Abstract
The aim of the study was to compare mRNA vaccine BNT162b2 with adenovirus vector- based vaccines in terms of presence of adverse reactions, immunogenicity, and protection against COVID-19. A total of 270 individuals were enrolled, of which 135 were vaccinated with adenovirus vector-based vaccines and compared with 135 age- and sex-matched participants who received the BNT162b2 mRNA vaccine. Serum sampling was performed on all participants on days 21, 42, 90, and 180 following the first dose, to evaluate anti-spike IgG and IgA responses. Antibodies were quantified by chemiluminescent microplate and ELISA assays. We demonstrate that both mRNA and adenovirus vector-based vaccines caused mild side-effects and were effective in inducing adequate antibody responses against SARS-CoV-2, although BNT162b2 was superior concerning the intensity of antibody responses and protection against severe COVID-19. Moreover, we identify that IgG and IgA responses depended primarily on both history of previous COVID-19 infection and vaccination platform used, with individuals immunized with a single-dose vaccine having lower antibody titers over time. Lastly, all vaccine platforms had limited side-effects, with the most frequent pain at the injection site. Our results provide useful information regarding antibody responses after vaccination with different vaccine platforms, which can be useful for public health vaccination strategies.
Keywords:
COVID-19; vaccination; IgG; IgA; antibody responses; BNT162b2; Ad26.COV2.S; ChAdOx1 nCoV-19 1. Introduction
Soon after its emergence, coronavirus disease 2019 (COVID-19) became a global pandemic leading to a loss of human life, as well as destabilization of the global economy and public health systems [1,2,3]. The development of vaccines to prevent infection became an effective tool against the pandemic. Thus, different vaccine platforms were developed and received emergency use authorization from the Food and Drug Administration (FDA) and/or European Medicines Agency (EMA), leading to their wide use [4,5,6,7,8]. In Greece, vaccination against COVID-19 began in late December 2020 with BNT162b2 vaccine (Comirnaty®; BioNTech/Pfizer, Mainz, Germany); a few months later, the ChAdOx1 nCoV-19 (AZD1222 of Oxford/AstraZeneca, University of Oxford, UK) and Ad26.COV2.S (Janssen Biontech, Inc., Janssen Pharmaceutical company, Johnson & Johnson, New Brunswick, NJ, USA) vaccines were added to the vaccination campaign.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which causes COVID-19 is a positive-stranded RNA virus, and its genome includes multiple regions encoding nonstructural, accessory, and structural proteins such as spike (S), membrane (M), nucleocapsid (N), and envelope (E) proteins. The S protein, especially the receptor-binding domain (RBD) of its S1 subunit, is crucial for virus entry into host cells through binding to human angiotensin-converting enzyme 2 receptor (ACE2) that is located on cell membranes [9,10]. In addition, protein S plays an important role in the host’s immune response due to its high antigenicity and ability to induce immune response [10]. Patients after COVID-19 develop anti-S antibodies with neutralizing activity [11,12]. Vaccines developed for SARS-CoV-1 and MERS-CoV were based on protein S-inducing neutralizing antibodies with a protective effect [12]. Therefore, the S protein was considered as a competent immunodominant target for most SARS-CoV-2 vaccines. Thus, most of them are either based on the S protein or on its immunogenetic region. The desired outcome for COVID-19 vaccines is to produce antibodies that will prevent the entrance of the virus into the host cell, thereby inhibiting its replication. The produced neutralizing antibodies are associated with epitopes of the S protein, and it was found, in the serum of patients after COVID-19, that the RBD region was the primary target of these antibodies [12]. Part of the success of vaccines is also the ability to prevent and protect against infection from new variants that may be more virulent, pathogenic, or capable of avoiding immunity due to mutations on S protein. Although studies suggest that vaccines can protect against severe COVID-19 from circulating variants of concern [13], the effectiveness of currently used vaccines against them is under evaluation [12,13,14].
The BNT162b2 messenger ribonucleic acid (mRNA) vaccine is a lipid nanoparticle-formulated RNA, encoding the SARS-CoV-2 full-length S protein, modified by two proline mutations to lock it in the prefusion conformation [5,8,15,16]. It requires cold storage and is administered intramuscularly in two doses, with an interval of 21 days [16]. The ChAdOx1 nCoV-19 vaccine is a recombinant, replication-deficient chimpanzee adenovirus vector also encoding the S protein. The vaccine is indicated for active immunization of individuals over 18 years of age and is also administered in two doses, with a recommended internal of 4–12 weeks after the first dose [5,17]. The Ad26.COV2.S vaccine is a recombinant, replication-incompetent adenovirus type 26 vector vaccine, constructed to encode the S protein. It is administered as a single intramuscular injection to individuals over 18 years of age [5,18].
Previous studies have reported the efficacy of the aforementioned vaccines in the prevention of COVID-19 morbidity and mortality [5,19,20]; however, comparisons in terms of their efficacy and tolerance in the “real world” are rather limited. Therefore, the aim of our study was to record side-effects and compare the efficacy of the vaccines in age- and sex-matched individuals that were randomly selected from the community. Vaccines efficacy was assessed against circulated variants during sample collection period. In this context, we also explored if the intensity of antibody responses was important for the development of COVID-19 following vaccination. We consider that our results may have significant implications on public health vaccination strategies.
2. Materials and Methods
2.1. Subjects
A total of 270 individuals were enrolled in the study from December 2020 to November 2021. Among these individuals, 135 were vaccinated with adenovirus vector-based vaccines (men/women: 81/54, median age: 49.0 years, range: 20.0–84.0); 67 individuals were vaccinated with two doses of ChAdOx1 nCoV-19 vaccine (AZD1222 of Oxford/AstraZeneca, University of Oxford, UK) (men/women; 39/28, median age: 64.0 years, range: 22.0–84.0) with an interval of 55–96 days, and 68 individuals were vaccinated with a single dose of Ad.COV2.S COVID-19 vaccine (Janssen Biotech, Inc., Janssen Pharmaceutical company, Johnson and Johnson, New Jersey, USA) (men/women: 42/26, median age: 46.5 years, range: 20.0–74.0). The humoral immune responses after adenovirus vector-based vaccines were compared with humoral responses in 135 age- and sex-matched individuals (men/women: 81/54, median age: 49.0 years, range: 27.0–84.0) receiving the BNT162b2 vaccine (Comirnaty®; BioNTech/Pfizer) in two doses with an interval of 21 days. Comparisons of antibody responses were performed between individuals of the entire group of viral vector vaccines and the entire group of mRNA BNT162b2 vaccinated participants, as well as between each group of viral vector vaccines and groups of BNT162b2 vaccinated individuals, who were age- and sex-matched.
A total of 17 individuals from the group of mRNA BNT2b2 vaccines and 10 from the Ad26.COV2.S COVID-19 vaccine exhibited a positive history of COVID-19 prior to vaccination. In addition, anti-N IgG antibodies were assessed as an indicator of recent SARS-CoV-2 infection, and positive titers were detected at enrollment in eight additional participants without a known history of COVID-19 infection.
Serum sampling was performed in all vaccinated individuals at (a) day 21, (b) day 42, (c) day 90, and (d) day 180 following the first dose of vaccination. During the study, participants were asked to complete a questionnaire regarding their medical history, adverse reactions after each dose of vaccination, and possible post-vaccination infection with SARS-CoV-2. Table 1 presents a detailed overview of participants’ medical history, including a history of COVID-19 before vaccination.

Table 1.
Overview of demographic and clinical data of SARS-CoV-2 vaccinated participants.
Each participant or their relatives, in the case of insomnia or mental disorders, provided signed informed consent. The study was conducted based on the principles of the Helsinki Declaration and was approved by the Ethical Committee of the Faculty of Medicine, University of Thessaly, Greece (No 2116).
2.2. Laboratory Tests
Anti-S and anti-N anti-SARS-CoV-2 IgG and IgA antibodies were quantified by chemiluminescent microparticle immunoassay (CMIA) and enzyme linked immunosorbent assay (ELISA), as described [21,22].
2.3. Statistical Analysis
Categorical variables are described using frequencies and relative frequencies, while continuous variables are described with medians and interquartile ranges (IQRs). The analysis of continuous variables was conducted using the Mann–Whitney U test and Spearman’s correlation coefficient, since the assumption of normal distribution was violated. Data were checked for deviation from normal distribution using the Shapiro–Wilk normality test. Multivariate analysis was performed in the form of multiple regression and binary logistic regression. Multiple regression was used to determine independent predictors of antibodies’ quantity/levels, and binary logistic regression was used to determine independent predictors of infection. For all analyses, a 5% significance level was set. Analysis was carried out with IBM Corp. Released 2019 - IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY: IBM Corp, USA) and GraphPad Prism for Windows, GraphPad Software, San Diego, California USA (version 9.2.0).
3. Results
3.1. Adverse Reactions after Vaccination
A total of 268 out of 270 participants (99.3%) were fully vaccinated according to the initial vaccination schedule. Two individuals did not receive the second dose; this included a 58 year old female from the BNT162b2 group due to severe facial flushing and electrocardiogram (ECG) changes and a 63 year old female from the ChAdOx1 nCoV-19 group due to tachycardia, facial flushing, and reactivation of herpes zoster. Complete records of all side-effects reported after each dose of vaccine were available for 239 individuals (89.2%), while, for the 31 remaining participants, the only information available was related to the presence of fever.
An overview and a comparison of the most common side-effects after vaccination are presented in Table 2 and Table 3, respectively. The presence of local pain—either with or without redness and swelling—at the injection site was the most common side-effect after the first dose of BNT162b2, compared to the Ad26.COV2.S vaccine (Table 2). Fever occurred more frequently in adenovirus vector-based vaccines (Table 2 and Table 3). Moreover, myalgias were reported more frequently after the second dose of BNT162b2 vaccine when compared to the Ad26.COV2.S group, as well as following the first dose of ChAdOx1 nCoV-19 vaccine in comparison to the BNT162b2 group. Reported side-effects were referred to as mild and limited in all study participants, except for two cases that did not receive the second dose as mentioned above (Table 2 and Table 3).

Table 2.
Comparison of adverse reactions after vaccination with BNT162b2 and Ad26.COV2.S vaccines.

Table 3.
Comparison of adverse reactions after vaccination with BNT162b2 and ChAdOx1 nCoV-19 vaccines.
3.2. Intensity and Dynamics of IgG and IgA Responses after Vaccination
All platforms achieved positive IgG antibody responses for the great majority of vaccinated individuals 21 days after vaccination; however, the intensity of responses differed (Table 4, Figure 1 and Figure 2). Specifically, the BNT162b2 vaccine resulted in significantly higher antibody titers when compared to the total number of adenovirus vector-based vaccines (p < 0.001) and to ChAdOx1nCoV-19 (p = 0.048). While differences were also present when BNT162b2 was compared with the Ad26.COV2.S vaccine, this difference was not considered significant (p = 0.077). In terms of IgA responses, most enrolled individuals failed to respond adequately (with antibody titers below the threshold of positivity); nevertheless, the intensity of IgA titers was higher after BNT162b2 vaccination (Table 4 and Figure 2).

Table 4.
IgG and IgA immune responses 21, 42, 90, and 180 days after vaccination.
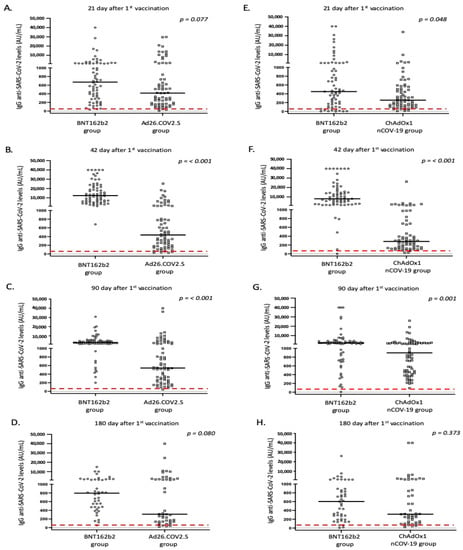
Figure 1.
IgG anti SARS-CoV-2 responses in the study participants according to vaccine platform: (A,E) day 21, (B,F) day 42, (C,G) day 90, and (D,H) day 180. Black lines indicate median values and red dotted lines represent the cutoff of positive anti-SARS-CoV-2 IgG (50 AU/mL) antibodies.
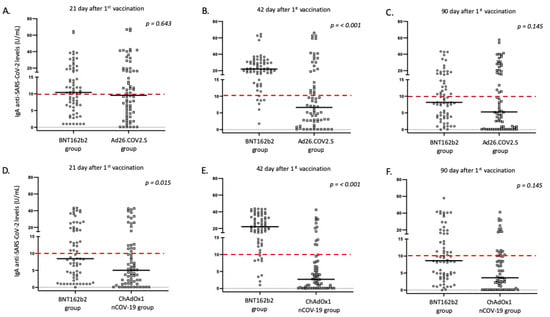
Figure 2.
IgA anti SARS-CoV-2 responses in the study participants according to vaccine platform: (A,D) day 21, (B,E) day 42, and (C,F) day 90. Black lines indicate median values, and red dotted lines represent the cutoff of positive anti-SARS-CoV-2 IgA (10 U/mL) antibodies.
Furthermore, on the 42nd day after the first dose, individuals who received the second dose of the mRNA vaccine displayed a significantly higher proportion of positive anti-S IgG and IgA antibodies, when compared to adenovirus vector-based vaccines (p < 0.001). These individuals also exhibited significantly higher levels of IgG and IgA anti-SARS-CoV-2 antibodies in their serum (Table 4 and Figure 1 and Figure 2). This pattern of expression was maintained for IgG responses at 90 days following the first vaccination dose, but was missed for IgA responses, where the majority of immunized individuals displayed IgA levels below the positivity cutoff (Table 4 and Figure 1 and Figure 2).
Unfortunately, not all individuals participated in blood sampling on day 180 following the first dose. Those who remained in the study (182 out of 270, 67.4%) continued to be age- and sex-matched. Interestingly, although anti-S IgG levels were higher in the BNT162b2 group, the statistical significance was marginally lost (especially comparing IgG levels between BNT162b2 and Ad26.COV2.S vaccines; Table 4, Figure 1). As previously mentioned, considering that most enrolled individuals displayed very low IgA levels on day 90, we did not measure IgA responses on day 180.
3.3. Correlation of IgG Responses with Demographic and Clinical Parameters of Vaccinated Individuals
According to multivariate analysis (Table 5) which included data from individuals vaccinated with all three vaccines, the main factors affecting IgG levels following vaccination were a history of COVID-19 infection (prior to or after vaccination) and the vaccine platform. Specifically, BNT162b2 vaccination was associated with higher IgG titers on sampling days 42 and 90 after vaccination, compared to adenovirus vector-based vaccines (p < 0.001 and p = 0.001, respectively). Moreover, SARS-CoV-2 infection confirmed by RT-PCR or detection of positive anti-N anti-SARS-CoV-2 antibodies was the constant factor with a positive effect on IgG antibody levels; no other factors from participants’ medical histories affected the intensity of antibody responses. When participants with a history of COVID-19 infection were excluded from analysis, the vaccine platform was the predominant factor associated with IgG antibody responses on sampling day 21 (p = 0.002), day 42 (p < 0.001), and day 90 (p = 0.002) following the first dose, with the BNT162b2 vaccine appearing superior compared to adenovirus vector-based vaccines. Age significantly affected the antibody titer only on sampling day 42 and was associated with lower IgG levels among older individuals. Considering the effect of adverse reactions on intensity of IgG responses, multivariate analysis revealed that the presence of fever after both doses, and fatigue after the second dose was associated with higher IgG antibody titers (Supplementary Table S1). When participants with a known history of COVID-19 were excluded from analysis, no side-effects appeared to have an effect on antibody responses.

Table 5.
Multivariate analyses of anti-SARS-CoV-2 IgG responses after vaccination with adenovirus vector-based (Ad26.COV2.S, ChAdOx1 nCoV-19) and mRNA (BNT162b2) vaccines.
By studying vaccine platforms in pairs, similar data were provided through multivariate analysis of IgG responses. In particular, previous COVID-19 infection always had a positive effect on the intensity of IgG responses; however, when participants with a known infection history were excluded from analysis, the vaccine platform played a significant role, which was more profound when compared to individuals receiving BNT162b2 and Ad26.COV2.S vaccines on sampling days 21, 42, and 90 (Supplementary Table S2). Further analysis revealed that participants receiving antihypertensive treatment and Ad26.COV2.S vaccine, displayed lower anti-SARS-CoV-2 levels on sampling day 42 (p = 0.019) (Supplementary Table S3). Lastly, individuals displaying headaches exhibited higher IgG levels on day 42, when compared to the BNT162b2 and ChAdOx1 nCoV-19 groups (p = 0.019).
3.4. Correlation of IgA Responses with Demographic and Clinical Parameters of Vaccinated Individuals
Similar to anti-S IgG responses, IgA levels were significantly affected by COVID-19 history before or after vaccination (p < 0.001). Multivariate analysis revealed that the type of vaccination significantly affected IgA levels on sampling days 42 and 90 following the first dose, regardless of COVID-19 history prior to or after vaccination (Table 6). Lastly, adverse side-effects did not affect the intensity of IgA responses, except for the presence of fever after the second immunization with BNT162b2 vaccine, especially when compared to BNT162b2 and ChAdOx1 nCoV-19 groups (p = 0.011).

Table 6.
Multivariate analyses of anti-SARS-CoV-2 IgA responses after vaccination with adenovirus vector-based (Ad26.COV2.S, ChAdOx1 nCoV-19) and mRNA (BNT162b2) vaccines.
3.5. Correlation of Antibody Titers and COVID-19 after Vaccination
A total of 18 participants (including two with prior SARS-CoV-2 infection, one from the BNT162b2 group, three from the ChAdOx1 nCoV-19 group, and 14 from the Ad26.COV2.S group) were infected by SARS-CoV-2 after vaccination, as confirmed by detection of the virus via RT-PCR on nasopharyngeal swabs or, in one case, by the detection of anti-N anti-SARS-CoV-2 antibodies in serum. The confirmed COVID-19 cases were distributed at different sampling times. Specifically, two participants were infected by SARS-CoV-2 between the 21st and 42nd day after the first dose, five between the 42nd and 90th day, and 11 between the 90th and 180th day. All COVID-19 patients displayed mild disease, and none required hospitalization.
A line chart was used to represent levels of anti-S IgG and IgA antibodies over time between infected and noninfected groups. It appears that those who were infected with SARS-Cov-2 had lower antibody titers throughout the study [23] (Figure 3).
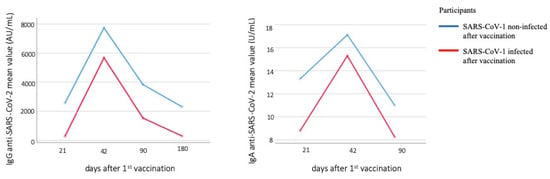
Figure 3.
Line chart of anti-S IgG and IgA antibodies levels between infected and noninfected with SARS-CoV-2 after vaccination individuals.
4. Discussion
Our study provides clear evidence that both mRNA and adenovirus vector-based vaccines are effective for inducing adequate antibody responses against SARS-CoV-2, while also demonstrating that the BNT162b2 mRNA vaccine is superior to adenovirus vector-based vaccines concerning the strength of antibody responses and protection against severe COVID-19.
As previously mentioned, all vaccine platforms were well tolerated, and side-effects were limited for most enrolled participants except two females, reported above, which did not receive a second dose due to severe adverse reactions. Similar to previous studies [24,25,26,27,28], local side-effects were more common after vaccination, while systemic adverse reactions (mild as a rule) were more common following adenovirus vector-based vaccines. Despite the presence of mild side-effects in the majority of individuals of our study, the number remains small to draw conclusions about the safety of vaccine platforms.
In our study, we compared the intensity of antibody responses between individuals receiving mRNA and adenovirus vector-based vaccines. Although similar studies have been published in the literature [29,30], our study has two significant differences which may represent important advantages. The first is that we compared antibody responses of age- and sex-matched participants from the community, while most previous studies compared only healthcare workers. The second was the inclusion of IgA response intensity as a significant parameter of immunization.
Both we and others have already reported that anti-S IgA responses after mRNA vaccination display a rapid decline compared to IgG responses, particularly 3 months following vaccination [25,31,32,33]. Although Zurac et al. reported a higher rate of positive IgA responses after vaccination, similar to our results, IgA levels were positively correlated only with a previous history of COVID-19 [32]. It is worth noting that IgA responses were evaluated in previous studies especially after mRNA vaccination, including a very small number of participants. To the best of our knowledge, our study is the largest in the literature analyzing IgA responses after vaccination with different vaccine platforms, and we identified that BNT162b2 vaccination resulted in the strongest positive effect on IgA antibody titers.
As with previous studies, we demonstrated that the BNT62b2 mRNA vaccine resulted in higher titers of anti-IgG levels compared to adenovirus vector-based vaccines [29,30,33,34]. In addition, serological data after the second dose of BNT162b2 vaccine resulted in boosting of anti-S IgG antibody titers, with a further waning over time [29]. Similar to previous studies, we also observed that age and a previous history of COVID-19 significantly affected intensity of IgG responses [22,29,35,36].
Interestingly, we identified that individuals immunized with BNT62b2 vaccine who displayed higher anti-S IgA and IgG antibody levels exhibited a lower incidence of COVID-19 after vaccination, compared to those immunized with adenovirus vector-based vaccines. In particular, 14 individuals (out of a total of 18 infected by SARS-CoV-2 after vaccination) were immunized with the Ad26.COV2.S vaccine, compared to three with the ChAdOx1 nCoV-19 vaccine and only one with the BNT162b2 vaccine. As mentioned above, the effectiveness of COVID-19 vaccination slowly decreases over time, which is more profound at 6 months following vaccination [29,37]. This observation is in line with Cohn et al., where protection against severe SARS-CoV-2 for individuals who received the BNT162b2 vaccine decreased from 87% to 45% 6 months after vaccination, while protection against severe SARS-CoV-2 for individuals who received the Ad26.COV2.S vaccine dropped from 86% to 13% [38]. Therefore, we could speculate that lower antibody levels, faster waning of antibody titers over time, and a higher rate of SARS-CoV-2 infection after Ad26.COV2.S immunization lead to the conclusion that a single-dose vaccine is less effective than others.
A limitation of our study is the relatively small number of enrolled participants. However, we conducted a real-world study in the community; a significant challenge was enrolling age- and sex-matched participants, considering the different politics concerning vaccination strategies among various age populations. For example, according to initial data of thrombotic events after adenovirus vector-based vaccines, this type of vaccination platform was contraindicated for women below 60 years of age and young individuals for a long period of time [39,40]. Moreover, it is always challenging to convince individuals who are not health workers to provide a blood donation. Thus, selecting individuals of different age groups who were age- and sex- matched was rather difficult, but we consider the number of enrolled individuals as adequate to provide conclusive remarks.
5. Conclusions
Our study provides supportive evidence about the effectiveness of COVID-19 vaccination. We identified that anti-S IgG and IgA responses depend primarily on both the presence of a previous COVID-19 infection history and the vaccination platform used, with the mRNA vaccine appearing superior to adenovirus vector-based vaccines. Furthermore, individuals who became infected following vaccination had lower antibody titers and were mainly from the Ad26.COV2.S group, suggesting that a single-dose vaccine is less effective than the other vaccines. We consider that our results will be useful for the implementation of further vaccination strategies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/vaccines10081268/s1: Table S1. Multivariate analyses of adverse side-effects and intensity of anti-SARS-CoV-2 IgG responses after vaccination with adenovirus vector-based (Ad26.COV2.S, ChAdOx1 nCoV-19) and mRNA (BNT162b2) vaccines on day 42 after vaccination; Table S2. Multivariate analyses of anti-SARS-CoV-2 IgG responses after vaccination with Ad26.COV.2.S and BNT162b2 vaccines; Table S3. Multivariate analysis of intensity of anti-SARS-CoV-2 IgG responses on sampling day 42 after the first dose of BNT162b2 and Ad26.COV2.S vaccination.
Author Contributions
Conceptualization, C.H., M.S. and I.V.; methodology, M.S., I.V., S.S., M.A.K., A.-M.P., A.N., I.O., I.A., D.P., A.-K.K., G.P. and C.H.; investigation, I.V., S.S., A.N., A.-M.P., A.T., V.A.M. and E.P.; formal analysis, C.H., M.S. and A.D.; data curation, C.H., M.S., I.V. and S.S.; funding acquisition, C.H.; writing—original draft preparation, M.S. and I.V.; writing—review and editing, C.H., M.S. and L.A.; supervision, C.H. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the ethical committee of the Faculty of Medicine, University of Thessaly, Greece (No. 2116, 22 April 2020).
Informed Consent Statement
All involved participants provided informed consent.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.
Acknowledgments
The authors want to thank all the participants for blood donation. We especially thank the Municipal Nursing Home of Larissa, Greece, the Saint Nicolas Elderly Health Care Unit of Metropolis of Tricca, Gardiki and Pyli, Megala Kalivia—Trikala, Greece, the Social Welfare Center, Region of Thessaly—branch of Trikala, Greece, and the Psychogeriatric Hospital “Ippokrateio Therapeutirio”, Larissa, Greece.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- World Health Organization. Novel Coronavirus (2019-nCoV) Situation Report-11. Available online: https://apps.who.int/iris/bitstream/handle/10665/330776/nCoVsitrep31Jan2020-eng.pdf (accessed on 11 June 2022).
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 Novel Coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the Coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Forni, G.; Mantovani, A. COVID-19 Vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Mascellino, M.T.; Di Timoteo, F.; De Angelis, M.; Oliva, A. Overview of the main anti-SARS-CoV-2 vaccines: Mechanism of action, efficacy and safety. Infect. Drug Resist. 2021, 14, 3459–3476. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef] [PubMed]
- WHO Issues Its First Emergency Use Validation for a COVID-19 Vaccine and Emphasizes Need for Equitable Global Access. Available online: https://www.who.int/news/item/31-12-2020-who-issues-its-first-emergency-use-validation-for-a-covid-19-vaccine-and-emphasizes-need-for-equitable-global-access (accessed on 9 June 2022).
- Lamb, Y.N. BNT162b2 mRNA COVID-19 vaccine: First approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef] [Green Version]
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Wu, J.; Liang, B.; Chen, C.; Wang, H.; Fang, Y.; Shen, S.; Yang, X.; Wang, B.; Chen, L.; Chen, Q.; et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat. Commun. 2021, 12, 1813. [Google Scholar] [CrossRef]
- Martinez-Flores, D.; Zepeda-Cervades, J.; Cruz-Resendiz, A.; Aguirre-Sampieri, S.; Sampieri, A.; Vaca, L. SARS-CoV-2 vaccines based on the Spike glycoprotein and implications of the new viral variants. Front. Immunol. 2021, 12, 701501. [Google Scholar] [CrossRef]
- Mistry, P.; Barmania, F.; Mellet, J.; Peta, K.; Strydom, A.; Viljoen, I.M.; James, W.; Gordon, S.; Petter, M.S. SARS-CoV-2 variants, vaccines, and host immunity. Front. Immunol. 2022, 12, 809244. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Zheng, Q.; Zhang, H.; Niu, Y.; Lou, Y.; Wang, H. The SARS-CoV-2 spike glycoprotein biosynthesis, structure, function, and antigenicity: Implications for the design of spike-based vaccine immunogens. Front. Immunol. 2020, 11, 576622. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency—Comirnaty. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/comirnaty (accessed on 29 June 2022).
- European Medicines Agency—Comirnaty: Annex I—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf (accessed on 11 June 2022).
- European Medicines Agency—Vaxzervia: Annex I—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/vaxzevria-previously-covid-19-vaccine-astrazeneca-epar-product-information_en.pdf (accessed on 11 June 2022).
- European Medicines Agency—JCOVDEN: Annex I—Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/jcovden-previously-covid-19-vaccine-janssen-epar-product-information_en.pdf (accessed on 11 June 2022).
- Xie, J.; Feng, S.; Li, X.; Gea-Mallorquí, E.; Prats-Uribe, A.; Prieto-Alhambra, D. Comparative effectiveness of the BNT162b2 and ChAdOx1 vaccines against COVID-19 in people over 50. Nat. Commun. 2022, 13, 1519. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Garcia-Beltran, W.F.; Chang, C.C.; Berrios Mairena, C.; Thierauf, J.C.; Kirkpatrick, G.; Onozato, M.L.; Cheng, J.; St Denis, K.J.; Lam, E.C.; et al. Comparative immunogenicity and effectiveness of MRNA-1273, BNT162b2, and Ad26.COV2.S COVID-19 vaccines. J. Infect. Dis. 2021, 225, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Speletas, M.; Kyritsi, M.A.; Vontas, A.; Theodoridou, A.; Chrysanthidis, T.; Hatzianastasiou, S.; Petinaki, E.; Hadjichristodoulou, C. Evaluation of two chemiluminescent and three ELISA immunoassays for the detection of SARS-CoV-2 IgG antibodies: Implications for disease diagnosis and patients’ management. Front. Immunol. 2020, 11, 609242. [Google Scholar] [CrossRef] [PubMed]
- Speletas, M.; Voulgaridi, I.; Sarrou, S.; Dadouli, A.; Mouchtouri, V.A.; Nikoulis, D.J.; Tsakona, M.; Kyritsi, M.A.; Peristeri, A.-M.; Avakian, I.; et al. Intensity and dynamics of anti-SARS-CoV-2 immune responses after BNT162b2 MRNA vaccination: Implications for public health vaccination strategies. Vaccines 2022, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, N.; Kuo, H.H.; Boucau, J.; Farmer, J.R.; Allard-Chamard, H.; Mahajan, V.S.; Piechocka-Trocha, A.; Lefteri, K.; Osborn, M.; Bals, J.; et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell 2020, 183, 143–175.e13. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomized, Controlled, Phase 2/3 Trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and efficacy of single-dose Ad26.COV2.S vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Mathioudakis, A.G.; Ghrew, M.; Ustianowski, A.; Ahmad, S.; Borrow, R.; Papavasileiou, L.P.; Petrakis, D.; Bakerly, N.D. Self-reported real-world safety and reactogenicity of COVID-19 vaccines: A vaccine recipient survey. Life 2021, 11, 249. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Khillan, R.; Mishra, Y.; Khurana, S. The safety profile of COVID-19 vaccinations in the United States. Am. J. Infect. Control 2022, 50, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Mateus, J.; Coelho, C.H.; Dan, J.M.; Moderbacher, C.R.; Gálvez, R.I.; Cortes, F.H.; Grifoni, A.; Tarke, A.; Chang, J.; et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022, 185, 2434–2451. [Google Scholar] [CrossRef] [PubMed]
- Szczepanek, J.; Skorupa, M.; Goroncy, A.; Jarkiewicz-Tretyn, J.; Wypych, A.; Sandomierz, D.; Jarkiewicz-Tretyn, A.; Dejewska, J.; Ciechanowska, K.; Pałgan, K.; et al. Anti-SARS-CoV-2 IgG against the S protein: A comparison of BNT162b2, mRNA-1273, ChAdOx1 nCoV-2019 and Ad26.COV2.S vaccines. Vaccines 2022, 10, 99. [Google Scholar] [CrossRef]
- Wisnewski, A.V.; Campillo Luna, J.; Redlich, C.A. Human IgG and IgA Responses to COVID-19 mRNA vaccines. PLoS ONE 2021, 16, e0249499. [Google Scholar] [CrossRef]
- Zurac, S.; Nichita, L.; Mateescu, B.; Mogodici, C.; Bastian, A.; Popp, C.; Cioplea, M.; Socoliuc, C.; Constantin, C.; Neagu, M. COVID-19 vaccination and IgG and IgA antibody dynamics in healthcare workers. Mol. Med. Rep. 2021, 24, 578. [Google Scholar] [CrossRef] [PubMed]
- Romero-Pinedo, S.; Quesada, M.; Horndler, L.; Álvarez-Fernández, S.; Olmo, A.; Abia, D.; Alarcón, B.; Delgado, P. Vaccine type-, age- and past infection-dependence of the humoral response to SARS-CoV-2 spike S protein. Front. Immunol. 2022, 13, 809285. [Google Scholar] [CrossRef]
- Kang, Y.M.; Minn, D.; Lim, J.; Lee, K.-D.; Jo, D.H.; Choe, K.-W.; Kim, M.J.; Kim, J.M.; Kim, K.N. Comparison of antibody response elicited by ChAdOx1 and BNT162b2 COVID-19 vaccine. J. Korean Med. Sci. 2021, 36, e311. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine 2021, 36, 100928. [Google Scholar] [CrossRef]
- Notarte, K.I.; Ver, A.T.; Velasco, J.V.; Pastrana, A.; Catahay, J.A.; Salvagno, G.L.; Yap, E.P.H.; Martinez-Sobrido, L.; Torrelles, J.B.; Lippi, G.; et al. Effects of age, sex, serostatus, and underlying comorbidities on humoral response post-SARS-CoV-2 Pfizer-BioNTech mRNA vaccination: A systematic review. Crit. Rev. Clin. Lab. Sci. 2022, 28, 1–18. [Google Scholar] [CrossRef]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.-J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Cohn, B.A.; Cirillo, P.M.; Murphy, C.C.; Krigbaum, N.Y.; Wallace, A.W. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science 2022, 375, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Perek, B.; Flisiak, R. Thrombotic thrombocytopenia after COVID-19 vaccination: In search of the underlying mechanism. Vaccines 2021, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Long, B.; Bridwell, R.; Gottlieb, M. Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines. Am. J. Emerg. Med. 2021, 49, 58–61. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).