Unseparated Olive Pruning Waste as a Sustainable Feedstock: DoE-Optimized Extracts with Antioxidant Activity Equivalent to Isolated Leaves
Abstract
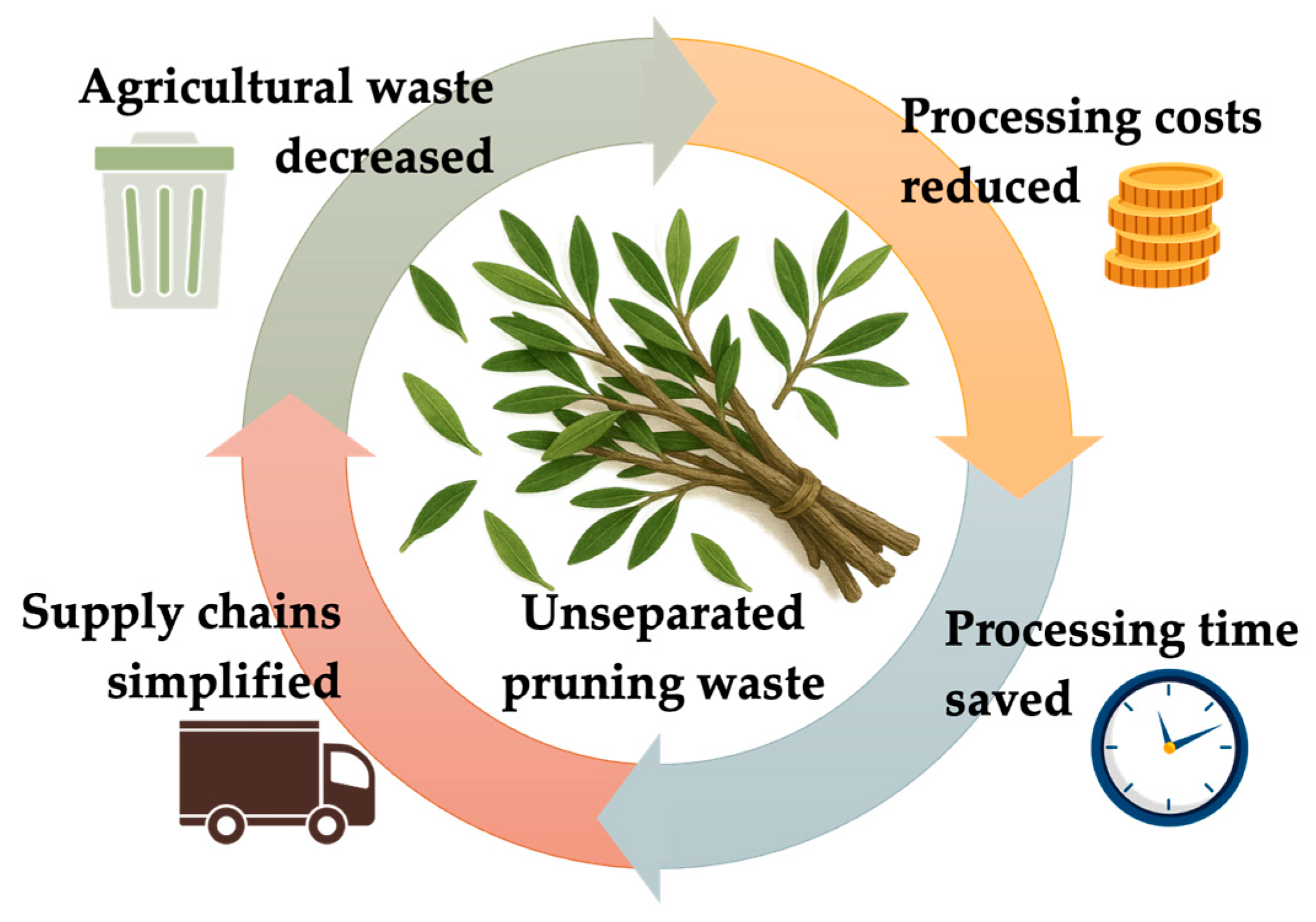
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Instruments
2.4. Extraction of Olea europaea Pruning Residues
- MAE extraction was carried out using a power of 80 Watts for the experiment at 90 °C, 25 Watts for all experiments at 40 °C, and 79 Watts for the experiment at 65 °C; a maximum pressure of 120 PSI; a ramp time of 2 min; and an extraction time of 5 min for each cycle under magnetic stirring.
- UAE extraction was carried out using a working frequency of 37 kHz and an electric power output of 250 W for experiments at 30 °C, 80 kHz and an electric power output of 450 W for experiments at 80 °C, and 55 Hz and 350 W for experiments at 55 °C. Each cycle lasted 10 min.
2.5. Chlorophyll Removal After Extraction
2.6. Extraction Scale-Up
2.7. HPLC-UV/PDA Analysis
2.8. Total Phenolic Content (TPC)
2.9. Free Radical Scavenging Activity
2.9.1. DPPH Assay
2.9.2. ORAC Assay
2.10. Functional In Vitro Activity
2.10.1. HaCaT Cell Line
2.10.2. Sample Preparation
2.10.3. MTT Assay
2.11. DCFH–DA Assay
2.12. Statistical Analysis
3. Results
3.1. Setup and Optimization of the Extraction Protocol for Pruning Residues of Olea europaea
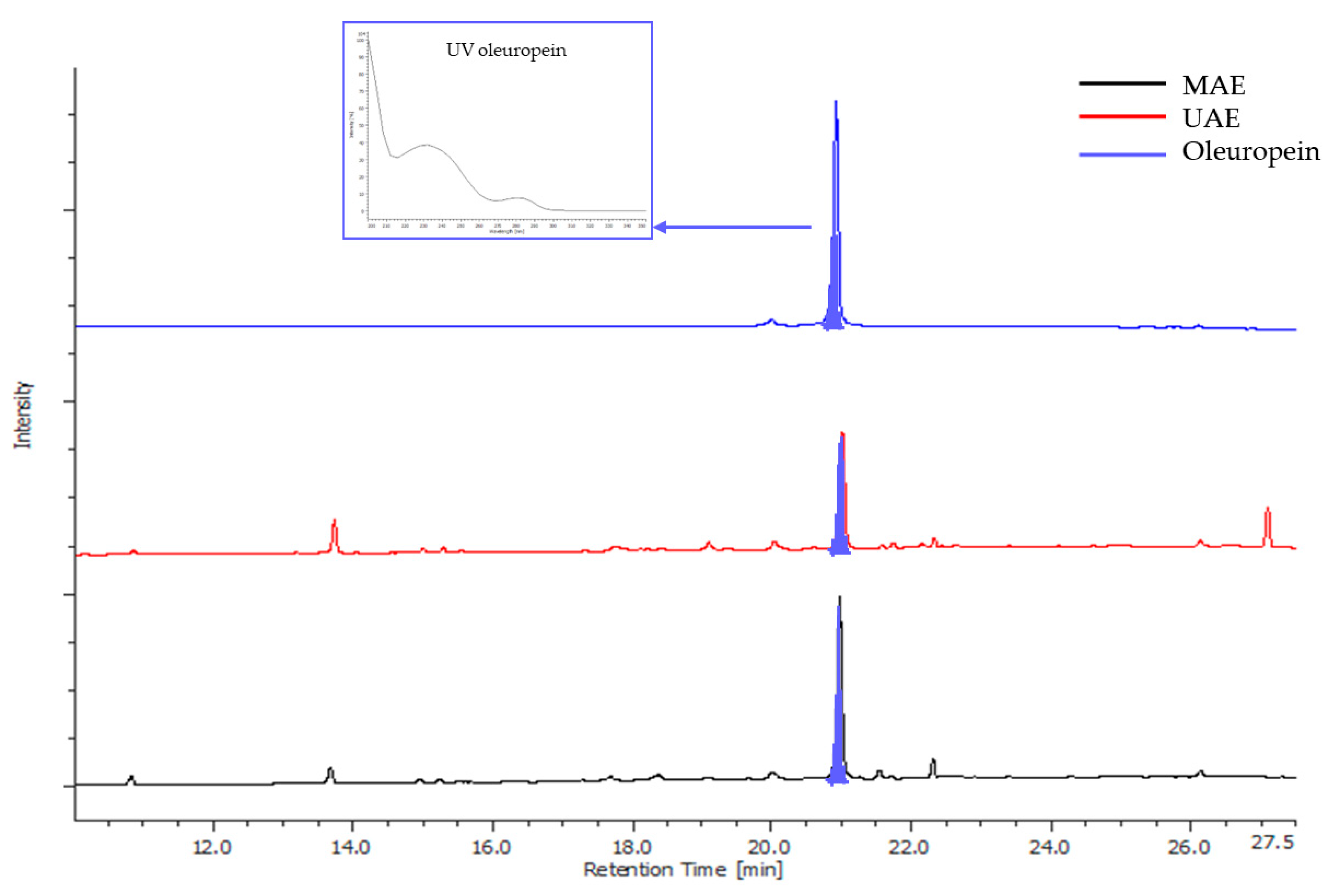
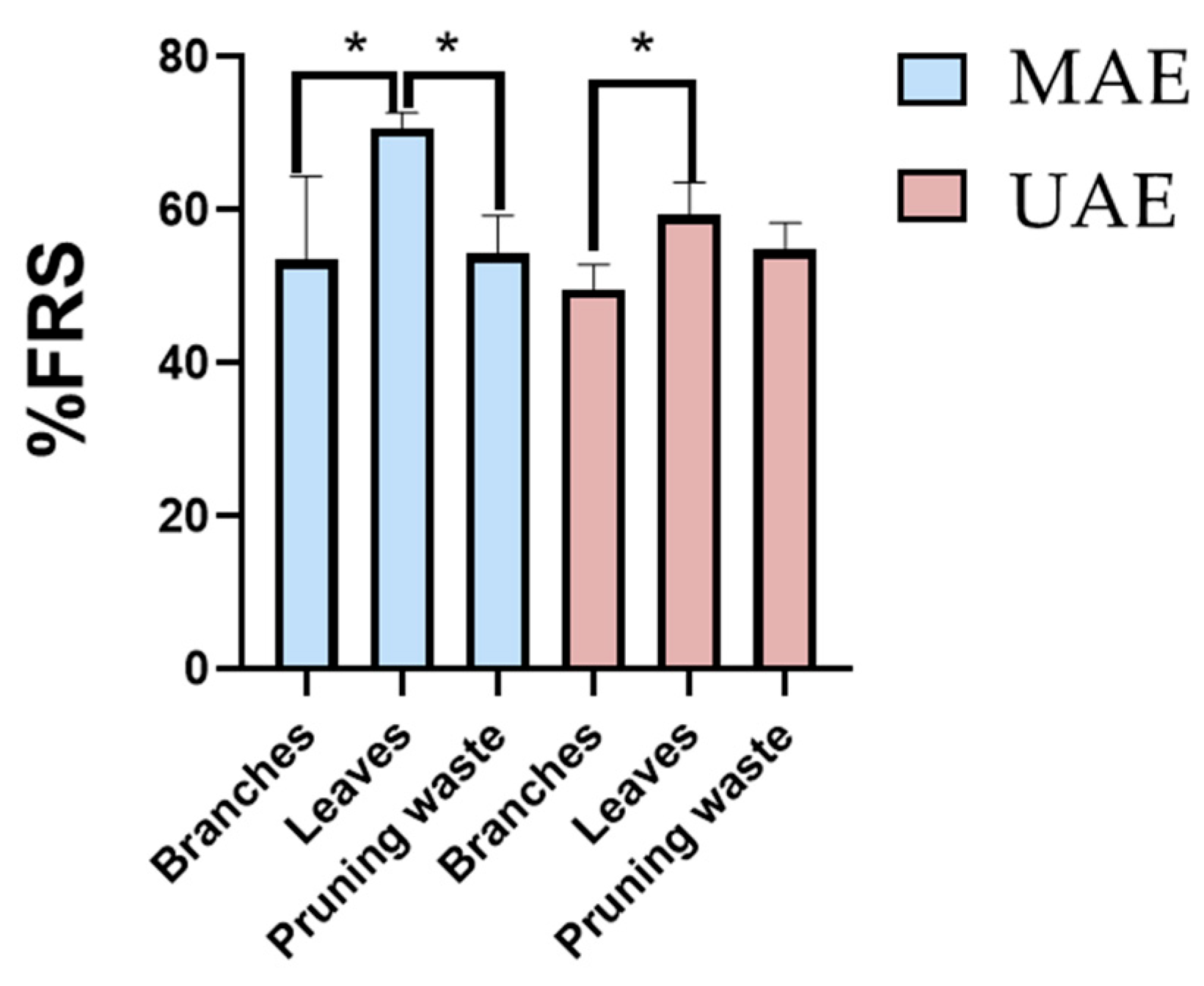
3.2. Evaluation of Extraction Method Scalability
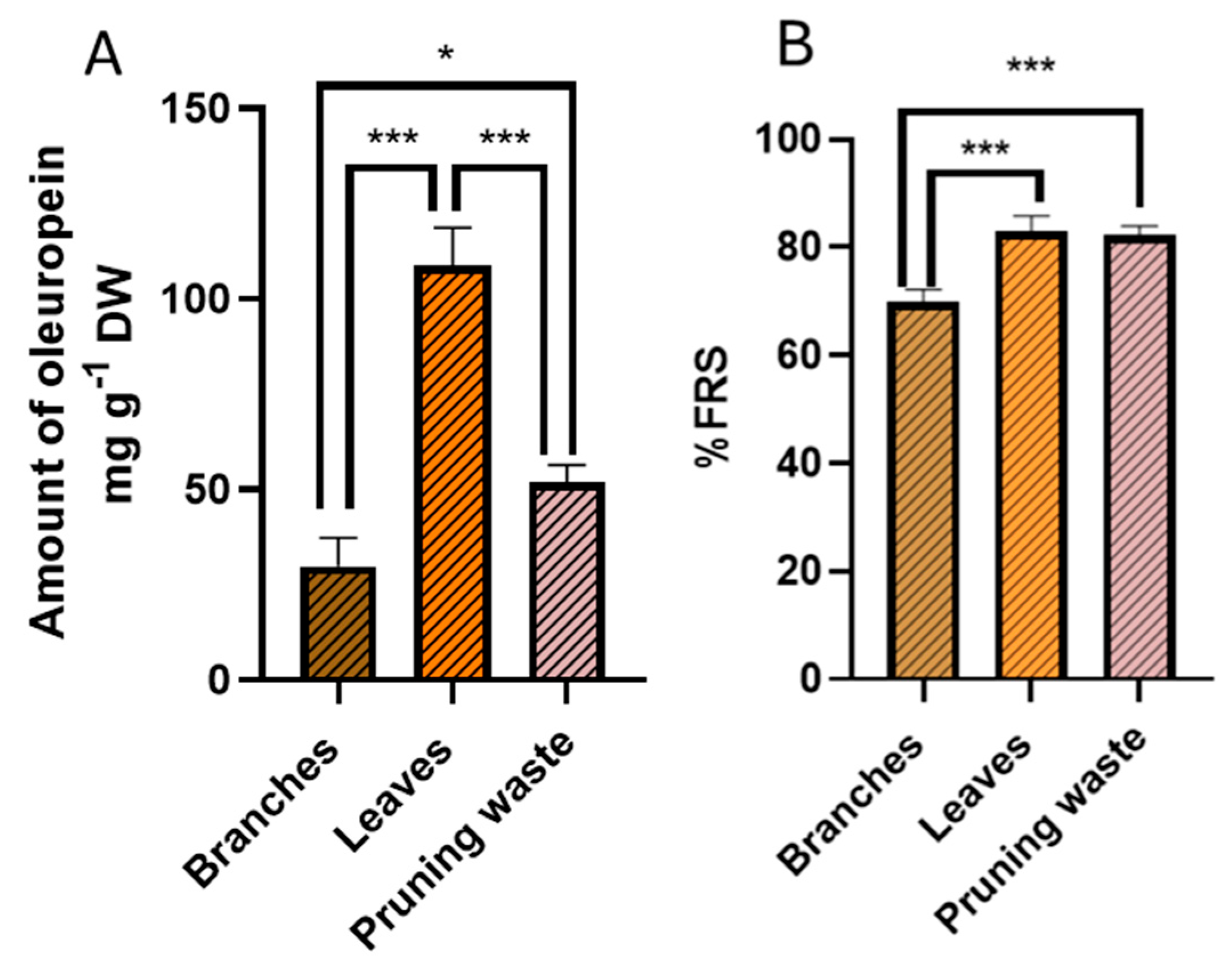
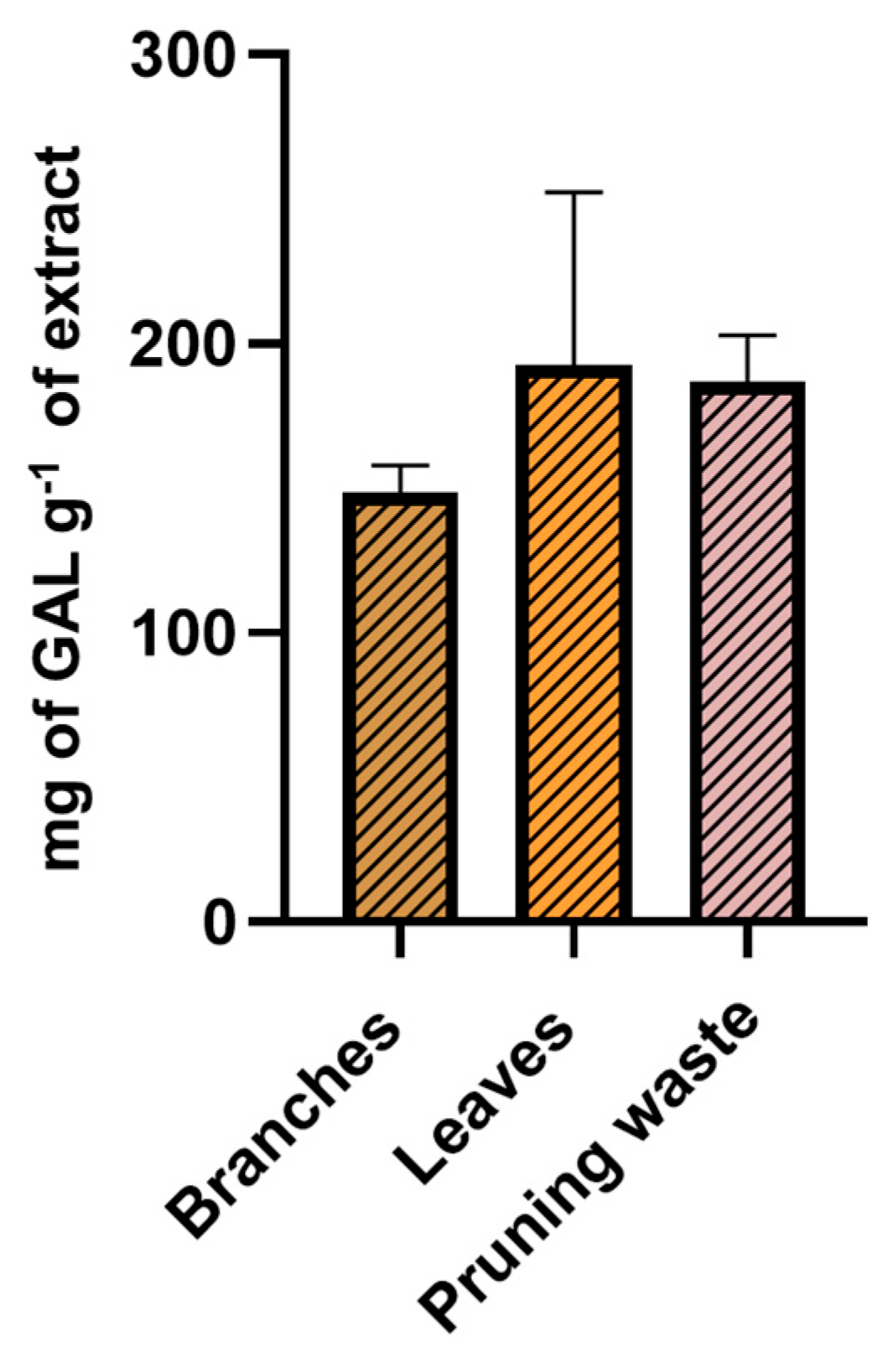
3.3. Total Phenolic Content (TPC) and Antioxidant Potential of the Extracts
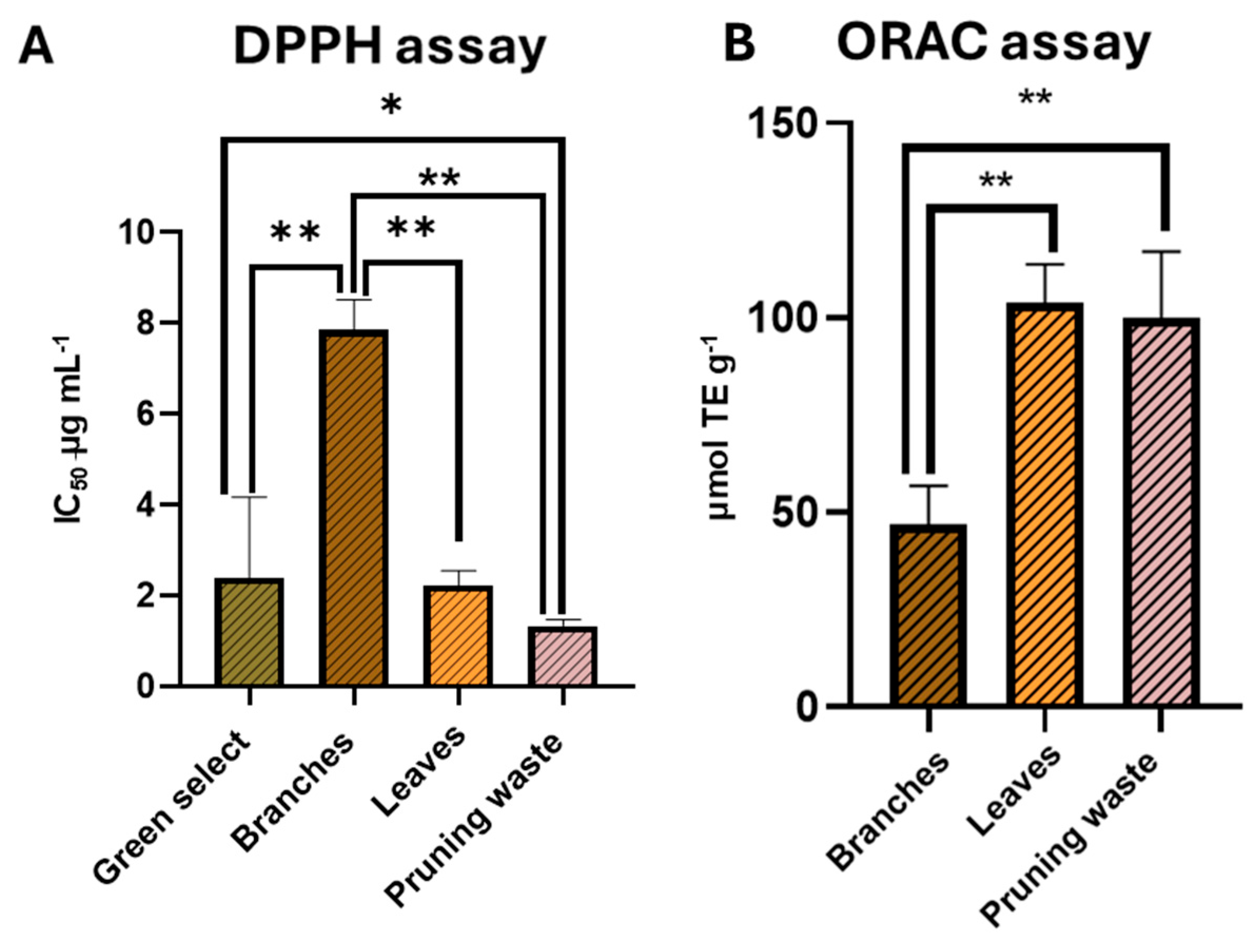
3.4. Evaluation of Free Radical Scavenging Activity of the Extracts
3.5. Evaluation of Antioxidant Effect in HaCaT Cell Line
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| % FRS | Percent Free Radical Scavenging |
| AAPH | 2,2′-Azobis(2-methylpropionamidine) dihydrochloride |
| ACN | Acetonitrile |
| AUC | Area Under the Curve |
| ANOVA | Analysis of Variance |
| CAT | Chemometric Agile Tool |
| CC (cc) | Cubic Centimeter |
| CI | Confidence Interval |
| CO2 | Carbon Dioxide |
| DAD/PDA | (UV-Vis) Photodiode Array Detector |
| DCFH–DA | 2′,7′-Dichlorodihydrofluorescein Diacetate |
| DCM | Dichloromethane |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DMSO | Dimethyl Sulfoxide |
| DoE | Design of Experiments |
| DW | Dry Weight |
| FBS | Fetal Bovine Serum |
| GA | Gallic Acid |
| H2O2 | Hydrogen Peroxide |
| HaCaT | Human Adult Keratinocyte Cell Line |
| HLB | Hydrophilic–Lipophilic Balanced |
| HPLC | High-Performance Liquid Chromatography |
| ICH Q2(R2) | International Council for Harmonisation, Q2(R2) |
| IC50 | Half-Maximal Inhibitory Concentration |
| MAE | Microwave-Assisted Solvent Extraction |
| MeOH | Methanol |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| Na2CO3 | Sodium Carbonate (anhydrous) |
| nm | Nanometer |
| OD | Optical Density |
| ORAC | Oxygen Radical Absorbance Capacity |
| PBS | Phosphate-Buffered Saline |
| PSI | Pound per Square Inch |
| QL | Quantification Limit |
| ROS | Reactive Oxygen Species |
| SD | Standard Deviation |
| SPE | Solid-Phase Extraction |
| TE | Trolox Equivalents |
| TPC | Total Phenolic Content |
| Trolox | (±)-6-Hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
| UAE | Ultrasound-Assisted Extraction |
| UV-Vis | Ultraviolet–Visible |
| W | Watt |
References
- Carrión, Y.; Ntinou, M.; Badal, E. Olea europaea L. in the North Mediterranean Basin during the Pleniglacial and the Early–Middle Holocene. Quat. Sci. Rev. 2010, 29, 952–968. [Google Scholar] [CrossRef]
- Espeso, J.; Isaza, A.; Lee, J.Y.; Sörensen, P.M.; Jurado, P.; Avena-Bustillos, R.d.J.; Olaizola, M.; Arboleya, J.C. Olive Leaf Waste Management. Front. Sustain. Food Syst. 2021, 5, 660582. [Google Scholar] [CrossRef]
- Rhizopoulou, S. Olea europaea L. A Botanical Contribution to Culture. Am.-Eurasian J. Agric. Environ. Sci. 2007, 2, 382–387. [Google Scholar]
- Mejri, R.; Dhraief, M.Z.; Souissi, A.; Dhehibi, B.; Oueslati, M.; Charry, A.C.; Frija, A.; Ouerghemmi, H.; Oumer, A.M.; Fendri, M.; et al. Empowering Smallholder Olive Growers in Northwest Tunisia through an Agroecological Business Model. Front. Sustain. Food Syst. 2025, 9, 1587318. [Google Scholar] [CrossRef]
- Deresse, T. Climate Change Financing: A Systematic Review 2023. SSRN Electron. J. 2023. [Google Scholar] [CrossRef]
- Caselli, A.; Petacchi, R. Climate Change and Major Pests of Mediterranean Olive Orchards: Are We Ready to Face the Global Heating? Insects 2021, 12, 802. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Baptista, P.; Pereira, J.A. A Review of Bactrocera oleae (Rossi) Impact in Olive Products: From the Tree to the Table. Trends Food Sci. Technol. 2015, 44, 226–242. [Google Scholar] [CrossRef]
- Buonaurio, R.; Almadi, L.; Famiani, F.; Moretti, C.; Agosteo, G.E.; Schena, L. Olive Leaf Spot Caused by Venturia Oleaginea: An Updated Review. Front. Plant Sci. 2023, 13, 1061136. [Google Scholar] [CrossRef]
- OLIMPO—Progetto. Available online: https://olimpo.unipv.it/progetto/ (accessed on 16 October 2025).
- OLIOP|Nord Ovest Digitale e Sostenibile. Available online: https://ecs-nodes.eu/6-agroindustria-primaria/progetti-imprese/oliop (accessed on 16 October 2025).
- Enaime, G.; Dababat, S.; Wichern, M.; Lübken, M. Olive Mill Wastes: From Wastes to Resources. Environ. Sci. Pollut. Res. 2024, 31, 20853–20880. [Google Scholar] [CrossRef]
- Dahdouh, A.; Khay, I.; Bouizi, Y.; Kervern, G.; Pontvianne, S.; El Maakoul, A.; Bakhouya, M.; Le Brech, Y. Hydrothermal Carbonization of Two-Phase Olive Mill Waste (Alperujo): Effect of Aqueous Phase Recycling. Biomass Bioenergy 2024, 184, 107205. [Google Scholar] [CrossRef]
- Alkhalidi, A.; Halaweh, G.; Khawaja, M.K. Recommendations for Olive Mills Waste Treatment in Hot and Dry Climate. J. Saudi Soc. Agric. Sci. 2023, 22, 361–373. [Google Scholar] [CrossRef]
- Abbattista, R.; Ventura, G.; Calvano, C.D.; Cataldi, T.R.I.; Losito, I. Bioactive Compounds in Waste By-Products from Olive Oil Production: Applications and Structural Characterization by Mass Spectrometry Techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef] [PubMed]
- García Martín, J.F.; Cuevas, M.; Feng, C.-H.; Álvarez Mateos, P.; Torres García, M.; Sánchez, S. Energetic Valorisation of Olive Biomass: Olive-Tree Pruning, Olive Stones and Pomaces. Processes 2020, 8, 511. [Google Scholar] [CrossRef]
- Masucci, F.; Serrapica, F.; De Luca, L.; Romano, R.; Garofalo, F.; Di Francia, A. Circular Economy on a Small Scale: The Sustainable Use of Olive Tree Biomass Residues as Feed for Lactating Cows in the Sorrento Peninsula. Sustainability 2025, 17, 845. [Google Scholar] [CrossRef]
- Mallamaci, R.; Budriesi, R.; Clodoveo, M.L.; Biotti, G.; Micucci, M.; Ragusa, A.; Curci, F.; Muraglia, M.; Corbo, F.; Franchini, C. Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation. Molecules 2021, 26, 1072. [Google Scholar] [CrossRef]
- Kabbash, E.M.; Abdel-Shakour, Z.T.; El-Ahmady, S.H.; Wink, M.; Ayoub, I.R. Comparative Metabolic Profiling of Olive Leaf Extracts from Twelve Different Cultivars Collected in Both Fruiting and Flowering Seasons. Sci. Rep. 2023, 13, 612. [Google Scholar] [CrossRef]
- Lo Giudice, V.; Faraone, I.; Bruno, M.R.; Ponticelli, M.; Labanca, F.; Bisaccia, D.; Massarelli, C.; Milella, L.; Todaro, L. Olive Trees By-Products as Sources of Bioactive and Other Industrially Useful Compounds: A Systematic Review. Molecules 2021, 26, 5081. [Google Scholar] [CrossRef]
- Kanakis, P.; Termentzi, A.; Michel, T.; Gikas, E.; Halabalaki, M.; Skaltsounis, A.-L. From Olive Drupes to Olive Oil. An HPLC-Orbitrap-Based Qualitative and Quantitative Exploration of Olive Key Metabolites. Planta Medica 2013, 79, 1576–1587. [Google Scholar] [CrossRef]
- Serrano-García, I.; Olmo-García, L.; Monago-Maraña, O.; de Alba, I.M.C.; León, L.; de la Rosa, R.; Serrano, A.; Gómez-Caravaca, A.M.; Carrasco-Pancorbo, A. Characterization of the Metabolic Profile of Olive Tissues (Roots, Stems and Leaves): Relationship with Cultivars’ Resistance/Susceptibility to the Soil Fungus Verticillium dahliae. Antioxidants 2023, 12, 2120. [Google Scholar] [CrossRef]
- Somantri, A.D.; Kurnia, D.; Zainuddin, A.; Dharsono, H.D.A.; Satari, M.H. Action Mode of Ursolic Acid as a Natural Antioxidant and Inhibitor of Superoxide Dismutase: In Vitro and in Silico Study. J. Adv. Pharm. Technol. Res. 2021, 12, 389. [Google Scholar] [CrossRef]
- Samsonowicz, M.; Kalinowska, M.; Gryko, K. Enhanced Antioxidant Activity of Ursolic Acid by Complexation with Copper (II): Experimental and Theoretical Study. Materials 2021, 14, 264. [Google Scholar] [CrossRef]
- Feldo, M.; Wójciak, M.; Ziemlewska, A.; Dresler, S.; Sowa, I. Modulatory Effect of Diosmin and Diosmetin on Metalloproteinase Activity and Inflammatory Mediators in Human Skin Fibroblasts Treated with Lipopolysaccharide. Molecules 2022, 27, 4264. [Google Scholar] [CrossRef]
- Garg, M.; Chaudhary, S.K.; Goyal, A.; Sarup, P.; Kumari, S.; Garg, N.; Vaid, L.; Shiveena, B. Comprehensive Review on Therapeutic and Phytochemical Exploration of Diosmetin: A Promising Moiety. Phytomedicine Plus 2022, 2, 100179. [Google Scholar] [CrossRef]
- Ahmad, T.; Khan, T.; Kirabo, A.; Shah, A.J. Antioxidant Flavonoid Diosmetin Is Cardioprotective in a Rat Model of Myocardial Infarction Induced by Beta 1-Adrenergic Receptors Activation. Curr. Issues Mol. Biol. 2023, 45, 4675–4686. [Google Scholar] [CrossRef]
- Oraibi, A.I.; Dawood, A.H.; Trabelsi, G.; Mahamat, O.B.; Chekir-Ghedira, L.; Kilani-Jaziri, S. Antioxidant Activity and Selective Cytotoxicity in HCT-116 and WI-38 Cell Lines of LC-MS/MS Profiled Extract from Capparis spinosa L. Front. Chem. 2025, 13, 1540174. [Google Scholar] [CrossRef]
- Degotte, G.; Frederich, M.; Francotte, P.; Franck, T.; Colson, T.; Serteyn, D.; Mouithys-Mickalad, A. Targeting Myeloperoxidase Activity and Neutrophil ROS Production to Modulate Redox Process: Effect of Ellagic Acid and Analogues. Molecules 2023, 28, 4516. [Google Scholar] [CrossRef]
- Han, D.H.; Lee, M.J.; Kim, J.H. Antioxidant and Apoptosis-Inducing Activities of Ellagic Acid. Anticancer. Res. 2006, 26, 3601–3606. [Google Scholar] [PubMed]
- Zhu, H.; Yan, Y.; Jiang, Y.; Meng, X. Ellagic Acid and Its Anti-Aging Effects on Central Nervous System. Int. J. Mol. Sci. 2022, 23, 10937. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Peng, L.; Fu, J.; Zou, L.; Zhao, G.; Zhao, J. Phytochemical, Antibacterial and Antioxidant Activity Evaluation of Rhodiola crenulata. Molecules 2020, 25, 3664. [Google Scholar] [CrossRef] [PubMed]
- Latos-Brozio, M.; Masek, A. Biodegradable Polyester Materials Containing Gallates. Polymers 2020, 12, 677. [Google Scholar] [CrossRef]
- Nagori, K.; Pradhan, M.; Nakhate, K.T. Ethyl Gallate Ameliorates Diabetes-Induced Alzheimer’s Disease-like Phenotype in Rats via Activation of A7 Nicotinic Receptors and Mitigation of Oxidative Stress. Biochem. Biophys. Res. Commun. 2024, 737, 150925. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Uccella, N. Biophenolic Components of Olives. Food Res. Int. 2000, 33, 475–485. [Google Scholar] [CrossRef]
- Jayakumar, T.; Huang, C.-J.; Yen, T.-L.; Hsia, C.-W.; Sheu, J.-R.; Bhavan, P.S.; Huang, W.-C.; Hsieh, C.-Y.; Hsia, C.-H. Activation of Nrf2 by Esculetin Mitigates Inflammatory Responses through Suppression of NF-κB Signaling Cascade in RAW 264.7 Cells. Molecules 2022, 27, 5143. [Google Scholar] [CrossRef]
- Kim, S.; Kang, K.; Zhang, R.; Piao, M.; Ko, D.; Wang, Z.; Chae, S.; Kang, S.; Lee, K.; Kang, H.; et al. Protective Effect of Esculetin against Oxidative Stress-Induced Cell Damage via Scavenging Reactive Oxygen Species. Acta Pharmacol. Sin. 2008, 29, 1319–1326. [Google Scholar] [CrossRef]
- Yoon, C.-S.; Lee, H.; Liu, Z.; Dong, L.; Lee, G.; Kim, N.; Oh, H.; Lee, D.-S. Cycloolivil Isolated from Nardostachys Jatamansi Inhibits TNF-α/IFN-γ-Induced Chemokine Production by Blocking NF-κB and JAK/STAT Activation in HaCaT Keratinocytes. Int. J. Mol. Sci. 2024, 25, 3342. [Google Scholar] [CrossRef]
- Pérez-Bonilla, M.; Salido, S.; van Beek, T.A.; Linares-Palomino, P.J.; Altarejos, J.; Nogueras, M.; Sánchez, A. Isolation and Identification of Radical Scavengers in Olive Tree (Olea europaea) Wood. J. Chromatogr. A 2006, 1112, 311–318. [Google Scholar] [CrossRef]
- Andjelković, M.; Van Camp, J.; De Meulenaer, B.; Depaemelaere, G.; Socaciu, C.; Verloo, M.; Verhe, R. Iron-Chelation Properties of Phenolic Acids Bearing Catechol and Galloyl Groups. Food Chem. 2006, 98, 23–31. [Google Scholar] [CrossRef]
- Christodoulou, A.; Nikolaou, P.-E.; Symeonidi, L.; Katogiannis, K.; Pechlivani, L.; Nikou, T.; Varela, A.; Chania, C.; Zerikiotis, S.; Efentakis, P.; et al. Cardioprotective Potential of Oleuropein, Hydroxytyrosol, Oleocanthal and Their Combination: Unravelling Complementary Effects on Acute Myocardial Infarction and Metabolic Syndrome. Redox Biol. 2024, 76, 103311. [Google Scholar] [CrossRef]
- Batarfi, W.A.; Mohd Yunus, M.H.; Hamid, A.A. The Effect of Hydroxytyrosol in Type II Epithelial-Mesenchymal Transition in Human Skin Wound Healing. Molecules 2023, 28, 2652. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Forbes-Hernández, T.Y.; Varela-López, A.; Puentes, J.G.; Sánchez-González, C.; Sumalla-Cano, S.; Battino, M.; García-Ruiz, R.; Sánchez, S.; et al. Effect of Olive Leaf Phytochemicals on the Anti-Acetylcholinesterase, Anti-Cyclooxygenase-2 and Ferric Reducing Antioxidant Capacity. Food Chem. 2024, 444, 138516. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Kader, M.S.; Abdel-Rahman, R.F.; Soliman, G.A.; Ogaly, H.A.; Alamri, M.A.; Alharbi, A.G. Oleuropein Relieves Pancreatic Ischemia Reperfusion Injury in Rats by Suppressing Inflammation and Oxidative Stress through HMGB1/NF-κB Pathway. Int. J. Mol. Sci. 2024, 25, 10171. [Google Scholar] [CrossRef]
- Quesada, G.J.M.; Santiago, M.R.M.; Casado, D.A. Oleuropein Compositions for Healing Wounds and Ulcers in Elderly People and/or Diabetics. WO2011141611, 13 May 2011. [Google Scholar]
- Kalaycıoğlu, Z.; Kopar, M.; Erim, F. Oleuropein Levels of Anatolian Olive Leaves and Correlated Antioxidant, Antidiabetic, and Anti-Inflammatory Activities. J. Chem. Metrol. 2020, 14, 133–141. [Google Scholar] [CrossRef]
- Martiny, T.R.; Raghavan, V.; Moraes, C.C.d.; Rosa, G.S.d.; Dotto, G.L. Bio-Based Active Packaging: Carrageenan Film with Olive Leaf Extract for Lamb Meat Preservation. Foods 2020, 9, 1759. [Google Scholar] [CrossRef]
- Wanitphakdeedecha, R.; Ng, J.N.C.; Junsuwan, N.; Phaitoonwattanakij, S.; Phothong, W.; Eimpunth, S.; Manuskiatti, W. Efficacy of Olive Leaf Extract–Containing Cream for Facial Rejuvenation: A Pilot Study. J. Cosmet. Dermatol. 2020, 19, 1662–1666. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, J.; Xu, Y.; Shi, N.; Lin, L.; Wang, R.; Dai, R.; Xu, L.; Hao, N.; Li, Q. Olea europaea Leaf Exosome-like Nanovesicles Encapsulated in a Hyaluronic Acid/Tannic Acid Hydrogel Dressing with Dual “Defense-Repair” Effects for Treating Skin Photoaging. Mater. Today Bio 2024, 26, 101103. [Google Scholar] [CrossRef] [PubMed]
- Ebauer, M.; Ott, R.; Walzel, B. Methods for the Prevention and/or Treatment of Age Spots. EP2218441B1, 25 May 2011. [Google Scholar]
- Holland, J.; Nikolovski, J.; Lyte, P.; Southall, M.; Zhu, V. Anti-inflammatory compositions and methods of use. EP1940432B1, 9 March 2011. [Google Scholar]
- Amari, G. Use of an Extract from the Leaves of Olea europea as an Antiradical. EP0937455A1, 25 August 1999. [Google Scholar]
- Pereira, A.P.; Ferreira, I.C.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic Compounds and Antimicrobial Activity of Olive (Olea europaea L. Cv. Cobrançosa) Leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Lee-Huang, S.; Zhang, L.; Lin Huang, P.; Chang, Y.-T.; Huang, P.L. Anti-HIV Activity of Olive Leaf Extract (OLE) and Modulation of Host Cell Gene Expression by HIV-1 Infection and OLE Treatment. Biochem. Biophys. Res. Commun. 2003, 307, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.; Keast, R. Biological Activities of Phenolic Compounds Present in Virgin Olive Oil. Int. J. Mol. Sci. 2010, 11, 458–479. [Google Scholar] [CrossRef]
- Petroni, A.; Blasevich, M.; Salami, M.; Papini, N.; Montedoro, G.F.; Galli, C. Inhibition of Platelet Aggregation and Eicosanoid Production by Phenolic Components of Olive Oil. Thromb. Res. 1995, 78, 151–160. [Google Scholar] [CrossRef]
- Silva, S.; Gomes, L.; Leitão, F.; Coelho, A.V.; Boas, L.V. Phenolic Compounds and Antioxidant Activity of Olea europaea L. Fruits and Leaves. Food Sci. Technol. Int. 2006, 12, 385–395. [Google Scholar] [CrossRef]
- Altarejos Caballero, J.; Salido Ruiz, S.; Linares Palomino, P.J.; Nogueras Montiel, M.; Sanchez Rodrigo, A.; Perez Bonilla, M. Procedure for the Extraction of Olive Wood and Extracts Enriched in Food Interest Products. ES2386860A1, 3 September 2012. [Google Scholar]
- Fossati, A.; Cavalloro, V.; Rossi, D.; Collina, S.; Martino, E. A Practical and Easy-to-Scale Protocol for Removing Chlorophylls from Leaf Extracts. Appl. Plant Sci. 2025, 13, e70018. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; Granados, M.; Saurina, J.; Sentellas, S. Olive Tree Leaves as a Great Source of Phenolic Compounds: Comprehensive Profiling of NaDES Extracts. Food Chem. 2024, 456, 140042. [Google Scholar] [CrossRef]
- ICH Q2(R2) Validation of Analytical Procedures—Scientific Guideline|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 16 October 2025).
- Q2(R2) Validation of Analytical Procedures|FDA. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2r2-validation-analytical-procedures (accessed on 12 November 2025).
- Attard, E. A Rapid Microtitre Plate Folin-Ciocalteu Method for the Assessment of Polyphenols. Open Life Sci. 2013, 8, 48–53. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Carvalho, J.R.B.; Meireles, A.N.; Marques, S.S.; Gregório, B.J.R.; Ramos, I.I.; Silva, E.M.P.; Barreiros, L.; Segundo, M.A. Exploiting Kinetic Features of ORAC Assay for Evaluation of Radical Scavenging Capacity. Antioxidants 2023, 12, 505. [Google Scholar] [CrossRef]
- Desiderio, A.; Pedrosa, M.C.; Heleno, S.A.; Carocho, M.; Rodrigues, D.B.; Buratti, S.; Soffientini, I.; Ratto, D.; Savino, E.; Rossi, P. Bio-Recycling Hazelnut Shells to Improve Antioxidant Properties of Lentinus Tigrinus Sporophore. Agriculture 2025, 15, 178. [Google Scholar] [CrossRef]
- Leardi, R.; Melzi, C.; Polotti, G. CAT (Chemometric Agile Tool). 2025. Available online: http://gruppochemiometria.it/index.php/software (accessed on 1 October 2025).
- Vo, T.P.; Nguyen, N.T.U.; Le, V.H.; Phan, T.H.; Nguyen, T.H.Y.; Nguyen, D.Q. Optimizing Ultrasonic-Assisted and Microwave-Assisted Extraction Processes to Recover Phenolics and Flavonoids from Passion Fruit Peels. ACS Omega 2023, 8, 33870–33882. [Google Scholar] [CrossRef]
- Sánchez-Gutiérrez, M.; Bascón-Villegas, I.; Rodríguez, A.; Pérez-Rodríguez, F.; Fernández-Prior, Á.; Rosal, A.; Carrasco, E. Valorisation of Olea europaea L. Olive Leaves through the Evaluation of Their Extracts: Antioxidant and Antimicrobial Activity. Foods 2021, 10, 966. [Google Scholar] [CrossRef]
- Soldo, B.; Bilušić, T.; Giacometti, J.; Ljubenkov, I.; Čikeš Čulić, V.; Bratanić, A.; Bošković, P.; Šola, I.; Ilić, K. A Comparative Study of Oleuropein Extraction from Wild Olive Leaves (Olea europea Subsp. Oleaster, Hoffmanns. & Link), Its Gastrointestinal Stability, and Biological Potential. Appl. Sci. 2024, 14, 869. [Google Scholar] [CrossRef]
- da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of Microwave, Ultrasonic and Conventional Techniques for Extraction of Bioactive Compounds from Olive Leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Ranjbar Nedamani, E.; Sadeghi Mahoonak, A.; Ghorbani, M.; Kashaninejad, M. Evaluation of Antioxidant Interactions in Combined Extracts of Green Tea (Camellia sinensis), Rosemary (Rosmarinus officinalis) and Oak Fruit (Quercus branti). J. Food Sci. Technol. 2015, 52, 4565–4571. [Google Scholar] [CrossRef] [PubMed]
- Alcántara, C.; Žugčić, T.; Abdelkebir, R.; García-Pérez, J.V.; Jambrak, A.R.; Lorenzo, J.M.; Collado, M.C.; Granato, D.; Barba, F.J. Effects of Ultrasound-Assisted Extraction and Solvent on the Phenolic Profile, Bacterial Growth, and Anti-Inflammatory/Antioxidant Activities of Mediterranean Olive and Fig Leaves Extracts. Molecules 2020, 25, 1718. [Google Scholar] [CrossRef]
- Popović, M.; Burčul, F.; Veršić Bratinčević, M.; Režić Mužinić, N.; Skroza, D.; Frleta Matas, R.; Nazlić, M.; Ninčević Runjić, T.; Jukić Špika, M.; Bego, A.; et al. In the Beginning Was the Bud: Phytochemicals from Olive (Olea europaea L.) Vegetative Buds and Their Biological Properties. Metabolites 2023, 13, 237. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’BRien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant Capacity as Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.d.l.L.; Pinto, D.; Delerue-Matos, C.; Rodrigues, F. Olive Fruit and Leaf Wastes as Bioactive Ingredients for Cosmetics—A Preliminary Study. Antioxidants 2021, 10, 245. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Han, D.-W.; Lee, J.H. The Cytoprotective Effects of Baicalein on H2O2-Induced ROS by Maintaining Mitochondrial Homeostasis and Cellular Tight Junction in HaCaT Keratinocytes. Antioxidants 2023, 12, 902. [Google Scholar] [CrossRef] [PubMed]
- Selim, S.; Albqmi, M.; Al-Sanea, M.M.; Alnusaire, T.S.; Almuhayawi, M.S.; AbdElgawad, H.; Al Jaouni, S.K.; Elkelish, A.; Hussein, S.; Warrad, M.; et al. Valorizing the Usage of Olive Leaves, Bioactive Compounds, Biological Activities, and Food Applications: A Comprehensive Review. Front. Nutr. 2022, 9, 1008349. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumminelli, E.; Cavalloro, V.; Ratto, D.; Marrubini, G.; Martino, E.; Rossi, P.; Rossi, D.; Collina, S. Unseparated Olive Pruning Waste as a Sustainable Feedstock: DoE-Optimized Extracts with Antioxidant Activity Equivalent to Isolated Leaves. Antioxidants 2025, 14, 1441. https://doi.org/10.3390/antiox14121441
Tumminelli E, Cavalloro V, Ratto D, Marrubini G, Martino E, Rossi P, Rossi D, Collina S. Unseparated Olive Pruning Waste as a Sustainable Feedstock: DoE-Optimized Extracts with Antioxidant Activity Equivalent to Isolated Leaves. Antioxidants. 2025; 14(12):1441. https://doi.org/10.3390/antiox14121441
Chicago/Turabian StyleTumminelli, Elisabetta, Valeria Cavalloro, Daniela Ratto, Giorgio Marrubini, Emanuela Martino, Paola Rossi, Daniela Rossi, and Simona Collina. 2025. "Unseparated Olive Pruning Waste as a Sustainable Feedstock: DoE-Optimized Extracts with Antioxidant Activity Equivalent to Isolated Leaves" Antioxidants 14, no. 12: 1441. https://doi.org/10.3390/antiox14121441
APA StyleTumminelli, E., Cavalloro, V., Ratto, D., Marrubini, G., Martino, E., Rossi, P., Rossi, D., & Collina, S. (2025). Unseparated Olive Pruning Waste as a Sustainable Feedstock: DoE-Optimized Extracts with Antioxidant Activity Equivalent to Isolated Leaves. Antioxidants, 14(12), 1441. https://doi.org/10.3390/antiox14121441










