Abstract
Diet is a potential modulator of telomere length (TL), but the impact of individual dietary components, such as nuts, on TL in young, healthy individuals remains underexplored. Peanuts are rich in bioactive compounds that may influence TL. Therefore, to fill this gap of knowledge, this study aimed to investigate the effect of peanut consumption on TL in this specific population. Fifty-eight young, healthy individuals were randomized to one of three different intervention groups for 6 months each: (1) 25 g/day of skin-roasted peanuts (SRP); (2) 32 g/day of peanut butter (PB); (3) 32 g/day of a control butter (CB) (based on peanut oil). TL was measured by quantitative real-time PCR in saliva at baseline and at the end of the intervention. Our findings revealed significant between-group differences in TL changes, particularly between the SRP and CB groups over 6 months (mean difference: 0.53; 95% CI: 0.01, 1.05; p-value = 0.048). No significant difference was observed between PB and CB groups (mean difference: 0.12; 95% CI: –0.42, 0.66; p-value = 0.66). This study provides novel insights into the impact of peanut consumption on TL maintenance in young and healthy individuals. The findings highlight the potential benefits of incorporating peanuts into the diet as a means of promoting cellular health and longevity. Further research is warranted to elucidate the underlying mechanisms and validate these findings across diverse populations and longer time frames.
1. Introduction
Peanuts (Arachis hypogaea) are a leguminous plant from South America that was introduced in Europe in the 18th century [1]. Although peanuts belong to the same family as legumes or beans, they are typically included in the group of nuts due to their similar nutritional composition [1]. Peanuts have a great nutritional profile, being rich in unsaturated fatty acids mainly, oleic, and linoleic acids, dietary fiber, B and E vitamins, potassium, phytonutrients such as (poly)phenols [2], and other bioactive compounds of potential interest for human health. These (poly)phenols, including flavonoids and stilbenes like resveratrol, exhibit potent antioxidant properties, counteracting oxidative stress by scavenging free radicals and reducing lipid peroxidation [3]. Extensive research supports that regular peanut consumption is associated with reduced risk of cardiovascular disease incidence and mortality [4], diabetes [5], certain types of cancer, and overall mortality [6,7]. Interestingly, these age-related conditions have been associated with shortened telomere length (TL) [8,9]. Within the ARISTOTLE trial, performed in young and healthy individual, Parrilli-Moser et al. described the improvement in vascular cognitive performance with the daily consumption of this peanuts [10,11].
Telomeres, comprised of repetitive sequences (TTAGGG), play a vital role in maintaining chromosome stability and integrity [12]. However, telomeres shorten with each cell division and their progressive attrition is linked to replicative senescence, a hallmark of aging [13]. While population-based studies revealed a decrease in TL with age [14], the rate of attrition is variable across individuals and life stages [15]. The length of telomeres in individuals is a dynamic process influenced by both initial TL at birth and the rate of erosion throughout life [16]. During infancy and childhood, TL shortens rapidly due to high cellular turnover [17,18], but the rate stabilizes to about 50–60 base pairs per year in adulthood. In older age, telomere shortening accelerates, with an estimated loss of 5–6 kilobases in individuals aged over 60 years [19]. This intricate process underscores the importance of analysing TL in healthy young individuals, as it not only provides insights into early aging markers but also helps identifying potential risk factors for age-related diseases, offering valuable opportunities for disease prevention.
TL dynamics are intricately influenced by various modifiable lifestyle factors such as physical activity, obesity, stress, or diet [20]. A growing body of research has shown that healthy diets rich in anti-inflammatory and antioxidant components could delay TL shortening [21]. Indeed, oxidative stress accelerates telomere shortening by increasing cellular DNA damage. In this context, antioxidants play a crucial role counteracting oxidative stress and protecting cells from damage. Peanuts, in particular, are rich in bioactive compounds such as resveratrol, flavonoids, and phytosterols, which have potent antioxidant and anti-inflammatory properties. Although studies specifically investigating “peanut and telomere” are scarce, the high content of these bioactive compounds in peanuts justifies further exploration of their impact on TL.
While epidemiological studies on TL in adults have provided valuable insights, there is a growing interest in investigating TL during early life stages due to its potential connection with adverse outcomes in later adulthood [22,23]. However, research on the effects of nut consumption on telomeres in young and healthy individuals remains limited [24], with no studies specifically addressing peanut consumption.
Therefore, building on data from the ARISTOTLE study (NCT04324749), a randomized controlled trial involving healthy young participants aged 18 to 33 years, this study aimed to explore the impact of daily consumption of skin-roasted peanuts (SRP) and peanut butter (PB) in preventing telomere shortening in young, healthy individuals.
2. Materials and Methods
2.1. Study Population and Study Design
The present study was carried out within the frame the ARISTOTLE study (NCT04324749), a three-arm parallel randomized controlled trial conducted in 2019–2020 in sixty-three healthy young adults (18–33 years) from the Food and Nutrition Torribera Campus of the University of Barcelona and surroundings, with the main objective to assess the impact of daily SRP and PB intake on the organism, evaluating their prebiotic and postbiotic effects. Exclusion criteria included a history of chronic diseases (e.g., cardiovascular diseases, cancer, diabetes), peanut allergy or intolerance, body mass index (BMI) over 25 kg/m2, active smoking, high alcohol consumption and other toxic habits. More details of the study design have been previously published [11]. In the present sub-study, five participants were excluded from the total sample who completed the study, due to incomplete data of telomere length. Finally, fifty-eight participants were included (Figure A1). The study protocol was approved by the Bioethics Commission of the University of Barcelona (Institutional Review Board: IRB 00003099) and carried out following the Declaration of Helsinki. Participants provided written informed consent prior to the start of the trial.
2.2. Intervention
After a two-week peanut-free run-in period, participants were randomly assigned to one of three 6-month duration interventions: 25 g/day of SRP or 2 tablespoons (32 g)/day of PB or 2 tablespoons (32 g)/day of a control butter (CB). The CB was made with peanut oil, free of phenolic compounds and fiber. Different amounts of peanuts and peanut butter were selected based on guidelines from official nutrition organizations, which define a standard portion size as 2 tablespoons (32 g) for peanut butter and 25–30 g for peanuts. Both peanuts (1 g of salt/100 g) and peanut butter (0.84 g of salt/100 g) contain salt as one of their ingredients. Participants maintained their usual dietary habits and consumed the assigned product at their convenience throughout the day. However, consumption of wine, grapes, dark chocolate (>70%), and berries was restricted due to their high resveratrol content, a phenolic compound which is also present in peanuts. Additionally, nuts (pistachio, walnuts, almonds, hazelnuts) were excluded from the diet due to their similar nutritional content to peanuts (see Parilli-Moser et al., 2021) [11].
2.3. Anthropometric, Biochemical, and Clinical Measurements
The following measurements were taken with the participants in fasting conditions at the beginning and end of the trial. Body weight and composition (body fat and muscle percentage) were assessed using a tetrapolar OMRON BF511 electronic scale, with participants wearing light clothes and no footwear. Height was measured in the standing position using a portable stadiometer. BMI was calculated as weight divided by height squared (kg/m2). Waist circumference was measured with a Holtain tape measure positioned at the midpoint between the lower margin of the last rib and the top of the iliac crest, while hip circumference was measured at the level of the upper trochanters. Both measurements were utilized to compute the waist-to-hip ratio. Blood pressure was recorded three times at two/three-minute intervals using an OMRON M6 digital monitor, with volunteers seated. Blood glucose and lipid parameters, including total cholesterol, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), and triglycerides (TG), were analysed at an external laboratory (Cerba internacional, Barcelona, Spain) employing enzymatic methodologies.
2.4. Dietary, Physical Activity, and Sociodemographic Variables
Covariate data were collected by professional staff members using general questionnaires about socio-demographic variables (i.e., sex, age, level of education), dietary intake and physical activity at baseline and at the end of the intervention. The diet was evaluated using a validated 151-item semi-quantitative food frequency questionnaire to quantify the usual food intake, estimated according to Spanish food composition tables [25]. Physical activity was measured as the metabolic equivalent of task-minutes per week (MET/week) using the Spanish validated version of the Minnesota Leisure-Time Physical Activity Questionnaire [26,27].
2.5. Telomere Length Assessment
Genomic DNA was isolated from frozen saliva samples (collected at 0 and 6 months) using lysis buffer and isopropanol extraction following the manufacturer’s instructions. This extraction method is vastly used in epidemiological studies [28]. TL can vary across different cells or tissues; however, salivary TL has been correlated with TL measured in whole blood [29], buffy coat [30], and leukocytes [31]. In this study, TL was measured by real-time PCR (qPCR) [32], using the SYBR Select Master Mix for CFX (Applied Biosystems, Waltham, MA, USA). The protocol was adapted from Nathan J O’Callaghan [33] and we used the following primers and standards: TeloF (CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT), TeloR (GGCTTGCCTTACCCTTACCCTTACCCTTACCCTTACCCT), 36b4F (CAGCAAGTGGGAAGGTGTAATCC), 36b4R (CCCATTCTATCATCAACGGGTACAA), Telomere standard (14 tandem copies of TTAGGG), and 36b4 standard (CAGCAAGTGGGAAGGTGTAATCCGTCTCCACAGACAAGGCCAGGACTCGTTTGTACCCGTTGATGATAGAATGGG). This approach employs 36b4 single-copy gene as a reference for each sample. The quantification of the relative copy numbers of telomeres (T) and a single copy gene (36b4; S) was performed in triplicates using a Bio-Rad CFX96 thermocycler with samples collected before and after the intervention. TL is expressed as a ratio of these two parameters (T/S ratio). A calibration curve with standards of Telomere (1.8 × 105 to 1.8 kb) and 36b4 (2.63 × 105 to 2.63 diploid copies) in 10-fold dilutions with a linearity agreement of R2 > 0.99 were included in each 96-well plate.
2.6. Statistical Anaysis
The normality of distribution was analysed by the Shapiro–Wilk test. Most of the variables did not follow a normal distribution, then differences in baseline characteristics between groups were assessed using the Kruskal–Wallis test for continuous variables, followed by the post hoc Dunn’s multiple comparisons test when significant differences were observed. The chi-square test was used to compare proportions among categorical variables. Means and standard deviations (SD) or numbers and percentages, as appropriate, are shown for the description of baseline characteristics according to the intervention group.
The primary outcome was TL change over 6-months by intervention group. Additional analyses included 6-month changes in anthropometric, biochemical, clinical, and dietary variables. The generalized estimating equation (GEE) model for repeated measurements (with identity link function, first-order autoregressive correlation, and robust standard error parameters) was used to estimate the effect of the interventions on the aforementioned variables. Two adjustment models were generated to avoid other factors influencing the outcomes. Model 1 was adjusted for sex and age. Model 2 was further adjusted for BMI (kg/m2), physical activity, and total energy intake (kcal/day). Data are expressed as adjusted mean differences and their 95% confidence intervals (CI). The proportion of participants in each group achieving an accelerated telomere shortening (ΔTL ≤ percentile 20) was also estimated.
In addition, linear regression models were used to evaluate associations between changes in anthropometric, biochemical, and dietary variables (which showed statistically significant differences between groups after 6 months of intervention) and changes in telomere length. Three adjustment models were used: Model 1 was adjusted for sex and age; model 2 was adjusted as for model 1 plus BMI (kg/m2), physical activity, and total energy intake (kcal/day); and model 3 was adjusted as for model 2 plus telomere length at baseline.
Due to the non-normality of most of the variables, their changes (difference between 6-months and baseline) were normalized and scaled in multiples of 1 SD using the Blom inverse normal transformation to perform this analysis [34].
All statistical analyses were performed with Stata software, version 16.0 (Stata Corp LP, College Station, TX, USA). Significance testing was considered for p-value < 0.05.
3. Results
3.1. Baseline Characteristics of Participants
A total of 58 participants from the ARISTOTLE study (mean age 22.74 ± 3.24 years; BMI was 22.45 ± 3.02 kg/m2, indicating young and healthy body weight), were included. The baseline characteristics (before the intervention) of participants by intervention group are presented in Table 1. No significant differences were observed among the study groups, except for plasmatic HDL-c (p-value = 0.016) and m-coumaric acid intake (p-value = 0.035).

Table 1.
General characteristics of study population at baseline by intervention group.
3.2. Effect of the Intervention on Telomere Length
The changes in TL after 6 months of the SRP or PB interventions compared with the CB intervention are shown in Table 2. The SRP group showed a significant increase in TL over time compared to the CB group (adjusted mean difference: 0.53; 95% CI: 0.01, 1.05; p-value = 0.048), after adjusting for sex, age, BMI, physical activity, and energy intake. No significant between-group differences in TL change from baseline were observed between the PB group and the CB group. Additionally, we evaluated the percentage of participants achieving an accelerated telomere shortening (defined as <20th percentile) and found that the participants consuming SRP were the only group not exhibiting accelerated telomere shortening. In contrast, 22% of those in the PB group and 38% in the CB group showed greater rates of telomere shortening (Figure 1).

Table 2.
Effect of intervention on telomere length after 6 months of intervention.
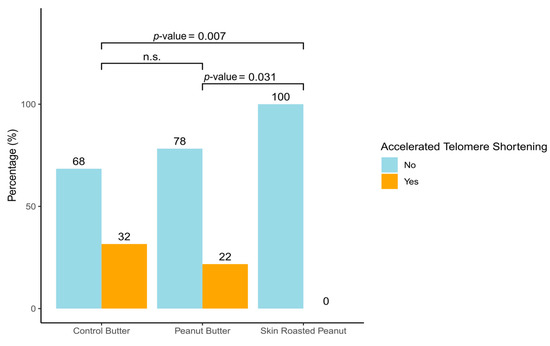
Figure 1.
Accelerated telomere shortening by intervention group. Accelerated telomere shortening was assessed by monitoring change in telomere length over a 6-month follow-up period, specifically targeting a threshold of percentile 20 or lower (ΔTL ≤ percentile 20; ΔTL ≤ p20).
3.3. Relationships Between Variables That Changed with the Intervention and Telomere Length
The between-group differences (SPR vs. CB, PB vs. CB) in anthropometric, biochemical, and clinical parameters over 6 months, analysed using the aforementioned models, are presented in Table A1. Regarding anthropometric measures, there were significant between-group differences (SRP vs. CB) in waist-to-hip ratio (adjusted mean difference: −0.03; 95%CI: −0.05, −0.01; p-value = 0.027), but no differences were observed between interventions in other related parameters such as body fat (%) or visceral fat. In terms of biochemical parameters, the SRP group exhibited a significantly greater increase in HDL cholesterol levels with the intervention (adjusted mean difference: 0.20; 95%CI: 0.02, 0.37; p-value = 0.030). It is worth noting that the SRP group already had higher baseline HDL cholesterol levels than the CB group at the beginning of the study.
Additionally, we examined between-group differences in changes in dietary intake over 6 months of intervention (Table A2). The SRP group showed a higher increase in the intake of sugars (adjusted mean difference: 11.59; 95%CI: 0.04, 23.15; p-value = 0.049) and fiber (adjusted mean difference: 4.88; 95%CI: 1.77, 7.99; p-value = 0.002) compared with the CB group. Fiber intake was also higher in the PB group (adjusted mean difference: 3.65; 95%CI: 0.45, 6.85; p-value = 0.025) than the CB group. Regarding micronutrients and phytochemicals, both SRP and PB groups had a higher intake of vitamin E (adjusted mean difference: 2.76; 95%CI: 0.85, 4.67; p-value = 0.005 and adjusted mean difference: 3.56; 95%CI: 1.25, 5.84; p-value = 0.002, respectively) than the CB group. Furthermore, the intake of phytochemicals specific to peanuts, such as coumaric acids and resveratrol, was notably higher in both intervention groups compared to the CB group, However, o-coumaric acid had a higher intake only in the SRP group (adjusted mean difference: 1.45; 95%CI: 0.85, 2.04; p-value < 0.001), as has been observed previously in this trial by Parilli-Moser et al. [11].
Following the observed modifications in anthropometric and biochemical variables throughout the intervention, linear regressions were carried out with TL as dependent variables, using three different models. Table 3 shows the linear regressions using the most adjusted model, while the rest are shown in Table A3. A positive association between monounsaturated fatty acids (MUFA) intake and TL was identified when adjusting for sex and age (β: 0.58; 95%CI: 0.11, 1.05; p-value = 0.016), This association remained significant after further adjustments for BMI, physical activity, and energy intake (β: 0.46; 95%CI: (0.02, 0.93; p-value = 0.048), and persisted when baseline TL was included in the model (β: 0.35 (0.01, 0.69), p-value = 0.046).

Table 3.
Association between anthropometric, biochemical, and dietary variables that changed with intervention and Δ telomere length.
Regarding specific phytochemicals, resveratrol and anthocyanins showed an initial positive association with TL (β: 1.52; 95%CI: 0.37, 2.67; p = 0.011 and β: 0.79; 95%CI: 0.02, 1.56; p-value = 0.044, respectively). However, these associations were no longer significant after adjusting for baseline TL (model 3). On the other hand, m-coumaric acid exhibited a significant positive association with TL only in model 3 (β: 0.42; 95%CI: 0.18, 0.67; p-value = 0.012).
4. Discussion
In the present sub-study, we have evaluated the impact of peanut consumption (SRP and PB), on TL in young, healthy individuals from the ARISTOTLE study. Our results revealed a positive impact of SRP on TL, whereas no significant differences were observed between the PB and CB groups (Table 2). Moreover, only SRP reveal a protective role against accelerated telomere shortening (Figure 1). While extensive research has explored dietary influences on TL, studies focusing on young, healthy populations remain scarce. To the best of our knowledge, this is the first randomized controlled trial investigating the specific impact of peanut consumption on TL in this demographic.
Telomere attrition, primarily driven by oxidative stress and inflammation, is a well-documented process [35]. Given that edible plants are rich in bioactive compounds with antioxidant and anti-inflammatory properties, there is a strong rationale to suggest that regular consumption of such foods, including peanuts, could help mitigate telomere shortening [36]. Recent systematic reviews have highlighted the positive association between TL and antioxidant-rich diets, emphasizing specific nutrients [21], increased fruits and vegetables intake [21], and adherence to plant-rich dietary patterns [36] such as the Mediterranean diet [21,37]. In this context, nut consumption has been associated with longer telomeres [38], suggesting a potential role delaying cellular aging and senescence.
Few randomized controlled trials [39,40,41,42] have examined the impact of nut consumption on TL across diverse populations. Consistent with our findings on SRP, previous studies have reported promising effects of walnuts consumption in elderly individuals [39] and pistachio intake in prediabetic individuals [40]. Specifically, walnuts have been associated with prevention of telomere shortening, and pistachios intake has been linked to increased expression of telomerase-related genes [39,40]. Moreover, the PREDIMED-Plus Study reported an increase in TL over time both groups following a Mediterranean diet, regardless of energy restriction and exercise intervention [41]. Notably, nut consumption significantly increased in both groups, which may have contributed to maintain TL. However, the PREDIMED study, conducted in adults at high cardiovascular risk, found that mixed nut supplementation (walnuts, hazelnuts, and almonds) accelerated telomere shortening compared to a low-fat control diet [42]. Discrepancies in results may stem from differences in participant characteristic, TL assessment methodologies, and the types of nuts consumed.
Our study focuses on young, healthy adults, suggesting that the effects of nut intake on TL might vary based on baseline health status. However, it is plausible that SRP could also exert a protective effect in elder population. Nevertheless, when transferring this study to older individuals, careful consideration should be given to salt intake from peanuts. Consuming unsalted peanuts would be advisable. Additionally, the distinct nutritional profile of peanuts compared to the mixed nuts in PREDIMED study could differentially influence telomere biology [43].
It is noteworthy that, while SRP consumption showed a beneficial effect on TL, no significant differences in TL changes were observed between the PB and CB groups. Although commercially available peanut butter often contains added fats, the peanut butter used in our study was composed solely of peanuts and salt. The key distinction between whole peanuts and peanut butter lies in their processing methods, such as grinding and homogenization. These processes modify the fiber matrix and may enhance the bioavailability of polyphenols, as suggested by findings from the ARISTOTLE study [11]. In contrast, whole peanuts, require grater digestive effort, particularly involving the gut microbiota, which may lead to increased production of short-chain fatty acids (SCFAs) [44]. Notably, our previous research identified elevated fecal SCFAs levels exclusively in the SRP group, with a negative correlation between SCFAs and biomarkers of depression and cortisol, factors closely linked to aging and telomere attrition [45].
Additionally, we explored specific peanut-derived compounds that may contribute to telomere maintenance. Our findings suggest that dietary intake of MUFA and m-coumaric acid may exert protective effect on telomere integrity. Previous studies have shown that MUFA [46] and hydroxycinnamic acid derivatives [47] such m-coumaric acid positively influence lipid metabolism, exhibit antioxidant activity, and reduce oxidative stress mechanisms that have been linked to improved metabolic health. These bioactive compounds are abundant in health-promoting dietary patterns like the Mediterranean diet, which has been widely associated with longevity and a reduced risk of chronic disease.
Despite these promising findings, previous studies have yielded inconsistent results regarding the effects of these compounds on TL. For instance, the Nurses’ Health Study reported an association between adherence to the Mediterranean diet and longer leukocyte telomeres; however, no significant correlations were observed with individual dietary components, including the MUFA-to-fatty-acid ratio were detected [48]. Similarly, research in older populations [49,50] has produced mixed findings, with some studies even noting negative correlations. This variability underscores the need for further research to determine how factors such as dietary sources, age, and lifestyle modulate the impact of these compounds on TL.
Regarding m-coumaric acid, no studies have directly examined its relationship with TL. However, its potent antioxidant and anti-inflammatory properties suggest a potential protective role in telomeres maintenance by mitigating oxidative stress and inflammation, two key drivers of telomere shortening [47]. Our findings highlight the need for further research to elucidate the role of m-coumaric acid in telomere biology.
More broadly, investigating the effects of these bioactive compounds, particularly their presence in peanuts and other plant-based foods, could provide valuable insights into their impact on TL regulation and cellular health. Given that nutrients influencing oxidative stress and inflammation are known to modulate telomere dynamics, nutrition represents a promising avenue for understanding aging and age-related diseases such as obesity, insulin resistance, and cardiovascular conditions, all of which are characterized by heightened inflammation and oxidative stress [51,52,53]. Future research should focus on elucidating the mechanisms underlying these effects and validating these findings in more diverse populations over extended study periods.
Our findings are particularly relevant, as telomere attrition, as previously mentioned, has been linked to metabolic dysfunction and increased risk of age-related diseases including cardiovascular diseases, diabetes, and cancer [54]. Peanut consumption may help mitigate these risks by reducing oxidative stress and inflammation, two key contributors to telomere shortening. Indeed, a recent meta-analysis reported that peanut intake was associated with a lower incidence of cardiovascular disease and mortality, as well as reduced rates of stroke and coronary heart disease [4]. Interestingly, no such associations were observed with peanut butter, possibly due to the addition of salt and fats as noted above. Moreover, another study found an inverse associated between peanut consumption and overall cancer risk [55].
One of the strengths of our study is the homogeneity of the participant pool, as all individuals were young (18–33 years) and recruited based on standardized criteria minimizing age-related confounding effects on TL. This reduces confounding bias related to age, a factor known to affect TL. Additionally, targeting a healthy population aligns with the goal of promoting preventive health strategies. However, this homogeneity also limits the generalizability of our findings to broader populations. Our study focused solely on a young population, where telomeres are inherently longer. It would be highly interesting to investigate the effects of peanut consumption in an older population, where telomere attrition is more pronounced.
Another potential limitation is the small sample size for each group. The ARISTOTLE trail was initially designed to achieve 80% of statistical power; however, due to a higher-than-expected dropout rate, this was reduced to 60%. Therefore, larger studies are needed to confirm these findings. Moreover, the participant self-selection is another limitation of the study because individuals who enrolled in the intervention may have been more health-conscious than the general population, potentially affecting the representativeness of the results. Furthermore, the absence of a peanut-free control group is a notable limitation, as the control group consumed a paste made from peanut oil, which may have introduced confounding effects.
5. Conclusions
In conclusion, this study underscores the potential benefits of peanut consumption, particularly whole peanuts (SRP), in supporting telomere maintenance and slowing cellular aging in young, healthy individuals. Rich in MUFA and m-coumaric acid, peanuts may contribute to healthy aging and lower risk of age-related diseases.
The observed differences between SRP and peanut butter (PB) highlight the impact of processing methods on fiber structure, nutrient bioavailability, and SCFA production, which may partially explain the distinct effects of both interventions. However, further research is needed to validate these findings in larger and more diverse populations over extended study periods, with a particular focus on elder populations. Moreover, it would be interesting to investigate the molecular mechanisms underlaying the effects of peanuts in a future mouse model study. Expanding this line of research could provide valuable insights into the role of dietary interventions in promoting longevity and preventing chronic diseases.
Author Contributions
S.C., J.R. and R.M.L.-R. designed and conducted the research. R.M.L.-R. and S.H.-B., conceptualized the study, while S.H.-B. and I.P.-M. were responsible for data collection. H.S.-L. and D.T.-O. processed the samples and D.T.-O. performed the TL analysis. I.P.-M. and E.P.L.S. analyzed the data. D.T.-O., S.C. and N.B.-T. wrote the paper. R.M.L.-R., D.T.-O., I.P.-M., E.P.L.S., N.B.-T., D.H., P.F.M., S.H.-B., J.R. and S.C. contributed to the research, provided critical revisions for intellectual content, and approved the final manuscript. J.R. and S.C. served as guarantors of this work, ensuring the integrity and accuracy of the data. They had full access to all study data and held primary responsibility for the final content. All authors have read and agreed to the published version of the manuscript.
Funding
The ARISTOTLE study was supported by the Peanut Institute 2019. D.T.-O. thanks the Ministerio de Ciencia, Innovación y Universidades for the FPU contract (FPU19/02601). E.P.L.S. thanks post-doctoral grant JDC2022-049842-I, funded by MICIU/AEI/10.13039/501100011033 and by “European Union NextGenerationEU/PRTR”. Nerea Becerra-Tomás was supported by the Beatriz Galindo program from the Spanish Ministry of Universities (BG22/00050).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, approved by the Ethics Committee of Clinical Investigation of the University of Barcelona (Barcelona, Spain, on 1 October 2019), and registered at https://clinicaltrials.gov/ct2/show/NCT04324749 (accessed on 27 March 2020).
Informed Consent Statement
According to the principles of the Declaration of Helsinki, informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request.
Acknowledgments
Thanks to INSA-UB Maria de Maeztu Unit of Excellence (Grant CEX2021-001234-M), funded by MICIN/AEI/FEDER, UE. The authors acknowledge all members of the Peanut study team, and those involved in the study in particular.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
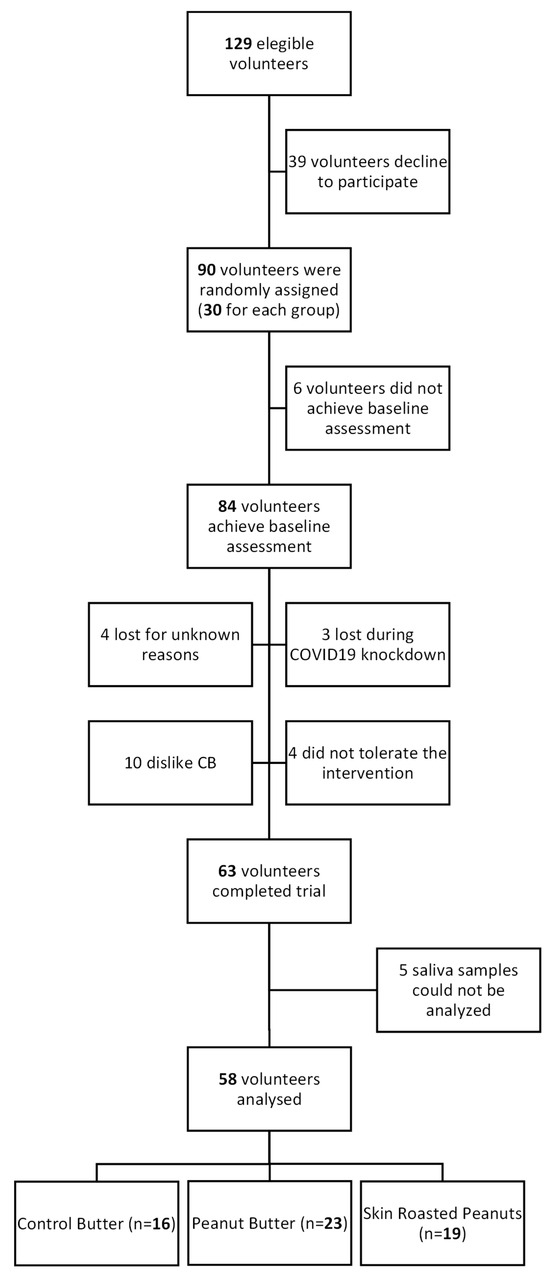
Figure A1.
Flowchart of ARISTOTLE study.

Table A1.
Intervention effect on anthropometric, biochemical, and clinical parameters at 6 months of intervention.
Table A1.
Intervention effect on anthropometric, biochemical, and clinical parameters at 6 months of intervention.
| Models | SRP vs. CB | p-Value | PB vs. CB | p-Value | |
|---|---|---|---|---|---|
| Mean Difference 6 Moth (95% CI) | Mean Difference 6 Moth (95% CI) | ||||
| Anthropometric measurements | |||||
| Weight (Kg) | Model 1 | −0.01 (−2.44, 2.42) | 0.991 | −0.69 (−2.94, 1.56) | 0.547 |
| Model 2 | 0.09 (−0.49, 0.68) | 0.752 | −0.24 (−0.73, 0.24) | 0.328 | |
| BMI (kg/m2) | Model 1 | −0.03 (−0.86, 0.79) | 0.935 | −0.16 (−0.91, 0.58) | 0.670 |
| Model 2 | −0.03 (−0.86, 0.79) | 0.939 | −0.20 (−0.95, 0.55) | 0.602 | |
| Waist circumference (cm) | Model 1 | −0.12 (−2.03, 1.79) | 0.902 | −0.14 (−2.36, 2.09) | 0.904 |
| Model 2 | −0.16 (−1.35, 1.04) | 0.797 | 0.19 (−1.72, 2.09) | 0.849 | |
| Waist-to-hip ratio | Model 1 | −0.03 (−0.07, 0.01) | 0.076 | −0.03 (−0.06, 0.01) | 0.086 |
| Model 2 | −0.03 (−0.05, −0.01) | 0.027 | −0.02 (−0.04, 0.01) | 0.139 | |
| Body fat (%) | Model 1 | −0.00 (−1.74, 1.74) | 0.999 | −0.34 (−2.10, 1.41) | 0.702 |
| Model 2 | 0.07 (−0.90, 1.05) | 0.880 | −0.11 (−0.99, 0.77) | 0.811 | |
| Visceral fat | Model 1 | −0.13 (−0.96, 0.70) | 0.762 | −0.11 (−0.86, 0.63) | 0.766 |
| Model 2 | −0.07 (−0.44, 0.30) | 0.707 | −0.03 (−0.39, 0.34) | 0.878 | |
| Muscle mass (%) | Model 1 | 0.37 (−0.73, 1.47) | 0.511 | 0.24 (−0.78,1.272) | 0.642 |
| Model 2 | 0.39 (−0.57, 1.33) | 0.436 | 0.15 (−0.60, 0.90) | 0.695 | |
| Blood pressure | |||||
| Systolic blood pressure (mmHg) | Model 1 | 0.74 (−7.86, 9.33) | 0.867 | −3.13 (−10.66, 4.39) | 0.415 |
| Model 2 | 0.80 (−7.19, 8.80) | 0.844 | −3.10 (−10.11, 3.90) | 0.385 | |
| Diastolic blood pressure (mmHg) | Model 1 | 2.60 (−4.61, 9.81) | 0.480 | 0.78 (−5.38, 6.93) | 0.805 |
| Model 2 | 2.64 (−4.20, 9.48) | 0.449 | 1.26 (−4.74, 7.25) | 0.681 | |
| Biochemical parameters | |||||
| Glucose (mmol/L) | Model 1 | 0.07 (−0.12, 0.27) | 0.456 | −0.05 (−0.23, 0.14) | 0.628 |
| Model 2 | 0.08 (−0.11, 0.27) | 0.387 | −0.03 (−0.21, 0.16) | 0.791 | |
| Triglyceride (mmol/L) | Model 1 | 0.08 (−0.13, 0.30) | 0.444 | −0.01 (−0.23, 0.21) | 0.938 |
| Model 2 | 0.09 (−0.13, 0.31) | 0.435 | 0.02 (−0.19, 0.23) | 0.857 | |
| Total cholesterol (mmol/L) | Model 1 | −0.06 (−0.73, 0.60) | 0.848 | −0.15 (−0.84, 0.54) | 0.676 |
| Model 2 | −0.05 (−0.72, 0.62) | 0.883 | −0.13 (−0.83, 0.57) | 0.717 | |
| HDL-cholesterol (mmol/L) | Model 1 | 0.20 (0.01, 0.39) | 0.035 | 0.16 (−0.06, 0.38) | 0.162 |
| Model 2 | 0.20 (0.02, 0.37) | 0.030 | 0.14 (−0.06, 0.34) | 0.168 | |
| LDL-cholesterol (mmol/L) | Model 1 | −0.07 (−0.55, 0.42) | 0.783 | −0.08 (−0.63, 0.47) | 0.771 |
| Model 2 | −0.04 (−0.53, 0.44) | 0.859 | −0.04 (−0.59, 0.50) | 0.877 | |
SRP: skin-roasted peanuts; PB: peanut butter; CB: control butter; BMI: body mass index. Generalized estimating equation (GEE) models were used to estimate effect (difference) of intervention among study groups. Model 1: adjusted by sex and age; Model 2: adjusted as in Model 1 plus BMI, physical activity, and energy intake. p-value: group × time interaction. Values < 0.05 are statistically significant and highlighted in bold.

Table A2.
Dietary intake after 6 months of intervention.
Table A2.
Dietary intake after 6 months of intervention.
| Dietary Intake | Models | SRP vs. CB | p-Value | PB vs. CB | p-Value |
|---|---|---|---|---|---|
| Difference Time- Exposure (95% CI) | Difference Time-Exposure (95% CI) | ||||
| Nutrient intake | |||||
| Carbohydrates (g/day) | Model 1 | 0.85 (−25.80, 27.49) | 0.950 | 2.85 (−24.73, 30.43) | 0.840 |
| Model 2 | 9.56 (−4.49, 23.63) | 0.182 | 7.79 (−7.99, 23.58) | 0.334 | |
| Sugars (g/day) | Model 1 | 7.95 (−6.13, 22.04) | 0.269 | 5.45 (−8.01, 18.92) | 0.427 |
| Model 2 | 11.59 (0.04, 23.15) | 0.049 | 6.42 (−3.66, 16.50) | 0.212 | |
| Fiber (g/day) | Model 1 | 3.38 (−1.17, 7.94) | 0.146 | 2.78 (−2.13, 7.70) | 0.267 |
| Model 2 | 4.88 (1.77, 7.99) | 0.002 | 3.65 (0.45, 6.85) | 0.025 | |
| Protein (g/day) | Model 1 | −4.57 (−13.69, 4.54) | 0.325 | −5.25 (−14.96, 4.47) | 0.290 |
| Model 2 | −0.78 (−7.94, 6.39) | 0.832 | −3.56 (−10.47, 3.35) | 0.313 | |
| Total fat (g/day) | Model 1 | 11.12 (−1.24, 23.48) | 0.078 | −5.97 (−19.18, 7.23) | 0.375 |
| Model 2 | 6.27 (−0.10, 12.63) | 0.054 | −3.13 (−10.21, 3.95) | 0.386 | |
| Saturated fat (g/day) | Model 1 | −0.87 (−4.86, 3.12) | 0.670 | −1.50 (−5.66, 2.67) | 0.481 |
| Model 2 | 0.60 (−1.77, 2.96) | 0.622 | −0.71 (−2.85, 1.43) | 0.515 | |
| Monounsaturated fat (g/day) | Model 1 | 7.41 (0.79, 14.02) | 0.028 | −5.41 (−12.36, 1.54) | 0.127 |
| Model 2 | 5.29 (0.12, 10.71) | 0.056 | −4.37 8 (10.71, 0.12) | 0.139 | |
| Polyunsaturated fat (g/day) | Model 1 | 0.60 (−3.11, 4.31) | 0.752 | 1.59 (−2.22, 5.40) | 0.413 |
| Model 2 | 1.34 (−1.31, 4.00) | 0.322 | 2.28 (−0.57, 5.13) | 0.117 | |
| Energy, kcal/d | Model 1 | −92.80 (−299, 114) | 0.379 | −52.09 (−269, 165) | 0.638 |
| Model 2 | |||||
| Food consumption | |||||
| Fruits (g/day) | Model 1 | 26.23 (−48.62, 101) | 0.492 | −19.05 (−67.13, 29.03) | 0.437 |
| Model 2 | 34.04 (−37.49, 106) | 0.351 | −21.80 (−66.30, 22.70) | 0.337 | |
| Vegetables (g/day) | Model 1 | −20.07 (−50.37, 10.21) | 0.194 | −9.79 (−44.11, 24.54) | 0.576 |
| Model 2 | −14.83 (−45.01, 15.36) | 0.336 | −8.79 (−42.36, 24.78) | 0.608 | |
| Cereals (g/day) | Model 1 | −20.40 (−57.86, 17.05) | 0.286 | −22.72 (−60.76) | 0.242 |
| Model 2 | −12.90 (−43.74, 17.95) | 0.412 | −15.70 (−47.94, 17.95) | 0.412 | |
| Potatoes (g/day) | Model 1 | −3.25 (−27.33, 20.82) | 0.791 | 10.30 (−12.33, 33.53) | 0.385 |
| Model 2 | −2.43 (−26.79, 21.93) | 0.845 | 12.28 (−12.24, 36.80) | 0.326 | |
| Legumes (g/day) | Model 1 | 4.28 (−8.27, 16.83) | 0.504 | −0.77 (−11.87, 10.34) | 0.892 |
| Model 2 | 5.02 (−7.10, 17.16) | 0.417 | −0.48 (−11.50, 10.54) | 0.932 | |
| Dairy products (g/day) | Model 1 | 45.74 (−38.52, 130) | 0.287 | 25.21 (−63.53, 113) | 0.578 |
| Model 2 | 51.76 (−30.19, 134) | 0.216 | 31.07 (−57.34, 119) | 0.491 | |
| Eggs (g/day) | Model 1 | 7.68 (−4.45, 19.81) | 0.214 | −12.11 (−38.11, 13.89) | 0.361 |
| Model 2 | 10.09 (−2.60, 22.77) | 0.119 | −11.25 (−35.84, 13.35) | 0.370 | |
| Fish and sea food (g/day) | Model 1 | −11.02 (−24.52, 2.48) | 0.109 | −0.76 (−12.30, 10.78) | 0.897 |
| Model 2 | −7.89 (−22.90, 7.12) | 0.303 | 0.21 (13.41, 13.83) | 0.976 | |
| Meat/meat products (g/day) | Model 1 | −0.39 (−20.89, 20.11) | 0.970 | −18.35 (−39.39, 2.68) | 0.087 |
| Model 2 | 3.05 (−18.52, 24.62) | 0.782 | −18.49 (−38.76, 1.78) | 0.074 | |
| Oils and butter (g/day) | Model 1 | 8.42 (−7.76, 24.59) | 0.308 | 1.23 (−10.36, 12.83) | 0.834 |
| Model 2 | 10.28 (−6.51, 27.06) | 0.230 | 2.34 (−8.51, 13.21) | 0.672 | |
| EVOO (g/day) | Model 1 | 0.82 (−6.99, 8.62) | 0.838 | −6.15 (−13.16, 0.86) | 0.086 |
| Model 2 | 1.23 (−6.97, 9.43) | 0.769 | −6.08 (−13.49, 1.33) | 0.108 | |
| Pastries (g/day) | Model 1 | 0.32 (−15.83, 16.47) | 0.969 | 3.14 (−12.53, 18.81) | 0.694 |
| Model 2 | 3.28 (−10.53, 17.10) | 0.641 | 5.51 (−6.15, 17.17) | 0.354 | |
| Micronutrients | |||||
| B-group vitamins | Model 1 | 10.06 (−41.38, 61.49) | 0.702 | 2.39 (−51.75, 56.52) | 0.931 |
| Model 2 | 26.51 (−15.94, 68.97) | 0.221 | 11.62 (−31.53, 54.76) | 0.598 | |
| Vitamin A | Model 1 | 67.44 (−195, 330) | 0.614 | −28.12 (−399, 344) | 0.882 |
| Model 2 | 129 (−129, 386) | 0.327 | −9.07 (−369, 351) | 0.961 | |
| Vitamin D | Model 1 | 0.25 (−0.59, 1.09) | 0.554 | 0.31 (−0.66, 1.28) | 0.533 |
| Model 2 | 0.50 (−0.49, 1.49) | 0.329 | 0.35 (−0.66, 1.37) | 0.499 | |
| Vitamin E | Model 1 | 3.38 (0.96, 5.80) | 0.006 | 3.97 (1.10, 6.85) | 0.007 |
| Model 2 | 2.76 (0.85, 4.67) | 0.005 | 3.56 (1.25, 5.84) | 0.002 | |
| Vitamin C | Model 1 | −16.78 (−45.88, 12.36) | 0.259 | −10.17 (−40.49, 20.15) | 0.511 |
| Model 2 | −12.33 (−42.59, 17.93) | 0.425 | −11.47 (−42.59, 19.65) | 0.470 | |
| Sodium | Model 1 | −45.05 (−395, 305) | 0.801 | −135 (−475, 203) | 0.432 |
| Model 2 | 59.28 (−257, 375) | 0.713 | −88 (−353, 176) | 0.512 | |
| Magnesium | Model 1 | −6.43 (−64.39, 51.53) | 0.828 | −15.74 (−74.86, 43.38) | 0.602 |
| Model 2 | 14.59 (−15.89, 45.07) | 0.348 | −1.38 (−31.28, 28.52) | 0.928 | |
| Potassium | Model 1 | −15.41 (−192, 162) | 0.865 | −68.76 (−243, 106) | 0.440 |
| Model 2 | 51.18 (−68.50, 171) | 0.402 | −30.80 (−147, 85.22) | 0.603 | |
| Iron | Model 1 | −0.02 (−1.56, 1.52) | 0.981 | −0.53 (−2.25, 1.19) | 0.546 |
| Model 2 | 0.61 (−0.39, 1.60) | 0.230 | −0.16 (−1.14, 0.82) | 0.748 | |
| Zinc | Model 1 | 0.05 (−1.09, 1.19) | 0.933 | −1.02 (−2.36, 0.32) | 0.136 |
| Model 2 | 0.54 (−0.22, 1.29) | 0.165 | −0.75 (−1.51, 0.01) | 0.053 | |
| Polyphenols | |||||
| Flavanols | Model 1 | 89.34 (−218, 397) | 0.570 | −122 (−493, 250) | 0.521 |
| Model 2 | 113 (−182, 409) | 0.451 | −113 (−460, 234) | 0.523 | |
| Flavonols | Model 1 | −3.94 (−11.54, 3.66) | 0.310 | 0.50 (−5.37, 6.37) | 0.868 |
| Model 2 | −2.57 (−9.25, 4.10) | 0.450 | 1.57 (−3.55, 6.68) | 0.548 | |
| Flavanones | Model 1 | −12.73 (−34.81, 9.35) | 0.258 | −3.34 (−19.88, 13.21) | 0.692 |
| Model 2 | −12.27 (−20.90, 13.39) | 0.274 | −3.76 (−20.90, 13.39) | 0.667 | |
| Flavones | Model 1 | −23.68 (−46.04, −1.33) | 0.038 | −24.84 (−48.34, −1.34) | 0.038 |
| Model 2 | −18.72 (−41.08, 3.65) | 0.101 | −20.41 (−41.66, 0.83) | 0.060 | |
| Isoflavonoids | Model 1 | −2.74 (−8.65, 3.16) | 0.362 | −2.49 (−5.56, 0.58) | 0.112 |
| Model 2 | −2.49 (−8.03, 3.05) | 0.378 | −2.11 (−4.83, 0.62) | 0.130 | |
| Anthocyanins | Model 1 | −6.48 (−12.46, −0.50) | 0.034 | −2.18 (−7.58, 3.22) | 0.429 |
| Model 2 | −5.71 (−11.43, 0.01) | 0.051 | −2.01 (−7.43, 3.42) | 0.468 | |
| Phenolic acids | Model 1 | 18.75 (−91.99, 129) | 0.740 | 18.86 (−100, 138) | 0.757 |
| Model 2 | 42.41 (−57.45, 142) | 0.405 | 30.13 (−67.03, 127) | 0.543 | |
| Stilbenes | Model 1 | 0.09 (−0.01, 0.28) | 0.351 | 0.10 (−0.07, 0.21) | 0.265 |
| Model 2 | −0.08 (−0.27, 0.11) | 0.424 | −0.09 (−0.27, 0.08) | 0.272 | |
| Lignans | Model 1 | −0.19 (−2.90, 2.53) | 0.892 | 0.23 (−2.96, 3.43) | 0.885 |
| Model 2 | −0.10 (−2.69, 2.49) | 0.938 | 0.45 (−2.60, 3.50) | 0.771 | |
| Present in peanuts | |||||
| m-coumaric acid | Model 1 | 0.40 (0.18, 0.63) | <0.001 | 0.41 (0.25, 0.57) | <0.001 |
| Model 2 | 0.43 (0.21, 0.66) | <0.001 | 0.41 (0.25, 0.57) | <0.001 | |
| o-coumaric acid | Model 1 | 1.43 (0.81, 2.04) | <0.001 | 0.04 (−0.05, 0.14) | 0.376 |
| Model 2 | 1.45 (0.85, 2.04) | <0.001 | 0.03 (−0.08, 0.13) | 0.631 | |
| p-coumaric acid | Model 1 | 6.15 (5.95, 6.36) | <0.001 | 12.99 (12.42, 13.56) | <0.001 |
| Model 2 | 6.19 (6.02, 6.37) | <0.001 | 13.01 (12.43, 13.59) | <0.001 | |
| Resveratrol | Model 1 | 0.07 (0.05,0.08) | <0.001 | 0.08 (0.06, 0.09) | <0.001 |
| Model 2 | 0.07 (0.05, 0.09) | <0.001 | 0.08 (0.07, 0.09) | <0.001 | |
SRP: skin-roasted peanuts; PB: peanut butter; CB: control butter; EVOO: extra virgin olive oil. Generalized estimating equation (GEE) models were used to estimate effect (difference) of intervention among study groups. Model 1: adjusted by sex and age; Model 2: adjusted as in Model 1 plus BMI, physical activity, and energy intake. p-value: group × time interaction. Values < 0.05 are statistically significant and highlighted in bold.

Table A3.
Association between anthropometric, biochemical, and dietary variables that changed with intervention and Δ telomere length.
Table A3.
Association between anthropometric, biochemical, and dietary variables that changed with intervention and Δ telomere length.
| n | Telomere Length | |||
|---|---|---|---|---|
| B (CI) | p-Value | |||
| Waist-to-hip ratio | Model 1 | 58 | −0.11 (−0.73;0.52) | 0.731 |
| Model 2 | 58 | −0.08 (−0.66, 0.50) | 0.791 | |
| HDL-cholesterol | Model 1 | 57 | 0.22 (−0.82; 1.32) | 0.643 |
| Model 2 | 57 | 0.03 (−0.94, 1.00) | 0.947 | |
| Carbohydrates intake | Model 1 | 58 | 0.08 (−0.66, 0.83) | 0.822 |
| Model 2 | 58 | 0.15 (−0.55, 0.85) | 0.660 | |
| Fiber intake | Model 1 | 58 | −0.01 (−0.78, 0.77) | 0.990 |
| Model 2 | 58 | 0.07 (−0.66,0.81) | 0.838 | |
| MUFA intake | Model 1 | 58 | 0.58 (0.11, 1.05) | 0.016 |
| Model 2 | 58 | 0.46 (0.02, 0.93) | 0.048 | |
| Vitamin E intake | Model 1 | 58 | −0.15 (−0.82, 0.52) | 0.663 |
| Model 2 | 58 | −0.09 (−0.65, 0.48) | 0.760 | |
| m-Coumaric acid | Model 1 | 58 | 0.24 (−0.31, 0.79) | 0.627 |
| Model 2 | 58 | 0.23 (−0.29, 0.75) | 0.630 | |
| o-Coumaric acid | Model 1 | 58 | −0.25 (−1.27, 0.76) | 0.619 |
| Model 2 | 58 | −0.27 (−1.21, 0.67) | 0.571 | |
| p-Coumaric acid | Model 1 | 58 | −0.06 (−1.90, 1.78) | 0.949 |
| Model 2 | 58 | 0.06 (−1.54, 1.67) | 0.936 | |
| Resveratrol | Model 1 | 58 | 1.20 (0.01, 2.39) | 0.048 |
| Model 2 | 58 | 1.52 (0.37, 2.67) | 0.011 | |
| Flavones | Model 1 | 58 | −0.07 (−0.80, 0.65) | 0.838 |
| Model 2 | 58 | −0.03 (−0.72, 0.66) | 0.929 | |
| Anthocyanins | Model 1 | 58 | 0.79 (0.02, 1.56) | 0.044 |
| Model 2 | 58 | 0.81 (−0.02, 1.66) | 0.057 | |
MUFA: monounsaturated fatty acids. B: Non-standardized coefficient. CI: Confidence interval. Model 1: sex and age. Model 2: adjusted as in model 1 plus body mass index, physical activity, and energy intake. p-values shown in bold are statistically significant p < 0.050.
References
- Ros, E.; Hu, F.B. Consumption of Plant Seeds and Cardiovascular Health: Epidemiological and Clinical Trial Evidence. Circulation 2013, 128, 553–565. [Google Scholar] [CrossRef]
- Toomer, O.T. Nutritional Chemistry of the Peanut (Arachis Hypogaea). Crit. Rev. Food Sci. Nutr. 2018, 58, 3042–3053. [Google Scholar] [CrossRef]
- Gebicki, J.M.; Nauser, T. Fast Antioxidant Reaction of Polyphenols and Their Metabolites. Antioxidants 2021, 10, 1297. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Paz-Graniel, I.; Kendall, C.; Kahleova, H.; Rahelić, D.; Sievenpiper, J.L.; Salas-Salvadó, J. Nut Consumption and Incidence of Cardiovascular Diseases and Cardiovascular Disease Mortality: A Meta-Analysis of Prospective Cohort Studies. Nutr. Rev. 2019, 77, 691–709. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Paz-Graniel, I.; Hernández-Alonso, P.; Jenkins, D.J.A.; Kendall, C.W.C.; Sievenpiper, J.L.; Salas-Salvadó, J. Nut Consumption and Type 2 Diabetes Risk: A Systematic Review and Meta-Analysis of Observational Studies. Am. J. Clin. Nutr. 2021, 113, 960–971. [Google Scholar] [CrossRef]
- Ros, E.; Singh, A.; O’keefe, J.H. Nuts: Natural Pleiotropic Nutraceuticals. Nutrients 2021, 13, 3269. [Google Scholar] [CrossRef]
- Rusu, M.E.; Mocan, A.; Ferreira, I.C.F.R.; Popa, D.S. Health Benefits of Nut Consumption in Middle-Aged and Elderly Population. Antioxidants 2019, 8, 302. [Google Scholar] [CrossRef]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte Telomere Length and Risk of Cardiovascular Disease: Systematic Review and Meta-Analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef]
- D’Mello, M.J.J.; Ross, S.A.; Briel, M.; Anand, S.S.; Gerstein, H.; Paré, G. Association between Shortened Leukocyte Telomere Length and Cardiometabolic Outcomes: Systematic Review and Meta-Analysis. Circ. Cardiovasc. Genet. 2015, 8, 82–90. [Google Scholar] [CrossRef]
- Parilli-Moser, I.; Domínguez-López, I.; Vallverdú-Queralt, A.; Hurtado-Barroso, S.; Lamuela-Raventós, R.M. Urinary Phenolic Metabolites Associated with Peanut Consumption May Have a Beneficial Impact on Vascular Health Biomarkers. Antioxidants 2023, 12, 698. [Google Scholar] [CrossRef]
- Parilli-Moser, I.; Domínguez-López, I.; Trius-Soler, M.; Castellví, M.; Bosch, B.; Castro-Barquero, S.; Estruch, R.; Hurtado-Barroso, S.; Lamuela-Raventós, R.M. Consumption of Peanut Products Improves Memory and Stress Response in Healthy Adults from the ARISTOTLE Study: A 6-Month Randomized Controlled Trial. Clin. Nutr. 2021, 40, 5556–5567. [Google Scholar] [CrossRef]
- Blackburn, E.H. Structure and Function of Telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Frenck, R.W.; Blackburn, E.H.; Shannon, K.M. The Rate of Telomere Sequence Loss in Human Leukocytes Varies with Age. Proc. Natl. Acad. Sci. USA 1998, 95, 5607–5610. [Google Scholar] [CrossRef]
- Aviv, A.; Chen, W.; Gardner, J.P.; Kimura, M.; Brimacombe, M.; Cao, X.; Srinivasan, S.R.; Berenson, G.S. Leukocyte Telomere Dynamics: Longitudinal Findings among Young Adults in the Bogalusa Heart Study. Am. J. Epidemiol. 2009, 169, 323–329. [Google Scholar] [CrossRef]
- Shalev, I.; Entringer, S.; Wadhwa, P.D.; Wolkowitz, O.M.; Puterman, E.; Lin, J.; Epel, E.S. Stress and Telomere Biology: A Lifespan Perspective. Psychoneuroendocrinology 2013, 38, 1835–1842. [Google Scholar] [CrossRef]
- Gorenjak, V.; Petrelis, A.M.; Stathopoulou, M.G.; Visvikis-Siest, S. Telomere Length Determinants in Childhood. Clin. Chem. Lab. Med. 2020, 58, 162–177. [Google Scholar] [CrossRef]
- Okuda, K.; Bardeguez, A.; Gardner, J.P.; Rodriguez, P.; Ganesh, V.; Kimura, M.; Skurnick, J.; Awad, G.; Aviv, A. Telomere Length in the Newborn. Pediatr. Res. 2002, 52, 377–381. [Google Scholar] [CrossRef]
- Calado, R.T.; Dumitriu, B. Telomere Dynamics in Mice and Humans. Semin. Hematol. 2013, 50, 165–174. [Google Scholar] [CrossRef]
- Vidaček, N.Š.; Nanić, L.; Ravlić, S.; Sopta, M.; Gerić, M.; Gajski, G.; Garaj-Vrhovac, V.; Rubelj, I. Telomeres, Nutrition, and Longevity: Can We Really Navigate Our Aging? J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2018, 73, 39–47. [Google Scholar] [CrossRef]
- Galiè, S.; Canudas, S.; Muralidharan, J.; García-Gavilán, J.; Bulló, M.; Salas-Salvadó, J. Impact of Nutrition on Telomere Health: Systematic Review of Observational Cohort Studies and Randomized Clinical Trials. Adv. Nutr. 2020, 11, 576–601. [Google Scholar] [CrossRef]
- Factor-Litvak, P.; Susser, E. The Importance of Early Life Studies of Telomere Attrition. Paediatr. Perinat. Epidemiol. 2015, 29, 144–145. [Google Scholar] [CrossRef]
- Blackburn, E.H.; Epel, E.S.; Lin, J. Human Telomere Biology: A Contributory and Interactive Factor in Aging, Disease Risks, and Protection. Science 2015, 350, 1193–1198. [Google Scholar] [CrossRef]
- Tucker, L.A. Dietary Fiber and Telomere Length in 5674 U.S. Adults: An NHANES Study of Biological Aging. Nutrients 2018, 10, 400. [Google Scholar] [CrossRef]
- Juton, C.; Castro-barquero, S.; Casas, R.; Freitas, T.; Ruiz-león, A.M.; Crovetto, F.; Domenech, M.; Crispi, F.; Vieta, E.; Gratacós, E.; et al. Reliability and Concurrent and Construct Validity of a Food Frequency Questionnaire for Pregnant Women at High Risk to Develop Fetal Growth Restriction. Nutrients 2021, 13, 1629. [Google Scholar] [CrossRef]
- Elosua, R.; Marrugat, J.; Molina, L.; Pons, S.; Pujol, E. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish Men. Am. J. Epidemiol. 1994, 139, 1197–1209. [Google Scholar] [CrossRef]
- Elosua, R.; Garcia, M.; Aguilar, A.; Molina, L. Validation of the Minnesota Leisure Time Physical Activity Questionnaire in Spanish Women. Med. Sci. Sports Exerc. 2000, 32, 1431–1437. [Google Scholar] [CrossRef]
- Lin, J.; Smith, D.L.; Esteves, K.; Drury, S. Telomere Length Measurement by QPCR—Summary of Critical Factors and Recommendations for Assay Design. Psychoneuroendocrinology 2019, 99, 271–278. [Google Scholar] [CrossRef]
- Stout, S.A.; Lin, J.; Hernandez, N.; Davis, E.P.; Blackburn, E.; Carroll, J.E.; Glynn, L.M. Validation of Minimally-Invasive Sample Collection Methods for Measurement of Telomere Length. Front. Aging Neurosci. 2017, 9, 397. [Google Scholar] [CrossRef]
- Goldman, E.A.; Eick, G.N.; Compton, D.; Kowal, P.; Snodgrass, J.J.; Eisenberg, D.T.A.; Sterner, K.N. Evaluating Minimally Invasive Sample Collection Methods for Telomere Length Measurement. Am. J. Hum. Biol. 2018, 30, e23062. [Google Scholar] [CrossRef]
- Mitchell, C.; Hobcraft, J.; McLanahan, S.S.; Siegeld, S.R.; Berg, A.; Brooks-Gunn, J.; Garfinkel, I.; Notterman, D. Social Disadvantage, Genetic Sensitivity, and Children’s Telomere Length. Proc. Natl. Acad. Sci. USA 2014, 111, 5944–5949. [Google Scholar] [CrossRef]
- Cawthon, R.M. Telomere Length Measurement by a Novel Monochrome Multiplex Quantitative PCR Method. Nucleic Acids Res. 2009, 37, e21. [Google Scholar] [CrossRef]
- O’Callaghan, N.J.; Fenech, M. A Quantitative PCR Method for Measuring Absolute Telomere Length. Biol. Proced. 2011, 13, 3. [Google Scholar] [CrossRef]
- Blom, G. Statistical Estimates and Transformed Beta Variables. Inc. Stat. 1960, 10, 53. [Google Scholar] [CrossRef]
- Saretzki, G. Telomeres, Telomerase and Ageing. Biochem. Cell Biol. Ageing Part I Biomed. Sci. 2018, 90, 221–308. [Google Scholar]
- Crous-Bou, M.; Molinuevo, J.L.; Sala-Vila, A. Plant-Rich Dietary Patterns, Plant Foods and Nutrients, and Telomere Length. Adv. Nutr. 2019, 10, S296–S303. [Google Scholar] [CrossRef]
- Canudas, S.; Becerra-Tomas, N.; Hernandez-Alonso, P.; Galie, S.; Leung, C.; Crous-Bou, M.; De Vivo, I.; Gao, Y.; Gu, Y.; Meinila, J.; et al. Mediterranean Diet and Telomere Length: A Systematic Review and Meta-Analysis. Adv. Nutr. 2020, 11, 1544–1554. [Google Scholar] [CrossRef]
- Tucker, L.A. Consumption of Nuts and Seeds and Telomere Length in 5,582 Men and Women of the National Health and Nutrition Examination Survey (NHANES). J. Nutr. Health Aging 2017, 21, 233–240. [Google Scholar] [CrossRef]
- Freitas-Simoes, T.M.; Cofán, M.; Blasco, M.A.; Soberón, N.; Foronda, M.; Serra-Mir, M.; Roth, I.; Valls-Pedret, C.; Doménech, M.; Ponferrada-Ariza, E.; et al. Walnut Consumption for Two Years and Leukocyte Telomere Attrition in Mediterranean Elders: Results of a Randomized Controlled Trial. Nutrients 2018, 10, 1907. [Google Scholar] [CrossRef]
- Canudas, S.; Hernández-Alonso, P.; Galié, S.; Muralidharan, J.; Morell-Azanza, L.; Zalba, G.; García-Gavilán, J.; Martí, A.; Salas-Salvadó, J.; Bulló, M. Pistachio Consumption Modulates DNA Oxidation and Genes Related to telomere Maintenance: A Crossover Randomized Clinical Trial. Am. J. Clin. Nutr. 2019, 109, 1738–1745. [Google Scholar] [CrossRef]
- Fernández de la Puente, M.; Hernández-Alonso, P.; Canudas, S.; Marti, A.; Fitó, M.; Razquin, C.; Salas-Salvadó, J. Modulation of Telomere Length by Mediterranean Diet, Caloric Restriction, and Exercise: Results from PREDIMED-Plus Study. Antioxidants 2021, 10, 1596. [Google Scholar] [CrossRef]
- García-Calzón, S.; Martínez-González, M.A.; Razquin, C.; Arós, F.; Lapetra, J.; Martínez, J.A.; Zalba, G.; Marti, A. Mediterranean Diet and Telomere Length in High Cardiovascular Risk Subjects from the PREDIMED-NAVARRA Study. Clin. Nutr. 2016, 35, 1399–1405. [Google Scholar] [CrossRef]
- Ros, E. Health Benefits of Nut Consumption. Nutrients 2010, 2, 652–682. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’toole, P.W.; O’connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Ridout, K.K.; Ridout, S.J.; Price, L.H.; Sen, S.; Tyrka, A.R. Depression and Telomere Length: A Meta-Analysis. J. Affect. Disord. 2016, 191, 237–247. [Google Scholar] [CrossRef]
- González-Becerra, K.; Ramos-Lopez, O.; Barrón-Cabrera, E.; Riezu-Boj, J.I.; Milagro, F.I.; Martínez-López, E.; Martínez, J.A. Fatty Acids, Epigenetic Mechanisms and Chronic Diseases: A Systematic Review. Lipids Health Dis. 2019, 18, 178. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic Acid Derivatives: A Potential Class of Natural Compounds for the Management of Lipid Metabolism and Obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Fung, T.T.; Prescott, J.; Julin, B.; Du, M.; Sun, Q.; Rexrode, K.M.; Hu, F.B.; De Vivo, I. Mediterranean Diet and Telomere Length in Nurses’ Health Study: Population Based Cohort Study. BMJ 2014, 349, g6674. [Google Scholar] [CrossRef]
- Song, Y.; You, N.C.Y.; Song, Y.; Kang, M.K.; Hou, L.; Wallace, R.; Eaton, C.B.; Tinker, L.F.; Liu, S. Intake of Small-to-Medium-Chain Saturated Fatty Acids Is Associated with Peripheral Leukocyte Telomere Length in Postmenopausal Women1-3. J. Nutr. 2013, 143, 907–914. [Google Scholar] [CrossRef]
- Tiainen, A.M.; Männistö, S.; Blomstedt, P.A.; Moltchanova, E.; Perälä, M.M.; Kaartinen, N.E.; Kajantie, E.; Kananen, L.; Hovatta, I.; Eriksson, J.G. Leukocyte Telomere Length and Its Relation to Food and Nutrient Intake in an Elderly Population. Eur. J. Clin. Nutr. 2012, 66, 1290–1294. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Goldfine, I.D.; Maddux, B.A.; Grodsky, G.M. Are Oxidative Stress−Activated Signaling Pathways Mediators of Insulin Resistance and β-Cell Dysfunction? Diabetes 2003, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Nut Consumption and Risk of Cardiovascular Disease, Total Cancer, All-Cause and Cause-Specific Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. BMC Med. 2016, 14, 207. [Google Scholar] [CrossRef]
- Naghshi, S.; Sadeghian, M.; Nasiri, M.; Mobarak, S.; Asadi, M.; Sadeghi, O. Association of Total Nut, Tree Nut, Peanut, and Peanut Butter Consumption with Cancer Incidence and Mortality: A Comprehensive Systematic Review and Dose-Response Meta-Analysis of Observational Studies. Adv. Nutr. 2021, 12, 793–808. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).