Rectal Microbiomes and Serum Metabolomics Reveal Changes in Serum Antioxidant Status and Immune Responses of Dezhou Donkeys in Late Gestation to Parturition
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval Declarations
2.2. Experimental Design and Treatments
2.3. Sample Collection
2.4. Rectal Microbiota
2.5. Serum Metabolome
2.6. Statistical Analysis
3. Results
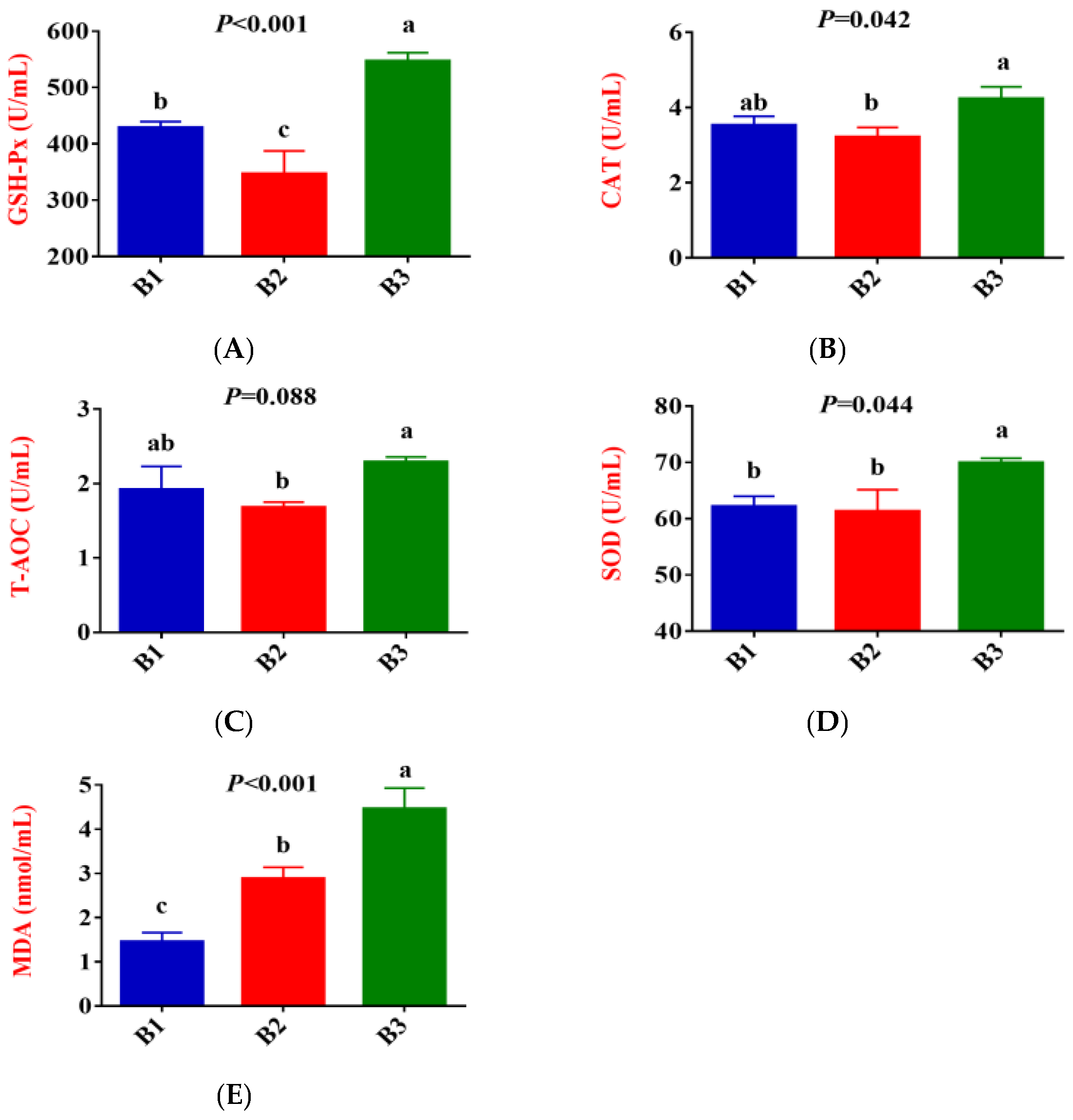
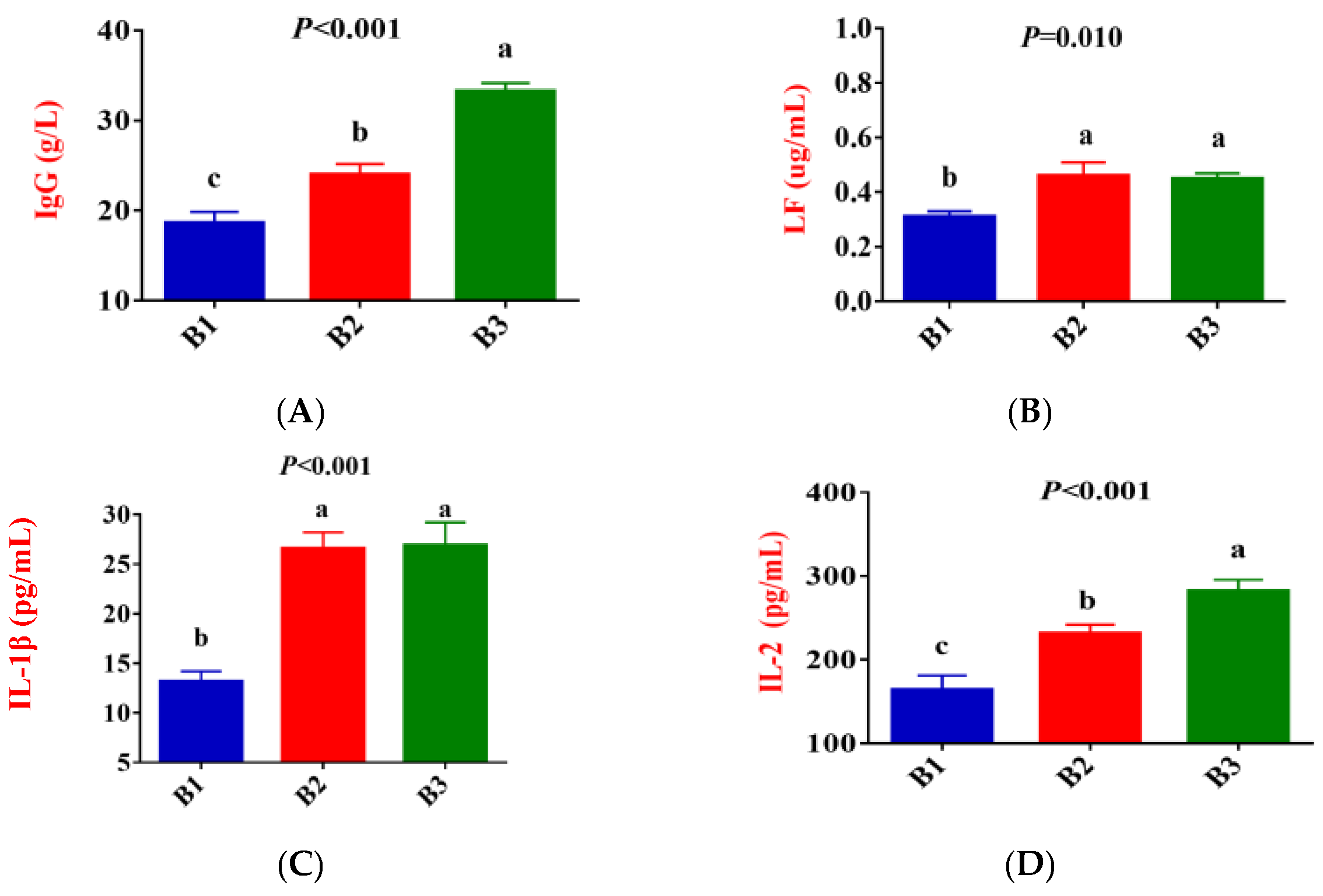
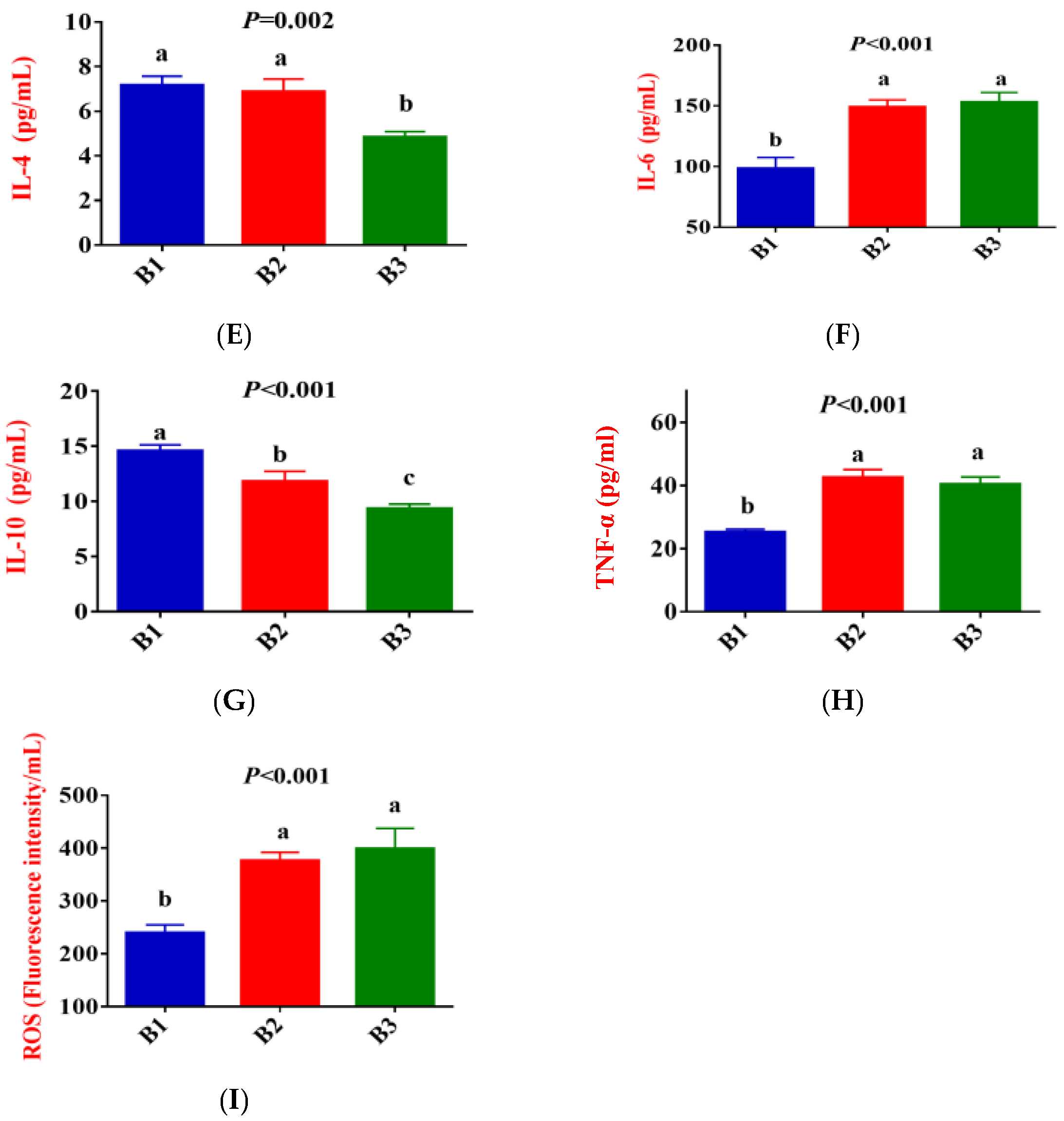
3.1. Serum Antioxidative Status, Inflammatory Cytokines, Immunoglobulin, and Biochemical Parameters
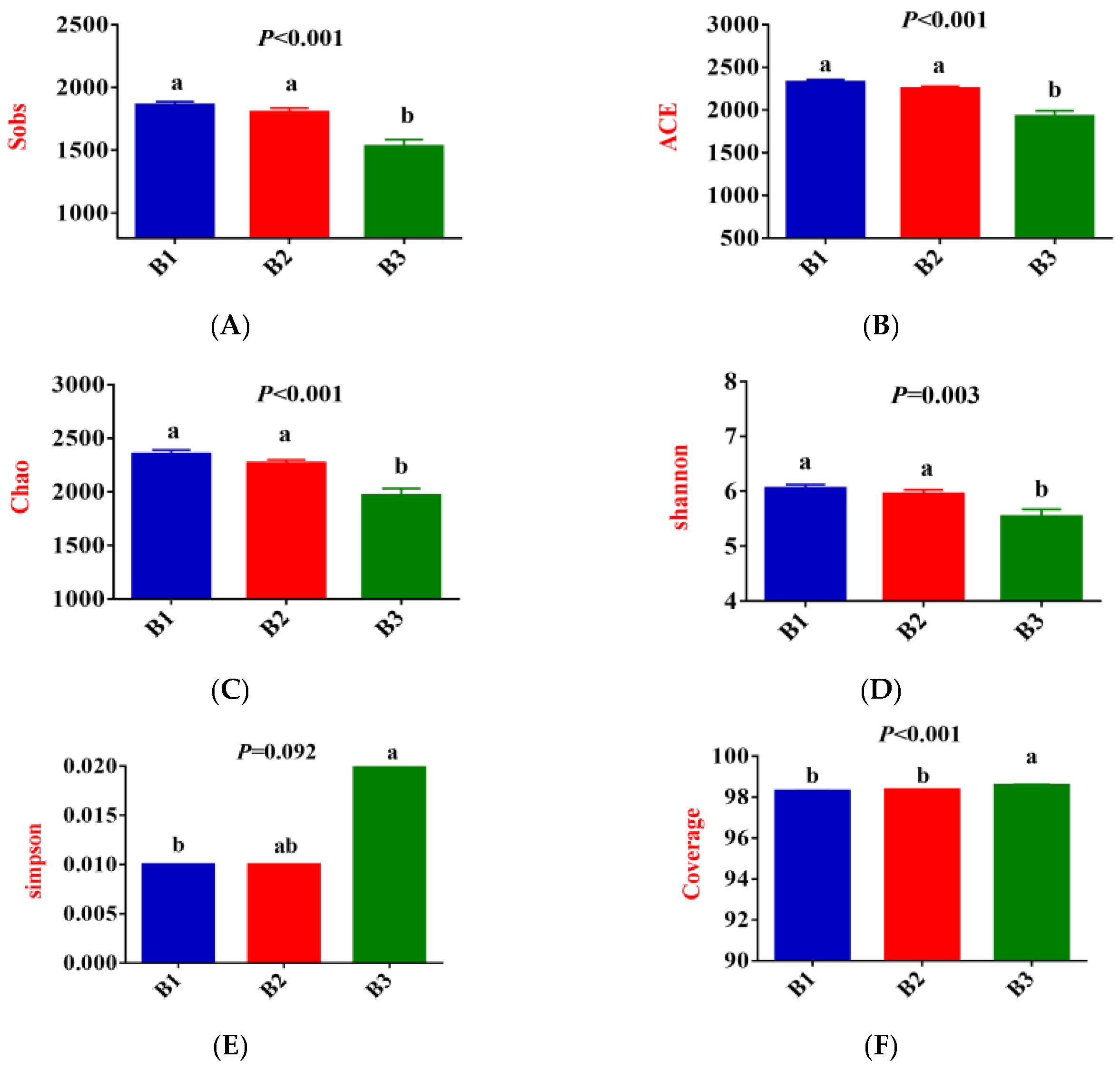
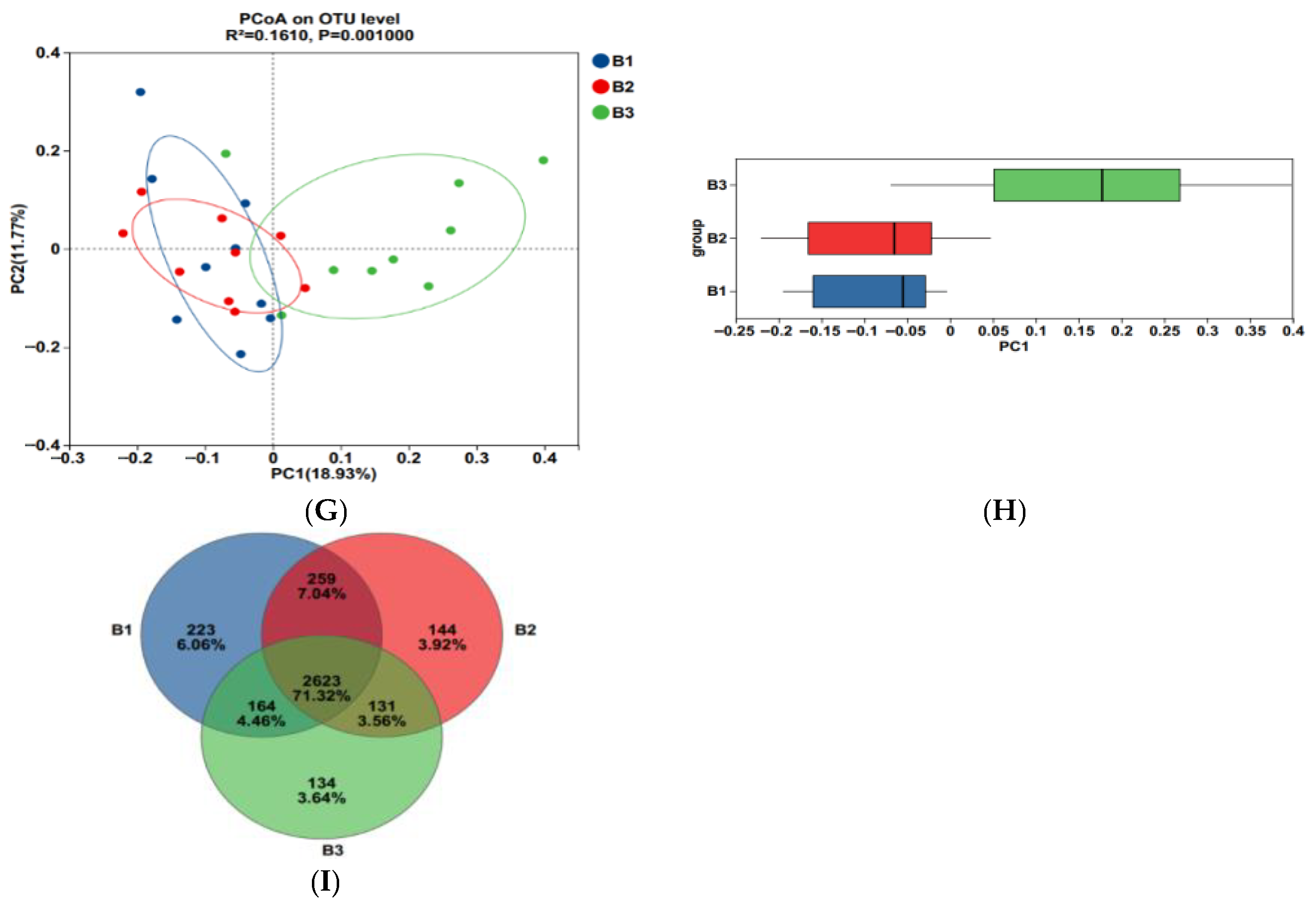
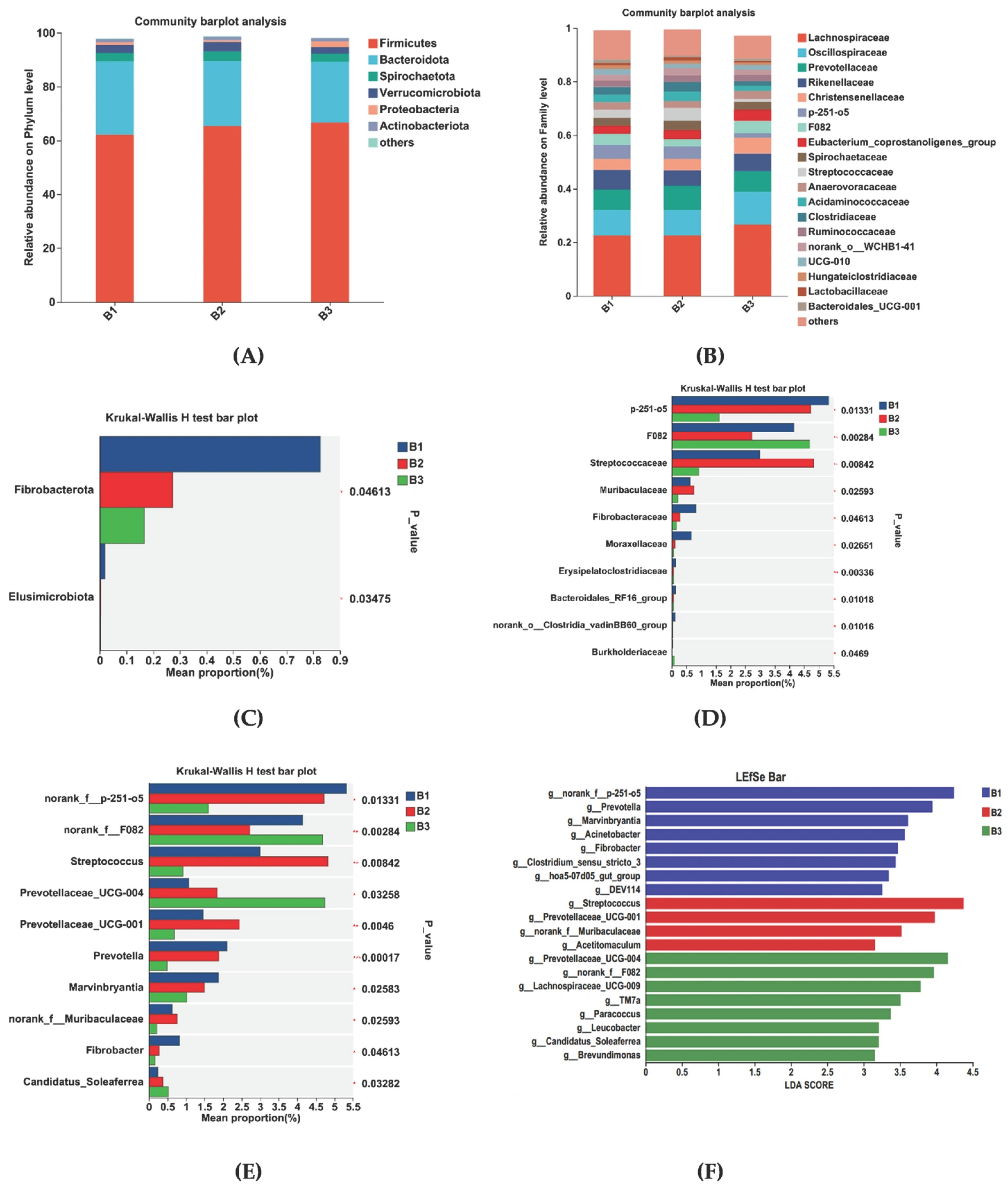
3.2. Rectal Bacterial Community Richness, Diversity, and Composition
3.3. Significantly Different Rectal Bacteria Among Groups B1, B2, and B3
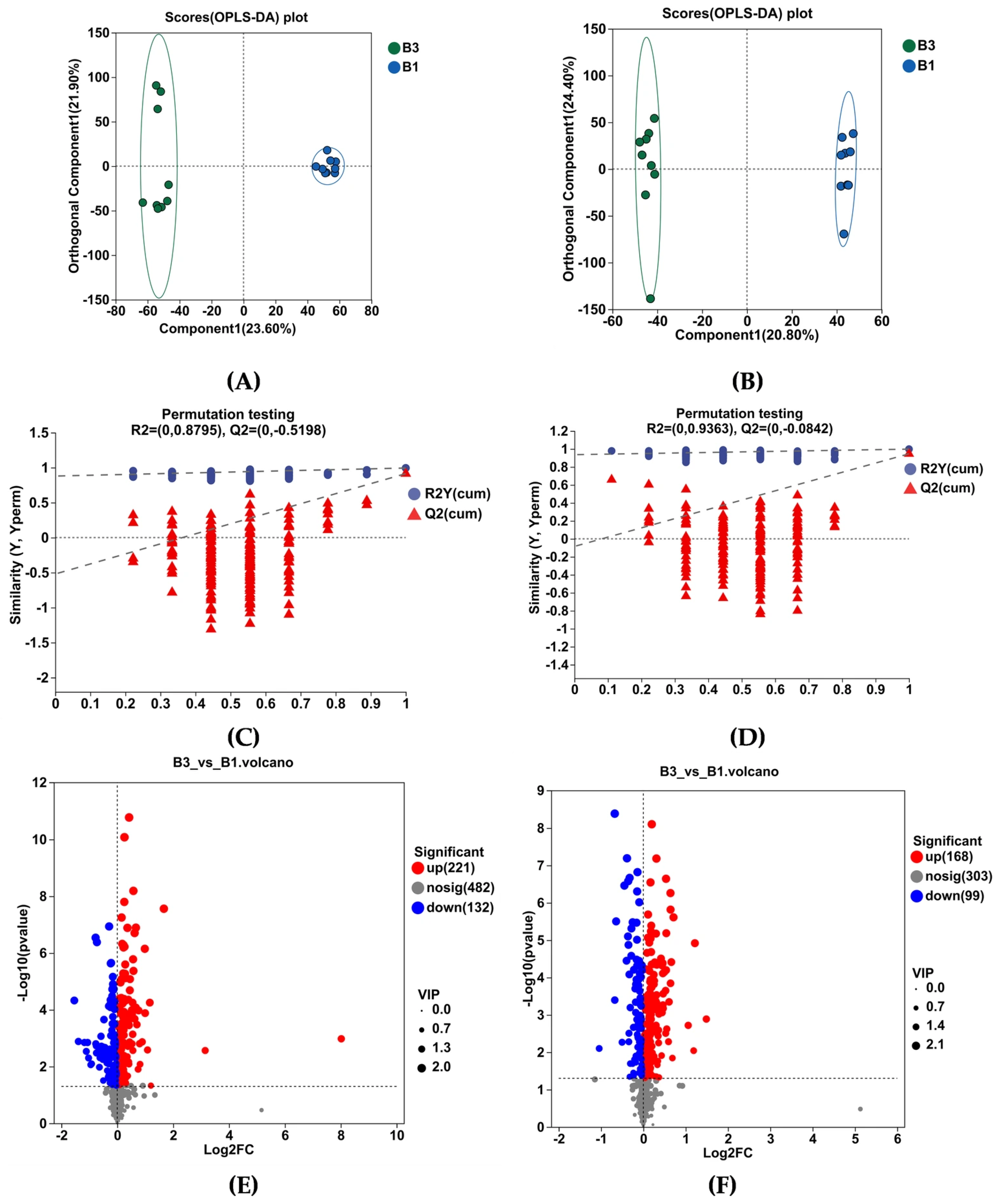
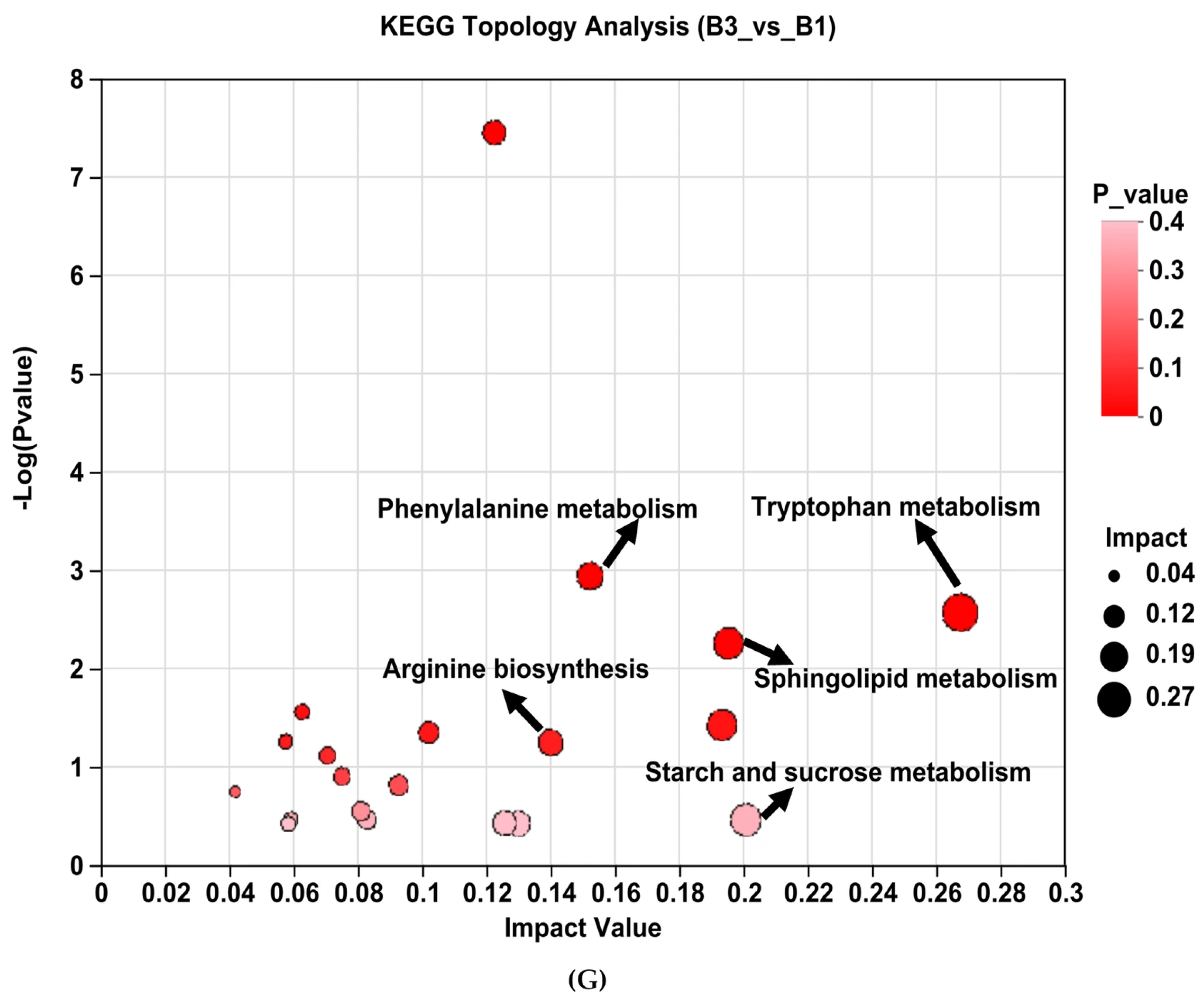
3.4. Metabolomic Profiles in the Serum and Identification of Metabolites
3.5. Correlation Analysis Between Serum Metabolites and Serum Antioxidant Indicators, Inflammatory Indicators, Serum Biochemical Indicators, and Rectal Bacteria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yue, Y.; Li, L.; Tong, M.; Li, S.; Zhao, Y.; Guo, X.; Guo, Y.; Shi, B.; Yan, S. Effect of Varying Dietary Crude Protein Level on Milk Production, Nutrient Digestibility, and Serum Metabolites by Lactating Donkeys. Animals 2022, 12, 2066. [Google Scholar] [CrossRef]
- Mulligan, F.J.; O’Grady, L.; Rice, D.A.; Doherty, M.L. A herd health approach to dairy cow nutrition and production diseases of the transition cow. Anim. Reprod. Sci. 2006, 96, 331–353. [Google Scholar] [CrossRef]
- Mulligan, F.J.; Doherty, M.L. Production diseases of the transition cow. Vet. J. 2008, 176, 3–9. [Google Scholar] [CrossRef]
- Szczubiał, M. Changes in oxidative stress markers in plasma of sows during periparturient period. Pol. J. Vet. Sci. 2020, 23, 185–190. [Google Scholar] [CrossRef]
- Yang, Z.; Luo, F.; Liu, G.; Luo, Z.; Ma, S.; Gao, H.; He, H.; Tao, J. Plasma Metabolomic Analysis Reveals the Relationship between Immune Function and Metabolic Changes in Holstein Peripartum Dairy Cows. Metabolites 2022, 12, 953. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, F.; Rota, A.; Corazza, M.; Serio, D.; Sgorbini, M. Hematological and biochemical findings in pregnant, postfoaling, and lactating jennies. Theriogenology 2016, 85, 1233–1238. [Google Scholar] [CrossRef]
- Liu, G.; Luo, X.; Zhao, X.; Zhang, A.; Jiang, N.; Yang, L.; Huang, M.; Xu, L.; Ding, L.; Li, M.; et al. Gut microbiota correlates with fiber and apparent nutrients digestion in goose. Poult. Sci. 2018, 97, 3899–3909. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ali, I.; Lei, Z.; Li, Y.; Yang, M.; Yang, C.; Li, L. Effect of a Multistrain Probiotic on Feline Gut Health through the Fecal Microbiota and Its Metabolite SCFAs. Metabolites 2023, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Goodrich, J.K.; Cullender, T.C.; Spor, A.; Laitinen, K.; Bäckhed, H.K.; Gonzalez, A.; Werner, J.J.; Angenent, L.T.; Knight, R.; et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012, 150, 470–480. [Google Scholar] [CrossRef]
- Cheng, C.; Wei, H.; Yu, H.; Xu, C.; Jiang, S.; Peng, J. Metabolic Syndrome During Perinatal Period in Sows and the Link with Gut Microbiota and Metabolites. Front. Microbiol. 2018, 9, 1989. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, J.; Xiong, X.; Zou, L.; Kong, X.; Tan, B.; Yin, Y. Differences in Gut Microbial and Serum Biochemical Indices Between Sows with Different Productive Capacities During Perinatal Period. Front. Microbiol. 2020, 10, 3047. [Google Scholar] [CrossRef]
- Hayakawa, T.; Masuda, T.; Kurosawa, D.; Tsukahara, T. Dietary administration of probiotics to sows and/or their neonates improves the reproductive performance, incidence of post-weaning diarrhea and histopathological parameters in the intestine of weaned piglets. Anim. Sci. J. 2016, 87, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Q.; Shi, X.; Liu, G.; Wang, C. Integrated multi-omics reveals novel microbe-host lipid metabolism and immune interactions in the donkey hindgut. Front. Immunol. 2022, 13, 1003247. [Google Scholar] [CrossRef]
- Guo, Y.; Yin, G.; Hui, F.; Guo, X.; Shi, B.; Zhao, Y.; Yan, S. Effects of dietary energy level on antioxidant capability, immune function and rectal microbiota in late gestation donkeys. Front. Microbiol. 2024, 15, 1308171. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Cheng, H.; Wang, H.; Tan, Y.; Feng, W.; Peng, C. Interactions between polysaccharides and gut microbiota: A metabolomic and microbial review. Food Res. Int. 2022, 160, 111653. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Tong, M.M.; Li, L.; Hui, F.; Meng, F.Z.; Zhao, Y.L.; Guo, Y.M.; Guo, X.Y.; Shi, B.L.; Yan, S.M. Rectal microbiomes and serum metabolomics reveal the improved effect of Artemisia ordosica crude polysaccharides on the lactation performance, antioxidant status, and immune responses of lactating donkeys. J. Dairy Sci. 2024, 107, 6696–6716. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Horses, 6th ed.; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef]
- Cao, M.; Li, Y.; Wu, Q.J.; Zhang, P.; Li, W.T.; Mao, Z.Y.; Wu, D.M.; Jiang, X.M.; Zhuo, Y.; Fang, Z.F.; et al. Effects of dietary Clostridium butyricum addition to sows in late gestation and lactation on reproductive performance and intestinal microbiota. J. Anim. Sci. 2019, 97, 3426–3439. [Google Scholar] [CrossRef] [PubMed]
- Mavangira, V.; Mangual, M.J.; Gandy, J.C.; Sordillo, L.M. 15-F2t-Isoprostane Concentrations and Oxidant Status in Lactating Dairy Cattle with Acute Coliform Mastitis. J. Vet. Intern. Med. 2016, 30, 339–347. [Google Scholar] [CrossRef]
- Mavangira, V.; Sordillo, L.M. Role of lipid mediators in the regulation of oxidative stress and inflammatory responses in dairy cattle. Res. Vet. Sci. 2018, 116, 4–14. [Google Scholar] [CrossRef]
- Gaál, T.; Ribiczeyné-Szabó, P.; Stadler, K.; Jakus, J.; Reiczigel, J.; Kövér, P.; Mézes, M.; Sümeghy, L. Free radicals, lipid peroxidation and the antioxidant system in the blood of cows and newborn calves around calving. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 143, 391–396. [Google Scholar] [CrossRef]
- Huang, P.F.; Mou, Q.; Yang, Y.; Li, J.M.; Xu, M.L.; Huang, J.; Li, J.Z.; Yang, H.S.; Liang, X.X.; Yin, Y.L. Effects of supplementing sow diets during late gestation with Pennisetum purpureum on antioxidant indices, immune parameters, and faecal microbiota. Vet. Med. Sci. 2021, 7, 1347–1358. [Google Scholar] [CrossRef]
- Mikulková, K.; Kadek, R.; Filípek, J.; Illek, J. Evaluation of oxidant/antioxidant status, metabolic profile and milk production in cows with metritis. Ir. Vet. J. 2020, 73, 8. [Google Scholar] [CrossRef]
- Li, Z. Study on the Changes of Serum Metabolites and Gut Microbiome Characteristics in Perinatal Mares Radin. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2022. (In Chinese). [Google Scholar] [CrossRef]
- Radin, L.; Šimpraga, M.; Vince, S.; Kostelić, A.; Milinković-Tur, S. Metabolic and oxidative status of Saanen goats of different parity during the peripartum period. J. Dairy Res. 2015, 82, 426–433. [Google Scholar] [CrossRef]
- He, Z.; Wang, W.; Chen, C.; Luo, J.; Li, C. Study on the changes of blood biochemistry, antioxidant status, milk composition, and milk yield in peripartum dairy goats. Chin. J. Anim. Husb. 2022, 58, 164–169. [Google Scholar] [CrossRef]
- Khan, M.Z.; Huang, B.; Kou, X.; Chen, Y.; Liang, H.; Ullah, Q.; Khan, I.M.; Khan, A.; Chai, W.; Wang, C. Enhancing bovine immune, antioxidant and anti-inflammatory responses with vitamins, rumen-protected amino acids, and trace minerals to prevent periparturient mastitis. Front. Immunol. 2024, 14, 1290044. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.O.; Bäckhed, F. Signals from the gut microbiota to distant organs in physiology and disease. Nat. Med. 2016, 22, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.W.; Kim, M.J.; Kim, J.; Lee, H.G.; Ryoo, S.B.; Ku, J.L.; Jeong, S.Y.; Park, K.J.; Kim, D.; Kim, J.F.; et al. Enterotypical prevotella and three novel bacterial biomarkers in preoperative stool predict the clinical outcome of colorectal cancer. Microbiome 2022, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Litvak, Y.; Byndloss, M.X.; Tsolis, R.M.; Bäumler, A.J. Dysbiotic Proteobacteria expansion: A microbial signature of epithelial dysfunction. Curr. Opin. Microbiol. 2017, 39, 1–6. [Google Scholar] [CrossRef]
- Wang, R.; Zhao, F.; Li, Y.; Zhu, J.; Liu, Y.; Li, J.; Yao, G.; Liu, H.; Guan, S.; Ma, S. The effects of chronic unpredicted mild stress on maternal negative emotions and gut microbiota and metabolites in pregnant rats. PeerJ 2023, 11, e15113. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Liu, G.; Zhang, Z.; Zhan, Y.; Zhu, M.; Wang, C. Metabolic Alternations During Gestation in Dezhou Donkeys and the Link to the Gut Microbiota. Front. Microbiol. 2022, 13, 801976. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, Y.; Guo, X.; Shi, B.; Ma, G.; Yan, S.; Zhao, Y. Effects of Artemisia ordosica Crude Polysaccharide on Antioxidant and Immunity Response, Nutrient Digestibility, Rumen Fermentation, and Microbiota in Cashmere Goats. Animals 2023, 13, 3575. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Alaniz, R.C.; Wood, T.K.; Jayaraman, A. The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Ortega, D.; Ugalde Muñiz, P.E.; Blanco Ayala, T.; Vázquez Cervantes, G.I.; Lugo Huitrón, R.; Pineda, B.; González Esquivel, D.F.; Pérez de la Cruz, G.; Pedraza Chaverrí, J.; Sánchez Chapul, L.; et al. On the Antioxidant Properties of L-Kynurenine: An Efficient ROS Scavenger and Enhancer of Rat Brain Antioxidant Defense. Antioxidants 2021, 11, 31. [Google Scholar] [CrossRef]
- Zhang, H.; Peng, A.; Yu, Y.; Guo, S.; Wang, M.; Wang, H. L-Arginine Protects Ovine Intestinal Epithelial Cells from Lipopolysaccharide-Induced Apoptosis through Alleviating Oxidative Stress. J. Agric. Food Chem. 2019, 67, 1683–1690. [Google Scholar] [CrossRef]
- Hirakawa, T.; Tanno, S.; Ohara, K. N-acetylglutamic acid alleviates oxidative stress based on histone acetylation in plants. Front. Plant Sci. 2023, 14, 1165646. [Google Scholar] [CrossRef]
- Aguayo, E.; Martínez-Sánchez, A.; Fernández-Lobato, B.; Alacid, F. L-Citrulline: A Non-Essential Amino Acid with Important Roles in Human Health. Appl. Sci. 2021, 11, 3293. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, F.; Liu, Y.; Liu, S.; An, Y.; Xue, H.; Wang, J.; Xia, F.; Chen, X.; Cao, Y. Microbiome-metabolome responses of Fuzhuan brick tea crude polysaccharides with immune-protective benefit in cyclophosphamide-induced immunosuppressive mice. Food Res. Int. 2022, 157, 111370. [Google Scholar] [CrossRef]
- Li, J.; Pang, B.; Yan, X.; Shang, X.; Hu, X.; Shi, J. Prebiotic properties of different polysaccharide fractions from Artemisia sphaerocephala Krasch seeds evaluated by simulated digestion and in vitro fermentation by human fecal microbiota. Int. J. Biol. Macromol. 2020, 162, 414–424. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Y.; Lin, C.; Peng, W. Editorial: Herbal medicines and their metabolites: Effects on lipid metabolic disorders via modulating oxidative stress. Front. Pharmacol. 2023, 14, 1279429. [Google Scholar] [CrossRef]
- Tall, A.R.; Yvan-Charvet, L.; Terasaka, N.; Pagler, T.; Wang, N. HDL, ABC transporters, and cholesterol efflux: Implications for the treatment of atherosclerosis. Cell Metab. 2008, 7, 365–375. [Google Scholar] [CrossRef]
- Arnone, D.; Vallier, M.; Hergalant, S.; Chabot, C.; Ndiaye, N.C.; Moulin, D.; Aignatoaei, A.M.; Alberto, J.M.; Louis, H.; Boulard, O.; et al. Long-Term Overconsumption of Fat and Sugar Causes a Partially Reversible Pre-inflammatory Bowel Disease State. Front. Nutr. 2021, 8, 758518. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, B.; Wang, Y.; Zhan, Y.; Zhu, M.; Wang, C. Dynamic alterations in the donkey fecal bacteria community and metabolome characteristics during gestation. Front. Microbiol. 2022, 13, 927561. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, M.; Gilkeson, G. Estrogen receptors in immunity and autoimmunity. Clin. Rev. Allergy Immunol. 2011, 40, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, D.; Marques, C.; Pestana, D.; Faria, A.; Norberto, S.; Calhau, C.; Monteiro, R. Effects of xenoestrogens in human M1 and M2 macrophage migration, cytokine release, and estrogen-related signaling pathways. Environ. Toxicol. 2016, 31, 1496–1509. [Google Scholar] [CrossRef] [PubMed]
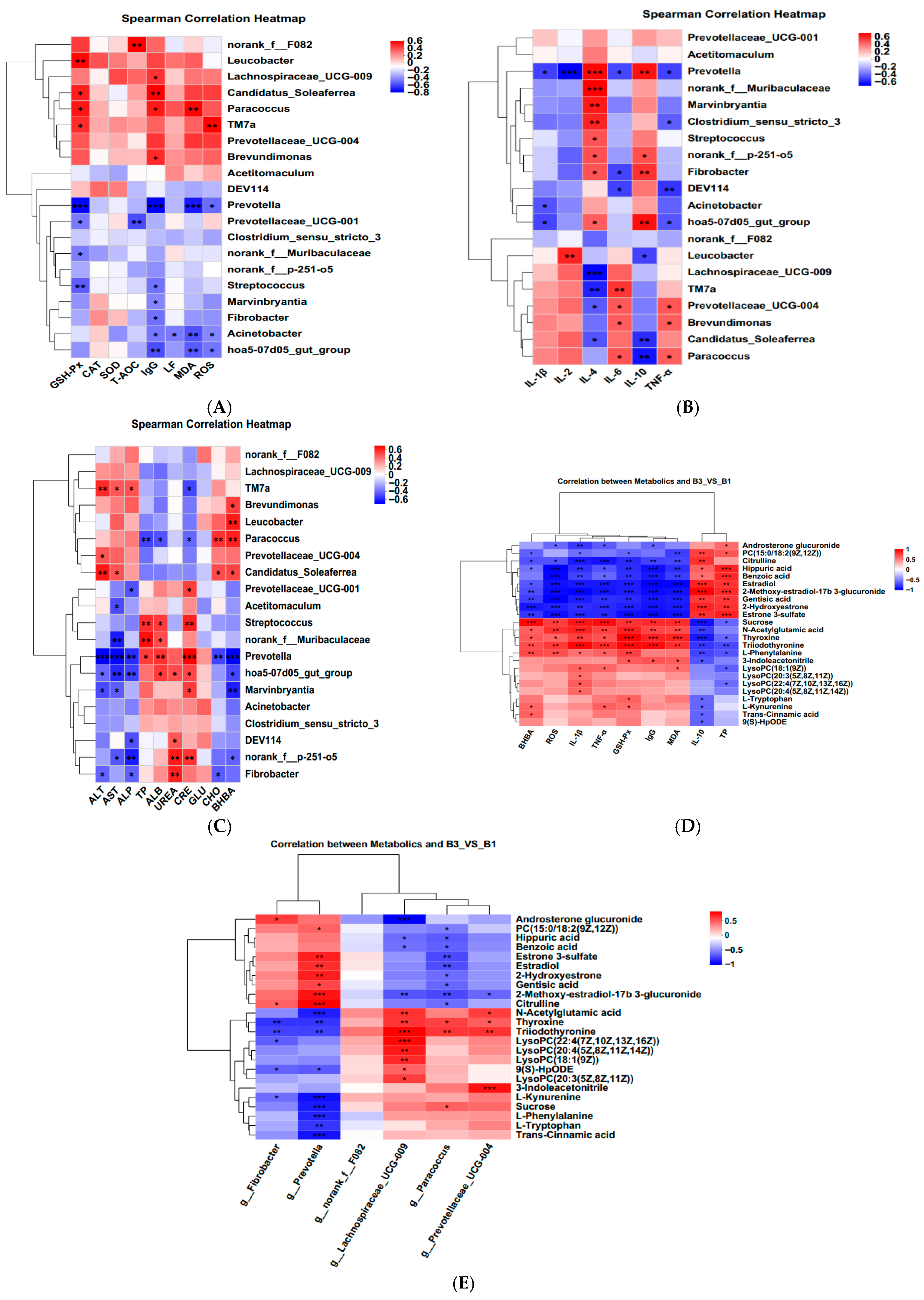
 ” represents a positive increase, “
” represents a positive increase, “ ” represents a negative decrease.
” represents a negative decrease.
 ” represents a positive increase, “
” represents a positive increase, “ ” represents a negative decrease.
” represents a negative decrease.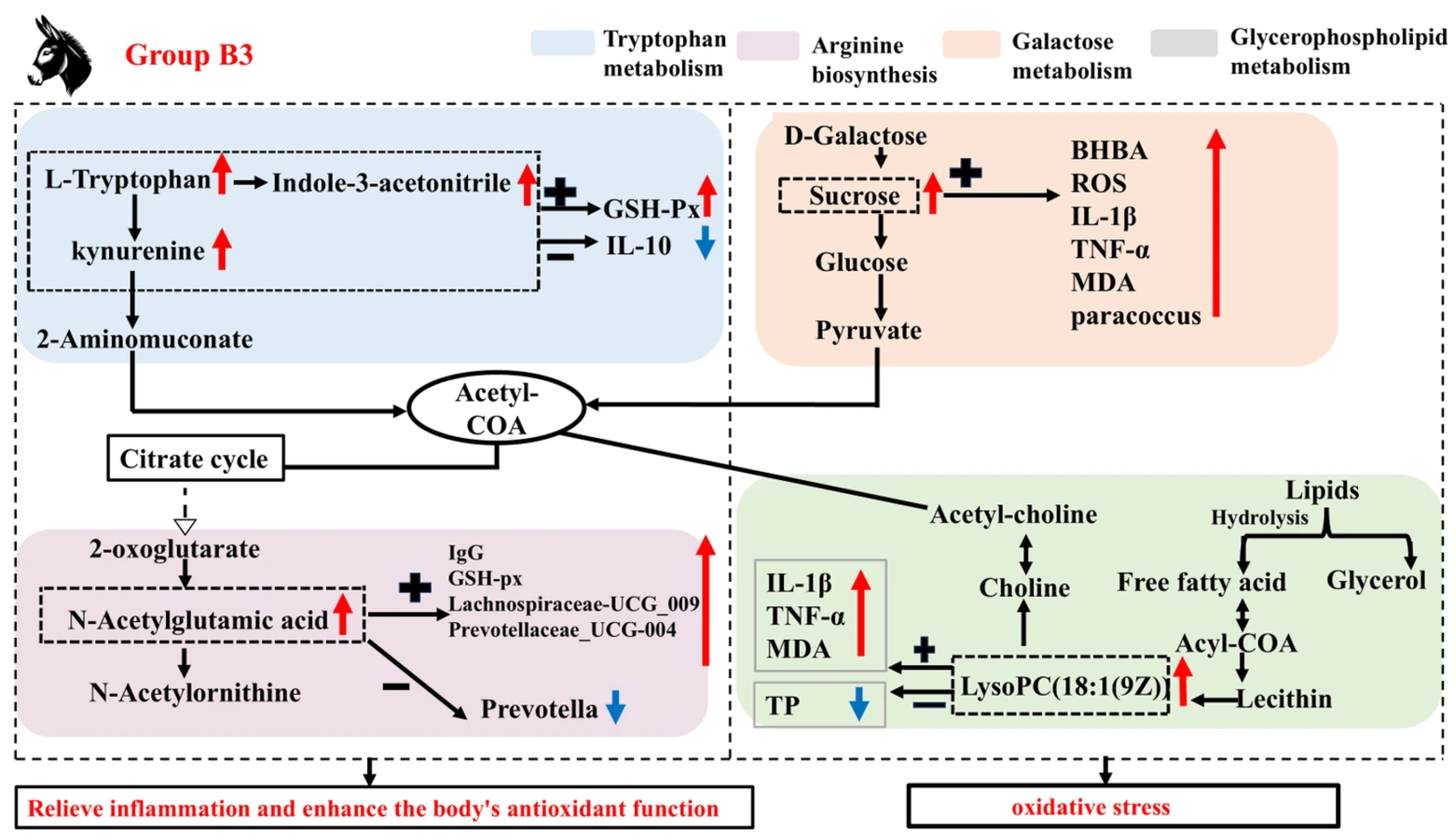
| Item | Content |
|---|---|
| Ingredients | |
| Millet straw | 44.63 |
| Alfalfa hay | 20.64 |
| Corn | 17.95 |
| Soybean meal | 8.27 |
| Corn gluten meal | 2.14 |
| Corn germ meal | 1.78 |
| Wheat bran | 1.71 |
| Distiller’s fried grains with solubles | 0.53 |
| Extruded full-fat soybean | 0.00 |
| Premix 1 | 0.63 |
| NaCl | 0.46 |
| CaCO3 | 0.41 |
| CaHPO4 | 0.83 |
| Total | 100.00 |
| Nutrient level, % | |
| Digestible energy, MJ/Kg 2 | 12.40 |
| Crude protein | 14.01 |
| Ether extract | 2.82 |
| Neutral detergent fiber | 52.48 |
| Acid detergent fiber | 31.15 |
| Calcium | 0.98 |
| Phosphorous | 0.31 |
| Item | B1 1 | B2 1 | B3 1 | SEM 2 | p-Value |
|---|---|---|---|---|---|
| ALT (U/L) | 5.72 a | 8.72 ab | 13.64 a | 2.262 | 0.062 |
| AST (U/L) | 240.49 b | 268.42 b | 346.00 a | 14.164 | <0.001 |
| ALP (U/L) | 83.67 b | 80.50 b | 116.77 a | 4.497 | <0.001 |
| TP (g/L) | 70.36 a | 67.96 ab | 62.68 b | 1.885 | 0.025 |
| ALB (g/L) | 35.02 a | 33.54 ab | 31.07 b | 0.977 | 0.028 |
| UREA (mmol/L) | 8.98 a | 8.31 ab | 8.16 b | 0.263 | 0.085 |
| CRE (μmol/L) | 63.44 a | 62.94 a | 22.49 b | 2.064 | <0.001 |
| GLU (mmol/L) | 4.48 a | 3.26 b | 3.76 b | 0.191 | 0.001 |
| CHO (mmol/L) | 1.72 b | 1.79 ab | 1.91 a | 0.044 | 0.018 |
| BHBA (mmol/L) | 0.20 b | 0.21 b | 0.27 a | 0.015 | 0.015 |
| Metabolic Pathways | KEGG ID | Hits 2 | p_Value 3 | Impact_Value | Upregulated | Downregulated |
|---|---|---|---|---|---|---|
| Tryptophan metabolism | map00380 | 6 | 0.003 | 0.267 | L-Tryptophan | |
| 3-(2-(methylamino) ethyl)-1H-indol-5-ol | ||||||
| L-Kynurenine | ||||||
| 3-Indoleacetic Acid | ||||||
| 3-Indoleacetonitrile | ||||||
| Kynurenine | ||||||
| Phenylalanine metabolism | map00360 | 5 | 0.001 | 0.152 | Trans-Cinnamic acid | Phenylacetylglycine |
| L-Phenylalanine | Hippuric acid | |||||
| Benzoic acid | ||||||
| Galactose metabolism | map00052 | 2 | 0.092 | 0.151 | Sucrose | Dulcitol |
| Arginine biosynthesis | map00220 | 2 | 0.056 | 0.140 | N-Acetylglutamic acid | Citrulline |
| Steroid hormone biosynthesis | map00140 | 12 | 0.000 | 0.122 | 18-Hydroxycorticosterone | Dehydroepiandrosterone |
| Cortisol | 5alpha-Pregnan-20alpha-ol-3-one | |||||
| Estrone sulfate | ||||||
| Androsterone glucuronide | ||||||
| Testosterone glucuronide | ||||||
| 2-Hydroxyestrone | ||||||
| Estrone 3-sulfate | ||||||
| Estradiol | ||||||
| Estrone 3-glucuronide | ||||||
| 2-Methoxy-estradiol-17b 3-glucuronide | ||||||
| Glycerophospholipid metabolism | map00564 | 10 | 0.044 | 0.102 | LysoPC (22:4(7Z, 10Z,13Z,16Z)) | PS (18:0/20:4(8Z,11Z,14Z,17Z)) |
| LysoPC (20:3(5Z,8Z,11Z)) | LysoPC (15:0) | |||||
| LysoPC (20:4(5Z,8Z,11Z,14Z)) | PC (15:0/18:2(9Z,12Z)) | |||||
| LysoPC (18:1(9Z)) | LPC (18:3) | |||||
| LysoPC (20:3(8Z, 11Z,14Z)) | ||||||
| LysoPC (16:1(9Z)/0:0) | ||||||
| Tyrosine metabolism | map00350 | 6 | 0.001 | 0.007 | Thyroxine | Dopaquinone |
| Phenol | Gentisic acid | |||||
| Metanephrine | ||||||
| Triiodothyronine | ||||||
| Linoleic acid metabolism | map00591 | 3 | 0.001 | 0.000 | 9(S)-HpODE | PC (15:0/18:2(9Z,12Z)) |
| 13(S)-HpODE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, F.; Zhao, Y.; Akonyani, Z.P.; Guo, Y.; Guo, X.; Zhang, Q.; Meng, F.; Li, L.; Shi, B.; Yan, S. Rectal Microbiomes and Serum Metabolomics Reveal Changes in Serum Antioxidant Status and Immune Responses of Dezhou Donkeys in Late Gestation to Parturition. Antioxidants 2025, 14, 1253. https://doi.org/10.3390/antiox14101253
Hui F, Zhao Y, Akonyani ZP, Guo Y, Guo X, Zhang Q, Meng F, Li L, Shi B, Yan S. Rectal Microbiomes and Serum Metabolomics Reveal Changes in Serum Antioxidant Status and Immune Responses of Dezhou Donkeys in Late Gestation to Parturition. Antioxidants. 2025; 14(10):1253. https://doi.org/10.3390/antiox14101253
Chicago/Turabian StyleHui, Fang, Yanli Zhao, Zaccheaus Pazamilala Akonyani, Yongmei Guo, Xiaoyu Guo, Qingyue Zhang, Fanzhu Meng, Li Li, Binlin Shi, and Sumei Yan. 2025. "Rectal Microbiomes and Serum Metabolomics Reveal Changes in Serum Antioxidant Status and Immune Responses of Dezhou Donkeys in Late Gestation to Parturition" Antioxidants 14, no. 10: 1253. https://doi.org/10.3390/antiox14101253
APA StyleHui, F., Zhao, Y., Akonyani, Z. P., Guo, Y., Guo, X., Zhang, Q., Meng, F., Li, L., Shi, B., & Yan, S. (2025). Rectal Microbiomes and Serum Metabolomics Reveal Changes in Serum Antioxidant Status and Immune Responses of Dezhou Donkeys in Late Gestation to Parturition. Antioxidants, 14(10), 1253. https://doi.org/10.3390/antiox14101253







