Prunus spinosa L. Branches as a New Source of Condensed Tannins: Phytochemical Profile and Antioxidant, Cytotoxic and Genotoxic In Vitro Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. The Biomass
2.2. Chemicals and Reagents
2.3. Characterization of Biomass
2.4. High Performance Thin Layer Chromatography (HPTLC) Analysis of Condensed Tannins from P. spinosa Crude Extract
- (a)
- Preparation of references
- (b)
- The HPTLC fingerprinting analysis
2.5. In Vitro Evaluation of the Antioxidant Activity of P. spinosa Crude Extract
- (a)
- HPTLC–DPPH bioautographic assay
- (b)
- DPPH assay
- (c)
- ABTS assay
2.6. In Vitro Evaluation of the Cytotoxic and Genotoxic Effects of P. spinosa Crude Extract
- (a)
- Cell culture and treatment
- (b)
- MTT assay
- (c)
- Comet assay
2.7. Data Analysis
3. Results
3.1. Characterization of Biomass
3.2. Characterization of P. spinosa Crude Extract
3.3. Antioxidant Activity of P. spinosa Crude Extract
- (a)
- HPTLC-DPPH bioautographic assay
- (b)
- DPPH assay
- (c)
- ABTS Assay
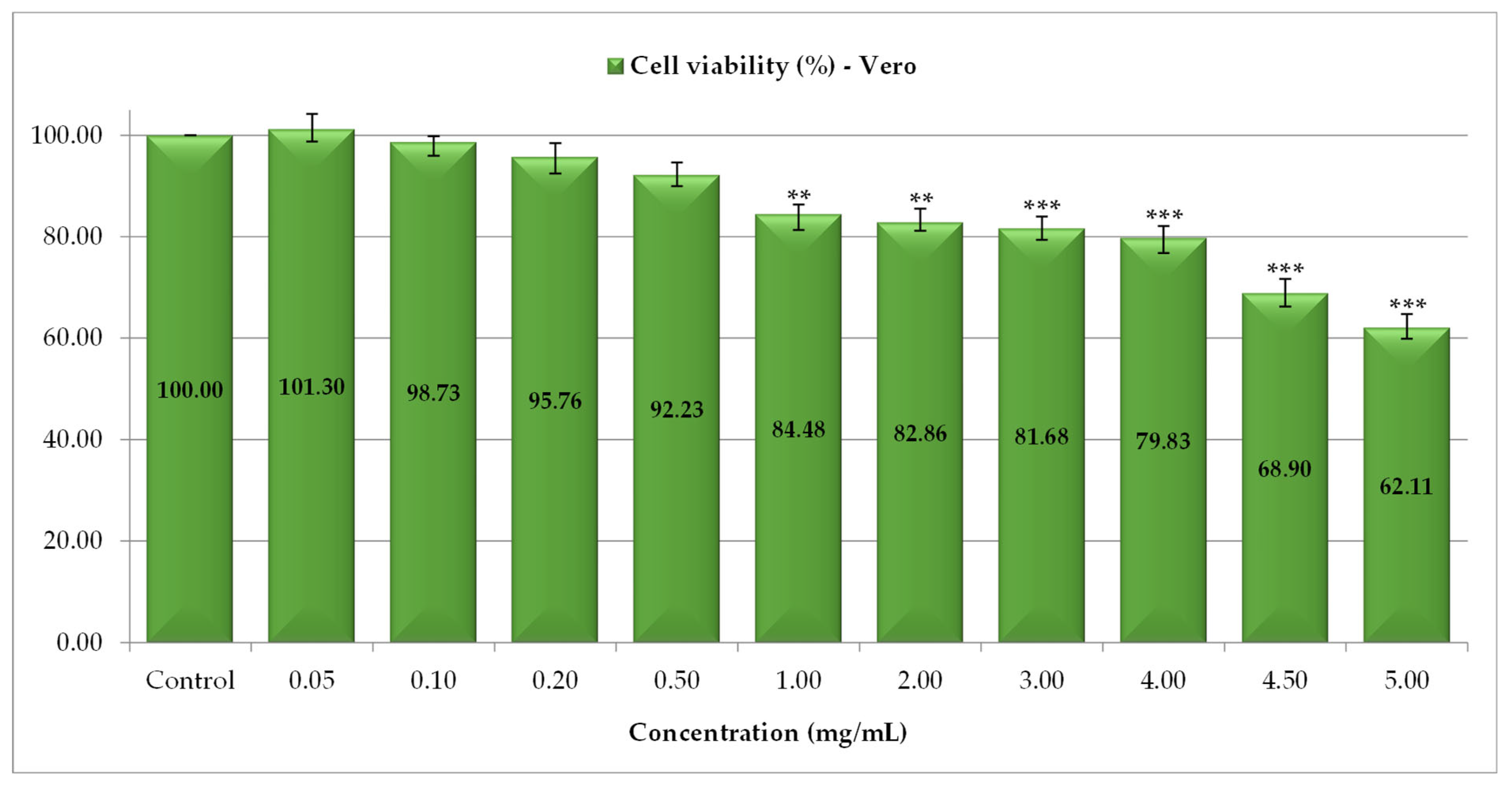
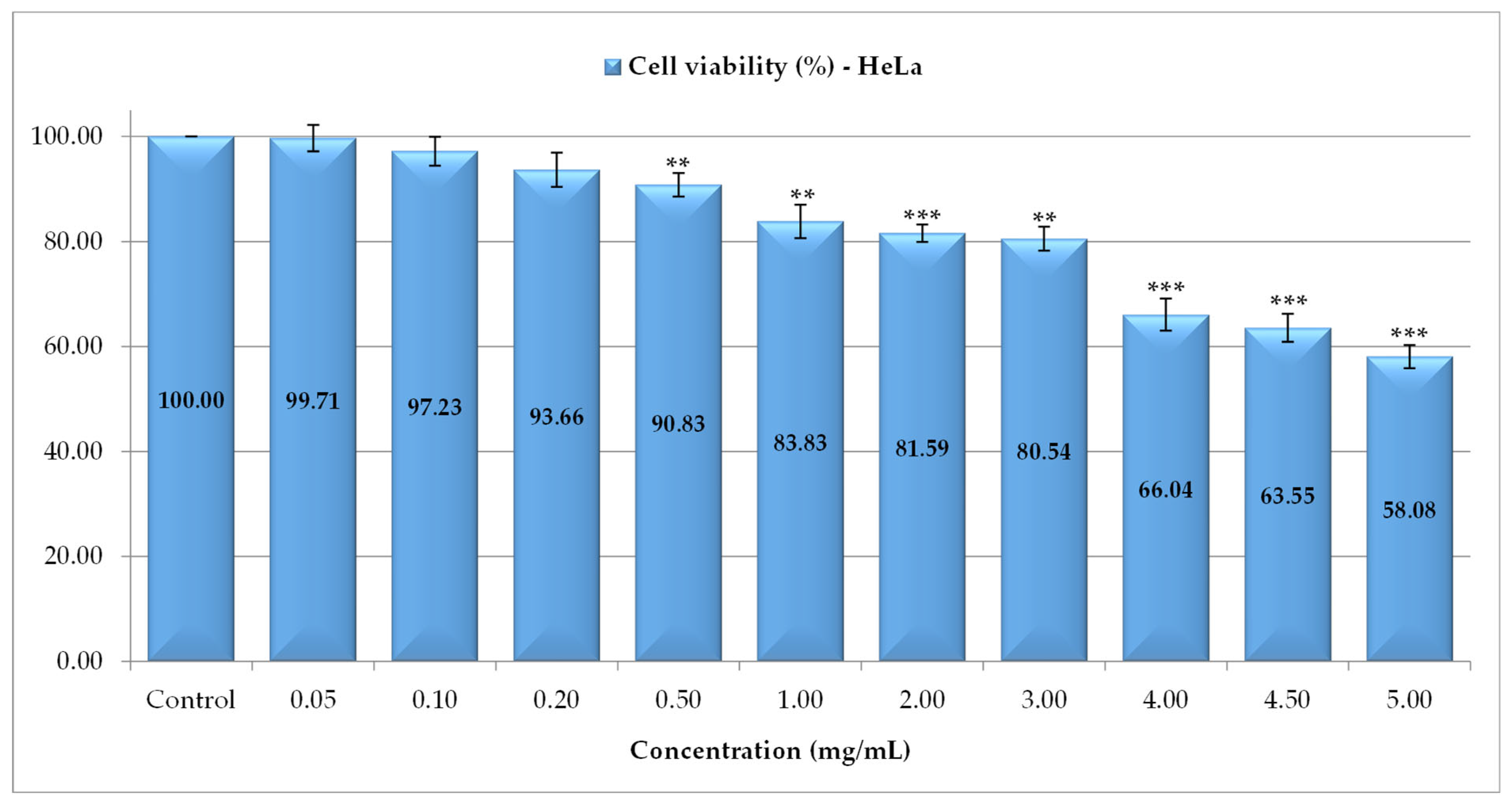
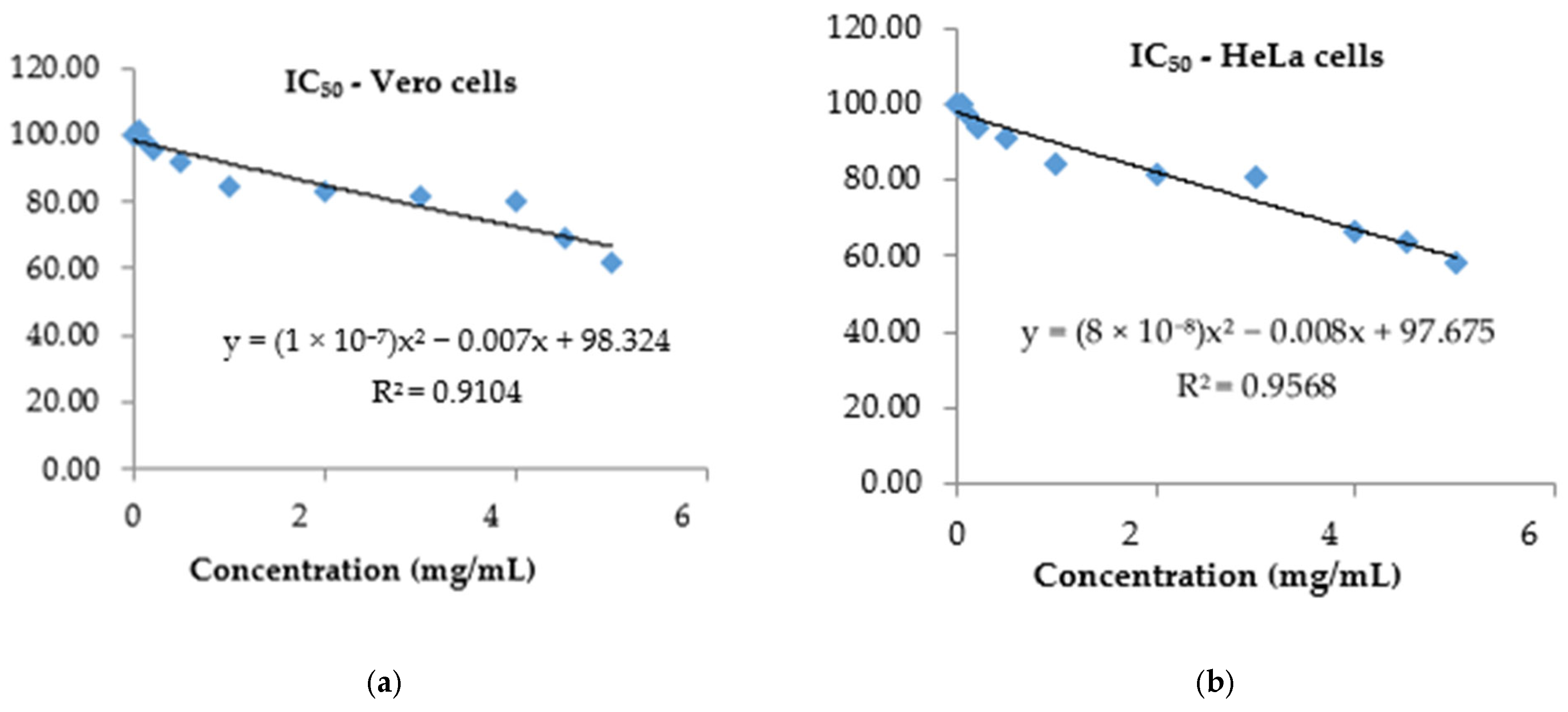
3.4. Assessment of Cytotoxic Effects of P. spinosa Crude Extract
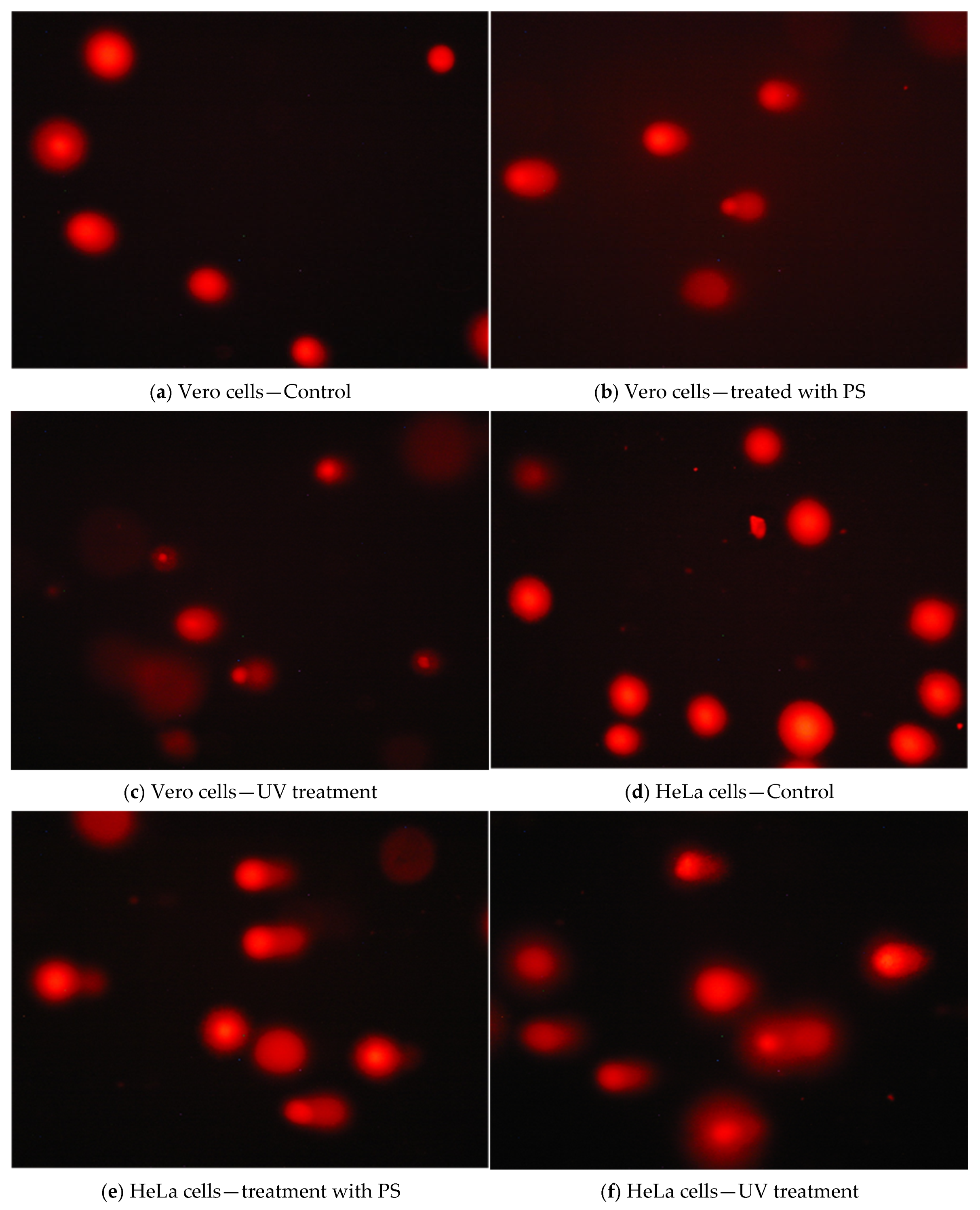
3.5. Assessment of Genotoxic Effects of P. spinosa Crude Extract
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Georgiev, M.I. Natural Products Utilization. Phytochem. Rev. 2014, 13, 339–341. [Google Scholar] [CrossRef][Green Version]
- Bernardini, S.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Natural Products for Human Health: An Historical Overview of the Drug Discovery Approaches. Nat. Prod. Res. 2018, 32, 1926–1950. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial Activity of Some Plant Extracts Against Bacterial Strains Causing Food Poisoning Diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef]
- Sabatini, L.; Fraternale, D.; Di Giacomo, B.; Mari, M.; Albertini, M.C.; Gordillo, B.; Rocchi, M.B.L.; Sisti, D.; Coppari, S.; Semprucci, F.; et al. Chemical Composition, Antioxidant, Antimicrobial and Anti-Inflammatory Activity of Prunus spinosa L. Fruit Ethanol Extract. J. Funct. Foods 2020, 67, 103885. [Google Scholar] [CrossRef]
- Marchelak, A.; Owczarek, A.; Matczak, M.; Pawlak, A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Olszewska, M.A. Bioactivity Potential of Prunus spinosa L. Flower Extracts: Phytochemical Profiling, Cellular Safety, Pro-Inflammatory Enzymes Inhibition and Protective Effects Against Oxidative Stress In Vitro. Front. Pharmacol. 2017, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Kour, A. Plants Exhibiting Potential for Cancer Treatment. Int. J. Pharm. Sci. Rev. Res. 2014, 6, 23–53. [Google Scholar]
- Takeoka, G.R.; Dao, L.T. Antioxidant Constituents of Almond [Prunus dulcis (Mill.) D.A. Webb] Hulls. J. Agric. Food Chem. 2003, 51, 496–501. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Chae, S.; Lee, J.; Park, S. Recent studies on flavonoids and their antioxidant activities. EXCLI J. 2013, 12, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Gourlay, G.; Constabel, C.P. Condensed Tannins Are Inducible Antioxidants and Protect Hybrid Poplar Against Oxidative Stress. Tree Physiol. 2019, 39, 345–355. [Google Scholar] [CrossRef]
- Berard, N.C.; Wang, Y.; Wittenberg, K.M.; Krause, D.O.; Coulman, B.E.; McAllister, T.A.; Ominski, K.H. Condensed Tannin Concentrations Found in Vegetative and Mature Forage Legumes Grown in Western Canada. Can. J. Plant Sci. 2011, 91, 669–675. [Google Scholar] [CrossRef]
- Kyamuhangire, W.; Krekling, T.; Reed, E.; Pehrson, R. The Microstructure and Tannin Content of Banana Fruit and Their Likely Influence on Juice Extraction. J. Sci. Food Agric. 2006, 86, 1908–1915. [Google Scholar] [CrossRef]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herderich, M.J.; Smith, P.A. Quantification of Condensed Tannins by Precipitation with Methyl Cellulose: Development and Validation of an Optimised Tool for Grape and Wine Analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Ortiz, J.; Marín-Arroyo, M.-R.; Noriega-Domínguez, M.-J.; Navarro, M.; Arozarena, I. Color, Phenolics, and Antioxidant Activity of Blackberry (Rubus glaucus Benth.), Blueberry (Vaccinium floribundum Kunth.), and Apple Wines from Ecuador: Fruit Wines from Ecuador. J. Food Sci. 2013, 78, C985–C993. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.C.; Zafimahova, A.; Alvarado, P.G.M.; Dubreucq, E.; Poncet-Legrand, C. Grape Seed and Apple Tannins: Emulsifying and Antioxidant Properties. Food Chem. 2015, 178, 38–44. [Google Scholar] [CrossRef]
- Terrill, T.H.; Rowan, A.M.; Douglas, G.B.; Barry, T.N. Determination of Extractable and Bound Condensed Tannin Concentrations in Forage Plants, Protein Concentrate Meals and Cereal Grains. J. Sci. Food Agric. 1992, 58, 321–329. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Y.; Iwaasa, A.D.; Xu, Z.; Schellenberg, M.P.; Zhang, Y.G.; Liu, X.L.; McAllister, T.A. Effect of Condensed Tannins on Ruminal Degradability of Purple Prairie Clover (Dalea purpurea Vent.) Harvested at Two Growth Stages. Anim. Feed. Sci. Technol. 2012, 176, 17–25. [Google Scholar] [CrossRef]
- Li, Y.; Iwaasa, A.D.; Wang, Y.; Jin, L.; Han, G.; Zhao, M. Condensed Tannins Concentration of Selected Prairie Legume Forages as Affected by Phenological Stages During Two Consecutive Growth Seasons in Western Canada. Can. J. Plant Sci. 2014, 94, 817–826. [Google Scholar] [CrossRef]
- Karakas, N.; Okur, M.E.; Ozturk, I.; Ayla, S.; Karadağ, A.E.; Polat, D.Ç. Antioxidant Activity and Cytotoxic Effects of Prunus spinosa L. Fruit Extract on Various Cancer Cell Lines. MMJ 2019, 34, 297–304. [Google Scholar] [CrossRef]
- Vokou, D.; Katradi, K.; Kokkini, S. Ethnobotanical Survey of Zagori (Epirus, Greece), a Renowned Centre of Folk Medicine in the Past. J. Ethnopharmacol. 1993, 39, 187–196. [Google Scholar] [CrossRef]
- Meschini, S.; Pellegrini, E.; Condello, M.; Occhionero, G.; Delfine, S.; Condello, G.; Mastrodonato, F. Cytotoxic and Apoptotic Activities of Prunus spinosa Trigno Ecotype Extract on Human Cancer Cells. Molecules 2017, 22, 1578. [Google Scholar] [CrossRef]
- Mohammed, S.B.; Upyr, T.V.; Shapoval, O.M.; Lenchyk, L.V.; Georgiev, K. Determination of phenolic compounds in Prunus domestica fruits extract and its pharmacological activity. JofIMAB 2019, 25, 2589–2594. [Google Scholar] [CrossRef]
- Uysal, S. Comparative Antioxidant Capacity and Enzyme Inhibitory Effect of Extracts from Prunus Avium Leaves. Kastamonu Üniversitesi Orman Fakültesi Derg. 2020, 20, 234–242. [Google Scholar] [CrossRef]
- Halarewicz, A. Tissue localization of the condensed tannins in the leaves of the black cherry, Prunus serotina ehrh. Electron. J. Pol. Agric. Univ. 2011, 14, 788–794. [Google Scholar]
- Song, W.; Qin, S.-T.; Fang, F.-X.; Gao, Z.-J.; Liang, D.-D.; Liu, L.-L.; Tian, H.-T.; Yang, H.-B. Isolation and Purification of Condensed Tannin from the Leaves and Branches of Prunus cerasifera and Its Structure and Bioactivities. Appl. Biochem. Biotechnol. 2018, 185, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Cesprini, E.; De Iseppi, A.; Giovando, S.; Tarabra, E.; Zanetti, M.; Šket, P.; Marangon, M.; Tondi, G. Chemical Characterization of Cherry (Prunus avium) Extract in Comparison with Commercial Mimosa and Chestnut Tannins. Wood Sci. Technol. 2022, 56, 1455–1473. [Google Scholar] [CrossRef]
- Roy, S.; Trinchieri, G. Microbiota: A Key Orchestrator of Cancer Therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Negrean, O.-R.; Farcas, A.C.; Pop, O.L.; Socaci, S.A. Blackthorn—A Valuable Source of Phenolic Antioxidants with Potential Health Benefits. Molecules 2023, 28, 3456. [Google Scholar] [CrossRef]
- Ciuperca (Apreutesei), O.T.; Ionescu, E.; Secula, M.S.; Volf, I. Microwave-Assisted Extraction of Condensed Tannins from Branches of Prunus spinosa L.: Response Surface Modeling and Optimization. Processes 2023, 11, 2024. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 7th ed.; Council of Europe: Strasbourg, France, 2012. [Google Scholar]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2009. [Google Scholar]
- Reich, E.; Schibli, A. High-Performance Thin-Layer Chromatography for the Analysis of Medicinal Plants; Thieme: New York, NY, USA, 2007. [Google Scholar]
- Cimpoiu, D.C. Analysis of Some Natural Antioxidants by Thin-Layer Chromatography and High-Performance Thin-Layer Chromatography. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 1125–1142. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT FoodSci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Molyneux, P. The Use of the Stable Free Radical Diphenylpicrylhydrazyl (DPPH) for Estimating Antioxidant. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Bujor, O.-C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal Variations of the Phenolic Constituents in Bilberry (Vaccinium myrtillus L.) Leaves, Stems and Fruits, and Their Antioxidant Activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical Scavenging and Antioxidant Activities of Methanolic Extracts from Hypericum Species Growing in Bulgaria. Phcog. Mag. 2010, 6, 74. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Laville, N.; Aït-Aïssa, S.; Gomez, E.; Casellas, C.; Porcher, J.M. Effects of Human Pharmaceuticals on Cytotoxicity, EROD Activity and ROS Production in Fish Hepatocytes. Toxicology 2004, 196, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid Colorimetric Assay for Cell Growth and Survival. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Berridge, M.V.; Herst, P.M.; Tan, A.S. Tetrazolium Dyes as Tools in Cell Biology: New Insights into Their Cellular Reduction. In Biotechnology Annual Review; Elsevier: Amsterdam, The Netherlands, 2005; Volume 11, pp. 127–152. [Google Scholar] [CrossRef]
- van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell Sensitivity Assays: The MTT Assay. In Cancer Cell Culture; Cree, I.A., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2011; Volume 731, pp. 237–245. [Google Scholar] [CrossRef]
- Stockert, J.C.; Blázquez-Castro, A.; Cañete, M.; Horobin, R.W.; Villanueva, Á. MTT Assay for Cell Viability: Intracellular Localization of the Formazan Product Is in Lipid Droplets. Acta Histochem. 2012, 114, 785–796. [Google Scholar] [CrossRef]
- Olive, P.L.; Banáth, J.P. The Comet Assay: A Method to Measure DNA Damage in Individual Cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Kumaravel, T.S.; Vilhar, B.; Faux, S.P.; Jha, A.N. Comet Assay Measurements: A Perspective. Cell Biol. Toxicol. 2009, 25, 53–64. [Google Scholar] [CrossRef]
- Singh, N.P.; Stephens, R.E.; Schneider, E.L. Modifications of Alkaline Microgel Electrophoresis for Sensitive Detection of DNA Damage. Int. J. Radiat. Biol. 1994, 66, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Murakami, C.; Hirakawa, Y.; Inui, H.; Nakano, Y.; Yoshida, H. Effect of Tea Catechins on Cellular Lipid Peroxidation and Cytotoxicity in HepG2 Cells. Biosci. Biotechnol. Biochem. 2002, 66, 1559–1562. [Google Scholar] [CrossRef]
- Damiani, E.; Solorio, J.A.; Doyle, A.P.; Wallace, H.M. How Reliable Are In Vitro IC50 Values? Values Vary with Cytotoxicity Assays in Human Glioblastoma Cells. Toxicol. Lett. 2019, 302, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Serpeloni, J.M.; Bisarro dos Reis, M.; Rodrigues, J.; Campaner dos Santos, L.; Vilegas, W.; Varanda, E.A.; Dokkedal, A.L.; Colus, I.M.S. In Vivo Assessment of DNA Damage and Protective Effects of Extracts from Miconia Species Using the Comet Assay and Micronucleus Test. Mutagenesis 2008, 23, 501–507. [Google Scholar] [CrossRef]
- Basri, D.F.; Alamin, Z.A.Z.; Chan, K.M. Assessment of Cytotoxicity and Genotoxicity of Stem Bark Extracts from Canarium odontophyllum Miq. (Dabai) Against HCT 116 Human Colorectal Cancer Cell Line. BMC Complement Altern. Med. 2015, 16, 36. [Google Scholar] [CrossRef]
- Al-Faifi, Z.; Masrahi, Y.; Aly, M.; Al-Turki, T.; Dardeer, T. Evaluation of Cytotoxic and Genotoxic Effects of Euphorbia triaculeata Forssk. Extract. Asian Pac. J. Cancer Prev. 2017, 18, 771. [Google Scholar] [CrossRef]
- Kahaliw, W.; Hellman, B.; Engidawork, E. Genotoxicity Study of Ethiopian Medicinal Plant Extracts on HepG2 Cells. BMC Complement Altern. Med. 2018, 18, 45. [Google Scholar] [CrossRef]
- Paul, S.; Chakraborty, S.; Mukherjee, A.; Kundu, R. Evaluation of Cytotoxicity and DNA Damaging Activity of Three Plant Extracts on Cervical Cancer Cell Lines. Int. J. Pharm. Sci. Rev. Res. 2015, 31, 183–189. [Google Scholar]
- Dibdiakova, J.; Wang, L.; Li, H. Characterization of Ashes from Pinus Sylvestris Forest Biomass. Energy Procedia 2015, 75, 186–191. [Google Scholar] [CrossRef][Green Version]
- Protásio, T.d.P.; Tonoli, G.H.D.; Guimarães Júnior, M.; Bufalino, L.; Couto, A.M.; Trugilho, P.F. Correlações Canônicas Entre as Características Químicas e Energéticas de Resíduos Lignocelulósicos. CERNE 2012, 18, 433–439. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Chemistry and Specialty Industrial Applications of Lignocellulosic Biomass. Waste Biomass Valor. 2021, 12, 2145–2169. [Google Scholar] [CrossRef]
- Dessie, W.; Luo, X.; He, F.; Liao, Y.; Duns, G.; Qin, Z. Lignin valorization: A crucial step towards full utilization of biomass, zero waste and circular bioeconomy. Biocatal. Agric. Biotechnol. 2023, 51, 102777. [Google Scholar] [CrossRef]
- Devi, A.; Bajar, S.; Kour, H.; Kothari, R.; Pant, D.; Singh, A. Lignocellulosic Biomass Valorization for Bioethanol Production: Circular Bioeconomy Approach. Bioenerg. Res. 2022, 15, 1820–1841. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, S.; Kaur, P.; Narang, J.; Mukherjee, G.; Thakur, B.; Kaur, S.; Tripathi, M. Fungal bioprocessing for circular bioeconomy: Exploring lignocellulosic waste valorization. Mycology 2024, 15, 538–563. [Google Scholar] [CrossRef]
- Yadav, A.; Sharma, V.; Tsai, M.; Chen, C.-W.; Sun, P.-P.; Nargotra, P.; Wang, J.-X.; Dong, C. Development of lignocellulosic biorefineries for the sustainable production of biofuels: Towards circular bioeconomy. Bioresour. Technol. 2023, 381, 129145. [Google Scholar] [CrossRef] [PubMed]
- Volf, I.; Popa, V.I. Integrated Processing of Biomass Resources for Fine Chemical Obtaining. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Elsevier: Amsterdam, The Netherlands, 2018; pp. 113–160. [Google Scholar] [CrossRef]
- Zhong, Y.; Ma, C.M.; Shahidi, F. Antioxidant and antiviral activities of lipophilic epigallocatechin gallate (EGCG) derivatives. Funct. Foods 2012, 4, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.R.; Nabavi, S.F.; Daglia, M.; Rastrelli, L.; Nabavi, S.M. Epigallocatechin gallate and mitochondria-A story of life and death. Pharmacol. Res. 2016, 104, 70–85. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Coșarcă, S.; Tanase, C.; Muntean, D.L. Therapeutic Aspects of Catechin and Its Derivatives—An Update. Acta Biol. Marisiensis 2019, 2, 21–29. [Google Scholar] [CrossRef]
- Agarwal, C.; Hofmann, T.; Vršanská, M.; Schlosserová, N.; Visi-Rajczi, E.; Voběrková, S.; Pásztory, Z. In vitro antioxidant and antibacterial activities with polyphenolic profiling of wild cherry, the European larch and sweet chestnut tree bark. Eur. Food Res. Technol. 2021, 247, 2355–2370. [Google Scholar] [CrossRef]
- Ortega-Vidal, J.; Ruiz-Martos, L.; Salido, S.; Altarejos, J. Proanthocyanidins in Pruning Wood Extracts of Four European Plum (Prunus domestica L.) Cultivars: Quantitative Analysis. Chem. Biodivers. 2023, 20, e202200931. [Google Scholar] [CrossRef]
- Uğur, Y.; Erdoğan, S.; Yılmaz, İ.; Başgel, S. Variation of Composition of Phenolic Compounds in the Apricot (Prunus armeniaca L.) Leaves by Seasons. J. Nat. Prod. Plant Resour. 2018, 8, 32–39. [Google Scholar]
- Amir, M.; Mujeeb, M.; Ahmad, S.; Akhtar, M.; Kamal, Y.T.; Ashraf, K. Simultaneous Quantitative HPLC Analysis of Ascorbic Acid, Gallic Acid, and Catechin in Punica granatum, Tamarindus indica and Prunus domestica. Planta Med. 2013, 79, 134. [Google Scholar] [CrossRef]
- Jasprica, I.; Bojić, M.; Mornar, A.; Bešić, E.; Bučan, K.; Medić-Šarić, M. Evaluation of Antioxidative Activity of Croatian Propolis Samples Using DPPH· and ABTS+ Stable Free Radical Assays. Molecules 2007, 12, 1006–1021. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, J.S.; Yang, B.; Lv, G.P.; Li, S.P. Free Radical Scavenging Activity and Characterization of Sesquiterpenoids in Four Species of Curcuma Using a TLC Bioautography Assay and GC–MS Analysis. Molecules 2017, 22, 7547–7557. [Google Scholar] [CrossRef]
- Wang, J.; Yue, Y.D.; Tang, F.; Sun, J. Screening and Analysis of the Potential Bioactive Components in Rabbit Plasma After Oral Administration of Hot-Water Extracts from Leaves of Bambusa textilis McClure. Molecules 2012, 17, 8872–8885. [Google Scholar] [CrossRef] [PubMed]
- Kusznierewicz, B.; Piekarska, A.; Mrugalska, B.; Konieczka, P.; Namieśnik, J.; Bartoszek, A. Phenolic Composition and Antioxidant Properties of Polish Blue-Berried Honeysuckle Genotypes by HPLC-DAD-MS, HPLC Post Column Derivatization with ABTS or FC, and TLC with DPPH Visualization. J. Agric. Food Chem. 2012, 60, 1755–1763. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Morton, D.W. HPTLC–Bioautographic Methods for Selective Detection of the Antioxidant and α-Amylase Inhibitory Activity in Plant Extracts. MethodsX 2018, 5, 797–802. [Google Scholar] [CrossRef]
- Ansari, H.I.; Dabhi, R.C.; Trivedi, P.G.; Thakar, M.S.; Maru, J.J.; Sindhav, G.M. Isolation and Characterization of an Undescribed Flavonoid from Abrus precatorius L. Based on HPTLC-DPPH Bioautography and Its Cytotoxicity Evaluation. Future J. Pharm. Sci. 2023, 9, 119. [Google Scholar] [CrossRef]
- Litewski, S.; Mróz, M.; Bartoszek, A.; Kusznierewicz, B. Post-Chromatographic Derivatization Coupled with Mass Spectrometry as a Method of Profiling and Identification of Antioxidants; Ligustrum vulgare Phytocomplex as an Example. Molecules 2023, 28, 8000. [Google Scholar] [CrossRef] [PubMed]
- Meriane, D.; Genta-Jouve, G.; Kaâbeche, M.; Michel, S.; Boutefnouchet, S. Rapid Identification of Antioxidant Compounds of Genista saharae Coss. & Dur. by Combination of DPPH Scavenging Assay and HPTLC-MS. Molecules 2014, 19, 4369–4379. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, R. Phytochemical Screening, HPTLC Finger Print and In Vitro Antioxidant Activity of Bark Extracts of Lannea coromandelica (Houtt.) Merr. Indian J. Pharm. Educ. Res. 2021, 55, 482–490. [Google Scholar] [CrossRef]
- Condello, M.; Pellegrini, E.; Spugnini, E.P.; Baldi, A.; Amadio, B.; Vincenzi, B.; Occhionero, G.; Delfine, S.; Mastrodonato, F.; Meschini, S. Anticancer Activity of “Trigno M”, Extract of Prunus spinosa Drupes, Against In Vitro 3D and In Vivo Colon Cancer Models. Biomed. Pharmacother. 2019, 118, 109281. [Google Scholar] [CrossRef]
- Nabende, P.; Karanja, S.; Mwatha, J.; Wachira, S. Anti-Proliferative Activity of Prunus Africana, Warburgia stuhlmannii and Maytenus senegalensis Extracts in Breast and Colon Cancer Cell Lines. Eur. J. Med. Plants 2015, 5, 366–376. [Google Scholar] [CrossRef]
- Onyancha, J.M.; Gikonyo, N.K.; Wachira, S.W.; Mwitari, P.G.; Gicheru, M.M. Anticancer Activities and Safety Evaluation of Selected Kenyan Plant Extracts Against Breast Cancer Cell Lines. J. Pharmacogn. Phytother. 2018, 10, 21–26. [Google Scholar] [CrossRef]
- Pinacho, R.; Cavero, R.Y.; Astiasarán, I.; Ansorena, D.; Calvo, M.I. Phenolic Compounds of Blackthorn (Prunus spinosa L.) and Influence of In Vitro Digestion on Their Antioxidant Capacity. J. Funct. Foods 2015, 19, 49–62. [Google Scholar] [CrossRef]
- Jang, G.H.; Kim, H.W.; Lee, M.K.; Jeong, S.Y.; Bak, A.R.; Lee, D.Y.; Kim, J.B. Characterization and Quantification of Flavonoid Glycosides in the Prunus Genus by UPLC-DAD-QTOF/MS. Saudi J. Biol. Sci. 2018, 25, 1622–1631. [Google Scholar] [CrossRef]
- Granica, S.; Krupa, K.; Kłębowska, A.; Kiss, A.K. Development and Validation of an HPLC-DAD-CAD-MS3 Method for Qualitative and Quantitative Standardization of Polyphenols in Agrimoniae eupatoriae herba. J. Pharm. Biomed. Anal. 2013, 86, 112–122. [Google Scholar] [CrossRef]
- Tomczyk, M.; Latté, K.P. Potentilla—A Review of Its Phytochemical and Pharmacological Profile. J. Ethnopharmacol. 2009, 122, 184–204. [Google Scholar] [CrossRef]
- Xie, Y.W.; Xu, H.X.; Dong, H.; Fiscus, R.R.; But, P.P.H. Role of Nitric Oxide in the Vasorelaxant and Hypotensive Effect of Extracts and Purified Tannins from Geum japonicum. J. Ethnopharmacol. 2007, 109, 128–133. [Google Scholar] [CrossRef]
- Crețu, R.; Mihăilescu, R.; Mitroi, G.; Iacob, E.; Ionescu, E. Cercetări Privind Obținerea Unor Produse Fitoterapeutice cu Acțiune Antioxidantă din Partea Lemnoasă de Vitis vinifera L., Rosa canina L., și Hippophae rhamnoides L. Obținerea și Caracterizarea Unui Extract Complex cu Acțiune Antioxidantă. Rev. Med. Chir. Soc. Med. Nat. Iași 2007, 111 (Suppl. S2), 233–236. [Google Scholar]
- Ciupercă, O.T.; Ţebrencu, C.E.; Lăzar, L.; Volf, I. Kinetic Study on Solid–Liquid Extraction of Condensed Tannins from Rosa canina Branches and Stems. Rev. Chim. 2020, 71, 153–161. [Google Scholar] [CrossRef]
- Apreutesei, O.T.; Ţebrencu, C.E.; Ionescu, E. Exploring the Phytochemical Profile and Quality Control of Hippophae rhamnoides (Sea buckthorn) Branches. Ann. Acad. Rom. Sci. Phys. Chem. Sci. 2024, 9, 2. [Google Scholar] [CrossRef]
- Sharangi, A.B. Medicinal and Therapeutic Potentialities of Tea (Camellia sinensis L.)—A Review. Food Res. Int. 2009, 42, 529–535. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, P.; Qu, H. Antioxidant and Anticancer Properties of Persimmon (Diospyros kaki) Condensed Tannins. Food Chem. 2011, 129, 387–392. [Google Scholar] [CrossRef]
- Wei, S.D.; Chen, R.Y.; Liao, M.M.; Tu, N.W.; Zhou, H.C.; Lin, Y.M. Antioxidant Condensed Tannins from Machilus pauhoi Leaves. J. Med. Plants Res. 2011, 5, 796–804. [Google Scholar]
- Miranda, I.; Lima, L.; Quilhó, T.; Knapic, S.; Pereira, H. The Bark of Eucalyptus sideroxylon as a Source of Phenolic Extracts with Antioxidant Properties. Ind. Crops Prod. 2016, 82, 81–87. [Google Scholar] [CrossRef]
- Diouf, P.N.; Stevanovic, T.; Cloutier, A. Study on Chemical Composition, Antioxidant and Anti-Inflammatory Activities of Hot Water Extract from Picea mariana Bark and Its Proanthocyanidin-Rich Fractions. Food Chem. 2009, 113, 897–902. [Google Scholar] [CrossRef]
- Ionescu, E.; Tebrencu, C.E.; Ciupercă, O.T.; Vochița, G. HPTLC Investigation of Phenolic Compounds from the Extracts of Ulmus glabra Huds. Bark. An. Ştiinţ. Univ. Al I Cuza Iaşi Biol. Veg. 2018, 64, 51–62. [Google Scholar]
- Vochiţa, G.; Ionescu, E.; Tebrencu, C.E.; Ciupercă, O.T.; Mihai, C.T.; Gherghel, D. In Vitro Investigation of Antitumoral Activity Mechanisms of Ulmus glabra Bark Extracts. In Proceedings of the 11th National Congress with International Participation and the 37th Annual Scientific Session of the Romanian Society of Cell Biology, Constanța, Romania, 20–23 June 2019. [Google Scholar]
- Ciupercă, O.T.; Ţebrencu, C.E.; Ionescu, E.; Iacob, E.; Volf, I. Studies on Polyphenols Isolated from Branches of Prunus spinosa L. Species. Rev. Chim. 2019, 70, 2897–2902. [Google Scholar] [CrossRef]
- Ţebrencu, C.E.; Ciupercă, O.T.; Ionescu, E. New Sources of Condensed Tannins—Investigation of Branches of Some Shrub Species Through HPTLC Analysis. Ann. Acad. Rom. Sci. Phys. Chem. Sci. 2020, 5, 49–58. [Google Scholar] [CrossRef]
- Gegiu, G.; Branza, A.-D.; Bucur, L.; Grigorian, M.; Tache, T.; Badea, V. Contributions to the Antimicrobial and Antifungal Study of the Aqueous Extract of Prunus spinosa L. Farmacia 2015, 63, 275–279. [Google Scholar]
- Veličković, I.; Žižak, Ž.; Rajčević, N.; Ivanov, M.; Soković, M.; Marin, P.D.; Grujić, S. Prunus spinosa L. Leaf Extracts: Polyphenol Profile and Bioactivities. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12137. [Google Scholar] [CrossRef]
- Kiran, S.R.; Deepika, D.S.; Babu, Y.T.R.; Chowdary, M.R.; Kumar, G.V. Evaluation of Antioxidant Potential and Selective Cytotoxicity of Endangered Medicinal Plant Prunus ceylanica (Wight) Miq. Ann. Biol. 2024, 40, 61–68. [Google Scholar]
- Hwang, D.; Kim, H.; Shin, H.; Jeong, H.; Kim, J.; Kim, D. Cosmetic Effects of Prunus padus Bark Extract. Korean J. Chem. Eng. 2014. [CrossRef]
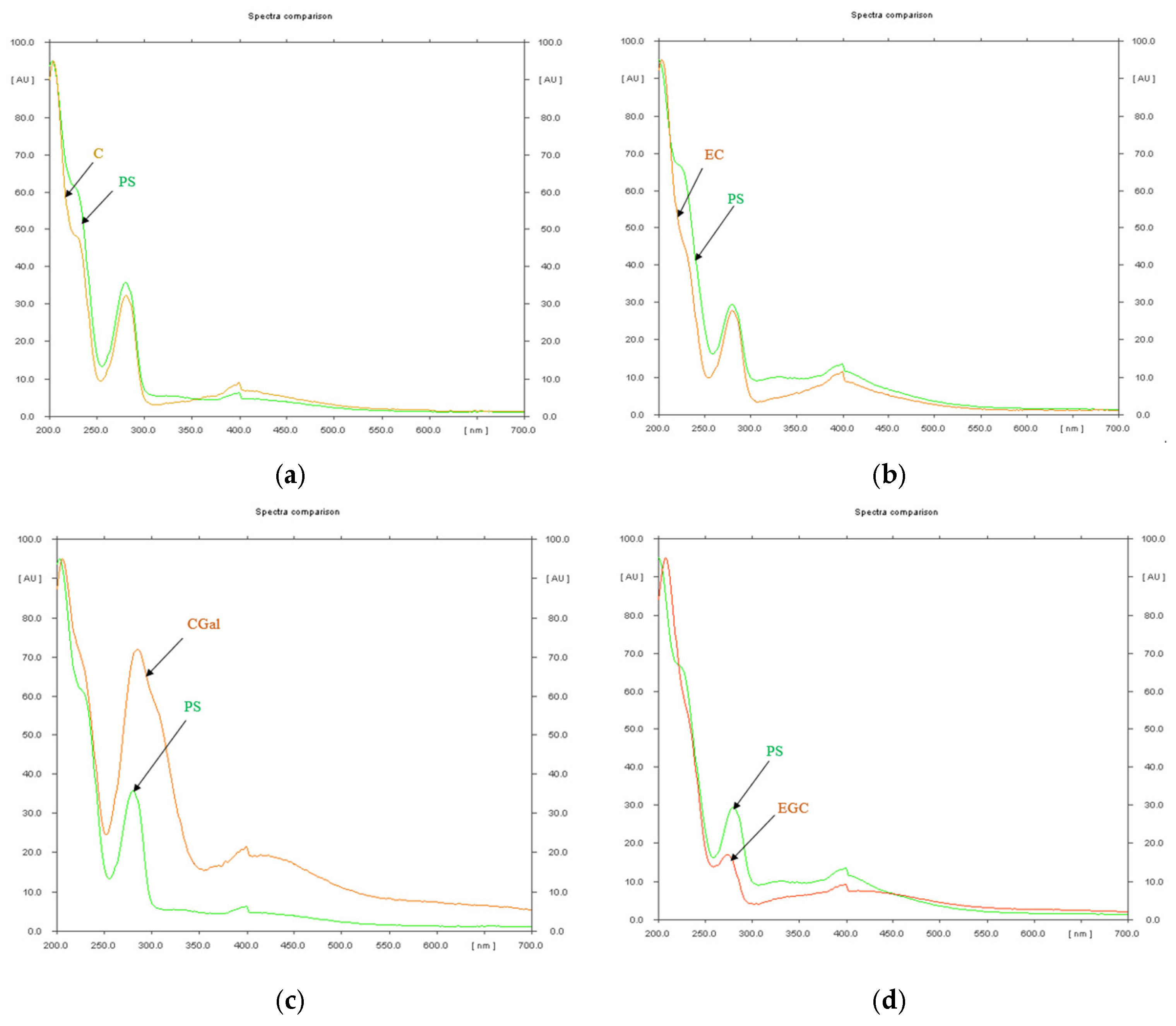
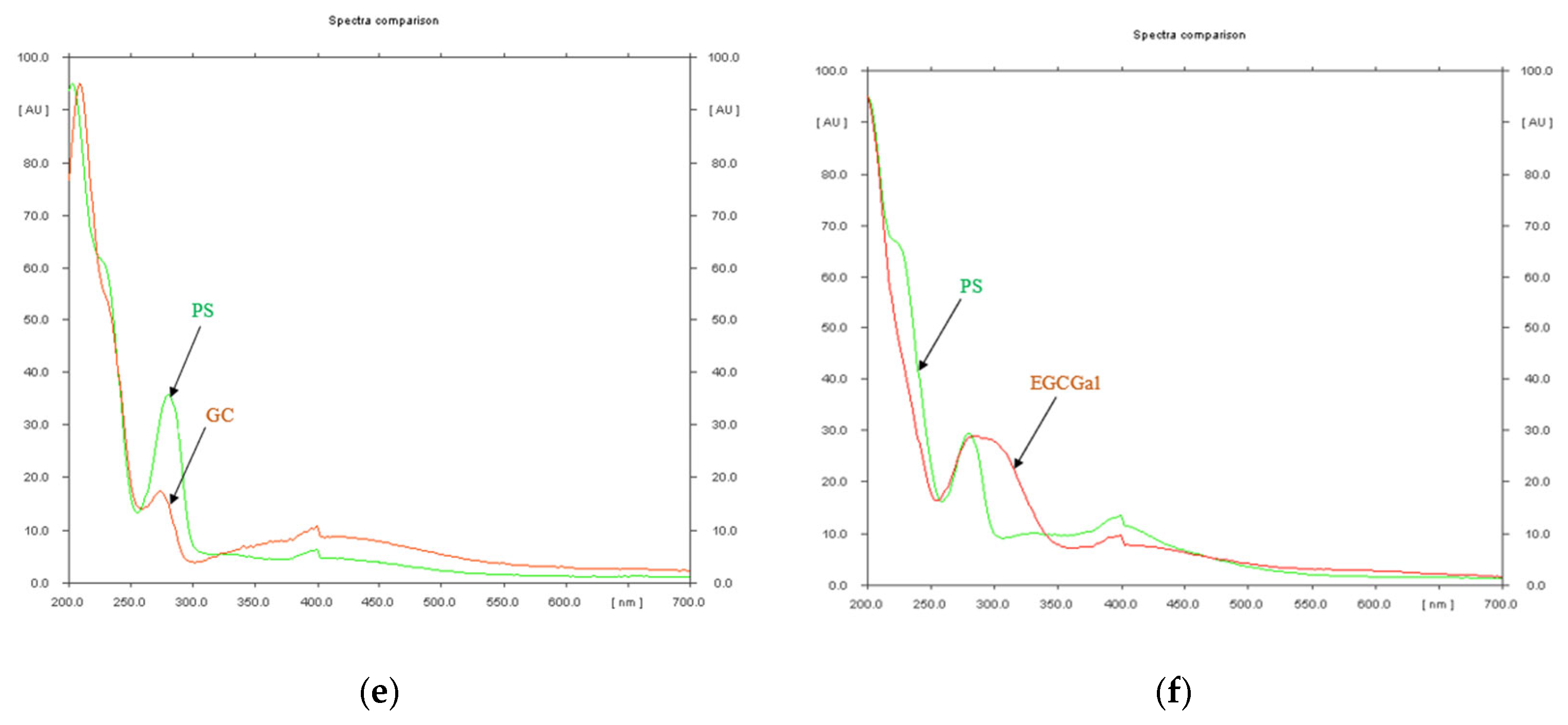
| Rf Value | Reference Substance | Assigned Substance in P. spinosa Catechin Crude Extract |
|---|---|---|
| 0.20, 0.23, 0.24 | Other tannins without correspondence in the applied standards | |
| 0.22 | Epigallocatechin gallate | Epigallocatechin gallate |
| 0.26 | Gallocatechin gallate | - |
| 0.26–0.27 | Epigallocatechin | Epigallocatechin |
| 0.29–0.30 | Gallocatechin | Gallocatechin |
| 0.32 | Other tannins without correspondence in the applied standards | |
| 0.35–0.36 | Epicatechin gallate | - |
| 0.38–0.39 | Catechin gallate | Catechin gallate |
| 0.40–0.41 | Epicatechin | Epicatechin |
| 0.43 | Catechin | Catechin |
| Extract/Control | IC50 (mg/mL) |
|---|---|
| PS | 1.02 ± 0.25 |
| Catechin—reference substance | 0.76 ± 0.0 |
| Extract/Control | IC50 (mg/mL) | ||
|---|---|---|---|
| 0 min | 5 min | 10 min | |
| PS | 0.81 ± 0.10 | 1.0188 ± 0.21 | 0.9255 ± 0.19 |
| Catechin | 0.023 ± 0.00 | 0.0424 ± 0.00 | 0.122 ± 0.04 |
| Vero | HeLa | |||||
|---|---|---|---|---|---|---|
| Head DNA% Mean ± SEM | Tail DNA% Mean ± SEM | p | Head DNA% Mean ± SEM | Tail DNA% Mean ± SEM | p | |
| Control | 86.59 ± 1.36 | 13.41 ± 1.36 | 81.13 ± 1.19 | 18.87 ± 1.19 | ||
| IC50 | 75.76 ± 1.24 | 24.76 ± 1.25 | <0.001 | 67.64 ± 1.27 | 32.36 ± 1.27 | <0.001 |
| UV | 79.45 ± 1.41 | 20.55 ± 1.41 | <0.001 | 72.53 ± 1.80 | 27.47 ± 1.80 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apreutesei, O.T.; Tebrencu, C.E.; Gherghel, D.; Oprică, L.A.; Volf, I.; Vochița, G. Prunus spinosa L. Branches as a New Source of Condensed Tannins: Phytochemical Profile and Antioxidant, Cytotoxic and Genotoxic In Vitro Evaluation. Antioxidants 2025, 14, 1408. https://doi.org/10.3390/antiox14121408
Apreutesei OT, Tebrencu CE, Gherghel D, Oprică LA, Volf I, Vochița G. Prunus spinosa L. Branches as a New Source of Condensed Tannins: Phytochemical Profile and Antioxidant, Cytotoxic and Genotoxic In Vitro Evaluation. Antioxidants. 2025; 14(12):1408. https://doi.org/10.3390/antiox14121408
Chicago/Turabian StyleApreutesei, Oana Teodora, Carmen Elena Tebrencu, Daniela Gherghel, Lăcrămioara Anca Oprică, Irina Volf, and Gabriela Vochița. 2025. "Prunus spinosa L. Branches as a New Source of Condensed Tannins: Phytochemical Profile and Antioxidant, Cytotoxic and Genotoxic In Vitro Evaluation" Antioxidants 14, no. 12: 1408. https://doi.org/10.3390/antiox14121408
APA StyleApreutesei, O. T., Tebrencu, C. E., Gherghel, D., Oprică, L. A., Volf, I., & Vochița, G. (2025). Prunus spinosa L. Branches as a New Source of Condensed Tannins: Phytochemical Profile and Antioxidant, Cytotoxic and Genotoxic In Vitro Evaluation. Antioxidants, 14(12), 1408. https://doi.org/10.3390/antiox14121408









