Lutein and Zeaxanthin Enhance, Whereas Oxidation, Fructosylation, and Low pH Damage High-Density Lipoprotein Biological Functionality
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and HDL Isolation
2.2. HDL Treatments
2.3. Glycosylation Modification Efficacy Determinations
2.4. Lutein/Zeaxanthin Incorporation Confirmation
2.5. Negative-Stain Transmission Electron Microscopy and Image Analysis
2.6. CEC Assay
2.7. LCAT Activity Assay
2.8. PON1 Activity Assay
2.9. Statistical Analysis
3. Results
3.1. HDL Particle Size and Distribution
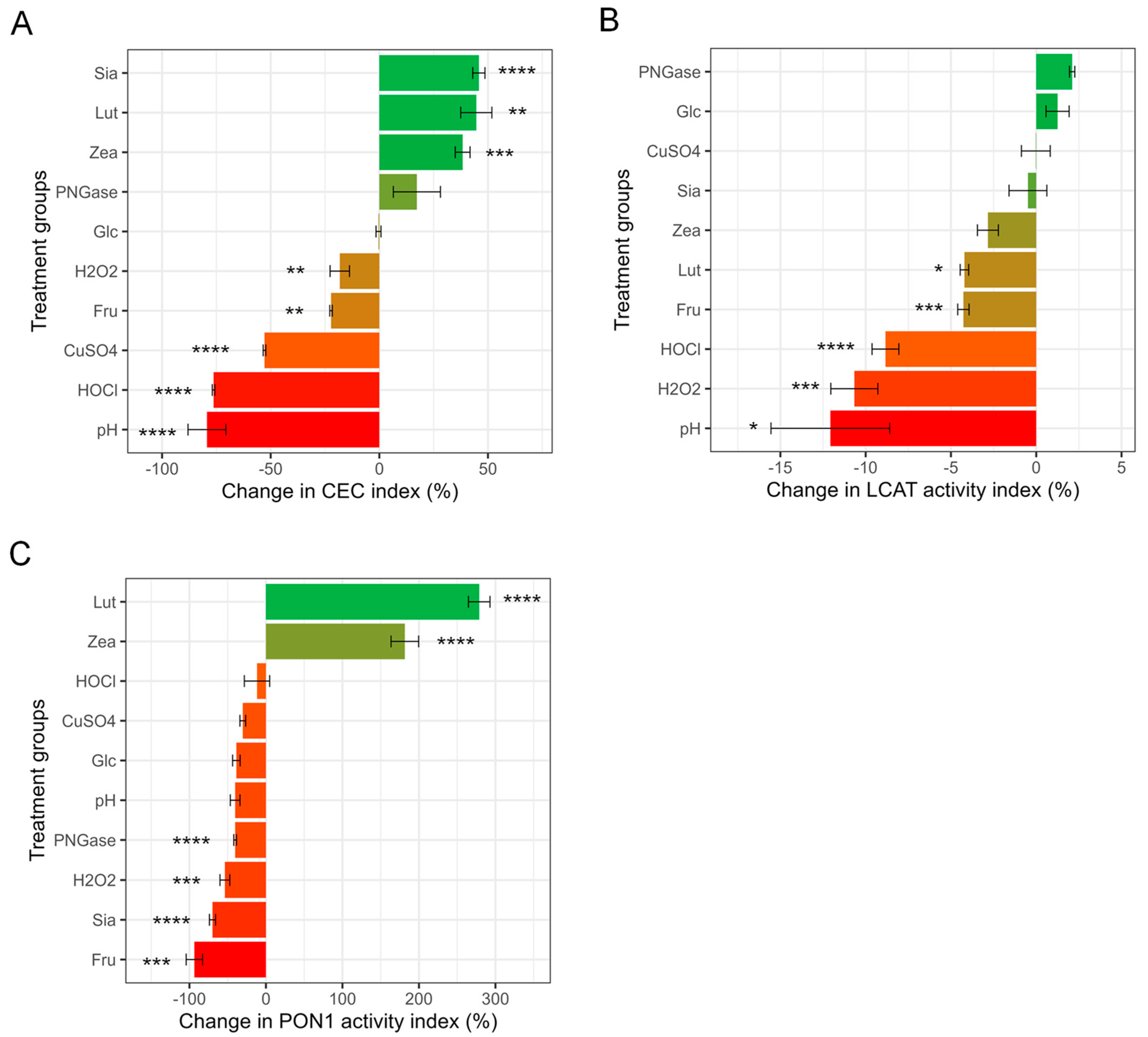
3.2. HDL CEC, LCAT Activity and PON1 Activity after Direct Treatments
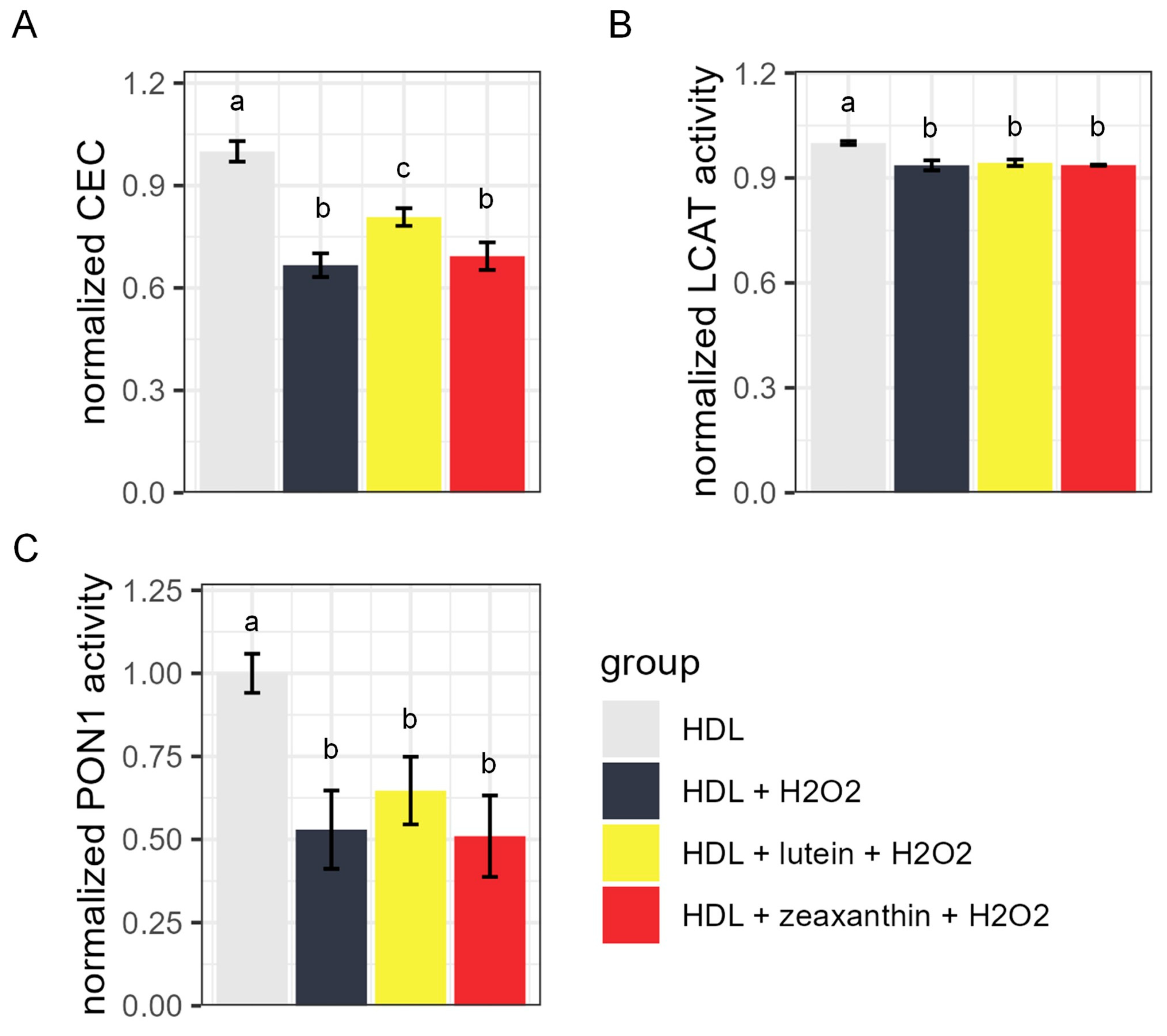
3.3. HDL CEC, LCAT Activity, and PON1 Activity after Lutein/Zeaxanthin Pre-Incubation Followed by Oxidation Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gofman, J.W.; Young, W.; Tandy, R. Ischemic heart disease, atherosclerosis, and longevity. Circulation 1966, 34, 679–697. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Castelli, W.P.; Hjortland, M.C.; Kannel, W.B.; Dawber, T.R. High density lipoprotein as a protective factor against coronary heart disease: The Framingham Study. Am. J. Med. 1977, 62, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.E.; Thelle, D.; Førde, O.; Mjøs, O. The Tromsøheart-Study: High-Density Lipoprotein and Coronary Heart-Disease: A Prospective Case-Control Study. Lancet 1977, 309, 965–968. [Google Scholar] [CrossRef] [PubMed]
- Voight, B.F.; Peloso, G.M.; Orho-Melander, M.; Frikke-Schmidt, R.; Barbalic, M.; Jensen, M.K.; Hindy, G.; Hólm, H.; Ding, E.L.; Johnson, T. Plasma HDL cholesterol and risk of myocardial infarction: A mendelian randomisation study. Lancet 2012, 380, 572–580. [Google Scholar] [CrossRef]
- Pedrini, S.; Chatterjee, P.; Hone, E.; Martins, R.N. High-density lipoprotein-related cholesterol metabolism in Alzheimer’s disease. J. Neurochem. 2020, 159, 343–377. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Tang, M.-X.; Schupf, N.; Manly, J.J.; Mayeux, R.; Luchsinger, J.A. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch. Neurol. 2010, 67, 1491–1497. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Calabresi, L.; Franceschini, G.; Baldassarre, D.; Amato, M.; Johansson, J.; Salvetti, M.; Monteduro, C.; Zulli, R.; Muiesan, M.L. Cardiovascular status of carriers of the apolipoprotein A-IMilano mutant: The Limone sul Garda study. Circulation 2001, 103, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- van Capelleveen, J.C.; Bochem, A.E.; Motazacker, M.M.; Hovingh, G.K.; Kastelein, J.J. Genetics of HDL-C: A causal link to atherosclerosis? Curr. Atheroscler. Rep. 2013, 15, 326. [Google Scholar] [CrossRef]
- Tall, A.R. HDL in Morbidity and Mortality: A 40+ Year Perspective. Clin. Chem. 2021, 67, 19–23. [Google Scholar] [CrossRef]
- Khera, A.V.; Cuchel, M.; De La Llera-Moya, M.; Rodrigues, A.; Burke, M.F.; Jafri, K.; French, B.C.; Phillips, J.A.; Mucksavage, M.L.; Wilensky, R.L. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011, 364, 127–135. [Google Scholar] [CrossRef]
- Lee, C.D.; Tse, W.; Smith, J.D.; Landreth, G.E. Apolipoprotein E promotes β-amyloid trafficking and degradation by modulating microglial cholesterol levels. J. Biol. Chem. 2012, 287, 2032–2044. [Google Scholar] [CrossRef] [PubMed]
- Marsche, G.; Stadler, J.T.; Kargl, J.; Holzer, M. Understanding myeloperoxidase-induced damage to HDL structure and function in the vessel wall: Implications for HDL-based therapies. Antioxidants 2022, 11, 556. [Google Scholar] [CrossRef]
- Holzer, M.; Zangger, K.; El-Gamal, D.; Binder, V.; Curcic, S.; Konya, V.; Schuligoi, R.; Heinemann, A.; Marsche, G. Myeloperoxidase-derived chlorinating species induce protein carbamylation through decomposition of thiocyanate and urea: Novel pathways generating dysfunctional high-density lipoprotein. Antioxid. Redox Signal. 2012, 17, 1043–1052. [Google Scholar] [CrossRef]
- Nakajima, T.; Origuchi, N.; Matsunaga, T.; Kawai, S.; Hokari, S.; Nakamura, H.; Inoue, I.; Katayama, S.; Nagata, A.; Komoda, T. Localization of oxidized HDL in atheromatous plaques and oxidized HDL binding sites on human aortic endothelial cells. Ann. Clin. Biochem. 2000, 37, 179–186. [Google Scholar] [CrossRef]
- Pennathur, S.; Bergt, C.; Shao, B.; Byun, J.; Kassim, S.Y.; Singh, P.; Green, P.S.; McDonald, T.O.; Brunzell, J.; Chait, A. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J. Biol. Chem. 2004, 279, 42977–42983. [Google Scholar] [CrossRef] [PubMed]
- Van Lenten, B.J.; Roheim, P.S. Changes in the concentrations and distributions of apolipoproteins of the aging rat. J. Lipid Res. 1982, 23, 1187–1195. [Google Scholar] [CrossRef]
- Bergt, C.; Pennathur, S.; Fu, X.; Byun, J.; O’Brien, K.; McDonald, T.O.; Singh, P.; Anantharamaiah, G.; Chait, A.; Brunzell, J. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. USA 2004, 101, 13032–13037. [Google Scholar] [CrossRef] [PubMed]
- Hoang, A.; Murphy, A.; Coughlan, M.; Thomas, M.; Forbes, J.; O’brien, R.; Cooper, M.; Chin-Dusting, J.; Sviridov, D. Advanced glycation of apolipoprotein AI impairs its anti-atherogenic properties. Diabetologia 2007, 50, 1770–1779. [Google Scholar] [CrossRef]
- Low, H.; Hoang, A.; Forbes, J.; Thomas, M.; Lyons, J.; Nestel, P.; Bach, L.A.; Sviridov, D. Advanced glycation end-products (AGEs) and functionality of reverse cholesterol transport in patients with type 2 diabetes and in mouse models. Diabetologia 2012, 55, 2513–2521. [Google Scholar] [CrossRef]
- Lê, Q.H.; El Alaoui, M.; Véricel, E.; Ségrestin, B.; Soulère, L.; Guichardant, M.; Lagarde, M.; Moulin, P.; Calzada, C. Glycoxidized HDL, HDL enriched with oxidized phospholipids and HDL from diabetic patients inhibit platelet function. J. Clin. Endocrinol. Metab. 2015, 100, 2006–2014. [Google Scholar] [CrossRef]
- Krishnan, S.; Huang, J.; Lee, H.; Guerrero, A.; Berglund, L.; Anuurad, E.; Lebrilla, C.B.; Zivkovic, A.M. Combined high-density lipoprotein proteomic and glycomic profiles in patients at risk for coronary artery disease. J. Proteome Res. 2015, 14, 5109–5118. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Shimoda, M.; Sacchi, R.; Kailemia, M.J.; Luxardi, G.; Kaysen, G.A.; Parikh, A.N.; Ngassam, V.N.; Johansen, K.; Chertow, G.M.; et al. HDL Glycoprotein Composition and Site-Specific Glycosylation Differentiates Between Clinical Groups and Affects IL-6 Secretion in Lipopolysaccharide -Stimulated Monocytes. Sci. Rep. 2017, 7, 43728. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.D.; Öörni, K.; Lee-Rueckert, M.; Pihlajamaa, T.; Metso, J.; Jauhiainen, M.; Kovanen, P.T. Spontaneous remodeling of HDL particles at acidic pH enhances their capacity to induce cholesterol efflux from human macrophage foam cells. J. Lipid Res. 2012, 53, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, N.; Woodside, J.V.; Kelly, S.; Allister, R.; Young, I.S.; McEneny, J. The two faces of α-and γ-tocopherols: An in vitro and ex vivo investigation into VLDL, LDL and HDL oxidation. J. Nutr. Biochem. 2012, 23, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Clevidence, B.A.; Bieri, J.G. [4] Association of carotenoids with human plasma lipoproteins. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1993; Volume 214, pp. 33–46. ISBN 0076-6879. [Google Scholar]
- Connor, W.E.; Duell, P.B.; Kean, R.; Wang, Y. The prime role of HDL to transport lutein into the retina: Evidence from HDL-deficient WHAM chicks having a mutant ABCA1 transporter. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4226–4231. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T.; Bone, R.A. Lutein, zeaxanthin, and the macular pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef]
- Ata, S.; Barona, J.; Kopec, R.; Jones, J.; Calle, M.; Schwartz, S.; Fernandez, M.L. Consumption of either one egg or lutein-enriched egg per day increases HDL cholesterol, reduces apolipoprotein B while increasing plasma carotenoids and macular pigment density in adult subjects. FASEB J. 2010, 24, 92–94. [Google Scholar] [CrossRef]
- Blesso, C.N.; Andersen, C.J.; Bolling, B.W.; Fernandez, M.L. Egg intake improves carotenoid status by increasing plasma HDL cholesterol in adults with metabolic syndrome. Food Funct. 2013, 4, 213–221. [Google Scholar] [CrossRef]
- Murillo, A.G.; Aguilar, D.; Norris, G.H.; DiMarco, D.M.; Missimer, A.; Hu, S.; Smyth, J.A.; Gannon, S.; Blesso, C.N.; Luo, Y. Compared with powdered lutein, a lutein nanoemulsion increases plasma and liver lutein, protects against hepatic steatosis, and affects lipoprotein metabolism in guinea pigs. J. Nutr. 2016, 146, 1961–1969. [Google Scholar] [CrossRef]
- Kang, J.W.; Tang, X.; Walton, C.J.; Brown, M.J.; Brewer, R.A.; Maddela, R.L.; Zheng, J.J.; Agus, J.; Zivkovic, A.M. Multi-omic Analyses Reveal Bifidogenic Effect and Metabolomic Shifts in Healthy Human Cohort Supplemented with a Prebiotic Dietary Fiber Blend. Front. Nutr. 2022, 9, 908534. [Google Scholar] [CrossRef]
- Zheng, J.J.; Agus, J.K.; Hong, B.V.; Tang, X.; Rhodes, C.H.; Houts, H.E.; Zhu, C.; Kang, J.W.; Wong, M.; Xie, Y. Isolation of HDL by sequential flotation ultracentrifugation followed by size exclusion chromatography reveals size-based enrichment of HDL-associated proteins. Sci. Rep. 2021, 11, 16086. [Google Scholar] [CrossRef] [PubMed]
- Jairajpuri, D.S.; Fatima, S.; Saleemuddin, M. Immunoglobulin glycation with fructose: A comparative study. Clin. Chim. Acta 2007, 378, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Goulinet, S.; Chapman, M.J. Plasma LDL and HDL subspecies are heterogenous in particle content of tocopherols and oxygenated and hydrocarbon carotenoids: Relevance to oxidative resistance and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Moschetti, A.; Fox, C.A.; McGowen, S.; Ryan, R.O. Lutein nanodisks protect human retinal pigment epithelial cells from UV light-induced damage. Front. Nanotechnol. 2022, 4, 955022. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.V.; Zheng, J.; Agus, J.K.; Tang, X.; Lebrilla, C.B.; Jin, L.-W.; Maezawa, I.; Erickson, K.; Harvey, D.J.; DeCarli, C.S. High-Density Lipoprotein Changes in Alzheimer’s Disease Are APOE Genotype-Specific. Biomedicines 2022, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Minami-Takano, A.; Iwata, H.; Miyosawa, K.; Shiozawa, T.; Hayashi, H.; Funamizu, T.; Ishii, K.; Nozaki, Y.; Tabuchi, H.; Sekita, G. The association between impairment of HDL cholesterol efflux capacity and atrial remodeling in atrial fibrillation. Sci. Rep. 2021, 11, 3547. [Google Scholar] [CrossRef]
- Gan, K.N.; Smolen, A.; Eckerson, H.W.; La Du, B.N. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab. Dispos. 1991, 19, 100–106. [Google Scholar]
- Kishimoto, Y.; Taguchi, C.; Saita, E.; Suzuki-Sugihara, N.; Nishiyama, H.; Wang, W.; Masuda, Y.; Kondo, K. Additional consumption of one egg per day increases serum lutein plus zeaxanthin concentration and lowers oxidized low-density lipoprotein in moderately hypercholesterolemic males. Food Res. Int. 2017, 99, 944–949. [Google Scholar] [CrossRef]
- Dwyer, J.H.; Navab, M.; Dwyer, K.M.; Hassan, K.; Sun, P.; Shircore, A.; Hama-Levy, S.; Hough, G.; Wang, X.; Drake, T. Oxygenated carotenoid lutein and progression of early atherosclerosis: The Los Angeles atherosclerosis study. Circulation 2001, 103, 2922–2927. [Google Scholar] [CrossRef]
- Severins, N.; Mensink, R.; Plat, J. Effects of lutein-enriched egg yolk in buttermilk or skimmed milk on serum lipids & lipoproteins of mildly hypercholesterolemic subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 210–217. [Google Scholar] [PubMed]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Jaouad, L.; de Guise, C.; Berrougui, H.; Cloutier, M.; Isabelle, M.; Fulop, T.; Payette, H.; Khalil, A. Age-related decrease in high-density lipoproteins antioxidant activity is due to an alteration in the PON1’s free sulfhydyl groups. Atherosclerosis 2006, 185, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.J. Protein glucosylation and its role in protein folding. Annu. Rev. Biochem. 2000, 69, 69–93. [Google Scholar] [CrossRef] [PubMed]
- Stanhope, K.L.; Schwarz, J.M.; Keim, N.L.; Griffen, S.C.; Bremer, A.A.; Graham, J.L.; Hatcher, B.; Cox, C.L.; Dyachenko, A.; Zhang, W. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J. Clin. Investig. 2009, 119, 1322–1334. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Li, Y.; Pan, A.; De Koning, L.; Schernhammer, E.; Willett, W.C.; Hu, F.B. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation 2019, 139, 2113–2125. [Google Scholar] [CrossRef]
- Lê, K.-A.; Tappy, L. Metabolic effects of fructose. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Feinman, R.D.; Fine, E.J. Fructose in perspective. Nutr. Metab. 2013, 10, 45. [Google Scholar] [CrossRef]
- Lewis, A.L.; Chen, X.; Schnaar, R.L.; Varki, A. Sialic acids and other nonulosonic acids. In Essentials of Glycobiology [Internet], 4th ed.; Cold Spring Harbor Press: Cold Spring Harbor, NY, USA, 2022. [Google Scholar]
- Draganov, D.I.; Teiber, J.F.; Speelman, A.; Osawa, Y.; Sunahara, R.; La Du, B.N. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J. Lipid Res. 2005, 46, 1239–1247. [Google Scholar] [CrossRef]
- Schindler, P.A.; Settineri, C.A.; Collet, X.; Fielding, C.J.; Burlingame, A.L. Site-specific detection and structural characterization of the glycosylation of human plasma proteins lecithin: Cholesterol acyltransferase and apolipoprotein D using HPLC/electrospray mass spectrometry and sequential glycosidase digestion. Protein Sci. 1995, 4, 791–803. [Google Scholar] [CrossRef]
- Anantharamaiah, G.; Hughes, T.A.; Iqbal, M.; Gawish, A.; Neame, P.J.; Medley, M.; Segrest, J. Effect of oxidation on the properties of apolipoproteins AI and A-II. J. Lipid Res. 1988, 29, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Strittmatter, W.J.; Weisgraber, K.H.; Huang, D.Y.; Dong, L.-M.; Salvesen, G.S.; Pericak-Vance, M.; Schmechel, D.; Saunders, A.M.; Goldgaber, D.; Roses, A.D. Binding of human apolipoprotein E to synthetic amyloid beta peptide: Isoform-specific effects and implications for late-onset Alzheimer disease. Proc. Natl. Acad. Sci. USA 1993, 90, 8098–8102. [Google Scholar] [CrossRef] [PubMed]
- Nagano, Y.; Arai, H.; Kita, T. High density lipoprotein loses its effect to stimulate efflux of cholesterol from foam cells after oxidative modification. Proc. Natl. Acad. Sci. USA 1991, 88, 6457–6461. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Ito, T. High-density lipoprotein (HDL) triglyceride and oxidized HDL: New lipid biomarkers of lipoprotein-related atherosclerotic cardiovascular disease. Antioxidants 2020, 9, 362. [Google Scholar] [CrossRef]
- Rocha, H.R.; Coelho, M.C.; Gomes, A.M.; Pintado, M.E. Carotenoids diet: Digestion, gut microbiota modulation, and inflammatory diseases. Nutrients 2023, 15, 2265. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.T.; Kwon, S.; Zheng, D.; Shaughnessy, S.; Wallace, P.; Hutto, A.; Pugh, K.; Jenkins, A.J.; Klein, R.L.; Liao, Y. Effects of insulin resistance and type 2 diabetes on lipoprotein subclass particle size and concentration determined by nuclear magnetic resonance. Diabetes 2003, 52, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Otvos, J.D.; Rosenson, R.S.; Pradhan, A.; Buring, J.E.; Ridker, P.M. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes 2010, 59, 1153–1160. [Google Scholar] [CrossRef]
- Dullaart, R.P.; Otvos, J.D.; James, R.W. Serum paraoxonase-1 activity is more closely related to HDL particle concentration and large HDL particles than to HDL cholesterol in Type 2 diabetic and non-diabetic subjects. Clin. Biochem. 2014, 47, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Sokooti, S.; Flores-Guerrero, J.L.; Kieneker, L.M.; Heerspink, H.J.; Connelly, M.A.; Bakker, S.J.; Dullaart, R.P. HDL particle subspecies and their association with incident type 2 diabetes: The PREVEND study. J. Clin. Endocrinol. Metab. 2021, 106, 1761–1772. [Google Scholar] [CrossRef]
- Wormald, M.R.; Dwek, R.A. Glycoproteins: Glycan presentation and protein-fold stability. Structure 1999, 7, R155–R160. [Google Scholar] [CrossRef]
- Solá, R.J.; Griebenow, K. Effects of glycosylation on the stability of protein pharmaceuticals. J. Pharm. Sci. 2009, 98, 1223–1245. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wong, M.; Tena, J.; Zhu, C.; Rhodes, C.; Zhou, Q.; Vinjamuri, A.; Oloumi, A.; Boddu, S.; Luxardi, G. Quantitative glycoproteomics of high-density lipoproteins. RSC Adv. 2022, 12, 18450–18456. [Google Scholar] [CrossRef] [PubMed]

| Treatment Name/Purpose | Treatment Reagent (Vendor) | Concentration | Treatment Time (h) | Reference |
|---|---|---|---|---|
| CuSO4/oxidation | copper (II) sulfate (CuSO4) (Cat. #: C988L31, Neta Scientific, Marlton, NJ, USA) | 10 µM | 18 | [14] |
| H2O2/oxidation | hydrogen peroxide (H2O2) (Cat. #: H1065, Spectrum Chemical, Gardena, CA, USA) | 160 µM | 2 | [17] |
| HOCl/oxidation | hypochlorous acid (HOCl) (Cat. #: S1316, Spectrum Chemical, Gardena, CA, USA) | 160 µM | 2 | [17] |
| Acidic pH/acidification | ammonium acetate buffer, pH 5.5 (Cat. #: 40100184-1, Spectrum Chemical, Gardena, CA, USA) | 20 mM | 18 | [23] |
| Glucose/glycation | Glucose (Cat. #: 40700008-1, Spectrum Chemical, Gardena, CA, USA) | 50 mM | 120 | [20] |
| Fructose/fructosylation | Fructose (Cat. #: 40600008-1, Spectrum Chemical, Gardena, CA, USA) | 100 mM | 192 | [33] |
| PNGase F/deglycosylation | PNGase F (Cat. #: P0705S, New England Biolabs, Ipswich, MA, USA) | 25,000 U/mL | 4 | [21] |
| Sialidase/desialylation | α2-3, 6, 8, 9 neuraminidase A (Cat. #: P722S, New England Biolabs, Ipswich, MA, USA) | 2000 U/mL | 2 | [21] |
| Lutein/antioxidation | Lutein (Cat. #: PHR1699, Sigma, St. Louis, MO, USA). | 1 mM | 18 | [24] |
| Zeaxanthin/antioxidation | Zeaxanthin (Cat. #: 1733119, Sigma, St. Louis, MO, USA) | 1 mM | 18 | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Hong, B.V.; Agus, J.K.; Tang, X.; Klebaner, N.R.; Chen, S.; Guo, F.; Harvey, D.J.; Lebrilla, C.B.; Zivkovic, A.M. Lutein and Zeaxanthin Enhance, Whereas Oxidation, Fructosylation, and Low pH Damage High-Density Lipoprotein Biological Functionality. Antioxidants 2024, 13, 616. https://doi.org/10.3390/antiox13050616
Zheng J, Hong BV, Agus JK, Tang X, Klebaner NR, Chen S, Guo F, Harvey DJ, Lebrilla CB, Zivkovic AM. Lutein and Zeaxanthin Enhance, Whereas Oxidation, Fructosylation, and Low pH Damage High-Density Lipoprotein Biological Functionality. Antioxidants. 2024; 13(5):616. https://doi.org/10.3390/antiox13050616
Chicago/Turabian StyleZheng, Jingyuan, Brian V. Hong, Joanne K. Agus, Xinyu Tang, Nola R. Klebaner, Siyu Chen, Fei Guo, Danielle J. Harvey, Carlito B. Lebrilla, and Angela M. Zivkovic. 2024. "Lutein and Zeaxanthin Enhance, Whereas Oxidation, Fructosylation, and Low pH Damage High-Density Lipoprotein Biological Functionality" Antioxidants 13, no. 5: 616. https://doi.org/10.3390/antiox13050616
APA StyleZheng, J., Hong, B. V., Agus, J. K., Tang, X., Klebaner, N. R., Chen, S., Guo, F., Harvey, D. J., Lebrilla, C. B., & Zivkovic, A. M. (2024). Lutein and Zeaxanthin Enhance, Whereas Oxidation, Fructosylation, and Low pH Damage High-Density Lipoprotein Biological Functionality. Antioxidants, 13(5), 616. https://doi.org/10.3390/antiox13050616







