Title Oxidative Stress in Age-Related Macular Degeneration: From Molecular Mechanisms to Emerging Therapeutic Targets
Abstract
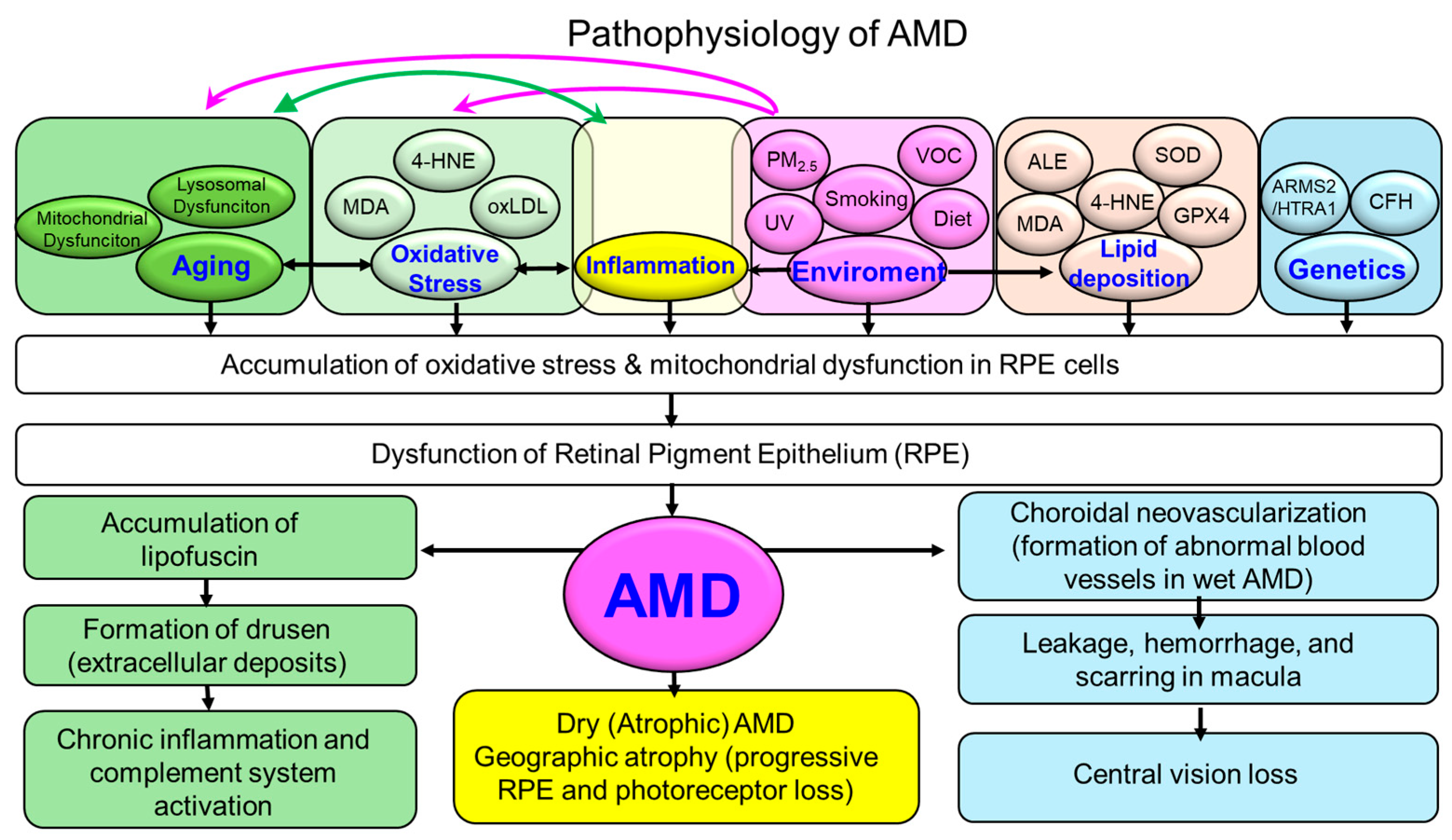
1. Introduction
2. Retinal Vulnerability to Oxidative Stress
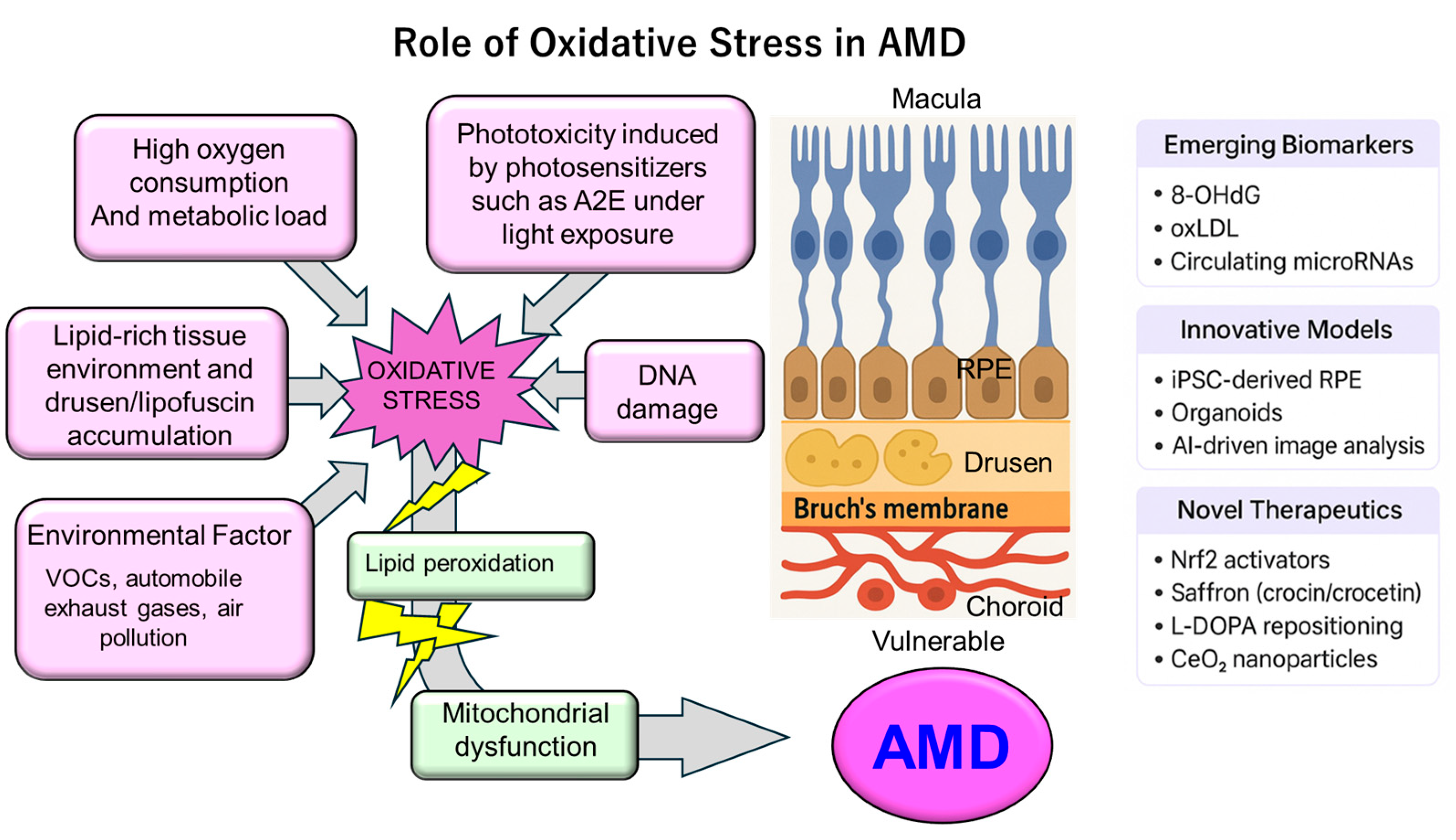
3. Sources of Oxidative Stress in AMD
3.1. Endogenous Factors
3.1.1. Mitochondrial Dysfunction in RPE and Photoreceptors
3.1.2. Imbalance Between ROS Production and Antioxidant Defense
3.1.3. Age-Related Decline in Redox Homeostasis and Accumulation of Oxidized Metabolites
3.2. Exogenous Factors
3.2.1. Light Exposure (Phototoxicity)
3.2.2. Smoking
3.2.3. Air Pollution Including VOCs
3.2.4. Diet and Oxidative Load
4. Molecular Mechanisms Linking Oxidative Stress to AMD Pathogenesis
4.1. Oxidative Damage in RPE Cells
4.2. Lipid Peroxidation and Advanced Lipoxidation End Products (ALEs)
4.3. Drusen Formation and Complement Activation
4.4. Crosstalk Among Oxidative Stress, Inflammation, and Autophagy
4.5. Nrf2 Pathway Dysregulation
4.6. Pathway Crosstalk and Emerging Multi-Target Therapeutic Strategies
4.7. Microglia and Macrophage Contributions to AMD Pathogenesis
4.8. Dose-Dependent Effects of ROS on AMD Progression
4.9. Integrative Perspective
- (1)
- metabolic and mitochondrial vulnerability in the RPE;
- (2)
- lipid peroxidation and ALE/AGE accumulation;
- (3)
- complement and immune activation driven by drusen;
- (4)
- impairment of autophagy, mitophagy, and lysosomal function;
- (5)
- downregulation of antioxidant gene networks such as Nrf2.
5. Clinical Evidence on Oxidative Stress
5.1. Oxidative Damage Markers in Serum, Aqueous Humor, and Retinal Tissue
5.2. Imaging Findings Reflecting Oxidative Damage
5.3. Oxidative Stress–Related Genetic Polymorphisms and Susceptibility
5.4. Conflicting Evidence on Oxidative Stress
5.5. Emerging Biomarkers and Personalized Therapeutic Implications
5.6. Summary
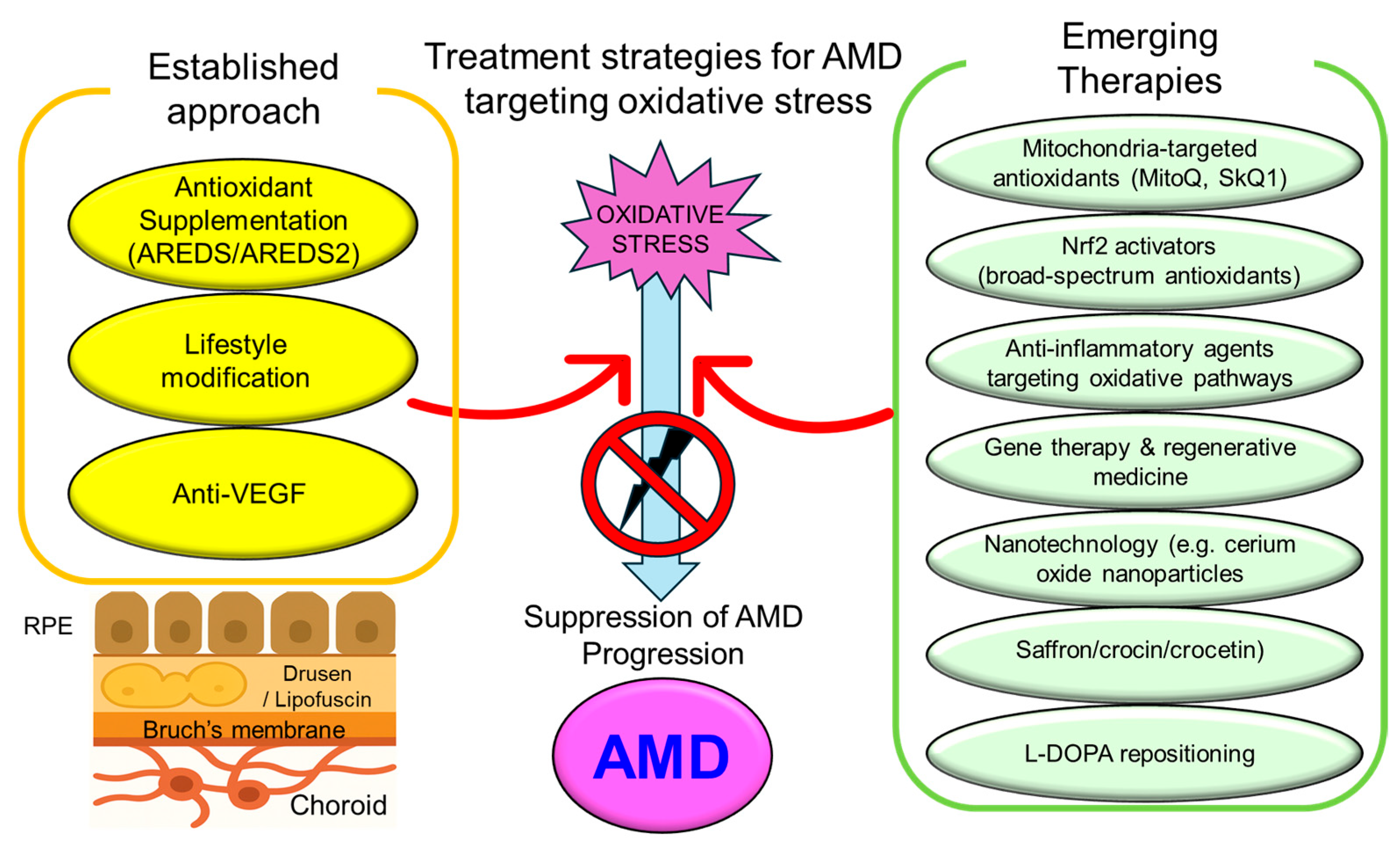
6. Therapeutic Strategies Targeting Oxidative Stress in AMD
6.1. Established Approaches
Antioxidant Supplementation Therapy (AREDS/AREDS2 Trials)
6.2. Emerging Therapeutic Trends
6.2.1. Mitochondria-Targeted Antioxidants
6.2.2. Nrf2 Activators (e.g., Bardoxolone Methyl, Sulforaphane)
6.2.3. Anti-Inflammatory Agents Modulating Oxidative Pathways
6.2.4. Gene Therapy and Regenerative Strategies
6.2.5. Saffron and Its Carotenoid Constituents
6.2.6. L-DOPA Repositioning in AMD Prevention and Treatment
6.2.7. Cerium Oxide Nanoparticles (CeO2-NPs) and Retinal Protection
6.2.8. Limitations of AREDS and Personalized Responses
6.3. Summary
7. Challenges and Future Perspectives
7.1. Limitations of Conventional Antioxidant Therapies
7.2. The Need for Personalized (Precision) Medicine Strategies
7.3. Integrative Approaches with Other AMD Therapies
7.4. Strategic Directions for Oxidative Stress Research in AMD
7.5. Selection of Oxidative Stress Models in In Vitro AMD Studies
7.6. Summary and Outlook
8. Discussion
8.1. Translational Potential of Novel Therapeutic Targets
8.2. Prospects for AMD Prevention Through Oxidative Stress Control
9. Conclusions (Final Remarks)
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxy-2-nonenal |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| A2E | N-retinylidene-N-retinylethanolamine |
| AGE | Advanced glycation end product |
| AI | Artificial intelligence |
| ALE | Advanced lipoxidation end product |
| AMD | Age-related macular degeneration |
| APOA1 | Apolipoprotein A1 |
| APOE | Apolipoprotein E |
| AREDS | Age-Related Eye Disease Study |
| ARMS2 | Age-related maculopathy susceptibility 2 |
| CDDO-Me | the methyl ester of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid |
| CeO2-NPs | Cerium oxide nanoparticles |
| CFB | Complement factor B |
| CFH | Complement factor H |
| DAMP | Danger-associated molecular pattern |
| DHA | Docosahexaenoic acid |
| DNA | Deoxyribonucleic acid |
| EPA | Eicosapentaenoic acid |
| GPX4 | Glutathione peroxidase 4 |
| GSH | γ-glutamyl-cysteinyl-glycine (glutathione) GSH) |
| GST | Glutathione S-transferase |
| HDL | High-density lipoprotein |
| HIF | Hypoxia-inducible factor |
| HO-1 | Heme oxygenase-1 |
| HTRA1 | HtrA serine peptidase 1 |
| IL | Interleukin |
| iPSC | Induced pluripotent stem cell |
| LCN1 | Lipocalin-1 |
| L-DOPA | Levodopa |
| LIPC | Hepatic Lipase (gene) |
| MDA | Malondialdehyde |
| MitoQ | Mitoquinone |
| mtDNA | Mitochondrial DNA |
| mTOR | Mammalian target of rapamycin |
| NAD+ | Nicotinamide adenine dinucleotide (oxidized form) |
| NADH | Nicotinamide adenine dinucleotide (reduced form) |
| NFE2L2 | Gene name of nuclear factor erythroid 2-related factor 2 (Nrf2) |
| NF-κB | Nuclear factor kappa B |
| NO2 | Nitrogen dioxide |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| O3 | Ozon |
| OCT | Optical coherence tomography |
| oxLDL | Oxidized low-density lipoprotein |
| OXYS rats | Oxidative stress-prone rats |
| PM2.5 | Particulate Matter with an aerodynamic diameter of less than 2.5 μm |
| PGC-1α | Peroxisome proliferator–activated receptor gamma coactivator-1 alpha |
| PUFAs | Polyunsaturated fatty acids |
| RAGE | Receptor for advanced glycation end products |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| RPE | Retinal pigment epithelium |
| SkQ1 | Skulachev Ion-1 |
| SMOC2 | Secreted protein, acidic and rich in cysteine (SPARC) related modular calcium binding 2 |
| SOD | Superoxide dismutase |
| SPARC | Secreted protein, acidic and rich in cysteine |
| SUCNR1 | Succinate receptor 1 |
| TAS | Total antioxidant status |
| TF | Serotransferrin |
| TPP+ | Triphenylphosphonium |
| UV | Ultraviolet |
| VEGF | Vascular endothelial growth factor |
| VOC | Volatile organic compound |
References
- Hadziahmetovic, M.; Malek, G. Age-related macular degeneration revisited: From pathology and cellular stress to potential therapies. Front. Cell Dev. Biol. 2021, 8, 612812. [Google Scholar] [CrossRef]
- Joachim, N.; Mitchell, P.; Burlutsky, G.; Kifley, A.; Wang, J.J. The incidence and progression of age-related macular degeneration over 15 years: The Blue Mountains Eye Study. Ophthalmology 2015, 122, 2482–2489. [Google Scholar] [CrossRef]
- Parmar, U.P.S.; Surico, P.L.; Mori, T.; Singh, R.B.; Cutrupi, F.; Premkishore, P.; Gallo Afflitto, G.; Di Zazzo, A.; Coassin, M.; Romano, F. Antioxidants in age-related macular degeneration: Lights and shadows. Antioxidants 2025, 14, 152. [Google Scholar] [CrossRef]
- Maurya, M.; Bora, K.; Blomfield, A.K.; Pavlovich, M.C.; Huang, S.; Liu, C.H.; Chen, J. Oxidative stress in retinal pigment epithelium degeneration: From pathogenesis to therapeutic targets in dry age-related macular degeneration. Neural Regen. Res. 2023, 18, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Ochoa Hernández, M.E.; Lewis-Luján, L.M.; Burboa Zazueta, M.G.; Del Castillo Castro, T.; De La Re Vega, E.; Gálvez-Ruiz, J.C.; Trujillo-López, S.; López Torres, M.A.; Iloki-Assanga, S.B. Role of oxidative stress and inflammation in age-related macular degeneration: Insights into the retinal pigment epithelium (RPE). Int. J. Mol. Sci. 2025, 26, 3463. [Google Scholar] [CrossRef] [PubMed]
- Abokyi, S.; To, C.H.; Lam, T.T.; Tse, D.Y. Central role of oxidative stress in age-related macular degeneration: Evidence from a review of the molecular mechanisms and animal models. Oxidative Med. Cell. Longev. 2020, 2020, 7901270. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Chen, W.; Schu, M.; Yaspan, B.L.; Yu, Y.; Thorleifsson, G.; Zack, D.J.; Arakawa, S.; Cipriani, V.; Ripke, S.; et al. Seven new loci associated with age-related macular degeneration. Nat. Genet. 2013, 45, 433–439.e2. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Cano, M.; Ebrahimi, K.; Wang, L.; Handa, J.T. The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD. Prog. Retin. Eye Res. 2017, 60, 201–218. [Google Scholar] [CrossRef]
- Heesterbeek, T.J.; Lorés-Motta, L.; Hoyng, C.B.; Lechanteur, Y.T.E.; den Hollander, A.I. Risk factors for progression of age-related macular degeneration. Ophthalmic Physiol. Opt. 2020, 40, 140–170. [Google Scholar] [CrossRef]
- Bell, C.M.; Zack, D.J.; Berlinicke, C.A. Human organoids for the study of retinal development and disease. Annu. Rev. Vis. Sci. 2020, 6, 91–114. [Google Scholar] [CrossRef]
- Ruan, Y.; Jiang, S.; Gericke, A. Age-related macular degeneration: Role of oxidative stress and blood vessels. Int. J. Mol. Sci. 2021, 22, 1296. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Nakanishi, K.; Parish, C.A. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1981–1989. [Google Scholar]
- Marie, M.; Bigot, K.; Angebault, C.; Barrau, C.; Gondouin, P.; Pagan, D.; Fouquet, S.; Villette, T.; Sahel, J.A.; Lenaers, G.; et al. Light action spectrum on oxidative stress and mitochondrial damage in A2E-loaded retinal pigment epithelium cells. Cell Death Dis. 2018, 9, 287. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, Z.; Wang, S. Role of mitochondria in retinal pigment epithelial aging and degeneration. Front. Aging 2022, 3, 926627. [Google Scholar] [CrossRef] [PubMed]
- Downie, L.E.; Busija, L.; Keller, P.R. Blue-light filtering intraocular lenses (IOLs) for protecting macular health. Cochrane Database Syst. Rev. 2018, 5, CD011977. [Google Scholar] [CrossRef] [PubMed]
- The Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar] [CrossRef]
- Wei, T.T.; Zhang, M.Y.; Zheng, X.H.; Xie, T.H.; Wang, W.; Zou, J.; Li, Y.; Li, H.Y.; Cai, J.; Wang, X.; et al. Interferon-γ induces retinal pigment epithelial cell ferroptosis by a JAK1-2/STAT1/SLC7A11 signaling pathway in age-related macular degeneration. FEBS J. 2022, 289, 1968–1983. [Google Scholar] [CrossRef]
- Grenell, A.; Wang, Y.; Yam, M.; Swarup, A.; Dilan, T.L.; Hauer, A.; Linton, J.D.; Philp, N.J.; Gregor, E.; Zhu, S.; et al. Loss of MPC1 Reprograms Retinal Metabolism to Impair Visual Function. Proc. Natl. Acad. Sci. USA 2019, 116, 3530–3535. [Google Scholar] [CrossRef]
- Fisher, C.R.; Ferrington, D.A. Perspective on AMD pathobiology: A bioenergetic crisis in the RPE. Investig. Ophthalmol. Vis. Sci. 2018, 59, AMD41–AMD47. [Google Scholar] [CrossRef]
- Mehrzadi, S.; Hemati, K.; Reiter, R.J.; Hosseinzadeh, A. Mitochondrial dysfunction in age-related macular degeneration: Melatonin as a potential treatment. Expert Opin. Ther. Targets 2020, 24, 359–378. [Google Scholar] [CrossRef]
- Bellezza, I. Oxidative stress in age-related macular degeneration: Nrf2 as therapeutic target. Front. Pharmacol. 2018, 9, 1280. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Aspects Med. 2012, 33, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Lenin, R.R.; Koh, Y.H.; Zhang, Z.; Yeo, Y.Z.; Parikh, B.H.; Seah, I.; Wong, W.; Su, X. Dysfunctional autophagy, proteostasis, and mitochondria as a prelude to age-related macular degeneration. Int. J. Mol. Sci. 2023, 24, 8763. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yang, K.; Chen, Y.; Li, Q.; Chen, J.; Li, S.; Wu, Y. Exposure of A2E to blue light promotes ferroptosis in the retinal pigment epithelium. Cell Mol. Biol. Lett. 2025, 30, 22. [Google Scholar] [CrossRef] [PubMed]
- Velilla, S.; García-Medina, J.J.; García-Layana, A.; Dolz-Marco, R.; Pons-Vázquez, S.; Pinazo-Durán, M.D.; Gómez-Ulla, F.; Arévalo, J.F.; Díaz-Llopis, M.; Gallego-Pinazo, R. Smoking and age-related macular degeneration: Review and update. J. Ophthalmol. 2013, 2013, 895147. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Y.; Xu, X. Association between ambient air pollution and age-related macular degeneration: A meta-analysis. BMC Ophthalmol. 2024, 24, 202. [Google Scholar] [CrossRef]
- Chua, S.Y.L.; Warwick, A.; Peto, T.; Balaskas, K.; Moore, A.T.; Reisman, C.; Desai, P.; Lotery, A.J.; Dhillon, B.; Khaw, P.T.; et al. UK Biobank Eye and Vision Consortium. Association of ambient air pollution with age-related macular degeneration and retinal thickness in UK Biobank. Br. J. Ophthalmol. 2022, 106, 705–711. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, D.; Peng, L.; Wang, X.; Ding, Q.; Li, L.; Xu, T. Exposure to volatile organic compounds is a risk factor for diabetes retinopathy: A cross-sectional study. Front. Public Health 2024, 12, 1347671. [Google Scholar] [CrossRef]
- Writing Committee for the OPTOS PEripheral RetinA (OPERA) Study Research Group; Domalpally, A.; Clemons, T.E.; Danis, R.P.; Sadda, S.R.; Cukras, C.A.; Toth, C.A.; Friberg, T.R.; Chew, E.Y. Peripheral retinal changes associated with age-related macular degeneration in the Age-Related Eye Disease Study 2: Age-Related Eye Disease Study 2 Report Number 12 by the Age-Related Eye Disease Study 2 Optos PEripheral RetinA (OPERA) Study Research Group. Ophthalmology 2017, 124, 479–487. [Google Scholar] [CrossRef]
- Broadhead, G.K.; Grigg, J.R.; McCluskey, P.; Hong, T.; Schlub, T.E.; Chang, A.A. Saffron therapy for the treatment of mild/moderate age-related macular degeneration: A randomised clinical trial. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 31–40. [Google Scholar] [CrossRef]
- Karimi, P.; Gheisari, A.; Gasparini, S.J.; Baharvand, H.; Shekari, F.; Satarian, L.; Ader, M. Crocetin Prevents RPE Cells from Oxidative Stress through Protection of Cellular Metabolic Function and Activation of ERK1/2. Int. J. Mol. Sci. 2020, 21, 2949. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Mokhosoev, I.M.; Mel’nikova, T.I.; Porozov, Y.B.; Terentiev, A.A. Oxidative stress and advanced lipoxidation and glycation end products (ALEs and AGEs) in aging and age-related diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3085756. [Google Scholar] [CrossRef] [PubMed]
- Mol, M.; Degani, G.; Coppa, C.; Baron, G.; Popolo, L.; Carini, M.; Aldini, G.; Vistoli, G.; Altomare, A. Advanced lipoxidation end products (ALEs) as RAGE binders: Mass spectrometric and computational studies to explain the reasons why. Redox Biol. 2019, 23, 101083. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.V.; Leitner, W.P.; Staples, M.K.; Anderson, D.H. Complement activation and inflammatory processes in drusen formation and age related macular degeneration. Exp. Eye Res. 2001, 73, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.G.; Park, Y.S.; Kim, I.B. Complement system and potential therapeutics in age-related macular degeneration. Int. J. Mol. Sci. 2021, 22, 6851. [Google Scholar] [CrossRef]
- Pivtoraiko, V.N.; Stone, S.L.; Roth, K.A.; Shacka, J.J. Oxidative stress and autophagy in the regulation of lysosome-dependent neuron death. AntiOxidative Redox Signal. 2009, 11, 481–496. [Google Scholar] [CrossRef]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Cheng, Y.; Ouyang, W.; Sang, X.; Liu, J.; Su, Y.; Liu, Y.; Li, C.; Yang, L.; et al. Autophagy dysfunction, cellular senescence, and abnormal immune-inflammatory responses in AMD: From mechanisms to therapeutic potential. Oxidative Med. Cell. Longev. 2019, 2019, 3632169. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. Interplay between reactive oxygen species and autophagy in the course of age-related macular degeneration. EXCLI J. 2020, 19, 1353–1371. [Google Scholar] [CrossRef]
- Brown, E.E.; Lewin, A.S.; Ash, J.D. Mitochondria: Potential targets for protection in age-related macular degeneration. Adv. Exp. Med. Biol. 2018, 1074, 11–17. [Google Scholar] [CrossRef]
- Lambros, M.L.; Plafker, S.M. Oxidative stress and the Nrf2 anti-oxidant transcription factor in age-related macular degeneration. Adv. Exp. Med. Biol. 2016, 854, 67–72. [Google Scholar] [CrossRef]
- Hu, Z.L.; Wang, Y.X.; Lin, Z.Y.; Ren, W.S.; Liu, B.; Zhao, H.; Qin, Q. Regulatory factors of Nrf2 in age-related macular degeneration pathogenesis. Int. J. Ophthalmol. 2024, 17, 1344–1362. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Yu, J. Salidroside alleviates ferroptosis in FAC-induced age-related macular degeneration models by activating Nrf2/SLC7A11/GPX4 axis. Int. Immunopharmacol. 2024, 142 Pt A, 113041. [Google Scholar] [CrossRef]
- Natoli, R.; Fernando, N.; Dahlenburg, T.; Jiao, H.; Aggio-Bruce, R.; Barnett, N.L.; de la Barca, J.M.C.; Tcherkez, G.; Reynier, P.; Fang, J.; et al. Obesity-induced metabolic disturbance drives oxidative stress and complement activation in the retinal environment. Mol. Vis. 2018, 24, 201–217. [Google Scholar]
- Combadière, C.; Feumi, C.; Raoul, W.; Keller, N.; Rodéro, M.; Pézard, A.; Lavalette, S.; Houssier, M.; Jonet, L.; Picard, E.; et al. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J. Clin. Investig. 2007, 117, 2920–2928. [Google Scholar] [CrossRef]
- Ozawa, Y. Oxidative stress in the light-exposed retina and its implication in age-related macular degeneration. Redox Biol. 2020, 37, 101779. [Google Scholar] [CrossRef]
- Li, H.; Li, B.; Zheng, Y. Role of microglia/macrophage polarisation in intraocular diseases: A review. Int. J. Mol. Med. 2024, 53, 45. [Google Scholar] [CrossRef]
- Khademi, S.; Yu, Z.; Zhou, T.; Song, B.; Xu, Z. Macrophages in age-related macular degeneration: A narrative review. Aging Adv. 2024, 1, 27–41. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Falcão, A.S.; Pedro, M.L.; Tenreiro, S.; Seabra, M.C. Targeting lysosomal dysfunction and oxidative stress in age-related macular degeneration. Antioxidants 2025, 14, 596. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, J.; Li, J.; Jiang, H.; Kong, J. Klotho levels are decreased and associated with enhanced oxidative stress and inflammation in the aqueous humor in patients with exudative age-related macular degeneration. Ocul. Immunol. Inflamm. 2022, 30, 630–637. [Google Scholar] [CrossRef]
- Hondur, G.; Göktas, E.; Yang, X.; Al-Aswad, L.; Auran, J.D.; Blumberg, D.M.; Cioffi, G.A.; Liebmann, J.M.; Suh, L.H.; Trief, D.; et al. Oxidative stress-related molecular biomarker candidates for glaucoma. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- Kersten, E.; Paun, C.C.; Schellevis, R.L.; Hoyng, C.B.; Delcourt, C.; Lengyel, I.; Peto, T.; Ueffing, M.; Klaver, C.C.W.; Dammeier, S.; et al. Systemic and ocular fluid compounds as potential biomarkers in age-related macular degeneration. Surv. Ophthalmol. 2018, 63, 9–39. [Google Scholar] [CrossRef]
- Huang, K.; Schofield, C.; Nguy, T.; Dere, R.; Wolowski, V.; Siebourg-Polster, J.; Dieckmann, A.; Garweg, J.G.; Chang, M.; Honigberg, L.; et al. Proteomics approach identifies aqueous humor biomarkers in retinal diseases. Commun. Med. 2025, 5, 134. [Google Scholar] [CrossRef]
- Shaw, P.X.; Zhang, L.; Zhang, M.; Du, H.; Zhao, L.; Lee, C.; Grob, S.; Lim, S.L.; Hughes, G.; Lee, J.; et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc. Natl. Acad. Sci. USA 2012, 109, 13757–13762. [Google Scholar] [CrossRef]
- Shaw, P.X.; Stiles, T.; Douglas, C.; Ho, D.; Fan, W.; Du, H.; Xiao, X. Oxidative stress, innate immunity, and age-related macular degeneration. AIMS Mol. Sci. 2016, 3, 196–221. [Google Scholar] [CrossRef]
- Javadzadeh, A.; Ghorbanihaghjo, A.; Bahreini, E.; Rashtchizadeh, N.; Argani, H.; Alizadeh, S. Plasma oxidized LDL and thiol-containing molecules in patients with exudative age-related macular degeneration. Mol. Vis. 2010, 16, 2578–2584. [Google Scholar]
- Klein, R.; Lee, K.E.; Tsai, M.Y.; Cruickshanks, K.J.; Gangnon, R.E.; Klein, B.E.K. Oxidized Low-density Lipoprotein and the Incidence of Age-related Macular Degeneration. Ophthalmology 2019, 126, 752–758. [Google Scholar] [CrossRef]
- Simonelli, F.; Zarrilli, F.; Mazzeo, S.; Verde, V.; Romano, N.; Savoia, M.; Testa, F.; Vitale, D.F.; Rinaldi, M.; Sacchetti, L. Serum oxidative and antioxidant parameters in a group of Italian patients with age-related maculopathy. Clin. Chim. Acta 2002, 320, 111–115. [Google Scholar] [CrossRef]
- Stradiotto, E.; Allegrini, D.; Fossati, G.; Raimondi, R.; Sorrentino, T.; Tripepi, D.; Barone, G.; Inforzato, A.; Romano, M.R. Genetic aspects of age-related macular degeneration and their therapeutic potential. Int. J. Mol. Sci. 2022, 23, 13280. [Google Scholar] [CrossRef] [PubMed]
- Favret, S.; Binet, F.; Lapalme, E.; Leboeuf, D.; Carbadillo, J.; Rubic, T.; Picard, E.; Mawambo, G.; Tetreault, N.; Joyal, J.S.; et al. Deficiency in the metabolite receptor SUCNR1 (GPR91) leads to outer retinal lesions. Aging (Albany NY) 2013, 5, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef] [PubMed]
- Cano, M.; Wang, L.; Wan, J.; Barnett, B.P.; Ebrahimi, K.; Qian, J.; Handa, J.T. Oxidative stress induces mitochondrial dysfunction and a protective unfolded protein response in RPE cells. Free Radic. Biol. Med. 2014, 69, 1–14. [Google Scholar] [CrossRef]
- Guo, H.; Li, J.; Lu, P. Systematic review and meta-analysis of mass spectrometry proteomics applied to ocular fluids to assess potential biomarkers of age-related macular degeneration. BMC Ophthalmol. 2023, 23, 507. [Google Scholar] [CrossRef]
- Acar, İ.E.; Lores-Motta, L.; Colijn, J.M.; Meester-Smoor, M.A.; Verzijden, T.; Cougnard-Grégoire, A.; Ajana, S.; Merle, B.M.J.; de Breuk, A.; Heesterbeek, T.J.; et al. Integrating metabolomics, genomics, and disease pathways in age-related macular degeneration: The EYE-RISK Consortium. Ophthalmology 2020, 127, 1693–1709. [Google Scholar] [CrossRef]
- Figueiredo, I.; Farinha, C.; Barreto, P.; Coimbra, R.; Pereira, P.; Marques, J.P.; Pires, I.; Cachulo, M.L.; Silva, R. Nutritional Genomics: Implications for Age-Related Macular Degeneration. Nutrients 2024, 16, 4124. [Google Scholar] [CrossRef]
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS Report No. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef]
- Chew, E.Y. Nutrition, genes, and age-related macular degeneration: What have we learned from the trials? Ophthalmologica 2017, 238, 1–5. [Google Scholar] [CrossRef]
- Muraleva, N.A.; Kozhevnikova, O.S.; Fursova, A.Z.; Kolosova, N.G. Suppression of AMD-like pathology by mitochondria-targeted antioxidant SkQ1 is associated with a decrease in the accumulation of amyloid β and in mTOR activity. Antioxidants 2019, 8, 177. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and other nutrigenomic Nrf2 activators: Can the clinician’s expectation be matched by the reality? Oxidative Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef]
- Szczesny-Malysiak, E.; Stojak, M.; Campagna, R.; Grosicki, M.; Jamrozik, M.; Kaczara, P.; Chlopicki, S. Bardoxolone Methyl Displays Detrimental Effects on Endothelial Bioenergetics, Suppresses Endothelial ET-1 Release, and Increases Endothelial Permeability in Human Microvascular Endothelium. Oxidative Med. Cell. Longev. 2020, 2020, 4678252. [Google Scholar] [CrossRef]
- Yagishita, Y.; Gatbonton-Schwager, T.N.; McCallum, M.L.; Kensler, T.W. Current Landscape of NRF2 Biomarkers in Clinical Trials. Antioxidants 2020, 9, 716. [Google Scholar] [CrossRef]
- Falsini, B.; Piccardi, M.; Minnella, A.; Savastano, C.; Capoluongo, E.; Fadda, A.; Balestrazzi, E.; Maccarone, R.; Bisti, S. Influence of saffron supplementation on retinal flicker sensitivity in early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6118–6124. [Google Scholar] [CrossRef]
- Piccardi, M.; Marangoni, D.; Minnella, A.M.; Savastano, M.C.; Valentini, P.; Ambrosio, L.; Capoluongo, E.; Maccarone, R.; Bisti, S.; Falsini, B. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: Sustained benefits to central retinal function. Evid. Based Complement. Alternat. Med. 2012, 2012, 429124. [Google Scholar] [CrossRef] [PubMed]
- Lashay, A.; Sadough, G.; Ashrafi, E.; Lashay, M.; Movassat, M.; Akhondzadeh, S. Short-term outcomes of saffron supplementation in patients with age-related macular degeneration: A double-blind, placebo-controlled, randomized trial. Med. Hypothesis Discov. Innov. Ophthalmol. 2016, 5, 32–38. [Google Scholar] [PubMed]
- Heitmar, R.; Brown, J.; Kyrou, I. Saffron (Crocus sativus L.) in ocular diseases: A narrative review of the existing evidence from clinical studies. Nutrients 2019, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Maccarone, R.; Di Marco, S.; Bisti, S. Saffron supplement maintains morphology and function after exposure to damaging light in mammalian retina. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1254–1261. [Google Scholar] [CrossRef]
- Heydari, M.; Zare, M.; Badie, M.R.; Watson, R.R.; Talebnejad, M.R.; Afarid, M. Crocin as a vision supplement. Clin. Exp. Optom. 2023, 106, 249–256. [Google Scholar] [CrossRef]
- Brilliant, M.H.; Vaziri, K.; Connor, T.B., Jr.; Schwartz, S.G.; Carroll, J.J.; McCarty, C.A.; Schrodi, S.J.; Hebbring, S.J.; Kishor, K.S.; Flynn, H.W., Jr.; et al. Mining retrospective data for virtual prospective drug repurposing: L-DOPA and age-related macular degeneration. Am. J. Med. 2016, 129, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Mathis, T.; Baudin, F.; Mariet, A.S.; Augustin, S.; Bricout, M.; Przegralek, L.; Roubeix, C.; Benzenine, É.; Blot, G.; Nous, C.; et al. DRD2 activation inhibits choroidal neovascularization in patients with Parkinson’s disease and age-related macular degeneration. J. Clin. Investig. 2024, 134, e174199. [Google Scholar] [CrossRef]
- Figueroa, A.G.; Boyd, B.M.; Christensen, C.A.; Javid, C.G.; McKay, B.S.; Fagan, T.C.; Snyder, R.W. Levodopa positively affects neovascular age-related macular degeneration. Am. J. Med. 2021, 134, 122–128.e3. [Google Scholar] [CrossRef] [PubMed]
- Kyosseva, S.V.; Chen, L.; Seal, S.; McGinnis, J.F. Nanoceria inhibit expression of genes associated with inflammation and angiogenesis in the retina of Vldlr null mice. Exp. Eye Res. 2013, 116, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef]
- Kong, L.; Cai, X.; Zhou, X.; Wong, L.L.; Karakoti, A.S.; Seal, S.; McGinnis, J.F. Nanoceria extend photoreceptor cell lifespan in tubby mice by modulation of apoptosis/survival signaling pathways. Neurobiol. Dis. 2011, 42, 514–523. [Google Scholar] [CrossRef]
- Cai, X.; Sezate, S.A.; Seal, S.; McGinnis, J.F. Sustained protection against photoreceptor degeneration in tubby mice by intravitreal injection of nanoceria. Biomaterials 2012, 33, 8771–8781. [Google Scholar] [CrossRef]
- Fiorani, L.; Passacantando, M.; Santucci, S.; Di Marco, S.; Bisti, S.; Maccarone, R. Cerium oxide nanoparticles reduce microglial activation and neurodegenerative events in light damaged retina. PLoS ONE 2015, 10, e0140387. [Google Scholar] [CrossRef]
- Maccarone, R.; Tisi, A.; Passacantando, M.; Ciancaglini, M. Ophthalmic applications of cerium oxide nanoparticles. J. Ocul. Pharmacol. Ther. 2020, 36, 376–383. [Google Scholar] [CrossRef]
- Dhall, A.; Self, W. Cerium oxide nanoparticles: A brief review of their synthesis methods and biomedical applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef]
- Corsi, F.; Deidda Tarquini, G.; Urbani, M.; Bejarano, I.; Traversa, E.; Ghibelli, L. The impressive anti-inflammatory activity of cerium oxide nanoparticles: More than redox? Nanomaterials 2023, 13, 2803. [Google Scholar] [CrossRef]
- Awh, C.C.; Lane, A.M.; Hawken, S.; Zanke, B.; Kim, I.K. CFH and ARMS2 genetic polymorphisms predict response to antioxidants and zinc in patients with age-related macular degeneration. Ophthalmology 2013, 120, 2317–2323. [Google Scholar] [CrossRef]
- Vavvas, D.G.; Small, K.W.; Awh, C.; Zanke, B.W.; Tibshirani, R.J.; Kustra, R. CFH and ARMS2 genetic risk determines progression to neovascular age-related macular degeneration after antioxidant and zinc supplementation. Proc. Natl. Acad. Sci. USA 2018, 115, E696–E704. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.-F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Schmidt-Erfurth, U.; et al. Intravitreal aflibercept (VEGF Trap-Eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P. Mitochondria-targeted antioxidants as promising drugs for treatment of age-related brain diseases. J. Alzheimers Dis. 2012, 28, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Gioscia-Ryan, R.A.; LaRocca, T.J.; Sindler, A.L.; Zigler, M.C.; Murphy, M.P.; Seals, D.R. Mitochondria-targeted antioxidant (MitoQ) ameliorates age-related arterial endothelial dysfunction in mice. J. Physiol. 2014, 592, 2549–2561. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Talalay, P. Induction of phase 2 genes by sulforaphane protects retinal pigment epithelial cells against photooxidative damage. Proc. Natl. Acad. Sci. USA 2004, 101, 10446–10451. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.M.; Cano, M.; Handa, J.T. Nrf2 signaling is impaired in the aging RPE given an oxidative insult. Exp. Eye Res. 2014, 119, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Hsu, J.C.; Chen, W.; Chew, E.Y.; Ding, Y. A simultaneous inference procedure to identify subgroups from RCTs with survival outcomes: Application to analysis of AMD progression studies. arXiv 2020, arXiv:2003.10528. [Google Scholar] [CrossRef]
- Xu, X.; Hang, L.; Huang, B.; Wei, Y.; Zheng, S.; Li, W. Efficacy of Ethanol Extract of Fructus lycii and Its Constituents Lutein/Zeaxanthin in Protecting Retinal Pigment Epithelium Cells against Oxidative Stress: In Vivo and In Vitro Models of Age-Related Macular Degeneration. J. Ophthalmol. 2013, 2013, 862806. [Google Scholar] [CrossRef] [PubMed]
- Brantley, M.A., Jr.; Osborn, M.P.; Sanders, B.J.; Rezaei, K.A.; Lu, P.; Li, C.; Milne, G.L.; Cai, J.; Sternberg, P., Jr. Plasma biomarkers of oxidative stress and genetic variants in age-related macular degeneration. Am. J. Ophthalmol. 2012, 153, 460–467.e1. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Moral, M.P.; Kannan, K. How stable is oxidative stress level? An observational study of intra- and inter-individual variability in urinary oxidative stress biomarkers of DNA, proteins, and lipids in healthy individuals. Environ. Int. 2019, 123, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Lains, I.; Mendez, K.; Nigalye, A.; Katz, R.; Douglas, V.P.; Kelly, R.S.; Kim, I.K.; Miller, J.B.; Vavvas, D.G.; Liang, L.; et al. Plasma metabolomic profiles associated with three-year progression of age-related macular degeneration. Metabolites 2022, 12, 32. [Google Scholar] [CrossRef]
- Golestaneh, N.; Chu, Y.; Cheng, S.K.; Cao, H.; Poliakov, E.; Berinstein, D.M. Repressed SIRT1/PGC-1α pathway and mitochondrial disintegration in iPSC-derived RPE disease model of age-related macular degeneration. J. Transl. Med. 2016, 14, 344. [Google Scholar] [CrossRef]
- Chang, Y.J.; Jenny, L.A.; Li, Y.S.; Cui, X.; Kong, Y.; Li, Y.; Sparrow, J.R.; Tsang, S.H. CRISPR editing demonstrates rs10490924 raised oxidative stress in iPSC-derived retinal cells from patients with ARMS2/HTRA1-related AMD. Proc. Natl. Acad. Sci. USA 2023, 120, e2215005120. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Rahman, H.S. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and antioxidants: Physiological and pathological role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]
- Markovets, A.M.; Fursova, A.Z.; Kolosova, N.G. Therapeutic Action of the Mitochondria-Targeted Antioxidant SkQ1 on Retinopathy in OXYS Rats Linked with Improvement of VEGF and PEDF Gene Expression. PLoS ONE 2011, 6, e21682. [Google Scholar] [CrossRef]
- Fairley, L.H.; Das, S.; Dharwal, V.; Amorim, N.; Hegarty, K.J.; Wadhwa, R.; Mounika, G.; Hansbro, P.M. Mitochondria-Targeted Antioxidants as a Therapeutic Strategy for Chronic Obstructive Pulmonary Disease. Antioxidants 2023, 12, 973. [Google Scholar] [CrossRef]
- Pan, H.; He, M.; Liu, R.; Brecha, N.C.; Yu, A.C.; Pu, M. Sulforaphane Protects Rodent Retinas against Ischemia-Reperfusion Injury through the Activation of the Nrf2/HO-1 Antioxidant Pathway. PLoS ONE 2014, 9, e114186. [Google Scholar] [CrossRef]
- Yang, P.M.; Cheng, K.C.; Huang, J.Y.; Wang, S.Y.; Lin, Y.N.; Tseng, Y.T.; Hsieh, C.W.; Wung, B.S. Sulforaphane Inhibits Blue Light-Induced Inflammation and Apoptosis by Upregulating the SIRT1/PGC-1α/Nrf2 Pathway and Autophagy in Retinal Pigment Epithelial Cells. Toxicol. Appl. Pharmacol. 2021, 421, 115545. [Google Scholar] [CrossRef]
- Matías-Pérez, D.; Varapizuela-Sánchez, C.F.; Pérez-Campos, E.L.; González-González, S.; Sánchez-Medina, M.A.; García-Montalvo, I.A. Dietary Sources of Antioxidants and Oxidative Stress in Age-Related Macular Degeneration. Front. Pharmacol. 2024, 15, 1442548. [Google Scholar] [CrossRef]
- de Jong, S.; Gagliardi, G.; Garanto, A.; de Breuk, A.; Lechanteur, Y.T.E.; Katti, S.; van den Heuvel, L.P.; Volokhina, E.B.; den Hollander, A.I. Implications of Genetic Variation in the Complement System in Age-Related Macular Degeneration. Prog. Retin. Eye Res. 2021, 84, 100952. [Google Scholar] [CrossRef]
- Jabbehdari, S.; Handa, J.T. Oxidative Stress as a Therapeutic Target for the Prevention and Treatment of Early Age-Related Macular Degeneration. Surv. Ophthalmol. 2021, 66, 423–440. [Google Scholar] [CrossRef]

| Therapy/Intervention | Mechanism of Action | Clinical Evidence | Development Stage | Key References |
|---|---|---|---|---|
| Established Therapies | ||||
| AREDS/AREDS2 supplementation | Antioxidants (vitamins C, E, zinc, lutein, zeaxanthin) reduce oxidative burden | Multiple large RCTs (AREDS, AREDS2) demonstrated reduced risk of progression to advanced AMD | Widely recommended in clinical guidelines | Age-Related Eye Disease Study Research Group, 2001; Age-Related Eye Disease Study 2 Research Group, 2013 [16,69] |
| Anti-VEGF therapy | Blocks VEGF-mediated neovascularization, indirectly reducing oxidative stress from hypoxia | Numerous RCTs show efficacy in wet AMD | Standard of care | Rosenfeld et al., 2006; Heier et al., 2012 [94,95] |
| Emerging Therapies | ||||
| Saffron (crocin, crocetin) | Antioxidant, neuroprotective, mitochondrial protection | Several small-to-moderate RCTs show improvements in visual function | Phase II/III clinical evaluation ongoing | Maccarone et al., 2008; Broadhead et al., 2019; Heydari et al., 2023 [30,79,80] |
| L-DOPA repositioning | Enhances melanin synthesis in RPE, antioxidant and cytoprotective effects | Epidemiological evidence (reduced AMD incidence in Parkinson’s patients on L-DOPA) | Repurposing under investigation | Brilliant et al., 2016 [81] |
| Cerium oxide nanoparticles (CeO2-NPs) | Regenerative catalytic antioxidant, anti-inflammatory, prevents drusen formation | Strong preclinical evidence in animal models | Preclinical, early translational | Fiorani et al., 2015; Maccarone et al., 2020 [88,89] |
| Mitochondria-targeted antioxidants (e.g., MitoQ, SkQ1) | Scavenge ROS directly in mitochondria | Preclinical and early-phase trials report efficacy | Early-phase human trials | Skulachev et al., 2012; Gioscia-Ryan et al., 2014 [96,97] |
| Nrf2 activators (e.g., sulforaphane) | Enhance endogenous antioxidant defense pathways | Preclinical neuroprotective evidence | Early-phase translational studies | Gao et al., 2004; Sachdeva et al., 2014 [98,99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mimura, T.; Noma, H. Title Oxidative Stress in Age-Related Macular Degeneration: From Molecular Mechanisms to Emerging Therapeutic Targets. Antioxidants 2025, 14, 1251. https://doi.org/10.3390/antiox14101251
Mimura T, Noma H. Title Oxidative Stress in Age-Related Macular Degeneration: From Molecular Mechanisms to Emerging Therapeutic Targets. Antioxidants. 2025; 14(10):1251. https://doi.org/10.3390/antiox14101251
Chicago/Turabian StyleMimura, Tatsuya, and Hidetaka Noma. 2025. "Title Oxidative Stress in Age-Related Macular Degeneration: From Molecular Mechanisms to Emerging Therapeutic Targets" Antioxidants 14, no. 10: 1251. https://doi.org/10.3390/antiox14101251
APA StyleMimura, T., & Noma, H. (2025). Title Oxidative Stress in Age-Related Macular Degeneration: From Molecular Mechanisms to Emerging Therapeutic Targets. Antioxidants, 14(10), 1251. https://doi.org/10.3390/antiox14101251







