Selenium-Binding Protein 1-Deficient Dendritic Cells Protect Mice from Sepsis by Increased Treg/Th17
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. SELENBP1 Analysis in Public Databases and Blood from Sepsis Patients
2.3. Mice
2.4. Septic Shock Model
2.5. Septic Model
2.6. HE Staining
2.7. Spleen Immunophenotype Assay
2.8. Immune Cell Detection in the Blood and Ascites of Septic Mice
2.9. DCs Preparation and Phenotypic Detection
2.10. Cell Viability and Apoptosis Assay
2.11. Migration Assay
2.12. Phagocytosis Assay
2.13. mDCs-Induced Differentiation of CD4+ T Cells
2.14. Redox Status Assay
2.15. T-BHP Treatment
2.16. Cytokine Detection
2.17. RT-PCR
2.18. Sepsis Treatment
2.19. Western Blotting
2.20. Statistical Analysis
3. Results
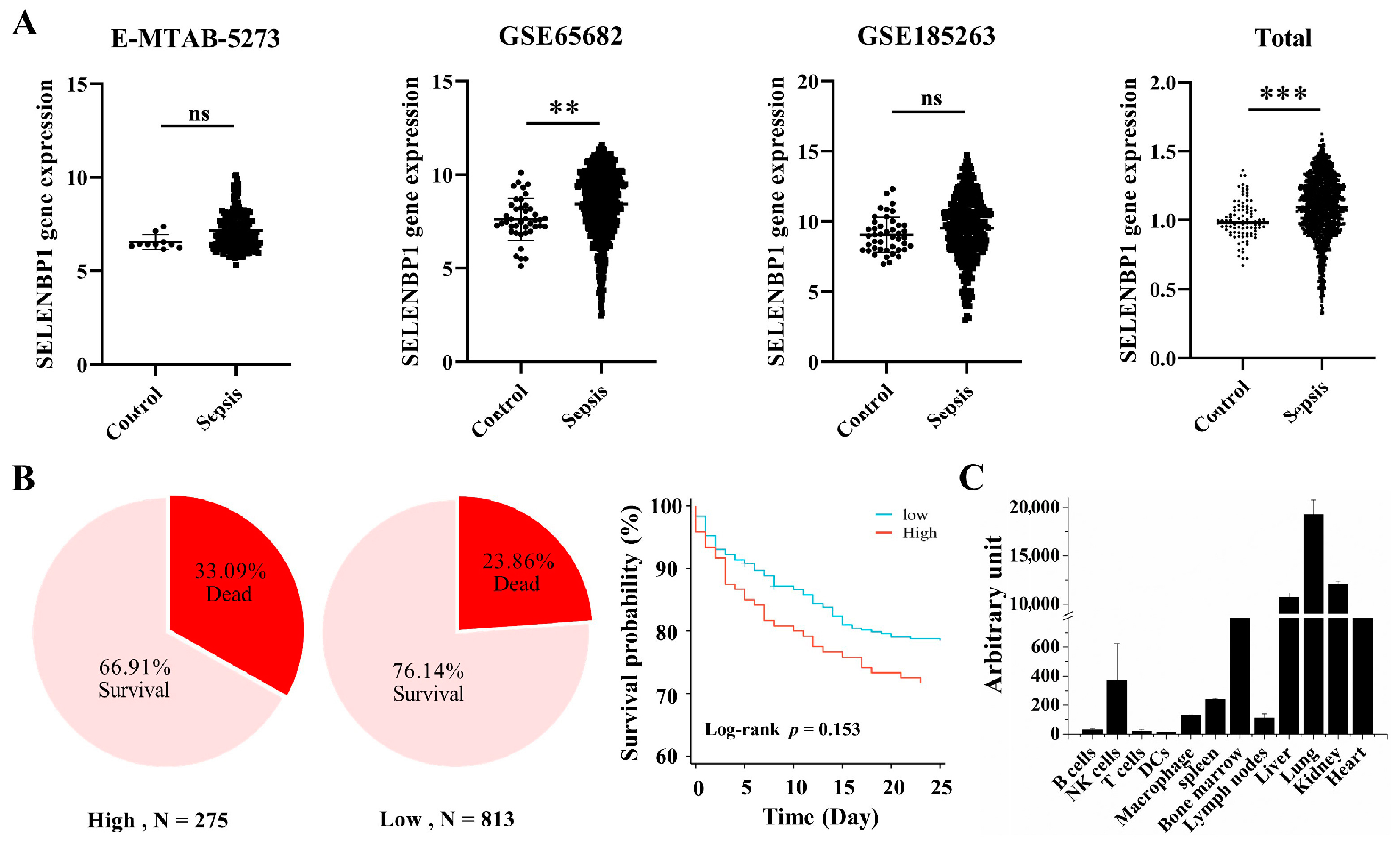
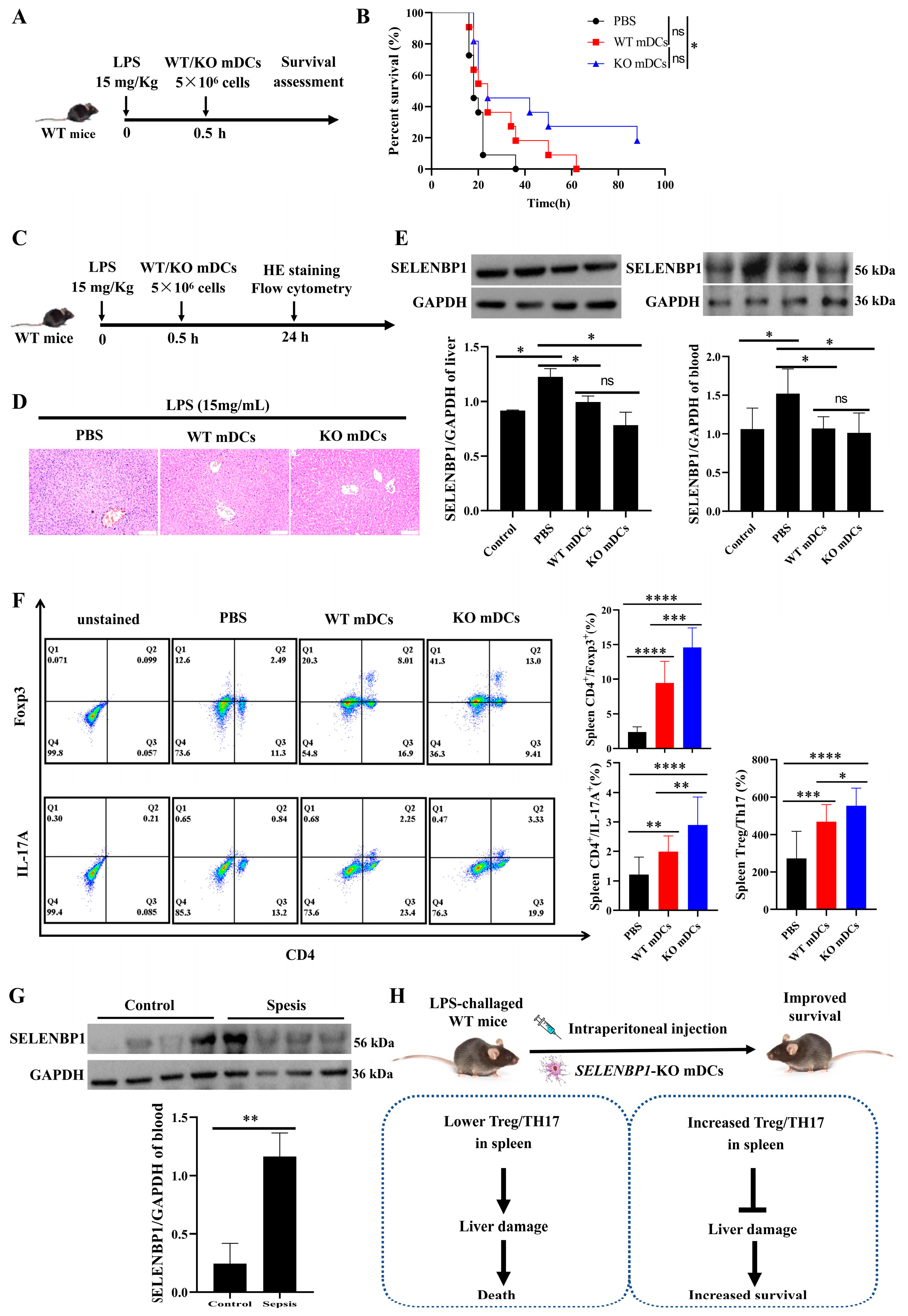
3.1. Lower SELENBP1 Expression Was Associated with Higher Survival in Sepsis
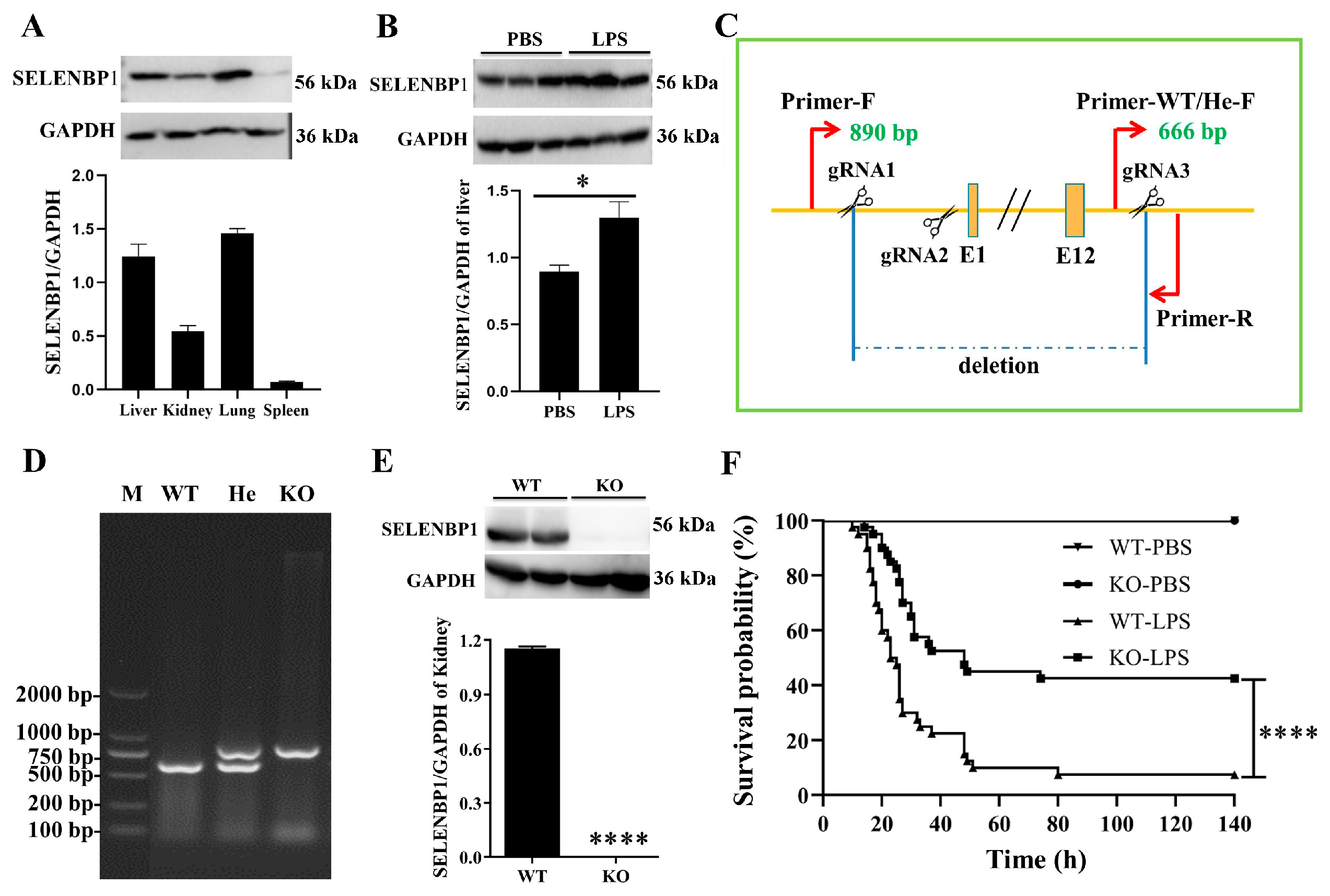
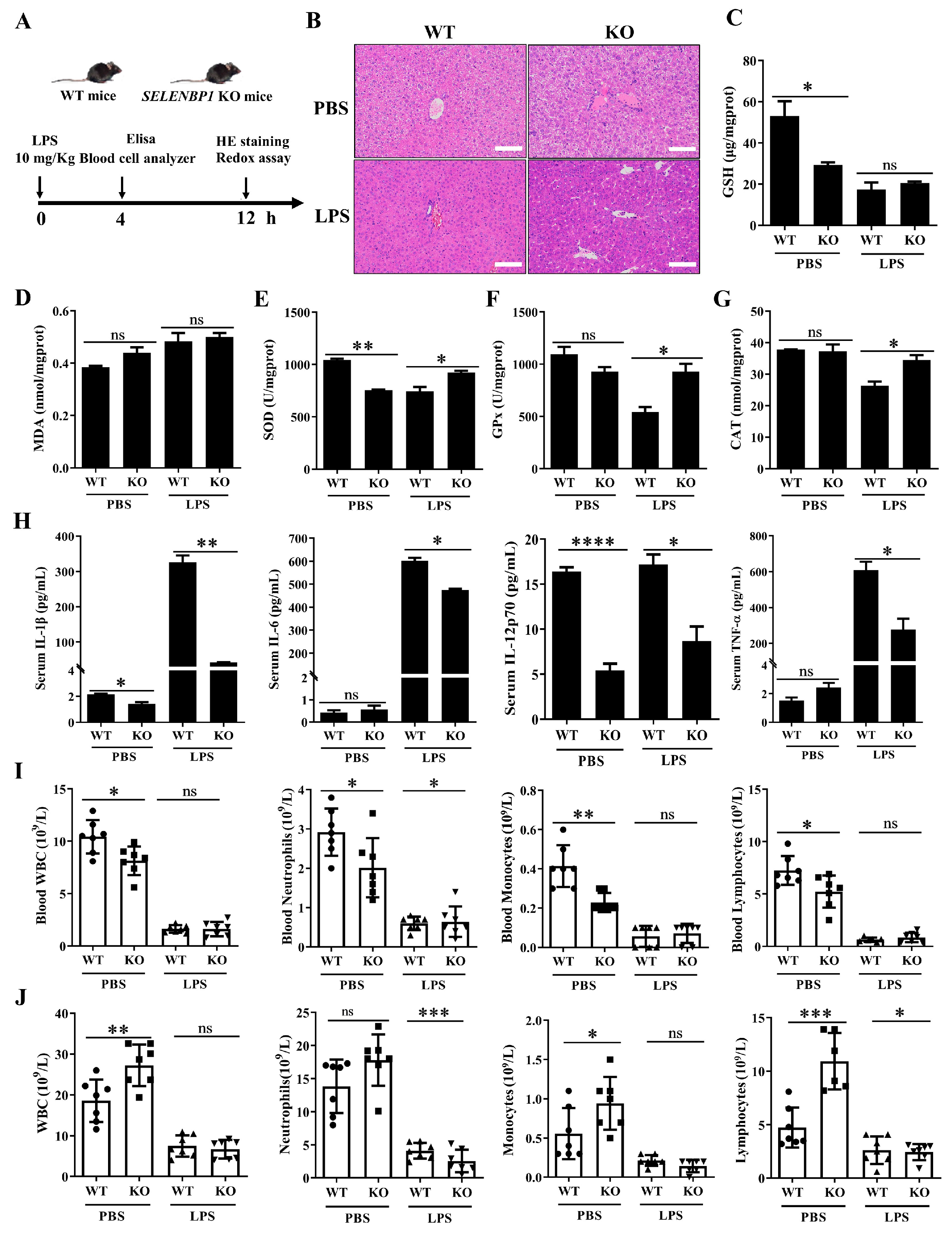
3.2. LPS-Challenged SELENBP1-KO Mice Had a Minor Liver Injury
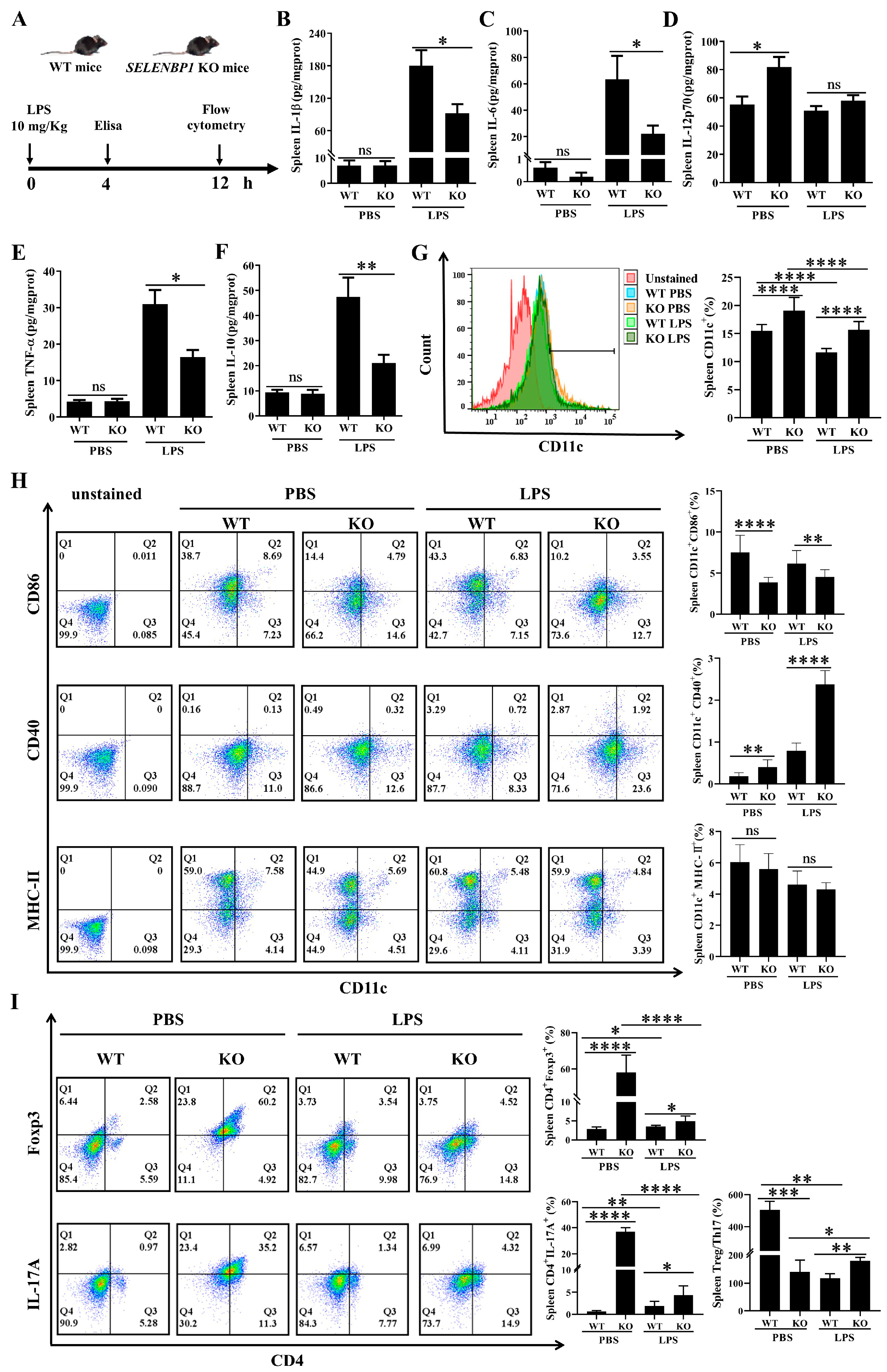
3.3. LPS-Challenged SELENBP1-KO Mice Showed Decreased Inflammation and Increased Immunosuppression in the Spleen
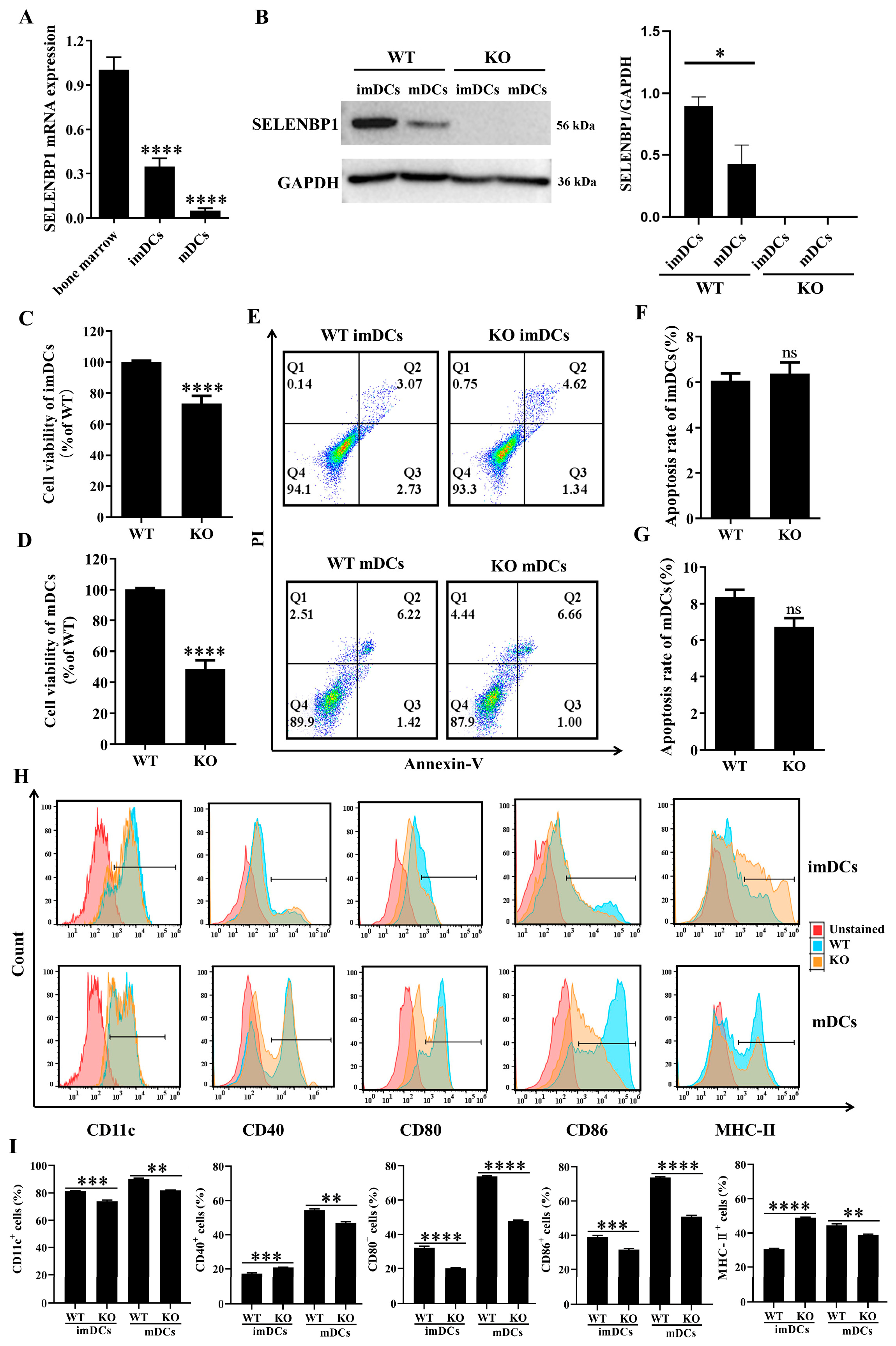
3.4. SELENBP1 Deficiency Inhibited DCs Maturation
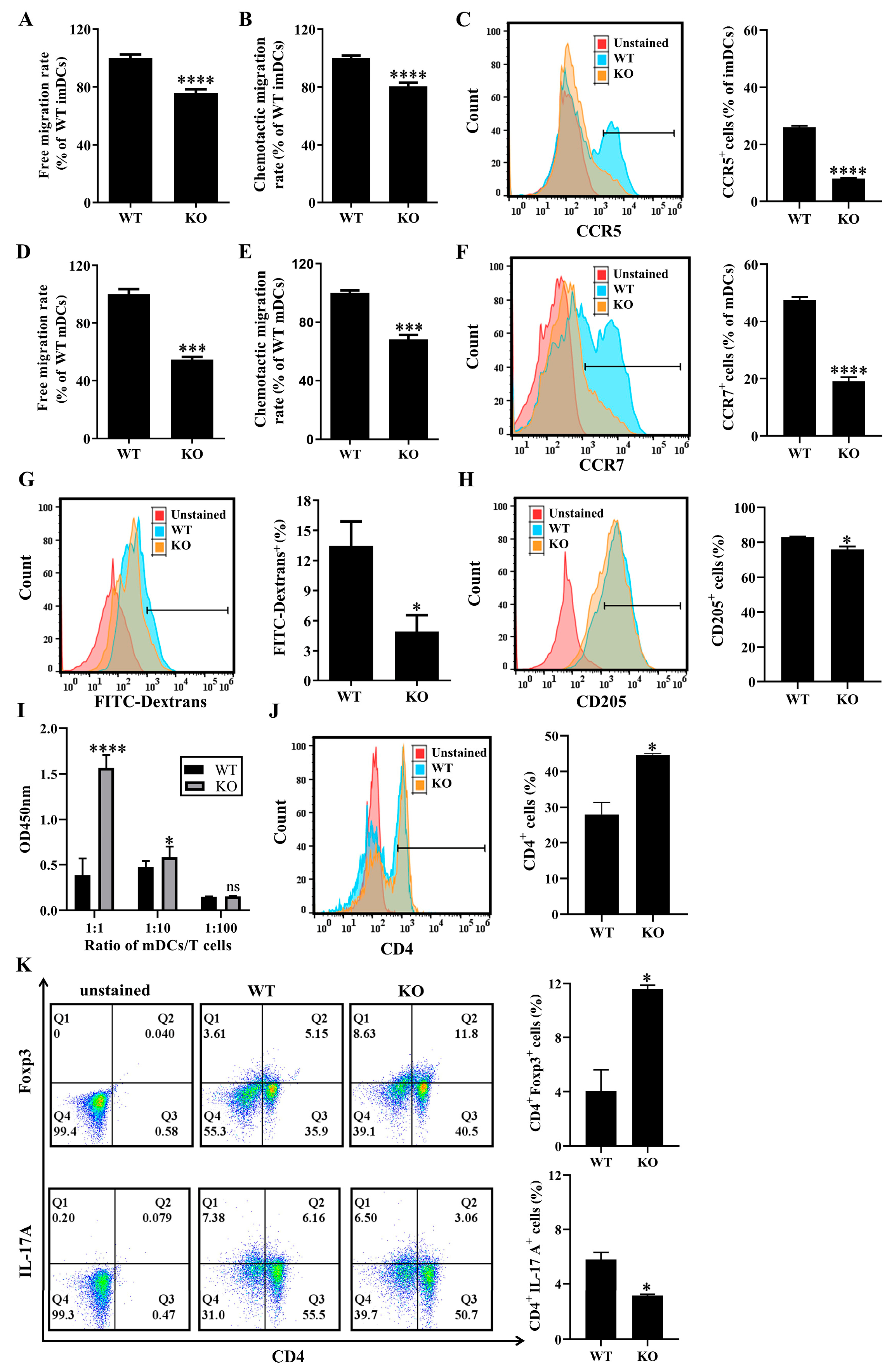
3.5. SELENBP1 Deficiency Altered the Immune Functions of DCs
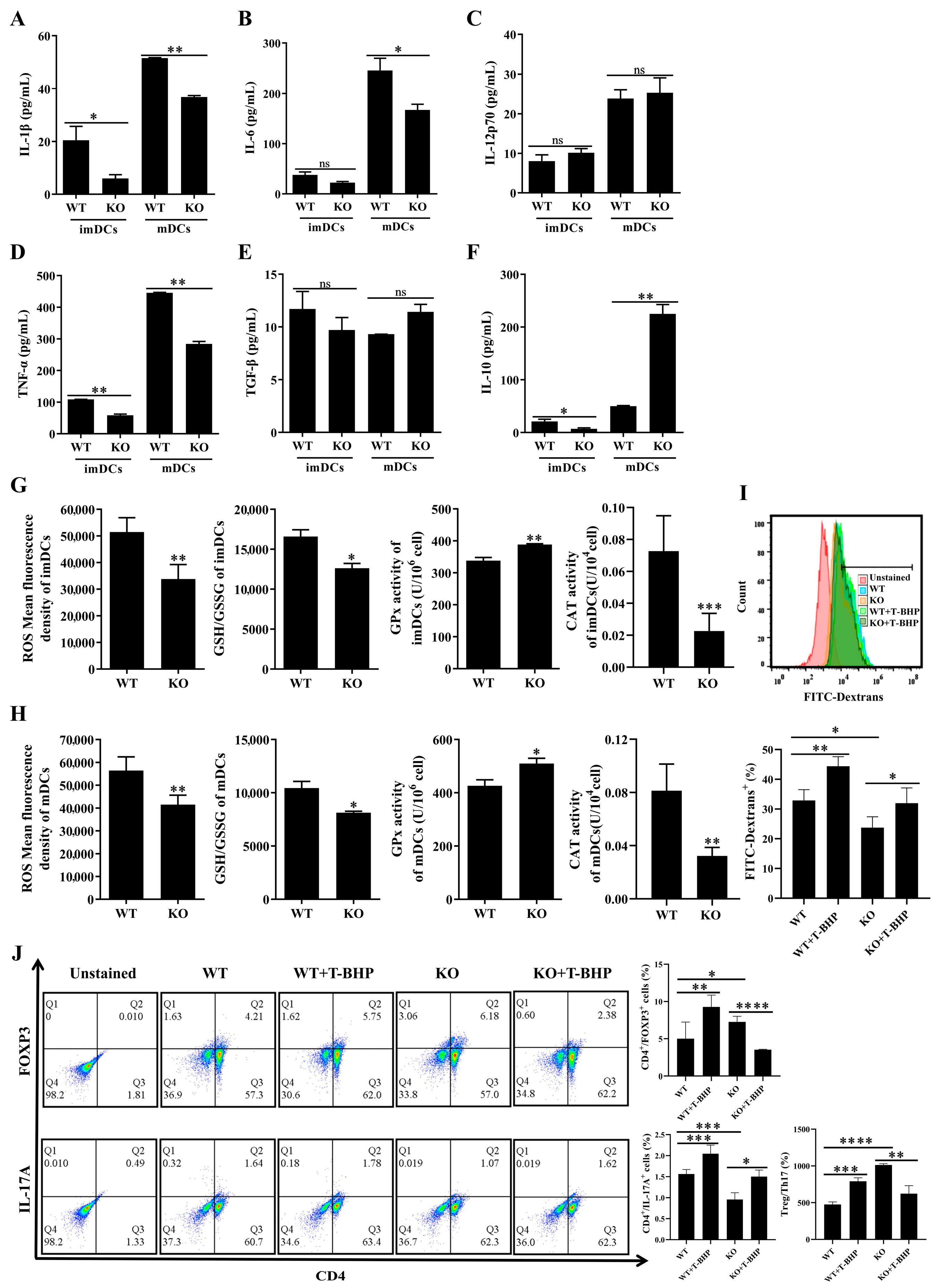
3.6. Redox Imbalance Involved the Immune Function of SELENBP1-Deficient DCs
3.7. Assessment of SELENBP1-KO mDCs for Sepsis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dervisi, I.; Valassakis, C.; Koletti, A.; Kouvelis, V.N.; Flemetakis, E.; Ouzounis, C.A.; Roussis, A. Evolutionary aspects of selenium binding protein (SBP). J. Mol. Evol. 2023, 91, 471–481. [Google Scholar] [CrossRef]
- Jia, Y.; Dai, J.; Zhang, L.; Xia, H. Biological functions of selenium-binding protein 1 and its relationship with diseases. Prog. Biochem. Biophys. 2019, 46, 128–137. [Google Scholar]
- Elhodaky, M.; Diamond, A.M. Selenium-binding protein 1 in human health and disease. Int. J. Mol. Sci. 2018, 19, 3437. [Google Scholar] [CrossRef] [PubMed]
- Koletti, A.; Dervisi, I.; Kalloniati, C.; Zografaki, M.E.; Rennenberg, H.; Roussis, A.; Flemetakis, E. Selenium-binding protein 1 (SBD1): A stress response regulator in Chlamydomonas reinhardtii. Plant Physiol. 2022, 189, 2368–2381. [Google Scholar] [CrossRef]
- Tobe, R.; Mihara, H. Delivery of selenium to selenophosphate synthetase for selenoprotein biosynthesis. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Goldberg, M.L.; Pohl, N.M.; Bi, X.; Tong, C.; Xiong, B.; Koh, T.J.; Diamond, A.M.; Yang, W. Functional and physical interaction between the selenium-binding protein 1 (SBP1) and the glutathione peroxidase 1 selenoprotein. Carcinogenesis 2010, 31, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Sasuclark, A.R.; Khadka, V.S.; Pitts, M.W. Cell-type specific analysis of selenium-related genes in brain. Antioxidants 2019, 8, 120. [Google Scholar] [CrossRef]
- Philipp, T.M.; Gernoth, L.; Will, A.; Schwarz, M.; Ohse, V.A.; Kipp, A.P.; Steinbrenner, H.; Klotz, L.O. Selenium-binding protein 1 (SELENBP1) is a copper-dependent thiol oxidase. Redox Biol. 2023, 65, 102807. [Google Scholar] [CrossRef]
- Pol, A.; Renkema, G.H.; Tangerman, A.; Winkel, E.G.; Engelke, U.F.; de Brouwer, A.P.M.; Lloyd, K.C.; Araiza, R.S.; van den Heuvel, L.; Omran, H.; et al. Mutations in SELENBP1, encoding a novel human methanethiol oxidase, cause extraoral halitosis. Nat. Genet. 2018, 50, 120–129. [Google Scholar] [CrossRef]
- Lee, E.K.; Shin, Y.J.; Park, E.Y.; Kim, N.D.; Moon, A.; Kwack, S.J.; Son, J.Y.; Kacew, S.; Lee, B.M.; Bae, O.N.; et al. Selenium-binding protein 1: A sensitive urinary biomarker to detect heavy metal-induced nephrotoxicity. Arch. Toxicol. 2017, 91, 1635–1648. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, A.; Li, C.; Zhang, P.; Su, X.; Li, Y.; Mu, C.; Li, T. The link between selenium binding protein from Sinonovacula constricta and environmental pollutions exposure. Fish Shellfish Immunol. 2013, 35, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Valassakis, C.; Livanos, P.; Minopetrou, M.; Haralampidis, K.; Roussis, A. Promoter analysis and functional implications of the selenium binding protein (SBP) gene family in Arabidopsis thaliana. J. Plant Physiol. 2018, 224–225, 19–29. [Google Scholar] [CrossRef]
- Ying, Q.; Ansong, E.; Diamond, A.M.; Lu, Z.; Yang, W.; Bie, X. Quantitative proteomic analysis reveals that anti-cancer effects of selenium-binding protein 1 in vivo are associated with metabolic pathways. PLoS ONE 2015, 10, e0126285. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, S.; Du, Y.; Dai, Y.; Huai, Q.; Li, X.; Du, Y.; Dai, H.; Yuan, W.; Yin, S.; et al. Tumor microenvironment-related gene selenium-binding protein 1 (SELENBP1) is associated with immunotherapy efficacy and survival in colorectal cancer. BMC Gastroenterol. 2022, 22, 437. [Google Scholar] [CrossRef]
- Ma, J.; Huang, X.; Xu, J.; Li, Z.; Lai, J.; Shen, Y.; Zhao, J.; Sun, X.; Ma, L. SBP1 promotes tumorigenesis of thyroid cancer through TXN/NIS pathway. Mol. Med. 2023, 29, 121. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Wang, J.; Wang, L.; Wang, Q.; Guo, Z.; Xu, M.; Jiang, N. Integrated proteomic and metabolomic profile analyses of cardiac valves revealed molecular mechanisms and targets in calcific aortic valve disease. Front. Cardiovasc. Med. 2022, 9, 944521. [Google Scholar] [CrossRef] [PubMed]
- Kühn, E.C.; Slagman, A.; Kühn-Heid, E.C.D.; Seelig, J.; Schwiebert, C.; Minich, W.B.; Stoppe, C.; Möckel, M.; Schomburg, L. Circulating levels of selenium-binding protein 1 (SELENBP1) are associated with risk for major adverse cardiac events and death. J. Trace Elem. Med. Biol. 2019, 52, 247–253. [Google Scholar] [CrossRef]
- Kühn-Heid, E.C.D.; Kühn, E.C.; Ney, J.; Wendt, S.; Seelig, J.; Schwiebert, C.; Minich, W.B.; Stoppe, C.; Schomburg, L. Selenium-binding protein 1 indicates myocardial stress and risk for adverse outcome in cardiac surgery. Nutrients 2019, 11, 2005. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, X.; Wang, Y.; Liu, Y.; Dai, J.; Zhang, L.; Wu, X.; Zhang, J.; Xiang, H.; Yang, Y.; et al. Knocking out selenium binding protein 1 induces depressive-like behavior in mice. Biol. Trace Elem. Res. 2024, 202, 3149–3162. [Google Scholar] [CrossRef]
- Philipp, T.M.; Gong, W.; Köhnlein, K.; Ohse, V.A.; Müller, F.I.; Priebs, J.; Steinbrenner, H.; Klotz, L.O. SEMO-1, a novel methanethiol oxidase in Caenorhabditis elegans, is a pro-aging factor conferring selective stress resistance. Biofactors 2022, 48, 699–706. [Google Scholar] [CrossRef]
- Köhnlein, K.; Urban, N.; Guerrero-Gómez, D.; Steinbrenner, H.; Urbánek, P.; Priebs, J.; Koch, P.; Kaether, C.; Miranda-Vizuete, A.; Klotz, L.O. A Caenorhabditis elegans ortholog of human selenium-binding protein 1 is a pro-aging factor protecting against selenite toxicity. Redox Biol. 2020, 28, 101323. [Google Scholar] [CrossRef]
- Bai, J.; Yu, J.; Wang, J.; Xue, B.; He, N.; Tian, Y.; Yang, L.; Wang, Y.; Wang, Y.; Tang, Q. DNA methylation of miR-122 aggravates oxidative stress in colitis targeting SELENBP1 partially by p65NF-κB signaling. Oxid. Med. Cell. Longev. 2019, 2019, 5294105. [Google Scholar] [CrossRef]
- Steinbrenner, H.; Micoogullari, M.; Hoang, N.A.; Bergheim, I.; Klotz, L.O.; Sies, H. Selenium-binding protein 1 (SELENBP1) is a marker of mature adipocytes. Redox Biol. 2019, 20, 489–495. [Google Scholar] [CrossRef]
- Li, T.; Yang, W.; Li, M.; Byun, D.S.; Tong, C.; Nasser, S.; Zhuang, M.; Arango, D.; Mariadason, J.M.; Augenlicht, L.H. Expression of selenium-binding protein 1 characterizes intestinal cell maturation and predicts survival for patients with colorectal cancer. Mol. Nutr. Food Res. 2008, 52, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Scheller, A.S.; Philipp, T.M.; Klotz, L.O.; Steinbrenner, H. Altered capacity for H2S production during the spontaneous differentiation of Caco-2 cells to colonocytes due to reciprocal regulation of CBS and SELENBP1. Antioxidants 2022, 11, 1957. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Cao, L.; Zhou, R.; Jiang, Z.; Xiao, K.; Kong, W.; Wang, H.; Deng, J.; Wen, B.; Tan, F.; et al. Identification of novel biomarkers for sepsis prognosis via urinary proteomic analysis using iTRAQ labeling and 2D-LC-MS/MS. PLoS ONE 2013, 8, e54237. [Google Scholar] [CrossRef] [PubMed]
- Joffre, J.; Hellman, J. Oxidative stress and endothelial dysfunction in sepsis and acute inflammation. Antioxid. Redox Signal. 2021, 35, 1291–1307. [Google Scholar] [CrossRef]
- Liu, D.; Huang, S.Y.; Sun, J.H.; Zhang, H.C.; Cai, Q.L.; Gao, C.; Li, L.; Cao, J.; Xu, F.; Zhou, Y.; et al. Sepsis-induced immunosuppression: Mechanisms, diagnosis and current treatment options. Mil. Med. Res. 2022, 9, 56. [Google Scholar] [CrossRef]
- van der Poll, T.; Shankar-Hari, M.; Wiersinga, W.J. The immunology of sepsis. Immunity 2021, 54, 2450–2464. [Google Scholar] [CrossRef]
- Venet, F.; Monneret, G. Advances in the understanding and treatment of sepsis-induced immunosuppression. Nat. Rev. Nephrol. 2018, 14, 121–137. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Yang, Y.; Luo, N.; Yang, J.; Zhong, L.; Guo, T.; Yuan, Z.; Wei, Q.; Wang, C. Protective role of the novel cytokine Metrnl/interleukin-41 in host immunity defense during sepsis by promoting macrophage recruitment and modulating Treg/Th17 immune cell balance. Clin. Immunol. 2023, 254, 109690. [Google Scholar] [CrossRef] [PubMed]
- Ness, S.; Lin, S.; Gordon, J.R. Regulatory dendritic cells, T cell tolerance, and dendritic cell therapy for immunologic disease. Front. Immunol. 2021, 12, 633436. [Google Scholar] [CrossRef] [PubMed]
- Morante-Palacios, O.; Fondelli, F.; Ballestar, E.; Martínez-Cáceres, E.M. Tolerogenic dendritic cells in autoimmunity and inflammatory diseases. Trends Immunol. 2021, 42, 59–75. [Google Scholar] [CrossRef]
- Ozeri, E.; Rider, P.; Rigbi, S.; Shahaf, G.; Nita, I.I.; Sekler, I.; Lewis, E.C.; Schuster, R. Differential signaling patterns of stimulated bone marrow-derived dendritic cells under α1-antitrypsin-enriched conditions. Cell. Immunol. 2021, 361, 104281. [Google Scholar] [CrossRef]
- Kumar, V. Dendritic cells in sepsis: Potential immunoregulatory cells with therapeutic potential. Mol. Immunol. 2018, 101, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, X.; Saredy, J.; Yuan, Z.; Yang, X.; Wang, H. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. 2020, 37, 101759. [Google Scholar] [CrossRef]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox regulation of the immune response. Cell. Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef]
- Deng, B.; He, X.; Wang, D.; Wang, Y.; Jiang, Y.; Chen, T.; Xu, L. Designing selenium nanoadjuvant as universal agent for live-killed virus-based vaccine. Small Methods 2023, 7, e2300293. [Google Scholar] [CrossRef]
- Xia, H.; Wang, Y.; Dai, J.; Zhang, X.; Zhou, J.; Zeng, Z.; Jia, Y. Selenoprotein K is essential for the migration and phagocytosis of immature dendritic cells. Antioxidants 2022, 11, 1264. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, H.; Xia, K.; Liu, X.; Zhang, X.; Dai, J.; Zeng, Z.; Jia, Y. Selenium regulation of the immune function of dendritic cells in mice through the ERK, Akt and RhoA/ROCK pathways. Biol. Trace Elem. Res. 2021, 199, 3360–3370. [Google Scholar] [CrossRef]
- Lee, H.J.; Park, J.S.; Yoo, H.J.; Lee, H.M.; Lee, B.C.; Kim, J.H. The selenoprotein MsrB1 instructs dendritic cells to induce T-helper 1 immune responses. Antioxidants 2020, 9, 1021. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, L.; Liu, X.; Zhang, S.; Dai, J.; Huang, J.; Chen, J.; Wang, Y.; Zhou, J.; Zeng, Z. Selenium can regulate the differentiation and immune function of human dendritic cells. Biometals 2021, 34, 1365–1379. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, L.; Dai, J.; Liu, X.; Zhang, X.; Zeng, Z.; Jia, Y. Effect of selenium and peroxynitrite on immune function of immature dendritic cells in humans. Med. Sci. Monit. 2021, 27, e929004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Xia, K.; Dai, J.; Huang, J.; Wang, Y.; Zhu, G.; Hu, Z.; Zeng, Z.; Jia, Y. Effects of dietary selenium on immune function of spleen in mice. J. Funct. Foods 2022, 89, 104914. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, C.; Pan, T.; Yao, H.; Li, S. Selenium accelerates chicken dendritic cells differentiation and affects selenoproteins expression. Dev. Comp. Immunol. 2017, 77, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Xu, Z.; Wang, D.; Yao, H.; Li, S. Selenium deficiency inhibits differentiation and immune function and imbalances the Th1/Th2 of dendritic cells. Metallomics 2018, 10, 759–767. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Höge, S.C.; Li, P.A.; Hoffmann, F.W.; Hashimoto, A.C.; Berry, M.J. The selenoproteome exhibits widely varying, tissue-specific dependence on selenoprotein P for selenium supply. Nucleic Acids Res. 2007, 35, 3963–3973. [Google Scholar] [CrossRef]
- Yu, J.; Xue, J.; Liu, C.; Zhang, A.; Qin, L.; Liu, J.; Yang, Y. MiR-146a-5p accelerates sepsis through dendritic cell activation and glycolysis via targeting ATG7. J. Biochem. Mol. Toxicol. 2022, 36, e23151. [Google Scholar] [CrossRef]
- Pierrakos, C.; Velissaris, D.; Bisdorff, M.; Marshall, J.C.; Vincent, J.L. Biomarkers of sepsis: Time for a reappraisal. Crit. Care 2020, 24, 287. [Google Scholar] [CrossRef]
- Komorowski, M.; Green, A.; Tatham, K.C.; Seymour, C.; Antcliffe, D. Sepsis biomarkers and diagnostic tools with a focus on machine learning. EBioMedicine 2022, 86, 104394. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ghimire, L.; Balasubramanian, A.; Hsu, A.Y.; Zhang, Z.; Yu, H.; Ma, F.; Luo, H.R. Neutrophil-specific depletion of gasdermin D does not protect against murine sepsis. Blood 2023, 141, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Xing, H.; Cao, S.; Long, X.; Liu, H.; Ma, J.; Guo, F.; Deng, Z.; Liu, X. Neutrophils restrain sepsis associated coagulopathy via extracellular vesicles carrying superoxide dismutase 2 in a murine model of lipopolysaccharide induced sepsis. Nat. Commun. 2022, 13, 4583. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Fu, Z.; Huang, W.; Huang, K. Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A meta-analysis. Am. J. Emerg. Med. 2020, 38, 641–647. [Google Scholar] [CrossRef]
- Zhang, Q.; Wei, J.; Liu, Z.; Huang, X.; Sun, M.; Lai, W.; Chen, Z.; Wu, J.; Chen, Y.; Guo, X.; et al. STING signaling sensing of DRP1-dependent mtDNA release in kupffer cells contributes to lipopolysaccharide-induced liver injury in mice. Redox Biol. 2022, 54, 102367. [Google Scholar] [CrossRef]
- Hafkamp, F.M.J.; Groot Kormelink, T.; de Jong, E.C. Targeting DCs for tolerance induction: Don’t lose sight of the neutrophils. Front. Immunol. 2021, 12, 732992. [Google Scholar] [CrossRef]
- Liu, X.; Yu, P.; Xu, Y.; Wang, Y.; Chen, J.; Tang, F.; Hu, Z.; Zhou, J.; Liu, L.; Qiu, W.; et al. Metformin induces tolerogenicity of dendritic cells by promoting metabolic reprogramming. Cell. Mol. Life Sci. 2023, 80, 283. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Aschenbrenner, A.C.; Bauer, M.; Bock, C.; Calandra, T.; Gat-Viks, I.; Kyriazopoulou, E.; Lupse, M.; Monneret, G.; Pickkers, P.; et al. The pathophysiology of sepsis and precision-medicine-based immunotherapy. Nat. Immunol. 2024, 25, 19–28. [Google Scholar] [CrossRef]
- Ramírez-Valle, F.; Maranville, J.C.; Roy, S.; Plenge, R.M. Sequential immunotherapy: Towards cures for autoimmunity. Nat. Rev. Drug Discov. 2024, 23, 501–524. [Google Scholar] [CrossRef]
- Mansilla, M.J.; Hilkens, C.M.U.; Martínez-Cáceres, E.M. Challenges in tolerogenic dendritic cell therapy for autoimmune diseases: The route of administration. Immunother. Adv. 2023, 3, ltad012. [Google Scholar] [CrossRef]
- Sheth, M.; Benedum, C.M.; Celi, L.A.; Mark, R.G.; Markuzon, N. The association between autoimmune disease and 30-day mortality among sepsis ICU patients: A cohort study. Crit. Care 2019, 23, 93. [Google Scholar] [CrossRef]
- Lai, H.; Xu, L.; Liu, C.; Shi, S.; Jiang, Y.; Yu, Y.; Deng, B.; Chen, T. Universal selenium nanoadjuvant with immunopotentiating and redox-shaping activities inducing high-quality immunity for SARS-CoV-2 vaccine. Signal Transduct. Target. Ther. 2023, 8, 88. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Zheng, R.; Lai, J.; Su, J.; Li, J.; Zhu, B.; Chen, T. Translational selenium nanoparticles boost GPx1 activation to reverse HAdV-14 virus-induced oxidative damage. Bioact. Mater. 2024, 38, 276–291. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Deng, B.; Zou, B.; Li, Y.; Bu, Q.; Tian, Y.; Chen, M.; Chen, W.; Kong, N.; Chen, T.; et al. Oral hydrogel microbeads-mediated in situ synthesis of selenoproteins for regulating intestinal immunity and microbiota. J. Am. Chem. Soc. 2023, 145, 12193–12205. [Google Scholar] [CrossRef]
- Bhattarai, U.; Xu, R.; He, X.; Pan, L.; Niu, Z.; Wang, D.; Zeng, H.; Chen, J.X.; Clemmer, J.S.; Chen, Y. High selenium diet attenuates pressure overload-induced cardiopulmonary oxidative stress, inflammation, and heart failure. Redox Biol. 2024, 76, 103325. [Google Scholar] [CrossRef]
- Liu, C.; Wang, W.; Lai, H.; Chen, Y.; Li, L.; Li, H.; Zhan, M.; Chen, T.; Cao, W.; Li, X. Biosynthesis of fungus-based oral selenium microcarriers for radioprotection and immuno-homeostasis shaping against radiation-induced heart disease. Bioact. Mater. 2024, 37, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.; Luo, M.; Liu, Z.; Liu, T.; Wang, X.; Huang, X.; Li, S.; Wu, H.; Pan, Q.; Chen, T.; et al. Selenium nanoparticles alleviate renal ischemia/reperfusion injury by inhibiting ferritinophagy via the XBP1/NCOA4 pathway. Cell Commun. Signal. 2024, 22, 376. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lai, H.; Chen, T. Boosting natural killer cell-based cancer immunotherapy with selenocystine/transforming growth factor-beta inhibitor-encapsulated nanoemulsion. ACS Nano 2020, 14, 11067–11082. [Google Scholar] [CrossRef]
- Huang, W.; Shi, S.; Lv, H.; Ju, Z.; Liu, Q.; Chen, T. Tellurium-driven maple leaf-shaped manganese nanotherapeutics reshape tumor microenvironment via chemical transition in situ to achieve highly efficient radioimmunotherapy of triple negative breast cancer. Bioact. Mater. 2023, 27, 560–573. [Google Scholar] [CrossRef]
- Liu, S.; Wei, W.; Wang, J.; Chen, T. Theranostic applications of selenium nanomedicines against lung cancer. J. Nanobiotechnology 2023, 21, 96. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Cai, F.; Guo, J.; Qin, X.; Zhang, H.; Chen, T.; Ma, L. Chirality-driven strong thioredoxin reductase inhibition. Biomaterials 2024, 311, 122705. [Google Scholar] [CrossRef] [PubMed]
- Bosteels, V.; Janssens, S. Striking a balance: New perspectives on homeostatic dendritic cell maturation. Nat. Rev. Immunol. 2025, 25, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J.H.; Jang, S.H.; Lee, E.Y.; Lee, M.; Park, S.; Moon, J.S. SBP1 contributes to mesangial proliferation and inflammation through mitochondrial respiration in glomerulus during IgA nephropathy. Free Radic. Biol. Med. 2024, 225, 711–725. [Google Scholar] [CrossRef]
- Liang, B.; Lin, W.; Tang, Y.; Li, T.; Chen, Q.; Zhang, W.; Zhou, X.; Ma, J.; Liu, B.; Yu, Z.; et al. Selenium supplementation elevated SELENBP1 to inhibit fibroblast activation in pulmonary arterial hypertension. iScience 2024, 27, 111036. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Han, S.; Zeng, Z.; Dai, J.; Jia, Y. Selenium-Binding Protein 1-Deficient Dendritic Cells Protect Mice from Sepsis by Increased Treg/Th17. Antioxidants 2025, 14, 468. https://doi.org/10.3390/antiox14040468
Zhang X, Han S, Zeng Z, Dai J, Jia Y. Selenium-Binding Protein 1-Deficient Dendritic Cells Protect Mice from Sepsis by Increased Treg/Th17. Antioxidants. 2025; 14(4):468. https://doi.org/10.3390/antiox14040468
Chicago/Turabian StyleZhang, Xin, Shuang Han, Zhu Zeng, Jie Dai, and Yi Jia. 2025. "Selenium-Binding Protein 1-Deficient Dendritic Cells Protect Mice from Sepsis by Increased Treg/Th17" Antioxidants 14, no. 4: 468. https://doi.org/10.3390/antiox14040468
APA StyleZhang, X., Han, S., Zeng, Z., Dai, J., & Jia, Y. (2025). Selenium-Binding Protein 1-Deficient Dendritic Cells Protect Mice from Sepsis by Increased Treg/Th17. Antioxidants, 14(4), 468. https://doi.org/10.3390/antiox14040468





