Immune Response of a Heterologous mRNA-1273 Second-Dose Immunization after a First Dose of ChadOx1 against SARS-CoV-2: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Setting
2.2. Data Collection
2.3. Ethical Consideration
2.4. Blood Sample Analysis
2.4.1. Serological Assay
2.4.2. Molecular Assay
2.5. Statistical Analysis
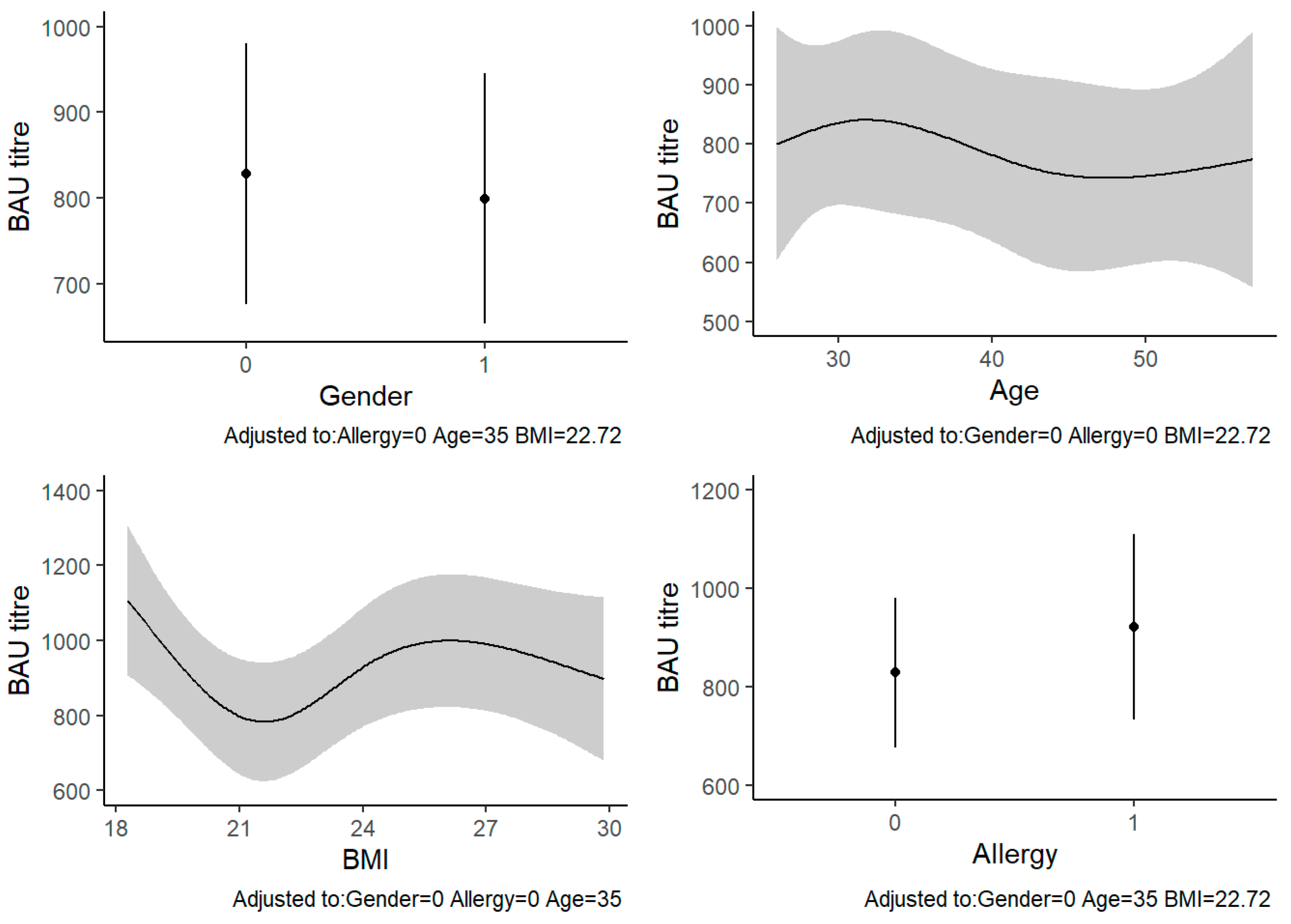
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Talic, S.; Shah, S.; Wild, H.; Gasevic, D.; Maharaj, A.; Ademi, Z.; Li, X.; Xu, W.; Mesa-Eguiagaray, I.; Rostron, J.; et al. Effectiveness of public health measures in reducing the incidence of COVID-19, SARS-CoV-2 transmission, and COVID-19 mortality: Systematic review and meta-analysis. BMJ 2021, 375, e068302. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef]
- Cavaleri, M.; Enzmann, H.; Straus, S.; Cooke, E. The European Medicines Agency’s EU conditional marketing authorisations for COVID-19 vaccines. Lancet 2021, 397, 355–357. [Google Scholar] [CrossRef]
- Sablerolles, R.S.G.; Goorhuis, A.; GeurtsvanKessel, C.H.; de Vries, R.D.; Huckriede, A.L.W.; Koopmans, M.P.G.; Lafeber, M.; Postma, D.F.; van Baarle, D.; Visser, L.G.; et al. Heterologous Ad26.COV2.S Prime and mRNA-Based Boost COVID-19 Vaccination Regimens: The SWITCH Trial Protocol. Front. Immunol. 2021, 12, 753319. [Google Scholar] [CrossRef]
- Lu, S. Heterologous prime-boost vaccination. Curr. Opin. Immunol. 2009, 21, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Venkatraman, N.; Ndiaye, B.P.; Bowyer, G.; Wade, D.; Sridhar, S.; Wright, D.; Powlson, J.; Ndiaye, I.; Dieye, S.; Thompson, C.; et al. Safety and Immunogenicity of a Heterologous Prime-Boost Ebola Virus Vaccine Regimen in Healthy Adults in the United Kingdom and Senegal. J. Infect. Dis. 2019, 219, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- Pozzetto, B.; Legros, V.; Djebali, S.; Barateau, V.; Guibert, N.; Villard, M.; Peyrot, L.; Allatif, O.; Fassier, J.B.; Massardier-Pilonchery, A.; et al. Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination. Nature 2021, 600, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Gross, R.; Zanoni, M.; Seidel, A.; Conzelmann, C.; Gilg, A.; Krnavek, D.; Erdemci-Evin, S.; Mayer, B.; Hoffmann, M.; Pohlmann, S.; et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine 2022, 75, 103761. [Google Scholar] [CrossRef] [PubMed]
- Westrop, S.J.; Whitaker, H.J.; Powell, A.A.; Power, L.; Whillock, C.; Campbell, H.; Simmons, R.; Warrener, L.; Ramsay, M.E.; Ladhani, S.N.; et al. Real-world data on immune responses following heterologous prime-boost COVID-19 vaccination schedule with Pfizer and AstraZeneca vaccines in England. J. Infect. 2022, 84, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef]
- Borobia, A.M.; Carcas, A.J.; Perez-Olmeda, M.; Castano, L.; Bertran, M.J.; Garcia-Perez, J.; Campins, M.; Portoles, A.; Gonzalez-Perez, M.; Garcia Morales, M.T.; et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef]
- Firinu, D.; Perra, A.; Campagna, M.; Littera, R.; Fenu, G.; Meloni, F.; Cipri, S.; Sedda, F.; Conti, M.; Miglianti, M.; et al. Evaluation of antibody response to BNT162b2 mRNA COVID-19 vaccine in patients affected by immune-mediated inflammatory diseases up to 5 months after vaccination. Clin. Exp. Med. 2022, 22, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Normark, J.; Vikstrom, L.; Gwon, Y.D.; Persson, I.L.; Edin, A.; Bjorsell, T.; Dernstedt, A.; Christ, W.; Tevell, S.; Evander, M.; et al. Heterologous ChAdOx1 nCoV-19 and mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 385, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Gram, M.A.; Nielsen, J.; Schelde, A.B.; Nielsen, K.F.; Moustsen-Helms, I.R.; Sorensen, A.K.B.; Valentiner-Branth, P.; Emborg, H.D. Vaccine effectiveness against SARS-CoV-2 infection, hospitalization, and death when combining a first dose ChAdOx1 vaccine with a subsequent mRNA vaccine in Denmark: A nationwide population-based cohort study. PLoS Med. 2021, 18, e1003874. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, D.; Ludwig, C.; Scholz, J.; Rode, I.; Tsamadou, C.; Jacobsen, E.M.; Winkelmann, M.; Grempels, A.; Lotfi, R.; Janda, A.; et al. mRNA Vaccines Enhance Neutralizing Immunity against SARS-CoV-2 Variants in Convalescent and ChAdOx1-Primed Subjects. Vaccines 2021, 9, 918. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021, 27, 1530. [Google Scholar] [CrossRef]
- Shaw, R.H.; Stuart, A.; Greenland, M.; Liu, X.; Nguyen Van-Tam, J.S.; Snape, M.D.; Com, C.O.V.S.G. Heterologous prime-boost COVID-19 vaccination: Initial reactogenicity data. Lancet 2021, 397, 2043–2046. [Google Scholar] [CrossRef]
- Sapkota, B.; Saud, B.; Shrestha, R.; Al-Fahad, D.; Sah, R.; Shrestha, S.; Rodriguez-Morales, A.J. Heterologous prime-boost strategies for COVID-19 vaccines. J. Travel. Med. 2021, 29, taab191. [Google Scholar] [CrossRef] [PubMed]
- Guiomar, R.; Santos, A.J.; Melo, A.M.; Costa, I.; Matos, R.; Rodrigues, A.P.; Kislaya, I.; Silva, A.S.; Roque, C.; Nunes, C.; et al. Monitoring of SARS-CoV-2 Specific Antibodies after Vaccination. Vaccines 2022, 10, 154. [Google Scholar] [CrossRef]
- Costa, C.; Migliore, E.; Galassi, C.; Scozzari, G.; Ciccone, G.; Coggiola, M.; Pira, E.; Scarmozzino, A.; La Valle, G.; Cassoni, P.; et al. Factors Influencing Level and Persistence of Anti SARS-CoV-2 IgG after BNT162b2 Vaccine: Evidence from a Large Cohort of Healthcare Workers. Vaccines 2022, 18, 474. [Google Scholar] [CrossRef] [PubMed]
- Raposo, F.; Lippi, G. Antibody response induced by the boost overdose during COVID-19 heterologous prime-boost vaccination strategy. Clin. Chim. Acta 2021, 523, 201–204. [Google Scholar] [CrossRef]
- Ilavska, S.; Horvathova, M.; Szabova, M.; Nemessanyi, T.; Jahnova, E.; Tulinska, J.; Liskova, A.; Wsolova, L.; Staruchova, M.; Volkovova, K. Association between the human immune response and body mass index. Hum. Immunol. 2012, 73, 480–485. [Google Scholar] [CrossRef]
- Dobner, J.; Kaser, S. Body mass index and the risk of infection—From underweight to obesity. Clin. Microbiol. Infect. 2018, 24, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Louie, J.K.; Acosta, M.; Winter, K.; Jean, C.; Gavali, S.; Schechter, R.; Vugia, D.; Harriman, K.; Matyas, B.; Glaser, C.A.; et al. Factors Associated With Death or Hospitalization Due to Pandemic 2009 Influenza A(H1N1) Infection in California. JAMA-J. Am. Med. Assoc. 2009, 302, 1896–1902. [Google Scholar] [CrossRef] [Green Version]
- Van der Wielen, M.; Van Damme, P.; Chlibek, R.; Smetana, J.; von Sonnenburg, F. Hepatitis A/B vaccination of adults over 40 years old: Comparison of three vaccine regimens and effect of influencing factors. Vaccine 2006, 24, 5509–5515. [Google Scholar] [CrossRef] [PubMed]
- Reuman, P.D.; Kubilis, P.; Hurni, W.; Brown, L.; Nalin, D. The effect of age and weight on the response to formalin inactivated, alum-adjuvanted hepatitis A vaccine in healthy adults. Vaccine 1997, 15, 1157–1161. [Google Scholar] [CrossRef]
- Perez-Perez, A.; Sanchez-Jimenez, F.; Vilarino-Garcia, T.; Sanchez-Margalet, V. Role of Leptin in Inflammation and Vice Versa. Int. J. Mol. Sci. 2020, 21, 5887. [Google Scholar] [CrossRef]
- Alwarawrah, Y.; Kiernan, K.; MacIver, N.J. Changes in Nutritional Status impact immune Cell Metabolism and Function. Front. Immunol. 2018, 9, 1055. [Google Scholar] [CrossRef] [Green Version]
- Bates, J.T.; Farmer, A.P.; Bierdeman, M.A.; Ederer, D.R.; Carney, L.S.; Montgomery, D.D.; Lirette, S.T.; Marshall, G.D. IgG Antibody Response to the Pfizer BNT162b2 SARS-CoV-2 Vaccine in Healthcare Workers with Healthy Weight, Overweight, and Obesity. Vaccines 2022, 10, 512. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine 2021, 36, 100928. [Google Scholar] [CrossRef] [PubMed]
- Malavazos, A.E.; Capitanio, G.; Chessa, M.; Matelloni, I.A.; Milani, V.; Stella, E.; Al Kassem, L.F.; Sironi, F.; Boveri, S.; Giamberti, A.; et al. Body mass index stratification in hospitalized Italian adults with congenital heart disease in relation to complexity, diagnosis, sex and age. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Mizoue, T.; Tanaka, A.; Oshiro, Y.; Inamura, N.; Konishi, M.; Ozeki, M.; Miyo, K.; Sugiura, W.; Sugiyama, H.; et al. Sex-associated differences between BMI and SARS-CoV-2 antibody titers following the BNT162b2 vaccine. Obesity 2022, 30, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.E.; Shapiro, J.R.; Dhakal, S.; Morgan, R.; Fink, A.L.; Liu, H.; Westerbeck, J.W.; Sylvia, K.E.; Park, H.S.; Ursin, R.L.; et al. Sex-specific effects of age and body mass index on antibody responses to seasonal influenza vaccines in healthcare workers. Vaccine 2022, 40, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Obesity may hamper Sars-CoV-2 vaccine immunogenicity. medRXiv, 2021; pre-print. [Google Scholar] [CrossRef]
| Characteristics | p-Value | |||
|---|---|---|---|---|
| Males (n = 161) | Females (n = 138) | All (n = 299) | ||
| Age (years; median, IQR) | 36.0 (30.0–47.0) | 35.0 (29.2–47.0) | 35.0 (30.0–47.0) | 0.712 |
| BMI (kg/m2; median, IQR) | 21.5 (19.5–24.0) | 23.9 (22.2–25.7) | 22.7 (20.7–25.1) | <0.001 |
| <25 (n, %) | 130 (81) | 93 (67) | 223 (75) | 0.009 |
| ≥25 (n, %) | 31 (19) | 45 (33) | 76 (25) | |
| Allergies (yes; n, %) | 41 (25) | 40 (29) | 81 (27) | 0.513 |
| Days from 2nd dose (median, IQR) | 173 (171–176) | 173 (171–178) | 173 (171–177) | 0.962 |
| Anti-SARS-CoV-2 IgG(BAU/mL; median, IQR) | 773.0 (508.0–1190.0) | 683.5 (411.0–1207.5) | 729.0 (455.0–1205.0) | 0.318 |
| Factors | β (SE) | 95% CI | p-Value |
|---|---|---|---|
| Sex | −44.5 (64.1) | −170.6–81.7 | 0.488 |
| Age * | −112.7 (88.5) | −287.0–61.5 | 0.649 |
| BMI * | 137.6 (87.0) | −33.5–308.7 | 0.007 |
| Allergies | 97.2 (71.7) | −44.0–238.3 | 0.176 |
| Days from 2nd dose | 11.8 (45.9) | −78.5–102.1 | 0.798 |
| Factors | β (SE) | 95% CI | p-Value |
|---|---|---|---|
| Sex | −29.3 (69.2) | −165.5–106.8 | 0.672 |
| Age * | −93.4 (88.0) | −266.5–79.7 | 0.789 |
| BMI * | 172.3 (86.0) | 2.9–341.6 | 0.007 |
| Allergies | 93.3 (71.7) | −47.9–234.5 | 0.194 |
| Factors | Males | Females | ||||
|---|---|---|---|---|---|---|
| β (SE) | 95% CI | p-Value | β (SE) | 95% CI | p-Value | |
| Age * | −84.1 (116.6) | −314.5–146.3 | 0.630 | −97.9 (129.4) | −353.9–158.1 | 0.671 |
| BMI * | 33.9 (114.4) | −192.1–259.9 | 0.079 | 277.0 (121.2) | 37.3–516.7 | 0.045 |
| Allergies | 130.6 (100.4) | −67.8–339.0 | 0.196 | 48.1 (106.3) | −162.1–254.4 | 0.651 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albanesi, B.; Godono, A.; Comoretto, R.I.; Casabona, E.; Curoso, G.; Leone, M.V.; Milanesio, N.; Mirra, I.; Montrucchio, G.; Pittaluga, F.; et al. Immune Response of a Heterologous mRNA-1273 Second-Dose Immunization after a First Dose of ChadOx1 against SARS-CoV-2: A Cross-Sectional Study. Vaccines 2022, 10, 1241. https://doi.org/10.3390/vaccines10081241
Albanesi B, Godono A, Comoretto RI, Casabona E, Curoso G, Leone MV, Milanesio N, Mirra I, Montrucchio G, Pittaluga F, et al. Immune Response of a Heterologous mRNA-1273 Second-Dose Immunization after a First Dose of ChadOx1 against SARS-CoV-2: A Cross-Sectional Study. Vaccines. 2022; 10(8):1241. https://doi.org/10.3390/vaccines10081241
Chicago/Turabian StyleAlbanesi, Beatrice, Alessandro Godono, Rosanna Irene Comoretto, Elena Casabona, Giuliano Curoso, Massimiliano Victor Leone, Nicolò Milanesio, Ilenia Mirra, Giulia Montrucchio, Fabrizia Pittaluga, and et al. 2022. "Immune Response of a Heterologous mRNA-1273 Second-Dose Immunization after a First Dose of ChadOx1 against SARS-CoV-2: A Cross-Sectional Study" Vaccines 10, no. 8: 1241. https://doi.org/10.3390/vaccines10081241
APA StyleAlbanesi, B., Godono, A., Comoretto, R. I., Casabona, E., Curoso, G., Leone, M. V., Milanesio, N., Mirra, I., Montrucchio, G., Pittaluga, F., Cavallo, R., Clari, M., & Ciocan, C. (2022). Immune Response of a Heterologous mRNA-1273 Second-Dose Immunization after a First Dose of ChadOx1 against SARS-CoV-2: A Cross-Sectional Study. Vaccines, 10(8), 1241. https://doi.org/10.3390/vaccines10081241






