Abstract
Estrogen deficiency is associated with endothelial dysfunction, vascular inflammation, increased lipoprotein oxidation, accumulation of lipid-rich material, and platelet activation. The absence of estrogen causes physiological, metabolic, and biochemical changes that increase the risk of cardiometabolic disease development caused by a deregulation in metabolic processes such as lipid metabolism and plasma lipoprotein levels. High-density lipoprotein (HDL) has cardioprotective properties related to the quality and the quantity of its components that can be modified by some nutritional factors. Guava (Psidium guajava L.), a widely cultivated fruit in Mexico, is notable for its high polyunsaturated fatty acid and dietary fiber content in its seeds, but its effect on health is understudied. This study aimed to evaluate the effect of guava-seed supplementation on body weight, blood pressure, lipid profile, HDL composition, and paraoxonase-1 (PON1) activity in an ovariectomized rat model (OVX). Four groups with six adult female Wistar rats each were classified as a SHAM group: rats with simulated ovariectomy; OVX group: rats with ovariectomy; OVX + GS group: ovariectomized rats supplemented with 6 g of guava seeds; OVX + DGS group: ovariectomized rats supplemented with 6 g of defatted guava seeds. Biochemical parameters, size, and lipid concentration of HDL subclasses, apolipoproteins, and PON1 activity were determined. A decrease in body weight gain, systolic blood pressure, mean arterial pressure, and triglycerides in plasma was observed at the end of the experiment in the supplemented groups. The supplementation of 6 g of guava seeds for 30 days decreased biochemical parameters in ovariectomized rats; these results could be attributed to the seed composition, suggesting a protective effect against the risk of developing diseases in menopausal states.
1. Introduction
The substantial reduction and deficiency in circulating levels of estrogen affects almost all organ systems, including the cardiovascular, immune, and digestive systems. In this context, menopause is characterized by depletion of the oocytes stored in the ovaries and a significant decrease in estrogen levels. Menopausal women experience significant physiological, metabolic, and biochemical changes such as an increase in total cholesterol, triglycerides, and low-density lipoprotein cholesterol (LDL-c), and a decrease in high-density lipoprotein cholesterol (HDL-c), as well as a shift in the distribution of lipid composition [1]. In this sense, estrogens and other ovarian hormones possess cardioprotective properties and play a critical regulatory role in glucose metabolism, lipid homeostasis, and inflammatory pathways [2,3], including insulin sensitivity, blood pressure, and prothrombotic processes [4].
The decrease in estrogen secretion leads to a reduced capacity of HDL to efflux cholesterol, which is mainly related to HDL core and surface lipid remodeling (free cholesterol, triglycerides, phospholipids, and sphingolipids), which has been described in women during menopause, suggesting indirect effects of estrogen signaling pathways on the lipid profile [5,6]. The lipidome and proteome have also been described to be altered with estrogen deficiency; this could potentially compromise the other cardioprotective properties of HDL. Therefore, this suggests that HDL may become dysfunctional when circulating estrogen levels are reduced, such as in a state of menopause, affecting their quality more than the quantity of these lipoproteins [7,8]. On the other hand, some nutritional factors and bioactive compounds have been shown to influence the metabolism of estrogen, HDL structure and composition, and cardiovascular health [9,10], so lowering cardiovascular risk factors during and after menopause through diet intervention is possible.
Guava (Psidium guajava L.) is a fruit from the Myrtaceae family and is widely cultivated in Mexico, ranking third worldwide for production [11]. During the processing of this fruit, pulp is obtained as the main product and seeds and peels as by-products [12]. Due to the presence of bioactive compounds, the pulp and the by-products have the potential to be used in healthy processed foods [13]. Guava is notable for its high vitamin C content, as well as for its seeds, for their polyunsaturated fatty acid (PUFAs) and dietary fiber content [14]. Guava seeds and oil have been characterized; however, their effect on health remains understudied. Significant improvements in lipid profile were also reported: total cholesterol, LDL-c, and triglycerides decreased, while HDL-c increased in diabetic mice supplemented with guava seed extract [15]. Also, guava seed oil has been shown to have antioxidant activity [16], anti-LDL peroxidation, and Gram-negative-bacteria inhibitory activity [17,18].
Therefore, in the present study, we aimed to evaluate the effect of guava-seed supplementation on body weight, blood pressure, lipid profile, HDL composition, and PON1 activity in an ovariectomized rat model.
2. Materials and Methods
2.1. Animals
Twenty-four adult female Wistar rats, weighing 200–250 g and aged 3 months, were maintained under temperature-controlled conditions with light–dark cycles (12 h) and received commercial feed (LabDiet 5008®, LabDiet, Richmond, IN, USA) and water ad libitum. Four groups were formed, with n = 6 experimental animals in each group, classified as follows: SHAM group: negative control, rats with simulated ovariectomy; OVX group: positive control, rats with ovariectomy; OVX + GS group: rats supplemented with 6 g of guava seeds; OVX + DGS group: rats supplemented with 6 g of defatted guava seeds. The treatment groups (OVX + GS and OVX + DGS) followed the same procedure as the OVX group and received a supplement of guava seeds prepared as described above and mixed with commercial LabDiet 5008 rat food in a 70–30% ratio (14 g of commercial food + 6 g of guava seeds) in pellet form for 30 days. The experimental protocols were carried out according to the Guidelines for the Protection of Animals Used for Scientific Purposes (2010/63/EU) and National Guidelines (NOM-O62-ZOO-1999) [19] and were approved by the Animal Ethics Committee of the Universidad Autónoma del Estado de Hidalgo (CICUAL/-V-I/02/2025).
2.2. Ovariectomy
To remove the ovaries, a dorsal ovariectomy was performed in which the rats were anesthetized for surgery using a combination of xylazine hydrochloride (2.5 mg/kg of body weight) and Zelazol® (Zoetis, Mexico City, Mexico) (10 mg/kg of body weight). To remove the ovaries, an incision was made on the left side of the vertebral column of the animal, the uterine horns were identified, fixed to the ovary at one end and to the uterus at the other. Ligatures were established on both sides of the ovary, sectioning and removing them. Once the ovaries were removed, the incision was sutured. The SHAM group underwent the same procedure, but the ovaries were not removed [20,21]. After surgery, meloxicam (0.3 mg/kg of body weight) was administered for 5 days for pain management.
2.3. Guava Seeds
For the supplement, ripe, undamaged guavas were obtained from a local market in the city of Pachuca, Hidalgo, which are harvested in the state of Michoacan. The fruits were washed and disinfected to remove the seeds. The guava seeds were washed with purified water to eliminate any pulp that could remain attached. Once clean, the guava seeds were air-dried at room temperature for 24 h and ground in a commercial coffee grinder; a portion was used in the group OVX + GS and stored until use in darkness at room temperature.
2.4. Seed Delipidation
The rest of the ground seeds were used for lipid extraction with dichloromethane at a ratio of 1:10 (w/v), and the mixture was stirred at 100 rpm/35 °C for 8 h. Following this, the mixture was centrifuged at 5000 rpm for 30 min to separate the solvent and then dried in an oven at 60 °C for 40 min to remove the solvent entirely, and stored for the group OVX + DGS.
2.5. Blood Pressure
Blood pressure levels were measured in the tail of rats after heating by a non-invasive method, and recorded in a CODA TM system from Kent Scientific Corporation (Torrington, CT, USA); this was performed with at least six blood pressure recordings for each animal, and the data were collected using CODA TM version 4.1 software for further processing.
2.6. Lipid Profile
Total cholesterol, triglycerides, and phospholipids levels in plasma, as well as HDL-c, HDL triglycerides (HDL-Tg), and HDL phospholipids (HDL-PPL) levels, were determined using commercial colorimetric enzymatic methods (Randox®, Crumlin, UK; Wako, Ltd., Osaka, Japan). Absorbance was read at a wavelength of 505 nm for cholesterol and triglycerides and 600 nm for phospholipids in a spectrophotometer (BECKMAN COULTER DU® UV/Vis Spectrophotometer, Krefeld, Germany). For the lipid composition of HDL, we used the phase containing the HDL fraction of plasma after ultracentrifugation, following the methodology described below.
2.7. HDL Size and Lipid Concentration of HDL Subclasses
HDL particles were separated via sequential ultracentrifugation in a Beckman Optima TLX table (Indianapolis, IN, USA) [22,23,24,25]. Total apo-B-containing lipoproteins were obtained by density < 1.063 mg/dL, whereas total HDL was 1.063 < density < 1.21 g/mL. The HDL particles were dialyzed against 0.09 M Tris/0.08 M boric acid/3 mM EDTA (TBE) buffer, pH 8.4. Then, HDL particles were separated further by their hydrodynamic diameters in non-denaturing 3–30% gradient polyacrylamide gel electrophoresis (PAGE). Gels were stained for total cholesterol, phospholipids, and triglycerides using enzymatic mixtures previously described [26,27,28]. The electrophoresis gels were then scanned (Bio-Rad GS-670 densitometer, Hercules, CA, USA) and stained again for proteins with Coomassie Blue R-250, before being scanned once more. The relative proportions of each HDL subclass were estimated via optical densitometry analysis, using as reference globular proteins (thyroglobulin, 17 nm; ferritin, 12.2 nm; catalase, 10.4 nm; lactate dehydrogenase, 8.2 nm; albumin, 7.1 nm; high-molecular-weight calibration kit, Amersham Pharmacia Biotech, Buckinghamshire, UK) [24,28]. The relative proportion of every HDL subclass is expressed as the percentage of the total HDL area under the curve, integrated from 7.9 to 12.36 nm. For the classification of the HDL subclasses, we considered the following size intervals: HDL3c, 7.94–8.45 nm; HDL3b, 8.45–8.98 nm; HDL3a, 8.98–9.94 nm; HDL2a, 9.94–10.58 nm; and HDL2b, 10.58–13.59 nm [28]. Enzymatic staining of total cholesterol, triglycerides, and phospholipids of HDL subclasses was performed according to the methodology previously described [27].
2.8. Determination of Apolipoproteins
Apolipoprotein (apo) composition was determined on an SDS-denaturing 4–21% PAGE gradient. Fifteen micrograms of previously isolated HDL protein was used. The bands of each apo were stained with Coomassie Blue and destained with a solution of methanol, acetic acid, and water (30/58/12, v/v/v). The apos were identified by comparison with molecular mass standards and subsequently analyzed by optical densitometry. The results are expressed as the percentage of each apo relative to total HDL protein. The apos CI and CII are expressed as a percentage of apo C [29].
2.9. Paraoxonase-1 Activity
PON1 was determined using the method described by Gan et al. [30]. The trial mixture included 1 mM phenylacetate as substrate and 0.9 mM CaCl2 in 20 mM Tris HCl, pH 8, and 10 μL of rat plasma heparin (diluted 1:10). Enzymatic hydrolysis of the substrate was measured spectrophotometrically at 270 nm (UVVIS Beckman Coulter, Brea, CA, USA). The absorbance at 270 nm for the reaction was 1310 M−1 cm−1. Enzyme activity was expressed as the number of micromoles of phenylacetate hydrolyzed per minute per milliliter of plasma.
2.10. Data Analysis
Normal distribution of all data was verified using the Shapiro–Wilk test. Since these data were normally distributed, they are presented as mean ± standard deviation (SD). Differences between groups were determined using analysis of variance (ANOVA) and post hoc analysis (Tukey’s test). A p-value < 0.05 was considered statistically significant. All analyses were performed using SPSS version 24 software (SPSS Inc. IBM, Chicago, IL, USA).
3. Results
3.1. Biochemical Parameters
Table 1 shows the biochemical changes observed after supplementation, with a final body weight decrease of 50% in the OVX + GS group compared to the OVX group. The OVX + DGS group showed a significantly lower blood pressure level than the OVX group. In the same direction, plasma glucose levels decreased with guava-seed supplementation: 38.2% in the OVX + GS group and 57% in the OVX + DSG. Concerning the results of the lipid profile in plasma of the supplemented groups compared to the OVX group, the OVX + DGS group had 48.4% fewer triglycerides and 40.2% fewer phospholipids, whereas in the OVX + GS group, there were significant reductions of 32% for triglycerides and 25.7% for phospholipids. In addition, there was a significant increase of 68.9% in non-HDL-c in the OVX + DGS group vs. the OVX + GS group.

Table 1.
Biochemical parameters post-supplementation in rats.
HDL particles mainly contain phospholipids, free cholesterol, esterified cholesterol, and triglycerides. In this context, our results showed that supplementation with guava seed was significantly associated with a 60% reduction in c-HDL plasma levels in the OVX + DGS group than in the other two groups. We further determined the HDL-Tg/HDL-PPL and HDL-c/HDL-PPL ratios as markers of HDL lipid composition [26]. The results of the OVX + DGS group showed a significant reduction of 20.5% in the HDL-c/HDL-PPL ratio after supplementation (Table 2).

Table 2.
HDL lipid profile post-supplementation in rats.
3.2. Size and Lipid Composition of HDL Subclasses
HDL is a mixture of different particles, which, depending on their lipid and protein composition, may impact the biological function and structure of these lipoproteins. Thereby, after 30 days of supplementation, changes were found in the size and composition of HDL subclasses. Significant reduction was observed in the protein composition of the HDL3c subclass in the OVX + DGS group compared to the OVX + GS group (Table 3). Moreover, an increase in cholesterol was found in the OVX + DGS group in the small subclasses 3b and 3c compared to the OVX group. According to the composition of HDL triglycerides and phospholipids, no significant differences were observed between groups, except for an increase in phospholipids in the OVX + DGS group compared with the OVX + GS group.

Table 3.
Size and composition of HDL subclasses.
3.3. Apolipoproteins
The apolipoprotein composition (protein fraction of HDL particles) in the experimental groups is listed in Table 4, showing a significant decrease in apo E content and a significant increase in apo C concentration in the OVX + DGS group compared to the other test groups.

Table 4.
Apolipoprotein percentage of the total HDL protein.
3.4. PON1 Activity
Finally, the antioxidant activity of PON1-dependent HDL is modified by the structural and chemical composition of these lipoproteins. Therefore, we determined the antioxidant activity of PON1. Interestingly, we found a significant decrease in the OVX + DGS group compared to the other test groups. Likewise, there was a significant decrease in the OVX group compared to the SHAM group (Figure 1).
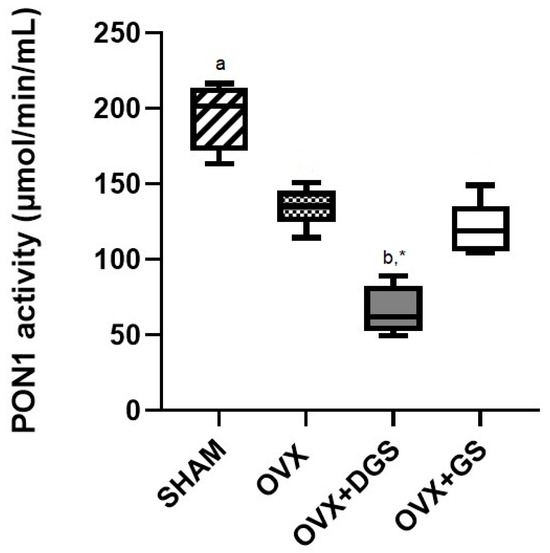
Figure 1.
Paraoxonase-1 activity in rat plasma. Data are expressed as median (horizontal lines) and interquartile range (boxes). n = 6. SHAM: SHAM-operated; OVX: ovariectomized; OVX + DGS: ovariectomized + defatted guava seeds; OVX + GS: ovariectomized + guava seeds. Tukey’s test (p < 0.05). OVX vs. a SHAM; OVX vs. b OVX + DGS; OVX + GS vs. * OVX + DGS.
4. Discussion
In the present study, we determined the effect of guava seed consumption in an ovariectomized animal model by simulating the biological processes due to a reduction in estrogen levels. In this model, estrogen depletion is related to metabolic effects and significant changes in body composition, including visceral fat accumulation, glucose and lipid metabolism disorders, and increased blood pressure, factors that significantly influence the high risk of developing cardiovascular diseases (CVD) [31].
An increase in body mass was observed in ovariectomized rats; nevertheless, a lower increase in body weight was observed in OVX + GS rats. A similar effect to this study was also found after administration of chia seed powder [32], as well as in the supplementation of pumpkin seed extract—high in omega-6 content [33]; in both studies, the effects are attributed to the high content of PUFAs that can help to reduce obesity by ameliorating oxidative stress, suppressing appetite, improving lipid oxidation and energy expenditure, as well as reducing fat deposition [32,33]. Therefore, in this work, PUFAs also could be responsible for the reduction in weight gain, given that the group supplemented with defatted seeds did not show significant differences compared to the ovariectomized group without supplementation.
In this sense, it has been mentioned that the composition of guava seed is highlighted by a content of fatty acids: 79% linoleic, 8% palmitic, 7% oleic, and 5% stearic [14]. Phytosterols have also been reported in guava seed oil, predominantly β-sitosterol (297.61 mg/100 g), stigmasterol (0.22 mg/100 g), and campesterol (11.04 mg/100 g) [16]. Also, they contain between 64 and 67% total dietary fiber, which comprises ~0.4% soluble dietary fiber, while insoluble dietary fiber covers most with 46–63% [14,34,35]. The last component may have an impact on improving blood pressure, but the mechanisms are not totally clear. Nevertheless, it has been suggested that these mechanisms could include a reduction in inflammation levels [36,37] and an improvement in endothelial function [38]. Additionally, studies have shown that long-term consumption of high-fiber diets improves glucose tolerance. Cellulose has been shown to inhibit starch digestion by binding to α-amylase [39], reducing glucose absorption, enhancing insulin sensitivity, and, as a result, lowering the risk of hypertension [40].
On the other hand, in the oil extraction process, a by-product obtained is protein paste. After the seeds were defatted, the guava seed paste had a higher content of protein (9.9% d.b.) and essential and nonessential amino acids (mainly leucine, arginine, glutamic acid, proline, among others), 12.0% (d.b.) of carbohydrate, a lower fat content (2.4% d.b.) and the highest values of raw fiber (69.3% d.b.) [41,42], this composition may be associated with the biological effects found in this study.
Plasma lipid depletion in rats from the OVX + DGS and OVX + GS groups suggests a reduction in hepatic cholesterol and triglyceride synthesis [43], as well as an increase in intestinal transit by insoluble fibers, which can create a physical barrier and, as a result, a decrease in lipid absorption [44].
Our results of the composition of HDL subclasses show a decrease in HDL 3c size in the OVX + DGS group in contrast to the OVX + GS group. In addition, there was an increase in the concentration of cholesterol in the smaller subclasses (HDL 3b and 3c) with respect to the OVX group, without observing significant changes in the composition of triglycerides between groups. A larger number of small HDL particles and their cholesterol efflux capacity could be related to the lowest HDL-c levels in the OVX + DGS group. Dietary intake is an important and modifiable factor that directly provides lipid precursors and modulates metabolic pathways. In particular, fatty acids and lipid-rich foods significantly alter lipid profile by changing the composition and metabolism of various species of lipids, such as triglycerides, sphingolipids, and PUFAs [45,46]. Also, diseases characterized by increased inflammatory processes are associated with changes in the lipidome of HDL, particularly a decrease in HDL phospholipid content and an increase in HDL triglycerides [47]. In a clinical study of menopausal women, HDL subfractions were evaluated; the results showed that, during the first 2 years after the decrease in estrogen secretion, the concentration and size of HDL2 subclasses decreased, while HDL3 and HDL-Tg increased [48]. There is controversy regarding whether the larger particles (HDL2) or the smaller ones (HDL3) are more atheroprotective. It has been suggested that the large HDL subclass is more prone to oxidative modification with respect to the small subclasses [49], whereas HDL3 plays a central role in the reverse cholesterol transport (RCT) by removing cholesterol from the periphery and maturing into HDL2 particles through progressive lipidation by the action of lecithin–cholesterol–acyltransferase (LCAT) [50].
Apolipoproteins are the most abundant group of proteins in HDL. In this work, the OVX + DGS group obtained the highest content of apo C and the lowest content of apo E in contrast to the other groups. Apo E is considered an atheroprotective protein because it removes more saturated than unsaturated lipids, and atherogenic plaques are rich in saturated lipids and cholesterol [51]. Likewise, apo E genotypes are viewed as key genetic determinants of inter-individual variations in postprandial lipemia. This interaction depends on the presence of certain fatty acids, such as PUFAs and phospholipids [52]. This is consistent with the significant changes observed in relation to the decrease in phospholipids and increase in non-HDL-c results in the plasma of this work. Furthermore, HDL particles containing apo E promote the efflux of cholesterol from extrahepatic cells [53] through ABCA1- and ABCG1-dependent processes, and this process is antagonized by the presence of apo CIII, which can negatively affect the antiatherogenic properties of HDL [54,55]. In a study where supplementation with PUFAs in rats was evaluated, a decrease in apo CIII was found, indicating a possible effect of these compounds on apolipoprotein gene expression [56].
It has been shown in some animal models that PON1 can prevent the harmful effects of oxidative stress on serum, and that serum levels of PON1 correlate with levels of HDL and apolipoprotein AI, although this correlation is not strong. We might suggest that increased PON1 activity is a consequence of higher HDL levels. In our study, the lower value of PON1 activity in the ovariectomized groups compared to SHAM rats can be attributed to estrogens, given that estradiol enhances PON1 activity [57]. In addition, a pro-oxidative environment could lead to an increase in the binding of free radicals to PON1, resulting in a less active enzyme in circulation [58]. It has been reported that dietary factors have a significant effect on PON1 activity, specifically polyunsaturated fatty acids [59], which could be associated with higher activity in the OVX + GS group compared to the OVX + DGS group. Furthermore, according to the observed results of apo and PON activity, the OVX + DGS group could be a population at risk for the development of cardiovascular disease.
Differences shown between the OVX + DGS and OVX + GS groups are related to the lipid composition of the seed. When supplementing with a defatted seed, the protective properties previously described were lost, indicating that such properties are associated with polyunsaturated fatty acids [60]—and probably other lipidic molecules contained in the whole seed, such as phytosterols [61]—which have been reported to benefit health owing to their cholesterol-lowering and anti-inflammatory effects [62,63]. Studies on biomodels fed a high-fat diet have proven the efficacy of supplementation with defatted seeds such as safflower (50–100 mg/kg of weight/day) [64] and poppy (33% fiber and 27% protein) [65] on plasma and hepatic lipid levels, reducing triglyceride and cholesterol levels. In addition, it has been described that defatted grape seed affects the 3T-L1 pre-adipocyte cells, showing a significant decrease in lipid accumulation, possibly due to a regulation of the mRNA expression of leptin and lipoprotein lipase (LPL), both of which have been demonstrated to regulate lipid metabolism [66].
5. Conclusions
The supplementation of guava seeds during 30 days limited increases in body weight, blood pressure, glucose, triglycerides, and non-HDL-c in ovariectomized rats. These results could be attributed to lipidic components and insoluble dietary fiber in the seed, suggesting a discrete protective effect against the risk of developing diseases during physiological cessation of estrogen secretion. Limitations of this study include the lack of estrogen level measurements, which future research could address, and the need for additional studies in order to further understand the role of whole and defatted guava seeds.
Author Contributions
Conceptualization: E.C.-T. and D.E.-L.; investigation, E.C.-T., D.E.-L., L.M.R.-M. and A.C.-O.; writing—original draft preparation, E.C.-T., D.E.-L. and L.M.R.-M.; writing—review and editing, E.C.-T., D.E.-L., A.C.-O., Ó.P.-M. and E.F.-M.; funding acquisition, D.E.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Generic State Fund of Universidad Autónoma del Estado de Hidalgo, PAO 2025-[Amp] 0246-Desarrollo de Programa Educativo de Posgrado.
Institutional Review Board Statement
The experimental protocols were approved by the Animal Ethics Committee of the Autonomous University of the State of Hidalgo (CICUAL/-V-I/02/2025).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
Special gratitude is extended to the university’s animal facility employees for their support in the animal handling and surgery processes. Lisette Monsibaez Ramírez Melo is a doctoral student from Programa de Doctorado en Ciencias de los Alimentos y Salud Humana de la Universidad Autónoma del Estado de Hidalgo and received fellowship from SECIHTI, with scholarship number 832973.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HDL | High-density lipoprotein |
| PON1 | Paraoxonase-1 |
| LDL | Low-density lipoprotein |
| SHAM | SHAM-operated rats |
| OVX | Ovariectomized rats |
| DGS | Defatted guava seeds |
| GS | Guava seeds |
| HDL-c | HDL cholesterol |
| HDL-Tg | HDL triglycerides |
| HDL-PPL | HDL phospholipids |
| Apo | Apolipoprotein |
| SD | Standard deviation |
| CVD | Cardiovascular diseases |
| RCT | Reverse cholesterol transport |
| LCAT | Lecithin–cholesterol–acyltransferase |
| E2 | Estradiol |
| LPL | Lipoprotein lipase |
References
- Anagnostis, P.; Stevenson, J.C.; Crook, D.; Johnston, D.G.; Godsland, I.F. Effects of gender, age and menopausal status on serum apolipoprotein concentrations. Clin. Endocrinol. 2016, 85, 733–740. [Google Scholar] [CrossRef]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef]
- Gold, E.B. The timing of the age at which natural menopause occurs. Obstet. Gynecol. Clin. 2011, 38, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Salpeter, S.R.; Walsh, J.M.E.; Ormiston, T.M.; Greyber, E.; Buckley, N.S.; Salpeter, E.E. Meta-analysis: Effect of hormone-replacement therapy on components of the metabolic syndrome in postmenopausal women. Diabetes Obes. Metab. 2006, 8, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Kostara, C.E.; Bairaktari, E.T.; Tsimihodimos, V. Effect of clinical and laboratory parameters on HDL particle composition. Int. J. Mol. Sci. 2023, 24, 1995. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Luna, D.; Carreón-Torres, E.; Bautista-Pérez, R.; Betanzos-Cabrera, G.; Dorantes-Morales, A.; Luna-Luna, M.; Vargas-Barrón, J.; Mejía, A.M.; Fragoso, J.M.; Carvajal-Aguilera, K.; et al. Microencapsulated pomegranate reverts high-density lipoprotein (HDL)-induced endothelial dysfunction and reduces postprandial triglyceridemia in women with acute coronary syndrome. Nutrients 2019, 11, 1710. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Chen, X.; Nasr, A.; Billheimer, J.; Brooks, M.M.; McConnell, D.; Orchard, T.J.; Crawford, S.L.; Matthews, K.A.; Rader, D.J. HDL (high-density lipoprotein) subclasses, lipid content, and function trajectories across the menopause transition: SWAN-HDL study. Arter. Thromb. Vasc. Biol. 2021, 41, 951–961. [Google Scholar] [CrossRef]
- Lehti, S.; Korhonen, T.M.; Soliymani, R.; Ruhanen, H.; Lähteenmäki, E.I.; Palviainen, M.; Siljander, P.; Lalowski, M.; Käkelä, R.; Lehti, M.; et al. The lipidome and proteome of high-density lipoprotein are altered in menopause. J. Appl. Physiol. 2025, 139, 308–324. [Google Scholar] [CrossRef]
- Hall, D.C. Nutritional influences on estrogen metabolism. Appl. Nutr. Sci. Rep. 2001, 1, 1–8. [Google Scholar]
- Gomez-Delgado, F.; Katsiki, N.; Lopez-Miranda, J.; Perez-Martinez, P. Dietary habits, lipoprotein metabolism and cardiovascular disease: From individual foods to dietary patterns. Crit. Rev. Food Sci. Nut. 2021, 61, 1651–1669. [Google Scholar] [CrossRef]
- Secretaría de Agricultura y Desarrollo Rural (SADER). Garantiza Agricultura Producción y Abasto de Guayaba para esta Temporada Decembrina. 2022. Available online: https://www.gob.mx/agricultura/prensa/garantiza-agricultura-produccion-y-abasto-de-guayaba-para-esta-temporada-decembrina#:~:text=En%202021%2C%20Michoac%C3%A1n%20registr%C3%B3%2010,y%2029%20mil%20982%20toneladas (accessed on 30 June 2025).
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- da Silva Lima, R.; Ferreira, S.R.S.; Vitali, L.; Block, J.M. May the superfruit red guava and its processing waste be a potential ingredient in functional foods? Food Res. Int. 2019, 115, 451–459. [Google Scholar] [CrossRef]
- Uchôa-thomaz, A.M.A.; Sousa, E.C.; Carioca, J.O.B.; Morais, S.M.D.; Lima, A.D.; Martins, C.G.; Alexandrino, C.D.; Ferreira, P.A.T.; Rodrigues, A.L.M.; Rodrigues, S.P.; et al. Chemical composition, fatty acid profile and bioactive compounds of guava seeds (Psidium guajava L.). Food Sci. Technol. 2014, 34, 485–492. [Google Scholar] [CrossRef]
- Shabbir, H.; Kausar, T.; Noreen, S.; Rehman, H.U.; Hussain, A.; Huang, Q.; Gani, A.; Su, S.; Nawaz, A. In vivo screening and antidiabetic potential of polyphenol extracts from guava pulp, seeds and leaves. Animals 2020, 10, 1714. [Google Scholar] [CrossRef]
- Prommaban, A.; Utama-Ang, N.; Chaikitwattana, A.; Uthaipibull, C.; Porter, J.B.; Srichairatanakool, S. Phytosterol, lipid and phenolic composition, and biological activities of guava seed oil. Molecules 2020, 25, 2474. [Google Scholar] [CrossRef]
- Huang, H.Y.; Chang, C.K.; Tso, T.K.; Huang, J.J.; Chang, W.W.; Tsai, Y.C. Antioxidant activities of various fruits and vegetables produced in Taiwan. Int. J. Food Sci. Nutr. 2004, 55, 423–429. [Google Scholar] [CrossRef]
- Pelegrini, P.B.; Murad, A.M.; Silva, L.P.; Dos Santos, R.C.; Costa, F.T.; Tagliari, P.D.; Bloch, C., Jr.; Noronha, E.F.; Miller, R.N.G.; Franco, O.L. Identification of a novel storage glycine-rich peptide from guava (Psidium guajava) seeds with activity against Gram-negative bacteria. Peptides 2008, 29, 1271–1279. [Google Scholar] [CrossRef]
- Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación. NORMA Oficial Mexicana NOM-062-ZOO-1999, Especificaciones Técnicas para la Producción, Cuidado y uso de los Animales de Laboratorio. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 30 June 2025).
- Brower, G.L.; Gardner, J.D.; Janicki, J.S. Gender mediated cardiac protection from adverse ventricular remodeling is abolished by ovariectomy. Mol. Cell Biochem. 2003, 251, 89–95. [Google Scholar] [CrossRef]
- Wronsky, T.J. The ovariectomized rat as an animal model for postmenopausal bone loss. Cell Mater. 1992, 1, S69–S74. [Google Scholar]
- Williams, P.T.; Krauss, R.M.; Nichols, A.V.; Vranizan, K.M.; Wood, P.D. Identifying the predominant peak diameter of high-density and low-density lipoproteins by electrophoresis. J. Lipid Res. 1990, 31, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Carreón-Torres, E.; Juárez-Meavepeña, M.; Cardoso-Saldaña, G.; Gómez, C.H.; Franco, M.; Fievet, C.; Luc, G.; Juárez-Oropeza, M.A.; Pérez-Méndez, O. Pioglitazone increases the fractional catabolic and production rates of high-density lipoproteins apo AI in the New Zealand White Rabbit. Atherosclerosis 2005, 181, 233–240. [Google Scholar] [CrossRef]
- Warnick, G.R.; McNamara, J.R.; Boggess, C.N.; Clendenen, F.; Williams, P.T.; Landolt, C.C. Polyacrylamide gradient gel electrophoresis of lipoprotein subclasses. Clin. Lab. Med. 2006, 26, 803–846. [Google Scholar] [CrossRef] [PubMed]
- Huesca-Gómez, C.; Carreón-Torres, E.; Nepomuceno-Mejía, T.; Sánchez-Solorio, M.; Galicia-Hidalgo, M.; Mejía, A.M.; Montaño, L.; Franco, M.; Posadas-Romero, C.; Pérez-Méndez, O. Contribution of cholesteryl ester transfer protein and lecithin: Cholesterol acyltransferase to HDL size distribution. Endocr. Res. 2004, 30, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Juárez-Meavepeña, M.; Carreón-Torres, E.; López-Osorio, C.; García-Sánchez, C.; Gamboa, R.; Torres-Tamayo, M.; Fragoso, J.M.; Rodríguez-Pérez, J.M.; Vargas-Alarcón, G.; Pérez-Méndez, O. The Srb1+ 1050T allele is associated with metabolic syndrome in children but not with cholesteryl ester plasma concentrations of high-density lipoprotein subclasses. Metab. Syndr. Relat. Disord. 2012, 10, 110–116. [Google Scholar] [CrossRef]
- García-Sánchez, C.; Torres-Tamayo, M.; Juárez-Meavepeña, M.; López-Osorio, C.; Toledo-Ibelles, P.; Monter-Garrido, M.; Cruz-Robles, D.; Carreón-Torres, E.; Vargas-Alarcón, G.; Pérez-Méndez, O. Lipid plasma concentrations of HDL subclasses determined by enzymatic staining on polyacrylamide electrophoresis gels in children with metabolic syndrome. Clin. Chim. Acta 2011, 412, 292–298. [Google Scholar] [CrossRef]
- Toledo-Ibelles, P.; García-Sánchez, C.; Ávila-Vazzini, N.; Carreón-Torres, E.; Posadas-Romero, C.; Vargas-Alarcón, G.; Pérez-Méndez, O. Enzymatic assessment of cholesterol on electrophoresis gels for estimating HDL size distribution and plasma concentrations of HDL subclasses. J. Lipid Res. 2010, 51, 1610–1617. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Gan, K.N.; Smolen, A.; Eckerson, H.W.; La Du, B.N. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab. Dispos. 1991, 19, 100–106. [Google Scholar] [CrossRef]
- Litwak, S.A.; Wilson, J.L.; Chen, W.; Garcia-Rudaz, C.; Khaksari, M.; Cowley, M.A.; Enriori, P.J. Estradiol prevents fat accumulation and overcomes leptin resistance in female high-fat diet mice. Endocrinology 2014, 155, 4447–4460. [Google Scholar] [CrossRef]
- Barakat, S.M.; El-Malah, M.M.; EL-Masry, H.G.; Haggag, M.H. The Effect of ChiaSeeds (Salvia hispanica L.) on Osteoporosis of Ovariectomized Rats. Egypt. J. Nutr. 2022, 3, 129–168. [Google Scholar] [CrossRef]
- Oh, J.; Hong, S.; Ko, S.H.; Kim, H.S. Evaluation of Antioxidant Effects of Pumpkin (Cucurbita pepo L.) Seed Extract on Aging-and Menopause-Related Diseases Using Saos-2 Cells and Ovariectomized Rats. Antioxidants 2024, 13, 241. [Google Scholar] [CrossRef]
- Fontanari, G.G.; Souza, G.R.; Batistuti, J.P.; Neves, V.A.; Pastre, I.A.; Fertonani, F.L. DSC studies on protein isolate of guava seeds Psidium guajava. J. Therm. Anal. Calorim. 2008, 93, 397–402. [Google Scholar] [CrossRef]
- El Anany, A.M. Nutritional composition, antinutritional factors, bioactive compounds and antioxidant activity of guava seeds (Psidium Myrtaceae) as affected by roasting processes. J. Food Sci. Technol. 2015, 52, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Griffith, J.A.; Chasan-Taber, L.; Olendzki, B.C.; Jackson, E.; Stanek, E.J., III; Li, W.; Pagoto, S.L.; Hafner, A.R.; Ockene, I.S.; et al. Association between dietary fiber and serum C-reactive protein. Am. J. Clin. Nutr. 2006, 83, 760–766. [Google Scholar] [CrossRef]
- Krishnamurthy, V.M.R.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High dietary fiber intake is associated with decreased inflammation and all-cause mortality in patients with chronic kidney disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef]
- Brock, D.W.; Davis, C.K.; Irving, B.A.; Rodriguez, J.; Barrett, E.J.; Weltman, A.; Taylor, A.G.; Gaesser, G.A. A high-carbohydrate, high-fiber meal improves endothelial function in adults with the metabolic syndrome. Diabetes Care 2006, 29, 2313–2315. [Google Scholar] [CrossRef]
- Dhital, S.; Gidley, M.J.; Warren, F.J. Inhibition of α-amylase activity by cellulose: Kinetic analysis and nutritional implications. Carbohydr. Polym. 2015, 123, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Quesada, O.; Claggett, B.; Rodriguez, F.; Cai, J.; Moncrieft, A.E.; Garcia, K.; Rivera, M.D.R.; Hanna, D.B.; Daviglus, M.L.; Talavera, G.A.; et al. Associations of insulin resistance with systolic and diastolic blood pressure: A study from the HCHS/SOL. Hypertension 2021, 78, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Nicanor, A.B.; Moreno, A.O.; Ayala, A.M.; Ortíz, G.D. Guava seed protein isolate: Functional and nutritional characterization. J. Food Biochem. 2001, 25, 77–90. [Google Scholar] [CrossRef]
- Abdeldaiem, M.H.; Hoda, G.M.; Nasr, E.H. Antioxidant activity of extract from gamma irradiated guava (Psidium guajava L.) seeds. FSQM 2014, 26, 13–25. [Google Scholar]
- Macho-González, A.; Garcimartín, A.; Naes, F.; López-Oliva, M.E.; Amores-Arrojo, A.; González-Muñoz, M.J.; Bastida, S.; Benedi, J.; Sanchez-Muniz, F.J. Effects of fiber purified extract of carob fruit on fat digestion and postprandial lipemia in healthy rats. J. Agric. Food Chem. 2018, 66, 6734–6741. [Google Scholar] [CrossRef]
- Wu, W.C.; Inui, A.; Chen, C.Y. Weight loss induced by whole grain-rich diet is through a gut microbiota-independent mechanism. World J. Diabetes 2020, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Quehenberger, O.; Dennis, E.A. The human plasma lipidome. N. Engl. J. Med. 2011, 365, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Beloribi-Djefaflia, S.; Vasseur, S.; Guillaumond, F. Lipid metabolic reprogramming in cancer cells. Oncogenesis 2016, 5, e189. [Google Scholar] [CrossRef] [PubMed]
- Kajani, S.; Curley, S.; McGillicuddy, F.C. Unravelling HDL—looking beyond the cholesterol surface to the quality within. Int. J. Mol. Sci. 2018, 19, 1971. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Nasr, A.; Billheimer, J.; Brooks, M.M.; McConnell, D.; Crawford, S.; Orchard, T.J.; Rader, D.J.; A Matthews, K. Associations of endogenous hormones with HDL novel metrics across the menopause transition: The SWAN HDL study. J. Clin. Endocrinol. Metab. 2022, 107, e303–e314. [Google Scholar] [CrossRef]
- Camont, L.; Chapman, M.J.; Kontush, A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol. Med. 2011, 17, 594–603. [Google Scholar] [CrossRef]
- Martin, S.S.; Khokhar, A.A.; May, H.T.; Kulkarni, K.R.; Blaha, M.J.; Joshi, P.H.; Toth, P.P.; Muhlestein, J.B.; Anderson, J.L.; Knight, S.; et al. HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: The Lipoprotein Investigators Collaborative. Eur. Heart J. 2015, 36, 22–30. [Google Scholar] [CrossRef]
- Kiskis, J.; Fink, H.; Nyberg, L.; Thyr, J.; Li, J.Y.; Enejder, A. Plaque-associated lipids in Alzheimer’s disease brain tissue visualized by nonlinear microscopy. Sci. Rep. 2015, 5, 13489. [Google Scholar] [CrossRef]
- Liang, S.; Steffen, L.M.; Steffen, B.T.; Guan, W.; Weir, N.L.; Rich, S.S.; Manichaikul, A.; Vargas, J.D.; Tsai, M.Y. APOE genotype modifies the association between plasma omega-3 fatty acids and plasma lipids in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2013, 228, 181–187. [Google Scholar] [CrossRef]
- Kypreos, K.E.; Zannis, V.I. Pathway of biogenesis of apolipoprotein E-containing HDL in vivo with the participation of ABCA1 and LCAT. Biochem. J. 2007, 403, 359–367. [Google Scholar] [CrossRef]
- Morton, A.M.; Koch, M.; Mendivil, C.O.; Furtado, J.D.; Tjønneland, A.; Overvad, K.; Wang, L.; Jensen, M.K.; Sacks, F.M. Apolipoproteins E and CIII interact to regulate HDL metabolism and coronary heart disease risk. JCI Insight 2018, 3, e98045. [Google Scholar] [CrossRef]
- Jensen, M.K.; Aroner, S.A.; Mukamal, K.J.; Furtado, J.D.; Post, W.S.; Tsai, M.Y.; Tjønneland, A.; Polak, J.F.; Rimm, E.B.; Overvad, K.; et al. High-density lipoprotein subspecies defined by presence of apolipoprotein C-III and incident coronary heart disease in four cohorts. Circulation 2018, 137, 1364–1373. [Google Scholar] [CrossRef]
- Asset, G.; Staels, B.; Wolff, R.L.; Baugé, E.; Madj, Z.; Fruchart, J.C.; Dallongeville, J. Effects of Pinus pinaster and Pinus koraiensis seed oil supplementation on lipoprotein metabolism in the rat. Lipids 1999, 34, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Scott, J.E. Estradiol enhances cell-associated paraoxonase 1 (PON1) activity in vitro without altering PON1 expression. Biochem. Biophys. Res. Commun. 2010, 397, 441–446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parra, S.; Alonso-Villaverde, C.; Coll, B.; Ferré, N.; Marsillach, J.; Aragonès, G.; Mackness, M.; Mackness, B.; Masana, L.; Joven, J.; et al. Serum paraoxonase-1 activity and concentration are influenced by human immunodeficiency virus infection. Atherosclerosis 2007, 194, 175–181. [Google Scholar] [CrossRef]
- Calabresi, L.; Villa, B.; Canavesi, M.; Sirtori, C.R.; James, R.W.; Bernini, F.; Franceschini, G. An ω-3 polyunsaturated fatty acid concentrate increases plasma high-density lipoprotein 2 cholesterol and paraoxonase levels in patients with familial combined hyperlipidemia. Metabolism 2004, 53, 153–158. [Google Scholar] [CrossRef]
- Yokoyama, M.; Origasa, H.; Matsuzaki, M.; Matsuzawa, Y.; Saito, Y.; Ishikawa, Y.; Oikawa, S.; Sasaki, J.; Hishida, H.; Itakura, H.; et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet 2007, 369, 1090–1098. [Google Scholar] [CrossRef]
- Thiyagarajan, A.; Rathnasamy, V.K.; Veerasamy, B.; Sangeetha, V.S.; Vellaichamy, J.; Subbian, M. Guava (Psidium guajava L.) Seed: A Review on Nutritional Profile, Bioactive Compounds, Functional Food Properties, Health Benefits and Industrial Applications. Fresenius Environ. Bull. 2024, 33, 703–710. [Google Scholar]
- Ikeda, I. Factors affecting intestinal absorption of cholesterol and plant sterols and stanols. J. Oleo Sci. 2015, 64, 9–18. [Google Scholar] [CrossRef]
- Mohamed, D.; Mohammed, S.; Hamed, I. Chia seeds oil enriched with phytosterols and mucilage as a cardioprotective dietary supplement towards inflammation, oxidative stress, and dyslipidemia. J. Herbmed Pharmacol. 2021, 11, 83–90. [Google Scholar] [CrossRef]
- Hwang, E.Y.; Yu, M.H.; Jung, Y.S.; Lee, S.P.; Shon, J.H.; Lee, S.O. Defatted safflower seed extract inhibits adipogenesis in 3T3-L1 preadipocytes and improves lipid profiles in C57BL/6J ob/ob mice fed a high-fat diet. Nutr. Res. 2016, 36, 995–1003. [Google Scholar] [CrossRef]
- Koza, J.; Jurgoński, A. Partially defatted rather than native poppy seeds beneficially alter lipid metabolism in rats fed a high-fat diet. Sci. Rep. 2023, 13, 14171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Shao, H.; Bi, Q.; Chen, J.; Ye, Z. Grape seed procyanidin B2 inhibits adipogenesis of 3T3-L1 cells by targeting peroxisome proliferator-activated receptor γ with miR-483-5p involved mechanism. Biomed. Pharmacother. 2017, 86, 292–296. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).