Anti-TNFα Treatment Impairs Long-Term Immune Responses to COVID-19 mRNA Vaccine in Patients with Inflammatory Bowel Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Procedure
2.3. Laboratory Methods
2.4. Statistical Analysis
3. Results
3.1. Study Population
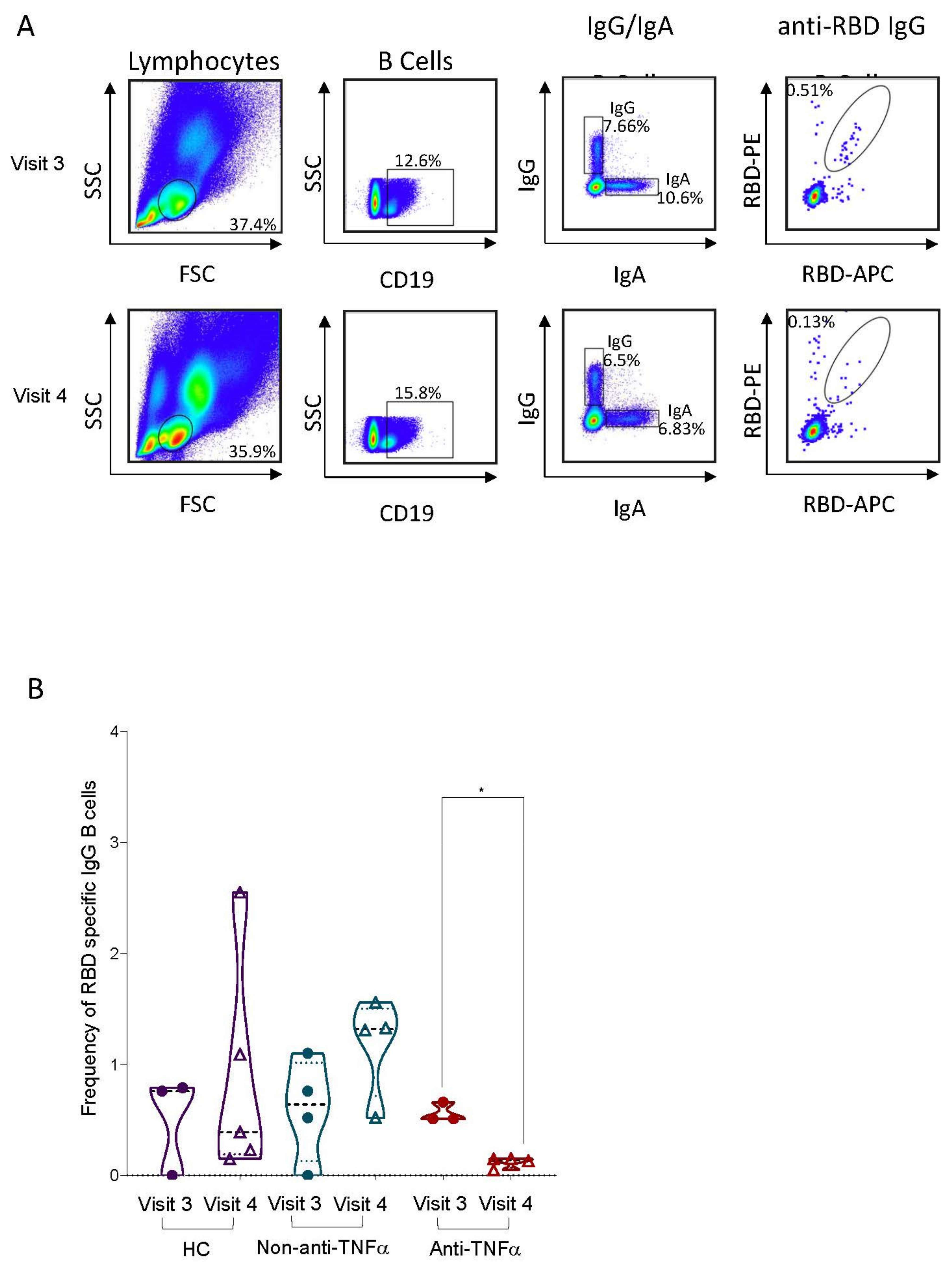
3.2. RBD-Specific Circulating B Cells Are Reduced in Patients with IBD Treated with Anti-TNFα
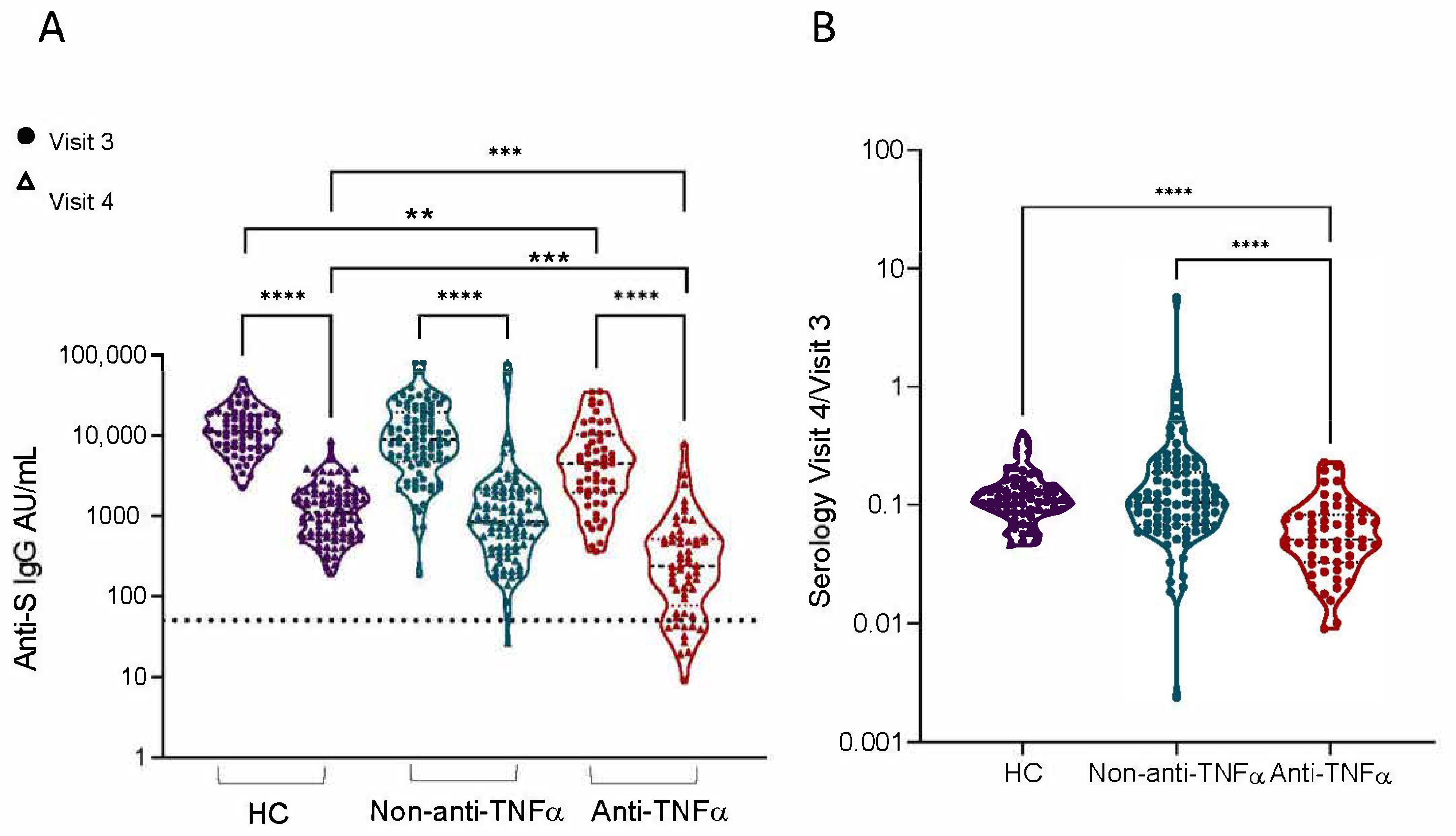
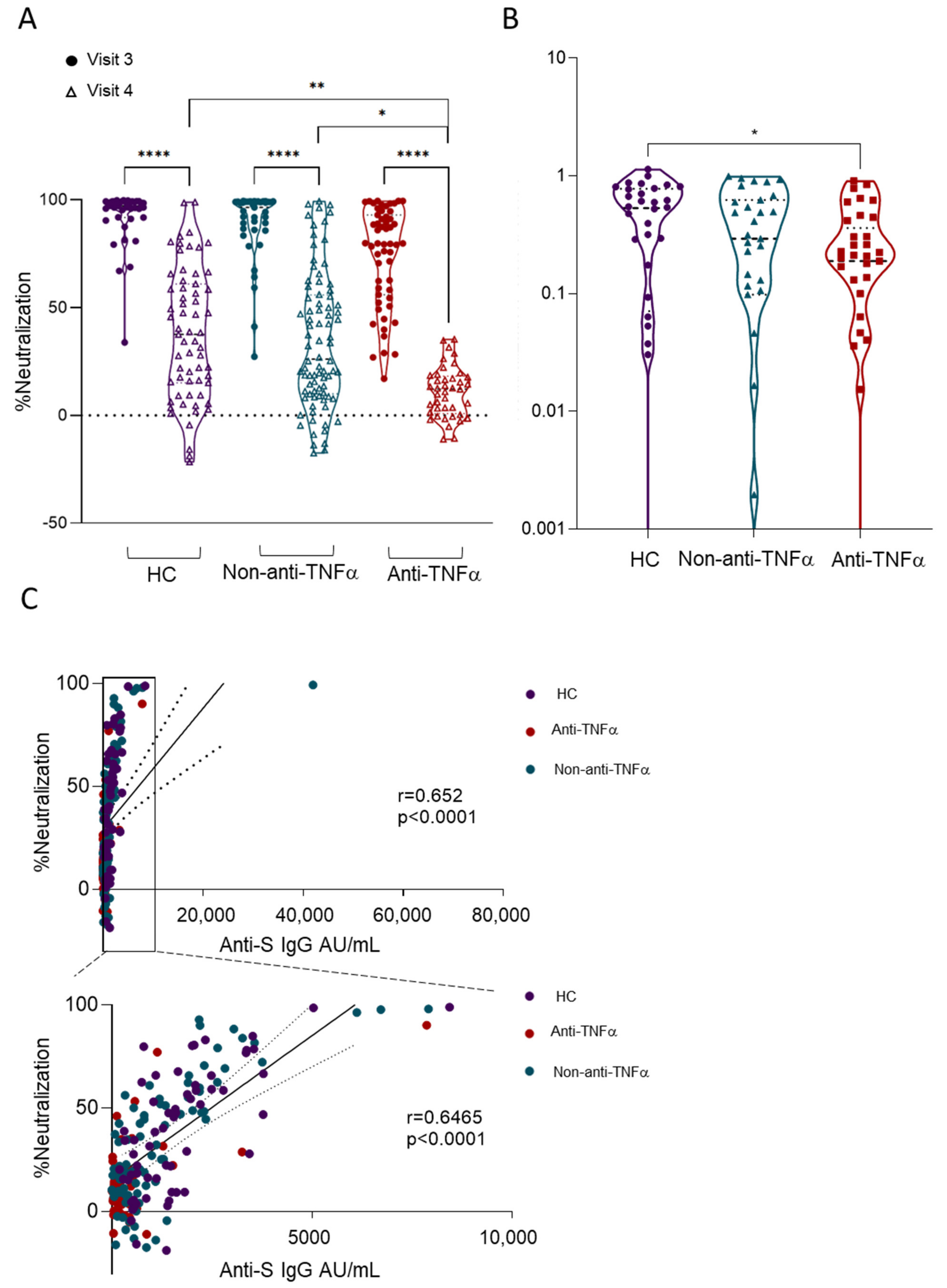
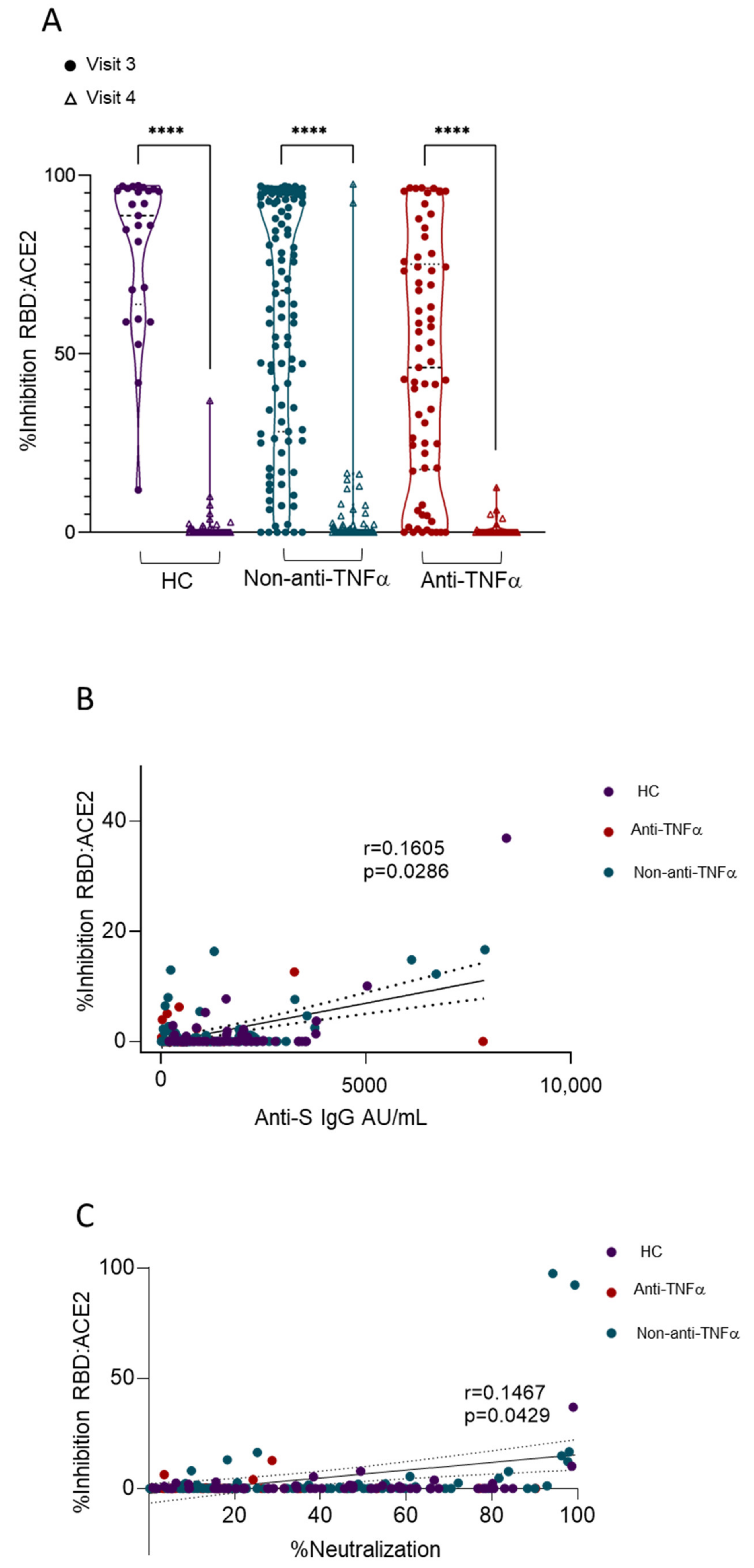
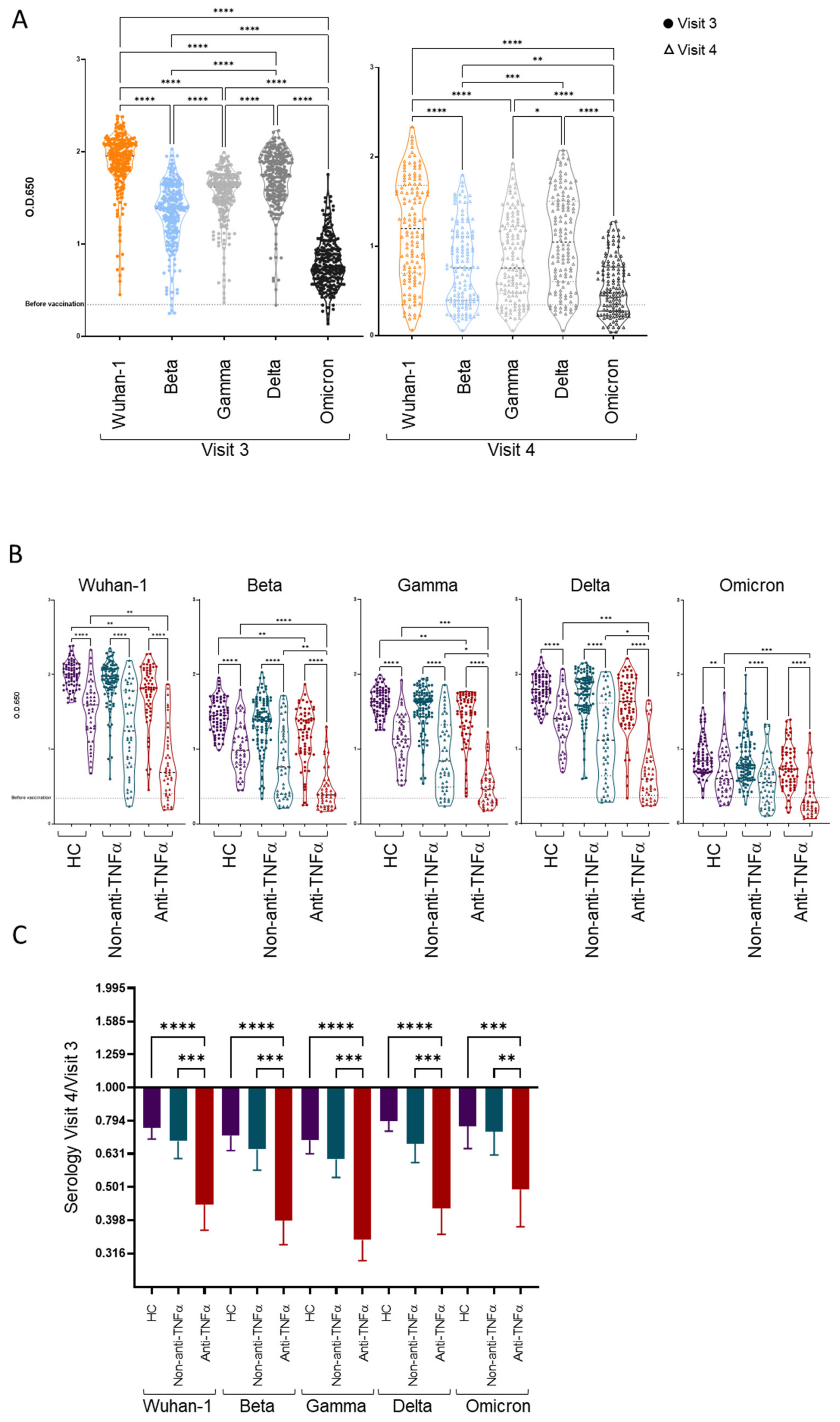
3.3. Patients with IBD Treated with Anti-TNFα Exhibit Decreased Reactivity to Beta VOCs
3.4. Additional Predictors of Lower Vaccine Responses
3.5. The Vaccine Is Safe in Patients with IBD and Is Not Associated with IBD Exacerbation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ford, A.C.; Peyrin-Biroulet, L. Opportunistic infections with anti-tumor necrosis factor-alpha therapy in inflammatory bowel disease: Meta-analysis of randomized controlled trials. Am. J. Gastroenterol. 2013, 108, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Spano, F.; Contatore, M.; Guastalla, A.; Penza, E.; Magnani, O.; Puppo, F. Infection risk associated with anti-TNF-alpha agents: A review. Expert Opin. Drug Saf. 2015, 14, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Rahier, J.F.; Magro, F.; Abreu, C.; Armuzzi, A.; Ben-Horin, S.; Chowers, Y.; Cottone, M.; de Ridder, L.; Doherty, G.; Ehehalt, R.; et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J. Crohns. Colitis. 2014, 8, 443–468. [Google Scholar] [CrossRef]
- Vulliemoz, M.; Brand, S.; Juillerat, P.; Mottet, C.; Ben-Horin, S.; Michetti, P. TNF-Alpha Blockers in Inflammatory Bowel Diseases: Practical Recommendations and a User’s Guide: An Update. Digestion 2020, 101, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Melmed, G.Y.; Agarwal, N.; Frenck, R.W.; Ippoliti, A.F.; Ibanez, P.; Papadakis, K.A.; Simpson, P.; Barolet-Garcia, C.; Ward, J.; Targan, S.R.; et al. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am. J. Gastroenterol. 2010, 105, 148–154. [Google Scholar] [CrossRef]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin. Infect. Dis. 2014, 58, e44–e100. [Google Scholar] [CrossRef] [Green Version]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Galmiche, S.; Luong Nguyen, L.B.; Tartour, E.; de Lamballerie, X.; Wittkop, L.; Loubet, P.; Launay, O. Immunological and clinical efficacy of COVID-19 vaccines in immunocompromised populations: A systematic review. Clin. Microbiol. Infect. 2021, 28, 163–177. [Google Scholar] [CrossRef]
- Hagin, D.; Freund, T.; Navon, M.; Halperin, T.; Adir, D.; Marom, R.; Levi, I.; Benor, S.; Alcalay, Y.; Freund, N.T. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J. Allergy Clin. Immunol. 2021, 148, 739–749. [Google Scholar] [CrossRef]
- Alexander, J.L.; Kennedy, N.A.; Ibraheim, H.; Anandabaskaran, S.; Saifuddin, A.; Castro Seoane, R.; Liu, Z.; Nice, R.; Bewshea, C.; D’Mello, A.; et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): A multicentre, prospective, case-control study. Lancet Gastroenterol. Hepatol. 2022, 7, 342–352. [Google Scholar] [CrossRef]
- Edelman-Klapper, H.; Zittan, E.; Bar-Gil Shitrit, A.; Rabinowitz, K.M.; Goren, I.; Avni-Biron, I.; Ollech, J.E.; Lichtenstein, L.; Banai-Eran, H.; Yanai, H.; et al. Lower Serologic Response to COVID-19 mRNA Vaccine in Patients With Inflammatory Bowel Diseases Treated With Anti-TNFalpha. Gastroenterology 2021, 162, 454–467. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Lin, S.; Goodhand, J.R.; Chanchlani, N.; Hamilton, B.; Bewshea, C.; Nice, R.; Chee, D.; Cummings, J.F.; Fraser, A.; et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut 2021, 70, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Corey, L.; Beyrer, C.; Cohen, M.S.; Michael, N.L.; Bedford, T.; Rolland, M. SARS-CoV-2 Variants in Patients with Immunosuppression. N. Engl. J. Med. 2021, 385, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, N.A.; Goodhand, J.R.; Bewshea, C.; Nice, R.; Chee, D.; Lin, S.; Chanchlani, N.; Butterworth, J.; Cooney, R.; Croft, N.M.; et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut 2021, 70, 865–875. [Google Scholar] [CrossRef]
- Harvey, R.F.; Bradshaw, J.M. A simple index of Crohn’s-disease activity. Lancet 1980, 315, 514. [Google Scholar] [CrossRef]
- Walmsley, R.S.; Ayres, R.C.; Pounder, R.E.; Allan, R.N. A simple clinical colitis activity index. Gut 1998, 43, 29–32. [Google Scholar] [CrossRef] [Green Version]
- Anon. SARS-CoV-2 Immunoassay|Abbott Core Laboratory. Available online: https://www.corelaboratory.abbott/us/en/offerings/segments/infectious-disease/sars-cov-2 (accessed on 8 June 2021).
- Mor, M.; Werbner, M.; Alter, J.; Safra, M.; Chomsky, E.; Lee, J.C.; Hada-Neeman, S.; Polonsky, K.; Nowell, C.J.; Clark, A.E.; et al. Multi-clonal SARS-CoV-2 neutralization by antibodies isolated from severe COVID-19 convalescent donors. PLoS Pathog 2021, 17, e1009165. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; Duda, S.N. The REDCap consortium: Building an international community of software platform partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef] [Green Version]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.R.; Painter, M.M.; Apostolidis, S.A.; Mathew, D.; Meng, W.; Rosenfeld, A.M.; Lundgreen, K.A.; Reynaldi, A.; Khoury, D.S.; Pattekar, A.; et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021, 374, abm0829. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Varona, J.F.; Madurga, R.; Penalver, F.; Abarca, E.; Almirall, C.; Cruz, M.; Ramos, E.; Castellano-Vazquez, J.M. kinetics of anti-SARS-CoV-2 antibodies over time. Results of 10 month follow up in over 300 seropositive Health Care Workers. Eur. J. Intern. Med. 2021, 89, 97–103. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Mizrahi, B.; Lotan, R.; Kalkstein, N.; Peretz, A.; Perez, G.; Ben-Tov, A.; Chodick, G.; Gazit, S.; Patalon, T. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat. Commun. 2021, 12, 6379. [Google Scholar] [CrossRef]
- Dispinseri, S.; Secchi, M.; Pirillo, M.F.; Tolazzi, M.; Borghi, M.; Brigatti, C.; De Angelis, M.L.; Baratella, M.; Bazzigaluppi, E.; Venturi, G.; et al. Neutralizing antibody responses to SARS-CoV-2 in symptomatic COVID-19 is persistent and critical for survival. Nat. Commun. 2021, 12, 2670. [Google Scholar] [CrossRef]
- Pang, N.Y.; Pang, A.S.; Chow, V.T.; Wang, D.Y. Understanding neutralising antibodies against SARS-CoV-2 and their implications in clinical practice. Mil. Med. Res. 2021, 8, 47. [Google Scholar] [CrossRef]
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 2020, 20, 229–238. [Google Scholar] [CrossRef]
- Viant, C.; Weymar, G.H.J.; Escolano, A.; Chen, S.; Hartweger, H.; Cipolla, M.; Gazumyan, A.; Nussenzweig, M.C. Antibody Affinity Shapes the Choice between Memory and Germinal Center B Cell Fates. Cell 2020, 183, 1298–1311.e1211. [Google Scholar] [CrossRef]
- Our World in DATA. Available online: https://ourworldindata.org/grapher/covid-variants-area?country=~GBR (accessed on 25 January 2022).
- GISAID. Available online: https://www.gisaid.org/hcov19-variants/ (accessed on 25 January 2022).
- Lucas, C.; Vogels, C.B.F.; Yildirim, I.; Rothman, J.E.; Lu, P.; Monteiro, V.; Gehlhausen, J.R.; Campbell, M.; Silva, J.; Tabachnikova, A.; et al. Impact of circulating SARS-CoV-2 variants on mRNA vaccine-induced immunity. Nature 2021, 600, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Zuckerman, N.; Nemet, I.; Atari, N.; Kliker, L.; Regev-Yochay, G.; Sapir, E.; Mor, O.; Alroy-Preis, S.; Mendelson, E.; et al. Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel. Eurosurveill 2021, 26, 2100557. [Google Scholar] [CrossRef]
- Alexander, J.L.; Moran, G.W.; Gaya, D.R.; Raine, T.; Hart, A.; Kennedy, N.A.; Lindsay, J.O.; MacDonald, J.; Segal, J.P.; Sebastian, S.; et al. SARS-CoV-2 vaccination for patients with inflammatory bowel disease: A British Society of Gastroenterology Inflammatory Bowel Disease section and IBD Clinical Research Group position statement. Lancet Gastroenterol. Hepatol. 2021, 6, 218–224. [Google Scholar] [CrossRef]
- Siegel, C.A.; Melmed, G.Y.; McGovern, D.P.; Rai, V.; Krammer, F.; Rubin, D.T.; Abreu, M.T.; Dubinsky, M.C.; International Organization for the Study of Inflammatory Bowel Disease. SARS-CoV-2 vaccination for patients with inflammatory bowel diseases: Recommendations from an international consensus meeting. Gut 2021, 70, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Vollenberg, R.; Tepasse, P.-R.; Kühn, J.E.; Hennies, M.; Strauss, M.; Rennebaum, F.; Schomacher, T.; Boeckel, G.; Lorentzen, E.; Bokemeyer, A.; et al. Humoral Immune Response in IBD Patients Three and Six Months after Vaccination with the SARS-CoV-2 mRNA Vaccines mRNA-1273 and BNT162b2. Biomedicines 2022, 10, 17. [Google Scholar] [CrossRef]
- Chen, R.E.; Gorman, M.J.; Zhu, D.Y.; Carreno, J.M.; Yuan, D.; VanBlargan, L.A.; Burdess, S.; Lauffenburger, D.A.; Kim, W.; Turner, J.S.; et al. Reduced antibody activity against SARS-CoV-2 B.1.617.2 delta virus in serum of mRNA-vaccinated individuals receiving tumor necrosis factor-alpha inhibitors. Medicine 2021, 2, 1327–1341.e1324. [Google Scholar] [CrossRef]
- Geisen, U.M.; Sumbul, M.; Tran, F.; Berner, D.K.; Reid, H.M.; Vullriede, L.; Ciripoi, M.; Longardt, A.C.; Hoff, P.; Morrison, P.J.; et al. Humoral protection to SARS-CoV2 declines faster in patients on TNF alpha blocking therapies. RMD Open 2021, 7, e002008. [Google Scholar] [CrossRef]
- Haberman, R.H.; Um, S.; Axelrad, J.E.; Blank, R.B.; Uddin, Z.; Catron, S.; Neimann, A.L.; Mulligan, M.J.; Herat, R.S.; Hong, S.J.; et al. Methotrexate and TNF inhibitors affect long-term immunogenicity to COVID-19 vaccination in patients with immune-mediated inflammatory disease. Lancet Rheumatol. 2022, 4, e384–e387. [Google Scholar] [CrossRef]
- Pasparakis, M.; Alexopoulou, L.; Episkopou, V.; Kollias, G. Immune and inflammatory responses in TNF alpha-deficient mice: A critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 1996, 184, 1397–1411. [Google Scholar] [CrossRef]
- Salinas, G.F.; De Rycke, L.; Barendregt, B.; Paramarta, J.E.; Hreggvidsdottir, H.; Cantaert, T.; van der Burg, M.; Tak, P.P.; Baeten, D. Anti-TNF treatment blocks the induction of T cell-dependent humoral responses. Ann. Rheum. Dis. 2013, 72, 1037–1043. [Google Scholar] [CrossRef]
- Boussiotis, V.A.; Nadler, L.M.; Strominger, J.L.; Goldfeld, A.E. Tumor necrosis factor alpha is an autocrine growth factor for normal human B cells. Proc. Natl. Acad. Sci. USA 1994, 91, 7007–7011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassese, G.; Arce, S.; Hauser, A.E.; Lehnert, K.; Moewes, B.; Mostarac, M.; Muehlinghaus, G.; Szyska, M.; Radbruch, A.; Manz, R.A. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J. Immunol. 2003, 171, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.E.; Dohos, D.; Sipos, Z.; Kiss, S.; Dembrovszky, F.; Kovacs, N.; Solymar, M.; Eross, B.; Hegyi, P.; Sarlos, P. Immune response to influenza and pneumococcal vaccines in adults with inflammatory bowel disease: A systematic review and meta-analysis of 1429 patients. Vaccine 2022, 40, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Kobie, J.J.; Zheng, B.; Bryk, P.; Barnes, M.; Ritchlin, C.T.; Tabechian, D.A.; Anandarajah, A.P.; Looney, R.J.; Thiele, R.G.; Anolik, J.H.; et al. Decreased influenza-specific B cell responses in rheumatoid arthritis patients treated with anti-tumor necrosis factor. Arthritis. Res. Ther. 2011, 13, R209. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Xu, A.; Mengesha, E.; Elyanow, R.; Gittelman, R.M.; Chapman, H.; Prostko, J.C.; Frias, E.C.; Stewart, J.L.; Pozdnyakova, V.; et al. The T-Cell Response to SARS-CoV-2 Vaccination in Inflammatory Bowel Disease is Augmented with Anti-TNF Therapy. Inflamm. Bowel Dis. 2022, 28, 1130–1133. [Google Scholar] [CrossRef]
- Lin, S.; Kennedy, N.A.; Saifuddin, A.; Sandoval, D.M.; Reynolds, C.J.; Seoane, R.C.; Kottoor, S.H.; Pieper, F.P.; Lin, K.M.; Butler, D.K.; et al. Antibody decay, T cell immunity and breakthrough infections following two SARS-CoV-2 vaccine doses in inflammatory bowel disease patients treated with infliximab and vedolizumab. Nat. Commun. 2022, 13, 1379. [Google Scholar] [CrossRef] [PubMed]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L. SARS-CoV-2 spike protein: A key target for eliciting persistent neutralizing antibodies. Signal Transduct. Target. Ther. 2021, 6, 95. [Google Scholar] [CrossRef]
- Ciabattini, A.; Pastore, G.; Fiorino, F.; Polvere, J.; Lucchesi, S.; Pettini, E.; Auddino, S.; Rancan, I.; Durante, M.; Miscia, M.; et al. Evidence of SARS-CoV-2-Specific Memory B Cells Six Months After Vaccination With the BNT162b2 mRNA Vaccine. Front. Immunol. 2021, 12, 740708. [Google Scholar] [CrossRef]
- Brenner, E.J.; Ungaro, R.C.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.F.; Reinisch, W.; Ruemmele, F.M.; et al. Corticosteroids, But Not TNF Antagonists, Are Associated With Adverse COVID-19 Outcomes in Patients With Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology 2020, 159, 481–491.e483. [Google Scholar] [CrossRef]
- Ungaro, R.C.; Brenner, E.J.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.F.; Reinisch, W.; Steinwurz, F.; et al. Effect of IBD medications on COVID-19 outcomes: Results from an international registry. Gut 2021, 70, 725–732. [Google Scholar] [CrossRef]
- Geers, D.; Shamier, M.C.; Bogers, S.; den Hartog, G.; Gommers, L.; Nieuwkoop, N.N.; Schmitz, K.S.; Rijsbergen, L.C.; van Osch, J.A.T.; Dijkhuizen, E.; et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci. Immunol. 2021, 6, abj1750. [Google Scholar] [CrossRef] [PubMed]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Iketani, S.; Liu, L.; Guo, Y.; Liu, L.; Chan, J.F.; Huang, Y.; Wang, M.; Luo, Y.; Yu, J.; Chu, H.; et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature 2022, 604, 553–556. [Google Scholar] [CrossRef]
- Lu, L.; Mok, B.W.; Chen, L.L.; Chan, J.M.; Tsang, O.T.; Lam, B.H.; Chuang, V.W.; Chu, A.W.; Chan, W.M.; Ip, J.D.; et al. Neutralization of SARS-CoV-2 Omicron variant by sera from BNT162b2 or Coronavac vaccine recipients. Clin. Infect. Dis. 2021, 2021, ciab104. [Google Scholar] [CrossRef]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Ben-Tov, A.; Banon, T.; Chodick, G.; Kariv, R.; Assa, A.; Gazit, S.; Collaborators of the Maccabi Institute for Research & Innovation COVID-19 Task Force. BNT162b2 Messenger RNA COVID-19 Vaccine Effectiveness in Patients With Inflammatory Bowel Disease: Preliminary Real-World Data During Mass Vaccination Campaign. Gastroenterology 2021, 161, 1715–1717.e1711. [Google Scholar] [CrossRef]
- Liang, W.; Liang, H.; Ou, L.; Chen, B.; Chen, A.; Li, C.; Li, Y.; Guan, W.; Sang, L.; Lu, J.; et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients With COVID-19. JAMA Intern. Med. 2020, 180, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mahmud, N. Effectiveness of SARS-CoV-2 Vaccination in a Veterans Affairs Cohort of Patients With Inflammatory Bowel Disease With Diverse Exposure to Immunosuppressive Medications. Gastroenterology 2021, 161, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Muhsen, K.; Maimon, N.; Mizrahi, A.; Varticovschi, B.; Bodenheimer, O.; Gelbshtein, U.; Grotto, I.; Cohen, D.; Dagan, R. Effects of BNT162b2 Covid-19 Vaccine Booster in Long-Term Care Facilities in Israel. N. Engl. J. Med. 2022, 386, 399–401. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schaffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-Scale Study of Antibody Titer Decay following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection. Vaccines 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Minn, D.; Lim, J.; Lee, K.D.; Kang, Y.M.; Choe, K.W.; Kim, K.N. Rapidly Declining SARS-CoV-2 Antibody Titers within 4 Months after BNT162b2 Vaccination. Vaccines 2021, 9, 1145. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yuan, Y.; Zhou, Y.; Deng, Z.; Zhao, J.; Feng, F.; Zou, H.; Sun, C. Safety of SARS-CoV-2 vaccines: A systematic review and meta-analysis of randomized controlled trials. Infect. Dis. Poverty 2021, 10, 94. [Google Scholar] [CrossRef]
- Sharif, N.; Alzahrani, K.J.; Ahmed, S.N.; Dey, S.K. Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 714170. [Google Scholar] [CrossRef]
- Akinosoglou, K.; Tzivaki, I.; Marangos, M. Covid-19 vaccine and autoimmunity: Awakening the sleeping dragon. Clin. Immunol. 2021, 226, 108721. [Google Scholar] [CrossRef]
- Bril, F.; Al Diffalha, S.; Dean, M.; Fettig, D.M. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J. Hepatol. 2021, 75, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Vojdani, A.; Kharrazian, D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin. Immunol. 2020, 217, 108480. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Bayas, A.; Hindi, F.; Rizvi, Z.; Espinosa, P.S. Neurological Complications of COVID-19: Guillain-Barre Syndrome Following Pfizer COVID-19 Vaccine. Cureus 2021, 13, e13426. [Google Scholar] [CrossRef]
- Lev-Tzion, R.; Focht, G.; Lujan, R.; Mendelovici, A.; Friss, C.; Greenfeld, S.; Kariv, R.; Ben-Tov, A.; Matz, E.; Nevo, D.; et al. COVID-19 Vaccine Is Effective in Inflammatory Bowel Disease Patients and Is Not Associated With Disease Exacerbation. Clin. Gastroenterol. Hepatol. 2021, 20, e1263–e1282. [Google Scholar] [CrossRef]
- Kappelman, M.D.; Weaver, K.N.; Boccieri, M.; Firestine, A.; Zhang, X.; Long, M.D.; Group, P.-C.S. Humoral Immune Response to Messenger RNA COVID-19 Vaccines Among Patients With Inflammatory Bowel Disease. Gastroenterology 2021, 161, 1340–1343.e1342. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, J.A.; Connolly, C.M.; Boyarsky, B.J.; Werbel, W.A.; Christopher-Stine, L.; Garonzik-Wang, J.; Segev, D.L.; Paik, J.J. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021, 80, 1351–1352. [Google Scholar] [CrossRef] [PubMed]
- Romero-Olmedo, A.J.; Schulz, A.R.; Hochstatter, S.; Das Gupta, D.; Virta, I.; Hirseland, H.; Staudenraus, D.; Camara, B.; Munch, C.; Hefter, V.; et al. Induction of robust cellular and humoral immunity against SARS-CoV-2 after a third dose of BNT162b2 vaccine in previously unresponsive older adults. Nat. Microbiol. 2022, 7, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Gao, G.F.; Wang, Q. The mysterious origins of the Omicron variant of SARS-CoV-2. Innovation 2022, 3, 100206. [Google Scholar] [CrossRef] [PubMed]
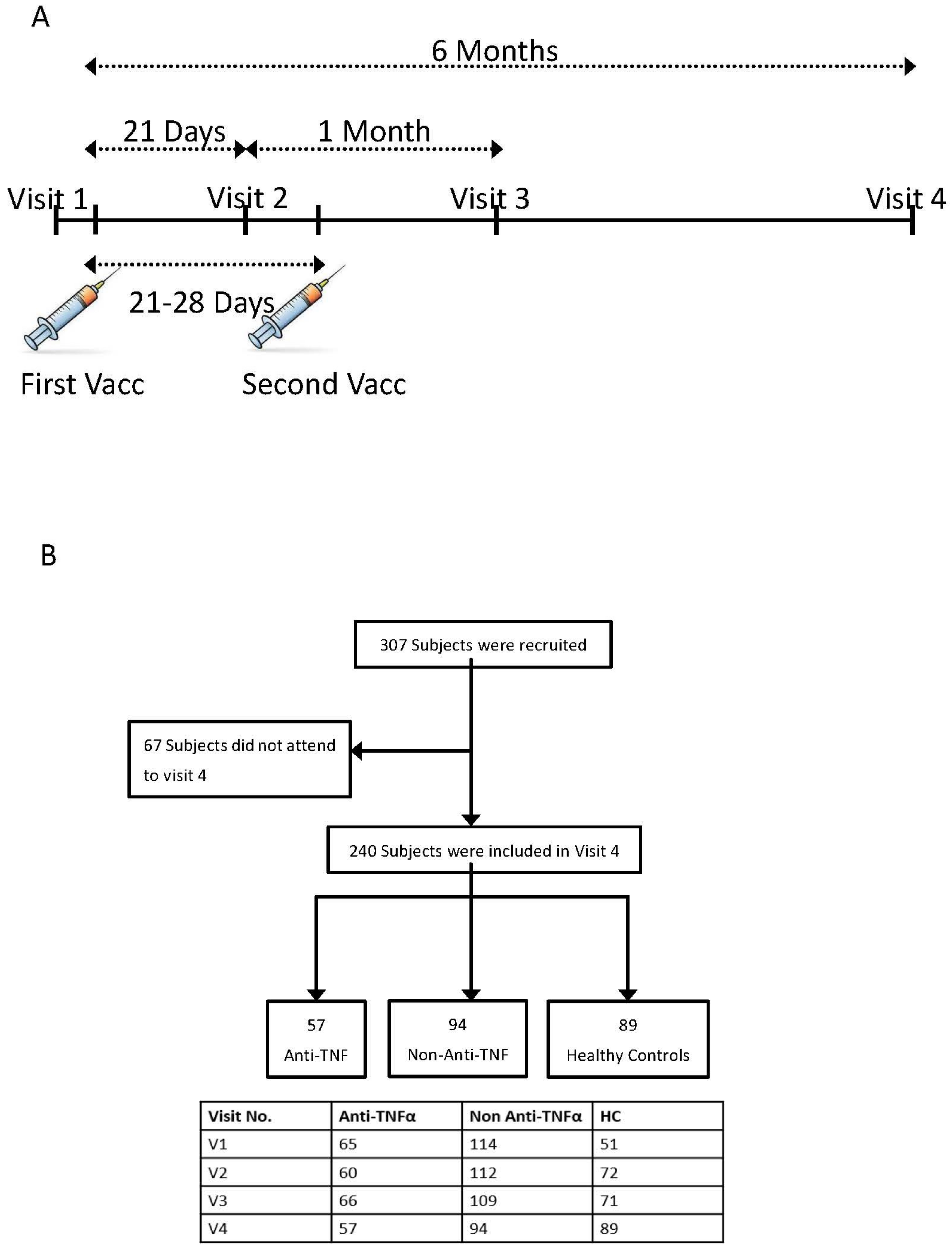
| Characteristics | Anti-TNFα N = 57 | Non-Anti-TNFα N = 94 | HC N = 89 | p Value |
|---|---|---|---|---|
| Mean age, years (SD) | 38.2 (14.1) | 39.3 (13.4) | 38.9 (12.2) | 0.888 |
| Female, n (%) | 20 (35.1) | 40 (42.6) | 62 (69.7) | <0.001 |
| Origin, n (%) | ||||
| Ashkenazi | 28 (49.1) | 42 (44.7) | 48 (53.9) | 0.457 |
| Non-Ashkenazi | 29 (50.9) | 52 (55.3) | 41 (46.1) | |
| Mean BMI, kg/m2 (SD) | 25.5 (4.1) | 24.3 (4.5) | 25.1 (5.3) | 0.329 |
| Smoking status, n (%) | ||||
| Present | 4 (7.0) | 8 (8.5) | 8 (9.0) | 0.140 |
| Past | 4 (7.0) | 7 (7.4) | 0 (0) | |
| No | 49 (86.0) | 79 (84.0) | 81 (91.0) | |
| Comorbidities a, n (%) | 5 (8.8) | 6 (6.4) | 5 (5.6) | |
| IBD phenotype, n (%) | ||||
| CD | 47 (82.5) | 50 (53.2) | ----- | <0.001 |
| UC | 7 (12.3) | 36 (38.3) | ----- | 0.001 |
| IPAA | 2 (3.5) | 5 (5.3) | ----- | |
| IBD-unclassified | 1 (1.8) | 3 (3.2) | ----- | |
| Disease activity b, n (%) | ||||
| Remission | 42 (75.0) | 54 (60.0) | ----- | 0.074 |
| Active | 14 (25.0) | 36 (40.0) | ----- | |
| Current medication, n (%) | ||||
| Infliximab | 29 (50.9) | ----- | ----- | |
| Adalimumab | 26 (45.6) | ----- | ----- | |
| Vedolizumab | ----- | 23 (24.5) | ----- | |
| Ustekinumab | ----- | 8 (8.5) | ||
| 5-ASA | 5 (8.8) | 33 (35.1) | ----- | |
| Steroids | 1 (1.8) | 7 (7.4) | ----- | |
| Immunomodulators c | 8 (14.0) | 4 (4.3) | ----- | |
| JAK inhibitor | ----- | 5 (5.3) | ||
| No medical treatment | ----- | 29 (30.9) | ----- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabinowitz, K.M.; Navon, M.; Edelman-Klapper, H.; Zittan, E.; Bar-Gil Shitrit, A.; Goren, I.; Avni-Biron, I.; Ollech, J.E.; Lichtenstein, L.; Banai-Eran, H.; et al. Anti-TNFα Treatment Impairs Long-Term Immune Responses to COVID-19 mRNA Vaccine in Patients with Inflammatory Bowel Diseases. Vaccines 2022, 10, 1186. https://doi.org/10.3390/vaccines10081186
Rabinowitz KM, Navon M, Edelman-Klapper H, Zittan E, Bar-Gil Shitrit A, Goren I, Avni-Biron I, Ollech JE, Lichtenstein L, Banai-Eran H, et al. Anti-TNFα Treatment Impairs Long-Term Immune Responses to COVID-19 mRNA Vaccine in Patients with Inflammatory Bowel Diseases. Vaccines. 2022; 10(8):1186. https://doi.org/10.3390/vaccines10081186
Chicago/Turabian StyleRabinowitz, Keren Masha, Michal Navon, Hadar Edelman-Klapper, Eran Zittan, Ariella Bar-Gil Shitrit, Idan Goren, Irit Avni-Biron, Jacob E. Ollech, Lev Lichtenstein, Hagar Banai-Eran, and et al. 2022. "Anti-TNFα Treatment Impairs Long-Term Immune Responses to COVID-19 mRNA Vaccine in Patients with Inflammatory Bowel Diseases" Vaccines 10, no. 8: 1186. https://doi.org/10.3390/vaccines10081186
APA StyleRabinowitz, K. M., Navon, M., Edelman-Klapper, H., Zittan, E., Bar-Gil Shitrit, A., Goren, I., Avni-Biron, I., Ollech, J. E., Lichtenstein, L., Banai-Eran, H., Yanai, H., Snir, Y., Pauker, M. H., Friedenberg, A., Levy-Barda, A., Segal, A., Broitman, Y., Maoz, E., Ovadia, B., ... on behalf of the Responses to COVID-19 Vaccine Israeli IBD Group. (2022). Anti-TNFα Treatment Impairs Long-Term Immune Responses to COVID-19 mRNA Vaccine in Patients with Inflammatory Bowel Diseases. Vaccines, 10(8), 1186. https://doi.org/10.3390/vaccines10081186








