Rose Oil Distillation Wastewater: By-Products of Essential Oil Extraction as Circular Biostimulants for Tomato Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Rose Distillation Wastewater (RDW)
2.2. Physico-Chemical Characterization of RDW
2.3. In Vitro Antioxidant Activity of RDW
2.4. In Vitro Tomato Germination Tests
2.5. Statistical Analysis
3. Results
3.1. Screening of Physico-Chemical and Antioxidant Properties of Rose Distillation Wastewater
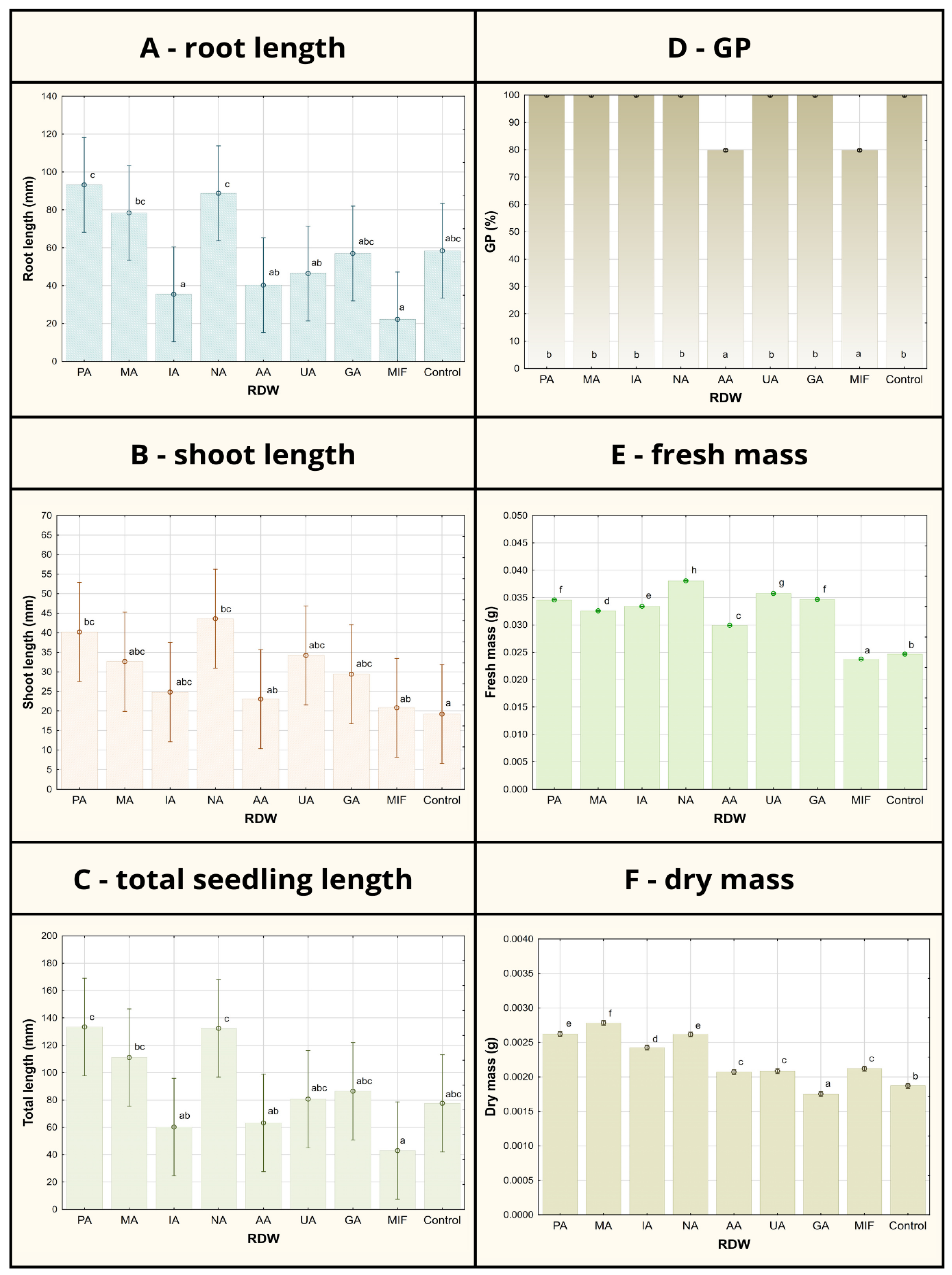
3.2. Tomato Growth Parameters After Seed Treatment Using Rose Distillation Wastewater Samples
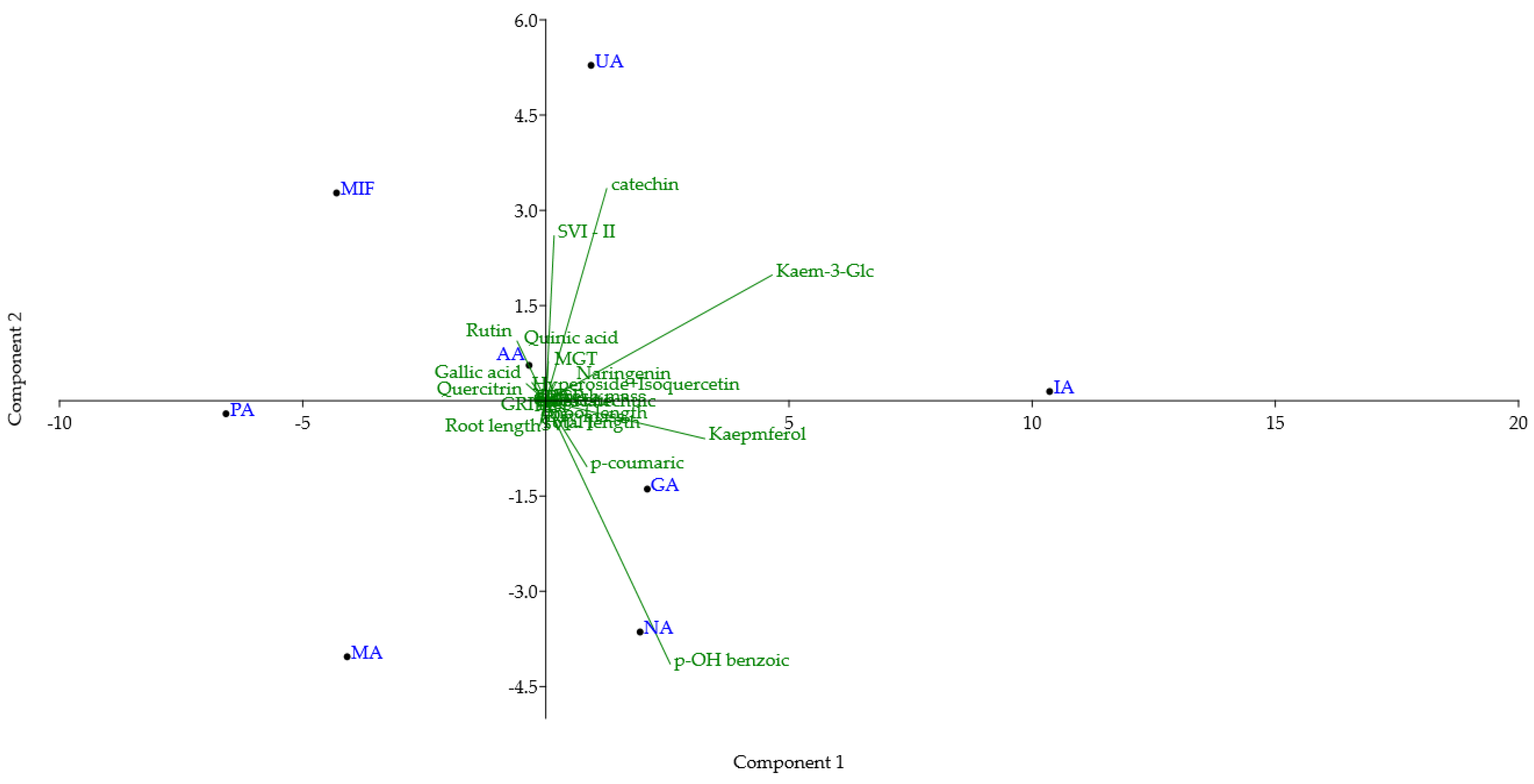
3.3. Principal Component Analysis
4. Discussion
4.1. Chemical Profiling and Antioxidant Potential of RDW Obtained from Various Rosa Cultivars
4.2. Plant Growth Modulating Capability of RDW
4.3. A Roadmap for Further Research and Validation Steps: The Proposed Mechanisms of Plant Growth Promotion in Relation to RDW Chemical and Antioxidant Composition
4.4. Commercial and Application Outlook on RDW as a Possible Biostimulant/Seed Treatment Agent
5. Conclusions and Future Research Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CE | cyanidin 3-O-glucoside |
| de | dry extract |
| GAE | gallic acid equivalents |
| AA | ‘Adore Aroma’ |
| GA | ‘Gentle Aroma’ |
| IA | ‘Intense Aroma’ |
| MA | ‘Magic Aroma’ |
| MIF | ‘Mina Frayla’ |
| NA | ‘Natural Aroma’ |
| PA | ‘Pure Aroma’ |
| UA | ‘Unique Aroma’ |
| TPC | total phenolic content |
| TFC | total flavonoid content |
| TAC | total monomeric anthocyanin content |
| QE | quercetin equivalents |
| RDW | rose distillation wastewater |
| PGP | plant growth promotion |
| GP | germination percent |
| MGT | mean germination time |
| GRI | germination rate index |
| SVI-I | seedling vigor index I |
| SVI-II | seedling vigor index II |
| RL | root length |
| SL | shoot length |
| TL | total seedling length |
| FM | fresh mass |
| DM | dry mass |
| PCA | principal component analysis |
References
- Verified Market Research®. Global Rose Oils Market Size by Type (Rose Otto, Rose Absolute), By Product (Organic, Conventional), by Application (Fragrance and Cosmetics, Pharmaceuticals, Food and Beverages), by Geographic Scope and Forecast. 2023. Available online: https://www.verifiedmarketresearch.com/product/rose-oils-market/ (accessed on 15 September 2025).
- Market Research Future (MRFR). Rose Oil Market Research Report. 2025. Available online: https://www.marketresearchfuture.com/reports/rose-oil-market-7243 (accessed on 15 September 2025).
- Kovacheva, N.; Rusanov, K.; Atanassov, I. Industrial Cultivation of Oil Bearing Rose and Rose Oil Production in Bulgaria During 21st Century, Directions and Challenges. Biotechnol. Biotechnol. Equip. 2010, 24, 1793–1798. [Google Scholar] [CrossRef]
- Gateva, S.; Jovtchev, G.; Angelova, T.; Dobreva, A.; Mileva, M. The Anti-Genotoxic Activity of Wastewaters Produced after Water-Steam Distillation of Bulgarian Rosa damascena Mill. and Rosa alba L. Essential Oils. Life 2022, 12, 455. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, Y.; Dimitrova, L.; Georgieva, A.; Vilhelmova-Ilieva, N.; Zaharieva, M.M.; Kokanova-Nedialkova, Z.; Dobreva, A.; Nedialkov, P.; Kussovski, V.; Kroumov, A.D.; et al. In Vitro Study of the Biological Potential of Wastewater Obtained after the Distillation of Four Bulgarian Oil-Bearing Roses. Plants 2022, 11, 1073. [Google Scholar] [CrossRef] [PubMed]
- Rusanov, K.; Garo, E.; Rusanova, M.; Fertig, O.; Hamburger, M.; Atanassov, I.; Butterweck, V. Recovery of Polyphenols from Rose Oil Distillation Wastewater Using Adsorption Resins-A Pilot Study. Planta Med. 2014, 80, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Solimine, J.; Garo, E.; Wedler, J.; Rusanov, K.; Fertig, O.; Hamburger, M.; Atanassov, I.; Butterweck, V. Tyrosinase Inhibitory Constituents from a Polyphenol Enriched Fraction of Rose Oil Distillation Wastewater. Fitoterapia 2016, 108, 13–19. [Google Scholar] [CrossRef]
- Boso, S.; Gago, P.; Santiago, J.-L.; Álvarez-Acero, I.; Martinez Bartolomé, M.-A.; Martínez, M.-C. Polyphenols in the Waste Water Produced during the Hydrodistillation of ‘Narcea Roses’ Cultivated in the Cibea River Valley (Northern Spain). Horticulturae 2022, 8, 376. [Google Scholar] [CrossRef]
- Simin, N.; Lesjak, M.; Živanović, N.; Božanić Tanjga, B.; Orčić, D.; Ljubojević, M. Morphological Characters, Phytochemical Profile and Biological Activities of Novel Garden Roses Edible Cultivars. Horticulturae 2023, 9, 1082. [Google Scholar] [CrossRef]
- Avitabile, M.; Aleksov, A.; Giosafatto, C.V.L.; Restaino, O.F.; Lesjak, M.; Živanović, N.; Mariniello, L.; Simin, N. Pectin-Based Bioplastics Functionalized with Polyphenols from Rose Oil Distillation Wastewater Exhibit Antioxidant Activity. Biomacromolecules 2024, 25, 7695–7703. [Google Scholar] [CrossRef]
- Jovanović, I.; Frantová, N.; Alba-Mejía, J.E.; Porčová, L.; Psota, V.; Asszonyi, J.; Cerkal, R.; Středa, T. Role of Total Polyphenol Content in Seed Germination Characteristics of Spring Barley Varieties Amidst Climate Change. Sci. Rep. 2024, 14, 23818. [Google Scholar] [CrossRef]
- Qiongqiong, Z.; Shaobo, W.; Liyuan, X.; Zhihui, W.; Xinying, Z.; Fen, Z.; Liping, W.; Jihua, T.; Renliang, Z.; Long, W. Molecular Mechanisms of Biostimulants in Promoting Tomato Seedling Growth: Linking Chemical Structure to Physiologic Function. J. Agric. Food Chem. 2025, 73, 7632–7644. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, X.; Lu, Y.; Wang, H.; Chen, D.; Luo, C.; Liu, H.; Gao, S.; Lei, T.; Huang, C.; et al. Gas Chromatography-Mass Spectrometry Analysis of Floral Fragrance-Related Compounds in Scented Rose (Rosa hybrida) Varieties and a Subsequent Evaluation on the Basis of the Analytical Hierarchy Process. Plant Physiol. Biochem. 2022, 185, 368–377. [Google Scholar] [CrossRef]
- Orčić, D.; Francišković, M.; Bekvalac, K.; Svirčev, E.; Beara, I.; Lesjak, M.; Mimica-Dukić, N. Quantitative Determination of Plant Phenolics in Urtica dioica Extracts by High-Performance Liquid Chromatography Coupled with Tandem Mass Spectrometric Detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Vlajkov, V.; Pajčin, I.; Loc, M.; Budakov, D.; Dodić, J.; Grahovac, M.; Grahovac, J. The Effect of Cultivation Conditions on Antifungal and Maize Seed Germination Activity of Bacillus-Based Biocontrol Agent. Bioengineering 2022, 9, 797. [Google Scholar] [CrossRef] [PubMed]
- Faur, C.A.; Zăhan, M.; Bunea, C.I.; Hârșan, E.; Bora, F.D.; Bunea, A. Antiproliferative and Biochemical Evaluation of Rose Extracts: Impact on Tumor and Normal Skin Cells. Front. Plant Sci. 2024, 15, 1477243. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Liu, X.; Li, J.; Zhang, J.; Liu, D. Chemical Constituents and Pharmacological Activities of Medicinal Plants from Rosa Genus. Chin. Herb. Med. 2022, 14, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, H. Beneficial Medicinal Effects and Material Applications of Rose. Heliyon 2023, 10, e23530. [Google Scholar] [CrossRef]
- Wedler, J.; Rusanov, K.; Atanassov, I.; Butterweck, V. A Polyphenol-Enriched Fraction of Rose Oil Distillation Wastewater Inhibits Cell Proliferation, Migration and TNF-α-Induced VEGF Secretion in Human Immortalized Keratinocytes. Planta Med. 2016, 82, 1000–1008. [Google Scholar] [CrossRef]
- El Malahi, S.; Irahoui, L.; Mokhtari, W.; Ennami, M.; Taimourya, H.; Zim, J.; Zayani, A.; Zakri, B.; Dhassi, K.; Mokhtari, M.; et al. Exploring the Synergistic Effect of Rose Distillation Waste and Biostimulant Beside Other Organic Amendments on Rosa damascena Seedlings’ Growth. Int. J. Recycl. Org. Waste Agric. 2024, 13, 1–12. [Google Scholar] [CrossRef]
- Slavov, A.; Vasileva, I.; Stefanov, L.; Stoyanova, A. Valorization of Wastes from the Rose Oil Industry. Rev. Environ. Sci. Biotechnol. 2017, 16, 309–325. [Google Scholar] [CrossRef]
- Sabahi, Z.; Farmani, F.; Mousavinoor, E.; Moein, M. Valorization of Waste Water of Rosa damascena Oil Distillation Process by Ion Exchange Chromatography. Sci. World J. 2020, 2020, 5409493. [Google Scholar] [CrossRef]
- Karami, M.; Bagheri, M.; Abbasi-Baharanchi, A.; Haghbeen, K.; Nouri, A.; Karkhane, A.A.; Ghorbanpour, M.; Farhadpour, M. Exploring the Capacity of Microorganism Treatment for Fermentation and Glycosidic Aroma Bioconversion from Rose Oil Distillation Wastewater. Chem. Biol. Technol. Agric. 2024, 11, 194. [Google Scholar] [CrossRef]
- Simin, N.; Živanović, N.; Božanić Tanjga, B.; Lesjak, M.; Narandžić, T.; Ljubojević, M. New Garden Rose (Rosa × hybrida) Genotypes with Intensely Colored Flowers as Rich Sources of Bioactive Compounds. Plants 2024, 13, 424. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Loypimai, P.; Moongngarm, A.; Chottanom, P. Thermal and pH Degradation Kinetics of Anthocyanins in Natural Food Colorant Prepared from Black Rice Bran. J. Food Sci. Technol. 2016, 53, 461–470. [Google Scholar] [CrossRef]
- Chroho, M.; Bouymajane, A.; Oulad El Majdoub, Y.; Cacciola, F.; Mondello, L.; Aazza, M.; Zair, T.; Bouissane, L. Phenolic Composition, Antioxidant and Antibacterial Activities of Extract from Flowers of Rosa damascena from Morocco. Separations 2022, 9, 247. [Google Scholar] [CrossRef]
- Mikanagi, Y.; Yokoi, M.; Ueda, Y.; Saito, N. Flower Flavonol and Anthocyanin Distribution in Subgenus Rosa. Biochem. Syst. Ecol. 1995, 23, 183–200. [Google Scholar] [CrossRef]
- Schieber, A.; Mihalev, K.; Berardini, N.; Mollov, P.; Carle, R. Flavonol Glycosides from Distilled Petals of Rosa damascena Mill. Z. Naturforsch. C 2005, 60, 379–384. [Google Scholar] [CrossRef]
- Mallick, S.R.; Hassan, J.; Hoque, M.A.; Sultana, H.; Kayesh, E.; Ahmed, M.; Ozaki, Y.; Al-Hashimi, A.; Siddiqui, M.H. Color, Proximate Composition, Bioactive Compounds and Antinutrient Profiling of Rose. Sci. Rep. 2024, 14, 21690. [Google Scholar] [CrossRef]
- Sohrabi, O.; Hatamzadeh, A.; Ghasemnezhad, A.; Samizadeh, H.; Erfani-Moghadam, V. Exploring the Effects of Medicinal Plant Extracts on Tomato (Solanum lycopersicum L.) Morphology, Biochemistry, and Plant Growth Regulators under Greenhouse Conditions. Int. J. Hortic. Sci. Technol. 2024, 11, 285–298. [Google Scholar]
- Kalisz, A.; Włodarczyk, Z.; Bieniasz, M.; Smoleń, S.; Neugebauerová, J.; Szewczyk-Taranek, B.; Pawłowska, B. Petals of Different Ornamental Rose Cultivars as a Rich Source of Bioactive Compounds for Functional Foods. Sci. Hortic. 2023, 321, 112240. [Google Scholar] [CrossRef]
- Alam, S.S.; Akhi, A.H.; Alam, F.; Hasanuzzaman, M.; Rohman, M. Enhancement of Plant Productivity and Stress Tolerance by the Application of an Exogenous Supply of Vitamins. In Biostimulants for Crop Production and Sustainable Agriculture; Hasanuzzaman, M., Hawrylak-Nowak, B., Islam, T., Fujita, M., Eds.; CAB International: Wallingford, UK, 2022; pp. 348–371. [Google Scholar]
- Dos Santos, A.M.P.; Silva, E.F.R.; dos Santos, W.N.L.; da Silva, E.G.P.; dos Santos, L.O.; da S. Santos, B.R.; da S. Sauthier, M.C.; dos Santos, W.P.C. Evaluation of Minerals, Toxic Elements and Bioactive Compounds in Rose Petals (Rosa spp.) Using Chemometric Tools and Artificial Neural Networks. Microchem. J. 2018, 138, 98–108. [Google Scholar] [CrossRef]
- Liu, X.; Wu, J.; Ji, F.; Cao, X.; Zhao, Q.; Cheng, C.; Ma, N.; Zhou, X.; Zhang, Z. Transcriptomic Profiling of Rose Flower under Treatment of Various Phytohormones and Plant Growth Regulators. Sci. Data 2022, 9, 669. [Google Scholar] [CrossRef]
- Han, M.; Kasim, S.; Yang, Z.; Deng, X.; Saidi, N.B.; Uddin, M.K.; Shuib, E.M. Plant Extracts as Biostimulant Agents: A Promising Strategy for Managing Environmental Stress in Sustainable Agriculture. Phyton-Int. J. Exp. Bot. 2024, 93, 2149–2166. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Plant Oxidative Stress: Biology, Physiology and Mitigation. Plants 2022, 11, 1185. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and Antagonism in Natural Product Extracts: When 1 + 1 Does Not Equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Chaachouay, N. Synergy, Additive Effects, and Antagonism of Drugs with Plant Bioactive Compounds. Drugs Drug Candidates 2025, 4, 4. [Google Scholar] [CrossRef]
- Godlewska, K.; Ronga, D.; Michalak, I. Plant Extracts—Importance in Sustainable Agriculture. Ital. J. Agron. 2021, 16, 1851. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, B.; Fan, J.; Yuan, Q.; He, F.; Liang, H.; Chen, F.; Liu, W. Gallic Acid Regulates Primary Root Elongation via Modulating Auxin Transport and Signal Transduction. Front. Plant Sci. 2024, 15, 1464053. [Google Scholar] [CrossRef] [PubMed]
- Negi, A.S.; Darokar, M.P.; Chattopadhyay, S.K.; Garg, A.; Bhattacharya, A.K.; Srivastava, V.; Khanuja, S.P. Synthesis of a Novel Plant Growth Promoter from Gallic Acid. Bioorg. Med. Chem. Lett. 2005, 15, 1243–1247. [Google Scholar] [CrossRef]
- Rahman, A.; Alam, M.U.; Hossain, M.S.; Mahmud, J.A.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Exogenous Gallic Acid Confers Salt Tolerance in Rice Seedlings: Modulation of Ion Homeostasis, Osmoregulation, Antioxidant Defense, and Methylglyoxal Detoxification Systems. Agronomy 2023, 13, 16. [Google Scholar] [CrossRef]
- Sami Ullah, S.; Shah, W.; Hafeez, A.; Ali, B.; Khan, S.; Ercisli, S.; Al-Ghamdi, A.A.; Elshikh, M.S. Biochar and Seed Priming Technique with Gallic Acid: An Approach toward Improving Morpho-Anatomical and Physiological Features of Solanum melongena L. under Induced NaCl and Boron Stresses. ACS Omega 2023, 8, 28207–28232. [Google Scholar] [CrossRef]
- Shao, Q.; Ren, L.; Ramzan, M.; Hussain, M.B.; Datta, R.; Almoallim, H.S.; Ansari, M.J.; Ehsan, A. Combined Effect of Gallic Acid and Zinc Ferrite Nanoparticles on Wheat Growth and Yield Under Salinity Stress. Sci. Rep. 2024, 14, 12854. [Google Scholar] [CrossRef]
- Zhang, X.; Ran, W.; Li, X.; Zhang, J.; Ye, M.; Lin, S.; Liu, M.; Sun, X. Exogenous Application of Gallic Acid Induces the Direct Defense of Tea Plant Against Ectropis obliqua Caterpillars. Front. Plant Sci. 2022, 13, 833489. [Google Scholar] [CrossRef] [PubMed]
- El-Nagar, A.; Elzaawely, A.A.; Taha, N.A.; Nehela, Y. The Antifungal Activity of Gallic Acid and Its Derivatives against Alternaria solani, the Causal Agent of Tomato Early Blight. Agronomy 2020, 10, 1402. [Google Scholar] [CrossRef]
- Tariq, H.; Asif, S.; Andleeb, A.; Hano, C.; Abbasi, B.H. Flavonoid Production: Current Trends in Plant Metabolic Engineering and De Novo Microbial Production. Metabolites 2023, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Ali, S.; Bao, G.; Bashir, K.; Hu, J.; Fan, C.; Hongwei, Z.; Li, G. Enhancing Tolerance of Rye (Secale cereale L.) Seedlings to Cadmium Chloride Stress and Freeze–Thaw Cycles through Protocatechuic Acid Application. Water Air Soil Pollut. 2025, 236, 424. [Google Scholar] [CrossRef]
- Xuan, T.D.; Khang, D.T. Effects of Exogenous Application of Protocatechuic Acid and Vanillic Acid to Chlorophylls, Phenolics and Antioxidant Enzymes of Rice (Oryza sativa L.) in Submergence. Molecules 2018, 23, 620. [Google Scholar] [CrossRef]
- Chen, W.; Xiao, Z.; Wang, Y.; Wang, J.; Zhai, R.; Lin-Wang, K.; Espley, R.; Ma, F.; Li, P. Competition between Anthocyanin and Kaempferol Glycosides Biosynthesis Affects Pollen Tube Growth and Seed Set of Malus. Hortic. Res. 2021, 8, 173. [Google Scholar] [CrossRef]
- Peer, W.A.; Bandyopadhyay, A.; Blakeslee, J.J.; Makam, S.N.; Chen, R.J.; Masson, P.H.; Murphy, A.S. Variation in Expression and Protein Localization of the PIN Family of Auxin Efflux Facilitator Proteins in Flavonoid Mutants with Altered Auxin Transport in Arabidopsis thaliana. Plant Cell 2004, 16, 1898–1911. [Google Scholar] [CrossRef]
- Soubeyrand, E.; Johnson, T.S.; Latimer, S.; Block, A.; Kim, J.; Colquhoun, T.A.; Butelli, E.; Martin, C.; Wilson, M.A.; Basset, G.J. The Peroxidative Cleavage of Kaempferol Contributes to the Biosynthesis of the Benzenoid Moiety of Ubiquinone in Plants. Plant Cell 2018, 30, 2910–2921. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Latimer, S.; Stutts, L.R.; Soubeyrand, E.; Block, A.K.; Basset, G.J. Kaempferol as a Precursor for Ubiquinone (Coenzyme Q) Biosynthesis: An Atypical Node between Specialized Metabolism and Primary Metabolism. Curr. Opin. Plant Biol. 2022, 66, 102165. [Google Scholar] [CrossRef] [PubMed]
- Ramzan, M.; Haider, S.T.A.; Hussain, M.B.; Ehsan, A.; Datta, R.; Alahmadi, T.A.; Ansari, M.J.; Alharbi, S.A. Potential of Kaempferol and Caffeic Acid to Mitigate Salinity Stress and Improving Potato Growth. Sci. Rep. 2024, 14, 21657. [Google Scholar] [CrossRef] [PubMed]
- Kotik, M.; Kulik, N.; Valentová, K. Flavonoids as Aglycones in Retaining Glycosidase-Catalyzed Reactions: Prospects for Green Chemistry. J. Agric. Food Chem. 2023, 71, 14890–14910. [Google Scholar] [CrossRef]
- Behr, M.; Neutelings, G.; El Jaziri, M.; Baucher, M. You Want it Sweeter: How Glycosylation Affects Plant Response to Oxidative Stress. Front. Plant Sci. 2020, 11, 571399. [Google Scholar] [CrossRef]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet Flavonoids”: Glycosidase-Catalyzed Modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Migut, D.; Piechowiak, T.; Buczek, J.; Balawejder, M. The Effect of Exogenous Application of Quercetin Derivative Solutions on the Course of Physiological and Biochemical Processes in Wheat Seedlings. Int. J. Mol. Sci. 2021, 22, 6882. [Google Scholar] [CrossRef]
- Jańczak-Pieniążek, M.; Migut, D.; Piechowiak, T.; Balawejder, M. Assessment of the Impact of the Application of a Quercetin-Copper Complex on the Course of Physiological and Biochemical Processes in Wheat Plants (Triticum aestivum L.) Growing Under Saline Conditions. Cells 2022, 11, 1141. [Google Scholar] [CrossRef]
- Parvin, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mohsin, S.M.; Fujita, M. Quercetin Mediated Salt Tolerance in Tomato through the Enhancement of Plant Antioxidant Defense and Glyoxalase Systems. Plants 2019, 8, 247. [Google Scholar] [CrossRef]
- Migut, D.; Sobaszek, M.; Jańczak-Pieniążek, M.; Skrobacz, K. Effect of the Aqueous Quercetin Solution on the Physiological Properties of Virginia Mallow (Ripariosida hermaphrodita) Grown Under Salt Stress Conditions. Int. J. Mol. Sci. 2025, 26, 1233. [Google Scholar] [CrossRef]
- Aslam, M.A.; Ahmed, S.; Saleem, M.; Shah, A.A.; Shah, A.N.; Tanveer, M.; Ali, H.M.; Ghareeb, R.Y.; Hasan, M.E.; Khan, J. Quercetin Ameliorates Chromium Toxicity Through Improvement in Photosynthetic Activity, Antioxidative Defense System; and Suppressed Oxidative Stress in Trigonella corniculata L. Front. Plant Sci. 2022, 13, 956249. [Google Scholar] [CrossRef]
- Gaonkar, S.S.; Sincinelli, F.; Balestrazzi, A.; Pagano, A. Quercetin and Rutin as Tools to Enhance Antioxidant Profiles and Post-Priming Seed Storability in Medicago truncatula. Agriculture 2024, 14, 738. [Google Scholar] [CrossRef]
- Ylstra, B.; Touraev, A.; Moreno, R.M.; Stöger, E.; van Tunen, A.J.; Vicente, O.; Mol, J.N.; Heberle-Bors, E. Flavonols Stimulate Development, Germination, and Tube Growth of Tobacco Pollen. Plant Physiol. 1992, 100, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, C.; Fini, A.; Sebastiani, F.; Gori, A.; Tattini, M. Modulation of Phytohormone Signaling: A Primary Function of Flavonoids in Plant–Environment Interactions. Front. Plant Sci. 2018, 9, 1042. [Google Scholar] [CrossRef]
- An, J.; Kim, S.H.; Bahk, S.; Pham, M.L.A.; Park, J.; Ramadany, Z.; Lee, J.; Hong, J.C.; Chung, W.S. Quercetin Induces Pathogen Resistance Through the Increase of Salicylic Acid Biosynthesis in Arabidopsis. Plant Signal. Behav. 2023, 18, 2270835. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Lee, H.; Ko, H.J.; Woo, E.R.; Lee, D.G. Fungicidal Effect of Isoquercitrin via Inducing Membrane Disturbance. Biochim. Biophys. Acta 2015, 1848, 695–701. [Google Scholar] [CrossRef]
- Yang, Q.; Song, Z.; Dong, B.; Niu, L.; Cao, H.; Li, H.; Du, T.; Liu, T.; Yang, W.; Meng, D.; et al. Hyperoside Regulates Its Own Biosynthesis via MYB30 in Promoting Reproductive Development and Seed Set in Okra. Plant Physiol. 2021, 185, 951–968. [Google Scholar] [CrossRef]
- Dong, B.; Yang, Q.; Song, Z.; Niu, L.; Cao, H.; Liu, T.; Du, T.; Yang, W.; Qi, M.; Chen, T.; et al. Hyperoside Promotes Pollen Tube Growth by Regulating the Depolymerization Effect of Actin-Depolymerizing Factor 1 on Microfilaments in Okra. Hortic. Res. 2021, 8, 145. [Google Scholar] [CrossRef]
- Su, L.; Zhang, M.; Zhang, Y.; Chen, Y.; Yang, L.; Wang, Y.; Song, Y.; Gong, L. Transcriptome Analysis Reveals the Crucial Function of Hyperoside in Inhibiting Anthocyanin Accumulation in Grape (Vitis vinifera L.) Fruits by Inducing VvMYB62. Front. Plant Sci. 2023, 14, 1119749. [Google Scholar] [CrossRef]
- Altansambar, N.; Sezgin Muslu, A.; Kadıoglu, A. The Combined Application of Rutin and Silicon Alleviates Osmotic Stress in Maize Seedlings by Triggering Accumulation of Osmolytes and Antioxidants’ Defense Mechanisms. Physiol. Mol. Biol. Plants 2024, 30, 513–525. [Google Scholar] [CrossRef]
- Dos Santos, C.A.L.; de Araújo Monteiro, A.A.; da Silva, P.A.G.; Kamdem, J.P.; Duarte, A.E.; Almutairi, M.M.; Ali, A.; Anwar, S.; Ibrahim, M. Protective Capacity of Rutin Against Oxidative Damage Induced by Saline Stress in the Roots of the Model Organism Allium cepa. Sci. Rep. 2025, 15, 24447. [Google Scholar] [CrossRef]
- Ismail, H.; Dragišic Maksimovic, J.; Maksimovic, V.; Shabala, L.; Živanovic, B.D.; Tian, Y.; Jacobsen, S.E.; Shabala, S. Rutin, a Flavonoid with Antioxidant Activity, Improves Plant Salinity Tolerance by Regulating K+ Retention and Na+ Exclusion from Leaf Mesophyll in Quinoa and Broad Beans. Funct. Plant Biol. 2015, 43, 75–86. [Google Scholar] [CrossRef]
- Sezgin Muslu, A.; Altuntaş, C.; Altansambar, N.; Demiralay, M.; Kadıoğlu, A. Combined Application of Rutin and Silicon Sustains Maize Seedlings Osmotic Stress Tolerance by Improving Photosynthetic Capacity and Chlorophyll Metabolism. Acta Bot. Croat. 2025, 84, 70–80. [Google Scholar] [CrossRef]
- Park, S.; Kim, D.H.; Yang, J.H.; Lee, J.Y.; Lim, S.H. Increased Flavonol Levels in Tobacco Expressing AcFLS Affect Flower Color and Root Growth. Int. J. Mol. Sci. 2020, 21, 1011. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, G.; Sutera, F.M.; Gutsch, A.; Berni, R.; Legay, S.; Sergeant, K.; Renaut, J.; Torabi-Pour, N.; Kargar, N.; Sully, R.E.; et al. Root-Applied Phyto-Courier Loaded with Rutin Translocates to Aerial Tissues Inducing Molecular and Anatomical Changes in Cannabis sativa Under Salinity. Nano Select 2025, e70036. [Google Scholar] [CrossRef]
- Yang, W.; Xu, X.; Li, Y.; Wang, Y.; Li, M.; Wang, Y.; Ding, X.; Chu, Z. Rutin-Mediated Priming of Plant Resistance to Three Bacterial Pathogens Initiating the Early SA Signal Pathway. PLoS ONE 2016, 11, e0146910. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Shen, H.; Zhang, R.; Yang, F.; Hu, J.; Che, J.; Dai, H.; Tong, H.; Wu, Q.; Zhang, Y.; et al. Seed Priming with Rutin Enhances Tomato Resistance Against the Whitefly Bemisia tabaci. Pestic. Biochem. Physiol. 2023, 194, 105470. [Google Scholar] [CrossRef]
- Market Data Forecast. Rose Market. Available online: https://www.marketdataforecast.com/market-reports/rose-market (accessed on 15 September 2025).
- Sujatha, S.; Tejaswini, P. Integrated Analysis of Energy and Resource Use Indicators in Rose Production Systems in Open-Field and Protected Condition in India. Cleaner Environ. Syst. 2021, 3, 100051. [Google Scholar] [CrossRef]
- Melnyk, H.; Yarnykh, T.; Buryak, M. Pharmacopeial Aspects of Preparation of Infusions and Decoctions in Pharmacies. EUREKA Health Sci. 2021, 4, 87–93. [Google Scholar] [CrossRef]
- Ghavam, M.; Afzali, A.; Manconi, M.; Bacchetta, G.; Manca, M.L. Variability in Chemical Composition and Antimicrobial Activity of Essential Oil of Rosa × damascena Herrm. from Mountainous Regions of Iran. Chem. Biol. Technol. Agric. 2021, 8, 22. [Google Scholar] [CrossRef]
- Önder, D. Variation in Antioxidant Capacity, Antioxidant Activity and Mineral Composition During Flower Development of Oil-Bearing Rose (Rosa damascena Mill.). Sci. Rep. 2023, 13, 17255. [Google Scholar] [CrossRef] [PubMed]



| PA | MA | IA | NA | AA | UA | GA | MIF | |
|---|---|---|---|---|---|---|---|---|
| pH value | ||||||||
| 100% | 4.11 | 4.31 | 4.43 | 4.21 | 4.67 | 4.30 | 4.92 | 4.11 |
| 25% | 4.21 | 4.38 | 4.46 | 4.23 | 4.79 | 4.35 | 4.95 | 4.18 |
| 10% | 4.35 | 4.40 | 4.54 | 4.32 | 4.89 | 4.39 | 4.98 | 4.22 |
| EC (µS/cm) | ||||||||
| 100% | 612.0 | 590.0 | 725.0 | 555.0 | 661.0 | 565.0 | 669.0 | 606.0 |
| 25% | 187.0 | 182.0 | 209.0 | 173.9 | 199.0 | 170.2 | 212.0 | 183.4 |
| 10% | 94.8 | 84.5 | 97.3 | 78.9 | 88.5 | 78.3 | 90.2 | 83.4 |
| PA | MA | IA | NA | AA | UA | GA | MIF | |
|---|---|---|---|---|---|---|---|---|
| TPC | ||||||||
| mg GAE/g de | 193.3 ± 6.309 a | 150.1 ± 6.762 b | 80.19 ± 2.702 d | 108.6 ± 1.209 c | 150.9 ± 9.834 b | 78.97 ± 3.752 d | 119.3 ± 1.949 b | 143.3 ± 2.876 b |
| mg GAE/L RDW | 3035 ± 99.04 a | 2101 ± 94.66 c | 1195 ± 40.26 e | 1477 ± 16.45 d | 2233 ± 145.5 bc | 1208 ± 57.41 e | 1503 ± 24.56 d | 2336 ± 46.89 b |
| TFC | ||||||||
| mg QE/g de | 24.64 ± 1.170 b | 16.22 ± 0.823 c | 12.54 ± 0.661 d | 10.73 ± 0.180 d | 26.64 ± 1.129 a | 11.93 ± 0.589 d | 12.31 ± 0.062 d | 16.61 ± 0.225 c |
| mg QE/L RDW | 386.8 ± 18.37 a | 227.1 ± 11.52 c | 186.9 ± 9.850 d | 145.9 ± 2.447 e | 394.3 ± 16.71 a | 182.6 ± 9.143 d | 155.1 ± 0.781 de | 270.8 ± 3.670 b |
| TAC | ||||||||
| mg CE/g de | 0.684 ± 0.043 a | <0.200 | <0.200 | <0.200 | <0.400 | <0.200 | <0.200 | 0.230 ± 0.004 b |
| mg CE/L RDW | 10.74 ± 0.670 a | <2.800 | <2.980 | <2.720 | <5.920 | <3.060 | <2.722 | 3.753 ± 0.068 b |
| PA | MA | IA | NA | AA | UA | GA | MIF | |
|---|---|---|---|---|---|---|---|---|
| p-OH Benzoic acid | ||||||||
| [μg/g de] | 7.796 ± 0.468 e | 58.63 ± 3.518 b ** | 66.82 ± 4.009 b | 81.58 ± 4.895 a | 30.04 ± 1.802 d | 24.96 ± 1.498 d | 50.30 ± 3.018 c | 6.823 ± 0.409 e |
| [mg/L RDW] | 0.122 ± 0.007 e | 0.821 ± 0.049 b | 0.996 ± 0.060 a | 1.109 ± 0.067 a | 0.455 ± 0.027 d | 0.382 ± 0.023 d | 0.634 ± 0.038 c | 0.111 ± 0.007 e |
| Protocatechuic acid | ||||||||
| [μg/g de] | 3379 ± 270.3 a | 80.88 ± 6.471 c | 20.92 ± 1.674 c | 18.65 ± 1.492 c | 2645 ± 211.4 b | 39.64 ± 3.171 c | 15.91 ± 1.273 c | 144.7 ± 11.58 c |
| [mg/L RDW] | 53.05 ± 4.244 a | 1.132 ± 0.091 c | 0.312 ± 0.025 c | 0.254 ± 0.020 c | 39.11 ± 3.129 b | 0.607 ± 0.049 c | 0.201 ± 0.016 c | 2.359 ± 0.189 c |
| p-Coumaric acid | ||||||||
| [μg/g de] | 7.859 ± 0.707 c | 26.96 ± 2.426 a | 31.63 ± 2.847 a | 19.59 ± 1.763 b | 31.75 ± 2.857 a | 10.05 ± 0.904 c | 20.58 ± 1.852 b | 6.637 ± 0.597 c |
| [mg/L RDW] | 0.123 ± 0.011 d | 0.377 ± 0.034 b | 0.471 ± 0.042 a | 0.266 ± 0.024 c | 0.470 ± 0.042 a | 0.154 ± 0.014 d | 0.259 ± 0.023 c | 0.108 ± 0.010 d |
| Vanillic acid | ||||||||
| [μg/g de] | <4.900 *** | <4.900 | 8.724 ± 2.617 a | Nd **** | <4.900 | 8.527 ± 2.558 a | <4.900 | 10.89 ± 3.268 a |
| [mg/L RDW] | <0.077 | <0.069 | 0.130 ± 0.039 a | nd | <0.073 | 0.130 ± 0.039 a | <0.062 | 0.178 ± 0.053 a |
| Gallic acid | ||||||||
| [μg/g de] | 76,479 ± 6883 d | 151,387 ± 13,625 ab | 43,632 ± 3927 e | 93,771 ± 8439 cd | 149,985 ± 13,499 ab | 114,868 ± 10,338 c | 121,277 ± 10,915 bc | 157,329 ± 14,160 a |
| [mg/L RDW] | 1201 ± 108.1 d | 2119 ± 190.7 ab | 650.1 ± 58.51 e | 1275 ± 114.8 d | 2220 ± 199.8 a | 1757 ± 158.2 bc | 1528 ± 255.2 cd | 2564 ± 230.8 a |
| Caffeic acid | ||||||||
| [μg/g de] | <2.450 | <2.450 | 3.247 ± 0.227 | <2.450 | <2.450 | <2.450 | <2.450 | <2.450 |
| [mg/L RDW] | <0.038 | <0.034 | 0.048 ± 0.003 | <0.033 | <0.036 | <0.037 | <0.031 | <0.040 |
| Quinic acid | ||||||||
| [μg/g de] | 20,701 ± 2070 e | 17,250 ± 1725 e | 25,231 ± 2523 de | 27,389 ± 2739 cde | 33,213 ± 3321 cd | 37,096 ± 3710 c | 81,751 ± 8175 a | 50,040 ± 5004 b |
| [mg/L RDW] | 325.0 ± 32.50 e | 241.5 ± 24.15 e | 375.9 ± 37.59 de | 372.5 ± 37.25 de | 491.6 ± 49.16 cd | 567.6 ± 56.76 d | 1030 ± 103.0 a | 815.6 ± 81.56 b |
| Ferulic acid | ||||||||
| [μg/g de] | <2.450 | <2.450 | 4.124 ± 0.412 b | <2.450 | 3.712 ± 0.371 b | 3.666 ± 0.367 b | 6.050 ± 0.605 a | 3.437 ± 0.344 b |
| [mg/L RDW] | <0.038 | <0.034 | 0.061 ± 0.006 ab | <0.033 | 0.055 ± 0.005 b | 0.056 ± 0.006 b | 0.076 ± 0.008 a | 0.056 ± 0.006 b |
| Sinapic acid | ||||||||
| [μg/g de] | <9.750 | <9.750 | 27.25 ± 2.725 a | nd | <9.750 | <9.750 | 28.12 ± 2.812 a | nd |
| [mg/L RDW] | <0.153 | <0.137 | 0.406 ± 0.041 a | nd | <0.144 | <0.149 | 0.354 ± 0.035 b | nd |
| Baicalein | ||||||||
| [μg/g de] | <39.05 | <39.05 | <39.05 | nd | <39.05 | <39.05 | 53.13 ± 15.94 | <39.05 |
| [mg/L RDW] | <0.613 | <0.547 | <0.582 | nd | <0.578 | <0.597 | 0.669 ± 0.201 | <0.637 |
| Naringenin | ||||||||
| [μg/g de] | 1.477 ± 0.103 d | 1.355 ± 0.095 d | 3.019 ± 0.211 c | 3.990 ± 0.279 b | 6.648 ± 0.465 a | 3.613 ± 0.253 bc | 4.123 ± 0.289 b | 1.185 ± 0.083 d |
| [mg/L RDW] | 0.023 ± 0.002 d | 0.019 ± 0.001 d | 0.045 ± 0.003 c | 0.054 ± 0.004 bc | 0.098 ± 0.007 a | 0.055 ± 0.004 b | 0.052 ± 0.004 bc | 0.019 ± 0.001 d |
| Kaempferol | ||||||||
| [μg/g de] | 115.8 ± 8.106 d | 57.35 ± 4.015 d | 1089 ± 76.23 a | 350.2 ± 24.52 c | 287.2 ± 20.11 c | 162.1 ± 11.35 d | 964.9 ± 67.54 b | 155.1 ± 10.86 d |
| [mg/L RDW] | 1.818 ± 0.127 de | 0.803 ± 0.056 e | 16.23 ± 1.136 a | 4.763 ± 0.333 c | 4.251 ± 0.298 c | 2.480 ± 0.174 d | 12.16 ± 0.851 b | 2.528 ± 0.177 d |
| Catechin | ||||||||
| [μg/g de] | nd | nd | 203.7 ± 20.37 a | 47.22 ± 4.722 c | 114.7 ± 11.47 b | 194.5 ± 19.45 a | 57.07 ± 5.707 c | 176.2 ± 17.62 a |
| [mg/L RDW] | nd | nd | 3.035 ± 0.304 a | 0.642 ± 0.064 c | 1.698 ± 0.170 b | 2.975 ± 0.298 a | 0.719 ± 0.072 c | 2.873 ± 0.287 a |
| Chrysoeriol | ||||||||
| [μg/g de] | <0.300 | <0.300 | <0.300 | <0.300 | <0.300 | <0.300 | <0.300 | 0.826 ± 0.025 |
| [mg/L RDW] | <0.005 | <0.004 | <0.004 | <0.004 | <0.004 | <0.005 | <0.004 | 0.014 ± 0.0004 |
| Quercetin | ||||||||
| [μg/g de] | 1639 ± 491.6 a | 239.8 ± 71.94 b | 112.1 ± 33.64 b | 61.37 ± 18.41 b | 358.9 ± 107.7 b | 92.97 ± 27.89 b | 63.27 ± 18.98 b | 429.9 ± 129.0 b |
| [mg/L RDW] | 25.73 ± 7.718 a | 3.357 ± 1.007 b | 1.671 ± 0.501 b | 0.835 ± 0.250 b | 5.312 ± 1.594 b | 1.423 ± 0.427 b | 0.797 ± 0.239 b | 7.007 ± 2.102 b |
| Chlorogenic acid | ||||||||
| [μg/g de] | <4.900 | <4.900 | 10.11 ± 0.506 a | <4.900 | <4.900 | <4.900 | <4.900 | 9.105 ± 0.455 a |
| [mg/L RDW] | <0. 77 | <0.069 | 0.151 ± 0.008 a | <0.067 | <0.073 | <0.075 | <0.062 | 0.148 ± 0.007 a |
| Kaempherol 3–O–Glc | ||||||||
| [μg/g de] | 3265 ± 130.6 f | 4500 ± 180.0 f | 44,295 ± 1772 a | 23,813 ± 952.5 c | 19,884 ± 795.4 d | 27,167 ± 1087 b | 23,631 ± 945.3 c | 9298 ± 371.9 e |
| [mg/L RDW] | 51.26 ± 2.050 e | 63.00 ± 2.520 e | 660.0 ± 26.40 a | 323.9 ± 12.95 c | 294.3 ± 11.77 c | 415.7 ± 16.63 b | 297.8 ± 11.91 c | 151.6 ± 6.062 d |
| Quercitrin | ||||||||
| [μg/g de] | 14,095 ± 845.7 b | 11,304 ± 678.2 c | 1208 ± 72.46 fg | 2411 ± 144.7 ef | 16,908 ± 1014 a | 2802 ± 168.1 e | 159.9 ± 9.591 g | 8535 ± 512.1 d |
| [mg/L RDW] | 221.3 ± 13.28 b | 158.3 ± 9.495 c | 17.99 ± 1.080 ef | 32.79 ± 1.968 de | 250.2 ± 15.01 a | 42.87 ± 2.572 d | 2.014 ± 0.121 f | 139.1 ± 8.347 c |
| Hyperoside + Isoquercetin | ||||||||
| [μg/g de] | 9202 ± 552.1 b | 7040 ± 422.4 c | 4785 ± 287.1 d | 1865 ± 111.9 e | 16,655 ± 999.3 a | 4076 ± 244.6 d | 587.7 ± 35.26 e | 9331 ± 559.8 b |
| [mg/L RDW] | 144.5 ± 8.668 b | 98.57 ± 5.914 c | 71.30 ± 4.278 d | 25.36 ± 1.522 e | 246.5 ± 14.79 a | 62.37 ± 3.742 d | 7.405 ± 0.444 e | 152.1 ± 9.125 b |
| Amenthoflavon | ||||||||
| [μg/g de] | 1.759 ± 0.053 | <1.200 | <1.200 | <1.200 | <1.200 | <1.200 | <1.200 | <1.200 |
| [mg/L RDW] | 0.028 ± 0.001 | <0.017 | <0.018 | <0.016 | <0.018 | <0.018 | <0.015 | <0.020 |
| Rutin | ||||||||
| [μg/g de] | 461.9 ± 13.86 b | 392.0 ± 11.76 cd | 190.1 ± 5.704 f | 43.10 ± 1.293 g | 412.3 ± 12.37 bc | 315.2 ± 9.457 e | 352.9 ± 10.59 de | 1428 ± 42.83 a |
| [mg/L RDW] | 7.252 ± 0.218 b | 5.487 ± 0.165 cd | 2.833 ± 0.085 f | 0.586 ± 0.018 g | 6.101 ± 0.183 c | 4.823 ± 0.145 de | 4.447 ± 0.133 e | 23.27 ± 0.698 a |
| Sum of quantified compounds | ||||||||
| [mg/g de] | 129.4 | 192.3 | 120.9 | 149.9 | 240.5 | 186.9 | 229.0 | 236.9 |
| [g/L RDW] | 2.031 | 2.692 | 1.802 | 2.037 | 3.560 | 2.677 | 2.885 | 3.861 |
| PA | MA | IA | NA | AA | UA | GA | MIF | |
|---|---|---|---|---|---|---|---|---|
| DPPH | ||||||||
| IC50 [μg de/mL] | 9.450 ± 0.028 de | 10.57 ± 0.665 c | 19.38 ± 0.531 a | 9.575 ± 0.120 d | 7.115 ± 0.403 f | 11.67 ± 0.106 b | 11.64 ± 0.226 b | 8.660 ± 0.099 e |
| FRAP | ||||||||
| [mg AAE/g de] | 182.0 ± 7.852 a | 145.2 ± 10.29 b | 83.31 ± 3.820 d | 101.4 ± 5.956 d | 140.0 ± 6.718 bc | 122.9 ± 8.604 c | 127.2 ± 4.135 bc | 183.2 ± 9.668 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Živanović, N.; Danilov, I.; Lesjak, M.; Dujković, T.; Simin, N.; Vlajkov, V.; Ljubojević, M.; Grahovac, J. Rose Oil Distillation Wastewater: By-Products of Essential Oil Extraction as Circular Biostimulants for Tomato Growth. Antioxidants 2025, 14, 1252. https://doi.org/10.3390/antiox14101252
Živanović N, Danilov I, Lesjak M, Dujković T, Simin N, Vlajkov V, Ljubojević M, Grahovac J. Rose Oil Distillation Wastewater: By-Products of Essential Oil Extraction as Circular Biostimulants for Tomato Growth. Antioxidants. 2025; 14(10):1252. https://doi.org/10.3390/antiox14101252
Chicago/Turabian StyleŽivanović, Nemanja, Ivana Danilov, Marija Lesjak, Tatjana Dujković, Nataša Simin, Vanja Vlajkov, Mirjana Ljubojević, and Jovana Grahovac. 2025. "Rose Oil Distillation Wastewater: By-Products of Essential Oil Extraction as Circular Biostimulants for Tomato Growth" Antioxidants 14, no. 10: 1252. https://doi.org/10.3390/antiox14101252
APA StyleŽivanović, N., Danilov, I., Lesjak, M., Dujković, T., Simin, N., Vlajkov, V., Ljubojević, M., & Grahovac, J. (2025). Rose Oil Distillation Wastewater: By-Products of Essential Oil Extraction as Circular Biostimulants for Tomato Growth. Antioxidants, 14(10), 1252. https://doi.org/10.3390/antiox14101252











