.; Tambe, A.; Gambhir, H.S. Immune-Mediated Thrombocytopenia Associated With Ad26.COV2.S (Janssen; Johnson & Johnson) Vaccine. Am. J. Ther. 2021, 28, e604–e606. [Google Scholar]
- Scanvion, Q.; Lambert, M.; Hachulla, E.; Terriou, L. Correspondence in reference to the previously published Epub manuscript: Immune thrombocytopenic purpura after SARS-CoV-2 vaccine. Br. J. Haematol. 2021, 194, e93–e95. [Google Scholar] [CrossRef]
- Candelli, M.; Rossi, E.; Valletta, F.; De Stefano, V.; Franceschi, F. Immune thrombocytopenic purpura after SARS-CoV-2 vaccine. Br. J. Haematol. 2021, 194, 547–549. [Google Scholar] [CrossRef]
- Paulsen, F.-O.; Schaefers, C.; Langer, F.; Frenzel, C.; Wenzel, U.; Hengel, F.E.; Bokemeyer, C.; Seidel, C. Immune Thrombocytopenic Purpura after vaccination with COVID-19 Vaccine (ChAdOx1 nCov-19). Blood 2021, 138, 996–999. [Google Scholar] [CrossRef]
- Gardellini, A.; Guidotti, F.; Maino, E.; Steffanoni, S.; Zancanella, M.; Turrini, M. Severe immune thrombocytopenia after COVID-19 vaccination: Report of four cases and review of the literature. Blood Cells Mol. Dis. 2021, 92, 102615. [Google Scholar] [CrossRef]
- Kim, G.; Choi, E.-J.; Park, H.-S.; Lee, J.-H.; Lee, J.-H.; Lee, K.-H. A Case Report of Immune Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. J. Korean Med. Sci. 2021, 36, e306. [Google Scholar] [CrossRef]
- Sivaramakrishnan, P.; Mishra, M. Vaccination-associated immune thrombocytopenia possibly due to ChAdOx1 nCoV-19 (Covishield) coronavirus vaccine. BMJ Case Rep. 2022, 15, e249237. [Google Scholar] [CrossRef]
- Al-Ahmad, M.; Al Rasheed, M.; Shalaby, N.; Rodriguez-Bouza, T.; Altourah, L. Immune Thrombocytopenia (ITP): Relapse Versus de novo After COVID-19 Vaccination. Clin. Appl. Thromb. Hemost. 2022, 28, 1–3. [Google Scholar] [CrossRef]
- Wong, J.S.Y.; Kang, J.H.-E.; Maw, K.Z. Acute immune thrombocytopenic purpura post first dose of COVID-19 vaccination. Postgrad. Med. J. 2022, 98, e129–e130. [Google Scholar] [CrossRef]
- Razzaq, A.K.; Al-Jasim, A. Oxford-AstraZeneca Coronavirus Disease-2019 Vaccine-Induced Immune Thrombocytopenia on Day Two. Case Rep. Hematol. 2021, 2021, 2580832. [Google Scholar] [CrossRef]
- Uaprasert, N.; Panrong, K.; Tungjitviboonkun, S.; Dussadee, K.; Decharatanachart, P.; Kaveevorayan, P.; Shoosanglertwijit, R.; Watanaboonyongcharoen, P.; Bunworasate, U.; Rojnuckarin, P. ChAdOx1 nCoV-19 vaccine-associated thrombocytopenia: Three cases of immune thrombocytopenia after 107 720 doses of ChAdOx1 vaccination in Thailand. Blood Coagul. Fibrinolysis 2022, 33, 67–70. [Google Scholar] [CrossRef]
- Liao, P.-W.; Teng, C.-L.J.; Chou, C.-W. Immune Thrombocytopenia Induced by the Chimpanzee Adenovirus-Vectored Vaccine against SARS-CoV-2 Infection. Vaccines 2021, 9, 1486. [Google Scholar] [CrossRef]
- Ganzel, C.; Ben-Chetrit, E. Immune Thrombocytopenia Following the Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine. Isr. Med. Assoc. J. 2021, 23, 341. [Google Scholar]
- Tarawneh, O.; Tarawneh, H. Immune thrombocytopenia in a 22-year-old post COVID-19 vaccine. Am. J. Hematol. 2021, 96, E133–E134. [Google Scholar] [CrossRef]
- Fueyo-Rodriguez, O.; Valente-Acosta, B.; Jimenez-Soto, R.; Neme-Yunes, Y.; Inclán-Alarcón, S.I.; Trejo-Gonzalez, R.; García-Salcido, M. Secondary immune thrombocytopenia supposedly attributable to COVID-19 vaccination. BMJ Case Rep. 2021, 14, e242220. [Google Scholar] [CrossRef]
- Jawed, M.; Khalid, A.; Rubin, M.; Shafiq, R.; Cemalovic, N. Acute Immune Thrombocytopenia (ITP) Following COVID-19 Vaccination in a Patient with Previously Stable ITP. Open Forum Infect. Dis. 2021, 8, ofab343. [Google Scholar] [CrossRef]
- King, E.R.; Towner, E. A Case of Immune Thrombocytopenia After BNT162b2 mRNA COVID-19 Vaccination. Am. J. Case Rep. 2021, 22, e931478. [Google Scholar] [CrossRef]
- Qasim, H.; Ali, E.; Yassin, M.A. Immune thrombocytopenia relapse post COVID-19 vaccine in young male patient. IDCases 2021, 26, e01344. [Google Scholar] [CrossRef]
- Shonai, T.; Kimura, F.; Watanabe, J. Severe Immune Thrombocytopenia after COVID-19 Vaccination: Two Case Reports and a Literature Review. Intern. Med. 2022, 61, 1581–1585. [Google Scholar] [CrossRef]
- Krajewski, P.K.; Szepietowski, J.C. Immune thrombocytopenic purpura associated with COVID-19 Pfizer-BioNTech BNT16B2b2 mRNA vaccine. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e626–e627. [Google Scholar] [CrossRef]
- Idogun, P.O.; Ward, M.C.; Teklie, Y.; Wiese-Rometsch, W.; Baker, J. Newly Diagnosed Idiopathic Thrombocytopenia Post COVID-19 Vaccine Administration. Cureus 2021, 13, e14853. [Google Scholar] [CrossRef]
- Hidaka, D.; Ogasawara, R.; Sugimura, S.; Fujii, F.; Kojima, K.; Nagai, J.; Ebata, K.; Okada, K.; Kobayashi, N.; Ogasawara, M.; et al. New-onset Evans syndrome associated with systemic lupus erythematosus after BNT162b2 mRNA COVID-19 vaccination. Int. J. Hematol. 2022, 115, 424–427. [Google Scholar] [CrossRef]
- Saito, K.; Ichikawa, S.; Hatta, S.; Katsuoka, Y.; Harigae, H.; Izumi, T. Vincristine therapy for severe and refractory immune thrombocytopenia following COVID-19 vaccination. Int. J. Hematol. 2022, 115, 424–427. [Google Scholar] [CrossRef]
- Pasin, F.; Calabrese, A.; Pelagatti, L. Immune thrombocytopenia following COVID-19 mRNA vaccine: Casuality or causality? Intern. Emerg. Med. 2022, 17, 295–297. [Google Scholar] [CrossRef]
- Nakamura, T.; Morodomi, Y.; Kanaji, S.; Okamura, T.; Nagafuji, K.; Kanaji, T. Detection of anti-GPIbα autoantibodies in a case of immune thrombocytopenia following COVID-19 vaccination. Thromb. Res. 2022, 209, 80–83. [Google Scholar] [CrossRef]
- Ogai, A.; Yoshida, R.; Yuasa, C.; Chin, K.; Fujimaki, K.; Nakajima, H. Acute immune thrombocytopenia following SARS-CoV-2 vaccination in chronic ITP patients and a healthy individual. Int. J. Hematol. 2022, 115, 293–295. [Google Scholar] [CrossRef]
- Battegay, R.; Istampoulouoglou, I.; Holbro, A.; Buser, A.; Hirsiger, J.R.; Eckstein, J.; Bergerg, C.T.; Koechlinbh, S.; Leuppi-Taegtmeyerbch, A.B. Immune thrombocytopenia associated with COVID-19 mRNA vaccine tozinameran—A clinical case and global pharmacovigilance data. Swiss Med. Wkly. 2021, 151, w30084. [Google Scholar]
- Ghosh, A.K.; Bhushan, S.; Lopez, L.D.R.; Sampat, D.; Salah, Z.; Hatoum, C.A. BNT162b2 COVID-19 Vaccine Induced Immune Thrombocytopenic Purpura. Case Rep. Med. 2022, 2022, 5603919. [Google Scholar] [CrossRef]
- Akiyama, H.; Kakiuchi, S.; Rikitake, J.; Matsuba, H.; Sekinada, D.; Kozuki, Y.; Iwata, N. Immune thrombocytopenia associated with Pfizer-BioNTech’s BNT162b2 mRNA COVID-19 vaccine. IDCases 2021, 25, e01245. [Google Scholar] [CrossRef]
- Jasaraj, R.B.; Shrestha, D.B.; Gaire, S.; Kassem, M. Immune Thrombocytopenic Purpura Following Pfizer-BioNTech COVID-19 Vaccine in an Elderly Female. Cureus 2021, 13, e16871. [Google Scholar] [CrossRef]
- Ghous, G.; Allahyar; Zafar, M.U.; Tarar, Z.I.; Shoukat, H.M.H. Immune Thrombocytopenic Purpura Associated With Pfizer-BioNTech COVID-19 Vaccine Refractory to Conventional Treatment. Am. J. Ther. 2021, 28, e521–e522. [Google Scholar] [CrossRef]
- Abuhelwa, Z.; Ning, Y.; Abdulsattar, W.; Ghazaleh, S.; Kahlon, N.; Elsayed, A. Romiplostim for SARS-CoV-2 Vaccine Induced Immune Thrombocytopenia. Am. J. Ther. 2021, 28, e685–e687. [Google Scholar] [CrossRef]
- Prasad, S.; Jariwal, R.; Adebayo, M.; Jaka, S.; Petersen, G.; Cobos, E. Immune Thrombocytopenia following COVID-19 Vaccine. Case Rep. Hematol. 2022, 2022, 6013321. [Google Scholar] [CrossRef]
- Chanut, M.; Jaidi, R.; Kohn, M.; Grange, T.; Brones, C.; Lombion, N.; Rousselot, P.; Longval, T. Successful mRNA SARS-Cov-2 vaccine rechallenge after a first episode of immune thrombocytopenic purpura. Platelets 2022, 33, 652–653. [Google Scholar] [CrossRef]
- Helms, J.M.; Ansteatt, K.T.; Roberts, J.C.; Kamatam, S.; Foong, K.S.; Labayog, J.-M.S.; Tarantino, M.D. Severe, Refractory Immune Thrombocytopenia Occurring After SARS-CoV-2 Vaccine. J. Blood Med. 2021, 12, 221–224. [Google Scholar] [CrossRef]
- Chong, K.-M.; Yang, C.-Y.; Lin, C.-C.; Lien, W.-C. Severe immune thrombocytopenia following COVID-19 vaccination (Moderna) and immune checkpoint inhibitor. Am. J. Emerg. Med. 2022, 56, 395.e1–395.e3. [Google Scholar] [CrossRef]
- Malayala, S.V.; Mohan, G.; Vasireddy, D.; Atluri, P. Purpuric Rash and Thrombocytopenia After the mRNA-1273 (Moderna) COVID-19 Vaccine. Cureus 2021, 13, e14099. [Google Scholar] [CrossRef]
- Julian, J.A.; Mathern, D.R.; Fernando, D. Idiopathic Thrombocytopenic Purpura and the Moderna Covid-19 Vaccine. Ann. Emerg. Med. 2021, 77, 654–656. [Google Scholar] [CrossRef]
- Toom, S.; Wolf, B.; Avula, A.; Peeke, S.; Becker, K. Familial thrombocytopenia flare-up following the first dose of mRNA-1273 Covid-19 vaccine. Am. J. Hematol. 2021, 96, E134–E135. [Google Scholar] [CrossRef]
- Hines, A.; Shen, J.G.; Olazagasti, C.; Shams, S. Immune thrombocytopenic purpura and acute liver injury after COVID-19 vaccine. BMJ Case Rep. 2021, 14, e242678. [Google Scholar] [CrossRef]
- Bennett, C.; Chambers, L.M.; Son, J.; Goje, O. Newly diagnosed immune thrombocytopenia in a pregnant patient after coronavirus disease 2019 vaccination. J. Obstet. Gynaecol. Res. 2021, 47, 4077–4080. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID Data Tracker; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2022; Available online: https://covid.cdc.gov/covid-data-tracker (accessed on 5 July 2022).
- Rosenblum, H.G.; Gee, J.; Liu, R.; Marquez, P.L.; Zhang, B.; Strid, P.; E Abara, W.; McNeil, M.M.; Myers, T.R.; Hause, A.M.; et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: An observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect. Dis. 2022, 22, 802–812. [Google Scholar] [CrossRef]
- Perricone, C.; Ceccarelli, F.; Nesher, G.; Borella, E.; Odeh, Q.; Conti, F.; Shoenfeld, Y.; Valesini, G. Immune thrombocytopenic purpura (ITP) associated with vaccinations: A review of reported cases. Immunol. Res. 2014, 60, 226–235. [Google Scholar] [CrossRef]
- Jefferson, T.; Price, D.; Demicheli, V.; Bianco, E. Unintended events following immunization with MMR: A systematic review. Vaccine 2003, 21, 3954–3960. [Google Scholar] [CrossRef]
- Cecinati, V.; Principi, N.; Brescia, L.; Giordano, P.; Esposito, S. Vaccine administration and the development of immune thrombocytopenic purpura in children. Hum. Vaccines Immunother. 2013, 9, 1158–1162. [Google Scholar] [CrossRef] [Green Version]
- Mithoowani, S.; Gregory-Miller, K.; Goy, J.; Miller, M.C.; Wang, G.; Noroozi, N.; Kelton, J.G.; Arnold, D.M. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: A systematic review and meta-analysis. Lancet Haematol. 2016, 3, e489–e496. [Google Scholar] [CrossRef]
- Watad, A.; Sharif, K.; Shoenfeld, Y. The ASIA syndrome: Basic concepts. Mediterr. J. Rheumatol. 2017, 28, 64–69. [Google Scholar] [CrossRef]
- Chi, X.; Gatti, P.; Papoian, T. Safety of antisense oligonucleotide and siRNA-based therapeutics. Drug Discov. Today 2017, 22, 823–833. [Google Scholar] [CrossRef]
- Lizbeth-Estefan, D.-P.; Pujol-Moix, N.; Jiménez, B.; Canals, C.; Barranco-Charris, E.; Muñiz-Díaz4, E.; Souto, J.-C. Shared Autoimmunity: A Case Series of 56 Patients with Immune Thrombocytopenia (ITP) Associated with Other Autoimmune Disorders. Open Access Libr. J. 2016, 3, 8. [Google Scholar]
- Kuter, D.J. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. Br. J. Haematol. 2021, 195, 365–370. [Google Scholar] [CrossRef]
- Simpson, C.R.; Shi, T.; Vasileiou, E.; Katikireddi, S.V.; Kerr, S.; Moore, E.; McCowan, C.; Agrawal, U.; Shah, S.A.; Ritchie, L.D.; et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 2021, 27, 1290–1297. [Google Scholar] [CrossRef]
- Cohen, Y.C.; Djulbegovic, B.; Shamai-Lubovitz, O.; Mozes, B. The Bleeding Risk and Natural History of Idiopathic Thrombocytopenic Purpura in Patients with Persistent Low Platelet Counts. Arch. Intern. Med. 2000, 160, 1630–1638. [Google Scholar] [CrossRef]
- Neunert, C.; Terrell, D.R.; Arnold, D.M.; Buchanan, G.; Cines, D.B.; Cooper, N.; Cuker, A.; Despotovic, J.M.; George, J.N.; Grace, R.F.; et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019, 3, 3829–3866. [Google Scholar] [CrossRef] [PubMed]
- Lal, L.S.; Said, Q.; Andrade, K.; Cuker, A. Second-line treatments and outcomes for immune thrombocytopenia: A retrospective study with electronic health records. Res. Pract. Thromb. Haemostasis. 2020, 4, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Grady, D.M. Death of a doctor who got Covid shot is being investigated. New York Times, 12 January 2021. [Google Scholar]
- Rodeghiero, F.; Stasi, R.; Gernsheimer, T.; Michel, M.; Provan, D.; Arnold, D.M.; Bussel, J.B.; Cines, D.B.; Chong, B.H.; Cooper, N.; et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 2009, 113, 2386–2393. [Google Scholar] [CrossRef]
- Welsh, K.J.; Baumblatt, J.; Chege, W.; Goud, R.; Nair, N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2021, 39, 3329–3332. [Google Scholar] [CrossRef]
- Gordon, S.F.; Clothier, H.J.; Morgan, H.; Buttery, J.P.; Phuong, L.K.; Monagle, P.; Chunilal, S.; Wood, E.M.; Tran, H.; Szer, J.; et al. Immune thrombocytopenia following immunisation with Vaxzevria ChadOx1-S (AstraZeneca) vaccine, Victoria, Australia. Vaccine 2021, 39, 7052–7057. [Google Scholar] [CrossRef]
- Hippisley-Cox, J.; Patone, M.; Mei, X.W.; Saatci, D.; Dixon, S.; Khunti, K.; Watkinson, P.; Shankar-Hari, M.; Harrison, D.A.; Sheikh, A. Risk of thrombocytopenia and thromboembolism after COVID-19 vaccination and SARS-CoV-2 positive testing: Self-controlled case series study. BMJ 2021, 374, n1931. [Google Scholar] [CrossRef]

| Author, Year | Age | Sex | Presenting Features | Time to Symptom Onset or Presentation after Vaccine (in Days) | Vaccine Dose | Comorbidities | Admission Platelet Counts or Nadir (Whichever Is Reported First) | Treatment Received | Outcomes | Second Dose, If Applicable |
|---|---|---|---|---|---|---|---|---|---|---|
| Cases due to Ad26.COV2-S (Johnson & Johnson vaccine) | ||||||||||
| Shah et al., 2021 [4] | 59 | F | Abdominal cramps, diarrhea | 2 | - | Chronic ITP and SLE (prior flare-up 2 years ago after Shingrix vaccine) | 64 × 103/μL | Dexamethasone | Discharged | No comment |
| Banerjee et al., 2021 [9] | 63 | F | Bleeding gums | 14 | - | Cervical cancer s/p hysterectomy | 2 × 103/μL | Platelets, Prednisone, IVIG, and dexamethasone | Discharged on day 5 | - |
| Cases due to ChAdOx1 nCoV-19 vaccine | ||||||||||
| Scanvion et al., 2021 [10] | 62 | F | Asymptomatic | 6 | First dose | ITP, overweight, HLD, HTN | 60 × 103/μL | None | No hospitalization | No comment |
| Scanvion et al., 2021 [10] | 45 | F | Asymptomatic | 3 | First dose | ITP | 53 × 103/μL | None | No hospitalization | No comment |
| Candelli et al., 2021 [11] | 28 | M | Petechiae, oral bleed, fatigue, headache, fever | 1 | First dose | None | 4 × 103/μL (Lupus anticoagulant positive) | Four-day Dexamethasone course, once during hospitalization and once 10 days after discharge | Discharged after 4 days | No comment |
| Paulsen et al., 2021 [12] | 72 | M | Petechiae, hematomas | 11 | First dose | Radioiodine-treated autoimmune thyroiditis | <5 × 103/μL | Prednisolone, IVIG | Discharged | No comment |
| Paulsen et al., 2021 [12] | 71 | F | Petechiae, headache | 11 | First dose | Latent hyperthyroidism, breast cancer, stroke | <5 × 103/μL | Prednisolone followed by dexamethasone, IVIG, TPO-RA | Discharged but readmitted 7 days after | No comment |
| Paulsen et al., 2021 [12] | 66 | M | Petechiae, hyposphagma | 1 | First dose | HTN, mild thrombocytopenia | < 5 × 103/μL | Prednisolone | Discharged | No comment |
| Paulsen et al., 2021 [12] | 64 | F | Petechiae, epistaxis | 15 | First dose | HTN, COPD, hepatic steatosis | 6 × 103/μL | Prednisolone | Discharged | No comment |
| Gardellini et al., 2021 [13] | 63 | M | Hematomas, epistaxis | 14 | First dose | DM, HTN, HLD | 2 × 103/μL | Prednisone | Discharged | Second dose given after 9 weeks |
| Kim et al., 2021 [14] | 66 | F | Bruising, gum bleeding | 2 | First dose | None | 4 × 103/μL | Dexamethasone and IVIG | Discharged | No comment |
| Sivaramakrishnan et al., 2022 [15] | - (middle-aged) | F | Hemoptysis and menorrhagia | 11 (had been evaluated 30 days after first dose for similar complaints and was given platelets) | First and second doses | None | 10 × 103/μL | Prednisolone | Discharged with relapse after 3 weeks | - |
| Al-Ahmad et al., 2022 [16] | 56 | M | Admitted for partial small bowel obstruction | 14 | First dose | Primary ITP s/p splenectomy | 9 × 103/μL | IVIG and TPO-RA (refused steroids) | Discharged with a repeat dose of TPO-RA for worsening counts | No comment |
| Al-Ahmad et al., 2022 [16] | 63 | F | Petechiae | 10 | Second dose | Chronic ITP | 35 × 103/μL | None | Not admitted | No comment |
| Al-Ahmad et al., 2022 [16] | 28 | F | Petechiae, gum, and nosebleed | 10 | First dose | Chronic ITP (maintained on romiplostim and prednisolone) | 30 × 103/μL | Romiplostim was continued and prednisolone dose was increased | Not admitted | No comment |
| Al-Ahmad et al., 2022 [16] | 54 | F | Ecchymoses | 13 | First dose | None | 10 × 103/μL | Prednisolone and IVIG | Discharged after 6 days – readmitted 3 days later with counts of 10 × 103/μL | No comment |
| Al-Ahmad et al., 2022 [16] | 33 | F | Ecchymoses | 21 | First dose | None | 3 × 103/μL | IVIG, Prednisolone, and Romiplostim | Discharged after 7 days, was readmitted 26 days later with recurrence | No comment |
| Wong et al., 2022 [17] | 86 | M | Gingival bleeding, ecchymosis, and tongue blisters | 2 | First dose | NA | 4 × 103/μL | Dexamethasone, platelets, IVIG and Rituximab | Discharged | NA |
| Wong et al., 2022 [17] | 38 | F | Petechiae and purpura | 10 | First dose | NA | 3 × 103/μL | Prednisone and IVIG | Discharged | NA |
| Razzaq et al., 2021 [18] | 26 | M | Asymptomatic | 2 | First dose | Mild thrombocytopenia | 64 × 103/μL | Methylprednisolone and IVIG | Discharged | No comment |
| Uaprasert et al., 2022 [19] | 80 | M | Bleeding from bitten tongue | 19 | First dose | None | 14 × 103/μL | Dexamethasone, prednisolone, IVIG, TPO-RA | Improved | No comment |
| Uaprasert et al., 2022 [19] | 84 | M | Dizziness | 9 | First dose | Adrenal insufficiency due to adrenal histoplasmosis, cirrhosis, past HBV infection | 36 × 103/μL (HCV and HIV positive) | None | Improved | No comment |
| Uaprasert et al., 2022 [19] | 55 | F | Purpura and oral bleeding | 24 | First dose | HLD | 41 × 103/μL | None | Improved | No comment |
| Liao et al., 2021 [20] | 79 | M | Asymptomatic | 8 | First dose | Stroke | 2 × 103/μL | Hydrocortisone followed by prednisolone | Discharged after 12 days | No comment |
| Cases due to BNT162b2 vaccine | ||||||||||
| Ganzel et al., 2021 [21] | 53 | M | Epistaxis, purpura, petechiae | 14 | First dose | Obesity, DM, HTN | 1 × 103/μL | Dexamethasone and IVIG | Improved | Second dose not given |
| Tarawneh et al., 2021 [22] | 22 | M | Petechiae, gum bleeding | 3 | First dose | None | 2 × 103/μL (mild transaminitis, SSA-antibody which later normalized) | Dexamethasone, platelet transfusion, and IVIG | Discharged after 5 days | No comment |
| Fueyo-Rodriguez et al., 2021 [23] | 41 | F | Fever, tachycardia, nausea | 1 | First dose | HTN, hypothyroidism, pre-DM | 65 × 103/μL (elevated IgE and CRP) | Methylprednisolone, dexamethasone, and IVIG | Discharge after 5 days | No comment |
| Shah et al.,2021 [4] | 53 | M | Fever, chills, myalgia, petechiae | 8 | Second dose | Crohn’s disease | 2 × 103/μL | Dexamethasone and IVIG | Discharged | No comment |
| Shah et al.,2021 [4] | 67 | M | Generalized weakness, melena, petechiae | 2 | First dose | Seizures, atrial fibrillation, chronic ITP in remission | 2 × 103/μL | Platelet, IVIG and dexamethasone | Discharged | Second dose not advised |
| Jawed et al., 2021 [24] | 47 | F | Gum bleeding, epistaxis | 18 | First dose | Chronic ITP, Hypothyroidism secondary to Hashimoto’s thyroiditis | 1 × 103/μL | Platelet, IVIG | Discharged | No comment |
| King et al., 2021 [25] | 39 | F | Petechiae | 1 | Second dose | PCOS | 1 × 103/μL (elevated ESR) | Platelet, methylprednisolone, and IVIG | Discharged after 3 days | No comment |
| Gardellini et al., 2021 [13] | 27 | M | Hematomas, epistaxis | 10 | First dose | None | 1 × 103/μL | IVIG, prednisolon, dexamethasone | Discharged | No comment |
| Gardellini et al., 2021 [13] | 39 | F | Petechiae, ecchymosis | 6 | Second dose | Chronic ITP | 1 × 103/μL | IVIG-prednisone, TPO-RA | Not reported | No comment |
| Qasim et al., 2021 [26] | 28 | M | Petechiae and epistaxis | 2 | Second dose | ITP | 1 × 103/μL | IVIG and dexamethasone | Discharged on prednisolone taper | Not reporter |
| Shonai et al., 2021 [27] | 69 | M | Oral bleeding and hemoptysis | 10 | Second dose (had asymptomatic thrombocytopenia after first dose) | Well-controlled postoperative intestinal obstruction and hypopharyngeal cancers s/p permanent tracheal fistula surgery | 6 × 103/μL (H pylori antibody positive) | Prednisolone | Improved (refused hospitalization) | No comment |
| Krajewski et a., 2021 [28] | 74 | M | Hemorrhagic mucosal blisters and purpura | 1 | First dose | HTN | 2 × 103/μL | Platelet and Dexamethasone | No comment | No comment |
| Al-Ahmad et al., 2022 [16] | 19 | M | Mouth and nosebleed | 4 | Second dose | Chronic ITP (maintained on eltrombopag) | 4 × 103/μL | Methylprednisolone, prednisolone, and increased dose of eltrombopag | Left against medical advice | No comment |
| Idogun et al., 2021 [29] | 54 | F | Petechiae, ecchymosis, mucosal bleeding | 7 days after first dose but presented 5 days after second dose (21 days after symptom onset) | First and second doses | HTN, congenital epidermal dysplasia, overactive bladder, mild cognitive impairment, CKD and anxiety | 0 | Platelet, dexamethasone, IVIG | Discharged but was readmitted after 4 days | - |
| Hidaka et al., 2022 [30] | 53 | F | Shortness of breath | 14 days after second dose but had transient wheezing and purpura after first dose | First and second doses | Asthma, Vogt-Koyanagi-Harada disease, Hashimoto disease | 39 × 103/μL (Also had AIHA, lupus anticoagulant and ANA positive, hypocomplementemia, COVID IgG positive) | Prednisolone, blood transfusion (for Evans syndrome associated with SLE post-COVID vaccination) | Discharged | - |
| Al-Ahmad et al., 2022 [16] | 30 | F | Petechiae | 7 | First dose | Chronic migraine, depression, chronic ITP | 40 × 103/μL | None | Not admitted | No comment |
| Saito et al., 2022 [31] | 66 | F | Malaise, lymphadenopathy, fever, hematuria, oral bleeding, and purpura | 2 | First dose | None | <1 × 103/μL (positive antiplatelet glycoprotein IIb/IIIa antibodies, elevated inflammatory markers) | Platelet, IVIG, prednisolone, pulsed methylprednisolone, TPO-RA, danazol and vincristine | Discharged on day 22 | No comment |
| Pasin et al., 2022 [32] | 84 | M | Petechiae, gum bleeding | 5 | First dose | Localized bladder cancer, tremors, mild CKD, Atrial fibrillation on apixaban | 3 × 103/μL (SARS-CoV-2 negative; positive antiplatelet glycoprotein IIb/IIIa antibodies) | Platelet, IVIG and prednisone | Improved | None |
| Nakamura et al., 2022 [33] | 32 | F | Petechiae, oral bleeding | 5 | Second dose | None | <1 × 103/μL (Platelet associated GPIbα IgG) | Prednisolone | Discharged on day 12 | No comment |
| Al-Ahmad et al., 2022 [16] | 37 | F | Petechiae | 10 | Second dose | Primary ITP | 25 × 103/μL | Prednisolone | Improved | No comment |
| Al-Ahmad et al., 2022 [16] | 30 | M | Fatigue, petechiae, gum bleeding, epistaxis | 7 | First dose | Primary ITP (on eltrombopag) | 11 × 103/μL | Prednisolone, IVIG and eltrombopag | Discharged on day 2 | Allowed to take second dose with close follow-up |
| Al-Ahmad et al., 2022 [16] | 56 | F | Gum and nose bleeding | 7 | Second dose | HTN, DM | 2 × 103/μL | IVIG, prednisolone and TPO-RA | Discharged after 3 days but readmitted 2 weeks later | No comment |
| Ogai et al., 2021 [34] | 64 | F | Oral bleeding and petechiae | 2 | First dose | Chronic ITP | 1 × 103/μL | Prednisolone and IVIG | Improvement | No comment |
| Ogai et al., 2021 [34] | 61 | F | Petechiae | 17 | Second dose | Chronic ITP, Scleroderma and Sjogren syndrome | 1 × 103/μL | Platelet, Prednisolone and TPO-RA | Improvement | No comment |
| Battegay et al., 2021 [35] | 77 | M | Asymptomatic; petechiae on buccal mucosa | 8 | First dose | CAD, Atrial fibrillation, HTN (Had mild thrombocytopenia pre-vaccination) | 28 × 103/μL | IVIG, prednisone and TPO-RA | Improvement | Second dose under eltrombopag taper |
| Ghosh et al., 2022 [36] | 63 | F | Rash and easy bruising | 1 | Second dose | COPD, HTN, DM | 0 (positive for SS-A and scleroderma antibodies) | Dexamethasone, IVIG, TPO-RA, rituximab | Discharged | - |
| Akiyama et al., 2021 [37] | 20 | F | Subcutaneous hemorrhage | 12 | First dose | None | 16 × 103/μL | Prednisolone | Improved | No comment |
| Jasaraj et al., 2021 [38] | 67 | F | Petechiae, gum bleeding, epistaxis, subconjunctival hemorrhage | 2 | Second dose (also had symptoms 14 days after the first dose) | HTN, DM, hypothyroidism, depression, b12 deficiency, headaches | 3 × 103/μL | Prednisone, IVIG, platelet, ACA, rituximab, TPO-RA | Discharged on day 14 (received rituximab and TPO-RA outpatient) | - |
| Ghous et al., 2021 [39] | 69 | F | Bruising and gum bleeding | 14 | First dose | Cataract, SCC, BCC | 5 × 103/μL (AST and LDH were high) | Platelet, IVIG, dexamethasone, TPO-RA, vincristine, prednisone | Discharged with return to ER for iatrogenic thrombocytosis | No comment |
| Cases due to mRNA-1273 vaccine | ||||||||||
| Abuhelwa et al., 2021 [40] | 65 | F | Epistaxis and rash | 1 | First dose | None | 3 × 103/μL | Platelets, IVIG, dexamethasone, Rho immunoglobulins and TPO-RA | Discharged on day 14 | No comment |
| Prasad et al., 2021 [41] | 58 | M | Mucosal bleed, petechiae | 21 | First dose | HTN, DM | 3 × 103/μL (PF4 antibody was weakly positive but SRA negative) | Dexamethasone, platelets, IVIG, second relapse treated with platelets, IVIG, TPO-RA and fostamatinib | Discharged in 6 days but presented 5 days later with recurrence, and then again 10 days later | Refused |
| Ogai et al., 2021 [34] | 73 | F | Petechiae | 11 | First dose | HTN, HLD | 2 × 103/μL | Prednisolone, IVIG, and TPO-RA | Improvement | No comment |
| Chanut et al., 2022 [42] | 73 | F | Epistaxis, intra-buccal hemorrhage, and bruises | 7 | First dose | IgA monoclonal gammopathy of undetermined significance, HTN, HLD, hypothyroidism, glaucoma | 2 × 103/μL | IVIG | Discharged | Rechallenged with BNT162b2 vaccine with no relapse |
| Helms et al., 2021 [43] | 74 | M | Epistaxis and diffuse cutaneous purpura | 1 | First dose | HTN, Gout, HLD and nonischemic cardiomyopathy | 10 × 103/μL | Dexamethasone, IVIG, rituximab, TPO-RA | Discharged on day 5; readmitted on day 13 | No comment |
| Chong et al., 2022 [44] | 75 | F | Hemoptysis | 3 | First dose | Refractory lung adenocarcinoma on durvalumab | 7 × 103/μL (prior hepatitis B infection) | Platelets, prednisolone | Discharged on day 5 | Second dose not advised |
| Malayala et al., 2021 [45] | 60 | M | Purpura, nausea, vomiting, shortness of breath, leg edema, chest and abdominal pain | 1 | First dose | Hepatitis C infection, CKD-stage IV, HTN, HFrEF, smoker | 84 × 103/μL (With deranged LFTs – heavy hepatitis C viral load and cirrhosis) | None | Left against medical advice on day 3 of hospitalization | No comment |
| Gardellini et al., 2021 [13] | 24 | M | Petechiae | 21 | Second dose | None | 2 × 103/μL | IVIG and prednisone | Discharged on steroid taper | No comment |
| Julian et al., 2021 [46] | 72 | F | Rash, spontaneous oral bleeding, headache | 1 | First dose | DM, seasonal contact dermatitis, gout | 12 × 103/μL (prior parvovirus infection) | Dexamethasone, IVIG, ACA, rituximab, and platelets | No comment | No comment |
| Toom et al., 2021 [47] | 36 | F | Petechiae, easy bruising, bleeding gum, headache | 7 | First dose | Familial ITP | 3 × 103/μL (unlikely due to vaginal estrogen ring) | Dexamethasone and IVIG | Discharged | No comment |
| Shonai et al., 2021 [27] | 34 | F | Purpura | 21 | Second dose | None | 11 × 103/μL | Initially not treated, however, on 1 week follow- up, had worsened platelet count for which prednisolone and TPO-RA were given | Improved | - |
| Hines et al., 2021 [48] | 26 | F | Petechiae | 7 | First dose o | Irregular menses on OCPs | 19 × 103/μL (transaminitis present) | Prednisone, dexamethasone, and IVIG | Discharged on day 5 | No comment |
| Bennett et al., 2021 [49] | 32 | F | Petechiae and bruising | 11 | First dose | None – was currently pregnant at 9 weeks | 1 × 103/μL | Prednisone | Discharged on day 3 | No comment |
| Variables | Post-Vaccination ITP Flare-Up (n = 29) | Post-Vaccination New ITP Diagnosis (n = 49) |
|---|---|---|
| Median age in years at presentation [42] | 43 (19–67) | 53 (20–86) |
| Females (%) | 41% | 57% |
| Most common symptom | Mucocutaneous bleed | Mucocutaneous bleed |
| Number of asymptomatic individuals | 3 | 3 |
| Median days to presentation | 7.5 | 12.5 |
| Number of cases/vaccine | 1—Ad26.COV2-S (Johnson & Johnson vaccine) 5—ChAdO × 1 nCoV-19 vaccine 10—BNT162b2 vaccine 1—mRNA-1273 vaccine | 1—Ad26.COV2-S (Johnson & Johnson) vaccine 18—ChAdO × 1 nCoV-19 vaccine 18—BNT162b2 vaccine 12—mRNA-1273 vaccine |
| Median platelet counts | 32.5 × 103/μL | 42 × 103/μL |
| % of patients needing escalation of treatment with second-line agents | 10% | 32.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saluja, P.; Amisha, F.; Gautam, N.; Goraya, H. A Systematic Review of Reported Cases of Immune Thrombocytopenia after COVID-19 Vaccination. Vaccines 2022, 10, 1444. https://doi.org/10.3390/vaccines10091444
Saluja P, Amisha F, Gautam N, Goraya H. A Systematic Review of Reported Cases of Immune Thrombocytopenia after COVID-19 Vaccination. Vaccines. 2022; 10(9):1444. https://doi.org/10.3390/vaccines10091444
Chicago/Turabian StyleSaluja, Prachi, FNU Amisha, Nitesh Gautam, and Harmeen Goraya. 2022. "A Systematic Review of Reported Cases of Immune Thrombocytopenia after COVID-19 Vaccination" Vaccines 10, no. 9: 1444. https://doi.org/10.3390/vaccines10091444
APA StyleSaluja, P., Amisha, F., Gautam, N., & Goraya, H. (2022). A Systematic Review of Reported Cases of Immune Thrombocytopenia after COVID-19 Vaccination. Vaccines, 10(9), 1444. https://doi.org/10.3390/vaccines10091444





