Increased Mild Vaccine-Related Side Effects and Higher Specific Antibody Titers in Health Care Workers with Previous SARS-CoV-2 Infection after the mRNA BNT162b2 Vaccine
Abstract
:1. Introduction
2. Materials and Methods
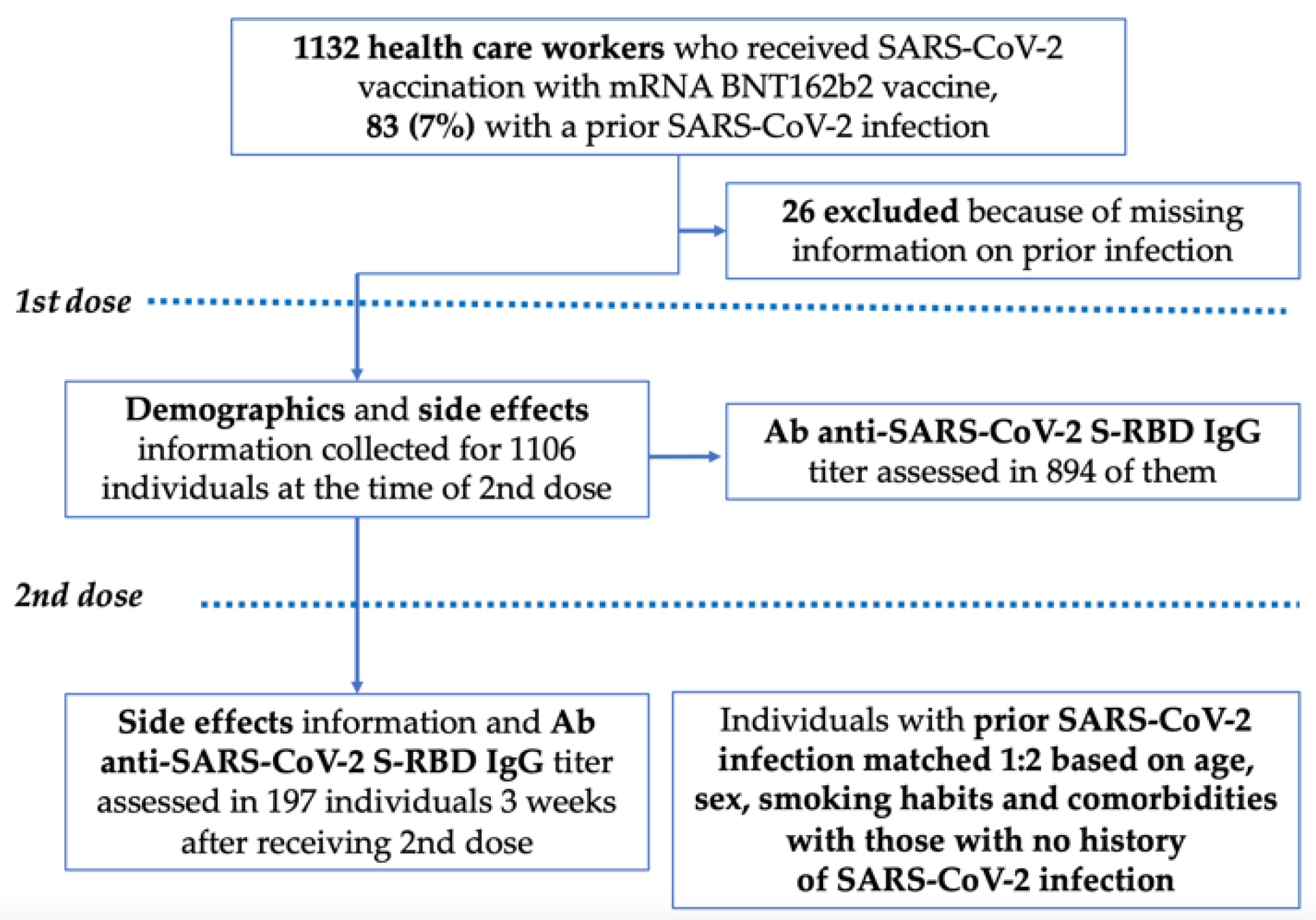
2.1. Study Design and Participants
2.2. Laboratory Test
2.3. Statistical Analysis
3. Results
3.1. Overall Population
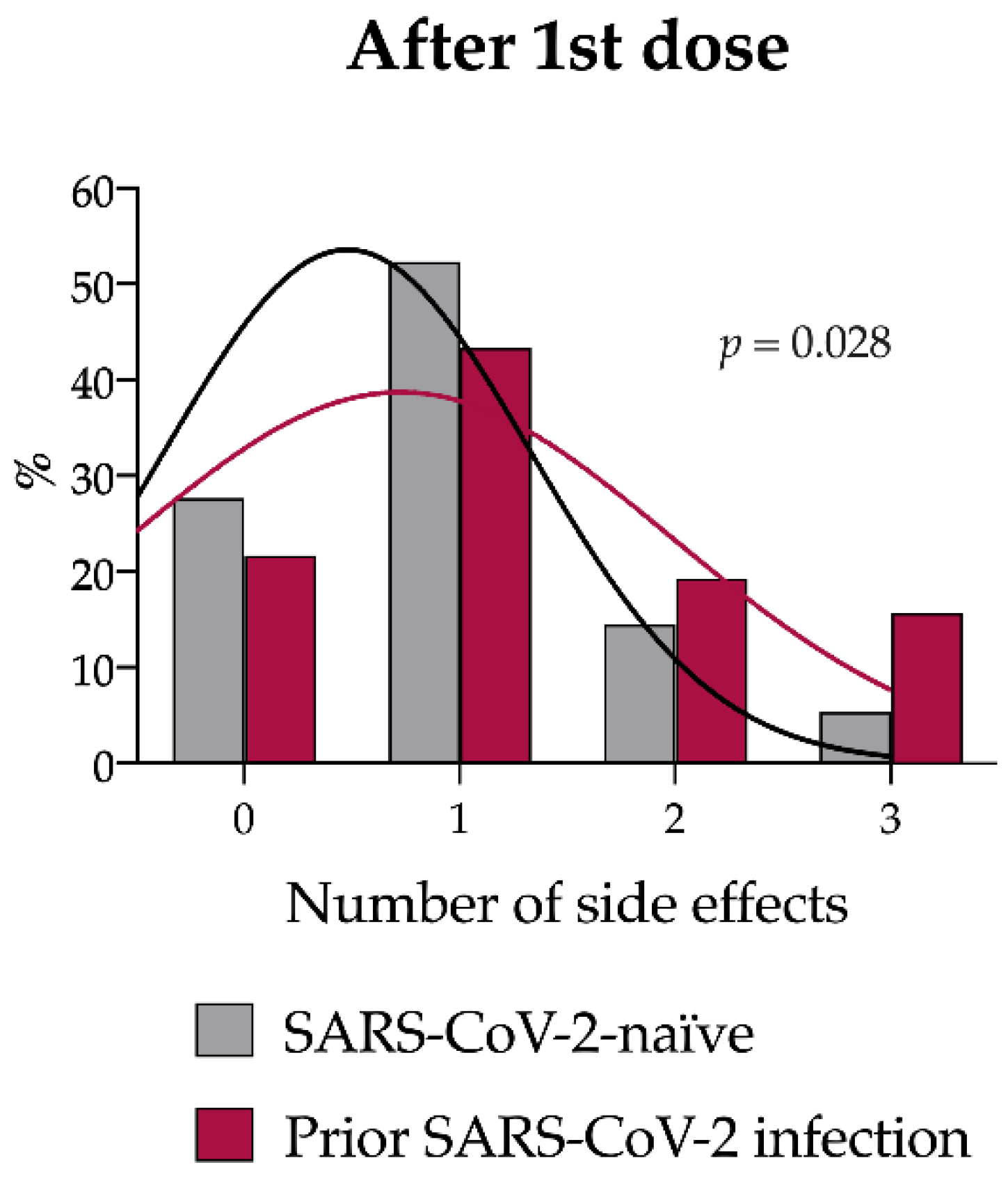
3.2. Number and Type of Solicited Side Effects in the Overall Population
3.3. Case–Control Analysis: Number and Type of Solicited Side Effects
3.4. Anti-SARS-CoV-2 RBD IgG Titer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emergency Use Authorization (EUA) for an Unapproved Product Review Memorandum. Available online: https://www.fda.gov/media/144416/download (accessed on 16 April 2022).
- Comirnaty Assessment Report. Available online: https://www.ema.europa.eu/en/documents/assessment-report/comirnaty-epar-public-assessment-report_en.pdf (accessed on 16 April 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2020-12-23&atto.codiceRedazionale=20A07197&elenco30giorni=false (accessed on 16 April 2022).
- Manisty, C.; Otter, A.D.; Treibel, T.A.; McKnight, A.; Altmann, D.M.; Brooks, T.; Noursadeghi, M.; Boyton, R.J.; Semper, A.; Moon, J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 2021, 397, 1057–1058. [Google Scholar] [CrossRef]
- Gobbi, F.; Buonfrate, D.; Moro, L.; Rodari, P.; Piubelli, C.; Caldrer, S.; Riccetti, S.; Sinigaglia, A.; Barzon, L. Antibody Response to the BNT162b2 mRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Viruses 2021, 13, 422. [Google Scholar] [CrossRef]
- Riad, A.; Pokorna, A.; Attia, S.; Klugarova, J.; Koscik, M.; Klugar, M. Prevalence of COVID-19 Vaccine Side Effects among Healthcare Workers in the Czech Republic. J. Clin. Med. 2021, 10, 1428. [Google Scholar] [CrossRef]
- Modenese, A.; Paduano, S.; Bargellini, A.; Bellucci, R.; Marchetti, S.; Bruno, F.; Grazioli, P.; Vivoli, R.; Gobba, F. Neutralizing Anti-SARS-CoV-2 Antibody Titer and Reported Adverse Effects, in a Sample of Italian Nursing Home Personnel after Two Doses of the BNT162b2 Vaccine Administered Four Weeks Apart. Vaccines 2021, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Izumo, T.; Kuse, N.; Awano, N.; Tone, M.; Sakamoto, K.; Takada, K.; Muto, Y.; Fujimoto, K.; Saiki, A.; Ito, Y.; et al. Side effects and antibody titer transition of the BNT162b2 messenger ribonucleic acid coronavirus disease 2019 vaccine in Japan. Respir. Investig. 2021, 59, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Van Eyk, J.E.; Braun, J.G.; et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021, 27, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Di Lauria, N.; Maggi, L.; Salvati, L.; Vanni, A.; Capone, M.; Lamacchia, G.; Mantengoli, E.; Spinicci, M.; Zammarchi, L.; et al. First-dose mRNA vaccination is sufficient to reactivate immunological memory to SARS-CoV-2 in subjects who have recovered from COVID-19. J. Clin. Investig. 2021, 131, e149150. [Google Scholar] [CrossRef] [PubMed]
- Hammerman, A.; Sergienko, R.; Friger, M.; Beckenstein, T.; Peretz, A.; Netzer, D.; Yaron, S.; Arbel, R. Effectiveness of the BNT162b2 Vaccine after Recovery from COVID-19. N. Engl. J. Med. 2022, 386, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermúdez-González, M.C.; Bielak, D.A.; Carreño, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Baldolli, A.; Michon, J.; Appia, F.; Galimard, C.; Verdon, R.; Parienti, J.J. Tolerance of BNT162b2 mRNA COVI-19 vaccine in patients with a medical history of COVID-19 disease: A case control study. Vaccine 2021, 39, 4410–4413. [Google Scholar] [CrossRef] [PubMed]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.; Xiao, S.; Debes, A.K.; Egbert, E.R.; Caturegli, P.; Colantuoni, E.; Milstone, A.M. Durability of Antibody Levels After Vaccination with mRNA SARS-CoV-2 Vaccine in Individuals with or without Prior Infection. JAMA 2021, 326, 2524–2526. [Google Scholar] [CrossRef] [PubMed]

| Overall (n = 1106) | Previous SARS-CoV-2 Infection | p | ||
|---|---|---|---|---|
| Yes (n = 83) | No (n = 1023) | |||
| Median age | 42 (31–51) | 42 (31–51) | 40 (30–48) | 0.260 |
| Males | 441 (39.8%) | 36 (43.4%) | 405 (39.6%) | 0.498 |
| Smokers | 327 (29.6%) | 14 (16.9%) | 313 (30.6%) | 0.008 |
| Comorbidities | ||||
| Hypertension | 113 (10.2%) | 6 (7.2%) | 107 (10.6%) | 0.350 |
| Cardiovascular disease | 12 (1.1%) | 0 (0%) | 12 (1.2%) | 0.321 |
| Obesity | 21 (1.9%) | 1 (1.2%) | 20 (2.0%) | 0.630 |
| Thyroid disorders | 110 (9.9%) | 6 (7.2%) | 104 (10.2%) | 0.390 |
| Diabetes | 22 (2.0%) | 0 (0%) | 22 (2.1%) | 0.177 |
| Neoplasm | 11 (1.0%) | 0 (0%) | 11 (1.1%) | 0.342 |
| Asthma | 15 (1.4%) | 1 (1.2%) | 14 (1.4%) | 0.901 |
| Other respiratory diseases | 14 (1.3%) | 2 (2.4%) | 12 (1.2%) | 0.332 |
| Autoimmune disorders | 30 (2.7%) | 1 (1.2%) | 29 (2.8%) | 0.379 |
| Dyslipidemia | 6 (0.5%) | 2 (2.4%) | 4 (0.4%) | 0.016 |
| Anemia | 4 (0.4%) | 1 (1.2%) | 3 (0.3%) | 0.183 |
| Other | 50 (4.5%) | 5 (6.0%) | 45 (4.4%) | 0.493 |
| 1st Vaccine Dose | ||||
|---|---|---|---|---|
| Overall (n = 1106) | Previous SARS-CoV-2 Infection | p | ||
| Yes (n = 83) | No (n = 1023) | |||
| Pain in site of injection | 704 (63.6%) | 63 (75.9%) | 641 (62.7%) | 0.016 |
| Muscle or joint pain | 133 (12.0%) | 14 (16.9%) | 119 (11.6%) | 0.158 |
| Weakness | 114 (10.3%) | 19 (22.9%) | 95 (9.3%) | <0.001 |
| Fever | 34 (3.1%) | 8 (9.6%) | 26 (2.5%) | <0.001 |
| Headache | 102 (9.2%) | 10 (12.0%) | 92 (9.0%) | 0.355 |
| Diarrhea | 6 (0.5%) | 0 (0%) | 6 (0.6%) | 0.484 |
| Nausea | 8 (0.8%) | 0 (0%) | 8 (0.8%) | 0.419 |
| Dyspnea | 4 (0.4%) | 1 (1.2%) | 3 (0.3%) | 0.183 |
| Skin rash | 2 (0.2%) | 0 (0%) | 2 (0.2%) | 0.687 |
| Lymphadenopathy | 3 (0.3%) | 0 (0%) | 3 (0.3%) | 0.621 |
| Paresthesia | 5 (0.5%) | 0 (0%) | 5 (0.5%) | 0.523 |
| Others | 17 (1.5%) | 2 (2.4%) | 15 (1.5%) | 0.502 |
| 1st Vaccine Dose | 2nd Vaccine Dose | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n = 249) | Previous SARS-CoV-2 Infection | p | Overall (n = 239) | Previous SARS-CoV-2 Infection | p | |||
| Yes (n = 83) | No (n = 166) | Yes (n = 82) | No (n = 157) | |||||
| Pain in site of injection | 176 | 63 | 113 | 0.201 | 126 | 36 | 90 | 0.048 |
| (70.7%) | (75.9%) | (68.1%) | (52.7%) | (43.9%) | (57.3%) | |||
| Muscle or joint pain | 34 | 14 | 20 | 0.296 | 84 | 24 | 60 | 0.169 |
| (13.7%) | (16.9%) | (12.0%) | (35.1%) | (29.3%) | (38.2%) | |||
| Weakness | 30 | 19 | 11 | <0.001 | 87 | 30 | 57 | 0.966 |
| (12.0%) | (22.9%) | (6.6%) | (34.4%) | (36.6%) | (36.3%) | |||
| Fever | 13 | 8 | 5 | 0.027 | 65 | 18 | 47 | 0.188 |
| (5.2%) | (9.6%) | (3.0%) | (27.2%) | (21.9%) | (29.9%) | |||
| Headache | 20 | 10 | 10 | 0.099 | 42 | 15 | 27 | 0.833 |
| (8.0%) | (12.0%) | (6.0%) | (17.6%) | (18.3%) | (17.2%) | |||
| Diarrhea | 0 | 0 | 0 | / | 2 | 0 | 2 | 0.305 |
| (0%) | (0%) | (0%) | (0.8%) | (0%) | (1.3%) | |||
| Nausea | 1 | 0 | 1 | 0.479 | 8 | 2 | 6 | 0.573 |
| (0.4%) | (0%) | (0.6%) | (3.3%) | (2.4%) | (3.8%) | |||
| Dyspnea | 1 | 1 | 0 | 0.156 | 2 | 1 | 1 | 0.639 |
| (0.4%) | (1.2%) | (0%) | (0.8%) | (1.2%) | (0.6%) | |||
| Skin rash | 1 | 0 | 1 | 0.479 | 1 | 1 | 0 | 0.166 |
| (0.4%) | (0%) | (0.6%) | (0.4%) | (1.2%) | (0%) | |||
| Lymphadeno-pathy | 0 | 0 | 0 | / | 4 | 1 | 3 | 0.692 |
| (0%) | (0%) | (0%) | (1.7%) | (1.2%) | (1.9%) | |||
| Paresthesia | 1 | 0 | 1 | 0.479 | 3 | 2 | 1 | 0.235 |
| (0.4%) | (0%) | (0.6%) | (1.2%) | (2.4%) | (0.6%) | |||
| Others | 4 | 2 | 2 | 0.476 | 17 | 3 | 14 | 0.133 |
| (1.6%) | (2.4%) | (1.2%) | (7.1%) | (3.7%) | (8.9%) | |||
| 1st Vaccine Dose | 2nd Vaccine Dose | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anti-SARS-CoV-2 RBD IgG (AU/mL) | Overall (%) n = 224 | Prior SARS-CoV-2 Infection | p | Anti-SARS-CoV-2 RBD IgG (AU/mL) | Overall (%) n = 197 | Prior SARS-CoV-2 Infection | p | ||
| Yes (n = 62) | No (n = 162) | Yes (n = 46) | No (n = 151) | ||||||
| Low (0–10) | 25 (11.2%) | 0 (0%) | 25 (15.4%) | <0.001 | Low (0–100) | 18 (9.1%) | 2 (4.3%) | 16 (10.6%) | 0.043 |
| Inter- mediate (10–100) | 132 (58.9%) | 12 (19.4%) | 120 (74.1%) | Inter- mediate (100–2000) | 167 (84.8%) | 38 (82.6%) | 129 (85.4%) | ||
| High (>100) | 67 (29.9%) | 50 (80.6%) | 17 (10.5%) | High (>2000) | 12 (6.1%) | 6 13.0%) | 6 (4.0%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, L.; Compagno, M.; Campogiani, L.; Teti, E.; Mulas, T.; Checchi, D.; Alessio, G.; Caldara, F.; Coppola, L.; De Simone, G.; et al. Increased Mild Vaccine-Related Side Effects and Higher Specific Antibody Titers in Health Care Workers with Previous SARS-CoV-2 Infection after the mRNA BNT162b2 Vaccine. Vaccines 2022, 10, 1238. https://doi.org/10.3390/vaccines10081238
Ferrari L, Compagno M, Campogiani L, Teti E, Mulas T, Checchi D, Alessio G, Caldara F, Coppola L, De Simone G, et al. Increased Mild Vaccine-Related Side Effects and Higher Specific Antibody Titers in Health Care Workers with Previous SARS-CoV-2 Infection after the mRNA BNT162b2 Vaccine. Vaccines. 2022; 10(8):1238. https://doi.org/10.3390/vaccines10081238
Chicago/Turabian StyleFerrari, Ludovica, Mirko Compagno, Laura Campogiani, Elisabetta Teti, Tiziana Mulas, Davide Checchi, Grazia Alessio, Federica Caldara, Luigi Coppola, Giuseppe De Simone, and et al. 2022. "Increased Mild Vaccine-Related Side Effects and Higher Specific Antibody Titers in Health Care Workers with Previous SARS-CoV-2 Infection after the mRNA BNT162b2 Vaccine" Vaccines 10, no. 8: 1238. https://doi.org/10.3390/vaccines10081238
APA StyleFerrari, L., Compagno, M., Campogiani, L., Teti, E., Mulas, T., Checchi, D., Alessio, G., Caldara, F., Coppola, L., De Simone, G., Ceccarelli, L., Spalliera, I., Vitale, P., Grelli, S., Andreoni, M., Sarmati, L., & Iannetta, M. (2022). Increased Mild Vaccine-Related Side Effects and Higher Specific Antibody Titers in Health Care Workers with Previous SARS-CoV-2 Infection after the mRNA BNT162b2 Vaccine. Vaccines, 10(8), 1238. https://doi.org/10.3390/vaccines10081238







