Early Administration of N-Acetylcysteine Provides Renal and Cardiac Mitochondrial and Redox Protection, Preventing the Development of Cardio-Renal Syndrome Type IV Induced by 5/6NX
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Design
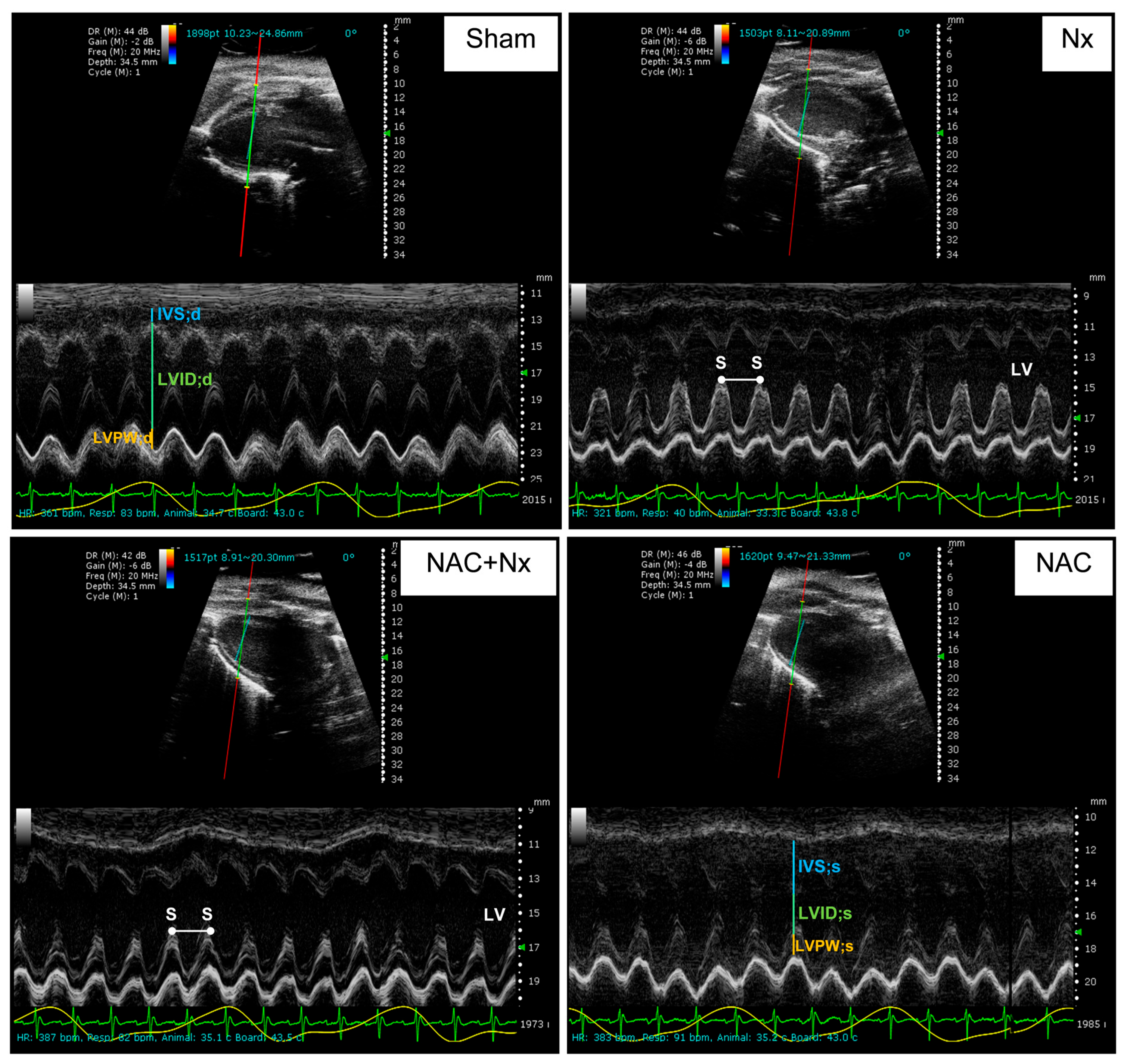
2.3. Cardio-Renal Damage Markers and Evaluation of Cardiac Function by Echocardiography
2.4. Kidney and Heart Histology
2.5. Protein Extraction and WB
2.6. Heart and Kidney Mitochondria Isolation
2.7. Mitochondrial Respiratory Parameters and Membrane Potential (ΔΨm)
2.8. Mitochondrial H2O2 Production Rates
2.9. Activity of Mitochondrial Respiratory Complexes and ATP Synthase, and NADP+/NADPH Ratio
2.10. Evaluation of Antioxidant Enzyme Activities
2.11. In Silico NAC Interaction with PPAR-α and Nuclear Respiratory Factor (NRF2)
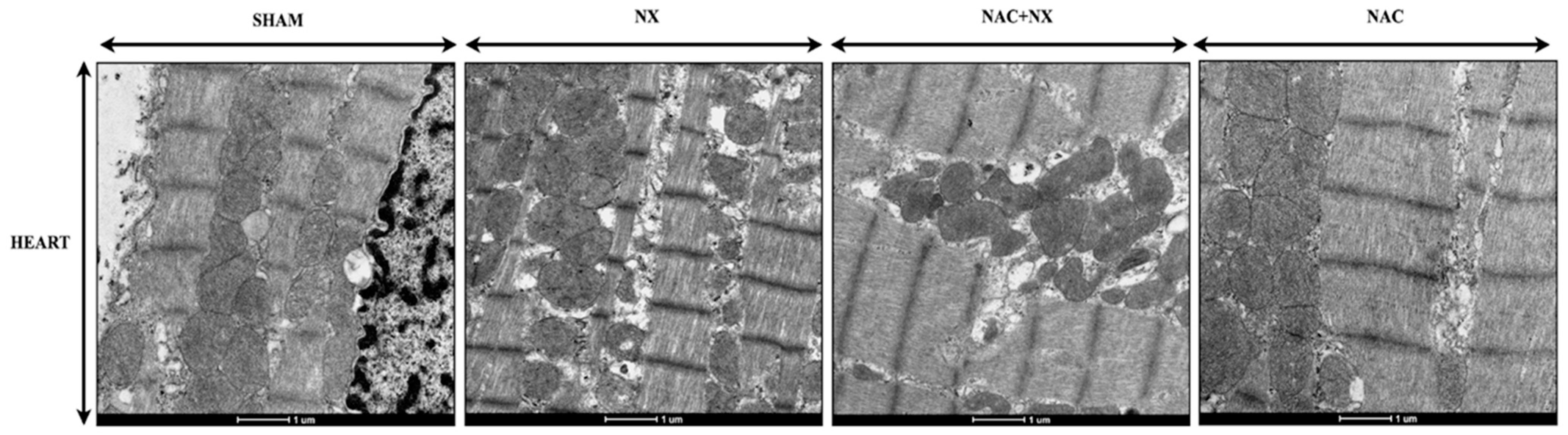
2.12. Electron Microscopy
2.13. Statistics
3. Results
3.1. NAC Prevents the Increase of Cardiorenal Syndrome
3.2. NAC Prevented Mitochondrial Bioenergetic Impairment in Kidney and Heart
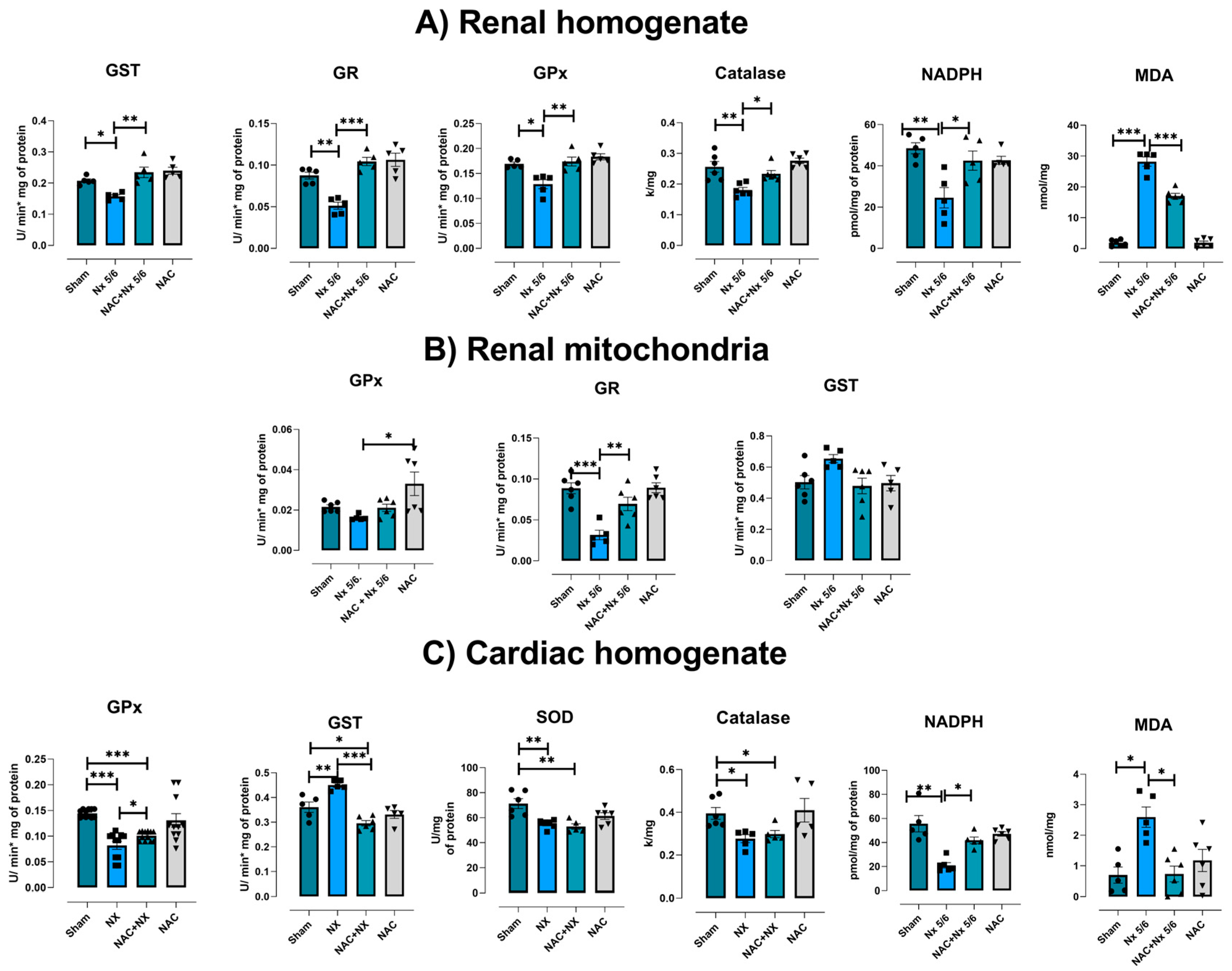
3.3. NAC Prevented Redox Imbalance in Renal and Heart
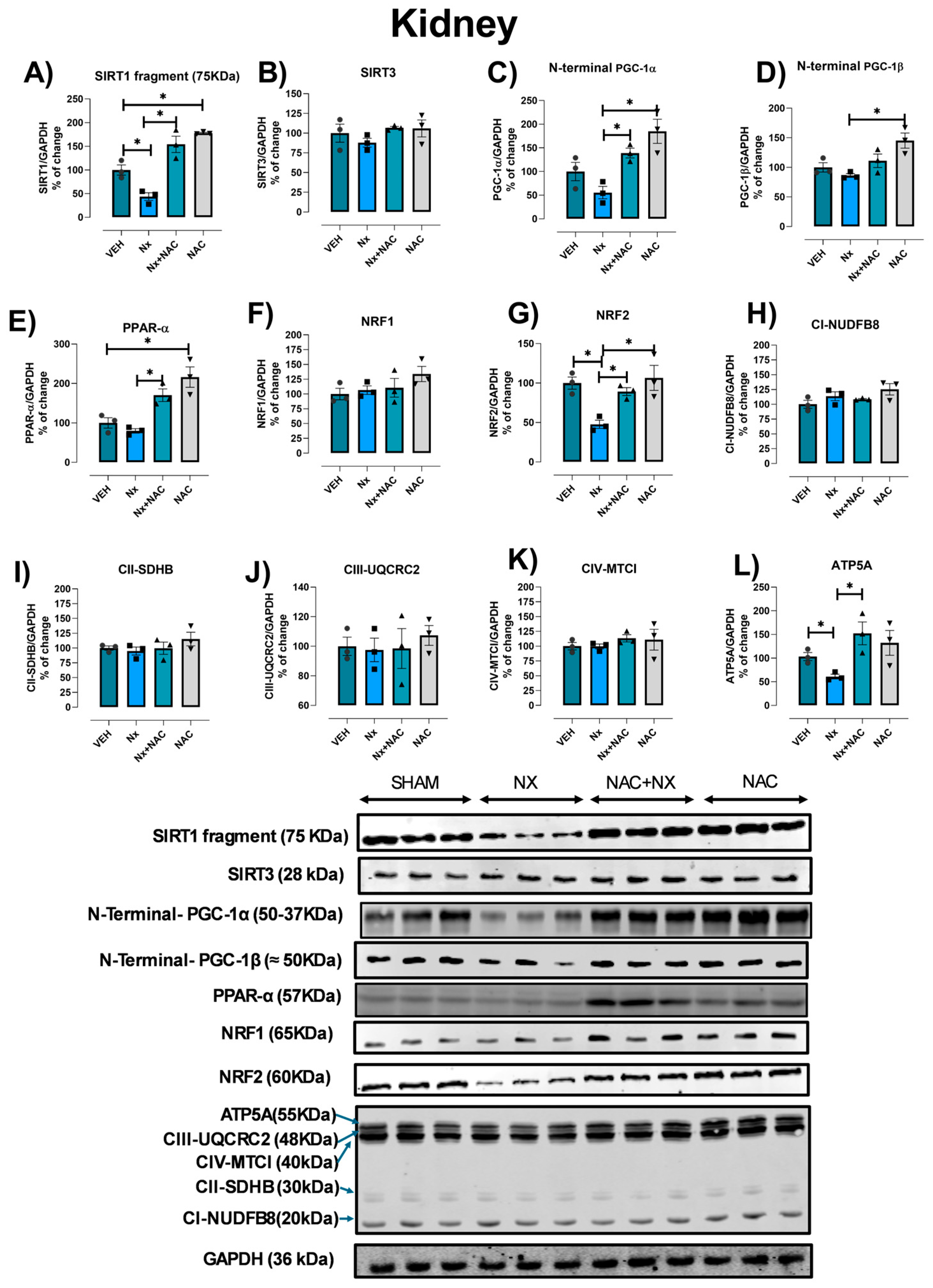
3.4. NAC Prevents Mitochondrial Biogenesis Reduction in Kidney
3.5. In Silico Activation of PPAR-α and NRF2 by NAC
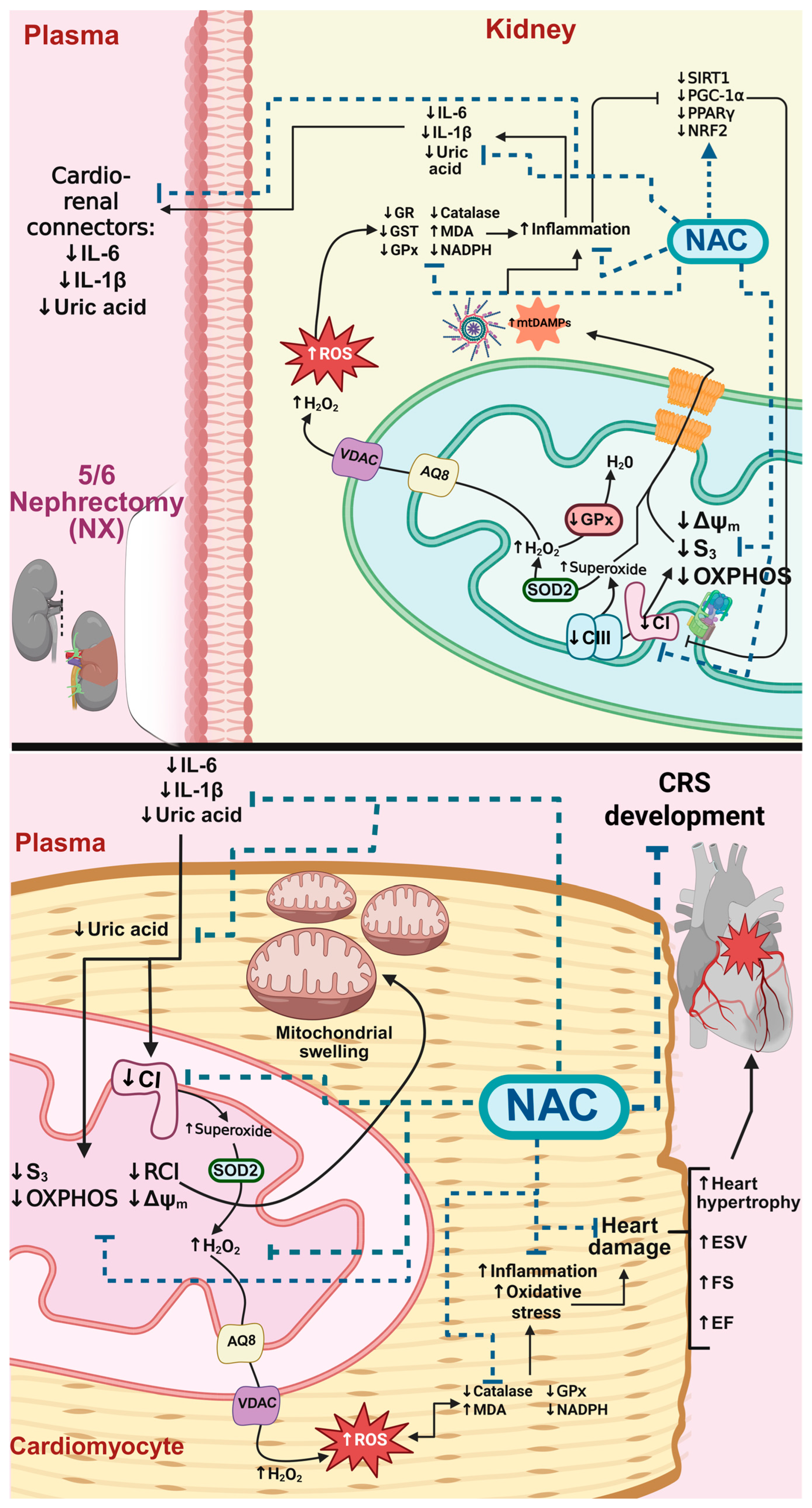
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lumpuy-Castillo, J.; Amador-Martínez, I.; Díaz-Rojas, M.; Lorenzo, O.; Pedraza-Chaverri, J.; Sánchez-Lozada, L.G.; Aparicio-Trejo, O.E. Role of Mitochondria in Reno-Cardiac Diseases: A Study of Bioenergetics, Biogenesis, and GSH Signaling in Disease Transition. Redox Biol. 2024, 76, 103340. [Google Scholar] [CrossRef] [PubMed]
- Szlagor, M.; Dybiec, J.; Młynarska, E.; Rysz, J.; Franczyk, B. Chronic Kidney Disease as a Comorbidity in Heart Failure. Int. J. Mol. Sci. 2023, 24, 2988. [Google Scholar] [CrossRef]
- Xue, Y.; Xu, B.; Su, C.; Han, Q.; Wang, T.; Tang, W. Cardiorenal Syndrome in Incident Peritoneal Dialysis Patients: What Is Its Effect on Patients’ Outcomes? PLoS ONE 2019, 14, e0218082. [Google Scholar] [CrossRef]
- Prothasis, M.; Varma, A.; Gaidhane, S.; Kumar, S.; Khatib, N.; Zahiruddin, Q.; Gaidhane, A. Prevalence, Types, Risk Factors, and Outcomes of Cardiorenal Syndrome in a Rural Population of Central India: A Cross-Sectional Study. J. Fam. Med. Prim. Care 2020, 9, 4127. [Google Scholar] [CrossRef]
- Suresh, H.; Arun, B.S.; Moger, V.; Swamy, M. Cardiorenal Syndrome Type 4: A Study of Cardiovascular Diseases in Chronic Kidney Disease. Indian Heart J. 2017, 69, 11–16. [Google Scholar] [CrossRef]
- Yu, A.S.; Pak, K.J.; Zhou, H.; Shaw, S.F.; Shi, J.; Broder, B.I.; Sim, J.J. All-Cause and Cardiovascular-Related Mortality in CKD Patients With and Without Heart Failure: A Population-Based Cohort Study in Kaiser Permanente Southern California. Kidney Med. 2023, 5, 100624. [Google Scholar] [CrossRef]
- GBD Chronic Kidney Disease Collaboration Global, Regional, and National Burden of Chronic Kidney Disease, 1990-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [CrossRef] [PubMed]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial Energetics in the Kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Hamzaoui, M.; Djerada, Z.; Brunel, V.; Mulder, P.; Richard, V.; Bellien, J.; Guerrot, D. 5/6 Nephrectomy Induces Different Renal, Cardiac and Vascular Consequences in 129/Sv and C57BL/6JRj Mice. Sci. Rep. 2020, 10, 1524. [Google Scholar] [CrossRef] [PubMed]
- Bigelman, E.; Cohen, L.; Aharon-Hananel, G.; Levy, R.; Rozenbaum, Z.; Saada, A.; Keren, G.; Entin-Meer, M. Pathological Presentation of Cardiac Mitochondria in a Rat Model for Chronic Kidney Disease. PLoS ONE 2018, 13, e0198196. [Google Scholar] [CrossRef]
- Ryan, J.; Treberg, J.R. Protein S-Glutathionlyation Links Energy Metabolism to Redox Signaling in Mitochondria. Redox Biol. 2016, 8, 110–118. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; MacK, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Ceballos-Picot, I.; Witko-Sarsat, V.; Merad-Boudia, M.; Nguyen, A.T.; Thévenin, M.; Jaudon, M.C.; Zingraff, J.; Verger, C.; Jungers, P.; Descamps-Latscha, B. Glutathione Antioxidant System as a Marker of Oxidative Stress in Chronic Renal Failure. Free Radic. Biol. Med. 1996, 21, 845–853. [Google Scholar] [CrossRef]
- Popkov, V.A.; Silachev, D.N.; Zalevsky, A.O.; Zorov, D.B.; Plotnikov, E.Y. Mitochondria as a Source and a Target for Uremic Toxins. Int. J. Mol. Sci. 2019, 20, 3094. [Google Scholar] [CrossRef]
- Shen, W.C.; Chou, Y.H.; Shi, L.S.; Chen, Z.W.; Tu, H.J.; Lin, X.Y.; Wang, G.J. Ast-120 Improves Cardiac Dysfunction in Acute Kidney Injury Mice via Suppression of Apoptosis and Proinflammatory Nf-Κb/Icam-1 Signaling. J. Inflamm. Res. 2021, 14, 505–518. [Google Scholar] [CrossRef]
- Tan, X.; Cao, X.S.; Zhang, P.; Xiang, F.F.; Teng, J.; Zou, J.Z.; Ding, X.Q. Endoplasmic Reticulum Stress Associated Apoptosis as a Novel Mechanism in Indoxyl Sulfate-Induced Cardiomyocyte Toxicity. Mol. Med. Rep. 2018, 18, 5117–5122. [Google Scholar] [CrossRef]
- Enoki, Y.; Watanabe, H.; Arake, R.; Fujimura, R.; Ishiodori, K.; Imafuku, T.; Nishida, K.; Sugimoto, R.; Nagao, S.; Miyamura, S.; et al. Potential Therapeutic Interventions for Chronic Kidney Disease-Associated Sarcopenia via Indoxyl Sulfate-Induced Mitochondrial Dysfunction. J. Cachexia Sarcopenia Muscle 2017, 8, 735–747. [Google Scholar] [CrossRef]
- Sun, C.Y.; Cheng, M.L.; Pan, H.C.; Lee, J.H.; Lee, C.C. Protein-Bound Uremic Toxins Impaired Mitochondrial Dynamics and Functions. Oncotarget 2017, 8, 77722–77733. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Tatebe, J.; Watanabe, I.; Yamazaki, U.; Ikeda, T.; Morita, T. Aryl Hydrocarbon Receptor Mediates Indoxyl Sulfate-Induced Cellular Senescence in Human Umbilical Vein Endothelial Cells. J. Atheroscler. Thromb. 2014, 21, 904–916. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Itoya, M.; Takemoto, N.; Matsuura, Y.; Tawa, M.; Matsumura, Y.; Ohkita, M. Indoxyl Sulfate Induces ROS Production via the Aryl Hydrocarbon Receptor-NADPH Oxidase Pathway and Inactivates NO in Vascular Tissues. Life Sci. 2021, 265, 118807. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Trejo, O.E.; Tapia, E.; Molina-Jijón, E.; Medina-Campos, O.N.; Macías-Ruvalcaba, N.A.; León-Contreras, J.C.; Hernández-Pando, R.; García-Arroyo, F.E.; Cristóbal, M.; Sánchez-Lozada, L.G.; et al. Curcumin Prevents Mitochondrial Dynamics Disturbances in Early 5/6 Nephrectomy: Relation to Oxidative Stress and Mitochondrial Bioenergetics. BioFactors 2017, 43, 293–310. [Google Scholar] [CrossRef]
- Aparicio-Trejo, O.E.; Rojas-Morales, P.; Avila-Rojas, S.H.; León-Contreras, J.C.; Hernández-Pando, R.; Jiménez-Uribe, A.P.; Prieto-Carrasco, R.; Sánchez-Lozada, L.G.; Pedraza-Chaverri, J.; Tapia, E. Temporal Alterations in Mitochondrial β-Oxidation and Oxidative Stress Aggravate Chronic Kidney Disease Development in 5/6 Nephrectomy Induced Renal Damage. Int. J. Mol. Sci. 2020, 21, 6512. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Carrasco, R.; García-Arroyo, F.E.; Aparicio-Trejo, O.E.; Rojas-Morales, P.; León-Contreras, J.C.; Hernández-Pando, R.; Sánchez-Lozada, L.G.; Tapia, E.; Pedraza-Chaverri, J. Progressive Reduction in Mitochondrial Mass Is Triggered by Alterations in Mitochondrial Biogenesis and Dynamics in Chronic Kidney Disease Induced by 5/6 Nephrectomy. Biology 2021, 10, 349. [Google Scholar] [CrossRef]
- Khan, S.A.; Campbell, A.M.; Lu, Y.; An, L.; Alpert, J.S.; Chen, Q.M. N-Acetylcysteine for Cardiac Protection During Coronary Artery Reperfusion: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2021, 8, 752939. [Google Scholar] [CrossRef]
- Cuevas-López, B.; Romero-Ramirez, E.I.; García-Arroyo, F.E.; Tapia, E.; León-Contreras, J.C.; Silva-Palacios, A.; Roldán, F.-J.; Campos, O.N.M.; Hernandez-Esquivel, L.; Marín-Hernández, A.; et al. NAC Pre-Administration Prevents Cardiac Mitochondrial Bioenergetics, Dynamics, Biogenesis, and Redox Alteration in Folic Acid-AKI-Induced Cardio-Renal Syndrome Type 3. Antioxidants 2023, 12, 1592. [Google Scholar] [CrossRef]
- Aparicio-Trejo, O.E.; Reyes-Fermín, L.M.; Briones-Herrera, A.; Tapia, E.; León-Contreras, J.C.; Hernández-Pando, R.; Sánchez-Lozada, L.G.; Pedraza-Chaverri, J. Protective Effects of N-Acetyl-Cysteine in Mitochondria Bioenergetics, Oxidative Stress, Dynamics and S-Glutathionylation Alterations in Acute Kidney Damage Induced by Folic Acid. Free Radic. Biol. Med. 2019, 130, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Amador-Martínez, I.; Aparicio-Trejo, O.E.; Aranda-Rivera, A.K.; Bernabe-Yepes, B.; Medina-Campos, O.N.; Tapia, E.; Cortés-González, C.C.; Silva-Palacios, A.; Roldán, F.J.; León-Contreras, J.C.; et al. Effect of N-Acetylcysteine in Mitochondrial Function, Redox Signaling, and Sirtuin 3 Levels in the Heart During Cardiorenal Syndrome Type 4 Development. Antioxidants 2025, 14, 367. [Google Scholar] [CrossRef]
- Aparicio-Trejo, O.E.; Avila-Rojas, S.H.; Tapia, E.; Rojas-Morales, P.; León-Contreras, J.C.; Martínez-Klimova, E.; Hernández-Pando, R.; Sánchez-Lozada, L.G.; Pedraza-Chaverri, J. Chronic Impairment of Mitochondrial Bioenergetics and β-Oxidation Promotes Experimental AKI-to-CKD Transition Induced by Folic Acid. Free Radic. Biol. Med. 2020, 154, 18–32. [Google Scholar] [CrossRef]
- Ojuka, E.; Andrew, B.; Bezuidenhout, N.; George, S.; Maarman, G.; Madlala, H.P.; Mendham, A.; Osiki, P.O. Measurement of β-Oxidation Capacity of Biological Samples by Respirometry: A Review of Principles and Substrates. Am. J. Physiol. Metab. 2016, 310, E715–E723. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Hernández-Reséndiz, S.; Correa, F.; García-Niño, W.R.; Buelna-Chontal, M.; Roldán, F.J.; Ramírez-Camacho, I.; Delgado-Toral, C.; Carbó, R.; Pedraza-Chaverrí, J.; Tapia, E.; et al. Cardioprotection by Curcumin Post-Treatment in Rats with Established Chronic Kidney Disease. Cardiovasc. Drugs Ther. 2015, 29, 111–120. [Google Scholar] [CrossRef]
- Chaudhary, K.; Malhotra, K.; Sowers, J.; Aroor, A. Uric Acid—Key Ingredient in the Recipe for Cardiorenal Metabolic Syndrome. Cardiorenal Med. 2013, 3, 208–220. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, B.; Li, Y.; Xu, X.; Lv, J.; Jia, Q.; Chai, R.; Xue, W.; Li, Y.; Wang, Y.; et al. Mitochondrial Dysfunction: An Emerging Link in the Pathophysiology of Cardiorenal Syndrome. Front. Cardiovasc. Med. 2022, 9, 837270. [Google Scholar] [CrossRef]
- Keshavarz-Bahaghighat, H.; Darwesh, A.M.; Sosnowski, D.K.; Seubert, J.M. Mitochondrial Dysfunction and Inflammaging in Heart Failure: Novel Roles of CYP-Derived Epoxylipids. Cells 2020, 9, 1565. [Google Scholar] [CrossRef]
- Stallons, L.J.; Whitaker, R.M.; Schnellmann, R.G. Suppressed Mitochondrial Biogenesis in Folic Acid-Induced Acute Kidney Injury and Early Fibrosis. Toxicol. Lett. 2014, 224, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhang, K.; Gong, Z.; Luo, T.; Li, J.; Wang, X.; Zou, H.; Song, R.; Zhu, J.; Ma, Y.; et al. N-Acetylcysteine Delayed Cadmium-Induced Chronic Kidney Injury by Activating the Sirtuin 1–P53 Signaling Pathway. Chem. Biol. Interact. 2023, 369, 110299. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xie, N.; Li, Y.; Liu, C.; Hou, F.F.; Wang, J. N-Acetylcysteine Ameliorates Cisplatin-Induced Renal Senescence and Renal Interstitial Fibrosis through Sirtuin1 Activation and P53 Deacetylation. Free Radic. Biol. Med. 2019, 130, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR Control of Metabolism and Cardiovascular Functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Li, K.; Peng, X.; Kan, Y.; Li, H.; Zhu, Y.; Wang, Z.; Li, Z.; Liu, H.Y.; Cai, D. Nuclear Receptor PPARα as a Therapeutic Target in Diseases Associated with Lipid Metabolism Disorders. Nutrients 2023, 15, 4772. [Google Scholar] [CrossRef]
- Rofaeil, R.R.; Abdellah, A.M.; Zenhom, N.M. Nephroprotective Effect of PPAR Agonists on Thioacetamide-Induced Nephrotoxicity in Rats. J. Pharmacol. Clin. Res. 2019, 6, 555698. [Google Scholar] [CrossRef]
- Bernardes, A.; Souza, P.C.T.; Muniz, J.R.C.; Ricci, C.G.; Ayers, S.D.; Parekh, N.M.; Godoy, A.S.; Trivella, D.B.B.; Reinach, P.; Webb, P.; et al. Molecular Mechanism of Peroxisome Proliferator-Activated Receptor α Activation by WY14643: A New Mode of Ligand Recognition and Receptor Stabilization. J. Mol. Biol. 2013, 425, 2878–2893. [Google Scholar] [CrossRef]
- Kamata, S.; Oyama, T.; Saito, K.; Honda, A.; Yamamoto, Y.; Suda, K.; Ishikawa, R.; Itoh, T.; Watanabe, Y.; Shibata, T.; et al. PPARα Ligand-Binding Domain Structures with Endogenous Fatty Acids and Fibrates. iScience 2020, 23, 101727. [Google Scholar] [CrossRef]
- Rosas-Martínez, L.; Rodríguez-Muñoz, R.; Namorado-Tonix del Carmen, M.; Missirlis, F.; del Valle-Mondragón, L.; Sánchez-Mendoza, A.; Reyes-Sánchez, J.L.; Cervantes-Pérez, L.G. Peroxisome Proliferator-Activated Receptor Alpha Stimulation Preserves Renal Tight Junction Components in a Rat Model of Early-Stage Diabetic Nephropathy. Int. J. Mol. Sci. 2024, 25, 13152. [Google Scholar] [CrossRef]
- Gao, J.; Gu, Z. The Role of Peroxisome Proliferator-Activated Receptors in Kidney Diseases. Front. Pharmacol. 2022, 13, 832732. [Google Scholar] [CrossRef]
- Barret, R. Importance and Evaluation of the Polar Surface Area (PSA and TPSA). In Medicinal Chemistry; ISTE Press Ltd.: London, UK, 2018; pp. 89–95. [Google Scholar] [CrossRef]
- Canning, P.; Sorrell, F.J.; Bullock, A.N. Structural Basis of Keap1 Interactions with Nrf2. Free Radic. Biol. Med. 2015, 88, 101. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Reddy, A.P.; Reddy, P.H.; Kshirsagar, S. Unraveling the NRF2 Confusion: Distinguishing Nuclear Respiratory Factor 2 from Nuclear Erythroid Factor 2. Ageing Res. Rev. 2024, 98, 102353. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-J.; Jeon, J.-H.; Fernandez-Alfonso, S.; Gil-Ortega, M.; Kim, M.-J.; Jeon, J.-H. Recent Advances in Understanding Nrf2 Agonism and Its Potential Clinical Application to Metabolic and Inflammatory Diseases. Int. J. Mol. Sci. 2022, 23, 2846. [Google Scholar] [CrossRef]
- Baldelli, S.; Aquilano, K.; Ciriolo, M.R. Punctum on Two Different Transcription Factors Regulated by PGC-1α: Nuclear Factor Erythroid-Derived 2-like 2 and Nuclear Respiratory Factor 2. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4137–4146. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.-J.; Hecking, M.; Ulasi, I.; Sola, L.; Thomas, B. Chronic Kidney Disease, Gender, and Access to Care: A Global Perspective. Semin. Nephrol. 2017, 37, 296–308. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation 2019, 139, E840–E878. [Google Scholar] [CrossRef] [PubMed]
- Aroor, A.R.; Mandavia, C.; Ren, J.; Sowers, J.R.; Pulakat, L. Mitochondria and Oxidative Stress in the Cardiorenal Metabolic Syndrome. Cardiorenal Med. 2012, 2, 87–109. [Google Scholar] [CrossRef]
- Correa, F.; Buelna-Chontal, M.; Hernández-Reséndiz, S.; García-Niño, W.R.; Roldán, F.J.; Soto, V.; Silva-Palacios, A.; Amador, A.; Pedraza-Chaverrí, J.; Tapia, E.; et al. Curcumin Maintains Cardiac and Mitochondrial Function in Chronic Kidney Disease. Free Radic. Biol. Med. 2013, 61, 119–129. [Google Scholar] [CrossRef]
- Moradi, H.; Sica, D.A.; Kalantar-Zadeh, K. Cardiovascular Burden Associated with Uremic Toxins in Patients with Chronic Kidney Disease. Am. J. Nephrol. 2013, 38, 136–148. [Google Scholar] [CrossRef]
- García-Arroyo, F.E.; Monroy-Sánchez, F.; Muñoz-Jiménez, I.; Gonzaga, G.; Andrés-Hernando, A.; Zazueta, C.; Juárez-Rojas, J.G.; Lanaspa, M.A.; Johnson, R.J.; Sánchez-Lozada, L.G. Allopurinol Prevents the Lipogenic Response Induced by an Acute Oral Fructose Challenge in Short-Term Fructose Fed Rats. Biomolecules 2019, 9, 601. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Zhang, C.; Song, Y.; Chen, L.; Chen, P.; Yuan, M.; Meng, Y.; Wang, Q.; Zheng, G.; Qiu, Z. Urolithin A Attenuates Hyperuricemic Nephropathy in Fructose-Fed Mice by Impairing STING-NLRP3 Axis-Mediated Inflammatory Response via Restoration of Parkin-Dependent Mitophagy. Front. Pharmacol. 2022, 13, 907209. [Google Scholar] [CrossRef]
- Lanaspa, M.A.; Sanchez-Lozada, L.G.; Choi, Y.J.; Cicerchi, C.; Kanbay, M.; Roncal-Jimenez, C.A.; Ishimoto, T.; Li, N.; Marek, G.; Duranay, M.; et al. Uric Acid Induces Hepatic Steatosis by Generation of Mitochondrial Oxidative Stress: Potential Role in Fructose-Dependent and -Independent Fatty Liver. J. Biol. Chem. 2012, 287, 40732–40744. [Google Scholar] [CrossRef]
- Choi, Y.J.; Shin, H.S.; Choi, H.S.; Park, J.W.; Jo, I.; Oh, E.S.; Lee, K.Y.; Lee, B.H.; Johnson, R.J.; Kang, D.H. Uric Acid Induces Fat Accumulation via Generation of Endoplasmic Reticulum Stress and SREBP-1c Activation in Hepatocytes. Lab. Investig. 2014, 94, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Zwaenepoel, B.; De Backer, T.; Glorieux, G.; Verbeke, F. Predictive Value of Protein-Bound Uremic Toxins for Heart Failure in Patients with Chronic Kidney Disease. ESC Heart Fail. 2024, 11, 466–474. [Google Scholar] [CrossRef]
- Caillard, P.; Bennis, Y.; Six, I.; Bodeau, S.; Kamel, S.; Choukroun, G.; Maizel, J.; Titeca-Beauport, D. The Role of Gut-Derived, Protein-Bound Uremic Toxins in the Cardiovascular Complications of Acute Kidney Injury. Toxins 2022, 14, 336. [Google Scholar] [CrossRef]
- Yang, K.; Wang, C.; Nie, L.; Zhao, X.; Gu, J.; Guan, X.; Wang, S.; Xiao, T.; Xu, X.; He, T.; et al. Klotho Protects against Indoxyl Sulphate-Induced Myocardial Hypertrophy. J. Am. Soc. Nephrol. 2015, 26, 2434–2446. [Google Scholar] [CrossRef]
- Capomolla, S.; Opasich, C.; Riccardi, G.; Febo, O.; Riccardi, R.; Cobelli, F.; Tavazzi, L. Beta Blockade Therapy in Chronic Heart Failure: Diastolic Function and Mitral Regurgitation Improvement by Carvedilol. J. Am. Coll. Cardiol. 1998, 31, 189. [Google Scholar] [CrossRef][Green Version]
- Dou, L.; Sallée, M.; Cerini, C.; Poitevin, S.; Gondouin, B.; Jourde-Chiche, N.; Fallague, K.; Brunet, P.; Calaf, R.; Dussol, B.; et al. The Cardiovascular Effect of the Uremic Solute Indole-3 Acetic Acid. J. Am. Soc. Nephrol. 2015, 26, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, Y.; Li, Y.; Huang, M.; Zhao, W. Geniposide Alleviates Diabetic Nephropathy of Mice through AMPK/SIRT1/NF-ΚB Pathway. Eur. J. Pharmacol. 2020, 886, 173449. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, S.; Wang, W.; Wang, Y.; Zhang, P.; Zhu, C.; Ding, G.; Liu, B.; Yang, T.; Zhang, A. Activation of Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α Ameliorates Mitochondrial Dysfunction and Protects Podocytes from Aldosterone-Induced Injury. Kidney Int. 2012, 82, 771–789. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Zhang, L.; Sui, M.X.; Zhu, Y.H.; Zeng, L. Protective Effects of Sirtuin 3 in a Murine Model of Sepsis-Induced Acute Kidney Injury. Sci. Rep. 2016, 6, 33201. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Zhou, J.; Liu, Y.; Du, C.; Yang, K.; Bi, X.; Liu, M.; Han, W.; Wang, K.; et al. IRF1-Mediated Downregulation of PGC1α Contributes to Cardiorenal Syndrome Type 4. Nat. Commun. 2020, 11, 4664. [Google Scholar] [CrossRef]
- Ergin, B.; Guerci, P.; Zafrani, L.; Nocken, F.; Kandil, A.; Gurel-Gurevin, E.; Demirci-Tansel, C.; Ince, C. Effects of N-Acetylcysteine (NAC) Supplementation in Resuscitation Fluids on Renal Microcirculatory Oxygenation, Inflammation, and Function in a Rat Model of Endotoxemia. Intensive Care Med. Exp. 2016, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.H.M.; Gois, P.H.F.; Volpini, R.A.; Canale, D.; Luchi, W.M.; Froeder, L.; Heilberg, I.P.; Seguro, A.C. N-Acetylcysteine Protects against Star Fruit-Induced Acute Kidney Injury. Ren. Fail. 2017, 39, 193–202. [Google Scholar] [CrossRef]
- Shen, Y.; Miao, N.J.; Xu, J.L.; Gan, X.X.; Xu, D.; Zhou, L.; Xue, H.; Zhang, W.; Lu, L.M. N-Acetylcysteine Alleviates Angiotensin II-Mediated Renal Fibrosis in Mouse Obstructed Kidneys. Acta Pharmacol. Sin. 2016, 37, 637–644. [Google Scholar] [CrossRef]
- Ware, K.; Qamri, Z.; Ozcan, A.; Satoskar, A.A.; Nadasdy, G.; Rovin, B.H.; Hebert, L.A.; Nadasdy, T.; Brodsky, S.V. N-Acetylcysteine Ameliorates Acute Kidney Injury but Not Glomerular Hemorrhage in an Animal Model of Warfarin-Related Nephropathy. Am. J. Physiol. Ren. Physiol. 2013, 304, 1421–1427. [Google Scholar] [CrossRef]
- Kizilgun, M.; Poyrazoglu, Y.; Oztas, Y.; Yaman, H.; Cakir, E.; Cayci, T.; Akgul, O.E.; Kurt, Y.G.; Yaren, H.; Kunak, Z.I.; et al. Beneficial Effects of N-Acetylcysteine and Ebselen on Renal Ischemia/Reperfusion Injury. Ren. Fail. 2011, 33, 512–517. [Google Scholar] [CrossRef]
- Ye, M.; Lin, W.; Zheng, J.; Lin, S. N-Acetylcysteine for Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Am. J. Transl. Res. 2021, 13, 2472–2485. [Google Scholar]
- Machado, J.T.; Iborra, R.T.; Fusco, F.B.; Castilho, G.; Pinto, R.S.; Machado-Lima, A.; Nakandakare, E.R.; Seguro, A.C.; Shimizu, M.H.; Catanozi, S.; et al. N-Acetylcysteine Prevents Endoplasmic Reticulum Stress Elicited in Macrophages by Serum Albumin Drawn from Chronic Kidney Disease Rats and Selectively Affects Lipid Transporters, ABCA-1 and ABCG-1. Atherosclerosis 2014, 237, 343–352. [Google Scholar] [CrossRef]
- Pereira, L.V.B.; Shimizu, M.H.M.; Rodrigues, L.P.M.R.; Leite, C.C.; Andrade, L.; Seguro, A.C. N-Acetylcysteine Protects Rats with Chronic Renal Failure from Gadolinium-Chelate Nephrotoxicity. PLoS ONE 2012, 7, e39528. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Hassan, M.H.; Saleem, T.H.; Mohamed, S.A.; El-Zeftawy, M.; Ahmed, E.A.; Mostafa, N.A.M.; Hetta, H.F.; Al Shaimaa, H.; Abdallah, A.A.M. KIM-1 and GADDI-153 Gene Expression in Paracetamol-Induced Acute Kidney Injury: Effects of N-Acetylcysteine, N-Acetylmethionine, and N-Acetylglucosamine. Turkish J. Biochem. 2022, 47, 409–416. [Google Scholar] [CrossRef]
- Song, L.; Yao, S.; Zheng, D.; Xuan, Y.; Li, W. Astaxanthin Attenuates Contrast-Induced Acute Kidney Injury in Rats via ROS/NLRP3 Inflammasome. Int. Urol. Nephrol. 2022, 54, 1355–1364. [Google Scholar] [CrossRef]
- Gu, Y.; Huang, F.; Wang, Y.; Chen, C.; Wu, S.; Zhou, S.; Hei, Z.; Yuan, D. Connexin32 Plays a Crucial Role in ROS-Mediated Endoplasmic Reticulum Stress Apoptosis Signaling Pathway in Ischemia Reperfusion-Induced Acute Kidney Injury. J. Transl. Med. 2018, 16, 117. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, G.; Jia, T.; Wang, C.; Lu, X.; Tian, L.; Yang, Q.; Zhu, C. Protection Against Post-Resuscitation Acute Kidney Injury by N-Acetylcysteine via Activation of the Nrf2/HO-1 Pathway. Front. Med. 2022, 9, 848491. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; You, J.; Wang, K.; Li, Y.; Zhang, Y.; Wei, H.; Liang, X.; Liu, Y. N -Acetylcysteine Attenuates Cisplatin-Induced Acute Kidney Injury by Inhibiting the C5a Receptor. Biomed Res. Int. 2019, 2019, 4805853. [Google Scholar] [CrossRef]
- Liu, C.; Shen, Y.; Huang, L.; Wang, J. TLR2/Caspase-5/Panx1 Pathway Mediates Necrosis-Induced NLRP3 Inflammasome Activation in Macrophages during Acute Kidney Injury. Cell Death Discov. 2022, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Amador-Martínez, I.; Aparicio-Trejo, O.E.; Bernabe-Yepes, B.; Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Sánchez-Lozada, L.G.; Pedraza-Chaverri, J.; Tapia, E. Mitochondrial Impairment: A Link for Inflammatory Responses Activation in the Cardiorenal Syndrome Type 4. Int. J. Mol. Sci. 2023, 24, 15875. [Google Scholar] [CrossRef]
- Lee, J.H.; Jo, Y.H.; Kim, K.; Lee, J.H.; Rim, K.P.; Kwon, W.Y.; Suh, G.J.; Rhee, J.E. Effect of N-Acetylcysteine (NAC) on Acute Lung Injury and Acute Kidney Injury in Hemorrhagic Shock. Resuscitation 2013, 84, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazik, E.A.; Hassan, H.M.M.; Mahmoud, Z.A.; Yousef, A.M.; Elsayed, E.A. Renoprotective Effect of N-Acetylcystein and Vitamin E in Bisphenol A-Induced Rat Nephrotoxicity; Modulators of Nrf2/ NF-ΚB and ROS Signaling Pathway. Acta Biomed. 2022, 93, e2022301. [Google Scholar] [CrossRef]
- Pedre, B.; Barayeu, U.; Ezeriņa, D.; Dick, T.P. The Mechanism of Action of N-Acetylcysteine (NAC): The Emerging Role of H2S and Sulfane Sulfur Species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef]
- Kong, X.; Wang, R.; Xue, Y.; Liu, X.; Zhang, H.; Chen, Y.; Fang, F.; Chang, Y. Sirtuin 3, a New Target of PGC-1α, Plays an Important Role in the Suppression of ROS and Mitochondrial Biogenesis. PLoS ONE 2010, 5, e11707. [Google Scholar] [CrossRef]
- Peerapanyasut, W.; Kobroob, A.; Palee, S.; Chattipakorn, N.; Wongmekiat, O. Activation of Sirtuin 3 and Maintenance of Mitochondrial Integrity by N-Acetylcysteine Protects against Bisphenol A-Induced Kidney and Liver Toxicity in Rats. Int. J. Mol. Sci. 2019, 20, 267. [Google Scholar] [CrossRef]
- Sharma, M.; Kaur, T.; Singla, S.K. Protective Effects of N-Acetylcysteine against Hyperoxaluria Induced Mitochondrial Dysfunction in Male Wistar Rats. Mol. Cell. Biochem. 2015, 405, 105–114. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, L.; Chen, R.; Lu, H.; Sui, M.; Zhu, Y.; Zeng, L. SIRT3 Protects against Acute Kidney Injury via AMPK/MTOR-Regulated Autophagy. Front. Physiol. 2018, 9, 1526. [Google Scholar] [CrossRef] [PubMed]
- Nam, B.Y.; Jhee, J.H.; Park, J.T.J.; Kim, S.; Kim, G.; Park, J.T.J.; Yoo, T.H.; Kang, S.W.; Yu, J.W.; Han, S.H. PGC-1α Inhibits the NLRP3 Inflammasome via Preserving Mitochondrial Viability to Protect Kidney Fibrosis. Cell Death Dis. 2022, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhu, H. The Overexpression of Sirtuin1 (SIRT1) Alleviated Lipopolysaccharide (LPS)-Induced Acute Kidney Injury (AKI) via Inhibiting the Activation of Nucleotide-Binding Oligomerization Domain-like Receptors (NLR) Family Pyrin Domain Containing 3 (NLRP3) Inflammas. Med. Sci. Monit. 2019, 25, 2718–2726. [Google Scholar] [CrossRef]
- Li, Q.; Liao, J.; Chen, W.; Zhang, K.; Li, H.; Ma, F.; Zhang, H.; Han, Q.; Guo, J.; Li, Y.; et al. NAC Alleviative Ferroptosis in Diabetic Nephropathy via Maintaining Mitochondrial Redox Homeostasis through Activating SIRT3-SOD2/Gpx4 Pathway. Free Radic. Biol. Med. 2022, 187, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Hostetter, T.H.; Olson, J.L.; Renke, H.G.; Venkatachalam, M.A.; Brenner, B.M. Hyperfiltration in Remnant Nephrons: A Potentially Adverse Response to Renal Ablation. J. Am. Soc. Nephrol. 2001, 12, 1315–1325. [Google Scholar] [CrossRef]
- Brenner, B.M. Nephron Adaptation to Renal Injury or Ablation. Am. J. Physiol. 1985, 249, F324–F337. [Google Scholar] [CrossRef]
- Taal, M.W.; Brenner, B.M. Adaptation to Nephron Loss and Mechanisms of Progression in Chronic Kidney Disease. In Brenner & Rector’s The Kidney; Skorecki, K., Chertow, G.M., Marsden, P.A., Taal, M.W., Yu, A.S.L., Eds.; Elsevier: Philadelphia, PA, USA, 2012; pp. 1918–1971. [Google Scholar]
- Sinuani, I.; Averbukh, Z.; Gitelman, I.; Rapoport, M.J.; Sandbank, J.; Albeck, M.; Sredni, B.; Weissgarten, J. Mesangial Cells Initiate Compensatory Renal Tubular Hypertrophy via IL-10-Induced TGF-Beta Secretion: Effect of the Immunomodulator AS101 on This Process. Am. J. Physiol. Renal Physiol. 2006, 291, F384–F394. [Google Scholar] [CrossRef][Green Version]
- Hauser, P.; Kainz, A.; Perco, P.; Bergmeister, H.; Mitterbauer, C.; Schwarz, C.; Regele, H.M.; Mayer, B.; Meyer, T.W.; Oberbauer, R. Transcriptional Response in the Unaffected Kidney after Contralateral Hydronephrosis or Nephrectomy. Kidney Int. 2005, 68, 2497–2507. [Google Scholar] [CrossRef]
- Wolf, G.; Ziyadeh, F.N. Molecular Mechanisms of Diabetic Renal Hypertrophy. Kidney Int. 1999, 56, 393–405. [Google Scholar] [CrossRef]
- Ceja-Galicia, Z.A.; García-Arroyo, F.E.; Aparicio-Trejo, O.E.; El-Hafidi, M.; Gonzaga-Sánchez, G.; León-Contreras, J.C.; Hernández-Pando, R.; Guevara-Cruz, M.; Tovar, A.R.; Rojas-Morales, P.; et al. Therapeutic Effect of Curcumin on 5/6Nx Hypertriglyceridemia: Association with the Improvement of Renal Mitochondrial β-Oxidation and Lipid Metabolism in Kidney and Liver. Antioxidants 2022, 11, 2195. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, Y.; Sun, S.; Yu, M.; Wang, C.; Pei, X.; Zhu, B.; Wu, J.; Zhao, W. Bone Marrow Stem Cells-Derived Microvesicles Protect against Renal Injury in the Mouse Remnant Kidney Model. Nephrology 2012, 17, 493–500. [Google Scholar] [CrossRef]
- Wu, M.; Xia, W.; Jin, Q.; Zhou, A.; Wang, Q.; Li, S.; Huang, S.; Zhang, A.; Zhang, Y.; Li, Y.; et al. Gasdermin E Deletion Attenuates Ureteral Obstruction- and 5/6 Nephrectomy-Induced Renal Fibrosis and Kidney Dysfunction. Front. Cell Dev. Biol. 2021, 9, 754134. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, M.; Miyashita, K.; Wakino, S.; Mitsuishi, M.; Hayashi, K.; Itoh, H. Chronic Kidney Disease Reduces Muscle Mitochondria and Exercise Endurance and Its Exacerbation by Dietary Protein through Inactivation of Pyruvate Dehydrogenase. Kidney Int. 2014, 85, 1330–1339. [Google Scholar] [CrossRef]
- Askari, H.; Seifi, B.; Kadkhodaee, M. Evaluation of Renal-Hepatic Functional Indices and Blood Pressure Based on the Progress of Time in a Rat Model of Chronic Kidney Disease. Nephrourol. Mon. 2016, 8, e37840. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, H.; Gabay, O.; Meir, H.; Haze, A.; Kandel, L.; Liebergall, M.; Gagarina, V.; Lee, E.J.; Dvir-Ginzberg, M. 75-Kd Sirtuin 1 Blocks Tumor Necrosis Factor α-Mediated Apoptosis in Human Osteoarthritic Chondrocytes. Arthritis Rheum. 2012, 64, 718–728. [Google Scholar] [CrossRef] [PubMed]
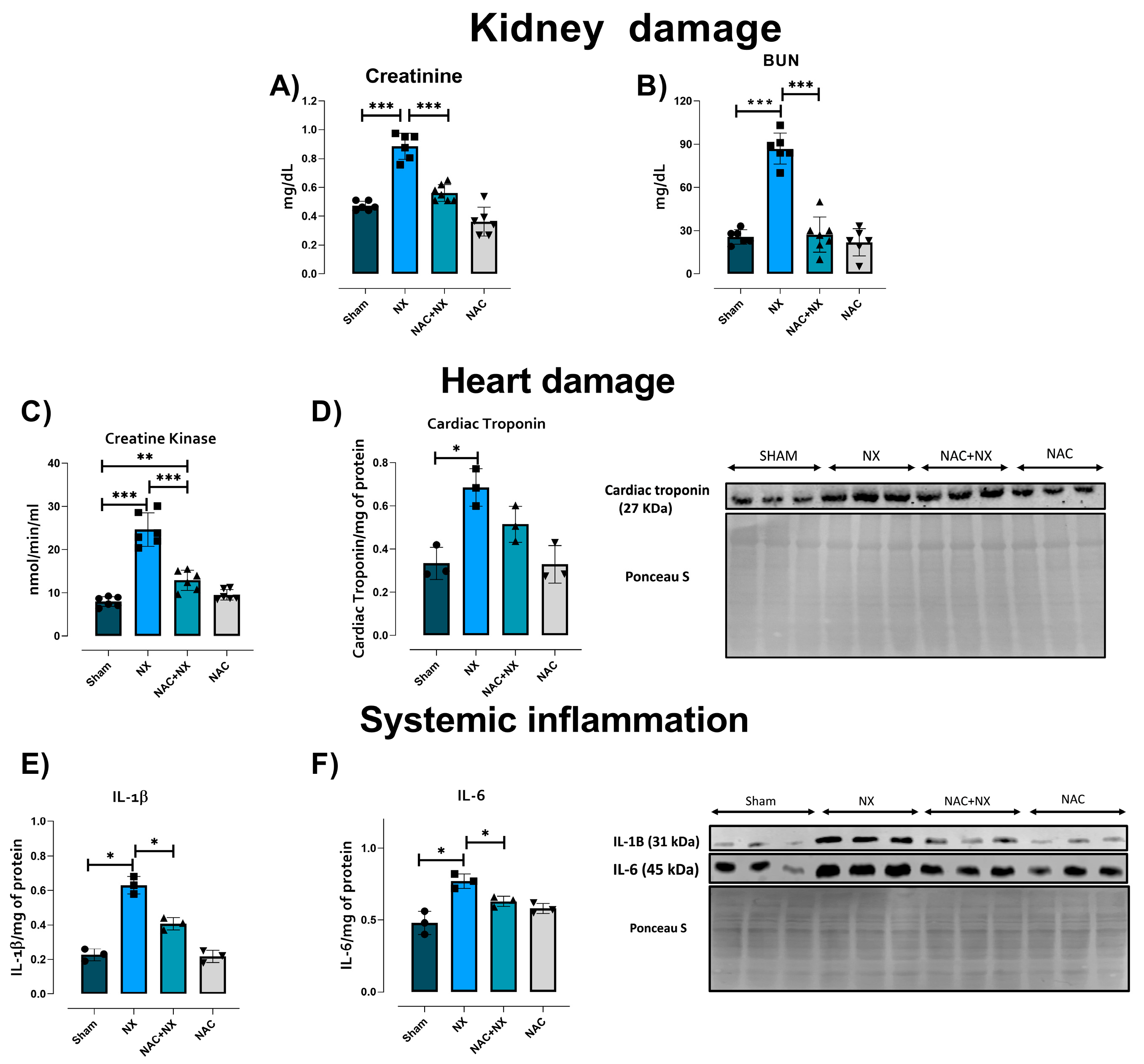
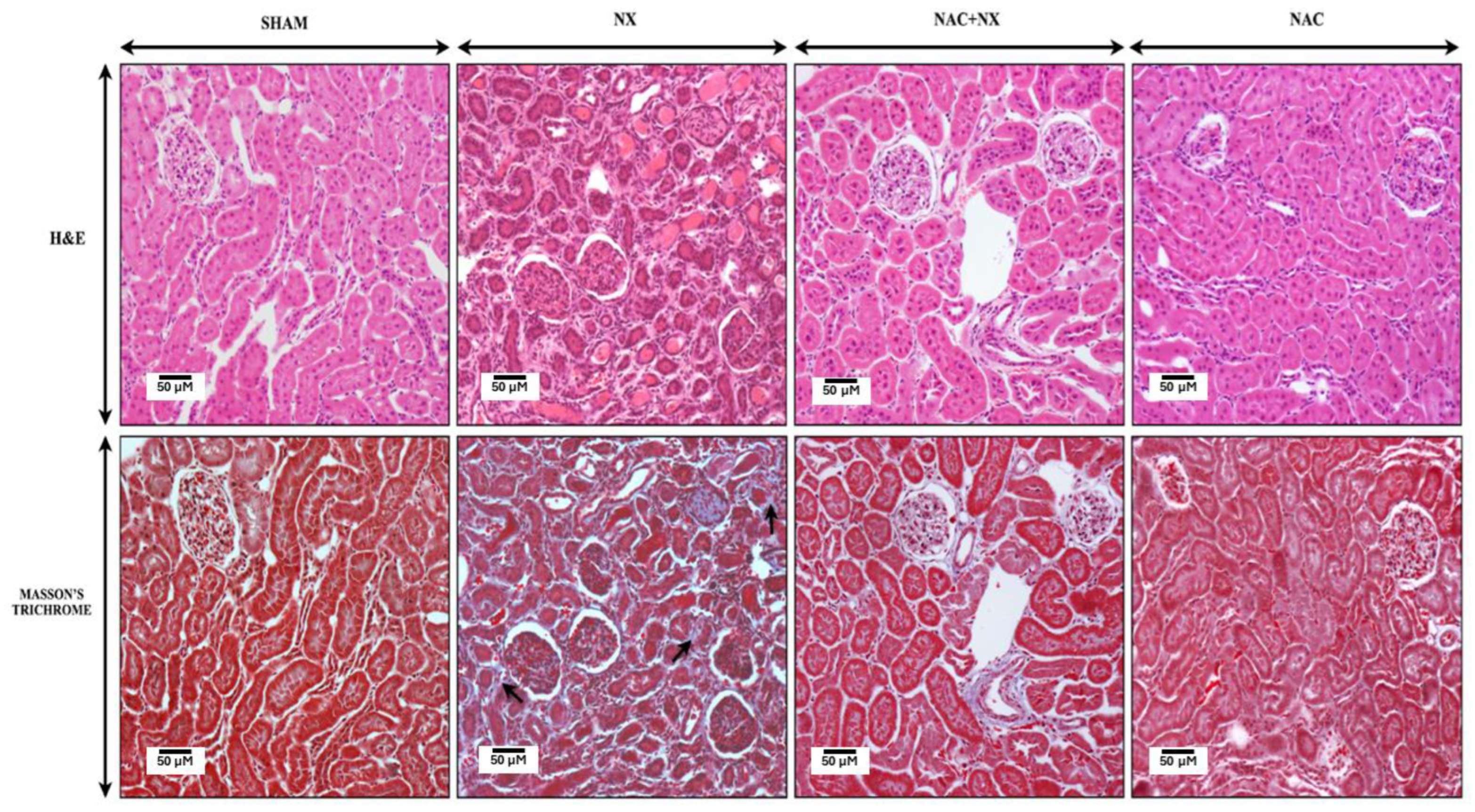

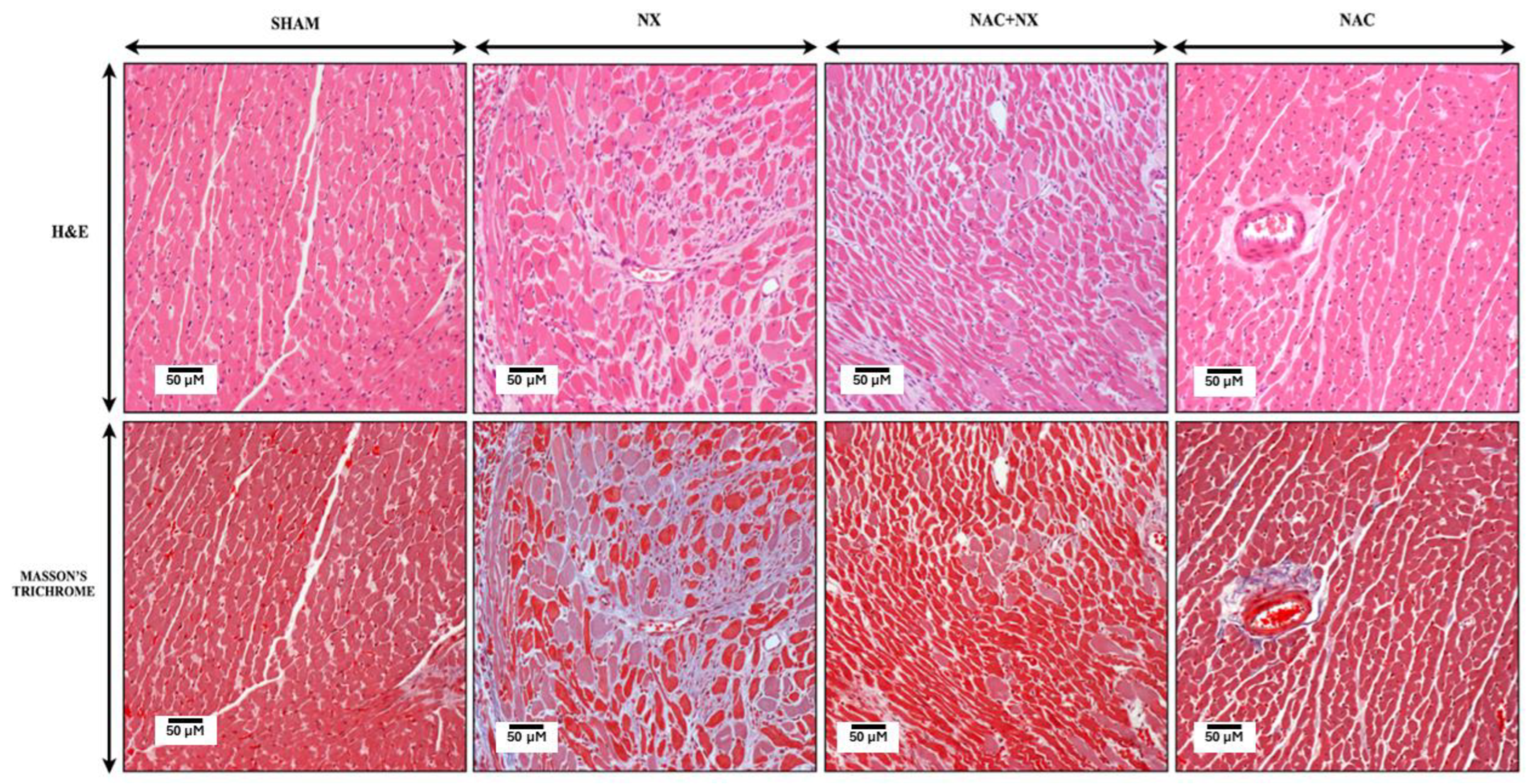
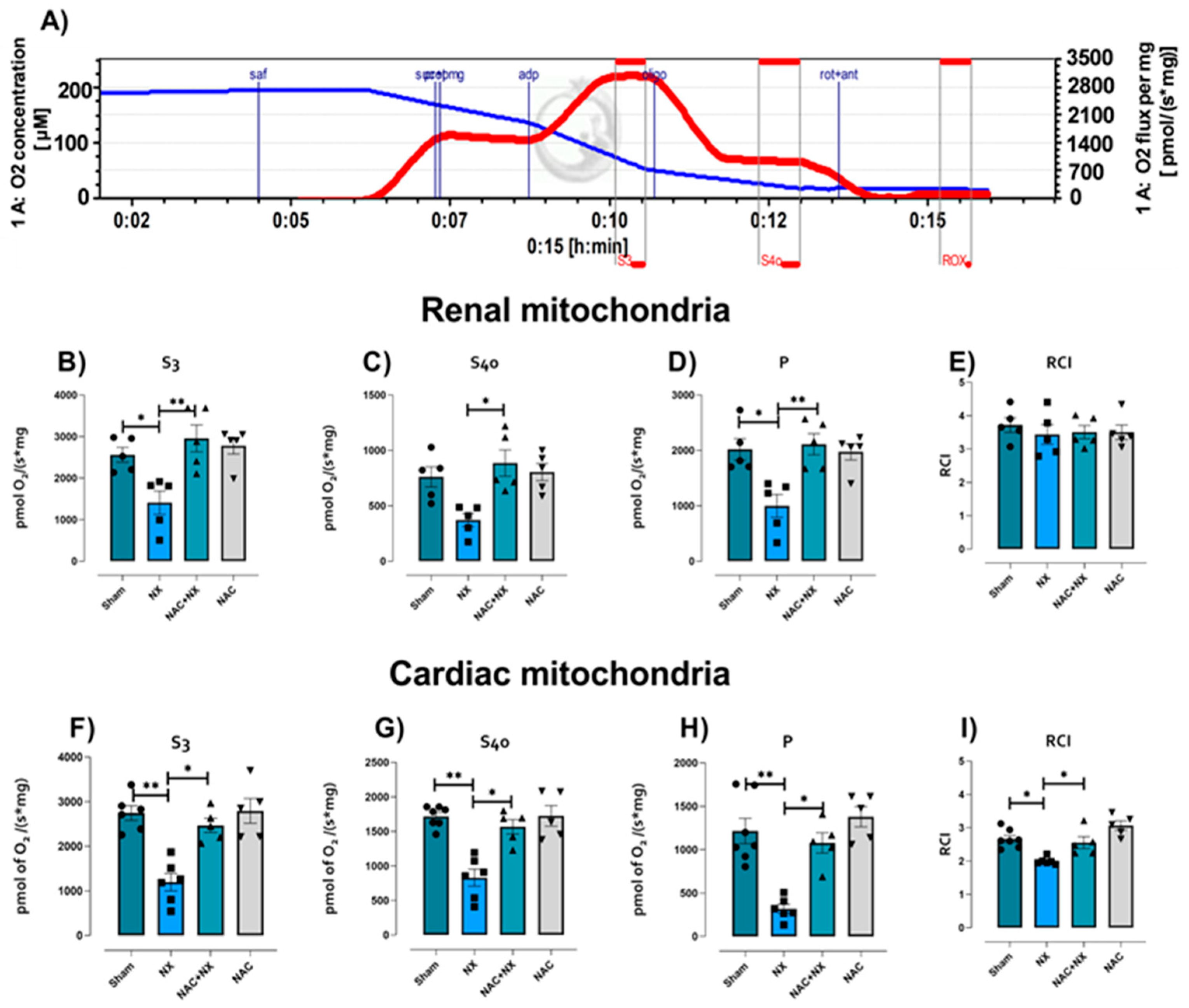
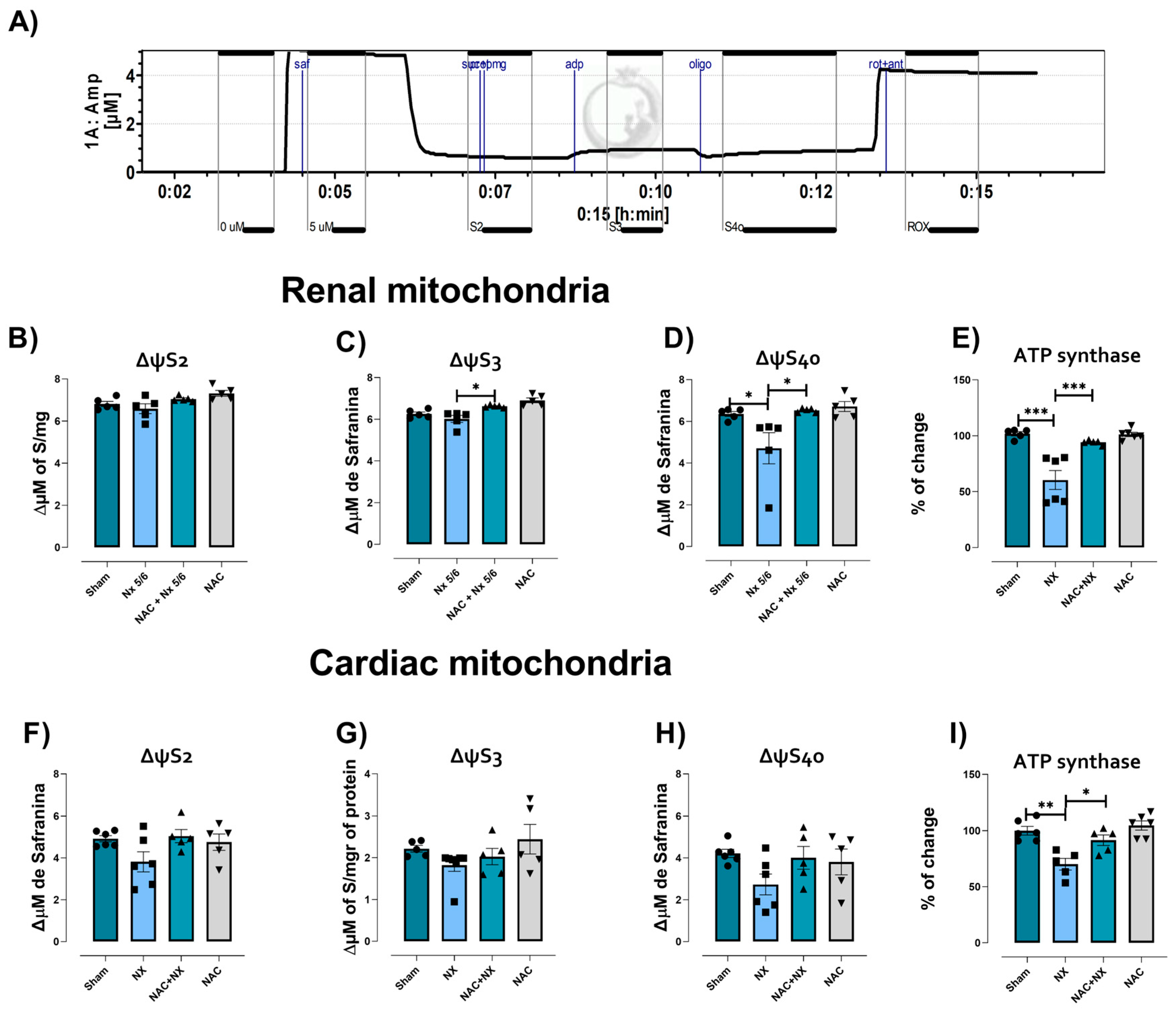
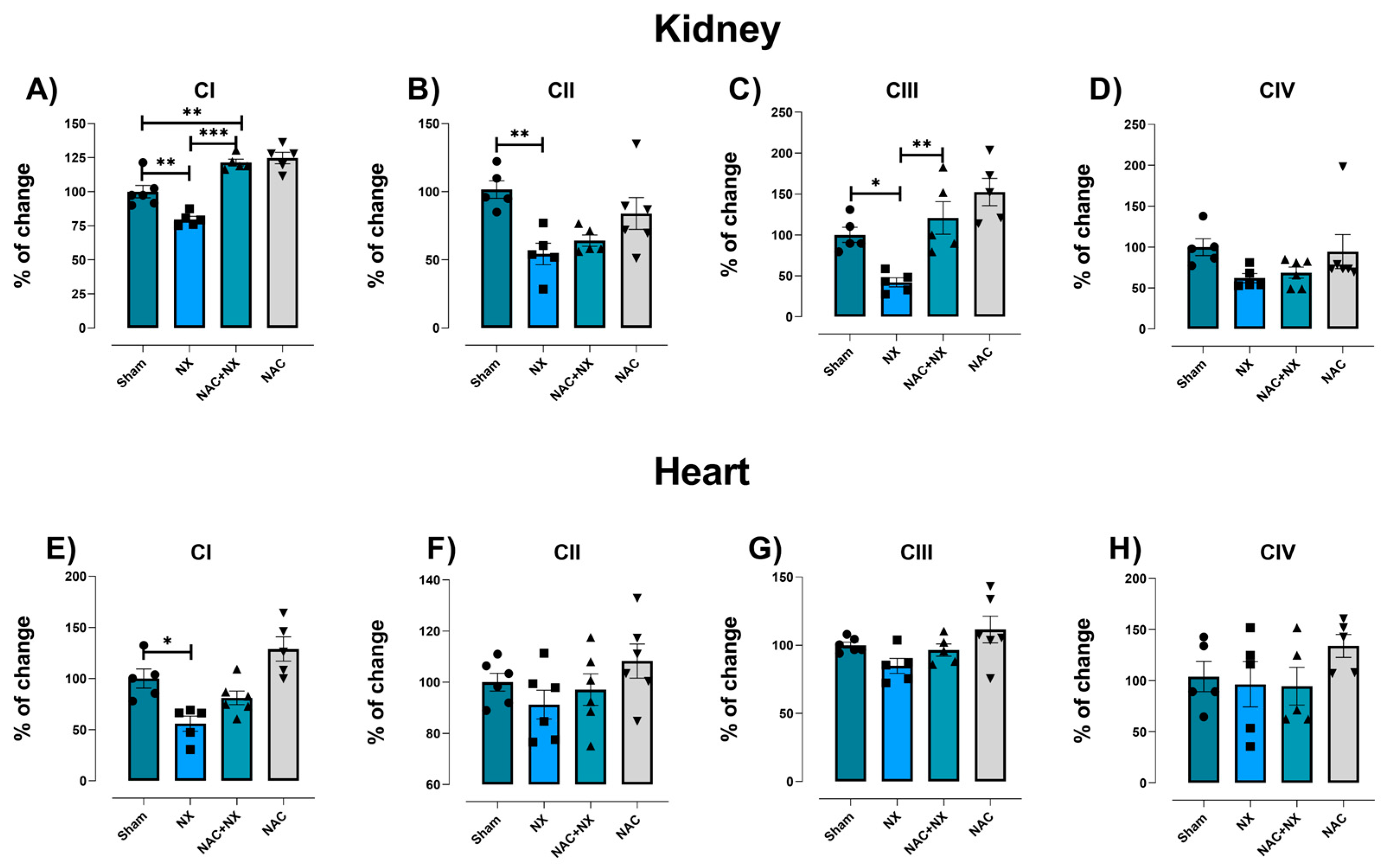
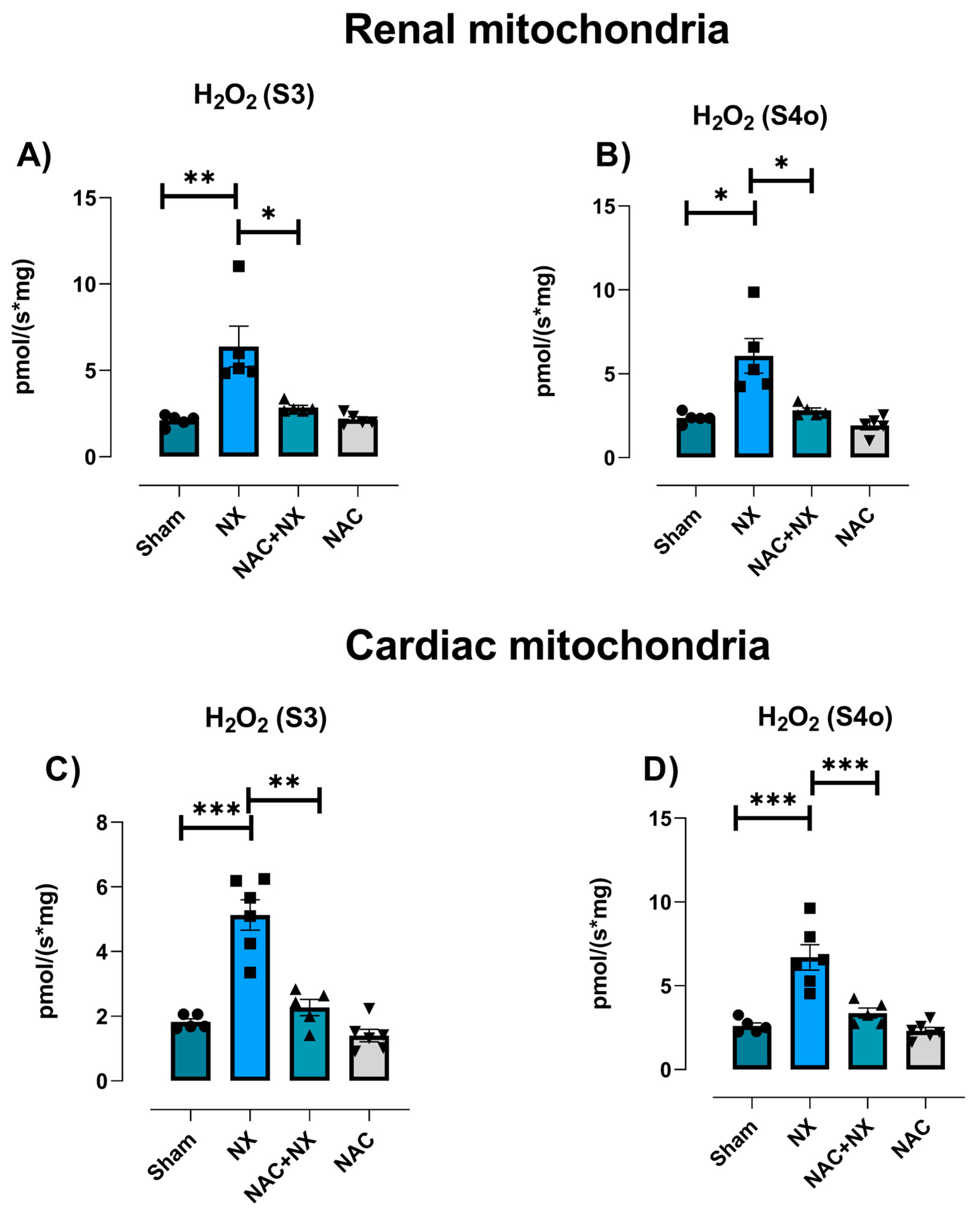
| Parameter | Sham | NX | NAC+NX | NAC |
|---|---|---|---|---|
| BW (g) | 229 ± 19 | 242 ± 9.9 | 235 ± 18 | 249 ± 25 |
| HW (g) | 0.93 ± 0.13 | 1.2 ± 0.13 * | 1.2 ± 0.11 * | 1.2 ± 0.14 * |
| LW (g) | 1.6 ± 0.39 | 2.1 ± 0.17 | 2.1 ± 0.32 | 2.2 ± 0.55 |
| KW (g) | 0.89 ± 0.09 | 1.2 ± 0.18 * | 1.2 ± 0.12 * | 1 ± 0.11 |
| HW/BW (g/Kg) | 4 ± 0.27 | 4.9 ± 0.37 | 5.1 ± 0.56 * | 4.7 ± 0.67 |
| LW/BW (g/Kg) | 7.1 ± 1.6 | 8.8 ± 0.71 | 9.1 ± 1.6 | 8.8 ± 2.6 |
| KW/BW (g/Kg) | 3.9 ± 0.48 | 5 ± 0.67 * | 5.1 ± 0.65 * | 4.2 ± 0.17 ** |
| HW/TL (g/cm) | 0.19 ± 0.03 | 0.25 ± 0.02 * | 0.25 ± 0.03 * | 0.27 ± 0.04 * |
| LW/TL (g/cm) | 0.34 ± 0.08 | 0.45 ± 0.04 | 0.45 ± 0.07 | 0.49 ± 0.14 * |
| KW/TL (g/cm) | 0.18 ± 0.02 | 0.25 ± 0.03 * | 0.25 ± 0.03 * | 0.24 ± 0.01 * |
| Parameter | Sham | NX | NAC+NX | NAC |
|---|---|---|---|---|
| HR (bpm) | 355.9 ± 19.3 | 365.2 ± 23.5 | 391 ± 24.6 | 365.9 ± 28.9 |
| IVS;d (mm) | 0.9 ± 0.1 | 1.1 ± 0.2 | 1.1 ± 0.3 | 1.1 ± 0.3 |
| LVID;d (mm) | 6.9 ± 0.8 | 6.7 ± 0.7 | 6 ± 0.6 | 6.8 ± 0.7 |
| LVPW;d (mm) | 1.5 ± 0.3 | 1.2 ± 0.2 | 1.3 ± 0.3 | 1.2 ± 0.1 |
| FS (%) | 57.8 ± 14.2 | 38.5 ± 7.9 * | 52 ± 9.9 *** | 52.1 ± 5.5 |
| EDV (µL) | 253.5 ± 65.9 | 236.2 ± 52.4 | 181.7 ± 39.9 | 245.6 ± 52.9 |
| ESV (µL) | 42.4 ± 37.1 | 78.2 ± 28.3 | 35.3 ± 17.5 *** | 48.3 ± 18.8 |
| EF (%) | 84.3 ± 10.1 | 66.5 ± 9.5 *,** | 80.1 ± 9.6 *** | 81.1 ± 4.6 |
| SV (µL) | 211.1 ± 42.9 | 158 ± 44.1 | 146.4 ± 36.2 * | 197.3 ± 36 |
| CO (mL/min) | 76.7 ± 17.7 | 57.2 ± 15.4 | 57.5 ± 15.1 | 72 ± 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peralta-Buendía, K.; Cuevas-López, B.; García-Arroyo, F.E.; Díaz-Rojas, M.; León-Contreras, J.C.; Silva-Palacios, A.; Gonzaga, G.; Tapia, E.; Saavedra, E.; Hernández-Pando, R.; et al. Early Administration of N-Acetylcysteine Provides Renal and Cardiac Mitochondrial and Redox Protection, Preventing the Development of Cardio-Renal Syndrome Type IV Induced by 5/6NX. Antioxidants 2025, 14, 1241. https://doi.org/10.3390/antiox14101241
Peralta-Buendía K, Cuevas-López B, García-Arroyo FE, Díaz-Rojas M, León-Contreras JC, Silva-Palacios A, Gonzaga G, Tapia E, Saavedra E, Hernández-Pando R, et al. Early Administration of N-Acetylcysteine Provides Renal and Cardiac Mitochondrial and Redox Protection, Preventing the Development of Cardio-Renal Syndrome Type IV Induced by 5/6NX. Antioxidants. 2025; 14(10):1241. https://doi.org/10.3390/antiox14101241
Chicago/Turabian StylePeralta-Buendía, Karen, Belén Cuevas-López, Fernando E. García-Arroyo, Miriam Díaz-Rojas, Juan Carlos León-Contreras, Alejandro Silva-Palacios, Guillermo Gonzaga, Edilia Tapia, Emma Saavedra, Rogelio Hernández-Pando, and et al. 2025. "Early Administration of N-Acetylcysteine Provides Renal and Cardiac Mitochondrial and Redox Protection, Preventing the Development of Cardio-Renal Syndrome Type IV Induced by 5/6NX" Antioxidants 14, no. 10: 1241. https://doi.org/10.3390/antiox14101241
APA StylePeralta-Buendía, K., Cuevas-López, B., García-Arroyo, F. E., Díaz-Rojas, M., León-Contreras, J. C., Silva-Palacios, A., Gonzaga, G., Tapia, E., Saavedra, E., Hernández-Pando, R., Pedraza-Chaverri, J., Sánchez-Lozada, L. G., & Aparicio-Trejo, O. E. (2025). Early Administration of N-Acetylcysteine Provides Renal and Cardiac Mitochondrial and Redox Protection, Preventing the Development of Cardio-Renal Syndrome Type IV Induced by 5/6NX. Antioxidants, 14(10), 1241. https://doi.org/10.3390/antiox14101241









