Biomedical Applications of Humic Substances: From Natural Biopolymers to Therapeutic Agents
Abstract
1. Introduction
2. Methods
3. Chemical Structure and Physicochemical Properties of HA and FA
4. Relationship Between Structure, Mechanism of Action and Application
5. Biomedical Applications
5.1. Antioxidant Properties
5.2. Anti-Inflammatory and Immunomodulatory Effects
5.3. Anticancer Properties
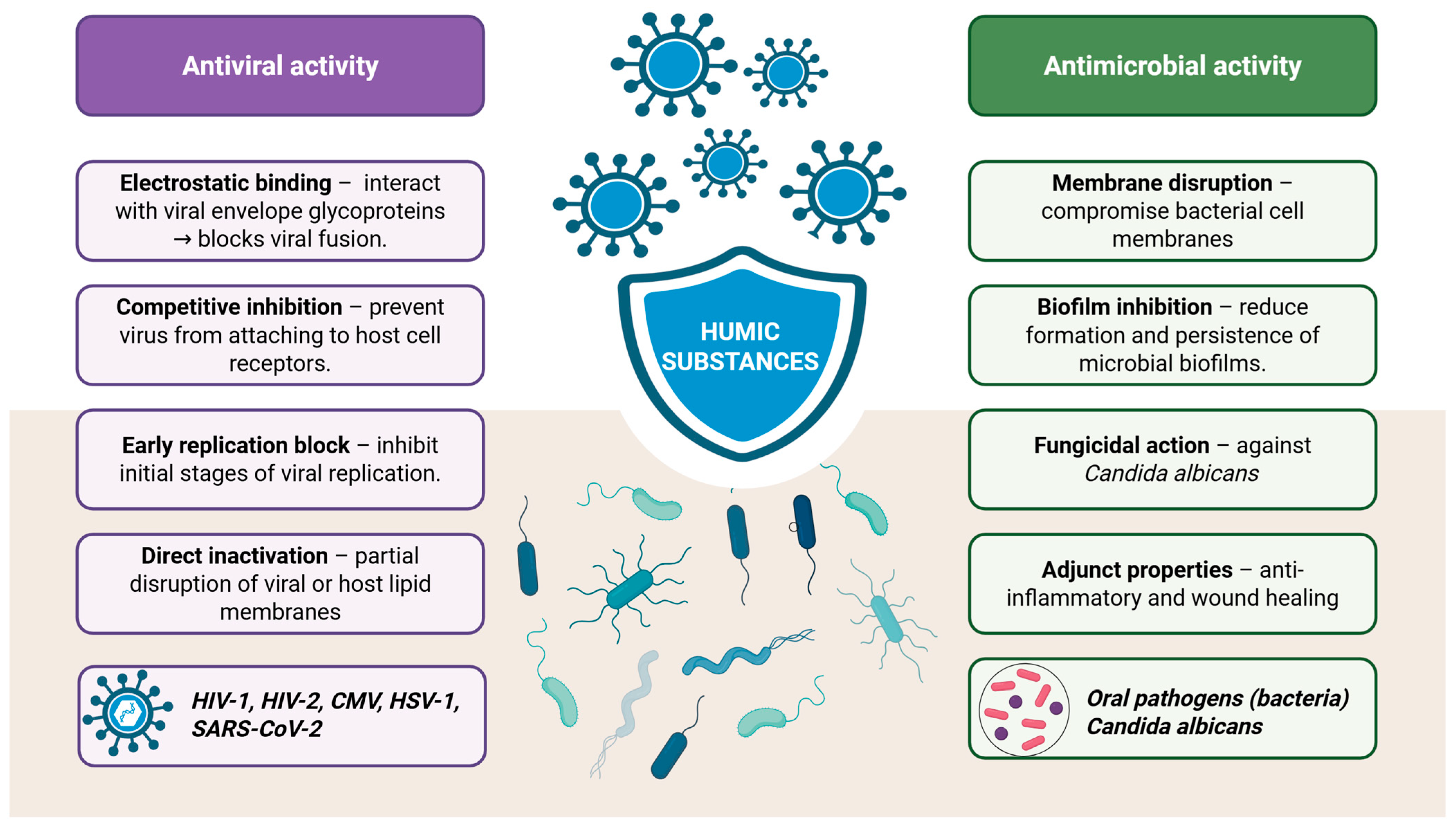
5.4. Antibacterial, Antifungal and Antiviral Activities
5.5. Wound Healing Properties
5.6. Detoxification (Adsorption Properties) and Heavy Metal Chelation
5.7. Gut Microbiota Modulation
5.8. Antiallergic Properties
5.9. Antihyperglycemia Activities by Type 2 Diabetes Mellitus
5.10. Neuroprotective Properties
5.11. Blood Properties
6. Cytotoxicity of Humic Substances
7. Drug Formulation and Drug Delivery
8. Limitations in Using Humic Substances
9. Future Perspectives
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| HS | Humic substances |
| HA | Humic acid |
| FA | Fulvic acid |
| HM | Humin |
| HSV | Herpes Simplex Virus |
| HIV | Human Immunodeficiency Virus |
| SARS-Cov | Coronavirus 2019 |
| TNF | Tumor necrosis factor |
| IL | Interleukin |
| ROS | Reactive oxygen species |
| AOK | Antioxidant capacity |
| ORAK | Oxygen radical absorbance capacity |
| CMV | Cytomegalovirus |
| TGF-β | Transforming growth factor beta |
| IBS | Irritable bowel syndrome |
| IBD | Irritable bowel disease |
| NCs | Nanoconjugates |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentrations |
| AgNPs | Silver nanoparticles |
References
- Yunusova, S.; Saidjon, S. World Experiments Dedicated to the Study of the Process of Formation of Humus in the Soil and the Composition of Humus Substances. Int. J. Virol. Mol. Biol. 2024, 13, 95–97. [Google Scholar]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–17. [Google Scholar] [CrossRef]
- Vašková, J.; Stupák, M.; Vidová Ugurbaş, M.; Žatko, D.; Vaško, L. Therapeutic Efficiency of Humic Acids in Intoxications. Life 2023, 13, 971. [Google Scholar] [CrossRef]
- Nebbioso, A.; Piccolo, A. Basis of a Humeomics Science: Chemical Fractionation and Molecular Characterization of Humic Biosuprastructures. Biomacromolecules 2011, 12, 1187–1199. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; Wiley: New York, NY, USA, 1994; ISBN 978-0-471-59474-1. [Google Scholar]
- Zhernov, Y.V.; Kremb, S.; Helfer, M.; Schindler, M.; Harir, M.; Mueller, C.; Hertkorn, N.; Avvakumova, N.P.; Konstantinov, A.I.; Brack-Werner, R.; et al. Supramolecular Combinations of Humic Polyanions as Potent Microbicides with Polymodal Anti-HIV-Activities. New J. Chem. 2017, 41, 212–224. [Google Scholar] [CrossRef]
- Olk, D.C.; Bloom, P.R.; Perdue, E.M.; McKnight, D.M.; Chen, Y.; Farenhorst, A.; Senesi, N.; Chin, Y.-P.; Schmitt-Kopplin, P.; Hertkorn, N.; et al. Environmental and Agricultural Relevance of Humic Fractions Extracted by Alkali from Soils and Natural Waters. J. Environ. Qual. 2019, 48, 217–232. [Google Scholar] [CrossRef]
- Sarlaki, E.; Sharif Paghaleh, A.; Kianmehr, M.H.; Asefpour Vakilian, K. Extraction and Purification of Humic Acids from Lignite Wastes Using Alkaline Treatment and Membrane Ultrafiltration. J. Clean. Prod. 2019, 235, 712–723. [Google Scholar] [CrossRef]
- Saito, B.; Seckler, M.M. Alkaline Extraction of Humic Substances from Peat Applied to Organic-Mineral Fertilizer Production. Braz. J. Chem. Eng. 2014, 31, 675–682. [Google Scholar] [CrossRef]
- Chi, M.; Wang, Z.; Xu, W.; Hou, R. Extraction and Characterization of Fulvic Acid from Corn Straw Compost by Alkali Solution Acid Precipitation. Ind. Crops Prod. 2023, 198, 116678. [Google Scholar] [CrossRef]
- Karak, N. Biodegradable Polymers. In Vegetable Oil-Based Polymers; Elsevier: Amsterdam, The Netherlands, 2012; pp. 31–53. ISBN 978-0-85709-710-1. [Google Scholar]
- Błońska-Sikora, E.M.; Klimek-Szczykutowicz, M.; Michalak, M.; Kulik-Siarek, K.; Wrzosek, M. Potential Possibilities of Using Peat, Humic Substances, and Sulfurous Waters in Cosmetology. Appl. Sci. 2024, 14, 6912. [Google Scholar] [CrossRef]
- Klučáková, M.; Krouská, J.; Kalina, M. Physico-Chemical Aspects of Metal–Fulvic Complexation. Processes 2024, 12, 989. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Bejger, R.; Debaene, G.; Smreczak, B. Characterization of Soil Organic Matter Individual Fractions (Fulvic Acids, Humic Acids, and Humins) by Spectroscopic and Electrochemical Techniques in Agricultural Soils. Agronomy 2021, 11, 1067. [Google Scholar] [CrossRef]
- Schnitzer, M. Recent Findings on the Characterization of Humic Substances Extracted from Soils from Widely Differing Climatic Zones; International Atomic Energy Agency: Braunschweig, Germany, 1977; Volume II, pp. 117–132. [Google Scholar]
- Von Wandruszka, R. Humic Acids: Their Detergent Qualities and Potential Uses in Pollution Remediation. Geochem. Trans. 2000, 1, 10. [Google Scholar] [CrossRef]
- Angelico, R.; Colombo, C.; Di Iorio, E.; Brtnický, M.; Fojt, J.; Conte, P. Humic Substances: From Supramolecular Aggregation to Fractal Conformation—Is There Time for a New Paradigm? Appl. Sci. 2023, 13, 2236. [Google Scholar] [CrossRef]
- Stancampiano, L.M.; Verrillo, M.; Cangemi, S.; Meignant, I.; Spaccini, R.; Piccolo, A.; Bridoux, M.C. The Molecular Composition of Humic Substances Extracted from Green Composts and Their Potential for Soil Remediation. Environ. Chem. Lett. 2023, 21, 2489–2498. [Google Scholar] [CrossRef]
- Tian, S.; Tan, W.; Wang, X.; Li, T.; Song, F.; Huang, N.; Bai, Y. Surface Activity of Humic Acid and Its Sub-Fractions from Forest Soil. Sustainability 2021, 13, 8122. [Google Scholar] [CrossRef]
- Gholami, S.; Behnami, A.; Hesami Arani, M.; Rezaei Kalantary, R. Impact of Humic Substances on the Bioremediation of Polycyclic Aromatic Hydrocarbons in Contaminated Soils and Sediments: A Review. Environ. Chem. Lett. 2024, 22, 889–918. [Google Scholar] [CrossRef]
- Urdiales, C.; Sandoval, M.P.; Escudey, M.; Pizarro, C.; Knicker, H.; Reyes-Bozo, L.; Antilén, M. Surfactant Properties of Humic Acids Extracted from Volcanic Soils and Their Applicability in Mineral Flotation Processes. J. Environ. Manag. 2018, 227, 117–123. [Google Scholar] [CrossRef]
- Chianese, S.; Fenti, A.; Iovino, P.; Musmarra, D.; Salvestrini, S. Sorption of Organic Pollutants by Humic Acids: A Review. Molecules 2020, 25, 918. [Google Scholar] [CrossRef]
- Ghabbour, E.A.; Davies, G.; Ghali, N.K.; Mulligan, M.D. The Effect of Temperature on Tight Metal Binding by Peat and Soil Derived Solid Humic Acids. Can. J. Soil. Sci. 2001, 81, 331–336. [Google Scholar] [CrossRef]
- Motta, F.L.; Melo, B.A.G.; Santana, M.H.A. Deprotonation and Protonation of Humic Acids as a Strategy for the Technological Development of pH-Responsive Nanoparticles with Fungicidal Potential. New Biotechnol. 2016, 33, 773–780. [Google Scholar] [CrossRef]
- Socol, D.C. Clinical Review of Humic Acid as an Antiviral: Leadup to Translational Applications in Clinical Humeomics. Front. Pharmacol. 2023, 13, 1018904. [Google Scholar] [CrossRef] [PubMed]
- Klöcking, R.; Helbig, B.; Schötz, G.; Schacke, M.; Wutzler, P. Anti-HSV-1 Activity of Synthetic Humic Acid-Like Polymers Derived from p-Diphenolic Starting Compounds. Antivir. Chem. Chemother. 2002, 13, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Cagno, V.; Donalisio, M.; Civra, A.; Cagliero, C.; Rubiolo, P.; Lembo, D. In Vitro Evaluation of the Antiviral Properties of Shilajit and Investigation of Its Mechanisms of Action. J. Ethnopharmacol. 2015, 166, 129–134. [Google Scholar] [CrossRef]
- Pant, K.; Yadav, A.K.; Gupta, P.; Rathore, A.S.; Nayak, B.; Venugopal, S.K. Humic Acid Inhibits HBV-Induced Autophagosome Formation and Induces Apoptosis in HBV-Transfected Hep G2 Cells. Sci. Rep. 2016, 6, 34496. [Google Scholar] [CrossRef]
- Bruccoleri, A. Positional Adaptability in the Design of Mutation-Resistant Nonnucleoside HIV-1 Reverse Transcriptase Inhibitors: A Supramolecular Perspective. AIDS Res. Hum. Retroviruses 2013, 29, 4–12. [Google Scholar] [CrossRef]
- Rege, A.A.; Ambaye, R.Y.; Deshmukh, R.A. Evaluation of in Vitro Inhibitory Effect of Selected Plants and Shilajit on HIV-Reverse Transcriptase. Indian J. Nat. Prod. Resour. 2012, 3, 145–151. [Google Scholar]
- Kornilaeva, G.V.; Siniavin, A.E.; Schultz, A.; Germann, A.; Moog, C.; Von Briesen, H.; Turgiev, A.S.; Karamov, E.V. The Differential Anti-HIV Effect of a New Humic Substance-Derived Preparation in Diverse Cells of the Immune System. Acta Naturae 2019, 11, 68–76. [Google Scholar] [CrossRef]
- Hafez, M.; Popov, A.I.; Zelenkov, V.N.; Teplyakova, T.V.; Rashad, M. Humic Substances as an Environmental- Friendly Organic Wastes Potentially Help as Natural Anti-Virus to Inhibit COVID-19. Sci. Arch. 2020, 1, 53–60. [Google Scholar] [CrossRef]
- Hajdrik, P.; Pályi, B.; Kis, Z.; Kovács, N.; Veres, D.S.; Szigeti, K.; Budán, F.; Hegedüs, I.; Kovács, T.; Bergmann, R.; et al. In Vitro Determination of Inhibitory Effects of Humic Substances Complexing Zn and Se on SARS-CoV-2 Virus Replication. Foods 2022, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- De Melo, B.A.G.; Motta, F.L.; Santana, M.H.A. Humic Acids: Structural Properties and Multiple Functionalities for Novel Technological Developments. Mater. Sci. Eng. C 2016, 62, 967–974. [Google Scholar] [CrossRef]
- Verrillo, M.; Parisi, M.; Savy, D.; Caiazzo, G.; Di Caprio, R.; Luciano, M.A.; Cacciapuoti, S.; Fabbrocini, G.; Piccolo, A. Antiflammatory Activity and Potential Dermatological Applications of Characterized Humic Acids from a Lignite and a Green Compost. Sci. Rep. 2022, 12, 2152. [Google Scholar] [CrossRef] [PubMed]
- Trofimova, E.S.; Zykova, M.V.; Danilets, M.G.; Ligacheva, A.A.; Sherstoboev, E.Y.; Tsupko, A.V.; Mikhalyov, D.A.; Belousov, M.V. Immunomodulating Properties of Humic Acids Extracted from Oligotrophic Sphagnum Magellanicum Peat. Bull. Exp. Biol. Med. 2021, 170, 461–465. [Google Scholar] [CrossRef]
- Jooné, G.K.; Van Rensburg, C.E.J. An In Vitro Investigation of the Anti-Inflammatory Properties of Potassium Humate. Inflammation 2004, 28, 169–174. [Google Scholar] [CrossRef]
- Vašková, J.; Veliká, B.; Pilátová, M.; Kron, I.; Vaško, L. Effects of Humic Acids in Vitro. Vitro Cell. Dev. Biol.-Anim. 2011, 47, 376–382. [Google Scholar] [CrossRef]
- Cudowski, A.; Pietryczuk, A.; Górniak, A. Effect of Humic Acid on the Growth and Metabolism of Candida Albicans Isolated from Surface Waters in North-Eastern Poland. Int. J. Environ. Res. Public Health 2022, 19, 9408. [Google Scholar] [CrossRef]
- Ansorg, R.; Rochus, W. Studies on the antimicrobial effect of natural and synthetic humic acids (author’s transl). Arzneimittelforschung 1978, 28, 2195–2198. [Google Scholar]
- Shang, E.; Li, Y.; Niu, J.; Zhou, Y.; Wang, T.; Crittenden, J.C. Relative Importance of Humic and Fulvic Acid on ROS Generation, Dissolution, and Toxicity of Sulfide Nanoparticles. Water Res. 2017, 124, 595–604. [Google Scholar] [CrossRef]
- Csicsor, A.; Tombácz, E.; Kulcsár, P. Antioxidant Potential of Humic Substances Measured by Folin-Ciocalteu, CUPRAC, QUENCHER-CUPRAC and ESR Methods. J. Mol. Liq. 2023, 391, 123294. [Google Scholar] [CrossRef]
- Piotrowska, D.; Długosz, A.; Witkiewicz, K.; Pajak, J. The Research on Antioxidative Properties of TOŁPA Peat Preparation and Its Fractions. Acta Pol. Pharm. 2000, 57, 127–129. [Google Scholar] [PubMed]
- Verrillo, M.; Cozzolino, V.; Spaccini, R. Antioxidant Features of Humic Products by ABTS Assay. In Immunosenescence; Amoriello, R., Ballerini, C., Mariottini, A., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2025; Volume 2857, pp. 223–227. ISBN 978-1-0716-4127-9. [Google Scholar]
- Klein, O.I.; Kulikova, N.A.; Konstantinov, A.I.; Zykova, M.V.; Perminova, I.V. A Systematic Study of the Antioxidant Capacity of Humic Substances against Peroxyl Radicals: Relation to Structure. Polymers 2021, 13, 3262. [Google Scholar] [CrossRef]
- Volikov, A.; Mareev, N.; Konstantinov, A.; Molodykh, A.; Melnikova, S.; Bazhanova, A.; Gasanov, M.; Nikolaev, E.; Zherebker, A.; Volkov, D.; et al. Directed Synthesis of Humic and Fulvic Derivatives with Enhanced Antioxidant Properties. Agronomy 2021, 11, 2047. [Google Scholar] [CrossRef]
- Wang, X.; Muhmood, A.; Ren, D.; Tian, P.; Li, Y.; Yu, H.; Wu, S. Exploring the Mechanisms of Humic Acid Mediated Degradation of Polystyrene Microplastics under Ultraviolet Light Conditions. Chemosphere 2023, 327, 138544. [Google Scholar] [CrossRef] [PubMed]
- Jacob, K.K.; Prashob, P.K.J.; Chandramohanakumar, N. Humic substances as a potent biomaterials for therapeutic and drug delivery system—A review. Int. J. Appl. Pharm. 2019, 11, 1–4. [Google Scholar] [CrossRef]
- Hriciková, S.; Kožárová, I.; Hudáková, N.; Reitznerová, A.; Nagy, J.; Marcinčák, S. Humic Substances as a Versatile Intermediary. Life 2023, 13, 858. [Google Scholar] [CrossRef]
- Tarasova, A.S.; Stom, D.I.; Kudryasheva, N.S. Antioxidant Activity of Humic Substances via Bioluminescent Monitoring in Vitro. Environ. Monit. Assess. 2015, 187, 89. [Google Scholar] [CrossRef]
- Goel, P.; Dhingra, M. Humic Substances: Prospects for Use in Agriculture and Medicine. In Humic Substances; Makan, A., Ed.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Winkler, J.; Ghosh, S. Therapeutic Potential of Fulvic Acid in Chronic Inflammatory Diseases and Diabetes. J. Diabetes Res. 2018, 2018, 5391014. [Google Scholar] [CrossRef]
- Alkan Ozdemir, S.; Ozdemir, N.; Aksan, O.; Kınalı, B.; Bilici Güler, G.; Erbil, G.; Ozer, E.; Ozer, E. Effect of Humic Acid on Oxidative Stress and Neuroprotection in Hypoxic-Ischemic Brain Injury: Part 1. J. Matern. Fetal Neonatal Med. 2022, 35, 4580–4589. [Google Scholar] [CrossRef]
- Santos, T.C.D.; Silva, H.P.; Lima, K.R.; Salvador, M.L.N.; Cândido, G.D.S.; Pimenta, L.C.J.P.; Bertolini, N.O.; Ribeiro, L.B.; Fagundes, F.G.; Orlando, D.R.; et al. Humic Acid Derived from Vermicompost Improves Bone Mineral Content and Alters Oxidative Stress Markers in Ovariectomized Mice. Biomedicines 2025, 13, 495. [Google Scholar] [CrossRef]
- Khuda, F.; Anjum, M.; Khan, S.; Khan, H.; Umar Khayam Sahibzada, M.; Khusro, A.; Jan, A.; Ullah, N.; Shah, Y.; Zakiullah; et al. Antimicrobial, Anti-Inflammatory and Antioxidant Activities of Natural Organic Matter Extracted from Cretaceous Shales in District Nowshera-Pakistan. Arab. J. Chem. 2022, 15, 103633. [Google Scholar] [CrossRef]
- Junek, R.; Morrow, R.; Schoenherr, J.I.; Schubert, R.; Kallmeyer, R.; Phull, S.; Klöcking, R. Bimodal Effect of Humic Acids on the LPS-Induced TNF-α Release from Differentiated U937 Cells. Phytomedicine 2009, 16, 470–476. [Google Scholar] [CrossRef]
- Yamada, P.; Isoda, H.; Han, J.K.; Talorete, T.P.N.; Yamaguchi, T.; Abe, Y. Inhibitory Effect of Fulvic Acid Extracted from Canadian Sphagnum Peat on Chemical Mediator Release by RBL-2H3 and KU812 Cells. Biosci. Biotechnol. Biochem. 2007, 71, 1294–1305. [Google Scholar] [CrossRef]
- Chien, S.-J.; Chen, T.-C.; Kuo, H.-C.; Chen, C.-N.; Chang, S.-F. Fulvic Acid Attenuates Homocysteine-Induced Cyclooxygenase-2 Expression in Human Monocytes. BMC Complement. Altern. Med. 2015, 15, 61. [Google Scholar] [CrossRef]
- Sabi, R.; Vrey, P.; Van Rensburg, C.E.J. Carbohydrate-derived Fulvic Acid (CHD-FA) Inhibits Carrageenan-induced Inflammation and Enhances Wound Healing: Efficacy and Toxicity Study in Rats. Drug Dev. Res. 2012, 73, 18–23. [Google Scholar] [CrossRef]
- Samiee-Rad, F.; Hosseini Sedighi, S.; Taherkhani, A.; Gheibi, N. Evaluation of Healing Effects of Poultice Containing 0.5% Fulvic Acid on Male White-Male Rats with Skin Ulcer. J. Cutan. Aesthetic Surg. 2022, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, N.; Samiee-Rad, F.; Sofiabadi, M.; Mosayebi, E.; Shalbaf, Z. The Effect of Combining Humic and Fulvic Acids Poultice on Wound Healing in Male Rats. J. Cutan. Aesthetic Surg. 2024, 17, 105–111. [Google Scholar] [CrossRef]
- Rahmani Barouji, S.; Saber, A.; Torbati, M.; Fazljou, S.M.B.; Yari Khosroushahi, A. Health Beneficial Effects of Moomiaii in Traditional Medicine. Galen Med. J. 2020, 9, e1743. [Google Scholar] [CrossRef]
- Pant, K.; Gupta, A.; Gupta, P.; Ashraf, A.; Yadav, A.; Venugopal, S. Anti-Proliferative and Anticancer Properties of Fulvic Acid on Hepatic Cancer Cells. J. Clin. Exp. Hepatol. 2015, 5, S2. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Chen, S.-C.; Chen, Y.-L.; Chen, J.-Y.; Lee, M.-L.; Lu, F.-J.; Wu, F.-Y.; Lai, J.-S.; Yang, H.-L. Humic Acid Induced Genotoxicity in Human Peripheral Blood Lymphocytes Using Comet and Sister Chromatid Exchange Assay. J. Hazard. Mater. 2008, 153, 784–791. [Google Scholar] [CrossRef]
- Aykac, A.; Becer, E.; Okcanoğlu, T.B.; Güvenir, M.; Süer, K.; Vatansever, S. The Cytotoxic Effects of Humic Acid on Human Breast Cancer Cells. Proceedings 2018, 2, 1565. [Google Scholar] [CrossRef]
- Zolghadr, L.; Behbehani, G.R.; Pakbin, B.; Hosseini, S.A.; Gheibi, N. A New Insight Into the Anti Proliferative and Apoptotic Effects of Fulvic and Humic Acids as Bio Product of Humus on Breast Cancer Cells, Optimized by Response Surface Methodology. Waste Biomass Valorization 2022, 14, 859–872. [Google Scholar] [CrossRef]
- Yang, E.J.; Chang, J.H. Humic Substances Suppresses the Proliferation of TC-1 Cells, the Lung Cancer Cell. Biomed. Sci. Lett. 2023, 29, 280–286. [Google Scholar] [CrossRef]
- Sherry, L.; Millhouse, E.; Lappin, D.F.; Murray, C.; Culshaw, S.; Nile, C.J.; Ramage, G. Investigating the Biological Properties of Carbohydrate Derived Fulvic Acid (CHD-FA) as a Potential Novel Therapy for the Management of Oral Biofilm Infections. BMC Oral Health 2013, 13, 47. [Google Scholar] [CrossRef]
- Sherry, L.; Jose, A.; Murray, C.; Williams, C.; Jones, B.; Millington, O.; Bagg, J.; Ramage, G. Carbohydrate Derived Fulvic Acid: An in Vitro Investigation of a Novel Membrane Active Antiseptic Agent Against Candida Albicans Biofilms. Front. Microbiol. 2012, 3, 116. [Google Scholar] [CrossRef]
- Yarkova, T.A. Chemical Modification of Humic Acids by the Introduction of Indole-Containing Fragments. Solid Fuel Chem. 2011, 45, 261–266. [Google Scholar] [CrossRef]
- Verrillo, M.; Salzano, M.; Savy, D.; Di Meo, V.; Valentini, M.; Cozzolino, V.; Piccolo, A. Antibacterial and Antioxidant Properties of Humic Substances from Composted Agricultural Biomasses. Chem. Biol. Technol. Agric. 2022, 9, 28. [Google Scholar] [CrossRef]
- Vanimuthu, K.; Kavitha, K.; Paul, J.A.J.; Kumar, P.; Gowrishankar, S.; Balachandar, R.; Prabhu, D.; Rajamanikandan, S.; Biruntha, M. Isolation, Characterization and Antifungal Behavior of Humic Acid and Fulvic Acid Fractions from Biowaste Derived Vermiproducts. 2024. Available online: https://www.researchsquare.com/article/rs-4221685/v1 (accessed on 11 September 2025).
- Dharejo, S.A.; Pirzada, T.; Shah, M.R.; Nadeem, A.; Thebo, K.H. In-Vitro Study of Hybrid Silver Nanoparticles with Humic Acid Extracted from Cow Dung against Pathogens. Heliyon 2025, 11, e41636. [Google Scholar] [CrossRef]
- Meerbach, A.; Neyts, J.; Balzarini, J.; Helbig, B.; De Clercq, E.; Wutzler, P. In Vitro Activity of Polyhydroxycarboxylates against Herpesviruses and Hiv. Antivir. Chem. Chemother. 2001, 12, 337–345. [Google Scholar] [CrossRef][Green Version]
- Ziemska, J.; Szynal, T.; Mazańska, M.; Solecka, J. Characterization of Humic Substances in Waters and Their Therapeutic Applications—A Review. Acta Balneol. 2024, 66, 60–68. [Google Scholar] [CrossRef]
- Zhernov, Y.V.; Konstantinov, A.I.; Zherebker, A.; Nikolaev, E.; Orlov, A.; Savinykh, M.I.; Kornilaeva, G.V.; Karamov, E.V.; Perminova, I.V. Antiviral Activity of Natural Humic Substances and Shilajit Materials against HIV-1: Relation to Structure. Environ. Res. 2021, 193, 110312. [Google Scholar] [CrossRef]
- Zhernov, Y. Natural Humic Substances Interfere with Multiple Stages of the Replication Cycle of Human Immunodeficiency Virus. J. Allergy Clin. Immunol. 2018, 141, AB233. [Google Scholar] [CrossRef]
- Çalışır, M.; Akpınar, A.; Talmaç, A.C.; Lektemur Alpan, A.; Göze, Ö.F. Humic Acid Enhances Wound Healing in the Rat Palate. Evid. Based Complement. Alternat. Med. 2018, 2018, 1783513. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, A.; Chen, X.; Che, X.; Zhou, K.; Wang, Z. Sodium Humate Accelerates Cutaneous Wound Healing by Activating TGF-β/Smads Signaling Pathway in Rats. Acta Pharm. Sin. B 2016, 6, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Liberek, I.; Galus, R.; Owczarek, W.; Włodarsk, K.; Zabielski, S.; Malejczyk, J.; Sladowski, D. Collagen based dressings in the treatment of wound healing. Pol. Merkur. Lek. Organ. Pol. Tow. Lek. 2013, 35, 51–54. [Google Scholar]
- Peña-Méndez, E.M.; Havel, J.; Patočka, J. Humic Substances—Compounds of Still Unknown Structure: Applications in Agriculture, Industry, Environment, and Biomedicine. J. Appl. Biomed. 2005, 3, 13–24. [Google Scholar] [CrossRef]
- Peng, X.-X.; Gai, S.; Cheng, K.; Yang, F. Roles of Humic Substances Redox Activity on Environmental Remediation. J. Hazard. Mater. 2022, 435, 129070. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, S.; Yuan, Y.; Lu, Q. Influence of Humic Acid Complexation with Metal Ions on Extracellular Electron Transfer Activity. Sci. Rep. 2015, 5, 17067. [Google Scholar] [CrossRef] [PubMed]
- Gerlach, A. Oral Application of Charcoal and Humic Acids to Dairy Cows Influences Clostridium Botulinum Blood Serum Antibody Level and Glyphosate Excretion in Urine. J. Clin. Toxicol. 2014, 4, 1000186. [Google Scholar] [CrossRef]
- Sahin, A.; Iskender, H.; Terim, K.K.; Altinkaynak, K.; Hayirli, A.; Gonultas, A.; Kaynar, O. The Effect of Humic Acid Substances on the Thyroid Function and Structure in Lead Poisoning. Rev. Bras. Ciênc. Avícola 2016, 18, 649–654. [Google Scholar] [CrossRef]
- Jusadi, D.; Aprilia, T.; Setiawati, M.; Agus Suprayudi, M.; Ekasari, J. Dietary Supplementation of Fulvic Acid for Growth Improvement and Prevention of Heavy Metal Accumulation in Nile Tilapia Fed with Green Mussel. Egypt. J. Aquat. Res. 2020, 46, 295–301. [Google Scholar] [CrossRef]
- Swidsinski, A.; Dörffel, Y.; Loening-Baucke, V.; Gille, C.; Reißhauer, A.; Göktas, O.; Krüger, M.; Neuhaus, J.; Schrödl, W. Impact of Humic Acids on the Colonic Microbiome in Healthy Volunteers. World J. Gastroenterol. 2017, 23, 885. [Google Scholar] [CrossRef]
- Schiefke, I. Humic Acids in Patients with Diarrhoea-Predominant Irritable Bowel Syndrome: Results from A Randomised Controlled Trial. Biomed. J. Sci. Tech. Res. 2021, 33, 25584–25591. [Google Scholar] [CrossRef]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, M.; Dai, W.; Xiang, C.; Li, B.; Jia, Q. Antidiarrhoeal Mechanism Study of Fulvic Acids Based on Molecular Weight Fractionation. Fitoterapia 2019, 137, 104270. [Google Scholar] [CrossRef]
- Yasar, S.; Gokcimen, A.; Altuntas, I.; Yonden, Z.; Petekkaya, E. Performance and Ileal Histomorphology of Rats Treated with Humic Acid Preparations*. J. Anim. Physiol. Anim. Nutr. 2002, 86, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Snyman, J.R.; Dekker, J.; Malfeld, S.C.K.; Van Rensburg, C.E.J. Pilot Study to Evaluate the Safety and Therapeutic Efficacy of Topical Oxifulvic Acid in Atopic Volunteers. Drug Dev. Res. 2002, 57, 40–43. [Google Scholar] [CrossRef]
- Motojima, H.; Yamada, P.; Han, J.; Ozaki, M.; Shigemori, H.; Isoda, H. Properties of Fulvic Acid Extracted from Excess Sludge and Its Inhibiting Effect on β-Hexosaminidase Release. Biosci. Biotechnol. Biochem. 2009, 73, 2210–2216. [Google Scholar] [CrossRef]
- Narayanan, V.; Kharkar, R. Fulvic acid—A natural and multifaceted approach to the management of inflammatory dermatosis. Indian Pract. 2019, 72, 28–31. [Google Scholar]
- Ozkan, A.; Sen, H.M.; Sehitoglu, I.; Alacam, H.; Guven, M.; Aras, A.B.; Akman, T.; Silan, C.; Cosar, M.; Karaman, H.I.O. Neuroprotective Effect of Humic Acid on Focal Cerebral Ischemia Injury: An Experimental Study in Rats. Inflammation 2015, 38, 32–39. [Google Scholar] [CrossRef]
- Klöcking, H.-P.; Klöcking, R. Studies on the Bimodal Effect of Humic Substances in the Blood Clotting System. In Proceedings of the 14th International Peat Congress, Stockholm, Sweden, 3–8 June 2012. [Google Scholar]
- Kübler, H. Differential Inhibitory Effects of Humic Acids on Coagulation Systems of Human Blood. In Humic Substances in the Aquatic and Terrestrial Environment; Allard, B., Borén, H., Grimvall, A., Eds.; Lecture Notes in Earth Sciences; Springer: Berlin/Heidelberg, Germany, 1991; Volume 33, pp. 429–436. ISBN 978-3-540-53702-1. [Google Scholar]
- Kihara, Y.; Yustiawati; Tanaka, M.; Gumiri, S.; Ardianor; Hosokawa, T.; Tanaka, S.; Saito, T.; Kurasaki, M. Mechanism of the Toxicity Induced by Natural Humic Acid on Human Vascular Endothelial Cells: Mechanism of the Toxicity of Humic Acid. Environ. Toxicol. 2014, 29, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-H.; Lu, F.-J.; Hung, H.-C.; Liu, G.-Y.; Lai, T.-J.; Lin, C.-L. Humic Acid Increases Amyloid β-Induced Cytotoxicity by Induction of ER Stress in Human SK-N-MC Neuronal Cells. Int. J. Mol. Sci. 2015, 16, 10426–10442. [Google Scholar] [CrossRef]
- Yang, H.; Huang, P.; Chen, S.; Cho, H.; Kumar, K.J.S.; Lu, F.; Chen, C.; Chang, C.; Hseu, Y. Induction of Macrophage Cell-cycle Arrest and Apoptosis by Humic Acid. Environ. Mol. Mutagen. 2014, 55, 741–750. [Google Scholar] [CrossRef]
- Mirza, M.A. Future of Humic Substances as Pharmaceutical Excipient. Pharm. Sci. 2018, 1, 180004. [Google Scholar]
- Mirza, M.A.; Agarwal, S.P.; Iqbal, Z. Effect of Fulvic Acid on Oral Delivery of Carbamazepine. Sci. Adv. Mater. 2011, 3, 223–232. [Google Scholar] [CrossRef]
- Khan, R.; Jain, P.; Zakir, F.; Aqil, M.; Alshehri, S.; Mirza, M.A.; Iqbal, Z. Quality and In Vivo Assessment of a Fulvic Acid Complex: A Validation Study. Sci. Pharm. 2022, 90, 33. [Google Scholar] [CrossRef]
- Khan, R.; Jain, P.; Aqil, M.; Agarwal, S.P.; Mirza, M.A.; Iqbal, Z. Pharmacokinetic Evaluation of Fulvic Acid-Ketoconazole Complexes: A Validation and Line Extension Study. J. Drug Deliv. Sci. Technol. 2020, 55, 101469. [Google Scholar] [CrossRef]
- Mirza, M.A.; Talegaonkar, S.; Ahmad, F.J.; Iqbal, Z. A Novel and Multifunctional Excipient for Vaginal Drug Delivery. Int. J. Pharm. Excip. 2016, 2, 98–112. [Google Scholar]
- Anwer, M.K.; Jamil, S.; Ahmad, M.; Ansari, M.N.; Khan, T.H. Inclusion Complex of Solid State Aspirin with Fulvic Acid: Dissolution, Permeability, Stability and Preliminary Pharmacological Studies. J. Biol. Sci. 2013, 13, 302–312. [Google Scholar] [CrossRef][Green Version]
- Khan, R.; Mirza, M.A.; Aqil, M.; Hassan, N.; Zakir, F.; Ansari, M.J.; Iqbal, Z. A Pharmaco-Technical Investigation of Thymoquinone and Peat-Sourced Fulvic Acid Nanoemulgel: A Combination Therapy. Gels 2022, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Konnova, M.A.; Volkov, A.A.; Solovyeva, A.G.; Peretyagin, P.V.; Melnikova, N.B. Anti-Inflammatory Property Establishment of Fulvic Acid Transdermal Patch in Animal Model. Sci. Pharm. 2023, 91, 45. [Google Scholar] [CrossRef]
- Savy, D.; Di Meo, V.; Verrillo, M.; Cangemi, S.; Cozzolino, V.; Piccolo, A. Novel Nanomaterials Made of Humic Substances from Green Composts and Chitosan Exerting Antibacterial Activity. ACS Sustain. Chem. Eng. 2023, 11, 9674–9683. [Google Scholar] [CrossRef]
- Acti Humic Acid. Available online: https://herbamedicabg.com/en/acti-humic-acid-350-mg-60-veggie-capsules (accessed on 11 September 2025).
- Dr. Mercola, Humic Fulvic Acid Complex. Available online: https://www.amazon.com/dr-mercola-servings-supplement-digestion/dp/b0cs1bf671 (accessed on 11 September 2025).
- Humuszuur. Available online: https://www.mattisson.nl/vegan-humuszuur-500-ml (accessed on 11 September 2025).
- Humigold. Available online: https://atavah.com/products/humigold (accessed on 11 September 2025).
- Humac Organic. Available online: https://www.humac-organic.ie/product-page/humac-nativ-test (accessed on 11 September 2025).
- Huminiqum. Available online: https://huminiqum.net (accessed on 11 September 2025).
- Fulvine Zuur. Available online: https://www.mattisson.nl/gefermenteerd-fulvine-zuur (accessed on 11 September 2025).
- Shilajit, FA. Available online: https://www.vegavero.com/products/shilajit-extract-capsules (accessed on 11 September 2025).
- Markova, R.M.; Tzotcheva, I.S.; Perenovska, P.; Mangarov, A.; Nikolaeva-Glomb, L.; Hadjiev, V. Efficacy and Safety of Aviron Rapid® in Adolescents and Children with Viral Acute Upper Respiratory Tract Infection: A Multi-Center, Randomized, Double Blind, Placebo-Controlled Clinical Trial. Folia Med. 2023, 65, 546–568. [Google Scholar] [CrossRef] [PubMed]
- Activomin. Available online: https://www.allergosan.com/de/produkte/activomin (accessed on 11 September 2025).
- Omni-Logic Humin. Available online: https://shop.omni-biotic.com/de-de/products/humin-at_de-99350?srsltid=afmboooerexmx1jj2oqpax6knrikf9ngzjy8jkclnq1i9fwnapfz20nl (accessed on 11 September 2025).
- Humic Acid Complex Sprej 50 mL. Available online: https://advancedhealth.cz/advanced-health-humic-acid-complex-sprej-50-ml (accessed on 11 September 2025).
- Pure Fulvic Facial Spray. Available online: https://humicsolution.eu/product/purefulvic-facial-spray-50-ml (accessed on 11 September 2025).
- Fulvic Mist. Available online: https://victoriahealth.com/fulvic-mist/?srsltid=afmboophk9wfhpiq8toz0v2dreystxgj9-vfazt0shx-wxd_hiv9okth (accessed on 11 September 2025).
- Organic-Minerals-Digestion-Hydration-Supplement. Available online: https://www.amazon.com/organic-minerals-digestion-hydration-supplement/dp/b0bl95bj7p (accessed on 11 September 2025).
- Jiang, L.; Zhu, J.; Wang, H.; Fu, Q.; Hu, H.; Huang, Q. Spatial Variability of the Molecular Composition of Humic Acids from Subtropical Forest Soils. J. Soils Sediments 2021, 21, 766–774. [Google Scholar] [CrossRef]
- Van Rensburg, C.E.J. The Antiinflammatory Properties of Humic Substances: A Mini Review. Phytother. Res. 2015, 29, 791–795. [Google Scholar] [CrossRef] [PubMed]




| Parameters | Alkaline Extraction of HS from Lignite Wastes | Alkaline Extraction of HS from Peat | Alkaline Extraction of HS from Corn Straw Compost |
|---|---|---|---|
| Alkaline solvent | NaOH | KOH | KOH |
| Temperature | 85 °C | 25 ± 2 °C | 20 °C |
| Agitating speed | 850 rpm | 300 rpm | - |
| Time | 4 h | 4 h | 24 h |
| Source | Sarlaki et al. (2019) [8] | Saito and Seckler (2014) [9] | Chi et al. (2023) [10] |
| Physicochemical Characteristics | Humic Acid | Fulvic Acid |
|---|---|---|
| Colour | Brown-black | Yellow-brown |
| Molecular size Solubility Functional groups | 10,000 Da–1,000,000 Da Insoluble < pH 2 Different proportion of carboxyl, phenolic hydroxyl, alcoholic hydroxyl, and carbonyl groups | 1000 Da–10,000 Da Water soluble in all pH Different proportion of carboxyl, phenolic hydroxyl, alcoholic hydroxyl, and carbonyl groups |
| Name/ Active Ingredient | Dosage Form | Activities | Manufacturer |
|---|---|---|---|
| Acti Humic Acid/ Membrane active HA | Veggie capsules 350 mg | Functions as an immunomodulator; Helps eliminate harmful metals from the body; Regulates oxidative stress levels; | Herbamedica Bulgaria [110] |
| Dr. Mercola, Humic Fulvic Acid Complex/ HA and FA | Capsules 90 mg HA and 33 mg FA | Promotes gut health; Enhances energy levels; Neutralizes free radicals; Aids in detoxification | Dr. Mercola USA [111] |
| Humuszuur/ HA, FA and HM | Suspension content 5% HS | Provides antioxidant protection to guard cells from oxidative stress, damage, and premature aging; Strengthens the immune system, helping prevent colds and viral infections, while alleviating existing symptoms; Helps maintain the body’s acid–base balance; Supports brain function and enhances memory; | Mattisson Healthstyle Netherlands [112] |
| Humigold/ Humic and Fulvic minerals | Natural ionic plant-derived humic and fulvic powder | Can be applied as a paste when mixed with water; Aids in the natural healing of rashes; Helps draw out toxins from insect bites or poison ivy; Supports lymphatic detoxification when applied to underarms; Soothes and nourishes facial skin; Assists in healing acne; | ALIVE India [113] |
| HUMAC® Nativ/ HA and HMs | Capsules 460 mg HA and 180 mg HM | Facilitates toxin absorption and removal; Exhibits anticarcinogenic properties; Demonstrates antioxidant activity; | HUMAC® Slovakia [114] |
| HUMINIQUM/ HA and FA | Syrup 20 mg HA and 48 mg FA | Provides antioxidant effects; Exhibits antiviral properties; Supports hair health and helps reduce hair loss; Assists in managing obesity and fatigue; Enhances enzyme activity; Acts against anemia; Promotes recovery and regeneration after illnesses; Supports bone health and may help prevent osteoporosis; | HYMATO PRODUCTS Kft.—Hungary [115] |
| Fulvinezuur/ fermented FA | Suspension FA 6% | Supports normal liver function; Promotes healthy excretory system activity | Mattisson Healthstyle Netherlands [116] |
| Shilajit mumijo/ FA | Capsules 250 mg FA | Helps maintain normal blood sugar levels and lipid profile; Acts as a powerful adaptogen; Supports tissue regeneration; Exhibits anti-inflammatory and regenerative properties; | Vegavero International GmbH Germany [117] |
| Aviron rapid/ HA | Tablets 250 mg HA | Promotes respiratory system health and function; Supports the immune system; Maintains overall respiratory tract wellness; | Neopharm Bulgaria [118] |
| ACTIVOMIN/ HA | Capsules 400 mg natural HA | Helps relieve digestive issues, including gas and bloating; Supports regular bowel movements and alleviates constipation or diarrhea; Assists in recovery from food poisoning; | INSTITUT ALLERGOSAN Deutschland (privat) GmbH—Germany [119] |
| OMNi-LOGiC®HUMIN/ HA of type WH67® | Capsules 400 mg HA | Binds pollutants in the intestine and promotes their excretion through stool; Protects the intestinal mucosa; Soothes irritated nerve endings in the gut; | INSTITUT ALLERGOSAN Deutschland (privat) GmbH—Germany [120] |
| Humic Acid 50 mL Spray/HA | Spray 1 dose (6 injections, 1 mL): HA: 60 mg | Supports digestive health: reflux, gastritis, Helicobacter pylori, histamine and food intolerance; Supports immune and respiratory health: allergies, asthma; Supports skin health: shingles, psoriasis, eczema, atopic eczema, acne; Supports metabolic and systemic health: diabetes, arthritis, chronic fatigue; Helps reduce inflammation | ADVANCED HEALTH SK—Slovakia [121] |
| PURE&FULVIC Facial spray/FA | skin spray | Restores cellular balance; Provides a calming and soothing effect; Promotes purification and detoxification; | Humic Solution EU [122] |
| Fulvic Mist/ FA | Hair, skin and nail spray | Nourishes hair follicles; Reduces hair thinning and shedding; Alleviates scalp inflammation; Promotes hair growth; Removes harmful toxins to support keratin production and hair renewal; | Ful.Vic.Health UK [123] |
| Organic Fulvic Acid + 72 Trace Minerals/ FA and HA | Drops FA & HA 22 mg | Enhances mineral and nutrient transport, improving the bioavailability of vitamins and minerals; Supports cellular energy production, overall energy levels, and mental alertness; Acts as a natural free-radical scavenger and binds heavy metals and toxins, aiding detoxification; Promotes gut microbiome balance by supporting beneficial bacteria growth; Supports digestion, reduces bloating, and assists nutrient breakdown; Strengthens the body’s response to pathogens and exhibits antimicrobial properties | The Healthy Life 4 ME USA [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gvozdeva, Y.; Peneva, P.; Katsarov, P. Biomedical Applications of Humic Substances: From Natural Biopolymers to Therapeutic Agents. Antioxidants 2025, 14, 1139. https://doi.org/10.3390/antiox14091139
Gvozdeva Y, Peneva P, Katsarov P. Biomedical Applications of Humic Substances: From Natural Biopolymers to Therapeutic Agents. Antioxidants. 2025; 14(9):1139. https://doi.org/10.3390/antiox14091139
Chicago/Turabian StyleGvozdeva, Yana, Petya Peneva, and Plamen Katsarov. 2025. "Biomedical Applications of Humic Substances: From Natural Biopolymers to Therapeutic Agents" Antioxidants 14, no. 9: 1139. https://doi.org/10.3390/antiox14091139
APA StyleGvozdeva, Y., Peneva, P., & Katsarov, P. (2025). Biomedical Applications of Humic Substances: From Natural Biopolymers to Therapeutic Agents. Antioxidants, 14(9), 1139. https://doi.org/10.3390/antiox14091139









