Abstract
Genomic instability markers are important hallmarks of aging, as previously evidenced within the European study of biomarkers of human aging, MARK-AGE; however, establishing the specific metabolic determinants of vascular aging is challenging. The objective of the present study was to evaluate the impact of the susceptibility to oxidation of serum LDL particles (LDLox) and the plasma metabolization products of nitric oxide (NOx) on relevant genomic instability markers. The analysis was performed on a MARK-AGE cohort of 1326 subjects (635 men and 691 women, 35–75 years old) randomly recruited from the general population. The Inverse Probability of Treatment Weighting causal inference algorithm was implemented in order to assess the potential causal relationship between the LDLox and NOx octile-based thresholds and three genomic instability markers measured in mononuclear leukocytes: the percentage of telomeres shorter than 3 kb, the initial DNA integrity, and the DNA damage after irradiation with 3.8 Gy. The results showed statistically significant telomere shortening for LDLox, while NOx yielded a significant impact on DNA integrity. Overall, the effect on the genomic instability markers was higher than for the confirmed vascular aging determinants, such as low HDL cholesterol levels, indicating a meaningful impact even for small changes in LDLox and NOx values.
1. Introduction
Establishing the specific biomarkers of vascular endothelium function is important for the complex evaluation of biological age in humans. According to Sir William Osler (1891), “Longevity is a vascular question which has been well expressed in the axiom that man is only as old as his arteries” [1]. This old axiom has been widely confirmed by epidemiological and observational studies establishing that vasculature aging is a complex process that in a large measure reflects the overall aging of the individual [2,3]. Although aging is a major risk factor for the incidence and progression of a wide range of pathologies, drawing a clear line between physiological and pathological aging using cellular and systemic specific biomarkers is still challenging. In this regard, at the systemic level, LDL oxidizability (LDLox) and nitric oxide metabolic pathway products (NOx) are currently recognized as representative biomarkers of oxidative stress, inflammation, and endothelial dysfunction, and are being assessed within the EU project MARK-AGE as candidate biomarkers of aging and cardiometabolic disease risk [4,5].
At the cellular level, it has been demonstrated that DNA repair capacity and the maintenance of genomic stability are both intimately linked to the aging process; therefore, relevant specific biomarkers were included in the MARK-AGE study [6]. Several older and more recent studies prove that telomere length, usually measured from the white blood cells, can be considered a marker of aging and of general health status [3,7,8,9,10]. Telomere shortening, which occurs due to the end-replication problem and reduced telomerase activity, is an important predictor of biological aging and can be accelerated by oxidative DNA damage [8,9]. Furthermore, critically short telomeres modify the immediate cellular response to DNA damage generated by endogenous and exogenous damaging agents, which can reduce DNA integrity [11].
With regard to the relationship between LDL oxidation (and NO metabolization products—NOx) and telomere length or DNA integrity, in vivo oxidized LDL (oxLDL) levels have been shown to be inversely correlated with telomere length in both smokers and non-smokers, after adjusting for age and sex [3]. In addition, oxLDL has been shown to induce oxidative DNA damage, while nitric oxide can sensitize the cells to ionizing radiation [12,13]. Nevertheless, no study has specifically analyzed the possible impact of NOx on telomere length, nor has any research evaluated in more detail, within a large population study or by means of multi-variable adjustment or machine learning approaches, the impact of LDL oxidation on these genomic instability markers or on the related DNA damage processes.
The MARK-AGE was an important population study that identified a specific combination of biomarkers that could explain biological age, including telomere length [6]. However, the systemic drivers of cellular telomere shortening and DNA damage through oxidative stress and other specific mechanisms have largely remained unexplored with regard to MARK-AGE data analysis, while machine learning approaches to such data are still scarce or focused rather on general machine learning recommendations or predictive modelling of cardiometabolic risk [5,14].
Therefore, given the scarcity of studies regarding the interrelationship between LDL oxidation, NOx, and telomere shortening or DNA integrity, as well as the complex information collected within the MARK-AGE dataset, the aim of the current research was to estimate the impact of LDL oxidizability and NO metabolization products on telomere length and DNA integrity by means of causal inference approaches.
2. Materials and Methods
2.1. Study Design
This cross-sectional observational study was carried out on a relevant population sample of 1326 subjects, comprising 635 men and 691 women, aged between 35 and 75 years old, and selected from among the participants included in the MARK-AGE group of randomly recruited age- and sex-stratified individuals from the general population (RASIG). Only MARK-AGE subjects with complete data for all studied parameters were included in the present study. Participants from the MARK-AGE cohort were enrolled, through the media, from seven European countries: Austria, Belgium, Finland, Germany, Greece, Italy, and Poland. Subjects who reported seropositivity for HIV or hepatitis (HBV and HCV), whose blood was tested positive for HBV or HCV, or who were being treated for cancer or receiving glucocorticoids were excluded from the study [5,6].
The biological samples (fasting blood) collected from participants were processed and stored within the MARK-AGE consortium, according to rigorous Standard Operating Procedures and quality control measures, as described [5,6]. Briefly, the double-coded blood samples (plasma and serum) were centrally stored in a biobank and distributed to each MARK-AGE partner for the independent measurement of the specific candidate biomarkers. All of the subjects’ clinical and biochemical data obtained from each partner were uploaded to a central database that also contained the demographic and anthropometric data. This phenotypic database could only be accessed and analyzed at the end of the MARK-AGE project [6,14].
The following parameters were selected from the central database and considered for the present analysis: serum glucose and insulin; cholesterol of the serum lipoprotein fractions LDL and HDL; LDL oxidation susceptibility (LDL oxidizability, LDLox); plasma-stable metabolic pathway products of NO (NOx); plasma tocopherols (α-/γ-tocopherol), carotenoids (α-/β-carotene, lycopene), retinol, and vitamin D; plasma iron (Fe), selenium (Se), copper (Cu), and zinc (Zn); DNA integrity and telomere length, assessed in peripheral blood mononuclear cells (PBMCs).
Standard demographic (age, sex) and anthropometric data (height, weight, waist circumference, body mass index, waist-to-hip ratio) were obtained from each participant. The resting systolic and diastolic blood pressures (SBP and DBP, mmHg) were recorded for all subjects, as well as whether there was currently a diagnosed high blood pressure problem. Participants completed a comprehensive questionnaire that included information on self-reported past and present diseases, hormone therapy (women), self-rated health status, and the following lifestyle characteristics: smoking status (never, former, or current smoker); number of years of smoking (smoking years); alcohol and other beverage consumption (whether the subjects never consume beer, wine, juice, or cola beverages); nutritional status (the quantitative consumption of meat, fish, eggs, bread, rice, fruits, vegetables, salty snacks, and sweets); educational background; marital status; information about residence, i.e., house or apartment and whether the subjects live with children, other relatives, or friends [5,6].
2.2. Laboratory Methods
Serum glucose, LDL cholesterol and HDL cholesterol were measured on the clinical auto-analyzer (LX20-Pro, Beckman–Coulter, Woerden, The Netherlands). Insulin was measured with an immuno-analyzer (Access-2, Beckman–Coulter, Woerden, The Netherlands).
Insulin resistance was evaluated using the Homeostasis Model Assessment—Insulin Resistance (HOMA-IR) as surrogate markers, and calculated as follows: [fasting insulin (mU/L) × fasting glucose (mg/dL)]/405 [15].
LDL oxidation susceptibility (LDL oxidizability, LDLox) was assessed in vitro using serum LDL isolated through selective precipitation and the measurement of the specific products of the lipid peroxidation chain reaction, after the exposure of LDL particles to a standard oxidative stress inducer, as previously described [5]. The results were expressed as nmol malondialdehyde (MDA) equivalent content/mL serum. The intra-assay CV was 6.5% and the inter-assay CV was 7.4%.
The total amount of plasma-stable metabolic pathway products of NO [NOx, the sum of nitrites and nitrates (NO2− + NO3−)] was determined using the Griess reagent, following the quantitative conversion of nitrates (NO3−) to nitrites (NO2−) with nitrate reductase (kit 23479, SIGMA). The results were expressed in μmols NOx/L plasma. Intra- and inter-assay CVs were below 7% and 9%, respectively.
Tocopherols (α-/γ-tocopherol), carotenoids (α-/β-carotene, lycopene), and retinol in plasma were simultaneously determined by HPLC and spectrophotometric and fluorescence detection as previously described [16].
Serum vitamin D was measured with an enzyme immunoassay (EIA): 25OHVitD (OCTEIA, AC57F1, IDS, Boldon, UK).
Plasma iron (Fe), selenium (Se), copper (Cu), and zinc (Zn) were determined using a Thermo XII Series ICP-MS (Thermo Electron Corporation, Waltham, MA, USA) according to previously described methods used for the measurement of trace elements in human plasma [17].
The DNA damage and the repair of DNA strand breaks (DNA integrity) were measured using a modified and automated version of the Fluorimetric Detection of Alkaline DNA Unwinding [18]. Cryopreserved isolated peripheral blood mononuclear cells (PBMCs) were immediately irradiated with a 3.8 Gy irradiation dose and incubated at 37 °C for 40 min to allow DNA repair.
The telomere length was determined in isolated PBMCs using an automated high-throughput quantitative fluorescence in situ hybridization (HT Q-FISH) telomere length analysis platform [19]. HT Q-FISH combines the labelling of telomeres in interphase nuclei, using a fluorescent peptide nucleic acid (PNA) probe against telomeric repeats, with automated HT microscopy in 96-well plates. The low detection limit of Q-FISH (<0.1 kb of telomere repeats) allows the quantification of critically short telomeres, the frequency of which is a determinant of telomere dysfunction.
2.3. Statistical Analysis
2.3.1. General Statistical Analysis—Causal Algorithm
The main aim of the statistical analysis was to estimate the impact of candidate vascular aging markers, namely LDLox and NOx, on telomere length and DNA integrity as well-established biomarkers with a critical impact on aging. The analysis was undertaken in Python Programming Language, version 3.9.2 [20]. In order to obtain a less biased effect estimate, a causal inference algorithm was used, namely the Inverse Probability of Treatment Weighting (IPTW) from the DoWhy package, which can take into account imbalanced binary interventions [21].
For each IPTW implementation, specific threshold values were used for LDLox and NOx, as well as for a narrow group of relevant biomarkers for the metabolic profile, cardiometabolic risk, and vascular aging, namely LDL cholesterol and HDL cholesterol, blood pressure (SBP and DBP), and HOMA-IR, respectively, which were considered as “interventions”. The percentage of telomeres shorter than 3 kb, the initial DNA integrity, and the DNA damage after irradiation with 3.8 Gy were the three “continuous outcomes”. The IPTW algorithm estimated the effect of each binary intervention on each continuous outcome [21].
2.3.2. Setting the Thresholds for the Intervention Variables
The thresholds were created based on the information from the updated clinical guidelines for HDL-C, LDL-C, blood pressure, and HOMA-IR [22,23,24,25], while for LDLox and NOx they were set based upon splitting the dataset into octiles (Q12.5; Q25; Q37.5; Q50; Q62.5; Q75; and Q87.5), since no standardized risk values are yet recommended for these two biomarkers. In addition to the seven octiles for LDLox and NOx, seven combined thresholds between each octile of LDLox and NOx were created (Risk_NOx+LDLox_Q12.5; Q25; Q37.5; Q50; Q62.5; Q75; and Q87.5, respectively, representing the concomitant status of LDLox and NOx above the specific octile based thresholds) in order to account for the cumulative impact of LDLox and NOx. Table 1 summarizes the threshold values, as well as the percentual frequency (% of subjects) for each threshold. Therefore, it should be mentioned that the IPTW algorithm was applied for each of the three outcomes and each threshold value: in each case, it estimated the increase (or decrease) in the outcome value when the specific marker value was above the mentioned threshold (or below the threshold, according to the mentioned sign), while also adjusting for specific confounders.

Table 1.
Thresholds for each intervention considered in IPTW algorithm.
2.3.3. Setting the Confounding Variables and Validating the Estimated Effects
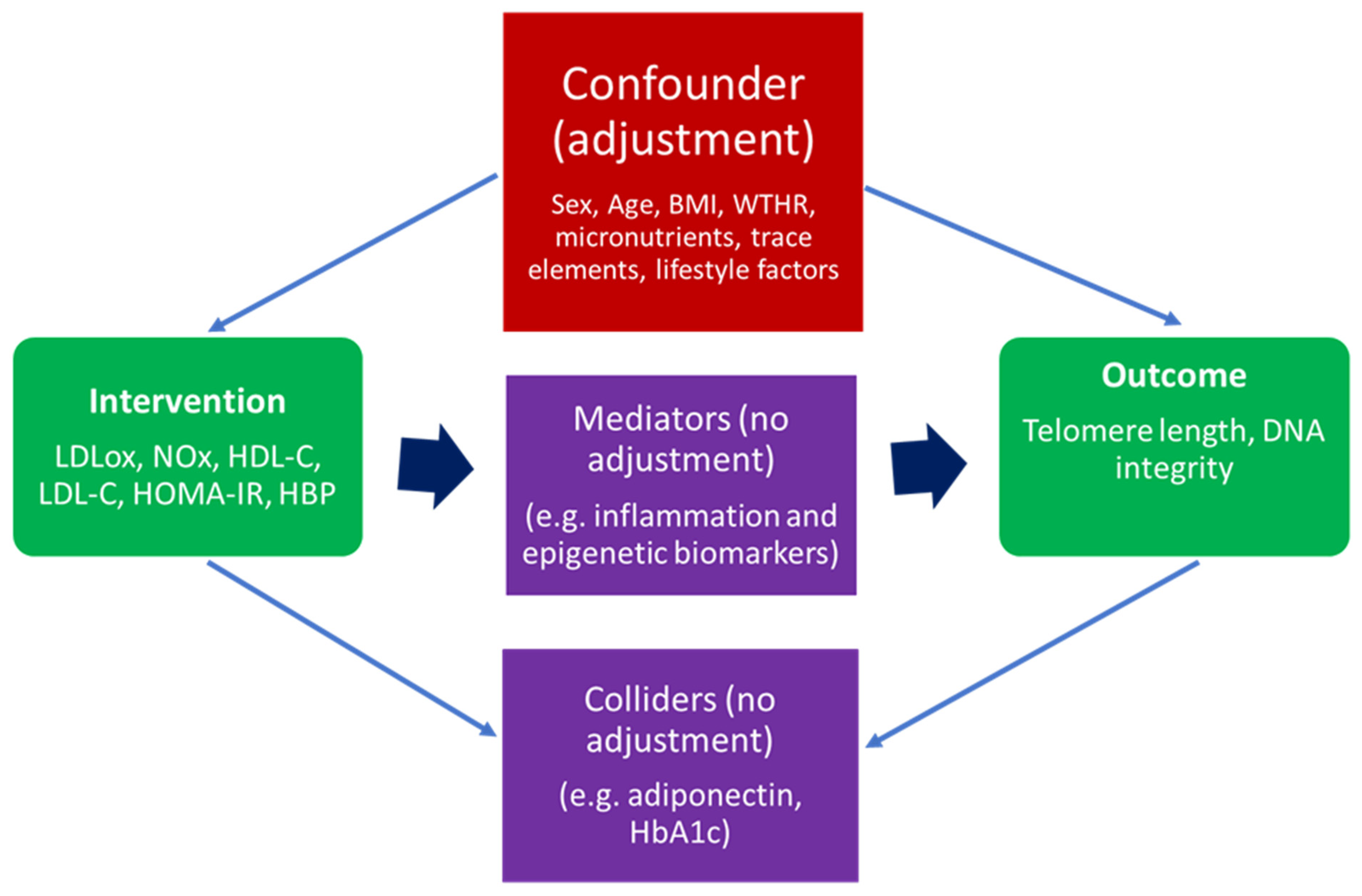
An essential step for the implementation of the IPTW algorithm was the careful selection of confounding variables. A confounder (or common cause) is a variable that influences both the intervention and the outcome. Therefore, when adjusting for specific parameters in such an analysis, reasonable distinctions have to be made between a confounder (which needs to be adjusted for) and a mediator (which lies on the causal path between intervention and outcome) or a collider (a variable influenced by both the intervention and the outcome). Hence, if one makes adjustments based on mediators or colliders, this could introduce bias in the effect estimate [21,26]. For a better understanding of the phenomenon, Figure 1 briefly presents a graphical causal diagram differentiating between confounders, mediators, and colliders.
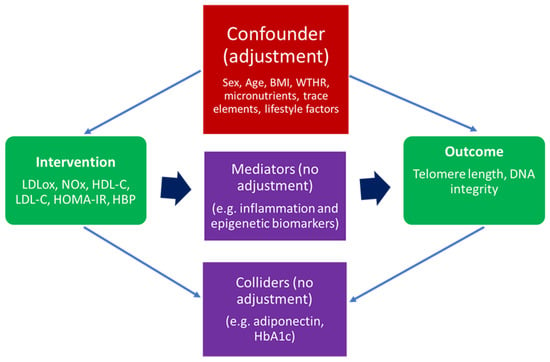
Figure 1.
A general causal diagram representing MARK-AGE parameters included in IPTW algorithm as an intervention, outcome confounder, mediator, and collider. Legend: BMI, body mass index; WTHR, waist-to-hip ratio; LDLox, low-density lipoprotein susceptibility to oxidation; NOx, nitric oxide metabolic pathway products; LDL-C, LDL cholesterol; HDL-C, HDL cholesterol; HOMA-IR, Homeostasis Model Assessment—Insulin Resistance; HbA1C, glycosylated hemoglobin A1C; HBP, high blood pressure.
In the current case, based on the structure of MARK-AGE data, previous studies involving the analysis of MARK-AGE data, and domain knowledge, the chosen confounders were sex, age, BMI, WTHR, relevant plasma levels of vitamins and trace elements, and specific lifestyle factors [4,5,6,27]. Prior to the effect estimation through IPTW, a Factor Analysis of Mixed Data (FAMD) dimensionality reduction technique was applied for an optimized convergence, since both continuous and categorical variables could be found in the list of confounders [6,20]. Table 2 presents the complete list of variables based on which the effect was adjusted.

Table 2.
Complete list of confounding variables for IPTW algorithm.
After applying the IPTW algorithm for all specified cases, the effect estimate was validated (refuted) based on the random common cause method from the DoWhy package, for which a random confounder (common cause) is added and the effect is recalculated with the updated data. Ideally, the estimated effect through random common cause should be identical (or almost the same) to the initial effect (without adding the random common cause), therefore testing the robustness of the estimated effect [21]. After applying the random common cause method, for each case, the percentual variation in the effect estimated through this refutation method was computed based on the formula presented in Equation (1) [20].
where Var(%) = percentual variation in the effect estimated through the random common cause method; Effectinitial = the initial estimated effect, adjusted for the confounders specified in Table 2; and EffectRCC = the estimated effect after adding a random common cause (confounder).
3. Results
Table 3 presents the general characteristics of the most important demographic, biochemical, clinical, and anthropometric parameters of the study population (1326 subjects). Since all parameters followed a non-Gaussian distribution, values are expressed as median (IQR).

Table 3.
General characteristics of study population in terms of most relevant demographic, biochemical, and clinical parameters.
In the first phase, we undertook a preliminary analysis of the age- and sex-related distributions of values, measured for the main studied parameters: LDLox, NOx, % of telomeres shorter than 3 kb (CST), initial DNA integrity (%), and DNA damage after 3.8 Gy (%). The results presented in the Supplementary Material (Figures S1–S10) show a high variability in terms of the parameter values (median, first quartile, third quartile, and outliers) for each sex and each of the four analyzed age decades (35–44, 45–54, 55–64, and 65–74). For example, Figure S1 (LDLox value representation per age decade for females) showed a steady increase in the median values from 35 to 44 until the 55–64 decade, followed by a decrease within the 65–74 decade; there were also more outliers within the 35–44 and 45–54 age decades. On the other hand, Figure S2 (LDLox value representation per age decade for males) highlighted a constant decrease from the first decade (35–44) until the last decade (65–74), with more outliers within the 55–64 and 65–74 groups. The graphical representation of plasma NOx (Figures S3 and S4) showed similar patterns, with the notable exception of the male sex, for which the median values were very similar for all four decades and the outliers were also more evenly distributed, even though the interquartile range and the number of outliers for the 65–74 group were lower than for the other three age groups. On the other hand, less standard patterns were observed for the graphical representations of the three outcomes from the IPTW algorithm (Figures S5 and S6—% of telomeres shorter than 3 kb; Figures S7 and S8—initial DNA integrity; Figures S9 and S10—DNA damage after 3.8 Gy), even though there was a constant high number of outliers for all age decades for the % of telomeres shorter than 3 kb, no outliers for the initial DNA integrity, and only a few outliers for the DNA damage after 3.8 Gy.
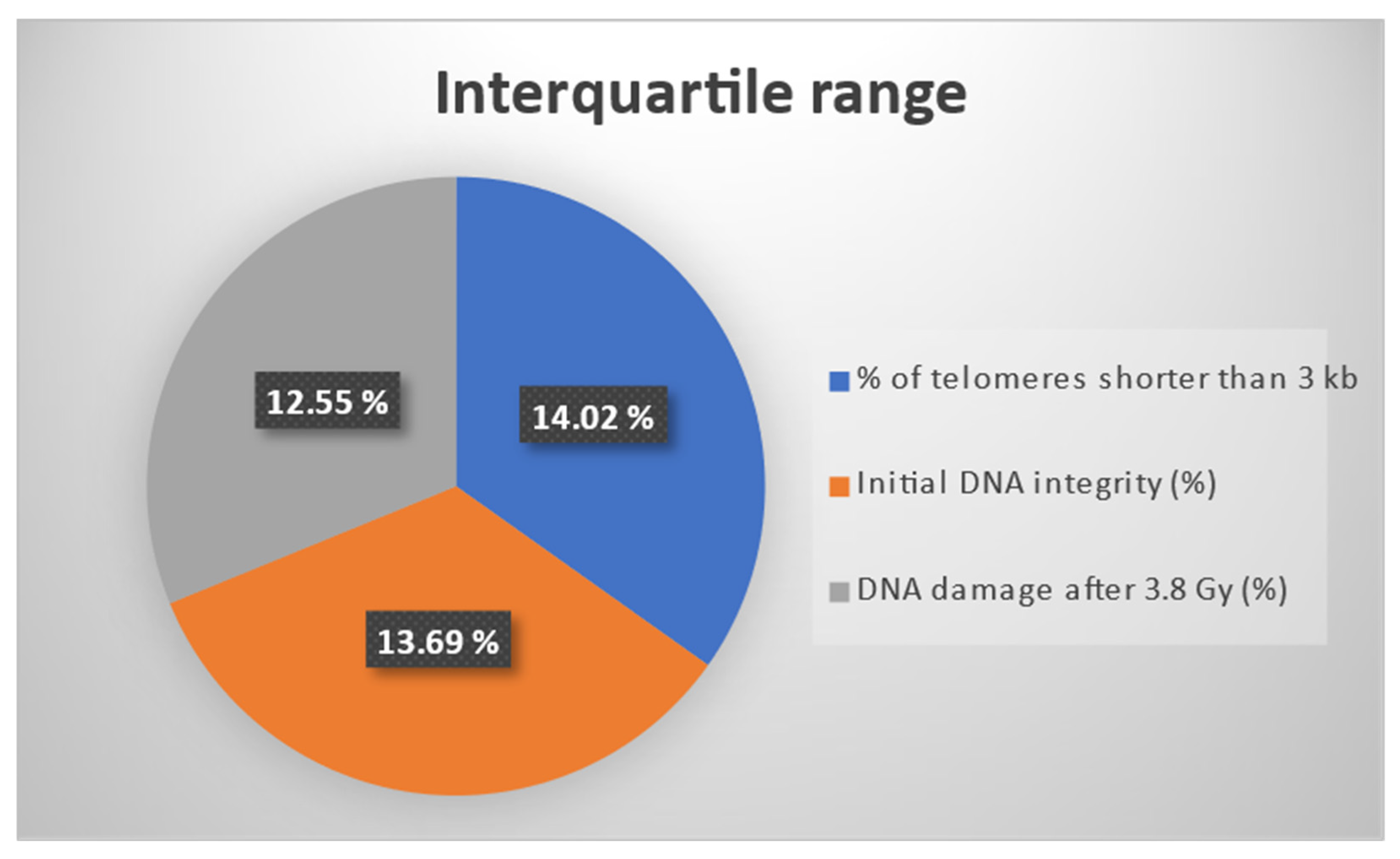
For an enhanced visualization of the three outcomes considered for the effect estimation through the IPTW algorithm, Figure 2 presents the interquartile ranges (IQRs) for the three biomarkers: % of telomeres shorter than 3 kb, initial DNA integrity, and DNA damage after 3.8 Gy. It should be mentioned that all three markers were percentage-type data. The IQR was computed as the difference between the third and the first quartile. It should be noted that the three IQRs had similar values.
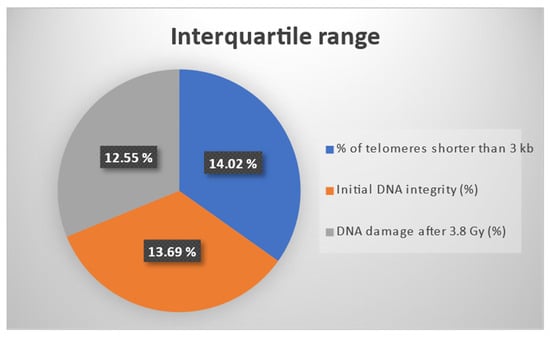
Figure 2.
Interquartile ranges for three percentage type outcomes: % of telomeres shorter than 3 kb, initial DNA integrity, and DNA damage after 3.8 Gy.
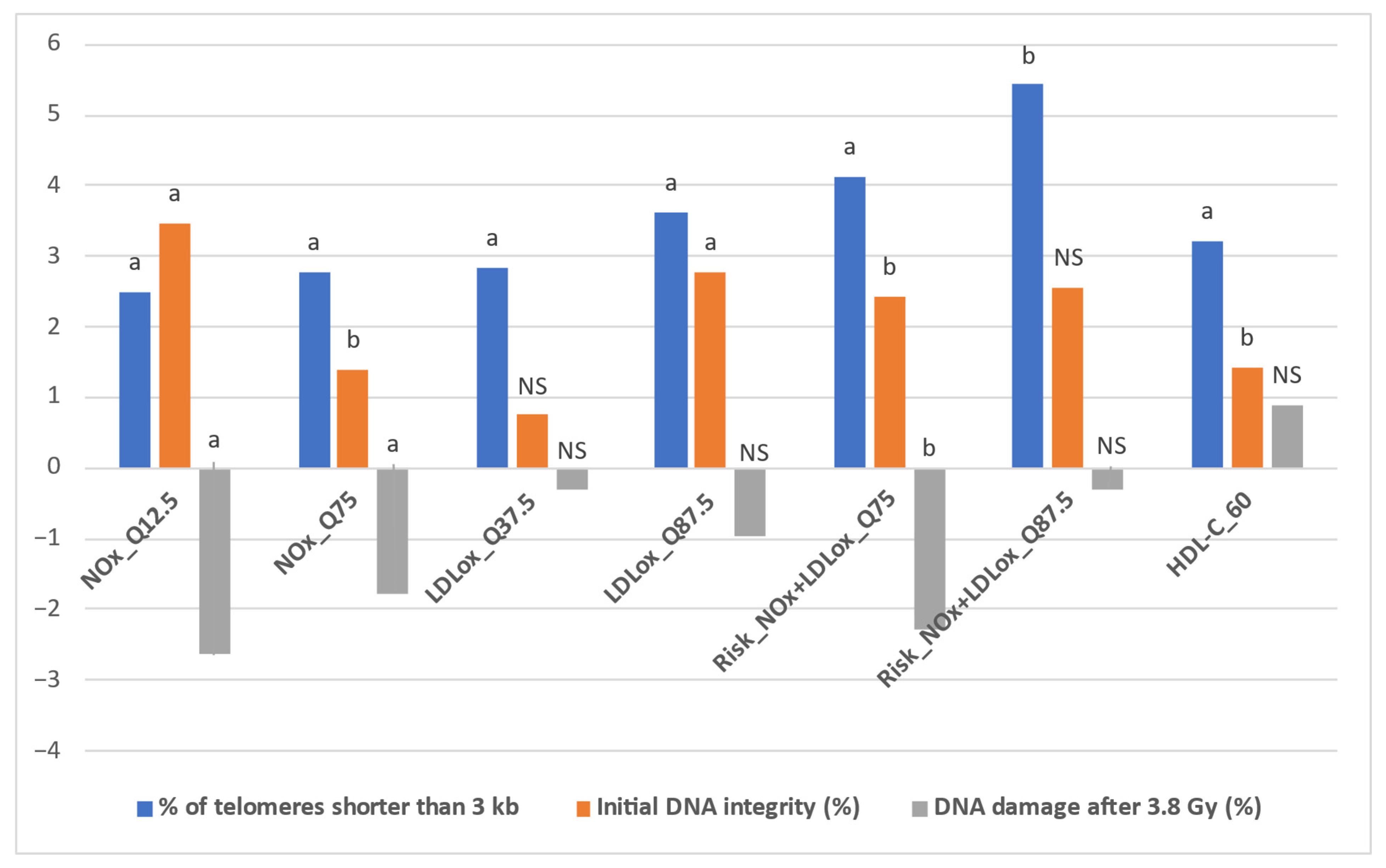
Table 4 presents the detailed results obtained after implementing the IPTW algorithm for all intervention thresholds and outcomes (estimated effect—the impact of the specific status, defined in Table 1, on each outcome, along with the p value for statistical significance, set at p < 0.05). In addition, Figure 3 depicts the representative situations for which the IPTW algorithm yielded relevant results. These results were obtained through the random common cause method, along with the percentual increase or decrease when compared to the initial effects. In this respect, Table S1 (Supplementary Material) presents the estimated effects of the intervention thresholds on the three outcomes obtained after implementing the IPTW algorithm with the addition of a random confounding variable to the initial confounder list.

Table 4.
Estimated effects of intervention thresholds on three outcomes, obtained after implementing IPTW algorithm.
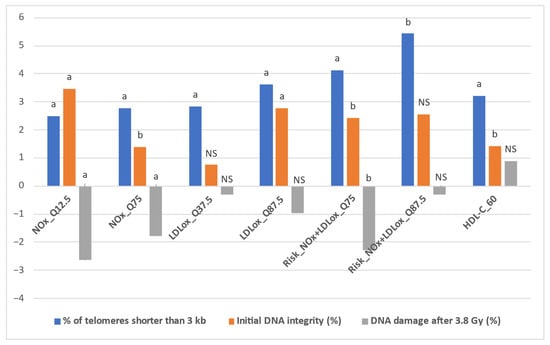
Figure 3.
Representative situations for IPTW comparatively estimated effects. Statistical significance: a p < 0.01; b p < 0.05; NS = non-significant.
First, when comparatively assessing the impact of LDLox and NOx thresholds on the % of telomeres shorter than 3 kb, NOx achieved an increase of over 2% in only two situations (2.492% for NOx_Q12.5 and 2.786% for NOx_Q75, both statistically significant, p < 0.01), while for LDLox a total of five such situations were identified (LDLox_Q12.5: 2.364%; LDLox_Q25: 2.45%; LDLox_Q37.5: 2.833%; LDLox_Q50: 2.705%; LDLox_Q87.5: 3.624%, all statistically significant, p < 0.02). However, when combining the LDLox and NOx thresholds, the increases in the % of telomeres shorter than 3 kb were more pronounced. All seven combined thresholds (based on the seven octiles: Q12.5, Q25, Q37.5, Q50, Q62.5, and Q87.5) led to a statistically significant increase of at least 2%, with a minimum of 2.335% for Risk_NOx + LDLox_Q62.5 (combined threshold—concomitant status of NOx over the Q62.5 octile of NOx and LDLox over the Q62.5 octile of LDLox), while the highest values were obtained for Risk_NOx + LDLox_Q75 (4.132%, p = 0.002) and Risk_NOx + LDLox_Q87.5 (5.434%, p = 0.03) (Table 4).
In terms of validation of the estimated effects (Table S1, Supplementary Material), robust values were obtained, with an absolute percentual variation in the effect lower than 2% in all situations where statistical significance was reached for the initial effect.
It is also relevant to display the age-related analysis of telomere shortening and DNA damage response (DDR) in association with LDLox and NOx. Table 5 highlights the relationships between the individual values of LDLox, NOx, and the three genomic instability markers, calculated per chronological age decade, in the MARK-AGE sample population (age range 35–75 years old). In addition, for a broader highlight of the associations, Table 6 shows the Spearman’s correlation coefficients between the individual values of LDLox, NOx and the three genomic instability markers on the entire cohort of 1326 individuals.

Table 5.
Spearman’s correlation coefficients (rho) between continuous values of LDLox, NOx, and three genomic instability markers, computed per chronological age decade, in RASIG participants.

Table 6.
Spearman’s correlation coefficients (rho) between continuous values of LDLox, NOx, and three genomic instability markers, computed on entire cohort of 1326 subjects, in RASIG participants.
As a general feature, the correlation coefficients displayed a similar pattern to that obtained within the causality analysis. An interesting observation was that there are more statistically significant associations in the case of younger subjects (35–44 years old). Moreover, in elderly subjects (65–74 years old), only plasma NOx was significantly positively correlated with the PBMC’s DNA integrity and inversely correlated with DNA damage response after irradiation. In the age decade 65–74 years, a significant but weak association was identified between LDLox and DNA integrity. The pattern was similar on the entire RASIG cohort, with small, positive, but significant interrelations between serum LDLox and the percentage of CST, as well as between plasma NOx and initial DNA integrity, while NOx levels were inversely correlated with the DNA damage response after irradiation.
4. Discussion
In a previous study conducted on the MARK-AGE sample population (age range 40–75 years old), we established that LDL susceptibility to oxidation (LDLox) is a valuable potential biomarker indicating the cardiometabolic risk associated with aging. In this respect, combined ratio- and threshold-based markers between LDLox and HDL cholesterol had a higher predictive value than individual HDL cholesterol values and thresholds in several situations [5]. Therefore, in the present study we focused on researching the possible specific causal effects of LDLox and metabolization products of nitric oxide (NOx), measured in plasma samples, on well-acknowledged genomic instability markers associated with aging, as assessed in peripheral blood mononuclear cells (PBMCs).
Causal inference approaches are more complex to implement when compared to the predictive analysis of the cardiometabolic risk outcomes, as the main aim of the causal inference is to estimate, with a low risk of bias, the impact (effect) of a treatment (intervention—in our case, LDLox and NOx) on an outcome (in our case, the three genomic instability markers: the % of critically short telomeres (CST), the initial DNA integrity and the DNA damage after irradiation). Taking into account the accurate and standardized methodology based on which the Inverse Probability of Treatment Weighting (IPTW) algorithm was applied, it is reasonable to state that, for the specific LDLox and NOx thresholds for which the impact on the genomic instability markers was statistically significant, the estimated effect was robust to unobserved confounders [6,21].
Overall, in the current research, we found significant causal relationships between LDLox levels (the degree of LDL damage susceptibility to induced oxidative stress) and the % of CST (LDLox increased the % of CST for all studied LDLox levels/thresholds). Conversely, almost all NOx levels (thresholds) were found to have a protective effect on both DNA markers, increasing the initial DNA integrity and decreasing the DNA damage after irradiation.
Recent research emphasizes that age, sex, and the complex interrelations between lifestyle factors have a significant impact on telomere length and, therefore, are also able to influence the percentage of critically short telomeres [28]. Hence, the specific observed patterns resulting from the age- and sex-related value distributions of the studied biomarkers seem to validate the reasoning behind choosing specific octile-based thresholds for LDLox and NOx while maintaining continuous values for the three outcomes (% of CST, initial DNA integrity, and DNA damage after 3.8 Gy), since LDLox and NOx showed clearer increases and decreases in terms of median values and interquartile ranges, reflecting the data dispersion per age decade.
In terms of the results obtained by applying the causal inference algorithm, it should be noted that different results were obtained for LDLox and NOx when comparing their impact on the three genomic instability markers.
The lower impact of NOx on telomere shortening when compared to LDLox might be explained by the fact that nitric oxide may have a beneficial effect in specific conditions, stimulating telomerases and delaying endothelial cell senescence [29], while in other situations it might contribute to telomere shortening and DNA damage through the production of reactive oxygen species, which could also create a favorable environment for LDL oxidation [4,30,31]. Indeed, after adjusting for age and sex, the study conducted by Nawrot et al. showed that in vivo circulating oxidized LDL (oxLDL) has an inverse relationship with telomere length [3]. Nevertheless, Hong et al. reported no involvement of nitric oxide in the modulation of telomerase activity, even though the research was not performed in vivo [32]. In addition, regarding the impact of high blood pressure, low HDL cholesterol (HDL-C), high LDL cholesterol (LDL-C), and HOMA-IR, it is worth mentioning that in the current study the only threshold for which statistical significance was reached in terms of impact on telomere length was the status of HDL-C < 60 mg/dL (HDL-C_60), with a percentual increase of 3.214 (p = 0.001), even though the effect was smaller than the highest values obtained for the LDLox-based threshold and the combined thresholds based on NOx and LDLox octiles. Indeed, in several studies there was a positive correlation between HDL cholesterol values and leukocyte telomere length; however, they were not undertaken on European cohorts [33,34]. Nevertheless, the vast majority of the studies, whether examining the relationship between nitric oxide, oxidized LDL, or HDL cholesterol and telomerase activity or telomere length, failed to adjust for a significant number of confounders [3,33,34]. Therefore, the novelty of the present study is the fact that we managed, due to the standardized approach employed during the MARK-AGE data collection process, to implement an adjusted analysis of the interventions and outcomes based on a significant number of relevant common causes.
Secondly, the results obtained after estimating the impact of LDLox- and NOx-based thresholds on initial DNA integrity and radiation-induced DNA damage, it is worth mentioning that NOx yielded a significantly higher effect when compared to LDLox. With regard to this, the impact of NOx on initial DNA integrity yielded significant increases in almost all situations excepting the highest threshold of NOx, thus suggesting a more pronounced protective effect for moderate values of plasma NOx. On the other hand, the impact of LDL oxidation on initial DNA integrity only reached statistical significance in the case of high levels of LDLox, with the highest effect being obtained for the maximum octile. The combined thresholds of NOx and LDLox octiles reached statistically significant intermediate values between the ones obtained for LDLox and NOx, with a maximum for Risk_NOx + LDLox_Q25. The status of HDL cholesterol under 60 mg/dl and the status of LDL cholesterol over 100 mg/dL were the only traditional vascular aging markers where statistical significance was reached in terms of an impact on a significant increase in initial DNA integrity (HDL-C_60) and a significant decrease in initial DNA integrity (LDL-C_100), even though the impact was smaller than for LDLox, NOx, or the combined LDLox- and NOx-based thresholds.
Nevertheless, the analysis of the impact of NOx- and LDLox-based thresholds on the DNA damage after 3.8 Gy yielded similar effects when compared to the impact on the initial DNA integrity, suggesting a decreased susceptibility to radiation-induced DNA damage with higher serum values of NOx and LDLox, respectively. Similarly to the impact on initial DNA integrity, NOx-based thresholds reached significantly higher effects in terms of magnitude compared to LDLox-based thresholds. Meanwhile, LDLox-based thresholds yielded lower effects and no threshold based on LDLox yielded statistical significance, while the combined LDLox and NOx thresholds had a similar impact to the ones of NOx. The more pronounced effect of NOx in reducing DNA damage after 3.8 Gy could be explained by the fact that nitric oxide might increase the production of tumor suppressor protein p53, which has the ability to interrupt the cell cycle, facilitating DNA repair mechanisms [35,36]. Nevertheless, in specific conditions, high levels of nitric oxide might exert DNA damage effects through peroxynitrite (ONOO−), the main NO-based oxidant in the majority of situations and a very deleterious free radical. It is acknowledged that the effects of DNA damage significantly depend on the concentration of the pro-oxidant NOx metabolites, the time of the exposure, and on the potential of the cell to trigger specific defense mechanisms against the deleterious impact of nitroxidative stress [37]. Therefore, these aspects might partly explain the fact that, in the current study, plasma NOx had a protective effect on both the initial DNA integrity and after irradiating the DNA with 3.8 Gy. On the other hand, in terms of the effect of LDL oxidation on DNA integrity, a study conducted by Inoue et al. reported that in dyslipidemic subjects oxLDL exerts an immunological response to DNA damage that is independent of serum LDL-C [38], possibly explainable by the mediation of endothelial dysfunction [39].
The present causality analysis is strengthened by the proof that the age-related analysis of telomere shortening and DNA damage response (DDR) in association with LDLox and NOx confirmed the high and exclusive impact of LDLox on telomere shortening, especially in young subjects, and the high and exclusive impact of NOx on DNA integrity and damage response in elderly subjects. With regard to the impact of LDL oxidation on telomeres, the present study confirms the effects reported in previous clinical studies [3]. Experimental studies suggest that the NOx-DDR interrelationship could be mediated (modulated) by cellular signaling pathways relevant to aging, such as SIRT1. Indeed, SIRT1 enhances endothelial nitric oxide synthase (eNOS) expression and NO bioavailability, but NO may also directly modulate SIRT1 expression [40]. To the same extent, it is acknowledged that SRT1 plays a crucial role in the DDR by promoting DNA repair [41].
The main biological outcome of the present study is the clear indication of the impact of LDL oxidation and NO metabolism on genomic instability markers in human subjects, evidenced within a large-scale population study. The novelty is that both parameters (evaluated at the systemic level), namely LDLox and NOx as key players involved in endothelial dysfunction and atherogenesis, were analyzed to explore their influence on cellular DNA. It is acknowledged that, from a physiopathological viewpoint, LDL oxidation and NO could have antagonistic actions within the vascular microenvironment [4]. In this sense, the present study demonstrated that LDLox and NOx also displayed distinct effects on specific genomic instability markers such as telomere length and DNA integrity. Although these DNA-based markers were evaluated in isolated PBMCs, we could extrapolate this situation to the adjacent endothelial cells. Therefore, this approach has special biological relevance by proving that LDLox and NOx exert a significant role on genome integrity, which is of critical importance in vascular cell function/senescence.
Hence, taking into account the results obtained by implementing the IPTW algorithm, one of the main findings of the current study is that the use of a wide range of thresholds could indicate that even small changes in LDLox and NOx values could have a meaningful effect on telomere length and DNA integrity. To our knowledge, this is the first study conducted on a European population that estimates the impact of specific oxidative stress parameters on genomic instability markers by adjusting for a relevant number of blood serum assays and sociodemographic and lifestyle factors. It should be noted that, due to the standardized approach during the MARK-AGE data collection process, we implemented an adjusted analysis based on a significant number of relevant common causes of the interventions and outcomes.
An important strength of the current research is the approach taken to vascular dysfunction/aging by considering multiple DNA-based markers as outcomes within the same analysis. Another element of originality is the fact that, within the MARK-AGE cohort, an in vitro method was employed for the evaluation of the susceptibility to oxidation of LDL (LDLox), which is a biochemical parameter that could more accurately indicate its intrinsic atherogenic properties, as compared to the circulating oxidized LDL (oxLDL) usually measured in vivo [5].
Nevertheless, given the main limitations of the analysis (the relatively small number of subjects, under 1500, despite being comparable to or higher than those in other similar studies [3,33,34,38], the presence of relevant unobserved confounders cannot be completely ruled out [6,21]), future studies must focus on designing randomized studies and enlarging the cohort size, consequently aiming to validate clinically relevant threshold combinations for LDLox and NOx, which could optimally explain vascular dysfunction/aging and other health issues related to telomere shortening and DNA damage.
5. Conclusions
In the present study, we managed to show the specific causal relationships between both the susceptibility to oxidation of LDL and the metabolization products of NO and genomic instability markers, defined as the fraction of critically short telomeres, DNA integrity in normal conditions, and radiation-induced DNA damage, through the use of a method with a low risk of bias on a representative MARK-AGE cohort from the general population. While LDLox showed significant harmful effects on telomeres, the analysis demonstrated protective effects of NOx in terms of native DNA integrity, with an additional reduction in DNA damage after applying a radiation level of 3.8 Gy. All effects were evidenced within a stratified analysis and were higher on average than the effects of traditional cardiometabolic risk/vascular aging markers/determinants such as high blood pressure, high insulin resistance index (HOMA-IR), high LDL cholesterol, and low HDL cholesterol. Therefore, the current study has the potential to open up future research aiming to define and validate the specific risk values of such non-traditional biomarkers and translate their complex role within the intricate mechanisms of vascular aging into a biomedical application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox14080933/s1.
Author Contributions
A.V., D.M., M.M.-V., M.B., E.S., G.M., M.C., N.B., J.B., C.S., O.T., F.D.-C., B.G.-L., B.W., S.F., E.S.G., A.H., E.P.S., A.d.C., M.E.T.D., E.H.J.M.J., E.M., R.G., F.P., M.M., D.W., W.S., T.G., C.F., A.B. and D.G. contributed to the design, data acquisition, and drafting and critically revising the article for important intellectual content. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the European Commission (Project Full Name: European Study to Establish Biomarkers of Human Ageing; Project Acronym: MARK-AGE; Project No. 200880). The current research and publication were undertaken with the support of “Carol Davila” University of Medicine and Pharmacy, Bucharest.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request.
Acknowledgments
The authors thank all study subjects for their willingness to participate, and extend their appreciations to all contributors from the different study centers in the MARK-AGE project. Furthermore, thanks are extended to all the working groups involved, particularly to Claudia Borsa, Cristina Ionescu, and Gianina Constantin, for their experimental support during the research carried out at “Ana Aslan” National Institute of Gerontology and Geriatrics, Bucharest, Romania.
Conflicts of Interest
Author Jürgen Bernhardt and Christiane Schön were employed by the company BioTeSys GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CST | Critically short telomeres |
References
- Kovacic, J.C.; Moreno, P.; Nabel, E.G.; Hachinski, V.; Fuster, V. Cellular Senescence, Vascular Disease, and Aging. Circulation 2011, 123, 1900–1910. [Google Scholar] [CrossRef]
- Dantas, A.P.; Jiménez-Altayó, F.; Vila, E. Vascular Aging: Facts and Factors. Front. Physiol. 2012, 3, 325. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, T.S.; Staessen, J.A.; Holvoet, P.; Struijker-Boudier, H.A.; Schiffers, P.; Van Bortel, L.M.; Fagard, R.H.; Gardner, J.P.; Kimura, M.; Aviv, A. Telomere Length and Its Associations with Oxidized-LDL, Carotid Artery Distensibility and Smoking. Front. Biosci. (Elite. Ed.) 2010, 2, 1164–1168. [Google Scholar] [CrossRef][Green Version]
- Gradinaru, D.; Borsa, C.; Ionescu, C.; Prada, G.I. Oxidized LDL and NO Synthesis—Biomarkers of Endothelial Dysfunction and Ageing. Mech. Ageing Dev. 2015, 151, 101–113. [Google Scholar] [CrossRef]
- Valeanu, A.; Margina, D.; Weber, D.; Stuetz, W.; Moreno-Villanueva, M.; Dollé, M.E.T.; Jansen, E.H.; Gonos, E.S.; Bernhardt, J.; Grubeck-Loebenstein, B.; et al. Development and Validation of Cardiometabolic Risk Predictive Models Based on LDL Oxidation and Candidate Geromarkers from the MARK-AGE Data. Mech. Ageing Dev. 2024, 222, 111987. [Google Scholar] [CrossRef]
- Bürkle, A.; Moreno-Villanueva, M.; Bernhard, J.; Blasco, M.; Zondag, G.; Hoeijmakers, J.H.J.; Toussaint, O.; Grubeck-Loebenstein, B.; Mocchegiani, E.; Collino, S.; et al. MARK-AGE Biomarkers of Ageing. Mech. Ageing Dev. 2015, 151, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Montpetit, A.J.; Alhareeri, A.A.; Montpetit, M.; Starkweather, A.R.; Elmore, L.W.; Filler, K.; Mohanraj, L.; Burton, C.W.; Menzies, V.S.; Lyon, D.E.; et al. Telomere Length. Nurs. Res. 2014, 63, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The Impact of Oxidative DNA Damage and Stress on Telomere Homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 2021, 11, 630186. [Google Scholar] [CrossRef]
- Yu, H.J.; Byun, Y.H.; Park, C.-K. Techniques for Assessing Telomere Length: A Methodological Review. Comput. Struct. Biotechnol. J. 2024, 23, 1489–1498. [Google Scholar] [CrossRef]
- Hewitt, G.; Jurk, D.; Marques, F.D.M.; Correia-Melo, C.; Hardy, T.; Gackowska, A.; Anderson, R.; Taschuk, M.; Mann, J.; Passos, J.F. Telomeres Are Favoured Targets of a Persistent DNA Damage Response in Ageing and Stress-Induced Senescence. Nat. Commun. 2012, 3, 708. [Google Scholar] [CrossRef]
- Thum, T.; Borlak, J. LOX-1 Receptor Blockade Abrogates OxLDL-Induced Oxidative DNA Damage and Prevents Activation of the Transcriptional Repressor Oct-1 in Human Coronary Arterial Endothelium. J. Biol. Chem. 2008, 283, 19456–19464. [Google Scholar] [CrossRef] [PubMed]
- Folkes, L.K.; O’Neill, P. Modification of DNA Damage Mechanisms by Nitric Oxide during Ionizing Radiation. Free Radic. Biol. Med. 2013, 58, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, E.; Remondini, D.; Bacalini, M.G.; Garagnani, P.; Pirazzini, C.; Yani, S.L.; Giuliani, C.; Menichetti, G.; Zironi, I.; Sala, C.; et al. Statistical Strategies and Stochastic Predictive Models for the MARK-AGE Data. Mech. Ageing Dev. 2015, 151, 45–53. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and ?—Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Stuetz, W.; Weber, D.; Dollé, M.E.T.; Jansen, E.; Grubeck-Loebenstein, B.; Fiegl, S.; Toussaint, O.; Bernhardt, J.; Gonos, E.S.; Franceschi, C.; et al. Plasma Carotenoids, Tocopherols, and Retinol in the Age-Stratified (35-74 Years) General Population: A Cross-Sectional Study in Six European Countries. Nutrients 2016, 8, 614. [Google Scholar] [CrossRef]
- Malavolta, M.; Piacenza, F.; Basso, A.; Giacconi, R.; Costarelli, L.; Pierpaoli, S.; Mocchegiani, E. Speciation of Trace Elements in Human Serum by Micro Anion Exchange Chromatography Coupled with Inductively Coupled Plasma Mass Spectrometry. Anal. Biochem. 2012, 421, 16–25. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Eltze, T.; Dressler, D.; Bernhardt, J.; Hirsch, C.; Wick, P.; von Scheven, G.; Lex, K.; Bürkle, A. The Automated FADU-Assay, a Potential High-Throughput in Vitro Method for Early Screening of DNA Breakage. ALTEX 2011, 28, 295–303. [Google Scholar] [CrossRef]
- Canela, A.; Vera, E.; Klatt, P.; Blasco, M.A. High-Throughput Telomere Length Quantification by FISH and Its Application to Human Population Studies. Proc. Natl. Acad. Sci. USA 2007, 104, 5300–5305. [Google Scholar] [CrossRef]
- Python Software Foundation Python Language Reference, Version 3.9.2. Available online: http://www.python.org (accessed on 17 July 2023).
- Sharma, A.; Kiciman, E. DoWhy: An End-to-End Library for Causal Inference. arXiv 2020, arXiv:2011.04216. [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Treatment of High Blood Cholesterol in Adults. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Jiménez-Maldonado, A.; García-Suárez, P.C.; Rentería, I.; Moncada-Jiménez, J.; Plaisance, E.P. Impact of High-Intensity Interval Training and Sprint Interval Training on Peripheral Markers of Glycemic Control in Metabolic Syndrome and Type 2 Diabetes. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165820. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- McEvoy, J.W.; McCarthy, C.P.; Bruno, R.M.; Brouwers, S.; Canavan, M.D.; Ceconi, C.; Christodorescu, R.M.; Daskalopoulou, S.S.; Ferro, C.J.; Gerdts, E.; et al. 2024 ESC Guidelines for the Management of Elevated Blood Pressure and Hypertension. Eur. Heart J. 2024, 45, 3912–4018. [Google Scholar] [CrossRef]
- MacKinnon, D.P.; Lamp, S.J. A Unification of Mediator, Confounder, and Collider Effects. Prev. Sci. 2021, 22, 1185–1193. [Google Scholar] [CrossRef]
- Pinchuk, I.; Kohen, R.; Stuetz, W.; Weber, D.; Franceschi, C.; Capri, M.; Hurme, M.; Grubeck-Loebenstein, B.; Schön, C.; Bernhardt, J.; et al. Do Low Molecular Weight Antioxidants Contribute to the Protection against Oxidative Damage? The Interrelation between Oxidative Stress and Low Molecular Weight Antioxidants Based on Data from the MARK-AGE Study. Arch. Biochem. Biophys. 2021, 713, 109061. [Google Scholar] [CrossRef] [PubMed]
- Moix, S.; Sadler, M.C.; Kutalik, Z.; Auwerx, C. Breaking down Causes, Consequences, and Mediating Effects of Telomere Length Variation on Human Health. Genome Biol. 2024, 25, 125. [Google Scholar] [CrossRef]
- Vasa, M.; Breitschopf, K.; Zeiher, A.M.; Dimmeler, S. Nitric Oxide Activates Telomerase and Delays Endothelial Cell Senescence. Circ. Res. 2000, 87, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Keefer, L.K.; Wink, D.A. DNA Damage and Nitric Oxid. Adv. Exp. Med. Biol. 1996, 387, 177–185. [Google Scholar] [PubMed]
- Compton, S.A.; Elmore, L.W.; Haydu, K.; Jackson-Cook, C.K.; Holt, S.E. Induction of Nitric Oxide Synthase-Dependent Telomere Shortening after Functional Inhibition of Hsp90 in Human Tumor Cells. Mol. Cell Biol. 2006, 26, 1452–1462. [Google Scholar] [CrossRef]
- Hong, Y.; Quintero, M.; Frakich, N.M.; Trivier, E.; Erusalimsky, J.D. Evidence against the Involvement of Nitric Oxide in the Modulation of Telomerase Activity or Replicative Capacity of Human Endothelial Cells. Exp. Gerontol. 2007, 42, 904–910. [Google Scholar] [CrossRef]
- Chen, W.; Gardner, J.P.; Kimura, M.; Brimacombe, M.; Cao, X.; Srinivasan, S.R.; Berenson, G.S.; Aviv, A. Leukocyte Telomere Length Is Associated with HDL Cholesterol Levels: The Bogalusa Heart Study. Atherosclerosis 2009, 205, 620–625. [Google Scholar] [CrossRef]
- Liu, X.; Ma, T.; Yang, C.; Li, J.; Zhang, Y.; Zhao, Y. Persistent Dyslipidemia Increases the Longitudinal Changes in Telomere Length. Lipids Health Dis. 2023, 22, 173. [Google Scholar] [CrossRef]
- Williams, A.B.; Schumacher, B. P53 in the DNA-Damage-Repair Process. Cold Spring Harb. Perspect. Med. 2016, 6, a026070. [Google Scholar] [CrossRef]
- Belenichev, I.; Popazova, O.; Bukhtiyarova, N.; Savchenko, D.; Oksenych, V.; Kamyshnyi, O. Modulating Nitric Oxide: Implications for Cytotoxicity and Cytoprotection. Antioxidants 2024, 13, 504. [Google Scholar] [CrossRef]
- Calcerrada, P.; Peluffo, G.; Radi, R. Nitric Oxide-Derived Oxidants with a Focus on Peroxynitrite: Molecular Targets, Cellular Responses and Therapeutic Implications. Curr. Pharm. Des. 2011, 17, 3905–3932. [Google Scholar] [CrossRef]
- Inoue, T.; Inoue, K.; Maeda, H.; Takayanagi, K.; Morooka, S. Immunological Response to Oxidized LDL Occurs in Association with Oxidative DNA Damage Independently of Serum LDL Concentrations in Dyslipidemic Patients. Clin. Chim. Acta 2001, 305, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhou, Y.; Nabavi, S.M.; Sahebkar, A.; Little, P.J.; Xu, S.; Weng, J.; Ge, J. Mechanisms of Oxidized LDL-Mediated Endothelial Dysfunction and Its Consequences for the Development of Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 925923. [Google Scholar] [CrossRef] [PubMed]
- Camici, G.G.; Shi, Y.; Cosentino, F.; Francia, P.; Lüscher, T.F. Anti-Aging Medicine: Molecular Basis for Endothelial Cell-Targeted Strategies—A Mini-Review. Gerontology 2011, 57, 101–108. [Google Scholar] [CrossRef]
- Jeong, J.; Juhn, K.; Lee, H.; Kim, S.-H.; Min, B.-H.; Lee, K.-M.; Cho, M.-H.; Park, G.-H.; Lee, K.-H. SIRT1 Promotes DNA Repair Activity and Deacetylation of Ku70. Exp. Mol. Med. 2007, 39, 8–13. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).