Abstract
In recent years, research on the discovery of natural compounds with potent antioxidant properties has resulted in growing interest in these compounds due to their potential therapeutic applications in oxidative-stress-related diseases. Argan oil, derived from the kernels of a native tree from Morocco, Argania spinosa, is renowned for its rich composition of bioactive compounds, prominently tocopherols, polyphenols, and fatty acids. Interestingly, a large body of data has shown that several components of argan oil activate the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, playing a crucial role in the cellular defense against oxidative stress. Activation of this Nrf2 pathway by argan oil components leads to the increased expression of downstream target proteins like NAD(P)H quinone oxidoreductase (NQO1), superoxide dismutase (SOD), heme oxygenase 1 (HO-1), and catalase (CAT). Such Nrf2 activation accounts for several health benefits related to antioxidant defense, anti-inflammatory effects, cardiovascular health, and neuroprotection in organisms. Furthermore, the synergistic action of the bioactive compounds in argan oil enhances the Nrf2 pathway. Accordingly, the modulation of the Kelch-like ECH associated protein 1 (Keap1)/Nrf2 signaling pathway by these components highlights the potential of argan oil in protecting cells from oxidative stress and underlines its relevance in dietetic prevention and therapeutic applications. This review aims to provide an overview of how major compounds in argan oil activate the Nrf2 pathway, updating our knowledge on their mechanisms of action and associated health benefits.
1. Introduction
Our body naturally generates antioxidants to combat the harmful effects of free radicals. However, an imbalance can result in excess reactive oxygen species (ROS) production, leading to oxidative stress [1]. ROS can be generated by the internal metabolism or after external exposure [2,3]. The excessive production of free radicals can lead to various disorders, including cardiovascular diseases, cancer, and inflammatory pathologies [4]. To combat this oxidative stress, organisms developed a complex system of antioxidants, which can be divided into enzymatic, such as superoxide dismutase (SOD), glutathione peroxidase (GPx), and catalase (CAT), and non-enzymatic, such as glutathione (GSH), ascorbic acid, and thioredoxin [5]. Nuclear factor erythroid 2-related factor 2 (Nrf2) is the main redox-sensitive transcription factor that plays a crucial role in regulating gene expression for molecules that have antioxidant properties [6]. In recent decades, there has been growing interest in bioactive compounds that have a range of biological activities, including anti-inflammatory, anticancer, immunomodulatory, and antioxidant effects [7,8]. In this context, argan oil has gained significant recognition for its unique composition and its numerous health benefits. It is obtained from Argania Spinosa, an endemic tree in the middle of Morocco [9]. Morocco produces 3000 to 4000 tons of argan oil annually, making it the world’s leading producer [10]. Argan oil has traditionally been used for culinary, medicinal, and cosmetic purposes. It is used for joint issues, skin, hair, and nails [11]. Our research team has focused on exploring the antioxidative effect of argan oil and some of its components [12,13,14,15,16,17,18]. The present work first provides a general review of the chemical composition of argan oil compared to other oils. Then, it delves into the antioxidant properties of argan oil as well as its effects on lipid metabolism. Moreover, it describes on the main redox-sensitive transcription factor, Nrf2, and its downstream signaling pathways. Finally, it focuses on the action mechanisms of argan oil and its components on the Nrf2 pathway, aiming to elucidate the antioxidative potential of compounds related to argan oil.
2. Composition of Argan Oil
Argan oil has a unique composition marked by elevated levels of linoleic and oleic acids. Moreover, it is rich in polyphenols and tocopherols, which confer antioxidant properties. Additionally, argan oil contains other minor compounds such as carotenoids, squalene, sterols, and xanthophylls. These compounds potentially contribute to the nutritional value of argan oil, its health benefits, its organoleptic characteristics, and its shelf-life [19,20,21].
2.1. Glyceride Saponifiable Fraction
The glyceride fraction represents 99% of argan oil, with the majority (95%) being triglycerides [22]. In contrast to other edible oils, an argan oil fatty acid analysis revealed a predominance of oleic acid and linoleic acid, about 80%, showing a balanced amount between monounsaturated and polyunsaturated fatty acids [23]. The proportion of oleic acid in argan oil (43–49%) is higher than that of the oil of sunflowers, soybeans, maize, grape seed, and sesame, whereas this content is lower than that of the oil of olives, almonds, and peanuts (Table 1). Unlike the level of linoleic acid in argan oil, which ranges from 29 to 36%, this concentration is lower than that of the oil of sunflowers, soybeans, maize, grape seed, and sesame, and higher than that of the oil of olives, almonds, and peanuts. Additionally, argan oil is abundant in saturated fatty acids relative to other natural oils and contains a small amount of linolenic acid.

Table 1.
Comparison of fatty acid composition of argan oil with other natural oils.
The table below displays a comparison of the main fatty acid compositions between argan oil and other natural oils. The values represent percentages of fatty acids in the total triglyceride fraction.
2.2. Unsaponifiable Fraction
The unsaponifiable fraction represents 1% of argan oil and is characterized by a rich composition of sterols and antioxidants such as polyphenols, particularly tocopherols [22]. The composition of the unsaponifiable fraction is mainly influenced by the geographical origin of the argan tree and the process of extraction of the argan oil [31].
2.2.1. Polyphenols
The phenolic composition of argan oil (Table 2) is characterized by the presence of four polyphenols, vanillic acid, syringic acid, ferulic acid, and tyrosol, with a predominance of ferulic acid, which represents more than 94% of the polyphenol fraction [32]. Nonetheless, the polyphenol content of argan oil (3263 μg/kg) is lower compared to olive oil (793 mg/kg); however, it exceeds that of other edible vegetable oils [33]. Polyphenols present in the oils are bioactive molecules that have antioxidant activity. They are primarily responsible for the prevention of auto-oxidation of unsaturated fatty acids, which increases the shelf life of these oils [34]. The pharmacological properties of argan oil are generally attributed to its phenolic compounds.

Table 2.
Comparison of polyphenol composition of argan oil with other natural oils.
The table below displays a comparison of the main polyphenol compositions of argan oil and other natural oils.
2.2.2. Sterols
Table 3 lists the five sterols found in argan oil. The two main sterols are schottenol and spinasterol, while stigmatasol, stigma-8,22-dien-3-ol, and stigma-7,24-dien-3-ol are found in trace amounts [31]. Both spinasterol and schottenol are absent from sunflower and olive oils [20]. Based on previous studies, the sterol content of argan oil (Table 3) remains unaffected by different extraction methods and fruit origins. However, seasonal and regional variations cannot be excluded due to their influences related to the climate and the soil characteristics, respectively [20].
2.2.3. Tocopherols
As natural antioxidants, four isoforms of tocopherols are found in vegetable oils: α-tocopherol (vitamin E), β-tocopherol, γ-tocopherol, and δ-tocopherol [37]. Argan oil contains double the amount of tocopherol as olive oil, with γ-tocopherol constituting the majority at over 75% of the total tocopherols [9].

Table 3.
Chemical composition of argan oil sterols and tocopherols by different extraction methods and in different geographical origins.
Table 3.
Chemical composition of argan oil sterols and tocopherols by different extraction methods and in different geographical origins.
| Extraction Method | Extraction by Mechanical Press | Artisanal Extraction | Extraction by Organic Solvents | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Roasting | Roasted Argan Seeds | Non-Roasted Argan Seeds | Roasted Argan Seeds | Non-Roasted Argan Seeds | Roasted Argan Seeds | ||||||||||||
| Depuling | Mechanical | Manual | Animal (Goat Dejections ) | Mechanical | |||||||||||||
| References | [38] | [39] | [31] | [40] | [20] | [41] | [31] | [31] | [31] | [31] | [41] | [41] | [40] | [40] | [40] | [40] | [40] |
| Geographical origin | Agadir | Taroudant | Tizdi Essaouira | Benaiznassen Chtouka ait baha | Tamanar Essaouira | Tiout Taroudant | Beniznassen Oujda | Ait mzal Chtouka ait baha | Ighrem Taroudant | Tizdi Essaouira | Tiout Taroudant | Tiout Taroudant | Tamanar Essaouira | Tamanar Essaouira | Tamanar Essaouira | Ighrem | Tamanar Essaouira |
| Tocopherols mg/kg of oil | |||||||||||||||||
| α-Tocopherol | 44.5 ± 6.2 | 42.23 ± 2.52 | 26.6 | 37.2 | 35 | 16 ± 1.6 | 37.2 | 33.2 | 49.3 | 32.7 | 13 ± 1.3 | 30 ± 3.0 | 29.6 | 33.0 | 32.0 | 49.3 | 29.6 |
| β-Tocopherol | 3.1 ± 0.8 | 3.07 ± 0.40 | 1.1 | 1.2 | |||||||||||||
| γ-Tocopherol | 616.9 ± 15.8 | 715.42 ± 7.8 | 631.3 | 701.1 | 480 | 382 ± 38.2 | 701.1 | 615.6 | 545.9 | 621.1 | 345 ± 34.5 | 283 ± 28.3 | 619.1 | 599.3 | 640.0 | 545.9 | 581.3 |
| δ-Tocopherol | 50.8 ± 6.8 | 103.22 ± 4.19 | 59.5 | 37.2 | 122 | 21 ± 2.1 | 37.2 | 38.0 | 38.7 | 50.9 | 32 ± 3.2 | 21 ± 2.1 | 50.2 | 46.4 | 45.4 | 38.7 | 56.3 |
| Sterol mg/100 g of oil | |||||||||||||||||
| Schottenol | 46.66 | 48.47 | 142 | 44 ± 2.2 | 48.47 | 44.99 | 46.12 | 43.39 | 46 ± 2.3 | 45 ± 2.3 | 46.03 | 47.43 | 44.62 | 46.12 | 45.39 | ||
| Spinasterol | 37.07 | 35.44 | 115 | 39 ± 2.0 | 35.44 | 39.17 | 39.29 | 38.50 | 38 ± 1.9 | 42 ± 2.1 | 36.11 | 38.54 | 37.05 | 39.29 | 36.91 | ||
| Stigmasta-8,22-dien-3-ol | 4.31 | 4.85 | 9 | 5 ± 0.3 | 4.85 | 4.77 | 5.40 | 4.57 | 5 ± 0.3 | 4 ± 0.2 | 4.08 | 3.01 | 4.21 | 5.40 | 4.99 | ||
| Stigma 7,24--dien-3-ol | 4.81 | 2.57 | 6 ± 0.3 | 2.57 | 4.71 | 3.55 | 5.94 | 5 ± 0.3 | 5 ± 0.3 | 4.48 | 4.67 | 6.89 | 3.55 | 4.48 | |||
| Campesterol | 0.20 | 0.11 | 0.11 | 0.24 | 0.31 | 0.17 | 0.16 | 0.14 | 0.16 | 0.31 | 0.20 | ||||||
Reported data reveal that the tocopherol content of argan oil can vary significantly between extraction methods and even between fruits grown in the same region (Table 3).
The primary goal of Table 3 is to emphasize the differences in the chemical composition (sterols and tocopherols) of argan oil based on its geographical origin and the extraction method used.
2.3. Antioxidant Properties of Argan Oil
The unsaponifiable fraction components have demonstrated antioxidative properties [42]. In mice, following bacterial lipopolysaccharides (LPS) administration, argan oil was able to restore the level of peroxisomal antioxidant enzyme activities, demonstrating its hepatoprotective effect. Accordingly, in both the liver and brain, the LPS-dependent increase in catalase activity, as well as in glutathione peroxidase (GPx) and superoxide dismutase (SOD) activities, can be successfully abrogated by argan oil treatment [14,15]. The LPS-dependent non-enzymatic formation of glutathione in the liver and brain was abolished by argan oil supply. Argan oil is also able to reduce the lipid peroxidation level through malondialdehyde (MDA) measurement during brain and liver injury [15]. Furthermore, a recent paper reported the antioxidant potential of argan oil in mice against iron-inducing oxidative stress in the liver, kidney, and brain. A similar effect was observed in the cultured protozoan Tetrahymena pyriformis [13]. Furthermore, exposure to argan oil modulates the peroxisomal antioxidant capacity by upregulating catalase and superoxide dismutase expressions at the translational and post-translational levels [15]. These findings underscore the protective role of argan oil against oxidative stress.
2.4. Effects on Lipid Metabolism
Fatty acid synthesis produces oils and fats, which are a source of energy [43]. They are essential to an organism’s growth and development and especially in the regulation of molecular signaling [44].
Indeed, altered levels and the metabolism of fatty acids can contribute to various health concerns in humans, including insulin resistance, obesity, hyperlipidemia, and cardiovascular disease. Therefore, it is critical that the body regulate its fatty acid levels [45]. This metabolic adaptation is performed mainly in the liver by the activation of the nuclear receptor peroxisome proliferator-activate receptor (PPAR)-α. This PPAR isotype is predominantly expressed in several tissues, such as the liver, heart, kidneys, brain, spleen, intestine, and stomach. PPARα target genes are related to fat metabolism and lipid transport and play an important role in fatty acid oxidation [46]. The ligand-dependent activation of this PPARα [47] leads to the recruitment of the coactivator peroxisome proliferator-activate receptor γ-Coactivator (PGC)-1α and then permits the regulation of the transcription of genes encoding peroxisomal and mitochondrial enzymes involved in the hepatic pathways of fatty acid β-oxidation (FAOx) [48,49]. Accordingly, it has been shown that argan oil has a protective effect against the decreased expression of genes involved in hepatic gluconeogenesis and FAOx. This effect might be associated with the recovery of gene expression of the nuclear receptor PPARα and its coactivator PCG-1α [50].
Among the classes of fatty acids identified as ligands for PPARs, unsaturated long-chain fatty acids stand out, constituting a significant part, exceeding 80% in both argan oil and olive oil. Indeed, these natural oils are rich in a monounsaturated oleic acid, which represents 46% and 76% of the total composition of argan oil and olive oil, respectively [23].
Fundamental studies have shown that oleic acid has a beneficial impact on the cardiovascular risk and lipid profile [51]. Administration of this fatty acid to humans decreases the concentration of LDL and TG [52]. Hence, the substitution of carbohydrates and saturated fat with oleic acid led to a reduction in blood sugar and blood pressure accompanied by an increase in HDL in diabetic patients [53]. These variations in the triglyceride levels are attributed to the increased oxidation of fatty acids through the induction of β-oxidation, a result of oleic acid’s activation of PPARα and the reduction in sterol regulatory element-binding protein (SREBP) activity, thereby decreasing lipogenesis. Moreover, this dietary fatty acid activates PPARα and PPARγ to promote fat oxidation and reduce insulin resistance, which consequently leads to a reduction in hepatic steatosis [54].
Fatty acid intake can effectively modulate liver lipid metabolism [51]. Thus, treatment of mice with olive oil leads to the accumulation of hepatic triglycerides, partly due to increased lipogenesis enzyme activity [55]. Conversely, inhibiting carnitine palmitoyl transferase I (CPT-I) [56] and carnitine/acylcarnitine translocase (CACT) [57] reduces fatty acid oxidation by impeding their transport from the cytosol to the mitochondria.
Polyunsaturated fatty acids are known to suppress hepatic lipogenesis, whereas saturated and monounsaturated fatty acids have minimal to no effect on fatty acid synthesis [44,58]. Additionally, an argan oil enriched diet prevents the hyperlipidemic effects associated with LPS by inducing the expression of hepatic nuclear receptors, including PPARα, Estrogen-related receptor (ERR) α, and their coactivator PGC-1α, thereby upregulating their mitochondrial and peroxisome target genes involved in fatty acid oxidation [59]. This highlights the beneficial impact of argan oil on lipid metabolism.
3. Nrf2
The main redox-sensitive transcription factor, the Nrf2 protein, is a master regulator for oxidative stress management in mammalian cells that maintains cellular homeostasis through controlling the redox balance, xenobiotic metabolism [60], the metabolism of carbohydrates [61], lipids and iron [62], antioxidant and anti-inflammatory responses, protein folding, and proliferation. Nrf2 was first discovered in 1994 [63]. It is transcribed from the human NFE2L2 gene and belongs to the cap‘n’collar subclass (CNC) of the basic leucine zipper transcription factor protein family (bZIP) along with five other nuclear factors: NF-E2, Nrf1, Nrf3, Bach1, and Bach2 [64]. It has been mapped to the long arm of human chromosome 2 (2q31.2) [65]. Nrf2 is expressed in all tissues, and especially in the organs involved in detoxification processes and metabolism [66,67]. Nrf2 is characterized by an extremely short cellular half-life of approximately 15 to 40 min through degradation by the ubiquitin proteasome arsenal [68]. This short-lived protein is known for its pivotal role in combating harmful ROS formation as well as associated inflammatory and metabolic responses. Nrf2 orchestrates the expression cytoprotective gene encoding phase II detoxifying enzymes [69,70] as well as the expression of genes involved in mitochondrial function and biogenesis [71]. Nrf2 translocates into the nucleus, heterodimerizes with the Maf or Jun protein, and then binds to its antioxidant response elements (AREs) to trigger the transcription of cytoprotective genes [72]. Interestingly, the Nrf2 signaling pathway was found necessary for hematopoietic cell differentiation [6], accelerating cell proliferation, neovascularization, and the repair of damaged tissues [73]. Moreover, it upregulates the expression of antioxidant genes, inhibits microglia-mediated inflammation, and improves mitochondrial function in neurodegenerative diseases [67,74,75]. In addition, it has been reported that Nrf2 expression loss is strongly associated with the metastatic behavior of cancer cells and tumor malignancy [76]. Altogether, Nrf2 is considered to be the pro-survival factor that orchestrates the production of cytoprotective machinery components.
3.1. Nrf2 Structure and Function
The Nrf2 protein consists of 605 amino acids and features seven highly conserved functional domains (Neh1-7), notably including the Neh2 domain at the N-terminal, which is responsible for interacting with the negative regulator Keap1 [77]. This domain contains DLG and ETGE motifs, with the latter exhibiting a higher affinity for Keap1 [78,79,80,81,82]. Disruption of the DLG motif suppresses Nrf2 ubiquitination [72,76], while lysine residues between DLG and ETGE motifs are crucial for ubiquitin conjugation and Nrf2 stability [79,83]. Additionally, serine residue ser40 facilitates Nrf2 nuclear translocation post-dissociation from Keap1 [79] (Figure 1).
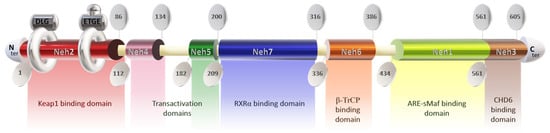
Figure 1.
Structure of the protein Nrf2. Keap1: Kelch-like ECH-associated protein 1; Neh domain: N-amino terminal end harbors; RXRα: Retinoic X receptor α; ARE: antioxidant response element; s-Maf: small musculoaponeurotic fibrosarcoma factor; CHD6: Chromodomain helicase DNA binding protein 6.
The Neh4 and Neh5 domains promote the interaction of Nrf2 with coactivators [80], enhancing the transactivation of target genes [84]. Neh5 also regulates Nrf2 cytoplasmic localization [85]. Neh7 serves as a repressive domain inhibiting Nrf2-RXRα binding [86], while Neh6 negatively regulates Nrf2 stability [87]. Neh1 facilitates DNA binding of Nrf2 to antioxidant response elements (ARE/EpRE) [88,89,90], promoting gene expression and downregulating pro-inflammatory mediators. The Neh1 domain contains a leucine zipper for heterodimerization with the small musculoaponeurotic fibrosarcoma factor (sMAF) and a nuclear localization signal for translocation to the nucleus [91,92,93]. The Neh3 domain in the C-terminal region mediates coactivator interactions [80,84].
Keap1, a dimeric cytoplasmic protein, functions as a major Nrf2 inhibitor (Figure 2). It contains an NTD, a BTB domain for Neh2 interaction, an IVR with an NES for cytoplasmic localization, a DGR with Kelch motifs essential for KEAP1-Nrf2 association, and a CTR [83,94,95,96,97]. Keap1’s structure and function are crucial in regulating the Nrf2 system.

Figure 2.
Structure of the protein Keap1. NTD: N-terminal domain; BTB: 2-a broad complex, tram-track, bric-a-brac; IVR: 3-a cysteine-rich intervening region; CTD: C-terminal domain.
3.2. Nrf2-KEAP1 Signaling Pathway
Nrf2 and KEAP1 are crucial for cellular defense mechanisms, maintaining redox homeostasis, and controlling cell fate [98]. In physiological conditions, Nrf2 is ubiquitinated by KEAP1/CUL3–RBX1 and degraded by proteasomes to keep basal gene expression [64,92,99]. KEAP1 cysteine residues (Cys151, Cys273, and Cys288) facilitate rapid Nrf2 degradation, while the ATP-dependent segregase p97 also promotes Nrf2 degradation [100]. Alternatively, proteins like β-TrCP and HRD1 trigger Nrf2 ubiquitination and degradation [101]. The oxidation of KEAP1 cysteines in noncanonical pathways leads to Nrf2 release [101]. Nrf2 contains redox-sensitive cysteines, preventing KEAP1 binding [102,103,104]. Several regulators, including p62, WTX, and DPP3, stimulate Nrf2 by binding KEAP1 [104,105,106]. Upon activation, Nrf2 translocates to the nucleus, heterodimerizes with sMaf proteins, and binds to ARE sequences, inducing cytoprotective gene expression [107,108]. Nrf2 regulates numerous genes related to vital cellular functions [109,110]. After activation, Nrf2 is phosphorylated, leading to nuclear export and degradation [70,77,111,112,113]. Interaction with RXRα inhibits Nrf2 activity [114]. Transcriptional and post-transcriptional regulation significantly affects Nrf2 activity [115].
3.3. Nrf2 and Oxidative Stress
Mammalian cells are regularly threatened by multiple stress sources within their immediate microenvironment. Thence, they are commonly armed with a strong arsenal of non-enzymatic compounds, such as glutathione (GSH), vitamin C (ascorbate), and vitamin E (tocopherols) [116,117,118], and antioxidant enzymes (SODs, catalase, thioredoxins, peroxiredoxins, and glutathione peroxidases) [119,120], participating in the adaptive regulatory mechanisms to maintain the cellular and tissue homeostasis [79]. The presence of oxidant molecules such as ROS and reactive nitrogen species (RNS) can generate an imbalance in the redox status, which usually causes damage to cellular macromolecules (lipids, proteins, and DNA). The consequences of this oxidative stress are cell death and the development of metabolic and chronic diseases, including atherosclerosis, autoimmune disorders, diabetes, osteoporosis, rheumatoid arthritis, and neurodegenerative diseases (Parkinson’s disease and Alzheimer’s disease), among others [68,121]. In 1985, Sies defined oxidative stress (OS) as “a disturbance in prooxidant-antioxidant balance in favor of the former” [122]. Dean Jones added to this definition that OS is also the disruption of redox signaling circuitries [123]. In addition to their cytotoxicity, ROS have an important role in vital cellular functions. At limited concentrations, they play a major role in proliferation, differentiation, inflammation, immune function, autophagy, and stress response by acting as a second messenger [124]. However, an elevated concentration of ROS may activate oncogenes, inactivate tumor suppressor genes, and promote mitochondrial malfunction [125]. As the redox master chief regulator, Nrf2 is activated by a plethora of ARE inducers, known as stressors, including oxidative and chemical stress provoked by hydrogen peroxide (H2O2) [126], NO [127], tertiary butylhydroquinone (tBHQ) [128], and fumarate (DMF) [129]. Amid the natural phytochemicals, silymarin, sulforaphane, curcumin, cinnamic aldehyde [130], and bardoxolone methyl are the most well studied. Numerous studies have reported the efficient effect of Nrf2 activation on the expression and production of several antioxidant enzymes to maintain the oxidative balance in eukaryotic cells (Table 4). In diabetic mice, the Nrf2/KEAP1 signaling pathway protects pancreatic β-cells by weakening oxidative damage through the induction of its antioxidant enzymatic system, which inhibits apoptosis and proliferation [131]. Accumulating evidence identifies the crosstalk between Nrf2 and NF-κB signaling pathways to maintain redox balance and to regulate the cellular response to stress and inflammation [132]. In age-related macular degeneration model, The H2O2 induced levels of ROS and MDA, while the Nrf2-related antioxidant enzymes SOD, GPx, and the reduced glutathione (GSH) were upregulated. These results were explained by the fact that Nrf2 induces the expression of heme oxygenase-1 (HO-1), a potent antioxidant enzyme [133]. Similarly, Jung and al. reported that Nrf2 activation increases the cellular HO-1 level, subsequently impeding the degradation of IκB-α [134], which inhibits NF-κB nuclear translocation [134,135]. Therefore, Nrf2 suppress the expression of NF-κB pro-inflammatory target genes. In addition, the administration of α-mangostin, an Nrf2 activator, enhanced antioxidant cell defense and reduced pro-inflammatory cytokines, including tumor necrosis factor (TNF-α) and interleukin-1β and -6 (IL-1β, IL-6), and contributed to the restoration of hepatic GSH, SOD, and catalase activities [136].

Table 4.
Nrf2 target genes and biochemical functions.
Table 4.
Nrf2 target genes and biochemical functions.
| Enzyme Name | Protein Symbol | Biochemical Function |
|---|---|---|
| Alcohol dehydrogenase | ADH | Antioxidant and detoxification enzymes [137,138] |
| Aldehyde dehydrogenase | ALDH | |
| Aldo-keto reductase family 1 | AKR1 | |
| ATP-binding cassette subfamily B/C | ABCB/ABCC | |
| Carbonyl reductase | CBR | |
| Catalase | CAT | |
| Cytochrome P450 | CYP1B1 | |
| Epoxide hydrolase, microsomal | EXPH | |
| Glutamate-cysteine ligase | GCL | |
| Glutathione peroxidase | GPx | |
| Glutathione reductase | GR | |
| Glutathione S-transferase | GST | |
| Glutathione synthase | GSS | |
| Heme oxygenase-1 | HO-1 | |
| NADPH-quinone oxidoreductase-1 | NQO-1 | |
| Peroxiredoxins | PRDX1 | |
| Prostaglandin reductase | PTGR | |
| Sodium independent cysteine glutamate antiporter | SLC7A11 | |
| Sulfiredoxin1 | SRXN1 | |
| Superoxide dismutase | SOD | |
| Thioredoxin | TXN | |
| Thioredoxin reductase | TXNRD | |
| UDP-glucuronosyl transferase | UGT | |
| Ferritin light/heavy chain | FTL /FTH | Iron metabolism [139] |
| Glucose-6-phosphate dehydrogenase | G6PD | Bioenergetic function [140] |
| 6-phophogluconate dehydrogenase | PGD | |
| Malic enzyme | ME | |
| NADP-dependent isocitrate dehydrogenase | IDH | |
| B-cell lymphoma | BCL | Apoptosis [141] |
| Autophagy protein | ATG | Autophagy [141] |
| Microtubule-associated protein1A/1B-light chain 3B | LC3B | |
| Activating transcription factor | ATF | Proteasomal degradation [142] |
| Proteasomes | PSM | |
| Sequestosome (p62) | SQSTM |
4. Nrf2 and Argan Oil Compounds
4.1. Nrf2 and Oleic and Linoleic Acids
Oleic acid (C18:1n-9) and linoleic acid (C18:2n-6) are common unsaturated fatty acids present in various dietary fat sources [143]. Argan oil contains a balanced ratio of oleic acid (46.3%) and linoleic acid (34%), whereas olive oil is predominantly composed of oleic acid (73%), with a lower linoleic acid content (11.3%). The oxidation of linoleic acid produces metabolites with conflicting roles as both beneficial and detrimental components [144,145]. One such product is 12,13-epoxy-9-keto-10(trans)-octadecenoic acid (EKODE), which activates ARE in primary cells and IMR-32 human neuroblastoma cells, mediated by the transcription factor Nrf2 [146]. A novel fatty acid metabolite, 10-oxo-trans-11-octadecenoic acid, generated by gut Lactobacillus plantarum, increases Nrf2 levels and induces antioxidant enzyme expression in mouse tissues and HepG2 cells [147].
Olive oil and its component hydroxytyrosol elevate Nrf2 activation and protein expression in fibroblasts, while oleic acid increases ROS production [148,149]. Roselle seed oil, rich in linolenic (30%), palmitic (25,8%), and oleic acids (14.45%), enhances Nrf2 levels in rat livers exposed to paracetamol [150]. Oleic acid treatment increases Nrf2 expression in HepG2 cells, but it does not affect Nrf2 in steatosis-induced HL-7702 cells [151,152]. In a rabbit model of acute respiratory distress syndrome, oleic acid does not alter Nrf2 levels [153]. However, oleic acid has been found to protect hepatic cells from H2O2-induced inflammation and oxidative stress. Accordingly, supplementation with oleic acid upregulates Nrf2 mRNA expression, contributing to its protective effects against hepatic ischemia-reperfusion injury in mice, possibly through the inhibition of AKT/mTOR pathways [154].
4.2. Nrf2 and Ferulic Acid
Ferulic acid, a common phenolic compound, is abundantly present in numerous fruits, vegetables, and vegetable oils such as argan oil [155] and olive oil [156] (Table 5).
Recently, several derivatives of ferulic acid with enhanced activity and improved stability and toxicity have been identified, making them promising candidates for various applications. Ferulic acid itself has demonstrated effectiveness in managing apoptosis, fibrosis, inflammation, platelet aggregation, oxidative stress, and vascular endothelial injury [157]. Studies consistently report the modulation of Nrf2 by ferulic acid at both mRNA and protein levels, although specific mechanisms may vary across cell models. For instance, in IPEC-J2 porcine enterocytes pretreated with deoxynivalenol, ferulic acid exposure decreased cytoplasmic Nrf2 content while increasing its nuclear translocation, accompanied by reduced Keap1 expression and elevated HO-1 expression, indicating Nrf2 pathway activation to mitigate oxidative stress [158]. Likewise, in irradiated human umbilical vein endothelial cells, ferulic acid facilitated nuclear Nrf2 translocation via extracellular signal-regulated kinase and phosphatidylinositol 3-kinase pathways, offering protection against radiation-induced oxidative stress [159].
An increased nuclear accumulation of Nrf2 was observed in hepatocytes treated with Nelumbo nucifera leaves, containing various phenolic compounds including ferulic acid, suggesting Nrf2-mediated upregulation of antioxidant enzymes (i.e., CAT, HO-1, and SOD-1) [160]. Ferulic acid also exhibited neuroprotective effects in SH-SY5Y neuroblastoma cells by inducing HO-1 expression and promoting Nrf2 nuclear translocation [161]. In PC12 cells (derived from rat pheochromocytoma) exposed to lead acetate, ferulic acid induced HO-1 gene expression and enhanced ARE promoter activity, indicating its potential for treating lead neurotoxicity in children [162]. In rat livers, the administration of ferulic acid increased the total Nrf2 protein expression and antioxidant gene expression, suggesting Nrf2 accumulation and ARE activation post-treatment [163,164]. In addition, the administration of mice with the ethyl acetate extract of purple rice was found to enhance the activities of GPx in mice livers and sera concomitantly to the increase in the Nrf2 expression. The characterization of this extract by ultra-high-performance liquid chromatography tandem mass spectrometry determined quercetin and ferulic acid as the primary phenolic compounds [165]. Moreover, the effect of the administration of ferulic acid was investigated against retinal photooxidative damage in pigmented rabbits. The HO-1 mRNA and protein levels were increased by ferulic acid after light exposure. In line with these modifications, the treatment with this phenolic compound reinforced the light-induced increase in both the Nrf2 protein and Nrf2 mRNA levels [166].
Ferulic acid treatment of carp resulted in a reduction in ROS formation caused by difenoconazole and restored CAT activity and successfully repressed the transcript levels of genes involved in the Nrf2 signaling pathway, including Keap 1, Nrf2, and HO-1. Consequently, FA restored the Nrf2 signaling pathway, thereby improving spleen function and reducing oxidative stress [167]. In another study investigating the effect of ferulic acid against methoxyethanol-induced testicular oxidative stress in rats, it was observed that ferulic acid increased the activities of GPx, CAT, and SOD in the testicles. By contrast, it significantly decreased the MDA level and Nrf2 expression [168]. Collectively, these studies provide diverse perspectives on the Keap1/Nrf2/ARE signaling pathway regulated by ferulic acid. While some studies support its activation, others contradict these findings, highlighting the need for further research to clarify the underlying mechanisms and factors governing ferulic acid’s interaction with the Nrf2 pathway.
In the case of increased antioxidant enzyme levels under oxidative stress, there are distinct scenarios. In one scenario, ROS oxidize the main cysteine residues of Keap1, triggering conformational changes that prevent Nrf2 binding and its transfer to the nucleus and subsequent activation of ARE-mediated gene expression, including GPx, CAT, and SOD. Another scenario occurs when the ROS attack exceeds the cellular defense capacity, resulting in the inadequate production of protective enzymes. Alternatively, when ROS attack and defense levels are balanced, cells increase the production of protective enzymes, leading to increased antioxidant enzyme levels. Subsequently, as oxidative stress diminishes due to ROS scavenging, the negative feedback mechanism reduces Nrf2 synthesis, resulting in a decline in antioxidant enzyme levels below the limit of detection.
4.3. Nrf2 and Phytosterols (Schottenol and Spinasterol)
In the last few years, much attention has been given to phytosterol-enriched foods [169]. Phytosterols are structurally related to cholesterol and are mainly C-28 and C-29 carbon steroid alcohols (Table 5) [170]. Schottenol and spinasterol (Figure 3) are two phytosterols mainly present in argan oil [15] and less in cactus seed oil [171]. To the best of our knowledge, there have been no studies investigating the direct impact of spinasterol or schottenol on the Nrf2 signaling pathway. However, we have recently explored how oxidative stress is influenced by these two phytosterols. Given that Nrf2 plays a crucial role as a transcription factor in cellular defense against oxidative stress, a potential association between the Nrf2 signaling pathway and both phytosterols could be suggested. Specifically, schottenol has been found to increase mitochondrial membrane potential, indicating mitochondrial hyperpolarization in BV2 cells [172] Additionally, phytosterol did not affect the cell growth of SK-N-BE human neuronal cells [17]. Furthermore, both schottenol and spinasterol reduced intracellular ROS and NO levels in the culture medium. Conversely, they enhanced ACOX1 activity in microglial BV-2 cells and normalized CAT activity in both wild-type and ACOX1-deficient microglial cells. These findings collectively suggest the potential of schottenol and spinasterol to provide protection against oxidative stress [16]. Another oil derived from cactus seed demonstrated hepatoprotective and neuroprotective effects against LPS-induced damage by restoring peroxisomal antioxidant and β-oxidative capacities in mouse livers, thereby maintaining brain peroxisomal antioxidant activities, including glutathione peroxidase and catalase [171].
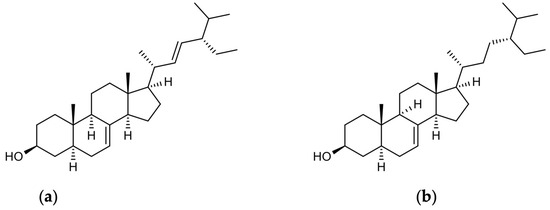
Figure 3.
Chemical structures of spinasterol and schottenol: (a) alpha-spinasterol; (b) schottenol.
4.4. Nrf2 and Tocopherols
Tocopherols (Table 5) available in nuts, vegetable oils, and some oilseeds, come in four lipid-soluble forms, α, β, γ, and δ, each with a distinct arrangement of methyl substituents on the chromanol ring and hydrocarbon side chain [173].
The antioxidant capacity of tocopherol isomers depends on the of hydroxyl groups and follows the order of α > β > γ > δ [174]. However, γ- and δ-tocopherols possess unique antioxidant properties not seen in α-tocopherol [175]. Argan oil contains mainly γ- and δ-tocopherol, with 81–92 g/100 g and 6.2–12.8 g/100g, respectively [176]. Interestingly, the unsaponifiable fraction of roselle seed oil, rich in γ-tocopherol (150 mg/100 g), α-tocopherol (58.7 mg/100 g), and δ-tocopherol (27.16 mg/100 g), raises the hepatic Nrf2 level in rats treated with paracetamol, concurrently reducing MDA levels and increasing glutathione (GSH) [150]. γ-Tocopherol treatment (25 µM) recovered cell viability in Hepa1c1c7 cells subjected to hydrogen peroxide [177]. Moreover, Rosa rubiginosa L., rich in α- and γ-tocopherols, was found to increase the hepatic Nrf2 level and increased its ARE binding capacity in rats subjected to ischemia followed by reperfusion. In addition, the mRNA expression of HO-1 was increased [178]. However, the elimination of α- and γ-tocopherols from Rosa rubiginosa L. prevented its protective effect against high-fat-diet induced Nrf2 depletion in the livers of mice, contrasting with the beneficial effects observed when both tocopherols were present [179]. In addition, it has been shown that γ-tocopherol decreased MDA levels without changing Nrf2 expression in mice [180]. Exposure of Human Retinal Pigment Epithelium to α-tocopherol followed by tert-butyl hydroperoxide increased Nrf2 expression 3.5-fold, while γ-tocopherol had no effect [181]. In rats, an enriched diet with γ-tocopherol mixtures increased liver Nrf2 protein levels while maintaining Keap1 levels. These findings collectively suggest that tocopherols modulate the Nrf2 signaling pathway differently depending on the dosage and cell model [182].

Table 5.
Biological activities of major argan oil compounds as Nrf2 pathway regulators in preclinical studies.
Table 5.
Biological activities of major argan oil compounds as Nrf2 pathway regulators in preclinical studies.
| Compound/Structure | Effective Concentration | Biological Activities | Nrf2 Downstream Genes | Disease(s) | Study Model | References | |
|---|---|---|---|---|---|---|---|
| 1 | Linoleic acid | 1–10 μM | Antioxidant | NQO1 | Oxidative stress | IMR-32 neuroblastoma cells and cerebro-cortical neurons | [146] |
| ˃1 µM | Antioxidant | NQO1, HO-1, and GCLM | Oxidative stress | HepG2 cells | [147] | ||
| ˃1 µM | Antioxidant | HO-1 | Oxidative stress | Murine dermal fibroblast | [148] | ||
| 0.6, 4 and 8 mL/kg | Antioxidant; anti-inflammatory | HO-1 | Inflammation | Rats | [150] | ||
| 1 mM | Antioxidant | HO-1 | Oxidative stress | Mice | [151] | ||
| 2 | Oleic acid | 100 µM | Antioxidant; anti-inflammatory | - | Hepatic ischemia-reperfusion (I/R) injury | Mice | [154] |
| 3 | Ferulic acid | 0–100 μM | Antioxidant; anti-inflammatory | SOD, CAT | Oxidative stress, inflammation, and apoptosis | IPEC-J2 cells | [158] |
| 0.2–5 µM | Antioxidant | NQO1, HO-1, GCLM, and GCLC | Oxidative stress | Human umbilical vein endothelial cells (HUVECs) | [159] | ||
| 50 mg/kg | Antioxidant | NQO1, HO-1, GST, SOD, CAT, and GPx | Oxidative stress | Rats | [168] | ||
| 4 | Alpha-tocopherol | 0.01 mL/g | Antioxidant; anti-inflammatory | HO-1 | Oxidative stress and obesity | Mice | [179] |
| 100 μM | Antioxidant | SOD | Oxidative stress | hTERT-RPE cells | [181] |
5. Conclusions
When compared to other edible oils, Argan oil shows an unique composition, with high levels of linoleic and oleic acids, as well as polyphenols, sterols, and tocopherols. Nrf2 is the main redox-sensitive transcription factor that regulates the expression of genes that encode antioxidant molecules. This pathway plays a crucial role in controlling the cellular antioxidant response against oxidative damage. Major argan oil constituents have been shown to regulate the Nrf2 pathway, and their antioxidant properties highlight the natural resource’s possible therapeutic uses in enhancing cellular defense systems and reducing oxidative stress. This pathway plays a crucial role in controlling the cellular antioxidant response against oxidative damage. The antioxidant qualities of argan oil are attributed to its constituents, including fatty acids, polyphenols, and tocopherols. Such structurally diverse chemicals can scavenge free radicals, prevent lipid peroxidation, and enhance cellular resilience. These findings underline the chemical basis that gives argan oil its antioxidant properties and highlight its promising medicinal uses. Of note, minor components of argan oil, such as alpha-linolenic acid (ALA), contribute to its nutritional profile and can activate the skn-1/Nrf2 transcription factor, offering potential benefits at different levels by inhibiting oxidative stress, promoting longevity, and exerting anti-inflammatory effects. Moreover, the activation of the Nrf2 signaling pathway by gamma-linolenic acid (GLA) can alleviate muscle atrophy and increase antioxidant defense mechanisms. Collectively, this research highlights the interesting interplay between natural compounds and cellular defense systems, making the natural argan oil a sustainable option for improving health by curing oxidative-stress-related diseases. Future studies will further explore the dietetic potential of argan oil, paving the way for comprehensive healthcare approaches.
Author Contributions
Conceptualization, R.E.K.; validation, L.S., P.A. and M.C.-M.; writing—original draft preparation, R.E.K., H.B., M.T.-J. and S.R.; writing—review and editing, R.E.K., M.C.-M., B.N., P.T., Y.L. and M.C.E.; funding acquisition, R.E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Higher Education, Scientific Research and Innovation of Morocco (MESRSI), the National Agency for Medicinal and Aromatic Plants (ANPMA), and the National Center for Scientific and Technical Research (CNRST) within the framework of the fourth edition of the research project in the field of Valorization of Medicinal and Aromatic Plants (VPMA4).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Finkel, T. Signal Transduction by Reactive Oxygen Species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Transcriptional Responses to Oxidative Stress: Pathological and Toxicological Implications. Pharmacol. Ther. 2010, 125, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P. Nutrients and Oxidative Stress: Friend or Foe? Oxid. Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Oxidants and Antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Marhoume, F.Z.; Aboufatima, R.; Zaid, Y.; Limami, Y.; Duval, R.E.; Laadraoui, J.; Belbachir, A.; Chait, A.; Bagri, A. Antioxidant and Polyphenol-Rich Ethanolic Extract of Rubia tinctorum L. Prevents Urolithiasis in an Ethylene Glycol Experimental Model in Rats. Molecules 2021, 26, 1005. [Google Scholar] [CrossRef]
- Ndayambaje, M.; Wahnou, H.; Sow, M.; Chgari, O.; Habyarimana, T.; Karkouri, M.; Limami, Y.; Naya, A.; Oudghiri, M. Exploring the Multifaceted Effects of Ammi visnaga: Subchronic Toxicity, Antioxidant Capacity, Immunomodulatory, and Anti-Inflammatory Activities. J. Toxicol. Environ. Health Part A 2024, 87, 150–165. [Google Scholar] [CrossRef]
- El Kharrassi, Y.; Maata, N.; Mazri, M.A.; El Kamouni, S.; Talbi, M.; El Kebbaj, R.; Moustaid, K.; Essamadi, A.K.; Andreoletti, P.; El Mzouri, E.H.; et al. Chemical and Phytochemical Characterizations of Argan Oil (Argania spinosa L. Skeels), Olive Oil (Olea europaea L. Cv. Moroccan Picholine), Cactus Pear (Opuntia Megacantha Salm-Dyck) Seed Oil and Cactus Cladode Essential Oil. Food Meas. 2018, 12, 747–754. [Google Scholar] [CrossRef]
- Taous, F.; Amenzou, N.; Marah, H.; Maia, R.; Maguas, C.; Bahmad, L.; Kelly, S. Stable Isotope Ratio Analysis as a New Tool to Trace the Geographical Origin of Argan Oils in Morocco. Forensic Chem. 2020, 17, 100198. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Nicoli, S.F.; Raffo, A.; Santini, A.; Novellino, E.; Souto, E.B.; Romani, A.; Belcaro, M.F.; Vita, C. Cold Pressed Argan (Argania spinose) Oil. In Cold Pressed Oils; Elsevier: Amsterdam, The Netherlands, 2020; pp. 459–465. [Google Scholar]
- Bouchab, H.; Essadek, S.; El Kamouni, S.; Moustaid, K.; Essamadi, A.; Andreoletti, P.; Cherkaoui-Malki, M.; El Kebbaj, R.; Nasser, B. Antioxidant Effects of Argan Oil and Olive Oil against Iron-Induced Oxidative Stress: In Vivo and In Vitro Approaches. Molecules 2023, 28, 5924. [Google Scholar] [CrossRef]
- Bouchab, H.; Ishaq, A.; El Kebbaj, R.; Nasser, B.; Saretzki, G. Protective Effect of Argan Oil on DNA Damage in Vivo and in Vitro. Biomarkers 2021, 26, 425–433. [Google Scholar] [CrossRef] [PubMed]
- El Kamouni, S.; El Kebbaj, R.; Andreoletti, P.; El Ktaibi, A.; Rharrassi, I.; Essamadi, A.; El Kebbaj, M.S.; Mandard, S.; Latruffe, N.; Vamecq, J.; et al. Protective Effect of Argan and Olive Oils against LPS-Induced Oxidative Stress and Inflammation in Mice Livers. Int. J. Mol. Sci. 2017, 18, 2181. [Google Scholar] [CrossRef]
- Essadek, S.; Bouchab, H.; El Kebbaj, R.; Gondcaille, C.; El Kamouni, S.; Savary, S.; Vamecq, J.; Essamadi, A.; Cherkaoui-Malki, M.; Nasser, B.; et al. Effects of a Short-Term Lipopolysaccharides Challenge on Mouse Brain and Liver Peroxisomal Antioxidant and β-Oxidative Functions: Protective Action of Argan Oil. Pharmaceuticals 2022, 15, 465. [Google Scholar] [CrossRef] [PubMed]
- Essadek, S.; Gondcaille, C.; Savary, S.; Samadi, M.; Vamecq, J.; Lizard, G.; Kebbaj, R.E.; Latruffe, N.; Benani, A.; Nasser, B.; et al. Two Argan Oil Phytosterols, Schottenol and Spinasterol, Attenuate Oxidative Stress and Restore LPS-Dysregulated Peroxisomal Functions in Acox1-/- and Wild-Type BV-2 Microglial Cells. Antioxidants 2023, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- Badreddine, A.; Zarrouk, A.; Nury, T.; El Kharrassi, Y.; Nasser, B.; Malki, M.C.; Lizard, G.; Samadi, M.; El Mostafa, K. An Expeditious Synthesis of Spinasterol and Schottenol, Two Phytosterols Present in Argan Oil and in Cactus Pear Seed Oil, and Evaluation of Their Biological Activities on Cells of the Central Nervous System. Steroids 2015, 99, 119–124. [Google Scholar] [CrossRef]
- Badreddine, A.; Zarrouk, A.; Karym, E.M.; Debbabi, M.; Nury, T.; Meddeb, W.; Sghaier, R.; Bezine, M.; Vejux, A.; Martine, L.; et al. Argan Oil-Mediated Attenuation of Organelle Dysfunction, Oxidative Stress and Cell Death Induced by 7-Ketocholesterol in Murine Oligodendrocytes 158N. Int. J. Mol. Sci. 2017, 18, 2220. [Google Scholar] [CrossRef]
- Cherki, M.; Berrougui, H.; Drissi, A.; Adlouni, A.; Khalil, A. Argan Oil: Which Benefits on Cardiovascular Diseases? Pharmacol. Res. 2006, 54, 1–5. [Google Scholar] [CrossRef]
- Khallouki, F.; Younos, C.; Soulimani, R.; Oster, T.; Charrouf, Z.; Spiegelhalder, B.; Bartsch, H.; Owen, R.W. Consumption of Argan Oil (Morocco) with Its Unique Profile of Fatty Acids, Tocopherols, Squalene, Sterols and Phenolic Compounds Should Confer Valuable Cancer Chemopreventive Effects. Eur. J. Cancer Prev. 2003, 12, 67–75. [Google Scholar] [CrossRef]
- Marfil, R.; Cabrera-Vique, C.; Giménez, R.; Bouzas, P.R.; Martínez, O.; Sánchez, J.A. Metal Content and Physicochemical Parameters Used as Quality Criteria in Virgin Argan Oil: Influence of the Extraction Method. J. Agric. Food Chem. 2008, 56, 7279–7284. [Google Scholar] [CrossRef]
- Guillaume, D.; Pioch, D.; Charrouf, Z. Argan [Argania spinosa (L.) Skeels] Oil. In Fruit Oils: Chemistry and Functionality; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 317–352. ISBN 978-3-030-12472-4. [Google Scholar]
- El Kebbaj, R.; El Kamouni, S.; El Hajj, H.I.; Andreoletti, P.; Gresti, J.; Latruffe, N.; El Kebbaj, M.S.; Vamecq, J.; Lizard, G.; Nasser, B.; et al. Modulation of Peroxisomes Abundance by Argan Oil and Lipopolysaccharides in Acyl-CoA Oxidase 1-Deficient Fibroblasts. Health 2013, 5, 26522. [Google Scholar] [CrossRef]
- Xu, W.; Yao, J.; Yi, Y.; Wang, H.-X.; Wang, L.-M. Effects of Storage Condition on the Physicochemical Characteristics of Sunflower Seed Oil. RSC Adv. 2019, 9, 42262–42271. [Google Scholar] [CrossRef]
- de Souza Aquino, J.; Batista, K.S.; Araujo-Silva, G.; dos Santos, D.C.; de Brito, N.J.N.; López, J.A.; da Silva, J.A.; das Graças Almeida, M.; Pincheira, C.G.; Magnani, M.; et al. Antioxidant and Lipid-Lowering Effects of Buriti Oil (Mauritia flexuosa L.) Administered to Iron-Overloaded Rats. Molecules 2023, 28, 2585. [Google Scholar] [CrossRef] [PubMed]
- Roncero, J.M.; Álvarez-Ortí, M.; Pardo-Giménez, A.; Rabadán, A.; Pardo, J.E. Influence of Pressure Extraction Systems on the Performance, Quality and Composition of Virgin Almond Oil and Defatted Flours. Foods 2021, 10, 1049. [Google Scholar] [CrossRef] [PubMed]
- Idrissi, Z.L.E.; El Moudden, H.; Mghazli, N.; Bouyahya, A.; Guezzane, C.E.; Alshahrani, M.M.; Al Awadh, A.A.; Goh, K.W.; Ming, L.C.; Harhar, H.; et al. Effects of Extraction Methods on the Bioactivities and Nutritional Value of Virginia and Valencia-Type Peanut Oil. Molecules 2022, 27, 7709. [Google Scholar] [CrossRef] [PubMed]
- Karoui, I.J.; Wannes, W.A.; Marzouk, B. Refined Corn Oil Aromatization by Citrus Aurantium Peel Essential Oil. Ind. Crops Prod. 2010, 32, 202–207. [Google Scholar] [CrossRef]
- Alves, A.Q.; da Silva, V.A.; Góes, A.J.S.; Silva, M.S.; de Oliveira, G.G.; Bastos, I.V.G.A.; de Castro Neto, A.G.; Alves, A.J. The Fatty Acid Composition of Vegetable Oils and Their Potential Use in Wound Care. Adv. Skin. Wound Care 2019, 32, 1–8. [Google Scholar] [CrossRef]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty Acids Composition of Vegetable Oils and Its Contribution to Dietary Energy Intake and Dependence of Cardiovascular Mortality on Dietary Intake of Fatty Acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Hilali, M.; Monfalouti, H.E.; Kartah, B.E. Evaluation of the Chemical Composition of Argan (Argania spinosa L.) Oil According to Its Extraction Method, Origin of Production and Altitude. Online J. Anim. Feed. Res. 2020, 10, 111–118. [Google Scholar] [CrossRef]
- Marfil, R.; Giménez, R.; Martínez, O.; Bouzas, P.R.; Rufián-Henares, J.A.; Mesías, M.; Cabrera-Vique, C. Determination of Polyphenols, Tocopherols, and Antioxidant Capacity in Virgin Argan Oil (Argania spinosa, Skeels). Eur. J. Lipid Sci. Technol. 2011, 113, 886–893. [Google Scholar] [CrossRef]
- Pellegrini, N.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F.; Serafini, M. Total Antioxidant Capacity of Plant Foods, Beverages and Oils Consumed in Italy Assessed by Three Different In Vitro Assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef]
- Cabrera-Vique, C.; Marfil, R.; Giménez, R.; Martínez-Augustin, O. Bioactive Compounds and Nutritional Significance of Virgin Argan Oil—An Edible Oil with Potential as a Functional Food. Nutr. Rev. 2012, 70, 266–279. [Google Scholar] [CrossRef]
- Cairone, F.; Cesa, S.; Ciogli, A.; Fabrizi, G.; Goggiamani, A.; Iazzetti, A.; Di Lena, G.; Sanchez del Pulgar, J.; Lucarini, M.; Cantò, L.; et al. Valorization of By-Products from Biofuel Biorefineries: Extraction and Purification of Bioactive Molecules from Post-Fermentation Corn Oil. Foods 2022, 11, 153. [Google Scholar] [CrossRef]
- Rueda, A.; Samaniego-Sánchez, C.; Olalla, M.; Giménez, R.; Cabrera-Vique, C.; Seiquer, I.; Lara, L. Combination of Analytical and Chemometric Methods as a Useful Tool for the Characterization of Extra Virgin Argan Oil and Other Edible Virgin Oils. Role of Polyphenols and Tocopherols. J. AOAC Int. 2016, 99, 489–494. [Google Scholar] [CrossRef]
- Adlouni, A. L’huile d’argan, de La Nutrition à La Santé. Phytothérapie 2010, 8, 89–97. [Google Scholar] [CrossRef]
- Harhar, H.; Gharby, S.; Kartah, B.; El Monfalouti, H.; Guillaume, D.; Charrouf, Z. Influence of Argan Kernel Roasting-Time on Virgin Argan Oil Composition and Oxidative Stability. Plant Foods Hum. Nutr. 2011, 66, 163–168. [Google Scholar] [CrossRef]
- Kamal, R.; Kharbach, M.; Vander Heyden, Y.; Doukkali, Z.; Ghchime, R.; Bouklouze, A.; Cherrah, Y.; Alaoui, K. In Vivo Anti-Inflammatory Response and Bioactive Compounds’ Profile of Polyphenolic Extracts from Edible Argan Oil (Argania spinosa L.), Obtained by Two Extraction Methods. J. Food Biochem. 2019, 43, e13066. [Google Scholar] [CrossRef]
- Hilali, M.; Charrouf, Z.; Aziz Soulhi, A.E.; Hachimi, L.; Guillaume, D. Influence of Origin and Extraction Method on Argan Oil Physico-Chemical Characteristics and Composition. J. Agric. Food Chem. 2005, 53, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Matthäus, B.; Guillaume, D.; Gharby, S.; Haddad, A.; Harhar, H.; Charrouf, Z. Effect of Processing on the Quality of Edible Argan Oil. Food Chem. 2010, 120, 426–432. [Google Scholar] [CrossRef]
- Badreddine, A.; Zarrouk, A.; Meddeb, W.; Nury, T.; Rezig, L.; Debbabi, M.; Bessam, F.Z.; Brahmi, F.; Vejux, A.; Mejri, M.; et al. Antioxidant and Neuroprotective Properties of Mediterranean Oils: Argan Oil, Olive Oil, and Milk Thistle Seed Oil. In Oxidative Stress and Dietary Antioxidants in Neurological Diseases; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–154. ISBN 978-0-12-817780-8. [Google Scholar]
- de Carvalho, C.; Caramujo, M. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef]
- Jump, D.B.; Botolin, D.; Wang, Y.; Xu, J.; Christian, B.; Demeure, O. Fatty Acid Regulation of Hepatic Gene Transcription. J. Nutr. 2005, 135, 2503–2506. [Google Scholar] [CrossRef] [PubMed]
- Ebbert, J.O.; Jensen, M.D. Fat Depots, Free Fatty Acids, and Dyslipidemia. Nutrients 2013, 5, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, T.; Ma, A. Genetic Mechanism of Tissue-Specific Expression of PPAR Genes in Turbot (Scophthalmus Maximus) at Different Temperatures. Int. J. Mol. Sci. 2022, 23, 12205. [Google Scholar] [CrossRef] [PubMed]
- Sundrani, D.P.; Karkhanis, A.R.; Joshi, S.R. Peroxisome Proliferator-Activated Receptors (PPAR), Fatty Acids and microRNAs: Implications in Women Delivering Low Birth Weight Babies. Syst. Biol. Reprod. Med. 2021, 67, 24–41. [Google Scholar] [CrossRef]
- Cherkaoui-Malki, M.; Meyer, K.; Cao, W.Q.; Latruffe, N.; Yeldandi, A.V.; Rao, M.S.; Bradfield, C.A.; Reddy, J.K. Identification of Novel Peroxisome Proliferator-Activated Receptor Alpha (PPARalpha) Target Genes in Mouse Liver Using cDNA Microarray Analysis. Gene Expr. 2001, 9, 291–304. [Google Scholar] [CrossRef]
- Ren, B.; Thelen, A.P.; Peters, J.M.; Gonzalez, F.J.; Jump, D.B. Polyunsaturated Fatty Acid Suppression of Hepatic Fatty Acid Synthase and S14 Gene Expression Does Not Require Peroxisome Proliferator-Activated Receptor Alpha. J. Biol. Chem. 1997, 272, 26827–26832. [Google Scholar] [CrossRef]
- El Kebbaj, R.; Andreoletti, P.; El Hajj, H.I.; El Kharrassi, Y.; Vamecq, J.; Mandard, S.; Saih, F.-E.; Latruffe, N.; El Kebbaj, M.S.; Lizard, G.; et al. Argan Oil Prevents Down-Regulation Induced by Endotoxin on Liver Fatty Acid Oxidation and Gluconeogenesis and on Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1α, (PGC-1α), Peroxisome Proliferator-Activated Receptor α (PPARα) and Estrogen Related Receptor α (ERRα). Biochim. Open 2015, 1, 51–59. [Google Scholar] [CrossRef]
- Marcelino, G.; Hiane, P.A.; Freitas, K.d.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; Guimarães, R.D.C.A. Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef]
- Williams, K.; Sniderman, A.D.; Sattar, N.; D’Agostino, R.; Wagenknecht, L.E.; Haffner, S.M. Comparison of the Associations of Apolipoprotein B and Low-Density Lipoprotein Cholesterol With Other Cardiovascular Risk Factors in the Insulin Resistance Atherosclerosis Study (IRAS). Circulation 2003, 108, 2312–2316. [Google Scholar] [CrossRef] [PubMed]
- Julius, U. Fat Modification in the Diabetes Diet. Exp. Clin. Endocrinol. Diabetes 2003, 111, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Soriguer, F.; Rojo-Martínez, G.; de Fonseca, F.R.; García-Escobar, E.; García Fuentes, E.; Olveira, G. Obesity and the Metabolic Syndrome in Mediterranean Countries: A Hypothesis Related to Olive Oil. Mol. Nutr. Food Res. 2007, 51, 1260–1267. [Google Scholar] [CrossRef]
- Portillo, M.P.; Chávarri, M.; Durán, D.; Rodríguez, V.M.; Macarulla, M.T. Differential Effects of Diets That Provide Different Lipid Sources on Hepatic Lipogenic Activities in Rats under Ad Libitum or Restricted Feeding. Nutrition 2001, 17, 467–473. [Google Scholar] [CrossRef]
- Ferramosca, A.; Savy, V.; Zara, V. Olive Oil Increases the Hepatic Triacylglycerol Content in Mice by a Distinct Influence on the Synthesis and Oxidation of Fatty Acids. Biosci. Biotechnol. Biochem. 2008, 72, 62–69. [Google Scholar] [CrossRef]
- Priore, P.; Stanca, E.; Gnoni, G.V.; Siculella, L. Dietary Fat Types Differently Modulate the Activity and Expression of Mitochondrial Carnitine/Acylcarnitine Translocase in Rat Liver. Biochim. Biophys. Acta 2012, 1821, 1341–1349. [Google Scholar] [CrossRef]
- Clarke, S.D. The Multi-Dimensional Regulation of Gene Expression by Fatty Acids: Polyunsaturated Fats as Nutrient Sensors. Curr. Opin. Lipidol. 2004, 15, 13–18. [Google Scholar] [CrossRef]
- Vamecq, J.; Andreoletti, P.; El Kebbaj, R.; Saih, F.-E.; Latruffe, N.; El Kebbaj, M.H.S.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Peroxisomal Acyl-CoA Oxidase Type 1: Anti-Inflammatory and Anti-Aging Properties with a Special Emphasis on Studies with LPS and Argan Oil as a Model Transposable to Aging. Oxid. Med. Cell. Longev. 2018, 2018, 6986984. [Google Scholar] [CrossRef]
- Lin, M.; Zhai, X.; Wang, G.; Tian, X.; Gao, D.; Shi, L.; Wu, H.; Fan, Q.; Peng, J.; Liu, K.; et al. Salvianolic Acid B Protects against Acetaminophen Hepatotoxicity by Inducing Nrf2 and Phase II Detoxification Gene Expression via Activation of the PI3K and PKC Signaling Pathways. J. Pharmacol. Sci. 2015, 127, 203–210. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Yaribeygi, H.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. The Effects of Ginsenosides on the Nrf2 Signaling Pathway. Adv. Exp. Med. Biol. 2021, 1328, 307–322. [Google Scholar] [CrossRef]
- Chambel, S.S.; Santos-Gonçalves, A.; Duarte, T.L. The Dual Role of Nrf2 in Nonalcoholic Fatty Liver Disease: Regulation of Antioxidant Defenses and Hepatic Lipid Metabolism. Biomed. Res. Int. 2015, 2015, 597134. [Google Scholar] [CrossRef]
- Moi, P.; Chan, K.; Asunis, I.; Cao, A.; Kan, Y.W. Isolation of NF-E2-Related Factor 2 (Nrf2), a NF-E2-like Basic Leucine Zipper Transcriptional Activator That Binds to the Tandem NF-E2/AP1 Repeat of the Beta-Globin Locus Control Region. Proc. Natl. Acad. Sci. USA 1994, 91, 9926–9930. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Gall Trošelj, K.; Tomljanović, M.; Jaganjac, M.; Matijević Glavan, T.; Čipak Gašparović, A.; Milković, L.; Borović Šunjić, S.; Buttari, B.; Profumo, E.; Saha, S.; et al. Oxidative Stress and Cancer Heterogeneity Orchestrate NRF2 Roles Relevant for Therapy Response. Molecules 2022, 27, 1468. [Google Scholar] [CrossRef]
- Deus, C.M.; Teixeira, J.; Raimundo, N.; Tucci, P.; Borges, F.; Saso, L.; Oliveira, P.J. Modulation of Cellular Redox Environment as a Novel Therapeutic Strategy for Parkinson’s Disease. Eur. J. Clin. Investig. 2022, 52, e13820. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Profumo, E.; Tucci, P.; Saso, L. A Perspective on Nrf2 Signaling Pathway for Neuroinflammation: A Potential Therapeutic Target in Alzheimer’s and Parkinson’s Diseases. Front. Cell Neurosci. 2021, 15, 787258. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic Targeting of the NRF2 and KEAP1 Partnership in Chronic Diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef]
- Farina, M.; Vieira, L.E.; Buttari, B.; Profumo, E.; Saso, L. The Nrf2 Pathway in Ischemic Stroke: A Review. Molecules 2021, 26, 5001. [Google Scholar] [CrossRef] [PubMed]
- Seminotti, B.; Grings, M.; Tucci, P.; Leipnitz, G.; Saso, L. Nuclear Factor Erythroid-2-Related Factor 2 Signaling in the Neuropathophysiology of Inherited Metabolic Disorders. Front. Cell Neurosci. 2021, 15, 785057. [Google Scholar] [CrossRef]
- Gray, N.E.; Farina, M.; Tucci, P.; Saso, L. The Role of the NRF2 Pathway in Maintaining and Improving Cognitive Function. Biomedicines 2022, 10, 2043. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules 2021, 27, 167. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; Çetinkaya, S.; Panieri, E.; Saha, S.; Buttari, B.; Profumo, E.; Saso, L. Regulatory Role of Nrf2 Signaling Pathway in Wound Healing Process. Molecules 2021, 26, 2424. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.A.; Johnson, D.A.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Vargas, M.R.; Chen, P.-C. The Nrf2-ARE Pathway: An Indicator and Modulator of Oxidative Stress in Neurodegeneration. Ann. N. Y. Acad. Sci. 2008, 1147, 61–69. [Google Scholar] [CrossRef]
- Lee, J.-M.; Calkins, M.J.; Chan, K.; Kan, Y.W.; Johnson, J.A. Identification of the NF-E2-Related Factor-2-Dependent Genes Conferring Protection against Oxidative Stress in Primary Cortical Astrocytes Using Oligonucleotide Microarray Analysis. J. Biol. Chem. 2003, 278, 12029–12038. [Google Scholar] [CrossRef]
- Rachakonda, G.; Sekhar, K.R.; Jowhar, D.; Samson, P.C.; Wikswo, J.P.; Beauchamp, R.D.; Datta, P.K.; Freeman, M.L. Increased Cell Migration and Plasticity in Nrf2-Deficient Cancer Cell Lines. Oncogene 2010, 29, 3703–3714. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2:INrf2 (Keap1) Signaling in Oxidative Stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Fukutomi, T.; Takagi, K.; Mizushima, T.; Ohuchi, N.; Yamamoto, M. Kinetic, Thermodynamic, and Structural Characterizations of the Association between Nrf2-DLGex Degron and Keap1. Mol. Cell Biol. 2014, 34, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Kansanen, E.; Kuosmanen, S.M.; Leinonen, H.; Levonen, A.-L. The Keap1-Nrf2 Pathway: Mechanisms of Activation and Dysregulation in Cancer. Redox Biol. 2013, 1, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Katoh, Y.; Itoh, K.; Yoshida, E.; Miyagishi, M.; Fukamizu, A.; Yamamoto, M. Two Domains of Nrf2 Cooperatively Bind CBP, a CREB Binding Protein, and Synergistically Activate Transcription. Genes Cells 2001, 6, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, B.; Tong, K.I.; Ohta, T.; Nakamura, Y.; Scharlock, M.; Ohtsuji, M.; Kang, M.-I.; Kobayashi, A.; Yokoyama, S.; Yamamoto, M. Structural Basis for Defects of Keap1 Activity Provoked by Its Point Mutations in Lung Cancer. Mol. Cell 2006, 21, 689–700. [Google Scholar] [CrossRef]
- Tong, K.I.; Padmanabhan, B.; Kobayashi, A.; Shang, C.; Hirotsu, Y.; Yokoyama, S.; Yamamoto, M. Different Electrostatic Potentials Define ETGE and DLG Motifs as Hinge and Latch in Oxidative Stress Response. Mol. Cell. Biol. 2007, 27, 7511–7521. [Google Scholar] [CrossRef]
- Taguchi, K.; Motohashi, H.; Yamamoto, M. Molecular Mechanisms of the Keap1–Nrf2 Pathway in Stress Response and Cancer Evolution. Genes Cells 2011, 16, 123–140. [Google Scholar] [CrossRef]
- Nioi, P.; Nguyen, T.; Sherratt, P.J.; Pickett, C.B. The Carboxy-Terminal Neh3 Domain of Nrf2 Is Required for Transcriptional Activation. Mol. Cell. Biol. 2005, 25, 10895–10906. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuźniak, V.; Paluszczak, J.; Baer-Dubowska, W. The Nrf2-ARE Signaling Pathway: An Update on Its Regulation and Possible Role in Cancer Prevention and Treatment. Pharmacol. Rep. 2017, 69, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, K.; Geng, M.; Gao, P.; Wu, X.; Hai, Y.; Li, Y.; Li, Y.; Luo, L.; Hayes, J.D.; et al. RXRα Inhibits the NRF2-ARE Signaling Pathway through a Direct Interaction with the Neh7 Domain of NRF2. Cancer Res. 2013, 73, 3097–3108. [Google Scholar] [CrossRef] [PubMed]
- Rada, P.; Rojo, A.I.; Evrard-Todeschi, N.; Innamorato, N.G.; Cotte, A.; Jaworski, T.; Tobón-Velasco, J.C.; Devijver, H.; García-Mayoral, M.F.; Van Leuven, F.; et al. Structural and Functional Characterization of Nrf2 Degradation by the Glycogen Synthase Kinase 3/β-TrCP Axis. Mol. Cell. Biol. 2012, 32, 3486–3499. [Google Scholar] [CrossRef] [PubMed]
- Ertel, W.; Kremer, J.P.; Kenney, J.; Steckholzer, U.; Jarrar, D.; Trentz, O.; Schildberg, F.W. Downregulation of Proinflammatory Cytokine Release in Whole Blood from Septic Patients. Blood 1995, 85, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, E.C.; Chan, J.Y.; Torti, F.M.; Torti, S.V. Nrf2 Mediates the Induction of Ferritin H in Response to Xenobiotics and Cancer Chemopreventive Dithiolethiones. J. Biol. Chem. 2003, 278, 2361–2369. [Google Scholar] [CrossRef]
- Sun, Z.; Chin, Y.E.; Zhang, D.D. Acetylation of Nrf2 by P300/CBP Augments Promoter-Specific DNA Binding of Nrf2 during the Antioxidant Response. Mol. Cell. Biol. 2009, 29, 2658–2672. [Google Scholar] [CrossRef]
- Theodore, M.; Kawai, Y.; Yang, J.; Kleshchenko, Y.; Reddy, S.P.; Villalta, F.; Arinze, I.J. Multiple Nuclear Localization Signals Function in the Nuclear Import of the Transcription Factor Nrf2. J. Biol. Chem. 2008, 283, 8984–8994. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 Represses Nuclear Activation of Antioxidant Responsive Elements by Nrf2 through Binding to the Amino-Terminal Neh2 Domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- Katsuoka, F.; Yamamoto, M. Small Maf Proteins (MafF, MafG, MafK): History, Structure and Function. Gene 2016, 586, 197–205. [Google Scholar] [CrossRef]
- Canning, P.; Sorrell, F.J.; Bullock, A.N. Structural Basis of Keap1 Interactions with Nrf2. Free Radic. Biol. Med. 2015, 88, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, S.B.; Gordan, J.D.; Jin, J.; Harper, J.W.; Diehl, J.A. The Keap1-BTB Protein Is an Adaptor That Bridges Nrf2 to a Cul3-Based E3 Ligase: Oxidative Stress Sensing by a Cul3-Keap1 Ligase. Mol. Cell. Biol. 2004, 24, 8477–8486. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, M.; Xiong, Y. BTB Protein Keap1 Targets Antioxidant Transcription Factor Nrf2 for Ubiquitination by the Cullin 3-Roc1 Ligase. Mol. Cell. Biol. 2005, 25, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Li, L.; Iwamoto, N.; Nakajima-Takagi, Y.; Kaneko, H.; Nakayama, Y.; Eguchi, M.; Wada, Y.; Kumagai, Y.; Yamamoto, M. The Antioxidant Defense System Keap1-Nrf2 Comprises a Multiple Sensing Mechanism for Responding to a Wide Range of Chemical Compounds. Mol. Cell. Biol. 2009, 29, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Telkoparan-Akillilar, P.; Panieri, E.; Cevik, D.; Suzen, S.; Saso, L. Therapeutic Targeting of the NRF2 Signaling Pathway in Cancer. Molecules 2021, 26, 1417. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kang, M.-I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative Stress Sensor Keap1 Functions as an Adaptor for Cul3-Based E3 Ligase to Regulate Proteasomal Degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef]
- Tao, S.; Liu, P.; Luo, G.; Rojo de la Vega, M.; Chen, H.; Wu, T.; Tillotson, J.; Chapman, E.; Zhang, D.D. P97 Negatively Regulates NRF2 by Extracting Ubiquitylated NRF2 from the KEAP1-CUL3 E3 Complex. Mol. Cell. Biol. 2017, 37, e00660-16. [Google Scholar] [CrossRef]
- Silva-Palacios, A.; Ostolga-Chavarría, M.; Zazueta, C.; Königsberg, M. Nrf2: Molecular and Epigenetic Regulation during Aging. Ageing Res. Rev. 2018, 47, 31–40. [Google Scholar] [CrossRef]
- Ichimura, Y.; Waguri, S.; Sou, Y.-S.; Kageyama, S.; Hasegawa, J.; Ishimura, R.; Saito, T.; Yang, Y.; Kouno, T.; Fukutomi, T.; et al. Phosphorylation of P62 Activates the Keap1-Nrf2 Pathway during Selective Autophagy. Mol. Cell 2013, 51, 618–631. [Google Scholar] [CrossRef]
- Komatsu, M.; Kurokawa, H.; Waguri, S.; Taguchi, K.; Kobayashi, A.; Ichimura, Y.; Sou, Y.-S.; Ueno, I.; Sakamoto, A.; Tong, K.I.; et al. The Selective Autophagy Substrate P62 Activates the Stress Responsive Transcription Factor Nrf2 through Inactivation of Keap1. Nat. Cell Biol. 2010, 12, 213–223. [Google Scholar] [CrossRef]
- Camp, N.D.; James, R.G.; Dawson, D.W.; Yan, F.; Davison, J.M.; Houck, S.A.; Tang, X.; Zheng, N.; Major, M.B.; Moon, R.T. Wilms Tumor Gene on X Chromosome (WTX) Inhibits Degradation of NRF2 Protein through Competitive Binding to KEAP1 Protein. J. Biol. Chem. 2012, 287, 6539–6550. [Google Scholar] [CrossRef]
- Hast, B.E.; Goldfarb, D.; Mulvaney, K.M.; Hast, M.A.; Siesser, P.F.; Yan, F.; Hayes, D.N.; Major, M.B. Proteomic Analysis of Ubiquitin Ligase KEAP1 Reveals Associated Proteins That Inhibit NRF2 Ubiquitination. Cancer Res. 2013, 73, 2199–2210. [Google Scholar] [CrossRef]
- Lu, K.; Alcivar, A.L.; Ma, J.; Foo, T.K.; Zywea, S.; Mahdi, A.; Huo, Y.; Kensler, T.W.; Gatza, M.L.; Xia, B. NRF2 Induction Supporting Breast Cancer Cell Survival Is Enabled by Oxidative Stress-Induced DPP3-KEAP1 Interaction. Cancer Res. 2017, 77, 2881–2892. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, Y.; Katsuoka, F.; Funayama, R.; Nagashima, T.; Nishida, Y.; Nakayama, K.; Engel, J.D.; Yamamoto, M. Nrf2-MafG Heterodimers Contribute Globally to Antioxidant and Metabolic Networks. Nucleic Acids Res. 2012, 40, 10228–10239. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/Small Maf Heterodimer Mediates the Induction of Phase II Detoxifying Enzyme Genes through Antioxidant Response Elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Villegas, J.; Ferraiuolo, L.; Mead, R.J.; Shaw, P.J.; Cuadrado, A.; Rojo, A.I. NRF2 as a Therapeutic Opportunity to Impact in the Molecular Roadmap of ALS. Free Radic. Biol. Med. 2021, 173, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal 2018, 29, 1727–1745. [Google Scholar] [CrossRef]
- David, J.A.; Rifkin, W.J.; Rabbani, P.S.; Ceradini, D.J. The Nrf2/Keap1/ARE Pathway and Oxidative Stress as a Therapeutic Target in Type II Diabetes Mellitus. J. Diabetes Res. 2017, 2017, 4826724. [Google Scholar] [CrossRef]
- Dodson, M.; de la Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in Disease: Timing Is Everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef]
- Pajares, M.; Jiménez-Moreno, N.; García-Yagüe, Á.J.; Escoll, M.; de Ceballos, M.L.; Van Leuven, F.; Rábano, A.; Yamamoto, M.; Rojo, A.I.; Cuadrado, A. Transcription Factor NFE2L2/NRF2 Is a Regulator of Macroautophagy Genes. Autophagy 2016, 12, 1902–1916. [Google Scholar] [CrossRef]
- Singh, A.; Bodas, M.; Wakabayashi, N.; Bunz, F.; Biswal, S. Gain of Nrf2 Function in Non-Small-Cell Lung Cancer Cells Confers Radioresistance. Antioxid. Redox Signal 2010, 13, 1627–1637. [Google Scholar] [CrossRef]
- Ghareghomi, S.; Ahmadian, S.; Zarghami, N.; Kahroba, H. Fundamental Insights into the Interaction between Telomerase/TERT and Intracellular Signaling Pathways. Biochimie 2021, 181, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Ahmadinejad, F.; Geir Møller, S.; Hashemzadeh-Chaleshtori, M.; Bidkhori, G.; Jami, M.-S. Molecular Mechanisms behind Free Radical Scavengers Function against Oxidative Stress. Antioxidants 2017, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Abreu, I.A.; Cabelli, D.E. Superoxide Dismutases-a Review of the Metal-Associated Mechanistic Variations. Biochim. Biophys. Acta 2010, 1804, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Cipak, A.; Jaganjac, M.; Tehlivets, O.; Kohlwein, S.D.; Zarkovic, N. Adaptation to Oxidative Stress Induced by Polyunsaturated Fatty Acids in Yeast. Biochim. Biophys. Acta 2008, 1781, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Medoro, A.; Intrieri, M.; Saso, L.; Scapagnini, G.; Kang, J.X. Targeting NRF2-KEAP1 Axis by Omega-3 Fatty Acids and Their Derivatives: Emerging Opportunities against Aging and Diseases. Free Radic. Biol. Med. 2022, 193, 736–750. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Introductory Remarks. In Oxidative Stress; Elsevier: Amsterdam, The Netherlands, 1985; pp. 1–8. ISBN 978-0-12-642760-8. [Google Scholar]
- Jones, D.P. Redefining Oxidative Stress. Antioxid. Redox Signal 2006, 8, 1865–1879. [Google Scholar] [CrossRef]
- Murphy, M.P.; Holmgren, A.; Larsson, N.-G.; Halliwell, B.; Chang, C.J.; Kalyanaraman, B.; Rhee, S.G.; Thornalley, P.J.; Partridge, L.; Gems, D.; et al. Unraveling the Biological Roles of Reactive Oxygen Species. Cell Metab. 2011, 13, 361–366. [Google Scholar] [CrossRef]
- Ogrunc, M.; Di Micco, R.; Liontos, M.; Bombardelli, L.; Mione, M.; Fumagalli, M.; Gorgoulis, V.G.; d’Adda di Fagagna, F. Oncogene-Induced Reactive Oxygen Species Fuel Hyperproliferation and DNA Damage Response Activation. Cell Death Differ. 2014, 21, 998–1012. [Google Scholar] [CrossRef]
- Covas, G.; Marinho, H.S.; Cyrne, L.; Antunes, F. Activation of Nrf2 by H2O2: De Novo Synthesis versus Nuclear Translocation. Methods Enzymol. 2013, 528, 157–171. [Google Scholar] [CrossRef]
- Li, C.-Q.; Kim, M.Y.; Godoy, L.C.; Thiantanawat, A.; Trudel, L.J.; Wogan, G.N. Nitric Oxide Activation of Keap1/Nrf2 Signaling in Human Colon Carcinoma Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14547–14551. [Google Scholar] [CrossRef]
- Wu, K.C.; McDonald, P.R.; Liu, J.; Klaassen, C.D. Screening of Natural Compounds as Activators of the Keap1-Nrf2 Pathway. Planta Med. 2014, 80, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Muri, J.; Wolleb, H.; Broz, P.; Carreira, E.M.; Kopf, M. Electrophilic Nrf2 Activators and Itaconate Inhibit Inflammation at Low Dose and Promote IL-1β Production and Inflammatory Apoptosis at High Dose. Redox Biol. 2020, 36, 101647. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxid. Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef]
- Yagishita, Y.; Fukutomi, T.; Sugawara, A.; Kawamura, H.; Takahashi, T.; Pi, J.; Uruno, A.; Yamamoto, M. Nrf2 Protects Pancreatic β-Cells from Oxidative and Nitrosative Stress in Diabetic Model Mice. Diabetes 2014, 63, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.P.; Seldon, M.P.; Gregoire, I.P.; Vassilevskaia, T.; Berberat, P.O.; Yu, J.; Tsui, T.-Y.; Bach, F.H. Heme Oxygenase-1 Modulates the Expression of Adhesion Molecules Associated with Endothelial Cell Activation. J. Immunol. 2004, 172, 3553–3563. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Su, T.; Qiu, X.; Mao, P.; Xu, Y.; Hu, Z.; Zhang, Y.; Zheng, X.; Xie, P.; Liu, Q. Protective Effect of Alpha-Mangostin against Oxidative Stress Induced-Retinal Cell Death. Sci. Rep. 2016, 6, 21018. [Google Scholar] [CrossRef]
- Chen, L.-G.; Zhang, Y.-Q.; Wu, Z.-Z.; Hsieh, C.-W.; Chu, C.-S.; Wung, B.-S. Peanut Arachidin-1 Enhances Nrf2-Mediated Protective Mechanisms against TNF-α-Induced ICAM-1 Expression and NF-κB Activation in Endothelial Cells. Int. J. Mol. Med. 2018, 41, 541–547. [Google Scholar] [CrossRef]
- Ganesh Yerra, V.; Negi, G.; Sharma, S.S.; Kumar, A. Potential Therapeutic Effects of the Simultaneous Targeting of the Nrf2 and NF-κB Pathways in Diabetic Neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef]
- Fu, T.; Li, H.; Zhao, Y.; Cai, E.; Zhu, H.; Li, P.; Liu, J. Hepatoprotective Effect of α-Mangostin against Lipopolysaccharide/d-Galactosamine-Induced Acute Liver Failure in Mice. Biomed. Pharmacother. 2018, 106, 896–901. [Google Scholar] [CrossRef]
- Ghareghomi, S.; Rahban, M.; Moosavi-Movahedi, Z.; Habibi-Rezaei, M.; Saso, L.; Moosavi-Movahedi, A.A. The Potential Role of Curcumin in Modulating the Master Antioxidant Pathway in Diabetic Hypoxia-Induced Complications. Molecules 2021, 26, 7658. [Google Scholar] [CrossRef]
- Chang, A.; Jeske, L.; Ulbrich, S.; Hofmann, J.; Koblitz, J.; Schomburg, I.; Neumann-Schaal, M.; Jahn, D.; Schomburg, D. BRENDA, the ELIXIR Core Data Resource in 2021: New Developments and Updates. Nucleic Acids Res. 2021, 49, D498–D508. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Saso, L. Potential Applications of NRF2 Inhibitors in Cancer Therapy. Oxid. Med. Cell. Longev. 2019, 2019, 8592348. [Google Scholar] [CrossRef] [PubMed]
- Panieri, E.; Pinho, S.A.; Afonso, G.J.M.; Oliveira, P.J.; Cunha-Oliveira, T.; Saso, L. NRF2 and Mitochondrial Function in Cancer and Cancer Stem Cells. Cells 2022, 11, 2401. [Google Scholar] [CrossRef]
- Song, M.-Y.; Lee, D.-Y.; Chun, K.-S.; Kim, E.-H. The Role of NRF2/KEAP1 Signaling Pathway in Cancer Metabolism. Int. J. Mol. Sci. 2021, 22, 4376. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Chattopadhyay, A. Nrf2-ARE Signaling in Cellular Protection: Mechanism of Action and the Regulatory Mechanisms. J. Cell. Physiol. 2020, 235, 3119–3130. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Kumar, S.A.; Iyer, A.; Waanders, J.; Ward, L.C.; Brown, L. Responses to Oleic, Linoleic and α-Linolenic Acids in High-Carbohydrate, High-Fat Diet-Induced Metabolic Syndrome in Rats. J. Nutr. Biochem. 2013, 24, 1381–1392. [Google Scholar] [CrossRef]
- Buchanan, M.R. Linoleic Acid Metabolites in Health and Disease. Adv. Exp. Med. Biol. 1999, 469, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Greene, J.F.; Hammock, B.D. Toxicity of Linoleic Acid Metabolites. Adv. Exp. Med. Biol. 1999, 469, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kern, J.T.; Goodfriend, T.L.; Ball, D.L.; Luesch, H. Activation of the Antioxidant Response Element by Specific Oxidized Metabolites of Linoleic Acid. Prostaglandins Leukot. Essent. Fat. Acids 2009, 81, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Furumoto, H.; Nanthirudjanar, T.; Kume, T.; Izumi, Y.; Park, S.-B.; Kitamura, N.; Kishino, S.; Ogawa, J.; Hirata, T.; Sugawara, T. 10-Oxo-Trans-11-Octadecenoic Acid Generated from Linoleic Acid by a Gut Lactic Acid Bacterium Lactobacillus Plantarum Is Cytoprotective against Oxidative Stress. Toxicol. Appl. Pharmacol. 2016, 296, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Romana-Souza, B.; Saguie, B.O.; Pereira De Almeida Nogueira, N.; Paes, M.; Dos Santos Valença, S.; Atella, G.C.; Monte-Alto-Costa, A. Oleic Acid and Hydroxytyrosol Present in Olive Oil Promote ROS and Inflammatory Response in Normal Cultures of Murine Dermal Fibroblasts through the NF-κB and NRF2 Pathways. Food Res. Int. 2020, 131, 108984. [Google Scholar] [CrossRef] [PubMed]
- C de S Ribeiro, B.; V de C Faria, R.; de S Nogueira, J.; Santos Valença, S.; Chen, L.; Romana-Souza, B. Olive Oil Promotes the Survival and Migration of Dermal Fibroblasts through Nrf2 Pathway Activation. Lipids 2023, 58, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.E.; El Senousy, A.S.; Abd-elsalam, W.H.; Ahmed, K.A.; El-askary, H.I.; Mouneir, S.M.; Fishawy, A.M. Roselle Seed Oil and Its Nano-Formulation Alleviated Oxidative Stress, Activated Nrf2 and Downregulated m-RNA Expression Genes of Pro-Inflammatory Cytokines in Paracetamol-Intoxicated Rat Model. Rec. Nat. Prod. 2019, 14, 1–17. [Google Scholar] [CrossRef]
- Wang, X.-H.; Cui, X.-X.; Sun, X.-Q.; Wang, X.-H.; Li, X.-C.; Qi, Y.; Li, W.; Han, M.-Y.; Muhammad, I.; Zhang, X.-Y. High Fat Diet-Induced Hepatic 18-Carbon Fatty Acids Accumulation Up-Regulates CYP2A5/CYP2A6 via NF-E2-Related Factor 2. Front. Pharmacol. 2017, 8, 233. [Google Scholar] [CrossRef]
- Qu, L.-L.; Yu, B.; Li, Z.; Jiang, W.-X.; Jiang, J.-D.; Kong, W.-J. Gastrodin Ameliorates Oxidative Stress and Proinflammatory Response in Nonalcoholic Fatty Liver Disease through the AMPK/Nrf2 Pathway. Phytother. Res. 2016, 30, 402–411. [Google Scholar] [CrossRef]
- Sun, Z.; Niu, Z.; Wu, S.; Shan, S. Protective Mechanism of Sulforaphane in Nrf2 and Anti-Lung Injury in ARDS Rabbits. Exp. Ther. Med. 2018, 15, 4911–4915. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, R.; Zhang, X.; Huang, L.; Sun, L.; Zhao, J.; Ma, J.; Han, C. Recent Progress in Rare Oncogenic Drivers and Targeted Therapy For Non-Small Cell Lung Cancer. Onco Targets Ther. 2019, 12, 10343–10360. [Google Scholar] [CrossRef]
- Rueda, A.; Cantarero, S.; Seiquer, I.; Cabrera-Vique, C.; Olalla, M. Bioaccessibility of Individual Phenolic Compounds in Extra Virgin Argan Oil after Simulated Gastrointestinal Process. LWT 2017, 75, 466–472. [Google Scholar] [CrossRef]
- Zarrouk, A.; Martine, L.; Grégoire, S.; Nury, T.; Meddeb, W.; Camus, E.; Badreddine, A.; Durand, P.; Namsi, A.; Yammine, A.; et al. Profile of Fatty Acids, Tocopherols, Phytosterols and Polyphenols in Mediterranean Oils (Argan Oils, Olive Oils, Milk Thistle Seed Oils and Nigella Seed Oil) and Evaluation of Their Antioxidant and Cytoprotective Activities. Curr. Pharm. Des. 2019, 25, 1791–1805. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Rui, Y.; Guo, S.; Luan, F.; Liu, R.; Zeng, N. Ferulic Acid: A Review of Its Pharmacology, Pharmacokinetics and Derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Yu, W.; Duan, N.; Wang, Z.; Shen, Y.; Wu, S. Protective Effects of Ferulic Acid on Deoxynivalenol-Induced Toxicity in IPEC-J2 Cells. Toxins 2022, 14, 275. [Google Scholar] [CrossRef]
- Ma, Z.-C.; Hong, Q.; Wang, Y.-G.; Tan, H.-L.; Xiao, C.-R.; Liang, Q.-D.; Zhang, B.-L.; Gao, Y. Ferulic Acid Protects Human Umbilical Vein Endothelial Cells from Radiation Induced Oxidative Stress by Phosphatidylinositol 3-Kinase and Extracellular Signal-Regulated Kinase Pathways. Biol. Pharm. Bull. 2010, 33, 29–34. [Google Scholar] [CrossRef]
- Je, J.-Y.; Lee, D.-B. Nelumbo Nucifera Leaves Protect Hydrogen Peroxide-Induced Hepatic Damage via Antioxidant Enzymes and HO-1/Nrf2 Activation. Food Funct. 2015, 6, 1911–1918. [Google Scholar] [CrossRef]
- Catino, S.; Paciello, F.; Miceli, F.; Rolesi, R.; Troiani, D.; Calabrese, V.; Santangelo, R.; Mancuso, C. Ferulic Acid Regulates the Nrf2/Heme Oxygenase-1 System and Counteracts Trimethyltin-Induced Neuronal Damage in the Human Neuroblastoma Cell Line SH-SY5Y. Front. Pharmacol. 2015, 6, 305. [Google Scholar] [CrossRef]
- Yu, C.-L.; Zhao, X.-M.; Niu, Y.-C. Ferulic Acid Protects Against Lead Acetate-Induced Inhibition of Neurite Outgrowth by Upregulating HO-1 in PC12 Cells: Involvement of ERK1/2-Nrf2 Pathway. Mol. Neurobiol. 2016, 53, 6489–6500. [Google Scholar] [CrossRef]
- Yeh, C.-T.; Ching, L.-C.; Yen, G.-C. Inducing Gene Expression of Cardiac Antioxidant Enzymes by Dietary Phenolic Acids in Rats. J. Nutr. Biochem. 2009, 20, 163–171. [Google Scholar] [CrossRef]
- Yeh, C.-T.; Yen, G.-C. Induction of Hepatic Antioxidant Enzymes by Phenolic Acids in Rats Is Accompanied by Increased Levels of Multidrug Resistance–Associated Protein 3 mRNA Expression. J. Nutr. 2006, 136, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, L.; Zhang, H.; Zhang, Y.; Zhang, H.; Wang, L.; Qian, H.; Qi, X.; Zhang, H. In Vitro and in Vivo Antioxidant Activity of Ethyl Acetate Extraction of Purple Rice. Cell. Mol. Biol. 2016, 62, 96–103. [Google Scholar] [PubMed]
- Wang, Y.; Huo, Y.; Zhao, L.; Lu, F.; Wang, O.; Yang, X.; Ji, B.; Zhou, F. Cyanidin-3-Glucoside and Its Phenolic Acid Metabolites Attenuate Visible Light-Induced Retinal Degeneration in Vivo via Activation of Nrf2/HO-1 Pathway and NF-κB Suppression. Mol. Nutr. Food Res. 2016, 60, 1564–1577. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Meng, Z.; Zhou, L.; Feng, H.; Wu, X.; Xin, Y.; Dong, J.; Li, Y. Ferulic Acid Attenuated Difenoconazole-Induced Immunotoxicity in Carp by Inhibiting TRAF/TAK1/NF-κB, Nrf2 and P53 Pathways. Ecotoxicol. Environ. Saf. 2023, 262, 115339. [Google Scholar] [CrossRef] [PubMed]
- Adeyi, O.E.; Somade, O.T.; James, A.S.; Adeyi, A.O.; Ogbonna-Eze, S.N.; Salako, O.Q.; Makinde, T.V.; Ajadi, O.M.; Nosiru, S.A. Ferulic Acid Mitigates 2-Methoxyethanol-Induced Testicular Oxidative Stress via Combined Downregulation of FoxO1, PTEN, and Modulation of Nrf2-Hmox1-NQO1 Signaling Pathway in Rats. Pharmacol. Res. Mod. Chin. Med. 2023, 7, 100257. [Google Scholar] [CrossRef]
- Lin, Y.; Knol, D.; Trautwein, E.A. Phytosterol Oxidation Products (POP) in Foods with Added Phytosterols and Estimation of Their Daily Intake: A Literature Review. Eur. J. Lipid Sci. Technol. 2016, 118, 1423–1438. [Google Scholar] [CrossRef]
- Otaegui-Arrazola, A.; Menéndez-Carreño, M.; Ansorena, D.; Astiasarán, I. Oxysterols: A World to Explore. Food Chem. Toxicol. 2010, 48, 3289–3303. [Google Scholar] [CrossRef]
- Tahri-Joutey, M.; Saih, F.-E.; El Kebbaj, R.; Gondcaille, C.; Vamecq, J.; Latruffe, N.; Lizard, G.; Savary, S.; Nasser, B.; Cherkaoui-Malki, M.; et al. Protective Effect of Nopal Cactus (Opuntia Ficus-Indica) Seed Oil against Short-Term Lipopolysaccharides-Induced Inflammation and Peroxisomal Functions Dysregulation in Mouse Brain and Liver. Int. J. Mol. Sci. 2022, 23, 11849. [Google Scholar] [CrossRef] [PubMed]
- El Kharrassi, Y.; Samadi, M.; Lopez, T.; Nury, T.; El Kebbaj, R.; Andreoletti, P.; El Hajj, H.I.; Vamecq, J.; Moustaid, K.; Latruffe, N.; et al. Biological Activities of Schottenol and Spinasterol, Two Natural Phytosterols Present in Argan Oil and in Cactus Pear Seed Oil, on Murine Miroglial BV2 Cells. Biochem. Biophys. Res. Commun. 2014, 446, 798–804. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols-Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef]
- Niki, E.; Abe, K. Vitamin E: Structure, Properties and Functions; The Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Jiang, Q. Natural Forms of Vitamin E: Metabolism, Antioxidant, and Anti-Inflammatory Activities and Their Role in Disease Prevention and Therapy. Free Radic. Biol. Med. 2014, 72, 76–90. [Google Scholar] [CrossRef]
- SNIMA, N. Service de Normalisation Industrielle Marocaine (Snima), Corps Gras d’origine Animale Ou Végétale- Huiles d’argan. Spécification. Norme Marocaine NM 2003, 8, 090. [Google Scholar]
- Wu, H.; Nakamura, T.; Guo, Y.; Matsumoto, R.; Munemasa, S.; Murata, Y.; Nakamura, Y. Cycloartenyl Ferulate Is the Predominant Compound in Brown Rice Conferring Cytoprotective Potential against Oxidative Stress-Induced Cytotoxicity. Int. J. Mol. Sci. 2023, 24, 822. [Google Scholar] [CrossRef] [PubMed]
- Dossi, C.G.; González-Mañán, D.; Romero, N.; Silva, D.; Videla, L.A.; Tapia, G.S. Anti-Oxidative and Anti-Inflammatory Effects of Rosa Mosqueta Oil Supplementation in Rat Liver Ischemia-Reperfusion. Food Funct. 2018, 9, 4847–4857. [Google Scholar] [CrossRef] [PubMed]
- Tapia, G.; Silva, D.; Romero, N.; Pettinelli, P.; Dossi, C.G.; De Miguel, M.; González-Mañán, D. Role of Dietary α- and γ-Tocopherol from Rosa Mosqueta Oil in the Prevention of Alterations Induced by High-Fat Diet in a Murine Model. Nutrition 2018, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Mah, E.; Li, J.; Jalili, T.; Symons, J.D.; Bruno, R.S. Improved Hepatic γ-Tocopherol Status Limits Oxidative and Inflammatory Stress-Mediated Liver Injury in Db/Db Mice with Nonalcoholic Steatohepatitis. J. Funct. Foods 2018, 40, 670–678. [Google Scholar] [CrossRef]
- Duncan, R.S.; Hurtado, D.T.; Hall, C.W.; Koulen, P. Differential Mechanisms of Action and Efficacy of Vitamin E Components in Antioxidant Cytoprotection of Human Retinal Pigment Epithelium. Front. Pharmacol. 2022, 12, 798938. [Google Scholar] [CrossRef]
- Smolarek, A.K.; So, J.Y.; Thomas, P.E.; Lee, H.J.; Paul, S.; Dombrowski, A.; Wang, C.-X.; Saw, C.L.-L.; Khor, T.O.; Kong, A.-N.T.; et al. Dietary Tocopherols Inhibit Cell Proliferation, Regulate Expression of ERα, PPARγ and Nrf2, and Decrease Serum Inflammatory Markers during the Development of Mammary Hyperplasia. Mol. Carcinog. 2013, 52, 514–525. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).