Optimizing Low Fishmeal Diets with Vitamin C Supplementation: A Comprehensive Study on Growth, Immunity, and Heat Stress Resistance in Largemouth Bass (Micropterus salmoides) Juveniles
Abstract
1. Introduction
2. Materials and Methods
2.1. Diet Preparation
2.2. Experimental Fish
2.3. Sample Collection
2.4. Heat Stress Experiment
2.5. Measurement of Growth Indicators
2.6. Body Composition and Biochemical Analysis
2.7. Methods of Analysing Apoptosis in Gills After Heat Stress
2.8. Real-Time PCR Analysis
2.9. Statistical Analyses
3. Results
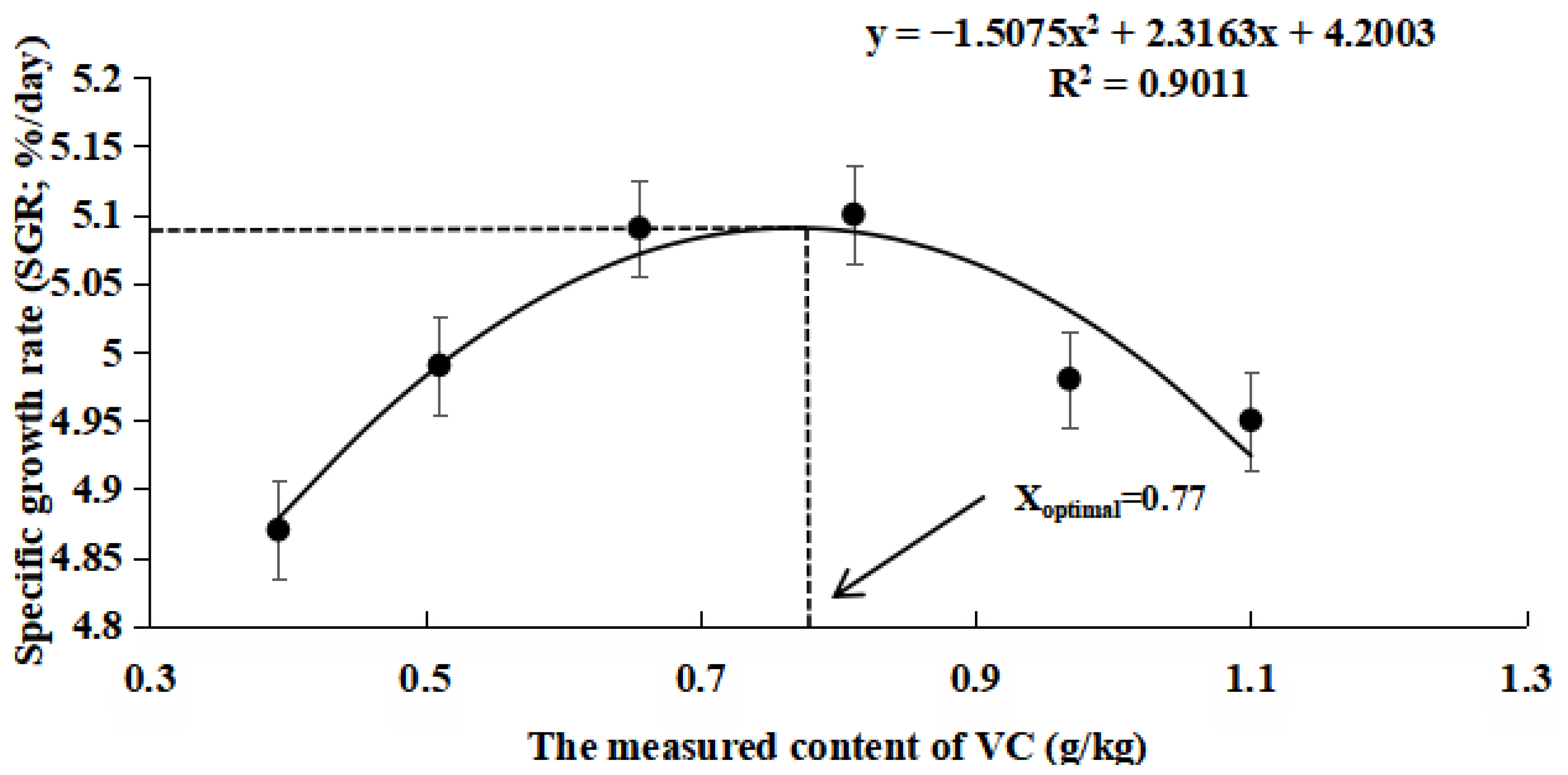
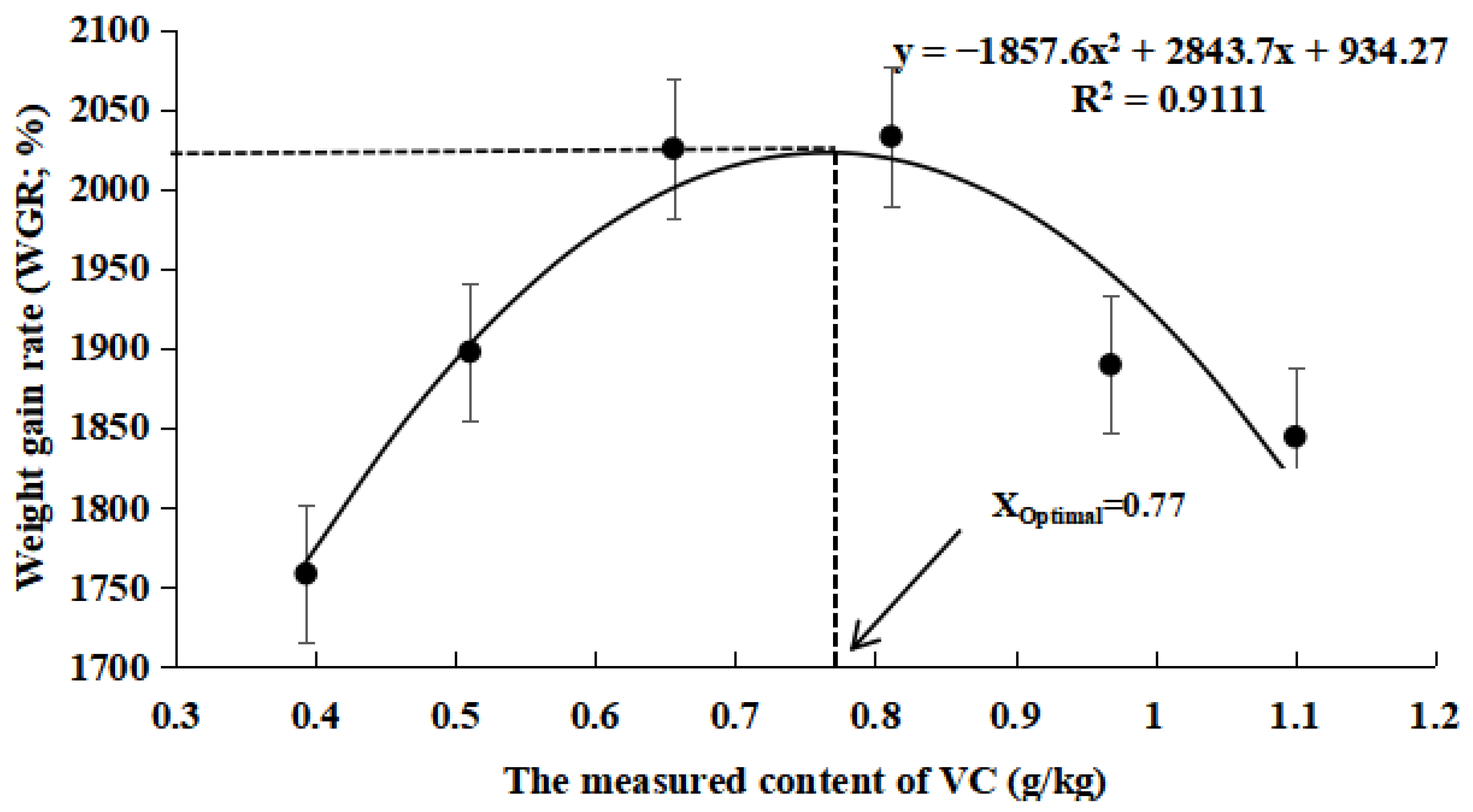
3.1. Growth Data
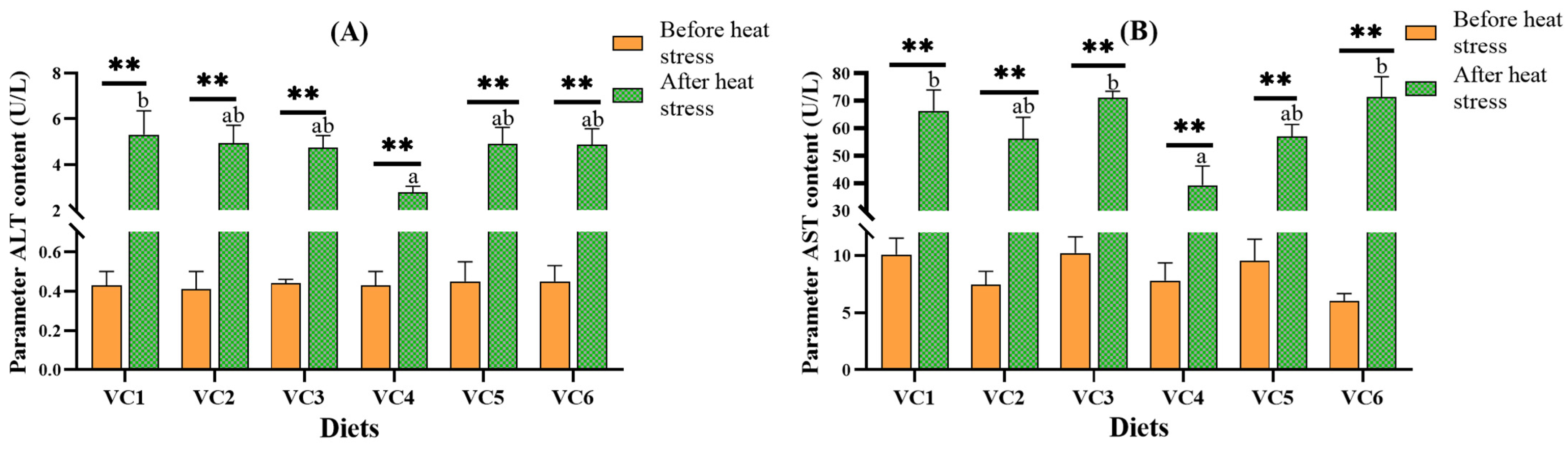
3.2. Plasma Biochemical Analysis Before and After Heat Stress
3.3. Analysis of Plasma Antioxidant Capacity
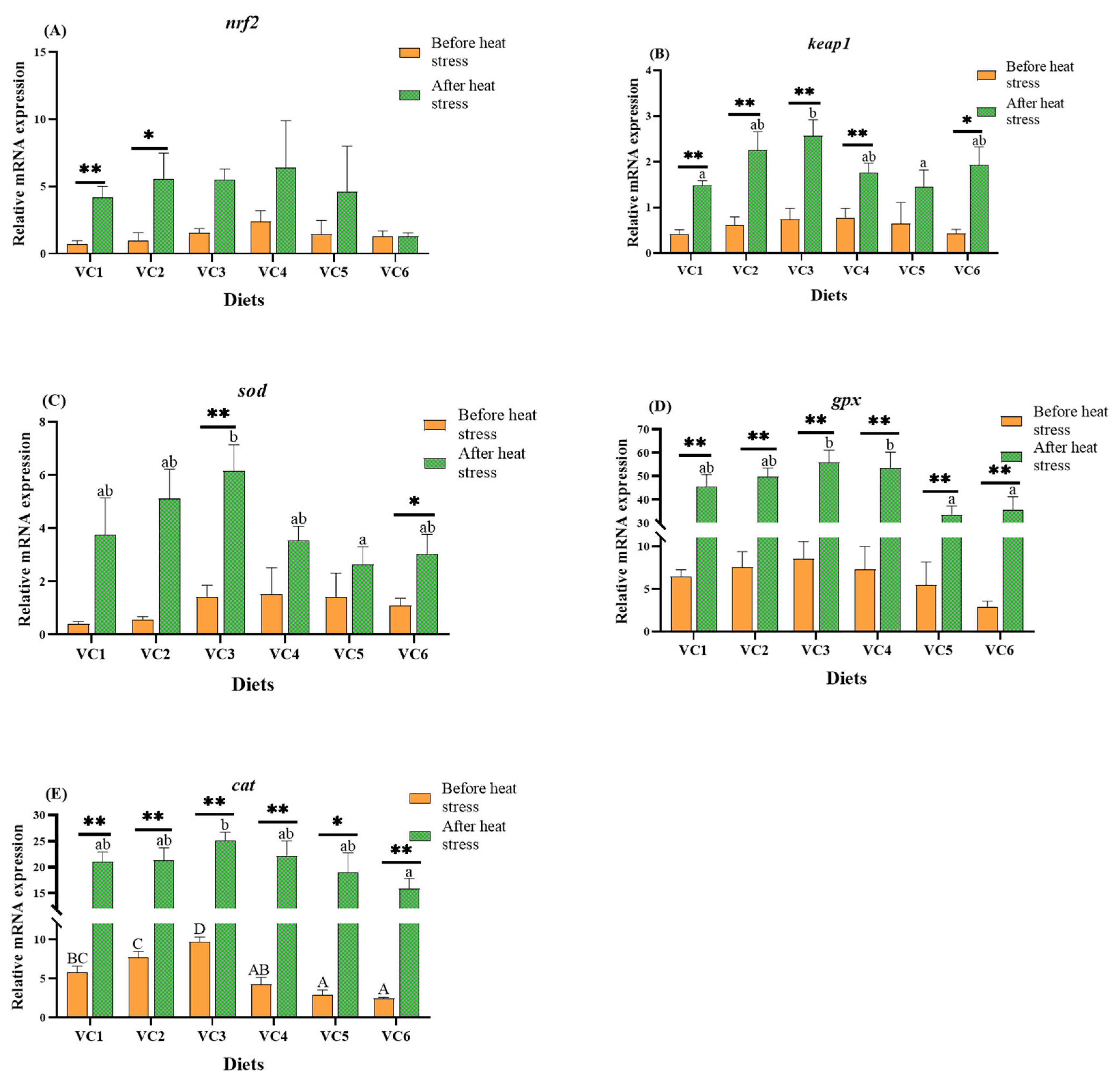
3.4. Analysis of Antioxidant-Related Genes in the Intestine
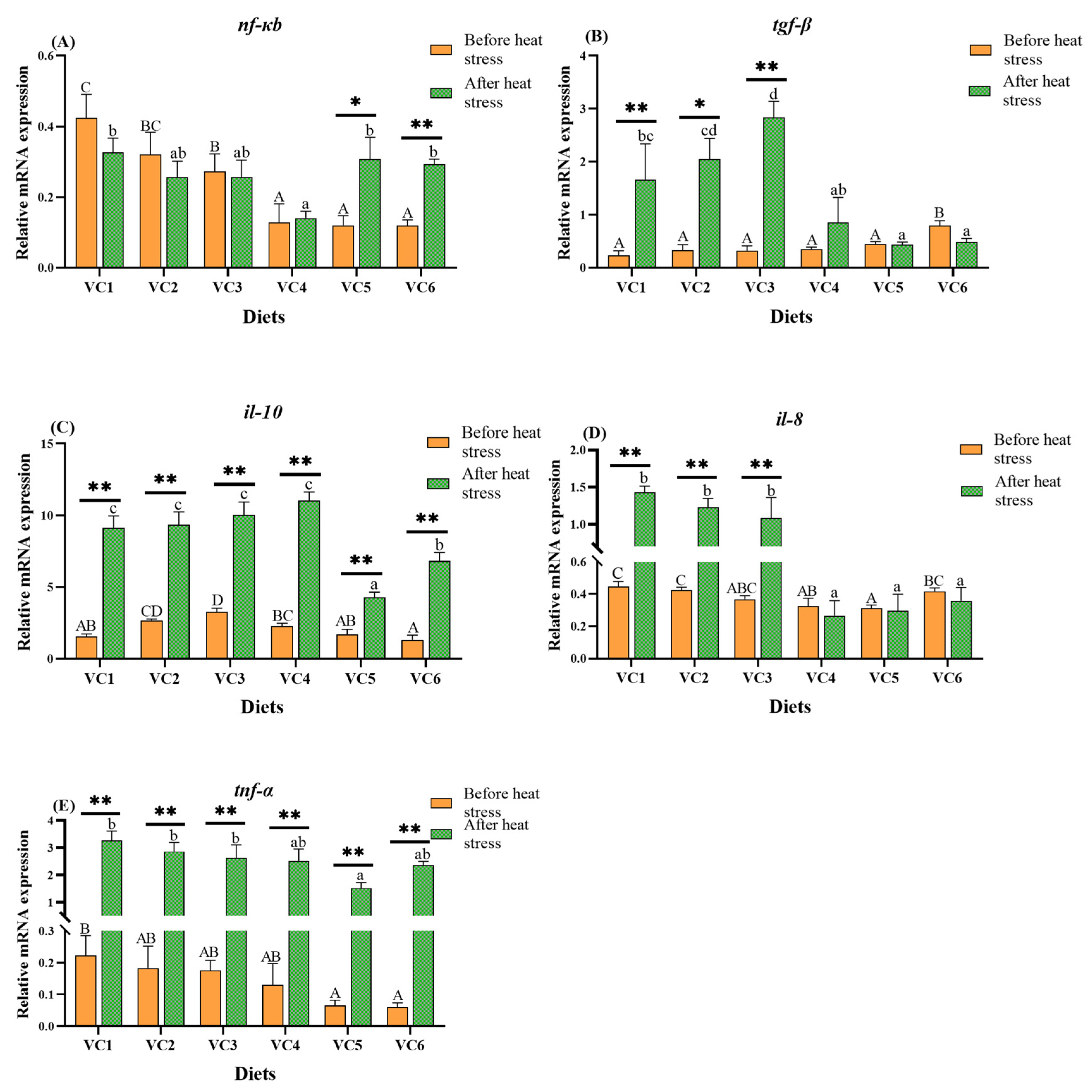
3.5. Analysis of Immune-Related Genes in the Intestine
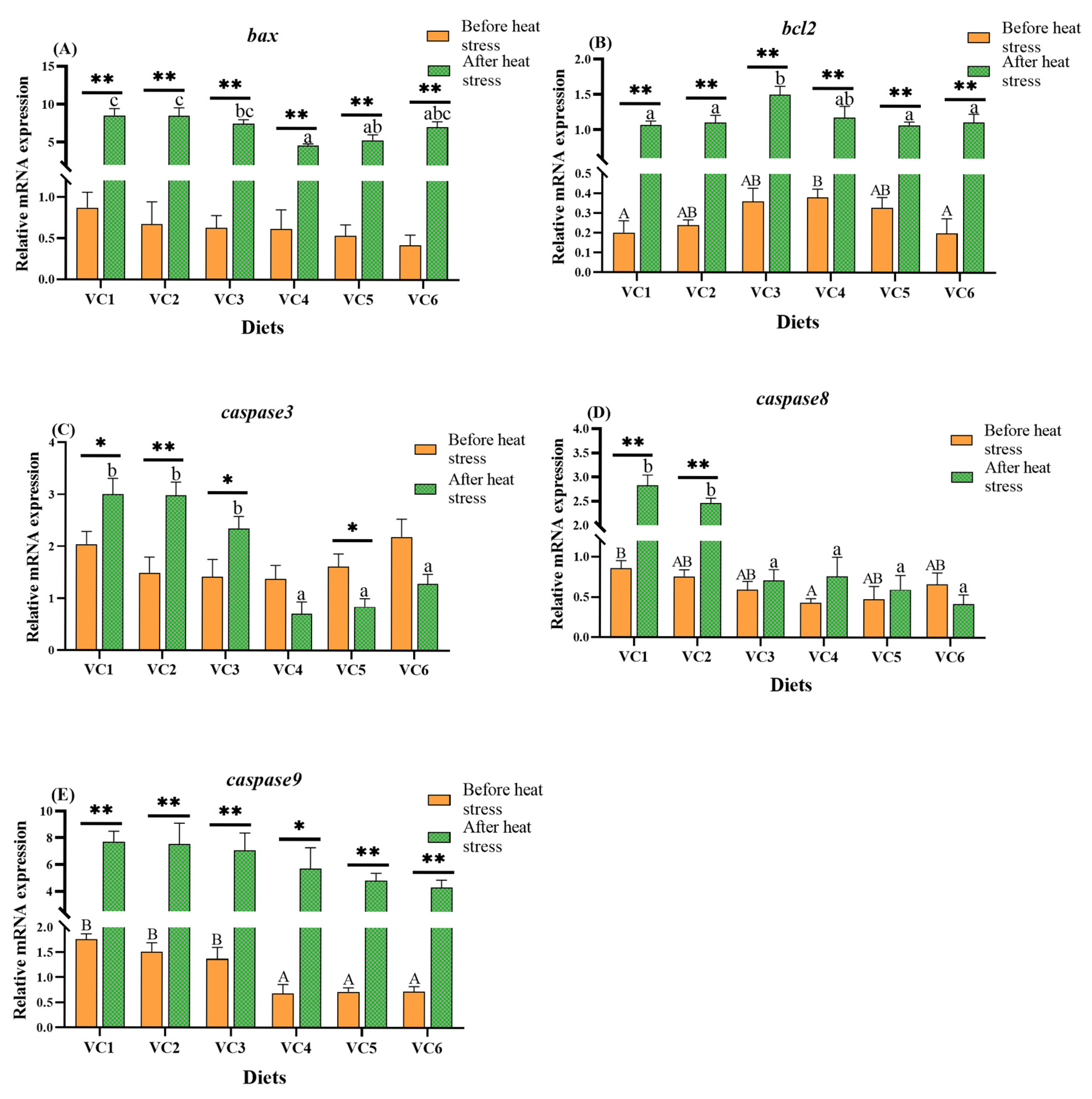
3.6. Expression of Apoptosis-Related Genes
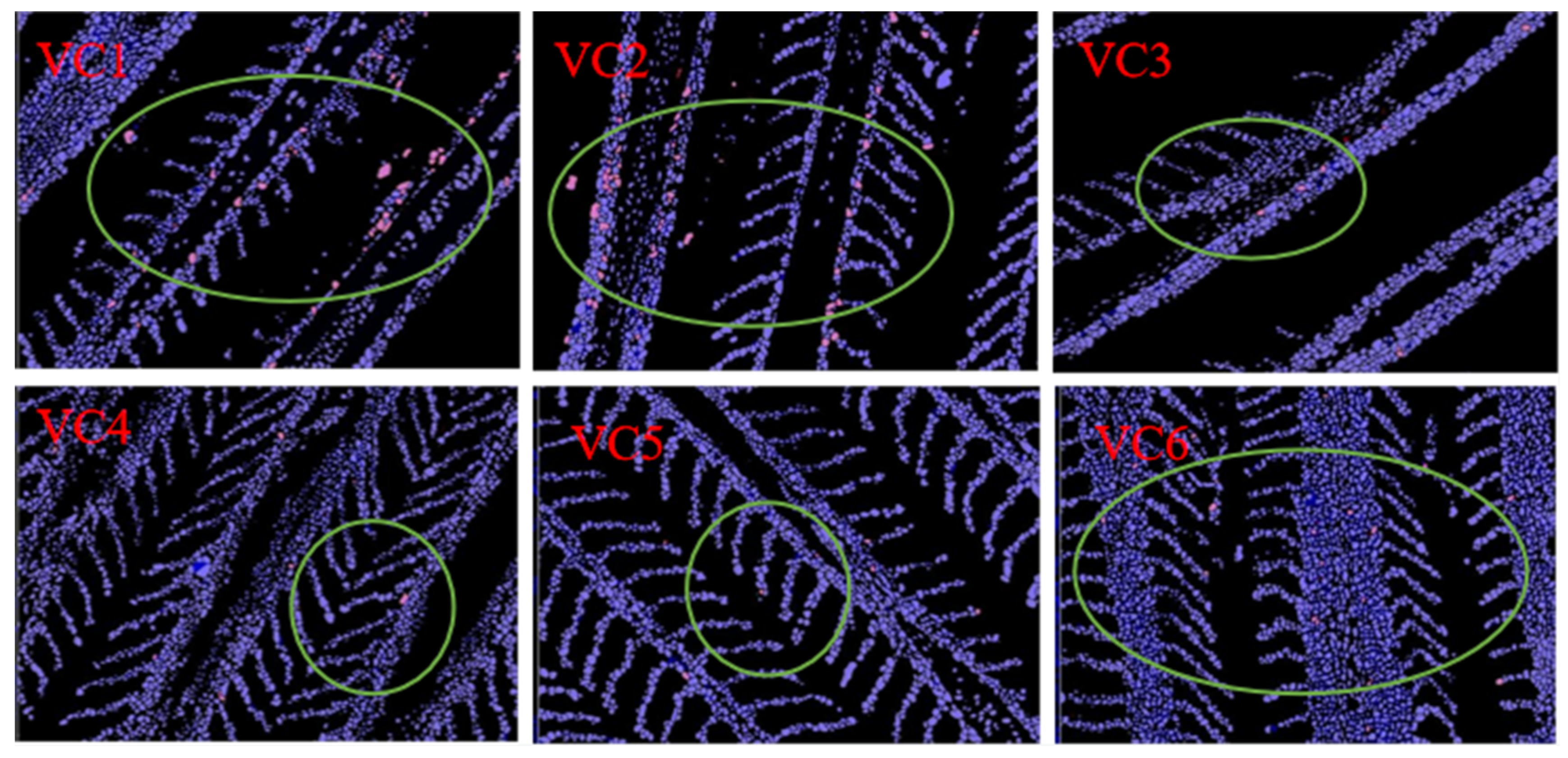
3.7. TUNEL (Red) Fluorescence Analysis
4. Discussion
4.1. Effects of VC Supplementation in Low Fishmeal Diets on the Growth Performance of M. salmoides
4.2. Effects of VC Supplementation in Low Fishmeal Diets on Plasma ALT and AST Levels in M. salmoides
4.3. Effects of VC Supplementation in Low Fishmeal Diets on Plasma and Intestinal Antioxidant Capacity of M. salmoides
4.4. Effects of VC Supplementation in Low Fishmeal Diets on Intestinal Immune Function in M. salmoides
4.5. Effects of VC Supplementation in Low Fishmeal Diets on Intestinal Apoptosis in M. salmoides
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jannathulla, R.; Rajaram, V.; Kalanjiam, R.; Ambasankar, K.; Muralidhar, M.; Dayal, J.S. Fishmeal availability in the scenarios of climate change: Inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquac. Res. 2019, 50, 3493–3506. [Google Scholar] [CrossRef]
- Dhar, V.; Singh, S.K.; Narsale, S.A.; Debbarma, S.; Saikia, P.; Yirang, Y. Fishmeal substitutions and their implications for aquatic animal immune and gut function: A review. Comp. Immunol. Rep. 2024, 7, 200171. [Google Scholar] [CrossRef]
- Macusi, E.D.; Cayacay, M.A.; Borazon, E.Q.; Sales, A.C.; Habib, A.; Fadli, N.; Santos, M.D. Protein Fishmeal Replacement in Aquaculture: A Systematic Review and Implications on Growth and Adoption Viability. Sustainability 2023, 15, 12500. [Google Scholar] [CrossRef]
- Bonvini, E.; Bonaldo, A.; Mandrioli, L.; Sirri, R.; Dondi, F.; Bianco, C.; Fontanillas, R.; Mongile, F.; Gatta, P.P.; Parma, L. Effects of feeding low fishmeal diets with increasing soybean meal levels on growth, gut histology and plasma biochemistry of sea bass. Animal 2018, 12, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.M.; Bano, A.A.; Ali, S.; Rizwan, M.; Adrees, M.; Zahoor, A.F.; Sarker, P.K.; Hussain, M.; Arsalan, M.Z.U.H.; Yong, J.W.H.; et al. Substitution of fishmeal: Highlights of potential plant protein sources for aquaculture sustainability. Heliyon 2024, 10, e26573. [Google Scholar] [CrossRef]
- Chen, S.; Maulu, S.; Wang, J.; Xie, X.; Liang, X.; Wang, H.; Wang, J.; Xue, M. The application of protease in aquaculture: Prospects for enhancing the aquafeed industry. Anim. Nutr. 2024, 16, 105–121. [Google Scholar] [CrossRef]
- Onomu, A.J.; Okuthe, G.E. The Role of Functional Feed Additives in Enhancing Aquaculture Sustainability. Fishes 2024, 9, 167. [Google Scholar] [CrossRef]
- Wang, X.; Shao, J. Editorial: Beneficial effects of functional ingredients in feed on immunity improvement and growth promotion of aquaculture animals. Front. Mar. Sci. 2023, 10, 1229367. [Google Scholar] [CrossRef]
- Cai, Q.; Wu, X.; Gatlin, D.M.; Zhang, L.; Zhai, H.; Zhou, Z.; Yin, H.; Geng, L.; Irm, M. Dietary vitamin C affects growth, antioxidant status and serum immune parameter of juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂) fed low fishmeal diets. Aquaculture 2022, 556, 738285. [Google Scholar] [CrossRef]
- Lin, M.; Shiau, S. Dietary l-ascorbic acid affects growth, nonspecific immune responses and disease resistance in juvenile grouper, Epinephelus malabaricus. Aquaculture 2005, 244, 215–221. [Google Scholar] [CrossRef]
- Al-Amoudi, M.M.; El-Nakkadi, A.M.N.; El-Nouman, B.M. Evaluation of optimum dietary requirement of vitamin C for the growth of Oreochromis spilurus fingerlings in water from the Red Sea. Aquaculture 1992, 105, 165–173. [Google Scholar] [CrossRef]
- Soliman, A.K.; Jauncey, K.; Roberts, R.J. The effect of varying forms of dietary ascorbic acid on the nutrition of juvenile tilapias (Oreochromis niloticus). Aquaculture 1986, 52, 1–10. [Google Scholar] [CrossRef]
- Maage, A.; Waagbø, R.; Olsson, P.E.; Julshamn, K.; Sandnes, K. Ascorbate-2-sulfate as a dietary vitamin C source for Atlantic salmon (Salmo salar): 2. Effects of dietary levels and immunization on the metabolism of trace elements. Fish Physiol. Biochem. 1990, 8, 429–436. [Google Scholar] [CrossRef]
- Shiau, S.Y.; Hsu, T.S. Quantification of vitamin C requirement for juvenile hybrid tilapia, Oreochromis niloticus × Oreochromis aureus, with l-ascorbyl-2-monophosphate-Na and l-ascorbyl-2-monophosphate-Mg. Aquaculture 1999, 175, 317–326. [Google Scholar] [CrossRef]
- Sandell, L.J.; Daniel, J.C. Effects of Ascorbic Acid on Collagen Mrna Levels in Short Term Chondrocyte Cultures. Connect. Tissue Res. 2009, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jiang, W.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Dietary vitamin C deficiency depressed the gill physical barriers and immune barriers referring to Nrf2, apoptosis, MLCK, NF-κB and TOR signaling in grass carp (Ctenopharyngodon idella) under infection of Flavobacterium columnare. Fish Shellfish Immunol. 2016, 58, 177–192. [Google Scholar] [CrossRef]
- Chien, L.T.; Hwang, D.F. Effects of thermal stress and vitamin C on lipid peroxidation and fatty acid composition in the liver of thornfish Terapon jarbua. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 128, 91–97. [Google Scholar] [CrossRef]
- Chien, L.T.; Hwang, D.F.; Jeng, S.S. Effect of Thermal Stress on Dietary Requirement of Vitamin C in Thornfish (Terapon jarbua). Fish. Sci. 1999, 65, 731–735. [Google Scholar] [CrossRef]
- Bai, J.; Lutz-Carrillo, D.J.; Quan, Y.; Liang, S. Taxonomic status and genetic diversity of cultured largemouth bass Micropterus salmoides in China. Aquaculture 2008, 278, 27–30. [Google Scholar] [CrossRef]
- Jiang, B.; Lu, G.; Du, J.; Wang, J.; Hu, Y.; Su, Y.; Li, A. First report of trypanosomiasis in farmed largemouth bass (Micropterus salmoides) from China: Pathological evaluation and taxonomic status. Parasitol. Res. 2019, 118, 1731–1739. [Google Scholar] [CrossRef]
- Yu, P.; Chen, H.; Liu, M.; Zhong, H.; Wang, X.; Wu, Y.; Sun, Y.; Wu, C.; Wang, S.; Zhao, C.; et al. Current status and application of largemouth bass (Micropterus salmoides) germplasm resources. Reprod. Breed. 2024, 4, 73–82. [Google Scholar] [CrossRef]
- Yin, Y.; Chen, X.; Gui, Y.; Zou, J.; Wang, Q.; Qiu, L.; Fan, L.; Meng, S.; Song, C. Risk and benefit assessment of potential neurodevelopment effect resulting from consumption of cultured largemouth bass (Micropterus salmoides) in China. Environ. Sci. Pollut. Res. 2022, 29, 89788–89795. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xie, Y.; Li, M.; Zhu, T.; Lei, C.; Song, H.; Han, L.; Li, S. Effects of chronic heat stress on growth performance, liver histology, digestive enzyme activities, and expressions of HSP genes in different populations of Largemouth bass (Micropterus salmoides). Aquac. Rep. 2024, 35, 101972. [Google Scholar] [CrossRef]
- Yusuf, A.; Huang, X.; Chen, N.; Li, S.; Apraku, A.; Wang, W.; David, M.A. Growth and metabolic responses of juvenile largemouth bass (Micropterus salmoides) to dietary vitamin c supplementation levels. Aquaculture 2021, 534, 736243. [Google Scholar] [CrossRef]
- Verlhac, V.; Gabaudan, J. Influence of vitamin C on the immune system of salmonids. Aquac. Res. 1994, 25, 21–36. [Google Scholar] [CrossRef]
- Yusuf, A.; Huang, X.; Chen, N.; Apraku, A.; Wang, W.; Cornel, A.; Rahman, M.M. Impact of dietary vitamin c on plasma metabolites, antioxidant capacity and innate immunocompetence in juvenile largemouth bass, Micropterus salmoides. Aquac. Rep. 2020, 17, 100383. [Google Scholar] [CrossRef]
- Ai, Q.; Mai, K.; Zhang, C.; Xu, W.; Duan, Q.; Tan, B.; Liufu, Z. Effects of Dietary Vitamin C on Growth and Immune Response of Japanese Seabass, Lateolabrax Japonicus. Aquaculture 2004, 242, 489–500. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, L.; Wang, H.; Xie, F.; Wang, T. Effect of Dietary Vitamin C on the Growth Performance and Innate Immunity of Juvenile Cobia (Rachycentron Canadum). Fish Shellfish Immunol. 2012, 32, 969–975. [Google Scholar] [CrossRef]
- Liu, H.P.; Wen, B.; Chen, Z.Z.; Gao, J.Z.; Liu, Y.; Zhang, Y.C.; Wang, Z.X.; Peng, Y. Effects of Dietary Vitamin C and Vitamin E on the Growth, Antioxidant Defence and Digestive Enzyme Activities of Juvenile Discus Fish (Symphysodon Haraldi). Aquac. Nutr. 2019, 25, 176–183. [Google Scholar] [CrossRef]
- Tewary, A.; Patra, B.C. Use of Vitamin C as an Immunostimulant. Effect on Growth, Nutritional Quality, and Immune Response of Labeo rohita (Ham.). Fish Physiol. Biochem. 2008, 34, 251–259. [Google Scholar] [CrossRef]
- Ai, Q.; Mai, K.; Tan, B.; Xu, W.; Zhang, W.; Ma, H.; Liufu, Z. Effects of Dietary Vitamin C on Survival, Growth, and Immunity of Large Yellow Croaker, Pseudosciaena crocea. Aquaculture 2006, 261, 327–336. [Google Scholar] [CrossRef]
- Liang, X.P.; Li, Y.; Hou, Y.M.; Qiu, H.; Zhou, Q.C. Effect of Dietary Vitamin C on the Growth Performance, Antioxidant Ability and Innate Immunity of Juvenile Yellow Catfish (Pelteobagrus fulvidraco richardson). Aquac. Res. 2017, 48, 149–160. [Google Scholar] [CrossRef]
- Ren, M.; Liao, Y.; Xie, J.; Liu, B.; Zhou, Q.; Ge, X.; Cui, H.; Pan, L.; Chen, R. Dietary arginine requirement of juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 2013, 414–415, 229–234. [Google Scholar] [CrossRef]
- Huang, D.; Zhu, J.; Zhang, L.; Ge, X.; Ren, M.; Liang, H. Dietary Supplementation with Eucommia ulmoides Leaf Extract Improved the Intestinal Antioxidant Capacity, Immune Response, and Disease Resistance against Streptococcus agalactiae in Genetically Improved Farmed Tilapia (GIFT.; Oreochromis niloticus). Antioxidants 2022, 11, 1800. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liang, J.; Chen, F.; Tang, X.; Liao, L.; Liu, Q.; Luo, J.; Du, Z.; Li, Z.; Luo, W.; et al. High carbohydrate diet induced endoplasmic reticulum stress and oxidative stress, promoted inflammation and apoptosis, impaired intestinal barrier of juvenile largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2021, 119, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, L.; Li, C.; Liu, E.; Zhu, H.; Ling, Q. Heat stress-induced endoplasmic reticulum stress promotes liver apoptosis in largemouth bass (Micropterus salmoides). Aquaculture 2022, 546, 737401. [Google Scholar] [CrossRef]
- Zhao, X.; Mao, W.; Lin, Z.; Ling, Q. Heat stress induced hepatocyte apoptosis in largemouth bass (Micropterus salmoides) via IRE1α/TRAF2/ASK1/JNK pathway. J. Oceanol. Limnol. 2024, 42, 988–1000. [Google Scholar] [CrossRef]
- Nasar, M.F.; Shah, S.Z.H.; Aftab, K.; Fatima, M.; Bilal, M.; Hussain, M. Dietary vitamin C requirement of juvenile grass carp (Ctenopharyngodon idella) and its effects on growth attributes, organ indices, whole-body composition and biochemical parameters. Aquac. Nutr. 2021, 27, 1903–1911. [Google Scholar] [CrossRef]
- Wu, L.; Xu, W.; Li, H.; Dong, B.; Geng, H.; Jin, J.; Han, D.; Liu, H.; Zhu, X.; Yang, Y.; et al. Vitamin C Attenuates Oxidative Stress, Inflammation, and Apoptosis Induced by Acute Hypoxia through the Nrf2/Keap1 Signaling Pathway in Gibel Carp (Carassius gibelio). Antioxidants 2022, 11, 935. [Google Scholar] [CrossRef]
- Kaur, R.; Batra, M.; Shah, T.K.; Saxena, A. Ameliorative effects of dietary Vitamin-C on growth performance and hemato-biochemical response of sodium fluoride-intoxicated Amur Carp, Cyprinus carpio haematopterus. Aquac. Res. 2022, 53, 2895–2909. [Google Scholar] [CrossRef]
- Grădinaru, A.C.; Popa, S. Vitamin C: From Self-Sufficiency to Dietary Dependence in the Framework of Its Biological Functions and Medical Implications. Life 2025, 15, 238. [Google Scholar] [CrossRef]
- Ebi, I.; Shapawi, R.; Lim, L.S.; Yong, A.S.K.; Mazlan, N.; Shah, M.D.; Basri, N.A.; Jaziri, A.A. Effects of dietary vitamins C and E on growth performance, hematological and biochemical parameters, skeletal abnormalities, and disease resistance against V. harveyi of hybrid groupers (Epinephelus fuscoguttatus ♀ × Epinephelus lanceolatus ♂). Aquac. Int. 2025, 33, 139. [Google Scholar] [CrossRef]
- Perera, A.D.; Bhujel, R.C. Potential role of L-ascorbic acid with Field cricket (Gryllus bimaculatus) meal in diets of Nile Tilapia (Oreochromis niloticus) during sex reversal and nursing. Isr. J. Aquac.—Bamidgeh 2021, 73, 1–17. [Google Scholar] [CrossRef]
- Lee, K.W.; Yoo, H.K.; Kim, S.S.; Han, G.S.; Jung, M.M.; Kim, H.S. Effect of Dietary Vitamin C Supplementation on Growth Performance and Biochemical Parameters in Grower Walleye Pollock, Gadus chalcogrammus. Animals 2024, 14, 1026. [Google Scholar] [CrossRef]
- Asztalos, B.; Nemcsók, J. Effect of pesticides on the LDH activity and isoenzyme pattern of carp (Cyprinus carpio L.) sera. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1985, 82, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Yu, H.; Li, L.; Li, M.; Qiu, X.; Fan, X.; Fan, Y.; Shan, L.; Xue, M. Effects of Dietary Vitamin C on the Growth Performance, Biochemical Parameters, and Antioxidant Activity of Coho Salmon Oncorhynchus kisutch (Walbaum, 1792) Postsmolts. Aquac. Nutr. 2022, 2022, 6866578. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wu, F.; Zhang, S.; Jiang, M.; Liu, W.; Tian, J.; Yang, C.; Wen, H. Dietary vitamin C requirement of juvenile Chinese sucker (Myxocyprinus asiaticus). Aquac. Res. 2017, 48, 37–46. [Google Scholar] [CrossRef]
- Daniel, N.; Muralidhar, A.P.; Srivastava, P.P.; Jain, K.K.; Pani Prasad, K.; Manish, J.; Sivaramakrishnan, T. Dietary ascorbic acid requirement for growth of striped catfish, Pangasianodon hypophthalmus(Sauvage, 1878) juveniles. Aquac. Nutr. 2018, 24, 616–624. [Google Scholar] [CrossRef]
- Ismail, H.T.H.; Mahboub, H.H.H. Effect of acute exposure to nonylphenol on biochemical, hormonal, and hematological parameters and muscle tissues residues of Nile tilapia; Oreochromis niloticus. Vet. World 2016, 9, 616–625. [Google Scholar] [CrossRef]
- Feng, L.; Xiao, W.; Liu, Y.; Jiang, J.; Hu, K.; Jiang, W.; Li, S.; Zhou, X. Methionine hydroxy analogue prevents oxidative damage and improves antioxidant status of intestine and hepatopancreas for juvenile Jian carp (Cyprinus carpio var. Jian). Aquac. Nutr. 2011, 17, 595–604. [Google Scholar] [CrossRef]
- Hou, D.X.; Korenori, Y.; Tanigawa, S.; Yamada-Kato, T.; Nagai, M.; He, X.; He, J. Dynamics of Nrf2 and Keap1 in ARE-Mediated NQO1 Expression by Wasabi 6-(Methylsulfinyl)hexyl Isothiocyanate. J. Agric. Food Chem. 2011, 59, 11975–11982. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. et Biophys. Acta (BBA)—Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, S.; Özoğul, F. Effects of selenium nanoparticles on growth performance, hematological, serum biochemical parameters, and antioxidant status in fish. Anim. Feed Sci. Technol. 2021, 281, 115099. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Wen, H.; Huan, Z.; Zhong, L.; Mao, X.; Li, J.; Xiao, T. Effect of vitamin C on growth, immunity and anti-ammonia-nitrite stress ability in juvenile black carp (Mylopharyngodon piceus). J. Fish. China 2013, 37, 565. [Google Scholar] [CrossRef]
- Luo, K.; Li, X.; Wang, L.; Rao, W.; Wu, Y.; Liu, Y.; Pan, M.; Huang, D.; Zhang, W.; Mai, K. Ascorbic Acid Regulates the Immunity, Anti-Oxidation and Apoptosis in Abalone Haliotis discus hannai Ino. Antioxidants 2021, 10, 1449. [Google Scholar] [CrossRef]
- Zeng, L.; Ai, C.; Wang, Y.; Zhang, J.; Wu, C. Abrupt Salinity Stress Induces Oxidative Stress via the Nrf2-Keap1 Signaling Pathway in Large Yellow Croaker Pseudosciaena crocea. Fish Physiol. Biochem. 2017, 43, 955–964. [Google Scholar] [CrossRef]
- Katoh, Y.; Iida, K.; Kang, M.-I.; Kobayashi, A.; Mizukami, M.; Tong, K.I.; McMahon, M.; Hayes, J.D.; Itoh, K.; Yamamoto, M. Evolutionary Conserved N-Terminal Domain of Nrf2 Is Essential for the Keap1-Mediated Degradation of the Protein by Proteasome. Arch. Biochem. Biophys. 2005, 433, 342–350. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell Survival Responses to Environmental Stresses via the Keap1-Nrf2-ARE Pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
- Chen, R.; Lochmann, R.; Goodwin, A.; Praveen, K.; Dabrowski, K.; Lee, K.J. Alternative Complement Activity and Resistance to Heat Stress in Golden Shiners (Notemigonus crysoleucas) Are Increased by Dietary Vitamin C Levels in Excess of Requirements for Prevention of Deficiency Signs. J. Nutr. 2003, 133, 2281–2286. [Google Scholar] [CrossRef]
- Yun, S.H.; Moon, Y.S.; Sohn, S.H.; Jang, I.S. Effects of Cyclic Heat Stress or Vitamin C Supplementation during Cyclic Heat Stress on HSP70, Inflammatory Cytokines, and the Antioxidant Defense System in Sprague Dawley Rats. Exp. Anim. 2012, 61, 543–553. [Google Scholar] [CrossRef]
- Bolzan, L.P.; Barroso, D.C.; Souza, C.F.; Oliveira, F.C.; Wagner, R.; Baldisserotto, B.; Val, A.L.; Baldissera, M.D. Dietary supplementation with nerolidol improves the antioxidant capacity and muscle fatty acid profile of Brycon amazonicus exposed to acute heat stress. J. Therm. Biol. 2021, 99, 103003. [Google Scholar] [CrossRef]
- Qiu, W.; Hu, J.; Magnuson, J.T.; Greer, J.; Yang, M.; Chen, Q.; Fang, M.; Zheng, C.; Schlenk, D. Evidence linking exposure of fish primary macrophages to antibiotics activates the NF-kB pathway. Environ. Int. 2020, 138, 105624. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Mokrani, A.; Ji, K.; Ge, X.; Ren, M.; Xie, J.; Liu, B.; Xi, B.; Zhou, Q. Dietary leucine modulates growth performance, Nrf2 antioxidant signaling pathway and immune response of juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2018, 73, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.L.; Davies, S.J.; Pulsford, A.L. The influence of ascorbic acid (vitamin C) on non-specific immunity in the turbot (Scophthalmus maximus L.). Fish Shellfish Immunol. 1995, 5, 27–38. [Google Scholar] [CrossRef]
- Xu, H.; Jiang, W.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.; Tang, L.; Tang, W.; Zhang, Y.; et al. Dietary vitamin C deficiency depresses the growth, head kidney and spleen immunity and structural integrity by regulating NF-κB, TOR, Nrf2, apoptosis and MLCK signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 52, 111–138. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Saini, V.P.; Meena, L.L. Heat stress induces oxidative stress and weakens the immune system in catfish Clarias magur: Evidence from physiological, histological, and transcriptomic analyses. Fish Shellfish Immunol. 2025, 161, 110294. [Google Scholar] [CrossRef]
- Raqib, R.; Ekberg, C.; Sharkar, P.; Bardhan, P.K.; Zychlinsky, A.; Sansonetti, P.J.; Andersson, J. Apoptosis in Acute Shigellosis Is Associated with Increased Production of Fas/Fas Ligand, Perforin, Caspase-1, and Caspase-3 but Reduced Production of Bcl-2 and Interleukin-2. Infect. Immun. 2002, 70, 3199–3207. [Google Scholar] [CrossRef]
- Ching, B.; Chen, X.L.; Yong, J.H.A.; Wilson, J.M.; Hiong, K.C.; Sim, E.W.L.; Wong, W.P.; Lam, S.H.; Chew, S.F.; Ip, Y.K. Increases in apoptosis, caspase activity and expression of p53 and bax, and the transition between two types of mitochondrion-rich cells, in the gills of the climbing perch, Anabas testudineus, during a progressive acclimation from freshwater to seawater. Front. Physiol. 2013, 4, 135. [Google Scholar] [CrossRef]
- Yabu, T.; Kishi, S.; Okazaki, T.; Yamashita, M. Characterization of zebrafish caspase-3 and induction of apoptosis through ceramide generation in fish fathead minnow tailbud cells and zebrafish embryo. Biochem. J. 2001, 360, 39–47. [Google Scholar] [CrossRef]
- Rahman, M.H.; Alam, M.A.; Flura; Sultana, S.; Islam, M.R. Effects of Dietary Vitamin C on the Growth Performance, Antioxidant Activity and Disease Resistance of Fish: A Review. Eur. J. Theor. Appl. Sci. 2023, 1, 744–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Zhao, X.; Li, C.; Wang, Y.; Li, L.; Zhu, H.; Ling, Q. Effects of acute heat stress on liver damage, apoptosis and inflammation of pikeperch (Sander lucioperca). J. Therm. Biol. 2022, 106, 103251. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xing, G.; Qian, Y.; Sun, X.; Zhong, J.; Chen, K. Dihydromyricetin attenuates heat stress-induced apoptosis in dairy cow mammary epithelial cells through suppressing mitochondrial dysfunction. Ecotoxicol. Environ. Saf. 2021, 214, 112078. [Google Scholar] [CrossRef] [PubMed]

| Ingredients | VC1 | VC2 | VC3 | VC4 | VC5 | VC6 |
|---|---|---|---|---|---|---|
| Fishmeal | 25 | 25 | 25 | 25 | 25 | 25 |
| Chicken meal 1 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 | 12.00 |
| Soy concentrated protein 1 | 13.41 | 13.41 | 13.41 | 13.41 | 13.41 | 13.41 |
| Soybean meal 1 | 15.70 | 15.70 | 15.70 | 15.70 | 15.70 | 15.70 |
| Wheat gluten 1 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Blood meal 1 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Wheat flour 1 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 | 8.00 |
| Tapioca starch | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Fish oil | 2.80 | 2.80 | 2.80 | 2.80 | 2.80 | 2.80 |
| Soybean oil | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 | 5.00 |
| Calcium dihydrogen phosphate | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 | 3.00 |
| Premix 2 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| Choline chloride | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| VC 3 (%) | 0.00 | 0.05 | 0.10 | 0.15 | 0.20 | 0.25 |
| Microcrystalline cellulose | 0.25 | 0.20 | 0.15 | 0.10 | 0.05 | 0.00 |
| L-Lysine 4 (98.5%) | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 |
| L-Methionine 4 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 | 0.11 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Analyzed proximate composition | ||||||
| Crude protein (%) | 48.85 | 48.26 | 48.87 | 49.11 | 48.65 | 48.50 |
| Crude lipid (%) | 12.73 | 12.32 | 12.02 | 12.24 | 12.02 | 12.47 |
| Actual VC content (g/kg) | 0.39 | 0.51 | 0.66 | 0.81 | 0.97 | 1.11 |
| Items | Full Name | Computational Formula |
|---|---|---|
| IBW (g) | initial body weight (g) | Total weight (g)/number of fish per bucket at the start of the culture |
| FBW (g) | final body weight (g) | Total weight (g)/number of fish per bucket at the end of the culture |
| FCR | feed conversion ratio | dry feed fed (g)/(final body weight (g) − initial body weight (g)) |
| WGR (%) | weight gain rate (%) | 100 × (final body weight (g) − initial body weight (g))/initial body weight (g) |
| SGR (%/day) | specific growth rate (%/day) | 100 × [(Ln (final body weight (g)) − Ln(initial body weight (g)))/days] |
| SR (%) | survival rate (%) | 100 × (survival fish number/total fish number) |
| Items | Methods | Assay Kits/Testing Equipment |
|---|---|---|
| Composition of diets/whole body | ||
| Moisture | Drying method (ID 920.36) | Electric blast drying oven (Shanghai Yiheng Scientific Instrument Co., Ltd., Shanghai, China) |
| Protein | Kjeldahl (ID984.13) | Auto kieldahl apparatus: Hanon K1100 (Jinan Hanon Instruments Co., Ltd., Jinan, China). |
| Lipid | Soxhlet (ID 991.36) | Auto fat analyzer: Hanon SOX606 (Jinan Hanon Instruments Co., Ltd., Jinan, China) |
| Ash | Combustion (ID 923.03) | Muffle: XL-2A (Hangzhou Zhuochi Instrument Co., Ltd., Hangzhou, China). |
| Plasma parameters | ||
| ALT AST | International Federation of Clinical Chemistry recommended | Assay kits (ALT: 105-000442-00.AST: 15-00443-000 purchased from Mindray Medical International Ltd. (Shenzhen, China); Mindray BS 400 automatic biochemical analyzer (Mindray Medical International Ltd., Shenzhen, China). |
| Plasma parameters related antioxidant capacity | ||
| MDA | TBA method Ammonium | |
| CAT | Molybdenum acid method | Assay kits (MDA: A003-1-2. CAT: A007-1-1. SOD: A001-3-2. GSH:A006-1-1. GPx: A005-1-2.T-AOC:A015-2-1) purchased from Jian Cheng Bioengineering Institute (Nanjing, China); Spectrophotometer (Thermo Fisher Multiskan GO, Shanghai, China). |
| SOD | WST-1 method | |
| GSH | Microplate method | |
| GSH-Px | Colorimetric method | |
| T-AOC | ABTS method | |
| Genes | Forward (5′-3′) | Reverse (5′-3′) | Primer Source |
|---|---|---|---|
| nrf2 | CACAGCAGCAGCAGGAAAAG | AAGATGCTGCCGTCTGTTGA | XM_038720536.1 |
| keap1 | CGTACGTCCAGGCCTTACTC | TGACGGAAATAACCCCCTGC | XP_018520553.1 |
| cat | CTATGGCTCTCACACCTTC | TCCTCTACTGGCAGATTCT | MK614708.1 |
| sod | GGTGTTTAAAGCCGTTTGTGTT | CCTCTGATTTCTCCTGTCACCT | XM_038708943.1 |
| gpx | ATGGCTCTCATGACTGATCCAAA | GACCAACCAGGAACTTCTCAAA | XM_038697220.1 |
| nf-κb | AGAAGACGACTCGGGGATGA | GCTTCTGCAGGTTCTGGTCT | XM_038699793.1 |
| tgf-β | CACCAAGGAGATGCTGATT | CGTATGTTAGAGATGCTGAAG | XM_038693206.1 |
| il-10 | CGGCACAGAAATCCCAGAGC | CAGCAGGCTCACAAAATAAACATCT | XM_038696252.1 |
| tnf-α | CTTCGTCTACAGCCAGGCATCG | TTTGGCACACCGACCTCACC | XM_038710731.1 |
| il-8 | GAGGGTACATGTCTGGGGGA | CCTTGAAGGTTTGTTCTTCATCGT | XM_038713529.1 |
| bax | ACTTTGGATTACCTGCGGGA | TGCCAGAAATCAGGAGCAGA | Ref. [36] |
| bcl-2 | TGTGGGGCTACTTTTTGGCA | TTCGACTGCCACCCCAATAC | Ref. [37] |
| caspase3 | GAGGCGATGGACAAGAGTCA | CACAGACGAATGAAGCGTGG | XM_038713063.1 |
| caspase8 | GAGACAGACAGCAGACAACCA | TTCCATTTCAGCAAACACATC | Ref. [38] |
| caspase9 | CTGGAATGCCTTCAGGAGACGGG | GGGAGGGGCAAGACAACAGGGTG | Ref. [36] |
| β-action | GGTGTGATGGTTGGTATGG | CTCGTTGTAGAAGGTGTGAT | MH018565.1 |
| Diets (VC mg/kg) | IBW (g) | FBW (g) | FCR | WGR(%) | SGR (%/Day) | SR (%) |
|---|---|---|---|---|---|---|
| VC1 | 2.22 ± 0.00 | 41.16 ± 0.20 a | 0.72 ± 0.01 c | 1758.10 ± 8.87 a | 4.87 ± 0.01 a | 96.70 ± 1.67 |
| VC2 | 2.22 ± 0.01 | 44.35 ± 0.55 b | 0.69 ± 0.01 a | 1897.50 ± 22.25 b | 4.99 ± 0.02 b | 100.00 ± 0.00 |
| VC3 | 2.22 ± 0.01 | 47.22 ± 1.14 c | 0.71 ± 0.01 c | 2025.20 ± 46.73 c | 5.09 ± 0.04 c | 96.70 ± 1.67 |
| VC4 | 2.22 ± 0.00 | 47.24 ± 0.19 c | 0.72 ± 0.00 c | 2032.80 ± 5.97 c | 5.10 ± 0.00 c | 98.30 ± 1.67 |
| VC5 | 2.21 ± 0.01 | 43.97 ± 0.48 b | 0.68 ± 0.00 ab | 1889.40 ± 21.64 b | 4.98 ± 0.02 b | 100.00 ± 0.00 |
| VC6 | 2.21 ± 0.00 | 42.90 ± 0.36 ab | 0.68 ± 0.00 ab | 1844.10 ± 15.41 b | 4.95 ± 0.01 b | 98.30 ± 1.67 |
| Diets (VC mg/kg) | Moisture (%) | Lipid (%) | Ash (%) | Protein (%) |
|---|---|---|---|---|
| VC1 | 69.58 ± 0.19 | 9.77 ± 0.23 | 3.04 ± 0.18 | 16.79 ± 0.19 |
| VC2 | 69.48 ± 0.49 | 9.47 ± 0.68 | 3.12 ± 0.18 | 16.84 ± 0.19 |
| VC3 | 69.35 ± 0.46 | 9.56 ± 0.37 | 3.03 ± 0.12 | 16.56 ± 0.09 |
| VC4 | 69.17 ± 0.62 | 9.82 ± 0.75 | 3.13 ± 0.15 | 17.39 ± 0.35 |
| VC5 | 69.35 ± 0.39 | 9.45 ± 0.41 | 3.26 ± 0.03 | 17.29 ± 0.48 |
| VC6 | 69.49 ± 0.20 | 10.59 ± 0.37 | 3.11 ± 0.06 | 16.46 ± 0.25 |
| Diets | VC1 | VC2 | VC3 | VC4 | VC5 | VC6 |
|---|---|---|---|---|---|---|
| Red Positive Cells% | 0.45 ± 0.01 c | 0.43 ± 0.02 c | 0.19 ± 0.02 a | 0.17 ± 0.04 a | 0.28 ± 0.02 b | 0.36 ± 0.05 bc |
| Red Positive Cells Density, number/104 pixels | 0.25 ± 0.02 b | 0.20 ± 0.03 ab | 0.21 ± 0.01 ab | 0.17 ± 0.04 a | 0.19 ± 0.01 ab | 0.22 ± 0.00 ab |
| Red Mean Density | 0.55 ± 0.06 b | 0.50 ± 0.04 ab | 0.49 ± 0.06 ab | 0.37 ± 0.03 a | 0.44 ± 0.01 ab | 0.39 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Liang, H.; Chen, X.; Zhang, L.; Huang, D.; Wang, Y.; Cheng, Z.; Ren, M. Optimizing Low Fishmeal Diets with Vitamin C Supplementation: A Comprehensive Study on Growth, Immunity, and Heat Stress Resistance in Largemouth Bass (Micropterus salmoides) Juveniles. Antioxidants 2025, 14, 1175. https://doi.org/10.3390/antiox14101175
Zhao S, Liang H, Chen X, Zhang L, Huang D, Wang Y, Cheng Z, Ren M. Optimizing Low Fishmeal Diets with Vitamin C Supplementation: A Comprehensive Study on Growth, Immunity, and Heat Stress Resistance in Largemouth Bass (Micropterus salmoides) Juveniles. Antioxidants. 2025; 14(10):1175. https://doi.org/10.3390/antiox14101175
Chicago/Turabian StyleZhao, Shengqi, Hualiang Liang, Xiaoru Chen, Lu Zhang, Dongyu Huang, Yongli Wang, Zhenyan Cheng, and Mingchun Ren. 2025. "Optimizing Low Fishmeal Diets with Vitamin C Supplementation: A Comprehensive Study on Growth, Immunity, and Heat Stress Resistance in Largemouth Bass (Micropterus salmoides) Juveniles" Antioxidants 14, no. 10: 1175. https://doi.org/10.3390/antiox14101175
APA StyleZhao, S., Liang, H., Chen, X., Zhang, L., Huang, D., Wang, Y., Cheng, Z., & Ren, M. (2025). Optimizing Low Fishmeal Diets with Vitamin C Supplementation: A Comprehensive Study on Growth, Immunity, and Heat Stress Resistance in Largemouth Bass (Micropterus salmoides) Juveniles. Antioxidants, 14(10), 1175. https://doi.org/10.3390/antiox14101175







