Dietary Supplements Derived from Food By-Products for the Management of Diabetes Mellitus
Abstract
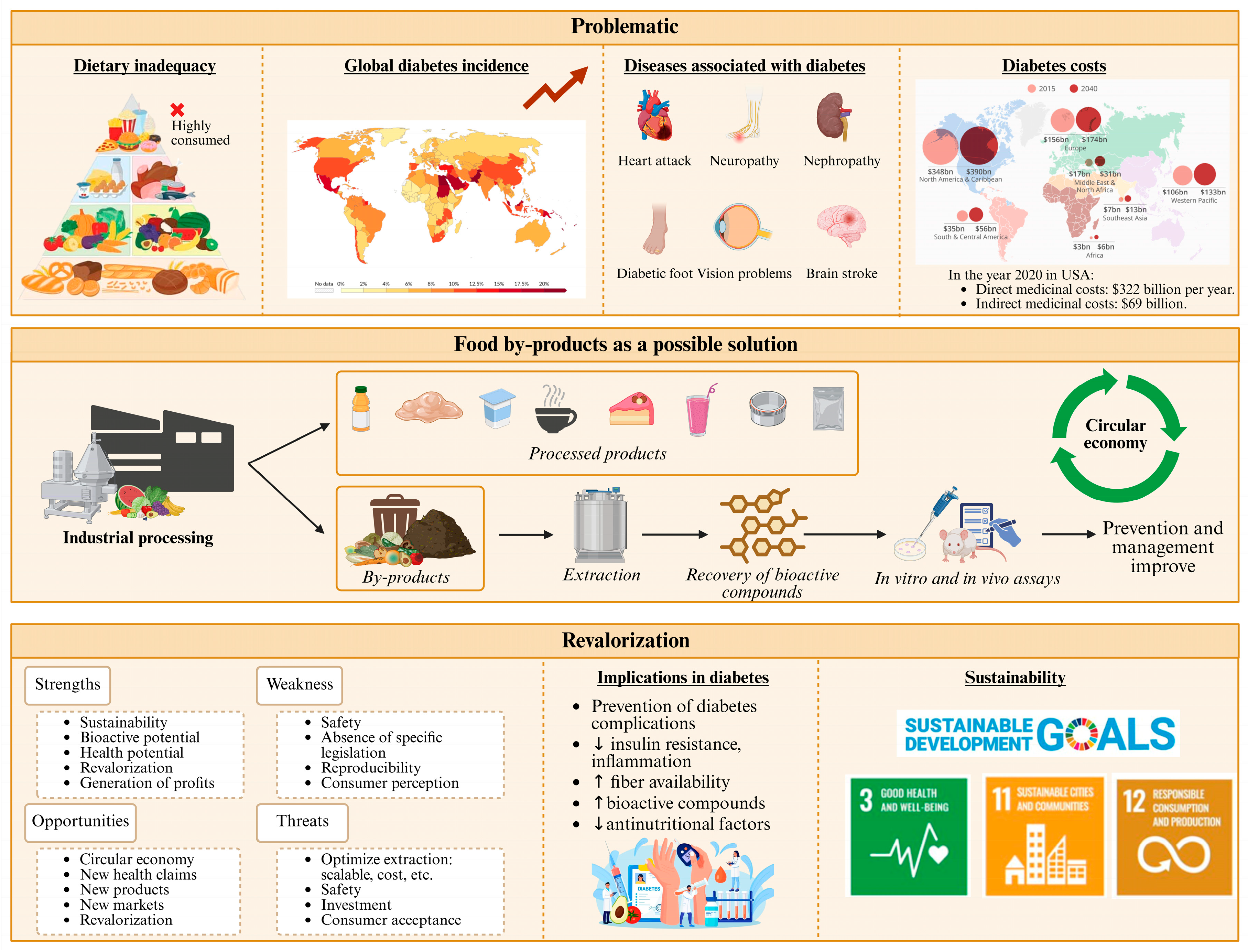
1. Introduction
2. Review Methodology
3. Food By-Products as Sources of Dietary Supplements for Diabetes Prevention
| By-Product | Compounds | Extraction | Concentration | AD Mechanism | Bioactivity | Applications | Ref. |
|---|---|---|---|---|---|---|---|
| Polyphenols | |||||||
| Orange peel | Naringin, naringenin | MA: EtOH 70%, 72 h | 100 mg/kg bw, 4 weeks | ↑ insulin, C-peptide, glycogen | AD, AO | PR, DS | [47] |
| Grapefruit peel | Polyphenols | MA: Acet 80% | 80–240 µg/mL | AG | AD, AO, AM, AH | PR, DS | [48] |
| Lemon peel | Limonoids | MA: EtOH, 24 h | 16.53 mg/mL | Join protein | AD, AO | PR, DS | [49] |
| Pear peel | Rutin, catechin, epicatechin | UAE: MetOH 60% | 25–75% | ↑ insulin SE, GU | AD, AO, AI | PR, DS | [50,51] |
| Pomegranate peel | Flavonoids, tannins | SE: MetOH 80% | 1 mg/mL | ↓ GL | AD, AO, OB | PR, DS | [52] |
| Orange peel | Flavonoids, phenolics | SE: MetOH 80% | 1 mg/mL | ↓ GL | AD, AO, OB | PR, DS | [52] |
| Mango peel | Flavonoids, anthocyanins | Powder | 5–10% | ↑ insulin SE, GU | AD, AO | PR, DS | [53] |
| Mango leaves | Polyphenols | SE | 80% | AG | AD, AO | CF | [54] |
| Grape seeds | Flavonoids, procyanidins | SE: EtOH 70%, 3 h | 52.01 and 152.18 mg/g dw | AG | AD, AO, OB | PR, DS | [55] |
| Apple pomace | Quercetin derivates | SE | 20 mg/kg | AG | AD, AO | PR, FI | [56] |
| Carrot pomace | Polyphenols | UAE: 30 °C, 80 min, 500 W | 150.6 mg/L | AG | AD, AO | PR, FI | [57] |
| Walnut husk | Polyphenols | SE | 74.08–166.44 mg/g | AG | AD, AO, AM | PR, FI | [58] |
| Fiber | |||||||
| Banana peel | Dietary fiber | DE, W | nd | ↓ FI, GU; ↑ insulin, GLP-1 | AD, AO | PR, DS | [59] |
| Mango peel | Dietary fiber | SE | 12.8–23% | ↓ FI, GU | AD, AO | PR, FI | [60,61] |
| Papaya peel | Pectin, lignin | SE: EtOH | nd | ↓ FI, GU | AD, MM, SA | PR, FI | [62] |
| Orange albedo | Pectin | SE: EtOH, 75 °C, 1 h | 18.73% | AG | AD, TP | PR, FI | [63] |
| Vitamins | |||||||
| Orange, grapefruit, and lemon peel | Vitamin C | MA: EtOH, 24 h | 110.4, 113.3, and 58.6 mg/100 g | ↑ glycemic control | AD, AO | PR, FI | [64] |
| Fatty acids | |||||||
| Walnut oil cake | Omega-3 | SE | 52–70% | AG | AD, AO, AI, CP | PR, FI | [65] |
| Fish waste | Omega-3 | UAE: hex, 60 °C, 80 min | 45.1% | AG | AD, AI, CP | PR, FI | [66,67] |
| Proteins | |||||||
| Fish processing waste | Bioactive peptides | EAE: papain, bromelain | 18.49% | ↑ GLP-1, ↑ insulin SE | AD, AO | PR, FI | [68,69] |
| Salmon co-products | Bioactive peptides | EAE: FoodPro, alcalase | 57–74% | AG | AD, AO | PR, FI | [70] |
| Cheese by-product | Whey proteins | SE | 85% | ↑ insulin SE, lipid metabolism | AD, TP | PR, FI | [71] |
| Other | |||||||
| Tomato waste | Lycopene | UEA: EtOH, 220 W, 25 min | 12–19 µg/g dw | ↓ GL | AD, AO | PR, FI | [72] |
| Tomato waste | Lycopene | SE: Acet:hex 1:3 | 5.32 mg/100 g fw | ↓ GL | AD, AO, AI | PR, FI | [73] |
| Carrot pomace | β-Carotene | UAE: 30 °C, 80 min, 500 W | 150.58 mg/L | ↓ GL | AD, AO | PR, FI | [57] |
| Carrot pomace | Carotenoids | UAE: EtOH 50%, 40 °C, 750 W | 51% | ↓ GL | AD, AO, AI | PR, FI | [74] |
| Orange albedo | Hesperidin | SE: MetOH, RT | 1.0–2.8% | ↓ GL | AD, AI, CP | PR, FI | [75] |
4. Mechanisms of Action of Dietary Supplements Derived from Food By-Products Involved in Diabetes Prevention
4.1. Regulation of Blood Glucose Levels
4.2. Improved Insulin Sensitivity
4.3. Reduction in Inflammation and Antioxidant Effects
4.4. Regulation of Lipid and Carbohydrate Metabolism
5. Efficacy of Dietary Supplements Derived from Food By-Products in Diabetes Prevention
5.1. Polyphenolic Compounds
5.2. Fiber
5.3. Vitamins and Minerals
5.4. Amino Acids and Proteins
5.5. Polyunsaturated Fatty Acids
| Study | Treatment | Mechanism Suggested | Therapeutic Effect | Ref. |
|---|---|---|---|---|
| Traditional treatments | ||||
| DBPC RCT (n = 41) | Met, 1 g, b.i.d., 26 weeks | N.S. | ↑ intestinal glucose uptake, glycemic control | [147] |
| DBPC RCT (n = 366) | Pio, Met, Dapa, 15–30 mg/day, 24 weeks | N.S. | ↓ in HbA1c of −0.38 to −0.83% | [177] |
| PUFAs | ||||
| DBPC RCT (n = 170) | DHA, 350 mg/day, 2 years | N.S. | Not slowing action in NPDR progression | [178] |
| RCT (n = 84) | EPA, DHA, 60 + 308 mg/day, 9 months | ↓ AA | ↑ maternal and fetal FA status (T1DM) | [179] |
| Mice (n = 56) | EPA, DHA, 1%, 15 days | ↓ OS, AI, ┤ β-cell apoptosis | Prevent pancreatic injury | [180] |
| PS (n = 47,663) ** | N.S. | AI, ↓ VLDL-C | ↓ CHD | [181] |
| DBPC RCT (n = 26) ** | EPA, DHA, PUFA, 1.8, 3.0 and 5.9 g/day, 9 weeks | Change energy use, ↓ glucose uptake | ↑ blood glucose; ↓ insulin sensitivity | [175] |
| DB (n = 100) | EPA, DHA, 500 and 200 mg/day, 12 weeks | AI | ↑ insulin levels; ↓ inflammatory markers, Chl | [174] |
| DBPC RCT (n = 44) | EPA and DHA, 310 and 210 mg/day, 10 weeks | N.S. | ↑ QUICKI levels; ↓ insulin, HOMA-IR levels (T2DM) | [176] |
| Amino acids | ||||
| RCT (n = 65) | EAA 11.7 g/day//Tyr 0.1 g/day//Cys 0.4 g/day, 12 weeks | N.S. | ↑ myocardial dysfunction (T2DM) | [166] |
| RCT (n = 34) | EAA 7.6 g/day//Tyr 0.1 g/day//Cys 0.3 g/day, 42 weeks | ↑ insulin secretion, postprandial glucose | ↑ insulin sensitivity, glycemic control (T2DM) | [167] |
| Vitamins and minerals | ||||
| DBPC RCT (n = 64) | Cr, 200 μg/day, 12 weeks | N.S. | ↑ insulin sensitivity; ↓ insulin resistance (T2DM) | [162] |
| RCT (n = 71) | Cr, 600 μg/day, 4 months | ↑ insulin signaling, GLUT4 translocation | ↓ fasting and postprandial glucose; ≈ lipid profile | [123] |
| DBPC RCT (n = 44) | Zn, 50 mg/day, 12 weeks | ↑ insulin signaling; ↓ insulin resistance | ↓ fasting glucose and HOMA-IR (T2DM) | [164] |
| RCT (n = 100) | Vitamin D, 125 μg/day (5000 IU/day), 12 weeks | N.S. | ≈ inflammatory and OS (T2DM) | [182] |
| DBPC RCT (n = 127) | Vitamin D, 100 μg/day (4000 IU/day), 48 weeks | N.S. | ≈ Met (T2DM) | [183] |
| Polyphenols | ||||
| RCT (n = 35) * | EGCG 120 mg/EGC 60 mg/ECG 25 mg EC; 2 caps./day; 8 weeks | AI | Regulation of weight gain | [155] |
| DBPC RCT (n = 64) * | EGCG, EGC, EGC, EC, 2 caps 530 mg/day, 6 weeks | ↑ FO: LKB1/AMP, modulation COMT | ↓ circulating SAA | [156] |
| DB (n = 100) | Cur, 80 mg nanocurcumin/day, 12 weeks | AI | ↑ insulin levels; ↓ inflammatory, Chl | [174] |
| DBPC RCT (n = 53) | Cur, 1500 mg, 10 weeks | ┤ protein replication, AI cytokines; ↑ RMR | ↓ fasting blood glucose and weight (T2DM) | [157] |
6. Safety and Toxicity of Food By-Product Derived Supplements
7. Regulatory Framework
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Generic. | |
| Cr | Chromium |
| DHA | Docosahexaenoic acid |
| DM | Diabetes mellitus |
| GLP-1 | Glucagon-like peptide-1 |
| HbA1c | Glycosylated hemoglobin |
| PUFAs | Polyunsaturated fatty acids |
| BGL | Blood glucose levels |
| COMT | Catechol-O-methyltransferase |
| FI | Food intake |
| FO | Fat oxidation |
| GL | Glucose levels |
| GLUT4 | Glucose transporter 4 |
| GU | Glucose uptake |
| HOMA-IR | Homeostatic model assessment of insulin resistance index |
| LKB1/AMP | Liver kinase B1/adenosine monophosphate activated protein kinase |
| NF-Κb | Nuclear factor κB |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| OS | Oxidative stress |
| PPAR | Peroxisome proliferator-activated receptors |
| QUICKI | Quantitative insulin sensitivity check index |
| RMR | Resting metabolic rate |
| ROS | Reactive oxygen species |
| SAA | Serum amyloid plasma |
| SE | Sensivity |
| VLDL-C | Very low-density lipoprotein cholesterol |
| Applications. | |
| CF | Commercial formulations |
| DS | Dietary supplementation |
| FI | Food ingredient |
| PR | Preservative |
| TP | Technological properties |
| Bioactivities. | |
| AA | Anti-aging |
| AD | Anti-diabetic |
| AG | α-amylase and α-glucosidase inhibition |
| AH | Anti-hypertensive |
| AI | Anti-inflammatory |
| AM | Antimicrobial |
| AO | Antioxidant activity |
| CP | Cardiovascular protection |
| MM | Microbiota modulation |
| OB | Anti-obesity |
| SA | Satiating |
| Compounds. | |
| AA | Arachidonic acids |
| AAs | Amino acids |
| AGEs | Advanced glycation end products |
| ALA | Alpha-lipoic acid |
| Chl | Cholesterol |
| Cr | Chromium |
| Cur | Curcumin |
| Cys | Cystine |
| Dapa | Dapagliflozin |
| DHA | Docosahexaenoic acid |
| EAA | Essential amino acids |
| EC | Epicatechin |
| ECG | Epicatechin gallate |
| EGC | Epigallocatechin |
| EGCG | Epigallocatechin gallate |
| EPA | Eicosapentaenoic acid |
| FA | Fatty acids |
| GLP-1 | Glucagon-like peptide-1 |
| HbA1c | Glycosylated hemoglobin |
| Met | Metformin |
| N.S. | Not specified |
| Pio | Pioglitazone |
| PUFAs | Polyunsaturated fatty acids |
| Tyr | Tyrosine |
| Diseases. | |
| CHD | Coronary heart disease |
| NPDR | Non-proliferative diabetic retinopathy |
| T2DM | Type 2 diabetic mellitus |
| Extraction. | |
| DE | Decoction |
| EAE | Enzyme-assisted extraction |
| MA | Maceration |
| SE | Solvent extraction |
| UAE | Ultrasound-assisted extraction |
| Solvents. | |
| Acet | Acetone |
| hex | Hexane |
| MetOH | Methanol |
| W | Deionized water |
| Study. | |
| DB | Double-blind controlled study |
| DBPC RCT | Double-blind placebo-controlled randomized controlled trial |
| n | Number of individuals |
| PS | Prospective study |
| RCT | Single-blind, placebo-controlled trial |
| RCT | Randomized controlled trial |
Appendix A
| Category | Parameter | Specification/Guideline | Notes |
|---|---|---|---|
| Source and Identity | Botanical/by-product identification | Include scientific name, plant part or food by-product, and origin | Ensures traceability and correct identification. |
| Extraction method | Detail solvents, temperature, duration, and yield | Extraction method influences chemical composition and safety. | |
| Composition | Marker compounds | Quantify key bioactive compounds (e.g., total polyphenols, fiber) | Allows standardization and comparison between batches or studies. |
| Additional constituents | Identify other relevant compounds (e.g., flavonoids, saponins, tannins) | Supports understanding of efficacy and potential toxicity. | |
| Dose Information | Human-relevant dose range | Express as mg/kg body weight or mg/day equivalent | Facilitates translation of experimental data to practical use. |
| Unit standardization | Consistently report per extract weight or per active compound | Avoids ambiguity in dosing and reporting. | |
| Purity and Safety | Residual solvents | Follow international guidelines | Prevents exposure to harmful solvents. |
| Heavy metals | Below internationally accepted limits | Ensures safety and regulatory compliance. | |
| Pesticides/Mycotoxins | Below internationally accepted limits | Minimizes health risks from contamination. | |
| Microbiological quality | Absence of pathogens; total microbial counts within accepted limits | Ensures hygienic quality of extracts. | |
| Variability and Reproducibility | Raw material variability | Document seasonal or batch variations | Accounts for differences in composition between raw material lots. |
| Batch-to-batch consistency | Use analytical profiling (e.g., HPLC, LC-MS) | Confirms reproducibility of results. | |
| Toxicity Data | Screening | Include in vitro cytotoxicity and, if available, in vivo safety data | Provides initial safety assessment for potential use. |
| Regulatory Compliance | Alignment with guidelines | All extracts should comply with the relevant legislation and regulatory requirements of the country in which they are intended to be marketed | Supports safe and regulated use of extracts. |
References
- Banday, M.Z.; Sameer, A.S.; Nissar, S. Pathophysiology of diabetes: An overview. Avicenna J. Med. 2020, 10, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef] [PubMed]
- Popović-Djordjević, J.B.; Katanić Stanković, J.S.; Mihailović, V.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Algae as a Source of Bioactive Compounds to Prevent the Development of Type 2 Diabetes Mellitus. Curr. Med. Chem. 2021, 28, 4592–4615. [Google Scholar] [CrossRef]
- Silva-Tinoco, R.; Cuatecontzi-Xochitiotzi, T.; Morales-Buenrostro, L.E.; Gracia-Ramos, A.E.; Aguilar-Salinas, C.A.; Castillo-Martínez, L. Prevalence of Chronic Kidney Disease in Individuals with Type 2 Diabetes Within Primary Care: A Cross-Sectional Study. J. Prim. Care Community Health 2024, 15, 21501319241259324. [Google Scholar] [CrossRef]
- Galaviz, K.I.; Narayan, K.M.V.; Lobelo, F.; Weber, M.B. Lifestyle and the Prevention of Type 2 Diabetes: A Status Report. Am. J. Lifestyle Med. 2018, 12, 4–20. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Beltrán-Velasco, A.I.; Rubio-Zarapuz, A.; Martínez-Guardado, I.; Valcárcel-Martín, R.; Tornero-Aguilera, J.F. Functional and Therapeutic Roles of Plant-Derived Antioxidants in Type 2 Diabetes Mellitus: Mechanisms, Challenges, and Considerations for Special Populations. Antioxidants 2025, 14, 725. [Google Scholar] [CrossRef]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef]
- Myhrstad, M.C.W.; Tunsjø, H.; Charnock, C.; Telle-Hansen, V.H. Dietary Fiber, Gut Microbiota, and Metabolic Regulation—Current Status in Human Randomized Trials. Nutrients 2020, 12, 859. [Google Scholar] [CrossRef]
- Dawi, J.; Misakyan, Y.; Affa, S.; Kades, S.; Narasimhan, A.; Hajjar, F.; Besser, M.; Tumanyan, K.; Venketaraman, V. Oxidative Stress, Glutathione Insufficiency, and Inflammatory Pathways in Type 2 Diabetes Mellitus: Implications for Therapeutic Interventions. Biomedicines 2024, 13, 18. [Google Scholar] [CrossRef]
- Viana, M.D.M.; Santos, S.S.; Cruz, A.B.O.; de Jesus, M.V.A.C.; Lauria, P.S.S.; Lins, M.P.; Villarreal, C.F. Probiotics as Antioxidant Strategy for Managing Diabetes Mellitus and Its Complications. Antioxidants 2025, 14, 767. [Google Scholar] [CrossRef]
- Cross, L.V.; Thomas, J.R. Safety and Efficacy of Dietary Supplements for Diabetes. Diabetes Spectr. 2021, 34, 67–72. [Google Scholar] [CrossRef]
- Hannon, B.A.; Fairfield, W.D.; Adams, B.; Kyle, T.; Crow, M.; Thomas, D.M. Use and abuse of dietary supplements in persons with diabetes. Nutr. Diabetes 2020, 10, 14. [Google Scholar] [CrossRef]
- Fagherazzi, G.; Ravaud, P. Digital diabetes: Perspectives for diabetes prevention, management and research. Diabetes Metab. 2019, 45, 322–329. [Google Scholar] [CrossRef]
- Schulze, M.B.; Hu, F.B. Primary prevention of diabetes: What Can Be Done and How Much Can Be Prevented? Annu. Rev. Public Health 2005, 26, 445–467. [Google Scholar] [CrossRef]
- Paschou, S.A.; Marina, L.V.; Spartalis, E.; Anagnostis, P.; Alexandrou, A.; Goulis, D.G.; Lambrinoudaki, I. Therapeutic strategies for type 2 diabetes mellitus in women after menopause. Maturitas 2019, 126, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.; Soliternik, J.; Mazzola, N. Nutritional supplements for the prevention of diabetes mellitus and its complications. J. Nutr. Intermed. Metab. 2018, 14, 16–21. [Google Scholar] [CrossRef]
- Reynolds, A.; Mitri, J. Dietary Advice For Individuals with Diabetes. Feingold 2024. [Google Scholar]
- Bergman, M.; Buysschaert, M.; Schwarz, P.E.; Albright, A.; Narayan, K.V.; Yach, D. Diabetes prevention: Global health policy and perspectives from the ground. Diabetes Manag. Lond. Engl. 2012, 2, 309–321. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Pereira, A.G.; Echave, J.; Jorge, A.O.S.; Nogueira-Marques, R.; Yuksek, E.N.; Barciela, P.; Perez-Vazquez, A.; Chamorro, F.; Oliveira, M.B.P.P.; Carpena, M.; et al. Therapeutic and Preventive Potential of Plant-Derived Antioxidant Nutraceuticals. Foods 2025, 14, 1749. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, S.; Khalid, W.; Kumar, P.; Benmebarek, I.E.; Rasool, I.F.U.; Trif, M.; Moreno, A.; Esatbeyoglu, T. Valorization of plant-based agro-industrial waste and by-products for the production of polysaccharides: Towards a more circular economy. Appl. Food Res. 2025, 5, 100954. [Google Scholar] [CrossRef]
- Kumar Gupta, R.; AE Ali, E.; Abd El Gawad, F.; Mecheal Daood, V.; Sabry, H.; Karunanithi, S.; Prakash Srivastav, P. Valorization of fruits and vegetables waste byproducts for development of sustainable food packaging applications. Waste Manag. Bull. 2024, 2, 21–40. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020, 11, 4853–4877. [Google Scholar] [CrossRef]
- Skwarek, P.; Karwowska, M. Fruit and vegetable processing by-products as functional meat product ingredients -a chance to improve the nutritional value. LWT 2023, 189, 115442. [Google Scholar] [CrossRef]
- Egbuna, C.; Awuchi, C.G.; Kushwaha, G.; Rudrapal, M.; Patrick-Iwuanyanwu, K.C.; Singh, O.; Odoh, U.E.; Khan, J.; Jeevanandam, J.; Kumarasamy, S.; et al. Bioactive Compounds Effective Against Type 2 Diabetes Mellitus: A Systematic Review. Curr. Top. Med. Chem. 2021, 21, 1067–1095. [Google Scholar] [CrossRef]
- Malik, F.; Iqbal, A.; Zia, S.; Ranjha, M.M.A.N.; Khalid, W.; Nadeem, M.; Selim, S.; Hadidi, M.; Moreno, A.; Manzoor, M.F.; et al. Role and mechanism of fruit waste polyphenols in diabetes management. Open Chem. 2023, 21, 20220272. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Pereira, A.G.; Lourenço-Lopes, C.; Garcia-Oliveira, P.; Cassani, L.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Main bioactive phenolic compounds in marine algae and their mechanisms of action supporting potential health benefits. Food Chem. 2021, 341, 128262. [Google Scholar] [CrossRef]
- Shahidi, F.; Vamadevan, V.; Oh, W.Y.; Peng, H. Phenolic compounds in agri-food by-products, their bioavailability and health effects. J. Food Bioact. 2019, 5, 57–119. [Google Scholar] [CrossRef]
- Fischer, M.; Timper, K.; Radimerski, T.; Dembinski, K.; Frey, D.M.; Zulewski, H.; Keller, U.; Müller, B.; Christ-Crain, M.; Grisouard, J. Metformin induces glucose uptake in human preadipocyte-derived adipocytes from various fat depots. Diabetes Obes. Metab. 2010, 12, 356–359. [Google Scholar] [CrossRef]
- Ruan, Y.; Sun, J.; He, J.; Chen, F.; Chen, R.; Chen, H. Effect of Probiotics on Glycemic Control: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. PLoS ONE 2015, 10, e0132121. [Google Scholar] [CrossRef]
- Echave, J.; Pereira, A.G.; Jorge, A.O.S.; Barciela, P.; Nogueira-Marques, R.; Yuksek, E.N.; Oliveira, M.B.P.P.; Barros, L.; Prieto, M.A. Grape Winemaking By-Products: Current Valorization Strategies and Their Value as Source of Tannins with Applications in Food and Feed. Molecules 2025, 30, 2726. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Toledo, R.T. Major Flavonoids in Grape Seeds and Skins: Antioxidant Capacity of Catechin, Epicatechin, and Gallic Acid. J. Agric. Food Chem. 2004, 52, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef]
- Wen, L.; Wu, D.; Tan, X.; Zhong, M.; Xing, J.; Li, W.; Li, D.; Cao, F. The Role of Catechins in Regulating Diabetes: An Update Review. Nutrients 2022, 14, 4681. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Chukwuma, C.I.; Nambooze, J.; Tripathy, S.; Salau, V.F.; Olofinsan, K.; Ogunlakin, A.D.; Ebuehi, O.A.T.; Unuofin, J.O. Tea Consumption and Diabetes: A Comprehensive Pharmacological Review of Black, White, Green, Oolong, and Pu-erh Teas. Plants 2025, 14, 1898. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Garcia-Perez, P.; Cassani, L.; Chamorro, F.; Cao, H.; Barba, F.J.; Simal-Gandara, J.; Prieto, M.A. Camellia japonica: A phytochemical perspective and current applications facing its industrial exploitation. Food Chem. X 2022, 13, 100258. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Fraga-Corral, M.; Garciá-Oliveira, P.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Carpena, M.; Otero, P.; Gullón, P.; Prieto, M.A.; Simal-Gandara, J. Culinary and nutritional value of edible wild plants from northern Spain rich in phenolic compounds with potential health benefits. Food Funct. 2020, 11, 8493–8515. [Google Scholar] [CrossRef]
- Donn, P.; Barciela, P.; Perez-Vazquez, A.; Cassani, L.; Simal-Gandara, J.; Prieto, M.A. Bioactive Compounds of Verbascum sinuatum L.: Health Benefits and Potential as New Ingredients for Industrial Applications. Biomolecules 2023, 13, 427. [Google Scholar] [CrossRef]
- Plakantonaki, S.; Roussis, I.; Bilalis, D.; Priniotakis, G. Dietary Fiber from Plant-Based Food Wastes: A Comprehensive Approach to Cereal, Fruit, and Vegetable Waste Valorization. Processes 2023, 11, 1580. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, G.; Pan, Y.; Chen, W.; Huang, W.; Chen, H.; Li, Y. Properties of soluble dietary fiber-polysaccharide from papaya peel obtained through alkaline or ultrasound-assisted alkaline extraction. Carbohydr. Polym. 2017, 172, 102–112. [Google Scholar] [CrossRef]
- Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A.; Aguilera, Y.; Esteban, R.M. Physicochemical properties and in vitro antidiabetic potential of fibre concentrates from onion by-products. J. Funct. Foods 2017, 36, 34–42. [Google Scholar] [CrossRef]
- Budhwar, S.; Chakraborty, M.; Sethi, K.; Chatterjee, A. Antidiabetic properties of rice and wheat bran—A review. J. Food Biochem. 2020, 44, e13424. [Google Scholar] [CrossRef]
- Kučuk, N.; Primožič, M.; Kotnik, P.; Knez, Ž.; Leitgeb, M. Mango Peels as an Industrial By-Product: A Sustainable Source of Compounds with Antioxidant, Enzymatic, and Antimicrobial Activity. Foods 2024, 13, 553. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Tan, C.; Zhang, B.; Wu, W.; Shang, N. The Valorization of Banana By-Products: Nutritional Composition, Bioactivities, Applications, and Future Development. Foods 2022, 11, 3170. [Google Scholar] [CrossRef] [PubMed]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Ahmed, O.M.; Hassan, M.A.; Abdel-Twab, S.M.; Abdel Azeem, M.N. Navel orange peel hydroethanolic extract, naringin and naringenin have anti-diabetic potentials in type 2 diabetic rats. Biomed. Pharmacother. 2017, 94, 197–205. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosum, A.O. Phenolic extracts from grapefruit peels (Citrus paradisi) inhibit key enzymes linked with type 2 diabetes and hypertension. J. Food Biochem. 2011, 35, 1703–1709. [Google Scholar] [CrossRef]
- El-Feky, A.M.; Aboulthana, W.M.; El-Rashedy, A.A. Assessment of the in vitro anti-diabetic activity with molecular dynamic simulations of limonoids isolated from Adalia lemon peels. Sci. Rep. 2024, 14, 21478. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-safi, I.; Haddad, H.; Bekkari, H.; Grafov, A.; Bousta, D. Combination of Catechin, Epicatechin, and Rutin: Optimization of a novel complete antidiabetic formulation using a mixture design approach. J. Nutr. Biochem. 2021, 88, 108520. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef]
- Smaniotto, F.A.; Dluzniewski, L.T.; Bortolazzo, P.C.; Monteiro, C.S.; Baranzelli, J.; da Silva, D.T.; Somacal, S.; Conterato, G.M.M.; Emanuelli, T. In vitro assessment of antidiabetic, anti-obesogenic, and antioxidant potential of pulp and seed extracts from Eugenia involucrata fruits. Food Res. Int. 2025, 202, 115693. [Google Scholar] [CrossRef]
- Gondi, M.; Basha, S.A.; Bhaskar, J.J.; Salimath, P.V.; Prasada Rao, U.J.S. Anti-diabetic effect of dietary mango (Mangifera indica L.) peel in streptozotocin-induced diabetic rats. J. Sci. Food Agric. 2015, 95, 991–999. [Google Scholar] [CrossRef]
- Yousefifar, H. Development of a Food Supplement for Diabetes Type II Management with Enhanced Antidiabetic and Antioxidant Properties from Selected Local Foods. Ph.D. Thesis, University of Nairobi, Nairobi, Kenya, 2019. [Google Scholar]
- Ivanov, Y.; Atanasova, M.; Godjevargova, T. Nutritional and Functional Values of Grape Seed Flour and Extract for Production of Antioxidative Dietary Supplements and Functional Foods. Molecules 2025, 30, 2029. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Kammerer, J.; Valet, R.; Carle, R. Recovery of polyphenols from the by-products of plant food processing and application as valuable food ingredients. Food Res. Int. 2014, 65, 2–12. [Google Scholar] [CrossRef]
- Salehi, L.; Dinani, S.T. Application of electrohydrodynamic—Ultrasonic procedure for extraction of β-carotene from carrot pomace. J. Food Meas. Charact. 2020, 14, 3031–3039. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comprehensive Review on the Chemical Constituents and Functional Uses of Walnut (Juglans spp.) Husk. Int. J. Mol. Sci. 2019, 20, 3920. [Google Scholar] [CrossRef]
- Wang, M.; Yang, F.; Yan, X.; Chao, X.; Zhang, W.; Yuan, C.; Zeng, Q. Anti-diabetic effect of banana peel dietary fibers on type 2 diabetic mellitus mice induced by streptozotocin and high-sugar and high-fat diet. J. Food Biochem. 2022, 46, e14275. [Google Scholar] [CrossRef]
- Tariq, A.; Sahar, A.; Usman, M.; Sameen, A.; Azhar, M.; Tahir, R.; Younas, R.; Issa Khan, M. Extraction of dietary fiber and polyphenols from mango peel and its therapeutic potential to improve gut health. Food Biosci. 2023, 53, 102669. [Google Scholar] [CrossRef]
- Ajila, C.M.; Prasada Rao, U.J.S. Mango peel dietary fibre: Composition and associated bound phenolics. J. Funct. Foods 2013, 5, 444–450. [Google Scholar] [CrossRef]
- Nieto-Calvache, J.E.; de Escalada Pla, M.; Gerschenson, L.N. Dietary fibre concentrates produced from papaya by—Products for agroindustrial waste valorisation. Int. J. Food Sci. Technol. 2019, 54, 1074–1080. [Google Scholar] [CrossRef]
- Figueira, O.; Pereira, V.; Castilho, P.C. A Two-Step Approach to Orange Peel Waste Valorization: Consecutive Extraction of Pectin and Hesperidin. Foods 2023, 12, 3834. [Google Scholar] [CrossRef]
- Sir Elkhatim, K.A.; Elagib, R.A.A.; Hassan, A.B. Content of phenolic compounds and vitamin C and antioxidant activity in wasted parts of Sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef]
- Song, H.; Cong, Z.; Wang, C.; He, M.; Liu, C.; Gao, P. Research progress on Walnut oil: Bioactive compounds, health benefits, extraction methods, and medicinal uses. J. Food Biochem. 2022, 46, e14504. [Google Scholar] [CrossRef]
- Ciftci, O.; Keskin Cavdar, H. Ultrasound—Assisted Extraction Improved the Extraction Rate, Omega-3, and Polyunsaturated Fatty Acid Content of Fish Oil From Atlantic Bonito Waste. Eur. J. Lipid Sci. Technol. 2025, 127, e202400131. [Google Scholar] [CrossRef]
- Rodrigues, M.; Rosa, A.; Almeida, A.; Martins, R.; Ribeiro, T.; Pintado, M.; Gonçalves, R.F.S.; Pinheiro, A.C.; Fonseca, A.J.M.; Maia, M.R.G.; et al. Omega-3 fatty acids from fish by-products: Innovative extraction and application in food and feed. Food Bioprod. Process. 2024, 145, 32–41. [Google Scholar] [CrossRef]
- Ishak, N.H.; Sarbon, N.M. A Review of Protein Hydrolysates and Bioactive Peptides Deriving from Wastes Generated by Fish Processing. Food Bioprocess Technol. 2018, 11, 2–16. [Google Scholar] [CrossRef]
- Gajanan, P.G.; Elavarasan, K.; Shamasundar, B.A. Bioactive and functional properties of protein hydrolysates from fish frame processing waste using plant proteases. Environ. Sci. Pollut. Res. 2016, 23, 24901–24911. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.M.; FitzGerald, R.J. Atlantic salmon (Salmo salar) co-product-derived protein hydrolysates: A source of antidiabetic peptides. Food Res. Int. 2018, 106, 598–606. [Google Scholar] [CrossRef]
- Ostojić, S.; Pavlović, M.; Živić, M.; Filipović, Z.; Gorjanović, S.; Hranisavljević, S.; Dojčinović, M. Processing of whey from dairy industry waste. Environ. Chem. Lett. 2005, 3, 29–32. [Google Scholar] [CrossRef]
- Mavridis, K.; Todas, N.; Kalompatsios, D.; Athanasiadis, V.; Lalas, S.I. Lycopene and Other Bioactive Compounds’ Extraction from Tomato Processing Industry Waste: A Comparison of Ultrasonication Versus a Conventional Stirring Method. Horticulturae 2025, 11, 71. [Google Scholar] [CrossRef]
- Poojary, M.M.; Passamonti, P. Extraction of lycopene from tomato processing waste: Kinetics and modelling. Food Chem. 2015, 173, 943–950. [Google Scholar] [CrossRef]
- Umair, M.; Jabbar, S.; Nasiru, M.M.; Lu, Z.; Zhang, J.; Abid, M.; Murtaza, M.A.; Kieliszek, M.; Zhao, L. Ultrasound-Assisted Extraction of Carotenoids from Carrot Pomace and Their Optimization through Response Surface Methodology. Molecules 2021, 26, 6763. [Google Scholar] [CrossRef]
- Victor, M.M.; David, J.M.; Cortez, M.V.M.; Leite, J.L.; da Silva, G.S.B. A High-Yield Process for Extraction of Hesperidin from Orange (Citrus sinensis L. osbeck) Peels Waste, and Its Transformation to Diosmetin, A Valuable and Bioactive Flavonoid. Waste Biomass Valorization 2021, 12, 313–320. [Google Scholar] [CrossRef]
- Afolayan, A.; Wintola, O. Dietary Supplements in the Management of Hypertension and Diabetes—A Review. African J. Tradit. Complement. Altern. Med. 2014, 11, 248. [Google Scholar] [CrossRef]
- Soares, T.F.; Oliveira, M.B.P.P. Cocoa By-Products: Characterization of Bioactive Compounds and Beneficial Health Effects. Molecules 2022, 27, 1625. [Google Scholar] [CrossRef]
- Scurria, A.; Lino, C.; Pitonzo, R.; Pagliaro, M.; Avellone, G.; Ciriminna, R. Vitamin D3 in fish oil extracted with limonene from anchovy leftovers. Chem. Data Collect. 2020, 25, 100311. [Google Scholar] [CrossRef]
- Lewgood, J.; Oliveira, B.; Korzepa, M.; Forbes, S.C.; Little, J.P.; Breen, L.; Bailie, R.; Candow, D.G. Efficacy of Dietary and Supplementation Interventions for Individuals with Type 2 Diabetes. Nutrients 2021, 13, 2378. [Google Scholar] [CrossRef] [PubMed]
- Radenkovs, V.; Püssa, T.; Juhnevica-Radenkova, K.; Kviesis, J.; Salar, F.J.; Moreno, D.A.; Drudze, I. Wild apple (Malus spp.) by-products as a source of phenolic compounds and vitamin C for food applications. Food Biosci. 2020, 38, 100744. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Martínez-Ballesta, M.C.; Carvajal, M.; García-Viguera, C.; Moreno, D.A. Broccoli-Derived By-Products—A Promising Source of Bioactive Ingredients. J. Food Sci. 2010, 75, C383–C392. [Google Scholar] [CrossRef]
- Fernández-López, J.; Fernández-Ginés, J.M.; Aleson-Carbonell, L.; Sendra, E.; Sayas-Barberá, E.; Pérez-Alvarez, J.A. Application of functional citrus by-products to meat products. Trends Food Sci. Technol. 2004, 15, 176–185. [Google Scholar] [CrossRef]
- Barba, F.J.; Putnik, P.; Bursać Kovačević, D.; Poojary, M.M.; Roohinejad, S.; Lorenzo, J.M.; Koubaa, M. Impact of conventional and non-conventional processing on prickly pear (Opuntia spp.) and their derived products: From preservation of beverages to valorization of by-products. Trends Food Sci. Technol. 2017, 67, 260–270. [Google Scholar] [CrossRef]
- Zhou, C.; Na, L.; Shan, R.; Cheng, Y.; Li, Y.; Wu, X.; Sun, C. Dietary Vitamin C Intake Reduces the Risk of Type 2 Diabetes in Chinese Adults: HOMA-IR and T-AOC as Potential Mediators. PLoS ONE 2016, 11, e0163571. [Google Scholar] [CrossRef]
- Montonen, J.; Knekt, P.; Jarvinen, R.; Reunanen, A. Dietary Antioxidant Intake and Risk of Type 2 Diabetes. Diabetes Care 2004, 27, 362–366. [Google Scholar] [CrossRef]
- Sluijs, I.; Cadier, E.; Beulens, J.W.J.; Spijkerman, A.M.W.; Van der Schouw, Y.T. Dietary intake of carotenoids and risk of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 376–381. [Google Scholar] [CrossRef]
- Watanabe, F.; Bito, T.; Koseki, K. Salmon meats and by-products as excellent sources of vitamin B12. Fish. Sci. 2025, 91, 405–415. [Google Scholar] [CrossRef]
- Zhou, Y.; He, A.; Xu, B. Natural resources, quantification, microbial bioconversion, and bioactivities of vitamin B12 for vegetarian diet. Food Chem. 2025, 463, 140849. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, J.; Wang, H.; Wang, X.; Liu, S. Vitamin D deficiency enhances insulin resistance by promoting inflammation in type 2 diabetes. Int. J. Clin. Exp. Pathol. 2019, 12, 1859–1867. [Google Scholar]
- Mazur-Bialy, A.I.; Pocheć, E. Vitamin B2 deficiency enhances the pro-inflammatory activity of adipocyte, consequences for insulin resistance and metabolic syndrome development. Life Sci. 2017, 178, 9–16. [Google Scholar] [CrossRef]
- Yao, L.; Wang, Y.; Qin, S.; Zhu, S.; Wu, L. The antidiabetic drug metformin aids bacteria in hijacking vitamin B12 from the environment through RcdA. Commun. Biol. 2023, 6, 96. [Google Scholar] [CrossRef]
- Cabrera, C.; Lorenzo, M.L.; De Mena, C.; Lopez, M.C. Chromium, copper, iron, manganese, selenium and zinc levels in dairy products: In vitro study of absorbable fractions. Int. J. Food Sci. Nutr. 1996, 47, 331–339. [Google Scholar] [CrossRef]
- Vasilyeva, V.T.; Sleptsova, T.V.; Lebedeva, U.M.; Abramov, A.F.; Bappagai, E.V. The content of zinc and selenium in local food products of Yakutia. Probl. Nutr. 2023, 92, 93–99. [Google Scholar] [CrossRef]
- Filippini, T.; Cilloni, S.; Malavolti, M.; Violi, F.; Malagoli, C.; Tesauro, M.; Bottecchi, I.; Ferrari, A.; Vescovi, L.; Vinceti, M. Dietary intake of cadmium, chromium, copper, manganese, selenium and zinc in a Northern Italy community. J. Trace Elem. Med. Biol. 2018, 50, 508–517. [Google Scholar] [CrossRef]
- Chen, C.; Chaudhary, A.; Mathys, A. Nutritional and environmental losses embedded in global food waste. Resour. Conserv. Recycl. 2020, 160, 104912. [Google Scholar] [CrossRef]
- Torres, M.D.; Seijo, J. By-Products from the Chestnut Industry Used to Produce Natural Potassium Soaps: Physicochemical Properties. J. Surfactants Deterg. 2016, 19, 381–387. [Google Scholar] [CrossRef]
- Picard, K.; Morris, A. Potassium Food Additives and Dietary Management of Serum Potassium: Proposed Best-Practice Recommendations. J. Ren. Nutr. 2025, 35, 221–228. [Google Scholar] [CrossRef]
- Sárközy, M.; Fekete, V.; Szűcs, G.; Török, S.; Szűcs, C.; Bárkányi, J.; Varga, Z.V.; Földesi, I.; Csonka, C.; Kónya, C.; et al. Anti-diabetic effect of a preparation of vitamins, minerals and trace elements in diabetic rats: A gender difference. BMC Endocr. Disord. 2014, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Kehinde, B.A.; Sharma, P. Recently isolated antidiabetic hydrolysates and peptides from multiple food sources: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 322–340. [Google Scholar] [CrossRef]
- Phadtare, I.; Vaidya, H.; Hawboldt, K.; Cheema, S.K. Shrimp Oil Extracted from Shrimp Processing By-Product Is a Rich Source of Omega-3 Fatty Acids and Astaxanthin-Esters, and Reveals Potential Anti-Adipogenic Effects in 3T3-L1 Adipocytes. Mar. Drugs 2021, 19, 259. [Google Scholar]
- Alfio, V.G.; Manzo, C.; Micillo, R. From Fish Waste to Value: An Overview of the Sustainable Recovery of Omega-3 for Food Supplements. Molecules 2021, 26, 1002. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional foods and lifestyle approaches for diabetes prevention and management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef]
- Vinayagam, R.; Xiao, J.; Xu, B. An insight into anti-diabetic properties of dietary phytochemicals. Phytochem. Rev. 2017, 16, 535–553. [Google Scholar] [CrossRef]
- Lacroix, I.M.E.; Li-Chan, E.C.Y. Overview of food products and dietary constituents with antidiabetic properties and their putative mechanisms of action: A natural approach to complement pharmacotherapy in the management of diabetes. Mol. Nutr. Food Res. 2014, 58, 61–78. [Google Scholar] [CrossRef]
- Jin, Y.; Arroo, R.R.J. Application of dietary supplements in the prevention of type 2 diabetes-related cardiovascular complications. Phytochem. Rev. 2021, 20, 181–209. [Google Scholar] [CrossRef]
- Sharkey, S.J.; Harnedy-Rothwell, P.A.; Allsopp, P.J.; Hollywood, L.E.; FitzGerald, R.J.; O’Harte, F.P.M. A Narrative Review of the Anti-Hyperglycemic and Satiating Effects of Fish Protein Hydrolysates and Their Bioactive Peptides. Mol. Nutr. Food Res. 2020, 64, 2000403. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, Q.; Shi, R.; Chang, J.; Chang, H.; Li, M. Food supplements could be an effective improvement of diabetes mellitus: A review. J. Futur. Foods 2021, 1, 67–81. [Google Scholar] [CrossRef]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, 19. [Google Scholar] [CrossRef]
- Dimitriadis, G.D.; Maratou, E.; Kountouri, A.; Board, M.; Lambadiari, V. Regulation of postabsorptive and postprandial glucose metabolism by insulin-dependent and insulin-independent mechanisms: An integrative approach. Nutrients 2021, 13, 159. [Google Scholar] [CrossRef]
- Rorsman, P.; Ashcroft, F.M. Pancreatic β-cell electrical activity and insulin secretion: Of mice and men. Physiol. Rev. 2018, 98, 117–214. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.L.; Udani, J.K. A proprietary alpha-amylase inhibitor from white bean (Phaseolus vulgaris): A review of clinical studies on weight loss and glycemic control. Nutr. J. 2011, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Farazi, M.; Houghton, M.J.; Nicolotti, L.; Murray, M.; Cardoso, B.R.; Williamson, G. Inhibition of human starch digesting enzymes and intestinal glucose transport by walnut polyphenols. Food Res. Int. 2024, 189, 114572. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.H.; Arshad, M.U.; Sultan, M.T. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017, 16, 84. [Google Scholar] [CrossRef]
- DasNandy, A.; Virge, R.; Hegde, H.V.; Chattopadhyay, D. A review of patent literature on the regulation of glucose metabolism by six phytocompounds in the management of diabetes mellitus and its complications. J. Integr. Med. 2023, 21, 226–235. [Google Scholar] [CrossRef]
- Gribble, F.M.; Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 2019, 15, 226–237. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Sailike, J.; Sun, X.; Abuduwaili, N.; Tuoliuhan, H.; Yusufu, M.; Nabi, X. hua Fourteen composite probiotics alleviate type 2 diabetes through modulating gut microbiota and modifying M1/M2 phenotype macrophage in db/db mice. Pharmacol. Res. 2020, 161, 105150. [Google Scholar]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of Probiotics on Gut Microbiota: An Overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Akilen, R.; Tsiami, A.; Devendra, D.; Robinson, N. Cinnamon in glycaemic control: Systematic review and meta analysis. Clin. Nutr. 2012, 31, 609–615. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Cefalu, W.T. Current concepts about chromium supplementation in type 2 diabetes and insulin resistance. Curr. Diab. Rep. 2010, 10, 145–151. [Google Scholar] [PubMed]
- Asbaghi, O.; Fatemeh, N.; Mahnaz, R.K.; Ehsan, G.; Elham, E.; Behzad, N.; Damoon, A.L.; Amirmansour, A.N. Effects of chromium supplementation on glycemic control in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 161, 105098. [Google Scholar] [CrossRef] [PubMed]
- Costello, R.B.; Dwyer, J.T.; Bailey, R.L. Chromium supplements for glycemic control in type 2 diabetes: Limited evidence of effectiveness. Nutr. Rev. 2016, 74, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Lewicki, S.; Zdanowski, R.; Krzyzowska, M.; Lewicka, A.; Debski, B.; Niemcewicz, M.; Goniewicz, M. The role of chromium III in the organism and its possible use in diabetes and obesity treatment. Ann. Agric. Environ. Med. 2014, 21, 331–335. [Google Scholar] [CrossRef]
- Paiva, A.N.; de Lima, J.G.; de Medeiros, A.C.; Figueiredo, H.A.; de Andrade, R.L.; Ururahy, M.A.; Rezende, A.A.; Brandão-Neto, J.; Almeida, M.d.G. Beneficial effects of oral chromium picolinate supplementation on glycemic control in patients with type 2 diabetes: A randomized clinical study. J. Trace Elem. Med. Biol. 2015, 32, 66–72. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, F.; Ge, X.; Yan, T.; Chen, X.; Shi, X.; Zhai, Q. SIRT1 Improves Insulin Sensitivity under Insulin-Resistant Conditions by Repressing PTP1B. Cell Metab. 2007, 6, 307–319. [Google Scholar] [CrossRef]
- Jardine, M.A.; Kahleova, H.; Levin, S.M.; Ali, Z.; Trapp, C.B.; Barnard, N.D. Perspective: Plant-Based Eating Pattern for Type 2 Diabetes Prevention and Treatment: Efficacy, Mechanisms, and Practical Considerations. Adv. Nutr. 2021, 12, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Le, Y.; Wang, B.; Xue, M. Nutraceuticals use and type 2 diabetes mellitus. Curr. Opin. Pharmacol. 2022, 62, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Pellegrini, M.V.; Gupta, V. Alpha-lipoic Acid. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Onaolapo, A.Y.; Onaolapo, O.J. Nutraceuticals and Diet-based Phytochemicals in Type 2 Diabetes Mellitus: From Whole Food to Components with Defined Roles and Mechanisms. Curr. Diabetes Rev. 2018, 16, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef]
- D’Esposito, V.; Di Tolla, M.F.; Lecce, M.; Cavalli, F.; Libutti, M.; Misso, S.; Cabaro, S.; Ambrosio, M.R.; Parascandolo, A.; Covelli, B.; et al. Lifestyle and Dietary Habits Affect Plasma Levels of Specific Cytokines in Healthy Subjects. Front. Nutr. 2022, 9, 913176. [Google Scholar] [CrossRef]
- Zwickey, H.; Horgan, A.; Hanes, D.; Schiffke, H.; Moore, A.; Wahbeh, H.; Jordan, J.; Ojeda, L.; McMurry, M.; Elmer, P.; et al. Effect of the Anti-Inflammatory Diet in People with Diabetes and Pre-Diabetes: A Randomized Controlled Feeding Study. J. Restor. Med. 2019, 8, e20190107. [Google Scholar] [CrossRef]
- Adefegha, S.A. Functional Foods and Nutraceuticals as Dietary Intervention in Chronic Diseases; Novel Perspectives for Health Promotion and Disease Prevention. J. Diet. Suppl. 2018, 15, 977–1009. [Google Scholar] [CrossRef]
- Sears, B.; Perry, M. The role of fatty acids in insulin resistance. Lipids Health Dis. 2015, 14, 121. [Google Scholar] [CrossRef]
- Naeini, Z.; Toupchian, O.; Vatannejad, A.; Sotoudeh, G.; Teimouri, M.; Ghorbani, M.; Nasli-Esfahani, E.; Koohdani, F. Effects of DHA-enriched fish oil on gene expression levels of p53 and NF-κB and PPAR-γ activity in PBMCs of patients with T2DM: A randomized, double-blind, clinical trial. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 441–447. [Google Scholar] [CrossRef]
- Shafras, M.; Sabaragamuwa, R.; Suwair, M. Role of dietary antioxidants in diabetes: An overview. Food Chem. Adv. 2024, 4, 100666. [Google Scholar] [CrossRef]
- Yousef, H.; Khandoker, A.H.; Feng, S.F.; Helf, C.; Jelinek, H.F. Inflammation, oxidative stress and mitochondrial dysfunction in the progression of type II diabetes mellitus with coexisting hypertension. Front. Endocrinol. 2023, 14, 1173402. [Google Scholar] [CrossRef]
- Obeme-Nmom, J.I.; Abioye, R.O.; Reyes Flores, S.S.; Udenigwe, C.C. Regulation of redox enzymes by nutraceuticals: A review of the roles of antioxidant polyphenols and peptides. Food Funct. 2024, 15, 10956–10980. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.E.; Eaton, S.E.M.; Cotter, M.A.; Tesfaye, S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 2001, 44, 1973–1988. [Google Scholar] [CrossRef]
- Kasznicki, J.; Kosmalski, M.; Sliwinska, A.; Mrowicka, M.; Stanczyk, M.; Majsterek, I.; Drzewoski, J. Evaluation of oxidative stress markers in pathogenesis of diabetic neuropathy. Mol. Biol. Rep. 2012, 39, 8669–8678. [Google Scholar] [CrossRef]
- Mhawish, R.; Komarnytsky, S. Small Phenolic Metabolites at the Nexus of Nutrient Transport and Energy Metabolism. Molecules 2025, 30, 1026. [Google Scholar] [CrossRef]
- Kato, M.; Nishikawa, S.; Ikehata, A.; Dochi, K.; Tani, T.; Takahashi, T.; Imaizumi, A.; Tsuda, T. Curcumin improves glucose tolerance via stimulation of glucagon-like peptide-1 secretion. Mol. Nutr. Food Res. 2017, 61, 1600471. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Mobasheri, A.; Busch, F.; Aldinger, C.; Stahlmann, R.; Montaseri, A.; Shakibaei, M. Curcumin modulates nuclear factor kappaB (NF-kappaB)-mediated inflammation in human tenocytes in vitro: Role of the phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 2011, 286, 28556–28566. [Google Scholar] [CrossRef]
- Peng, Z.; Zeng, Y.; Tan, Q.; He, Q.; Wang, S.; Wang, J. 6-Gingerol alleviates ectopic lipid deposition in skeletal muscle by regulating CD36 translocation and mitochondrial function. Biochem. Biophys. Res. Commun. 2024, 708, 149786. [Google Scholar] [CrossRef]
- Piera-Mardemootoo, C.; Lambert, P.; Faillie, J.L. Efficacy of metformin on glycemic control and weight in drug-naive type 2 diabetes mellitus patients: A systematic review and meta-analysis of placebo-controlled randomized trials. Therapies 2021, 76, 647–656. [Google Scholar] [CrossRef]
- Bailey, C.J. Metformin: Historical overview. Diabetologia 2017, 60, 1566–1576. [Google Scholar] [CrossRef] [PubMed]
- Koffert, J.P.; Mikkola, K.; Virtanen, K.A.; Andersson, A.M.D.; Faxius, L.; Hällsten, K.; Heglind, M.; Guiducci, L.; Pham, T.; Silvola, J.M.U.; et al. Metformin treatment significantly enhances intestinal glucose uptake in patients with type 2 diabetes: Results from a randomized clinical trial. Diabetes Res. Clin. Pract. 2017, 131, 208–216. [Google Scholar] [CrossRef] [PubMed]
- De Pablos-Velasco, P. Pioglitazone: Beyond glucose control. Expert Rev. Cardiovasc. Ther. 2010, 8, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- Nesti, L.; Tricò, D.; Mengozzi, A.; Natali, A. Rethinking pioglitazone as a cardioprotective agent: A new perspective on an overlooked drug. Cardiovasc. Diabetol. 2021, 20, 109. [Google Scholar] [CrossRef]
- Mittermayer, F.; Caveney, E.; De Oliveira, C.; Gourgiotis, L.; Puri, M.; Tai, L.-J.; Turner, J.R. Addressing Unmet Medical Needs in Type 2 Diabetes: A Narrative Review of Drugs under Development. Curr. Diabetes Rev. 2015, 11, 17. [Google Scholar] [CrossRef]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary Polyunsaturated Fatty Acids (PUFAs): Uses and Potential Health Benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef]
- Flores-Hernández, M.N.; Martínez-Coria, H.; López-Valdés, H.E.; Arteaga-Silva, M.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R. Efficacy of a High-Protein Diet to Lower Glycemic Levels in Type 2 Diabetes Mellitus: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 10959. [Google Scholar] [CrossRef]
- Suksomboon, N.; Poolsup, N.; Yuwanakorn, A. Systematic review and meta-analysis of the efficacy and safety of chromium supplementation in diabetes. J. Clin. Pharm. Ther. 2014, 39, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Meuffels, F.M.; Isenmann, E.; Strube, M.; Lesch, A.; Oberste, M.; Brinkmann, C. Exercise Interventions Combined with Dietary Supplements in Type 2 Diabetes Mellitus Patients—A Systematic Review of Relevant Health Outcomes. Front. Nutr. 2022, 9, 817724. [Google Scholar] [CrossRef]
- Basu, A.; Du, M.; Sanchez, K.; Leyva, M.J.; Betts, N.M.; Blevins, S.; Wu, M.; Aston, C.E.; Lyons, T.J. Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition 2011, 27, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Lane, J.; Holyoak, C.; Nicol, B.; Mayes, A.E.; Dadd, T. Health effects of green tea catechins in overweight and obese men: A randomised controlled cross-over trial. Br. J. Nutr. 2011, 106, 1880–1889. [Google Scholar] [CrossRef]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: A randomized, double-blind clinical trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef]
- Kutan Fenercioglu, A.; Saler, T.; Genc, E.; Sabuncu, H.; Altuntas, Y. The effects of polyphenol-containing antioxidants on oxidative stress and lipid peroxidation in Type 2 diabetes mellitus without complications. J. Endocrinol. Investig. 2010, 33, 118–124. [Google Scholar] [CrossRef]
- Jovanovski, E.; Khayyat, R.; Zurbau, A.; Komishon, A.; Mazhar, N.; Sievenpiper, J.L.; Blanco Mejia, S.; Ho, H.V.T.; Li, D.; Jenkins, A.L.; et al. Should Viscous Fiber Supplements Be Considered in Diabetes Control? Results From a Systematic Review and Meta-analysis of Randomized Controlled Trials. Diabetes Care 2019, 42, 755–766. [Google Scholar] [CrossRef]
- O’Connell, B.S. Select Vitamins and Minerals in the Management of Diabetes. Diabetes Spectr. 2001, 14, 133–148. [Google Scholar] [CrossRef]
- Bartlett, H.E.; Eperjesi, F. Nutritional supplementation for type 2 diabetes: A systematic review. Ophthalmic Physiol. Opt. 2008, 28, 503–523. [Google Scholar] [CrossRef]
- Farrokhian, A.; Mahmoodian, M.; Bahmani, F.; Amirani, E.; Shafabakhsh, R.; Asemi, Z. The Influences of Chromium Supplementation on Metabolic Status in Patients with Type 2 Diabetes Mellitus and Coronary Heart Disease. Biol. Trace Elem. Res. 2020, 194, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, W.; Zheng, W.; Fang, X.; Chen, L.; Rink, L.; Min, J.; Wang, F. Zinc supplementation improves glycemic control for diabetes prevention and management: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2019, 110, 76–90. [Google Scholar] [CrossRef]
- Khan, M.I.; Siddique, K.U.; Ashfaq, F.; Ali, W.; Reddy, H.D.; Mishra, A. Effect of high-dose zinc supplementation with oral hypoglycemic agents on glycemic control and inflammation in type-2 diabetic nephropathy patients. J. Nat. Sci. Biol. Med. 2013, 4, 336. [Google Scholar] [CrossRef]
- Aquilani, R. Oral amino acid administration in patients with diabetes mellitus: Supplementation or metabolic therapy? Am. J. Cardiol. 2004, 93, 21–22. [Google Scholar] [CrossRef]
- Scognamiglio, R.; Negut, C.; Piccolotto, R.; Dioguardi, F.S.; Tiengo, A.; Avogaro, A. Effects of oral amino acid supplementation on myocardial function in patients with type 2 diabetes mellitus. Am. Heart J. 2004, 147, 1106–1112. [Google Scholar] [CrossRef]
- Solerte, S.B.; Fioravanti, M.; Locatelli, E.; Bonacasa, R.; Zamboni, M.; Basso, C.; Mazzoleni, A.; Mansi, V.; Geroutis, N.; Gazzaruso, C. Improvement of Blood Glucose Control and Insulin Sensitivity During a Long-Term (60 Weeks) Randomized Study with Amino Acid Dietary Supplements in Elderly Subjects with Type 2 Diabetes Mellitus. Am. J. Cardiol. 2008, 101, S82–S88. [Google Scholar] [CrossRef] [PubMed]
- Görgüç, A.; Gençdağ, E.; Yılmaz, F.M. Bioactive peptides derived from plant origin by-products: Biological activities and techno-functional utilizations in food developments—A review. Food Res. Int. 2020, 136, 109504. [Google Scholar] [CrossRef] [PubMed]
- Abish, Z.A.; Alibekov, R.S.; Tarapoulouzi, M.; Bakhtybekova, A.R.; Kobzhasarova, Z.I. Review in deep processing of whey. Cogent Food Agric. 2024, 10, 2415380. [Google Scholar] [CrossRef]
- Djuricic, I.; Calder, P.C. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Zaloga, G.P. Narrative Review of n-3 Polyunsaturated Fatty Acid Supplementation upon Immune Functions, Resolution Molecules and Lipid Peroxidation. Nutrients 2021, 13, 662. [Google Scholar] [CrossRef]
- Banaszak, M.; Dobrzyńska, M.; Kawka, A.; Górna, I.; Woźniak, D.; Przysławski, J.; Drzymała-Czyż, S. Role of Omega-3 fatty acids eicosapentaenoic (EPA) and docosahexaenoic (DHA) as modulatory and anti-inflammatory agents in noncommunicable diet-related diseases—Reports from the last 10 years. Clin. Nutr. ESPEN 2024, 63, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.P.C.; Tseng, P.T.; Zeng, B.S.; Chang, C.H.; Su, H.; Chou, P.H.; Su, K.P. Safety of Supplementation of Omega-3 Polyunsaturated Fatty Acids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Asghari, K.M.; Saleh, P.; Salekzamani, Y.; Dolatkhah, N.; Aghamohammadzadeh, N.; Hashemian, M. The effect of curcumin and high-content eicosapentaenoic acid supplementations in type 2 diabetes mellitus patients: A double-blinded randomized clinical trial. Nutr. Diabetes 2024, 14, 14. [Google Scholar] [CrossRef] [PubMed]
- Mostad, I.L.; Bjerve, K.S.; Bjorgaas, M.R.; Lydersen, S.; Grill, V. Effects of n−3 fatty acids in subjects with type 2 diabetes: Reduction of insulin sensitivity and time-dependent alteration from carbohydrate to fat oxidation. Am. J. Clin. Nutr. 2006, 84, 540–550. [Google Scholar] [CrossRef]
- Farsi, P.F.; Djazayery, A.; Eshraghian, M.R.; Koohdani, F.; Saboor-Yaraghi, A.A.; Derakhshanian, H.; Zarei, M.; Javanbakht, M.H.; Djalali, M. Effects of supplementation with omega-3 on insulin sensitivity and non-esterified free fatty acid (NEFA) in type 2 diabetic patients. Arq. Bras. Endocrinol. Metabol. 2014, 58, 335–340. [Google Scholar] [CrossRef]
- Cho, Y.K.; Kim, K.S.; Lee, B.W.; Hong, J.H.; Yu, J.M.; Lim, S.; Kim, Y.A.; Lee, C.B.; Kim, S.S.; Kwak, S.H.; et al. Efficacy and Safety of Pioglitazone Add-on in Patients with Type 2 Diabetes Mellitus Inadequately Controlled with Metformin and Dapagliflozin: A Multicenter, Randomized, Double-blind, and Placebo-controlled Study. Clin. Ther. 2024, 46, 662–669. [Google Scholar] [CrossRef]
- García, P.P.; Martínez, F.J.H.; López, N.A.; Torre, L.C.; Sempere, M.E.T. Supplementation with a Highly Concentrated Docosahexaenoic Acid (DHA) in Non-Proliferative Diabetic Retinopathy: A 2-Year Randomized Double-Blind Placebo-Controlled Study. Antioxidants 2022, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Ivanisevic, M.; Horvaticek, M.; Delmis, K.; Delmis, J. Supplementation of EPA and DHA in pregnant women with type 1 diabetes mellitus. Ann. Med. 2021, 53, 848–859. [Google Scholar] [CrossRef]
- Zou, H.Y.; Zhang, H.J.; Zhao, Y.C.; Li, X.Y.; Wang, Y.M.; Zhang, T.T.; Xue, C.H. N-3 PUFA Deficiency Aggravates Streptozotocin-Induced Pancreatic Injury in Mice but Dietary Supplementation with DHA/EPA Protects the Pancreas via Suppressing Inflammation, Oxidative Stress and Apoptosis. Mar. Drugs 2023, 21, 39. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Wan, X.; Zhuang, P.; Wu, Y.; Zhang, L.; Ao, Y.; Yao, J.; Zhang, Y.; Jiao, J. Association of Fish Oil Supplementation with Risk of Coronary Heart Disease in Individuals with Diabetes and Prediabetes: A Prospective Study in the UK Biobank. Nutrients 2023, 15, 3176. [Google Scholar] [CrossRef]
- Yiu, Y.F.; Yiu, K.H.; Siu, C.W.; Chan, Y.H.; Li, S.W.; Wong, L.Y.; Lee, S.W.L.; Tam, S.; Wong, E.W.K.; Lau, C.P.; et al. Randomized controlled trial of vitamin D supplement on endothelial function in patients with type 2 diabetes. Atherosclerosis 2013, 227, 140–146. [Google Scholar] [CrossRef]
- Angellotti, E.; D’Alessio, D.; Dawson-Hughes, B.; Nelson, J.; Cohen, R.M.; Gastaldelli, A.; Pittas, A.G. Vitamin D Supplementation in Patients with Type 2 Diabetes: The Vitamin D for Established Type 2 Diabetes (DDM2) Study. J. Endocr. Soc. 2018, 2, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Malik, K.; Moore, J.M.; Kamboj, B.R.; Malik, S.; Malik, V.K.; Arya, S.; Singh, K.; Mahanta, S.; Bishnoi, D.K. Valorisation of Agri-Food Waste for Bioactive Compounds: Recent Trends and Future Sustainable Challenges. Molecules 2024, 29, 2055. [Google Scholar] [CrossRef] [PubMed]
- Ben-Othman, S.; Joudu, I.; Bhat, R. Bioactives from Agri-Food Wastes: Present Insights. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Wijngaard, H.; Hossain, M.B.; Rai, D.K.; Brunton, N. Techniques to extract bioactive compounds from food by-products of plant origin. Food Res. Int. 2012, 46, 505–513. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Opara, U.L. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Complement. Altern. Med. 2016, 16, 143. [Google Scholar] [CrossRef]
- López-Santiago, J.; García García, A.I.; Villarino, A.G.; Som, A.M.; Gómez-Villarino, M.T. Assessing wineries’ performance in managing critical control points for arsenic, lead, and cadmium contamination risk in the wine-making industry: A survey-based analysis utilizing performance indicators as a results tool. Heliyon 2024, 10, e22962. [Google Scholar] [CrossRef]
- Schilter, B.; Andersson, C.; Anton, R.; Constable, A.; Kleiner, J.; O’Brien, J.; Renwick, A.G.; Korver, O.; Smit, F.; Walker, R. Guidance for the safety assessment of botanicals and botanical preparations for use in food and food supplements. Food Chem. Toxicol. 2003, 41, 1625–1649. [Google Scholar] [CrossRef]
- Schiebel, C.S.; Bueno, L.R.; Pargas, R.B.; de Mello Braga, L.L.V.; da Silva, K.S.; Fernandes, A.C.V.U.; dos Santos Maia, M.H.; de Oliveira, N.M.T.; Bach, C.; Maria-Ferreira, D. Exploring the biological activities and potential therapeutic applications of agro-industrial waste products through non-clinical studies: A systematic review. Sci. Total Environ. 2024, 950, 175317. [Google Scholar] [CrossRef]
- Gurley, B.J. Pharmacokinetic Herb-Drug Interactions (Part 1): Origins, Mechanisms, and the Impact of Botanical Dietary Supplements. Planta Medica 2012, 78, 1478–1489. [Google Scholar] [CrossRef]
- Mei, S.; Perumal, M.; Battino, M.; Kitts, D.D.; Xiao, J.; Ma, H.; Chen, X. Mangiferin: A review of dietary sources, absorption, metabolism, bioavailability, and safety. Crit. Rev. Food Sci. Nutr. 2023, 63, 3046–3064. [Google Scholar] [CrossRef]
- Vilas-Boas, A.A.; Pintado, M.; Oliveira, A.L.S. Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods 2021, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, B.; Munro, I.; Abbot, P.; Baldwin, N.; Lopez-Garcia, R.; Ly, K.; McGirr, L.; Roberts, A.; Socolovsky, S. Review of the regulation and safety assessment of food substances in various countries and jurisdictions. Food Addit. Contam. Part A 2013, 30, 1147–1220. [Google Scholar] [CrossRef] [PubMed]
- Socas-Rodríguez, B.; Álvarez-Rivera, G.; Valdés, A.; Ibáñez, E.; Cifuentes, A. Food by-products and food wastes: Are they safe enough for their valorization? Trends Food Sci. Technol. 2021, 114, 133–147. [Google Scholar] [CrossRef]
- Devkota, L.; Montet, D.; Anal, A.K. Regulatory and Legislative Issues for Food Waste Utilization. In Food Processing by—Products and their Utilization; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 535–548. ISBN 9781118432921. [Google Scholar]
- Raak, N.; Symmank, C.; Zahn, S.; Aschemann-Witzel, J.; Rohm, H. Processing- and product-related causes for food waste and implications for the food supply chain. Waste Manag. 2017, 61, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.B.; Oliveira, A.; Coelho, M.; Veiga, M.; Costa, E.M.; Silva, S.; Nunes, J.; Vicente, A.A.; Pintado, M. Are olive pomace powders a safe source of bioactives and nutrients? J. Sci. Food Agric. 2021, 101, 1963–1978. [Google Scholar] [CrossRef]
- European Commission. European Parliament and the Council of the EU Directive of The European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed 2002/32. Off. J. Eur. Communities 2002, L140, 1–15. [Google Scholar]
- European Commission. European Parliament Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food saf. Off. J. Eur. Communities 2002, L031, 1–24. [Google Scholar]
- Castro-Muñoz, R.; Yáñez-Fernández, J.; Fíla, V. Phenolic compounds recovered from agro-food by-products using membrane technologies: An overview. Food Chem. 2016, 213, 753–762. [Google Scholar] [CrossRef]
- Rebitzer, G.; Ekvall, T.; Frischknecht, R.; Hunkeler, D.; Norris, G.; Rydberg, T.; Schmidt, W.-P.; Suh, S.; Weidema, B.P.; Pennington, D.W. Life cycle assessment: Part 1: Framework, goal and scope definition, inventory analysis, and applications. Environ. Int. 2004, 30, 701–720. [Google Scholar] [CrossRef]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Onyeji, C.O.; Igbinoba, S.I.; Olayiwola, G. Therapeutic Potentials and Cytochrome P450-Mediated Interactions Involving Herbal Products Indicated for Diabetes Mellitus. Drug Metab. Lett. 2017, 11, 74–85. [Google Scholar] [CrossRef]
- Martemucci, G.; Portincasa, P.; Centonze, V.; Mariano, M.; Khalil, M.; D’ALessandro, A.G. Prevention of Oxidative Stress and Diseases by Antioxidant Supplementation. Med. Chem. 2023, 19, 509–537. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Stoeva, S.; Hvarchanova, N.; Georgiev, K.D.; Radeva-Ilieva, M. Green Tea: Antioxidant vs. Pro-Oxidant Activity. Beverages 2025, 11, 64. [Google Scholar] [CrossRef]
- Acunha, T.; García-Cañas, V.; Valdés, A.; Cifuentes, A.; Simó, C. Metabolomics study of early metabolic changes in hepatic HepaRG cells in response to rosemary diterpenes exposure. Anal. Chim. Acta 2018, 1037, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef]
- Djaoudene, O.; Romano, A.; Bradai, Y.D.; Zebiri, F.; Ouchene, A.; Yousfi, Y.; Amrane-Abider, M.; Sahraoui-Remini, Y.; Madani, K. A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects. Nutrients 2023, 15, 3320. [Google Scholar] [CrossRef] [PubMed]
- Deluyker, H. Is scientific assessment a scientific discipline? EFSA J. 2017, 15, e15111. [Google Scholar] [CrossRef]
- Chatzopoulou, S.; Eriksson, N.L.; Eriksson, D. Improving Risk Assessment in the European Food Safety Authority: Lessons From the European Medicines Agency. Front. Plant Sci. 2020, 11, 349. [Google Scholar] [CrossRef]
- Coppens, P.; da Silva, M.F.; Pettman, S. European regulations on nutraceuticals, dietary supplements and functional foods: A framework based on safety. Toxicology 2006, 221, 59–74. [Google Scholar] [CrossRef]
- Silano, V.; Coppens, P.; Larrañaga-Guetaria, A.; Minghetti, P.; Roth-Ehrang, R. Regulations applicable to plant food supplements and related products in the European Union. Food Funct. 2011, 2, 710–719. [Google Scholar] [CrossRef]
- Anadón, A.; Ares, I.; Martínez-Larrañaga, M.-R.; Martínez, M.-A. Evaluation and regulation of food supplements: European perspective. In Nutraceuticals, 2nd ed.; Gupta, R.C., Lall, R., Srivastava, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1241–1271. ISBN 978-0-12-821038-3. [Google Scholar]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Knutsen, H.K.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; et al. Scientific advice related to nutrient profiling for the development of harmonised mandatory front-of-pack nutrition labelling and the setting of nutrient profiles for restricting nutrition and health claims on foods. EFSA J. 2022, 20, e07259. [Google Scholar]
- Hoffman, F.A.N.N. Regulation of Dietary Supplements in the United States: Understanding the Dietary Supplement Health and Education Act. Clin. Obstet. Gynecol. 2001, 44, 780–788. [Google Scholar] [CrossRef]
- Wallace, T.C. Twenty Years of the Dietary Supplement Health and Education Act—How Should Dietary Supplements Be Regulated? J. Nutr. 2015, 145, 1683–1686. [Google Scholar] [CrossRef]
- Soyombo, D.A. Navigating regulatory challenges and safety considerations in lactation supplement development: A Nigeria and US Comparison. World J. Biol. Pharm. Health Sci. 2024, 18, 1–11. [Google Scholar] [CrossRef]
- Smith, A.; Jogalekar, S.; Gibson, A. Regulation of natural health products in Canada. J. Ethnopharmacol. 2014, 158, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, A.; Tutelyan, V. Codex Alimentarius and transparency of the global food safety system. In Food System Transparency; Steier, G., Friedlander, A., Eds.; CRC Press: Boca Raton, FL, USA, 2021; p. 28. [Google Scholar]
- van der Meulen, B.; Card, M.M.; Din, A.; Fortin, N.D.; Mahmudova, A.; Maister, B.; Türkoğlu, H.G.; Bilgin, F.K.; Lederman, J.; Poto, M.P.; et al. Food regulation around the world. In Ensuring Global Food Safety, 2nd ed.; Martinović, A., Oh, S., Lelieveld, H., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 11–137. ISBN 978-0-12-816011-4. [Google Scholar]
- Miyazaki, Y.; Omori, T.; Fujitani, K.; Fujita, J.; Kawabata, R.; Imamura, H.; Okada, K.; Moon, J.-H.; Hirao, M.; Matsuyama, J.; et al. Oral nutritional supplements versus a regular diet alone for body weight loss after gastrectomy: A phase 3, multicenter, open-label randomized controlled trial. Gastric Cancer 2021, 24, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Sciuto, S.; Martello, E.; Pezzolato, M.; Bozzetta, E. Disclosing Frauds in Herbal Food Supplements Labeling: A Simple LC-MS/MS Approach to Detect Alkaloids and Biogenic Amines. J. Food Prot. 2023, 86, 100152. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Woodlee, J.; Marriott, R.; Meyer, C. Dietary supplements. In An Overview of FDA Regulated Products; Pacifici, E., Bain, S., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 237–256. ISBN 978-0-443-23780-5. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuksek, E.N.; Pereira, A.G.; Prieto, M.A. Dietary Supplements Derived from Food By-Products for the Management of Diabetes Mellitus. Antioxidants 2025, 14, 1176. https://doi.org/10.3390/antiox14101176
Yuksek EN, Pereira AG, Prieto MA. Dietary Supplements Derived from Food By-Products for the Management of Diabetes Mellitus. Antioxidants. 2025; 14(10):1176. https://doi.org/10.3390/antiox14101176
Chicago/Turabian StyleYuksek, Ezgi Nur, Antia G. Pereira, and Miguel A. Prieto. 2025. "Dietary Supplements Derived from Food By-Products for the Management of Diabetes Mellitus" Antioxidants 14, no. 10: 1176. https://doi.org/10.3390/antiox14101176
APA StyleYuksek, E. N., Pereira, A. G., & Prieto, M. A. (2025). Dietary Supplements Derived from Food By-Products for the Management of Diabetes Mellitus. Antioxidants, 14(10), 1176. https://doi.org/10.3390/antiox14101176








