Abstract
Background: The novel SARS-CoV-2 vaccines partially exploit intrinsic DNA or RNA adjuvanticity, with dysregulation in the metabolism of both these nucleic acids independently linked to triggering experimental autoimmune diseases, including lupus and myositis. Methods: Herein, we present 15 new onset autoimmune myositis temporally associated with SARS-CoV-2 RNA or DNA-based vaccines that occurred between February 2021 and April 2022. Musculoskeletal, pulmonary, cutaneous and cardiac manifestations, laboratory and imaging data were collected. Results: In total, 15 cases of new onset myositis (11 polymyositis/necrotizing/overlap myositis; 4 dermatomyositis) were identified in the Yorkshire region of approximately 5.6 million people, between February 2021 and April 2022 (10 females/5 men; mean age was 66.1 years; range 37–83). New onset disease occurred after first vaccination (5 cases), second vaccination (7 cases) or after the third dose (3 cases), which was often a different vaccine. Of the cases, 6 had systemic complications including skin (3 cases), lung (3 cases), heart (2 cases) and 10/15 had myositis associated autoantibodies. All but 1 case had good therapy responses. Adverse event following immunization (AEFI) could not be explained based on the underlying disease/co-morbidities. Conclusion: Compared with our usual regional Rheumatology clinical experience, a surprisingly large number of new onset myositis cases presented during the period of observation. Given that antigen release inevitably follows muscle injury and given the role of nucleic acid adjuvanticity in autoimmunity and muscle disease, further longitudinal studies are required to explore potential links between novel coronavirus vaccines and myositis in comparison with more traditional vaccine methods.
1. Introduction
The “Severe Acute Respiratory Syndrome-related Coronavirus type 2” (SARS-CoV-2) infection has resulted in over 5 million deaths and numerous other medical and societal issues. In addition to natural resistance and infection-acquired immunity, vaccines represented a fundamental element in mitigating against severe “Coronavirus Disease 2019” (COVID-19) [1,2,3]. The authorised COVID-19 vaccines have shown efficacy, safety, and tolerability in both randomised clinical trials (RCTs) and in the real-world setting [1,2,3,4,5,6]. Many of these vaccines are based on novel strategies based around DNA and RNA technology and some rare—though potentially serious—autoimmune diseases have emerged within days or weeks of vaccine utilisation, including vaccine-induced immune thrombotic thrombocytopenia (VITT) with DNA vaccines [7] and myopericarditis with RNA vaccines [8].
Many immune-mediated diseases (IMDs) are characterised by the emergence of autoantibodies several months or even years before the clinical onset and presentation [9]. The adjuvanticity of the available SARS-CoV-2 vaccines is novel and at least in part depends on the intrinsic vaccine messenger RNA (mRNA) or DNA. Both stimulate innate immunity through endosolic and cytoplasmic nucleic acid receptors—such as Toll-Like Receptors (TLRs) 3, 7, 8, and 9—as well as components of the inflammasome, including Retinoic acid-Inducible Gene I (RIG-I) and Melanoma Differentiation-Associated Gene 5 (MDA5) [10,11].
A role for altered nucleic acid metabolism with aberrant TLR pathway activation has been postulated in both experimental and human autoimmune connective tissue diseases [12,13]. Given that intramuscular vaccination is capable of releasing muscle antigens and the RNA/DNA vaccine components may get taken up in the muscle [14], then a theoretical basis for emergent muscle autoimmunity exists which is distinct from conventional vaccines that are thought to act mainly on antigen presenting cells and other immune cells. Bearing these theoretical considerations in mind, we noted emergent autoimmunity in our region and especially an unexpected high number of new onset myositis cases temporally linked to SARS-CoV-2 vaccination in the Yorkshire region of the United Kingdom (UK).
2. Methods
This study was reported according to the “CAse REports” (CARE) guidelines [15]. All participants recruited granted verbal or written consent to the local treating physicians for the use of their anonymised data. Six NHS Trusts from Yorkshire and Humber region in the UK, (Bradford Teaching Hospitals NHS Foundation trust; Harrogate and District NHS Foundation Trust; Hull University Teaching Hospitals NHS Trust, Leeds Teaching Hospitals NHS Trust; Mid Yorkshire Hospitals NHS Trust and York and Scarborough Teaching Hospitals NHS Foundation Trust—see map in Figure 1) provided data. The authors evaluated patients presenting and diagnosed with: (a) myositis; (b) occurring in plausible temporal relationship with any agent authorised in UK for the SARS-CoV2 vaccination programme.
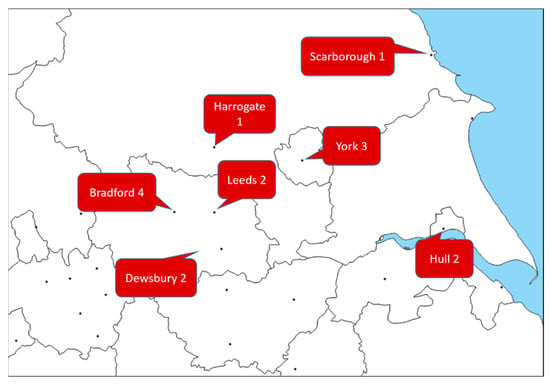
Figure 1.
Map highlighting the distribution of new-onset myositis cases across the Yorkshire region, adapted from https://d-maps.com/carte.php?num_car=18610&lang=en (accessed on 12 May 2022). Dewsbury and District Hospital is part of The Mid Yorkshire Hospitals NHS Trust, alongside Pinderfiels Hospital of Wakefield.
Diagnosis of myositis was based on referring consultant overall clinical opinion, informed by presentation, symptoms, laboratory findings (creatine-kinases (CK); C-reactive protein, CRP; auto-antibodies), magnetic resonance imaging (MRI) and biopsy—where available. No specific time frame after vaccination agent exposure was specified, as it was felt as too narrow to restrict myositis onset to one month or less since it is well established that autoantibodies precede the clinical onset of IMIDs by several months or years [16].
As suggested by World Health Organization (WHO) guidance [17], Adverse Events Following Immunization (AEFI) represent untoward medical events following vaccination. According to WHO, vaccines could represent the primary factor linked to AEFI or cofactors within complex events [18], although this approach has shortcomings, but robust alternatives are lacking [19]. After validating myositis diagnosis and excluding non-vaccination-related causes, biological plausibility and temporal compatibility between the immunization and the occurrence of the AEFIs were assessed as previously described [20]. Descriptive statistics were used to summarize variables including age, gender, history of IMIDs, average time to onset of symptoms, severity of disease course, therapeutics administered, and key clinical and laboratory findings.
3. Results
The estimated Yorkshire population size is 5.6 million and is ethnically diverse. Of 19 cases referred for evaluation, 15 new cases fulfilled the selection criteria (new presentation of myositis—as diagnosed by the treating clinician—occurring in plausible temporal relationship with any COVID-19 vaccine). The remaining 4 cases were excluded for the following reasons: post-vaccine flare of already established myositis (3 cases); myositis onset potentially preceding vaccine exposure.
Overall, 10 cases were females, 5 were males. The average age at myositis symptoms onset was 66.1 years (standard deviation 13.7; range 37–83). Vaccination agents recorded before the onset of myositis were AZD1222/ChAdOx1 (9 cases) and BNT162b2 (6 cases). The median time between exposure to vaccination agent and myositis onset was 5 weeks (interquartile range 4–9; absolute range 1–34 weeks). Myositis occurred in 5 cases after dose 1; in 7 cases after dose 2; in 3 cases after dose 3. In total, 6 out of 15 cases were on statins at the time of myositis onset.
Myositis symptoms are described in Table 1. In one case, CKs were normal and biopsy normal (amyopathic variant). No cases had evidence of a paraneoplastic myositis. The most common non-muscular clinical manifestation was skin involvement (only in 3 cases) and interstitial lung disease (3 cases, representative case in Figure 2). All 4 cases of DM had received DNA vaccination.

Table 1.
Clinical, laboratory and Imaging Features of Myositis Cases.
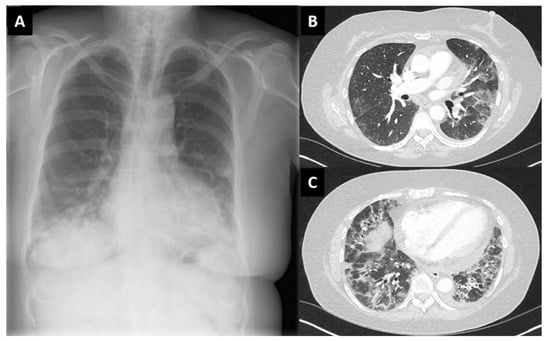
Figure 2.
Chest X-ray (A) and high-resolution computed tomography scan (B,C) of case 3 (female, 58 years old) with findings compatible with interstitial lung disease. Lung biopsy not performed.
Anti-nuclear autoantibodies (ANA) were positive in 8 cases and negative in 7 cases. Myositis-associated autoantibodies were positive in 10 cases and negative in 5 cases. Case 9 was reported as positive for anti-Ro/RNP and anti-chromatin autoantibodies prior to exposure to vaccines and myositis development. This patient had previously been treated as an inflammatory arthritis and was treated with azathioprine years before vaccination and could represent an undefined connective tissue disease prior to definitive myositis diagnosis post vaccination.
Muscle biopsy (case 10 sample is shown in Figure 3) was performed in 10 cases: 5 were compatible with polymyositis, 3 with necrotising features, 1 compatible with dermatomyositis and 1 histologically suggestive of mitochondrial myopathy, but the clinical picture was of autoimmune myositis. Overall, 4 out of 15 patients had classical DM rash pointing towards a predominant DM picture. Of note, in 3 cases (20%), investigations revealed anti 3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies (all on statins). Magnetic resonance imaging (MRI) of thigh muscle masses (Figure 3) was performed in 11 cases and intramuscular oedema was the most prominent imaging feature (11/11 MRI).
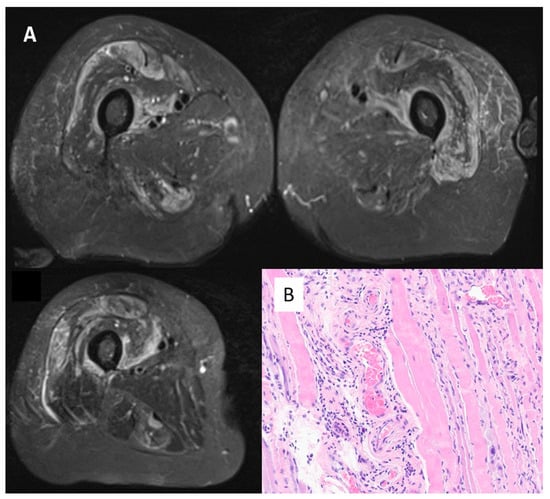
Figure 3.
Panel (A)—MRI scan of thighs from Case 1 (female, 68 years old). Oedema in the quadriceps (vastus intermedium femuri; vasti medialis and rectum femuri) bilaterally, pointing to inflammation in the muscle masses explored. Panel (B)—Muscle biopsy from case 10 (female, 71 years old). Haematoxylin/Eosin, magnification 400×. Perimyseal pathology with local thrombosis consistent with a vasculopathy and macrophages present consistent with immune-mediated necrotising myositis. Anti 3-hydroxy-3-methylglutaryl-coenzyme A reductase antibodies were positive.
In total, 13 cases were treated with corticosteroids and/or immunosuppressants (12 cases) as detailed in Table 1. With the exception of case 12, patients described in this series improved (both in amelioration of strength and CK level reductions after treatment, although time-to-recovery and degree of recovery differed across cases (Table 2). Complications such as aspiration pneumonia, cataract, pulmonary embolism, anuria, respiratory arrest and stroke occurred in 5 cases (Table 1).

Table 2.
Myositis Cases Therapy and Responses.
4. Discussion
The incidence of autoimmune myositis ranges in 1.16 to 19/million/year according to estimates [21]. Herein, we report a cluster of autoimmune myositis that was temporally related to SARS-CoV-2 vaccination in the Yorkshire region of UK with 40% of cases on statins and with three of these associated with Anti-HMGCR autoantibodies. In this series, myositis followed immunization with both DNA-based and RNA-based vaccines. Time-to-symptoms onset was variable across the cases reported, as well as the association to vaccine cycles timings (that is, occurrence after dose one; dose two or occurrence after dose three—implemented in UK since November 2021). Some of the cases reported could be classified as polymyositis—historically the most common of idiopathic inflammatory myositidies [22].
Our voluntary reporting strategy has limitations, including selection bias (namely, reporting of more severe and early-stage cases). It is also important to acknowledge that the temporal links between vaccination doses administered and the development of myositis-related symptoms was unclear. In Yorkshire, there was relatively little or no exposure to mRNA-1273 (Moderna) or JNJ-78436735 (Ad26.COV2.S) SARS-CoV-2 vaccines. Furthermore, it is unclear what the optimal cut off time is for establishing putative links between vaccines and potential autoimmunity. In the context of musculoskeletal disorders, reactive arthritis typically occurs within weeks to a month of infection or vaccine challenge, but it is important to recognise that muscle autoantibodies can precede autoimmune disease by several years [15]. Nevertheless, most of our cases occurred in a short timeframe following vaccination, but active surveillance in the coming years for evidence of autoimmune myositis development at a later timeframe may be warranted.
Our findings could represent mere coincidence, but collectively, the number of new myositis cases was in considerable excess of our normal clinical expectations. Given that autoimmune myositis is associated with autoantigens that mostly often bind to native RNA, whereas autoimmunity in systemic lupus erythematosus (SLE) is linked to autoantigens bound to DNA, we were interested to see whether a particular association between DNA or RNA vaccination and myositis was evident. We found numerically more cases of myositis in cases exposed to the AZD1222/ChAdOx1 vaccine; however, this finding could be due to the prominent role that the AZD1222/ChAdOx1 agent had in the initial vaccine rollout in UK (in other countries—such as Germany and Israel—the BNT162b2 agent was more commonly administered). Our data do not show specific tendency of vaccine cycle positioning, as myositis cases occurred almost equally after dose 1 or 2. No signal was detected in regards with potential role of dose 3 (3 cases in total), which argues against the idea of autoimmunity that might be expected to be worse with repeated booster injections following earlier breakage of immune tolerance.
One small series of 3 auto-immune myositis occurring after immunization with agent AZD1222/ChAdOx1 is available in the medical literature [23]. In addition, there has also been sporadic case reports of myositis following DNA and RNA vaccines [24,25]. Patients described in our series underwent vaccination cycles with agents approved in UK at the time of clinical presentation and data collection (that is, BNT162b2; AZD1222/ChAdOx1). Given that the intra-muscular injection technique may cause muscle injury, the consequent release of naturally segregated intra-cellular, muscle-specific (auto) antigens could ultimately lead to abundant presentation activity in regional lymph node draining sites of vaccine inoculation (Figure 4A). Combined with potential uptake of RNA/DNA into myocytes and other cell types, antigen presentation by myocytes might favor autoimmunity over conventional adjuvants with the emergence of T-cells and their return to the muscle compartment (Figure 4B) [26]. This mechanism would offer a plausible explanation for muscle autoimmunity, especially polymyositis, which is more muscle-centric compared with dermatomyositis which is more muscle-interstitium- and vascular-centric. We noted numerically more polymyositis cases, but these findings need confirmation in larger cohorts and with robust longitudinal epidemiological surveys. Both RNA and DNA vaccines are also associated with excellent potentiation of both CD4+ and CD8+ T-cell reactivity, with both cell types being incriminated in autoimmune myositis [27] (Figure 4B).
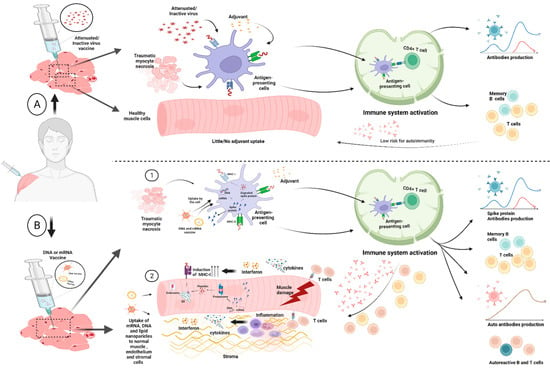
Figure 4.
Panel (A): Conventional vaccines involve the delivery of a killed pathogen, attenuated pathogen or a protein subunit with adjuvants—including alum and others—to the inoculation site. This is associated with local muscle and endothelial and supporting tissue injury at the site of vaccination. It is thought that uptake of both the antigens and adjuvants by the antigen presenting cells leads to activation of such cells and migration to the regional lymph nodes where lymphocyte priming takes place and robust antibody responses to antigen takes place. In theory, vaccine-associated injury from the injecting needle might release self-antigens that are also taken up by the Antigen-Presenting Cells (APCs) and could theoretically lead to tolerance failure and autoimmunity. However, this is not something that is recognised in the clinical setting with conventional vaccines; hence, it seems unlikely to occur. Panel (B): Like Conventional vaccines, the novel DNA- and RNA-based vaccines are also taken up by antigen-presenting cells and migrate to regional lymph nodes and likewise damaged self-muscle, endothelial and stromal tissue elements from sites of injury could undergo pinocytosis and likewise be transported. Copying elements of the viral life cycle within APCs facilitates CD4 and cytotoxic CD8 T-cell responses, that are superior to conventional vaccines and could thus contribute also to the development of better cell-mediated immune responses [28,29]. There is actually a very limited amount of data about the uptake and processing of nucleic acid vaccines following intramuscular injection; however, direct uptake of RNA and DNA into the muscle has been reported [26,30,31]. Further experimental studies and epidemiological surveys are needed to test this hypothesis. Image created with BioRender.com.
To conclude, it is our impression that a surprisingly high rate of new onset myositis following novel RNA and DNA SARS-CoV-2 vaccination recently emerged in the Yorkshire region. We have reported a temporal association between SARS-CoV-2 vaccines and myositis onset and in the light of a mass vaccine campaign, this does not imply causality. Proof of a causal association is lacking. However, whilst this could be mere co-incidence, the link between muscle injury and novel adjuvants priming of CD4+ and CD8+ T-cells that is known to occur after vaccination merits consideration. A specific epidemiological evaluation for autoimmune muscle disease is needed in the post SARS-CoV-2 era and mechanistic studies of novel vaccines and muscle autoimmunity may address this issue further.
Author Contributions
Conceptualization, G.D.M. (Gabriele De Marco) and D.G.M. (Dennis Gerald McGonagle); methodology, G.D.M. (Gabriele De Marco) and D.G.M. (Dennis Gerald McGonagle); validation, all authors; formal analysis, G.D.M. (Gabriele De Marco); writing—original draft preparation, G.D.M. (Gabriele De Marco) and D.G.M. (Dennis Gerald McGonagle); writing—review and editing, all authors; visualization, S.G.; supervision, D.G.M. (Dennis Gerald McGonagle); project administration, G.D.M. (Gabriele De Marco). All authors have read and agreed to the published version of the manuscript.
Funding
This article presents independent research supported by the National Institute for Health and Care Research (NIHR) Leeds Biomedical Research Centre (BRC). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Institutional Review Board Statement
Not required.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank Hector Chinoy and Federico Roncaroli, of Salford Royal NHS Foundation Trust, for their role in assessing several muscle biopsies taken from the patients described in this series and Aruna Chakrabarty, Leeds Teaching Hospitals NHS Trust, for muscle biopsies taken from patient 10 described in this series. Tran is Wellcome Trust Institutional Strategic Support Fund Early Career Research Fellow and Honorary Lecturer, at the University of Leeds.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 637–646. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zeng, G.; Pan, H.X.; Li, C.G.; Hu, Y.L.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef]
- Kim, H.W.; Jenista, E.R.; Wendell, D.C.; Azevedo, C.F.; Campbell, M.J.; Darty, S.N.; Parker, M.A.; Kim, R.J. Patients with Acute Myocarditis following mRNA COVID-19 Vaccination. JAMA Cardiol. 2021, 6, 1196–1201. [Google Scholar] [CrossRef]
- McGonagle, D.; McDermott, M.F. A proposed classification of the immunological diseases. PLoS Med. 2006, 3, e297. [Google Scholar] [CrossRef] [Green Version]
- Tatematsu, M.; Funami, K.; Seya, T.; Matsumoto, M. Extracellular RNA Sensing by Pattern Recognition Receptors. J. Innate Immun. 2018, 10, 398–406. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Krug, A. Nucleic acid recognition receptors in autoimmunity. In Handbook Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 129–151. [Google Scholar]
- Rodero, M.P.; Crow, Y.J. Type I interferon-mediated monogenic autoinflammation: The type I interferonopathies, a conceptual overview. J. Exp. Med. 2016, 213, 2527–2538. [Google Scholar] [CrossRef] [PubMed]
- Heine, A.; Juranek, S.; Brossart, P. Clinical and immunological effects of mRNA vaccines in malignant diseases. Mol. Cancer 2021, 20, 52. [Google Scholar] [CrossRef]
- Gagnier, J.J.; Kienle, G.; Altman, D.G.; Moher, D.; Sox, H.; Riley, D. The CARE guidelines: Consensus-based clinical case reporting guideline development. J. Med. Case Rep. 2013, 7, 223. [Google Scholar] [CrossRef] [Green Version]
- Miller, F.W.; Waite, K.A.; Biswas, T.; Plotz, P.H. The role of an autoantigen, histidyl-tRNA synthetase, in the induction and maintenance of autoimmunity. Proc. Natl. Acad. Sci. USA 1990, 87, 9933–9937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Manual on Surveillance of Adverse Events Following Immunization, 2016 Update ed.; World Health Organization: Geneva, Switzerland, 2014.
- Bellavite, P. Causality assessment of adverse events following immunization: The problem of multifactorial pathology. F1000Research 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Kang, H.Y.; Cho, S.; Park, S.; Kim, A.Y.; Jung, S.Y.; Seong, B.L.; Lee, Y.M. Causality Assessment Guidelines for Adverse Events Following Immunization with a Focus on Guillain-Barre Syndrome. Vaccines 2020, 8, 101. [Google Scholar] [CrossRef] [Green Version]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following mRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef]
- Meyer, A.; Meyer, N.; Schaeffer, M.; Gottenberg, J.E.; Geny, B.; Sibilia, J. Incidence and prevalence of inflammatory myopathies: A systematic review. Rheumatology 2015, 54, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Lundberg, I.E.; Fujimoto, M.; Vencovsky, J.; Aggarwal, R.; Holmqvist, M.; Christopher-Stine, L.; Mammen, A.L.; Miller, F.W. Idiopathic inflammatory myopathies. Nat. Rev. Dis. Primers 2021, 7, 86. [Google Scholar] [CrossRef]
- Maramattom, B.V.; Philips, G.; Thomas, J.; Santhamma, S.G.N. Inflammatory myositis after ChAdOx1 vaccination. Lancet Rheumatol. 2021, 3, e747–e749. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Woo, C.G. Clinicopathological Characteristics of Inflammatory Myositis Induced by COVID-19 Vaccine (Pfizer-BioNTech BNT162b2): A Case Report. J. Korean Med. Sci. 2022, 37, e91. [Google Scholar] [CrossRef] [PubMed]
- Gouda, W.; Albasri, A.; Alsaqabi, F.; Al Sabah, H.Y.; Alkandari, M.; Abdelnaby, H.J. Dermatomyositis Following BNT162b2 mRNA COVID-19 Vaccination. Korean Med. Sci. 2022, 37, e32. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, S.; Giovani, C.; Mangiavacchi, S.; Magini, D.; Maione, D.; Baudner, B.; Geall, A.J.; De Gregorio, E.; D’Oro, U.; Buonsanti, C. CD8 T-cell priming upon mRNA vaccination is restricted to bone-marrow-derived antigen-presenting cells and may involve antigen transfer from myocytes. Immunology 2015, 146, 312–326. [Google Scholar] [CrossRef]
- Sheridan, C. First COVID-19 DNA vaccine approved, others in hot pursuit. Nat. Biotechnol. 2021, 39, 1479–1482. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Chavda, V.P.; Hossain, M.K.; Beladiya, J.; Apostolopoulos, V. Nucleic Acid Vaccines for COVID-19: A Paradigm Shift in the Vaccine Development Arena. Biologics 2021, 1, 337–356. [Google Scholar] [CrossRef]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. npj Vaccines 2021, 6, 28. [Google Scholar] [CrossRef]
- Kurteva, E.; Vasilev, G.; Tumangelova-Yuzeir, K.; Ivanova, I.; Ivanova-Todorova, E.; Velikova, T.; Kyurkchiev, D. Interferon-gamma release assays outcomes in healthy subjects following BNT162b2 mRNA COVID-19 vaccination. Rheumatol. Int. 2022, 42, 449–456. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).