Abstract
Osteoarthritis (OA) is the most common type of arthritis and chronic joint disease, affecting more than 240 million people worldwide. Although there are numerous advances in using drugs in treating OA, the use of natural compounds has aroused much interest among researchers due to their safety margin. Recent discovery shows that natural compounds play an extensive role in the oxidative stress signaling pathway in treating OA. Thus, this review summarizes the commonly used natural compounds for treating OA focusing on the oxidative stress signaling pathway and its downstream mediators. Selected databases—such as Scopus, Web of Science, Nature, and PubMed—were used to search for potentially relevant articles. The search is limited to the last 15 years and the search was completed using the Boolean operator’s guideline using the keywords of natural product AND oxidative stress AND osteoarthritis OR natural extract AND ROS AND degenerative arthritis OR natural plant AND free radicals AND degenerative joint disease. In total, 37 articles were selected for further review. Different downstream mechanisms of oxidative stress involved in the usage of natural compounds for OA treatment and anabolic and catabolic effects of natural compounds that exhibit chondroprotective effects have been discussed with the evidence of in vitro and in vivo trials in this review.
1. Introduction
Osteoarthritis (OA) is the most common type of arthritis that affects joints and is one of the chief sources of disability [1]. As claimed by Katz et al. (2021), it is estimated that OA affects more than 240 million people globally and up to 32 million in the United States alone [2]. According to Musumeci et al. (2015), OA affects roughly half of the population over 65 years old with a more noteworthy rate in females after menopause (18%) compared to males (9.6%) [3]. This draws public concern due to its negative impact on irreversible physical disability [4]. The progression of OA is usually slow and persistent for a longer period [5]. OA usually damages the joints and their surrounding tissues, which play a crucial role in the movement. This includes bones, meniscus, tendons, ligaments, synovium, and articular cartilage. OA can affect any joint in the body but predominantly affects the knees, hands, feet, and hips [2]. Permanent articular cartilage degradation, osteophyte formation, inflammation of synovium with various degrees, remodeling of subchondral bone, ligament and meniscus degeneration, cartilage calcification, angiogenesis and joint capsule hypertrophy [6,7,8], changes in local fat pads, nerves, periarticular muscles, and the bursa [6] are some of the pathological changes that are commonly notable in OA affected joints.
OA can be classified into two types, which are primary and secondary OA. Primary OA—also known as idiopathic OA—refers to the deteriorating changes of joints that occur due to genetic conditions but without known underlying causes. The primary OA can be further classified into localized OA, which affects only one joint while the generalized OA affects three or more joints. Secondary OA is associated with risk factors or injuries. Some of the relatable condition of secondary OA includes obesity, diet, diabetes, physical activities, rheumatoid arthritis, and other diseases related to bone or metabolism [3,9].
Hitherto, there is no effective cure to stop or reverse the progression of OA. The current goals for OA management are mainly to alleviate painful symptoms, reduce disability, and improve the overall quality of life. Some of the non-pharmacological management methods for OA include weight management, exercise, and strength training [10,11,12]. The treatment for OA is always a lifelong therapy. However, long-term usage of OA-relieving medicines could cause negative effects on various organs specifically in the kidney, gastrointestinal tract, and cardiovascular system [13,14]. Thus, there is an immediate demand in clinical practice for an OA symptom-relieving treatment strategy that is desirable for long-term use with minimum adverse effects.
In consideration of the side effects of OA medicines, the possibility of natural compounds that are able to exhibit chondroprotective effects in the oxidative stress signaling pathway has gained researchers’ attention. This is because these natural compounds have been proven to act as osteoprotective and chondroprotective capabilities by acting as antioxidants and anti-inflammatory agents, preventing chondrocyte catabolism, and suppressing chondrocyte differentiation, thus improving bone health [5,15]. Besides that, natural compounds are considered a safer alternative for OA treatment due to their lesser or non-existent side effects [16,17]. Therefore, this review recapitulated the chondroprotective effects of the natural compound on OA from the oxidative stress signaling pathway perspective.
2. Materials and Methods
2.1. Search Strategy
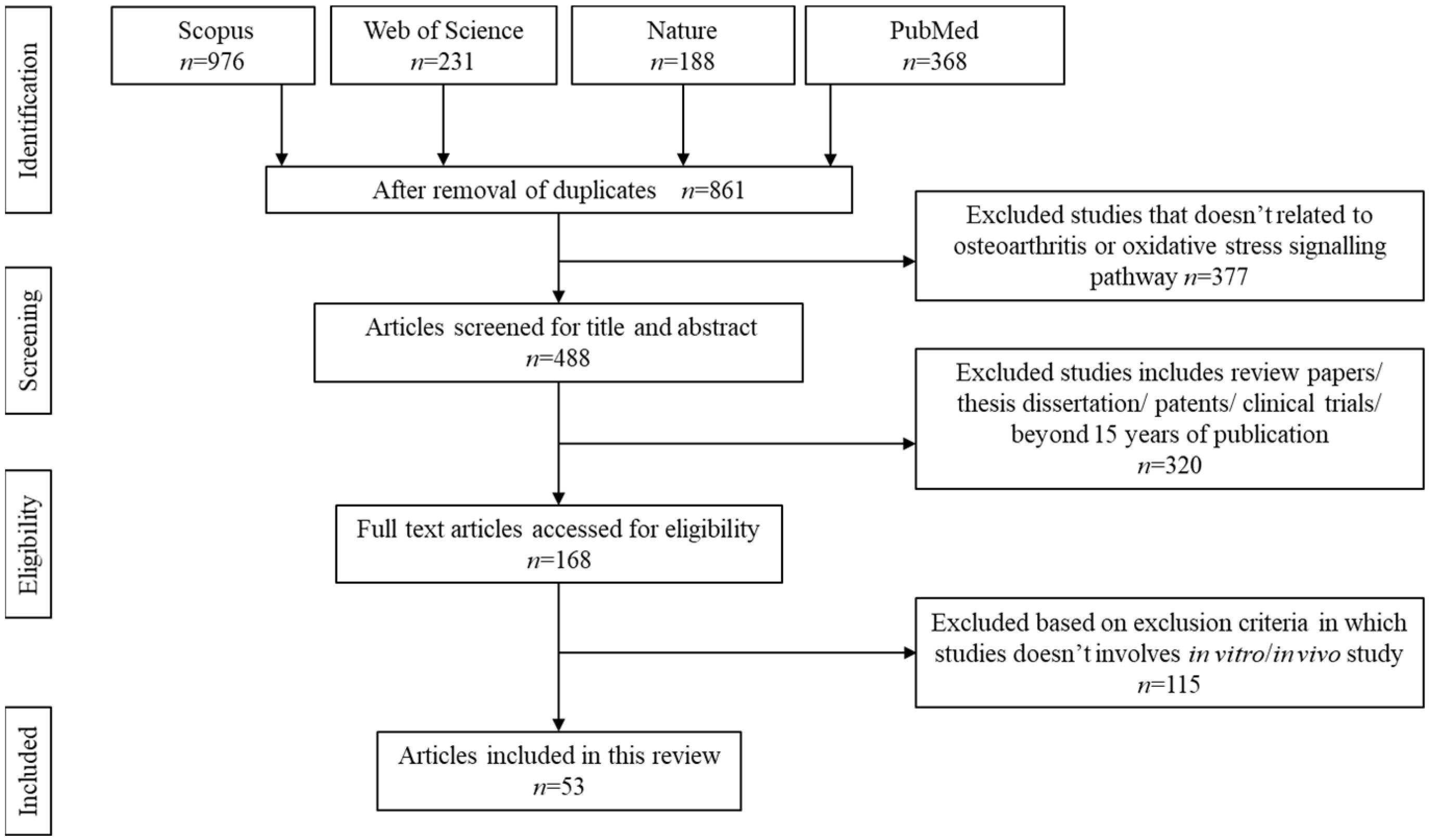
The search was carried out systematically using Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline for scoping review as described elsewhere. Boolean operator guideline was used to select the appropriate keywords for article search. The selected keywords are: natural product AND oxidative stress AND osteoarthritis OR natural extract AND ROS AND degenerative arthritis OR natural plant AND free radicals AND degenerative joint disease. The articles were searched using Nature, PubMed, Scopus, and Web of Science databases. Figure 1 summarises the search strategy.
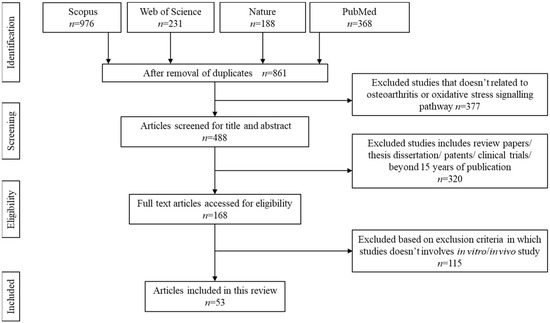
Figure 1.
Article search and selection process.
2.2. Inclusion Criteria
All original articles that are based on in vitro or in vivo studies were chosen to be further reviewed. Only articles written in the English language were selected. The article selections were limited to the last 15 years starting from 2007 onwards to obtain the latest published research papers. The articles must contain either four of the following parameters: (1) subject, (2) active compounds, (3) type of model used, (4) dosage, (5) duration of treatment, and (6) outcome observed.
2.3. Exclusion Criteria
Articles that did not meet the inclusion criteria, theses, secondary articles, review papers, clinical trials, conference proceedings, and patents were excluded from being further reviewed. In addition, any articles that do not provide clear information on dosage or duration of treatment were excluded. Data extraction was carried out independently by three authors (L.Y.T., A.U., and M.D.Y.) and any disagreements were resolved by consulting the fourth author (M.H.M.Y.). No conflict of interest arises among the authors during the discussion or the data extraction process.
3. Results
The initial search resulted in 1763 articles. Upon screening, 861 articles were removed due to duplication, unmatched content, or absence of an abstract. Another 377 articles were further removed due to unreliable keywords or articles discussing stiffness or knee injuries apart from osteoarthritis. Additionally, 320 articles were removed upon confirming these papers as review papers, technical papers, and retrospective studies. A total of 53 articles that met the inclusion criteria were chosen to be further reviewed.
4. Pathogenesis of OA
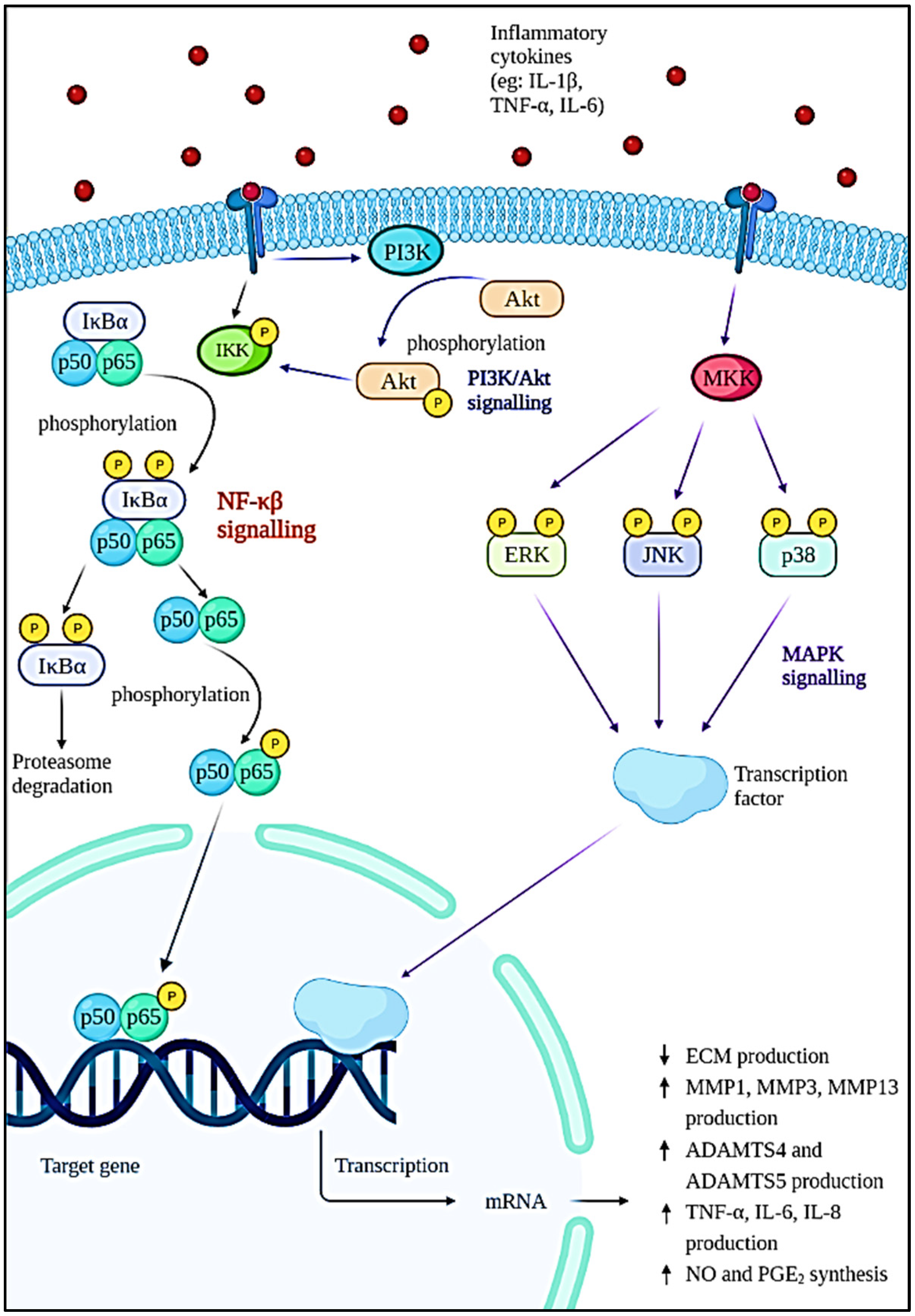
The pathophysiology of OA is complex and poorly understood. Generally, normal articular cartilage consists of chondrocytes and extracellular matrices (ECM) such as collagen, proteoglycans, and water to provide the shock-absorbing capability [18,19]. Proteoglycan consists of aggrecan and hyaluronan that interact with each other to form glycosaminoglycan (GAG) [20]. The failure of chondrocytes to establish equilibrium between ECM synthesis and degradation will result in osteoarthritis. In these cases, the wear particles produced in joints will be phagocytosed by the immune cells, resulting in the increased secretion and activation of lysozymes during injury [21]. When the wear particles’ formation outpaces the system’s ability to remove them, they will mediate the inflammation process, thus causing the chondrocytes to produce degradative enzymes to break down collagen and proteoglycan in the joint [22]. As a natural defence of immune response, various immune and pro-inflammatory cells—including neutrophils, megakaryocytes, macrophages, lymphocytes, leukocytes, and dendritic cells—will be produced [23]. A network of factors—including proinflammatory cytokines, chemokines, and lipid mediators—is responsible for bind to chondrocytes and coordinating the immune cell function by activating signal transduction pathways (Figure 2), especially the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κβ) pathway [24]. As a result, more metalloproteinases will be formed while type II collagen production is inhibited. Resultantly, degradation of cartilage is accelerated, thus increasing chondrocyte apoptosis [18]. On the contrary, various cytokines and chemokines play an important role in the pathogenesis of OA. This includes inflammatory cytokines such as interleukin (IL)-1β (IL-1β), tumour necrosis factor-alpha (TNF-α), IL-6, IL-15, IL-17, and IL-18 [25,26]. Among them, IL-1β, TNF-α, and IL-6 are the most significant inflammatory mediators in the etiology of OA, as they trigger a variety of signaling pathways, which in turn activate other cytokines and pathogenic processes. Apart from that, cytokines are also able to stimulate chemokine production and this will attract more inflammatory cells to the joint, thereby further enhancing the secretion of inflammatory substances and accelerating the progression of the disease [27].
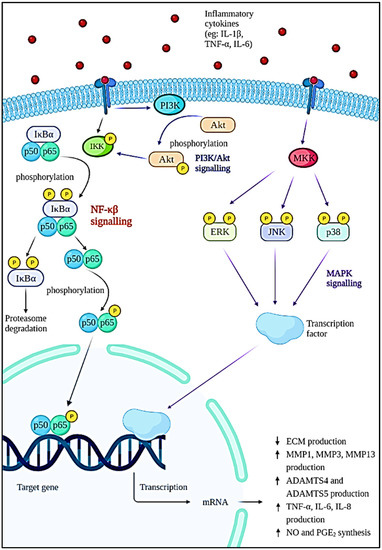
Figure 2.
Intracellular signaling pathways and downstream effects involved in the progression of OA.
The NF-κβ transcription factor is a flexible and multifunctional transcription factor that is involved in immunological responses, cellular differentiation, inflammatory responses, and also normal and malignant cell survival [28]. It has a role in a variety of biological activities. In OA, the NF-κβ transcription factor is abnormally activated and it is known to be one of the disease-causing factors. This factor is initiated by various inflammatory cytokines such as IL-1β, TNF-α, and IL-6; severe mechanical stressors; or the breakdown products of ECM that can promote the transcription of the catabolic gene and also stimulate the production of inflammatory mediators through a positive feedback loop [29,30]. The activation of IκB kinase (IKK) leads to IκBα phosphorylation and degradation by the proteasome, which is the first step in the NF-κβ signaling cascade. The p65 protein is liberated from the cytoplasm, phosphorylated, and translocated to the nucleus. The genes of degradative enzymes and aggrecanases, a disintegrin and metalloproteinase domain with thromboplastin motifs’ (ADAMTS), including matrix metalloproteinases (MMP)-1, MMP3, MMP-13, ADAMTS4, and ADAMTS5 are activated as a result of these processes. This will eventually lead to the breakdown of collagen and aggrecan [31,32]. The expression of cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS), which are the major proinflammatory and destructive mediators of OA, will also be stimulated. The upregulation of iNOS and COX-2 increased nitric oxide (NO) and prostaglandin E2 (PGE2) production [29,33,34]. The increase in NO will modulate the expression of cytokines and homeostasis of ECM by inhibiting the production of proteoglycan and collagen type II, which will result in oxidative damage and chondrocyte death. The elevation of PGE2 lowers the synthesis of ECM, inhibits the proliferation of chondrocytes, and leads to osteophyte formation [26,35,36].
According to Xie et al. (2019), the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling is known to have a synergistic effect on the NF-κβ signaling pathway. This pathway is activated when cytokines such as IL-1β bind to their receptors on the cell surface. The phosphorylation of Akt by membrane protein PI3K will occur in response to the stimulation and cause the cell to synthesize more MMPs [37]. In addition, the mitogen-activated protein kinase (MAPK) pathway is one of the multiple signal transduction pathways in the OA progression [38]. The three signal cascades pathway of the MAPK pathway includes p38, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK) [39]. These are the mediator that controls the downstream MMPs and pro-inflammatory cytokines expression. Cytokines, matrix proteins, and growth factors can activate MAP kinases (MKKs) by binding to integrin, cytokine receptors, G-protein coupled receptors, and receptor tyrosine kinases. Upon binding to the cell surface receptors, signals known as ‘upstream activators’ are generated and will cause phosphorylation of specific MKKs by MAP kinase. The activated MKKs will then trigger the activation of transcriptional regulatory proteins resulting in the increased expression of inflammatory genes such as TNF-α, IL-1, and MMPs. The cytokines produced can then keep JNK activated, eventually resulting in increased production of MMPs and cytokines [40].
Inflammatory Pathway in Osteoarthritis Pathogenesis
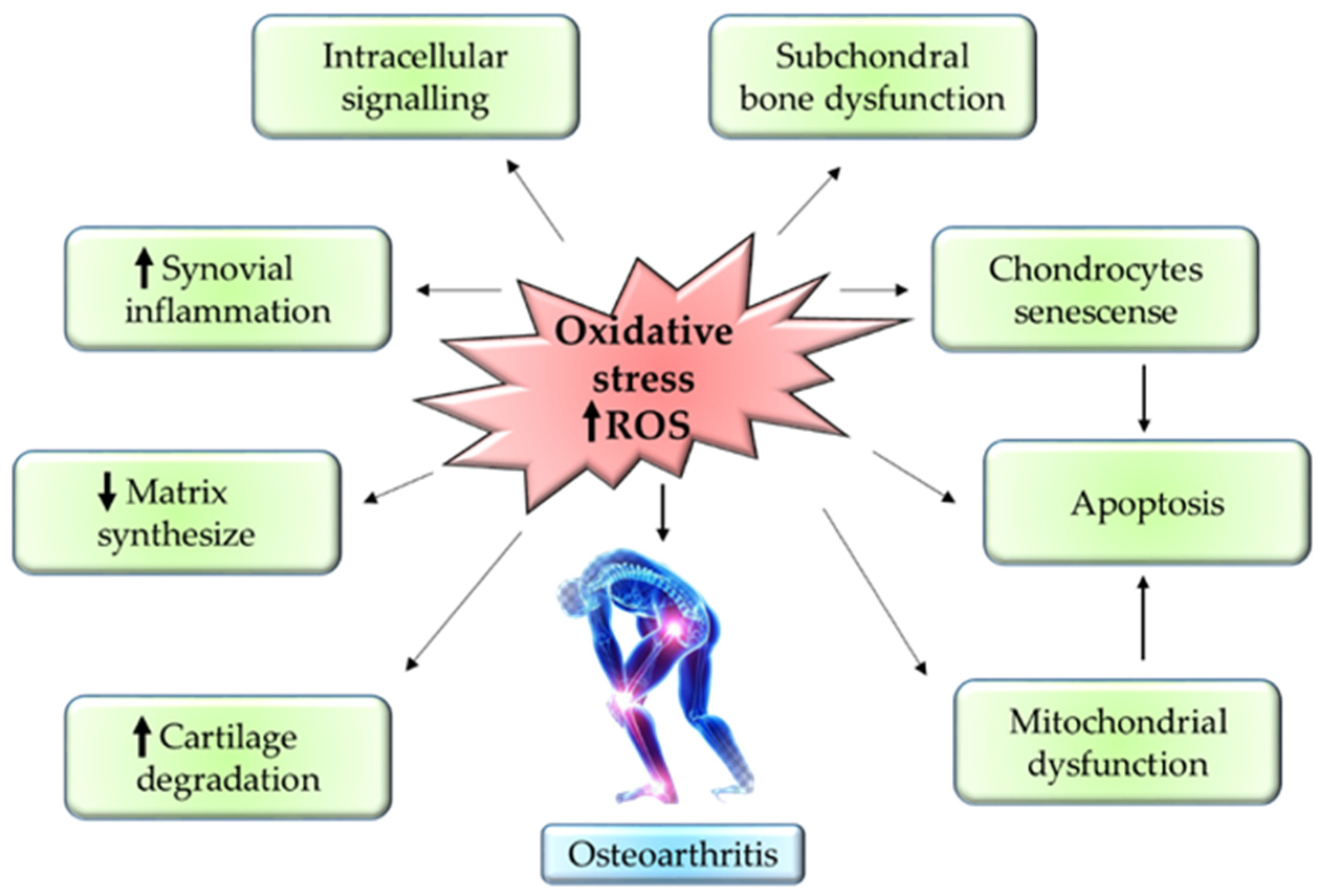
The progression of OA mainly depends on the reactive oxygen species (ROS) and oxidative stress. ROS production is one of the essential steps for the regulation of homeostasis in the chondrocytes. ROS is produced in the cells via NADPH oxidase and mitochondria in the chondrocytes. ROS usually modulates the normal expression of genes, apoptosis, synthesis of ECM, and production of inflammatory factors such as cytokines. The overexpression of NADPH oxidase enhances the build-up of oxidative stress in the joints, thereby accelerating the progression of OA [41].
Besides that, the upregulation of inflammatory cytokines—such as IL-1β, IL-17, interferon-γ (IFN-γ), and TNF-α—via iNOS further worsens the OA condition. Protein synthesis of iNOS is known as one of the nitrogen metabolites, the key enzyme for NO formation, and a primary mediator of inflammation in skeletal muscles [42]. Overexpression of iNOS will stimulate abnormally high production of NO in cartilages which then will cause damage to the cartilage due to its role as an endogenous modulator in cartilage matrix turnover. Thus, the cartilage tends to lose chondrocyte phenotypes through the dedifferentiation of fibroblastoid [43]. Aside from this, NO together with IL-1 could inhibit proliferation in the chondrocytes and stimulate apoptosis. This is mainly because IL-1 increases the vulnerability of NO toward oxygen radical-mediated cell apoptosis in chondrocytes [44]. Therewithal, NO suppresses the synthesis of proteoglycan by preventing the attachment of chondrocytes to fibronectin in the ECM. This will compromise the cell survival, hence causing chondrocyte apoptosis [45].
Into the bargain, the formation of hydrogen peroxide (H2O2) by hypoxanthine-xanthine oxidase induces apoptosis in chondrocytes through ROS in a dose-dependent manner. In such cases, the membrane integrity of the mitochondria is compromised, thus causing the release of cytochome c into the cytoplasm [46]. The elevation of cytochome c in the cytoplasm will eventually stimulate the activation of apoptotic protease-activating factor-1. This will then configure the apoptosome through oligomerisation with Apaf-1, thus initiating caspase-9 [47]. Stimulation of caspase-9 will cleave caspase-3, also known as ‘executioner caspase’, leading to chondrocyte apoptosis [48]. Conversely, sodium nitroprusside (SNP), a donor of NO, triggers an apoptotic mechanism by suppressing the action of BCL-2. SNP induces apoptotic mechanisms in chondrocytes by stimulating DNA fragmentation, dysfunctional mitochondria, and remodeling of the cytoskeleton [41]. Figure 3 summarizes the inflammatory pathway related to pathogenesis in OA. Figure 3 summarizes the inflammatory pathway related pathogenesis in OA.
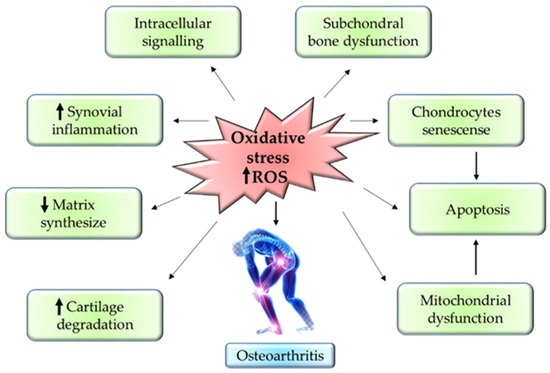
Figure 3.
Mechanism of oxidative stress in OA pathogenesis.
5. In Vitro and In Vivo Evidence of Natural Compounds Affecting Inflammatory Pathways of Osteoarthritis
The findings from this review show that 100% of the natural compounds can slow down the progression of OA by targeting the oxidative stress signaling pathway as shown in Table 1 and Table 2. Out of 37 studies, 32 studies were conducted in vitro only or in vitro followed by in vivo study with a 100% success rate in cartilage regeneration or inhibition of the chondrocytes damage. This involves various pathways under the oxidative stress signaling mechanism. The most common discussed pathway in the in vitro studies includes inhibition of p65, AKT, MAPK (ERK1/2 and p38), apoptosis signal-regulating kinase 1 (ASK1), JNK, TNF, NF-κβ, and IκBα phosphorylation. Generally, ROS—being a second messenger—is able to activate ROS/MAPK, ROS/AKT, and TNF/ NF-κβ in OA pathogenesis [49]. In vitro study proves that natural compounds such as galangin [50,51], propolis [52], astaxanthin [53], anthriscus sylvestris [54], cynaroside [55], and rhizoma coptidis [56] are able to suppress the production of ROS and its downstream pathway to prevent the pathological progression of OA.

Table 1.
In vitro evidence of the effects of natural compound on osteoarthritis.

Table 2.
In vivo evidence of the effects of natural compounds on osteoarthritis.
Conversely, cytoprotective genes become stimulated due to the upregulation of nuclear factor (erythroid-derived 2)-like 2 (Nrf-2) in proper regulation of oxidative stress [57]. Nrf-2 plays a vital role in the regulation of redox homeostasis via the modulation of the antioxidant response element (ARE). In such conditions—under excessive production of ROS—Nrf-2 will be downregulated leading to the depletion of SOD, GPx, and GSH. However, in a controlled oxidative stress environment, Nrf2 exhibits cytoprotective activity thus causing the chondrocytes to remain ‘young’ [58]. Another study supports the statements by indicating that chondrocytes treated with astaxanthin show an upregulation of Nrf2, which in turn increases the expression of heme oxygenase-1 (HO-1) and reduces the activity of NADPH and nicotinamide adenine dinucleotide phosphate (NADPH):quinone oxidoreductase (NQO1) [59]. The stimulation of HO-1 will in turn repress the expression of the MMP gene in the chondrocytes [60]. Similarly, a reduction in NADPH is able to enhance proteoglycan synthesis in the cartilage [61]. NQO1 act as detoxifying enzyme or defence mechanisms for unregulated antioxidants [62]. Aside from this, in vitro analysis of chondrocytes treated with curcumin [63] and EGCG [64] shows gradual elevation of CITED 2 gene. This proves that natural extracts such as curcumin and EGCG are able to act as chondroprotectants by suppressing the activity of MMP through the expression of CITED 2 gene via the JAK/STAT pathway. CITED 2 gene is also known as a transcriptional regulator that plays an important role in the downregulation of MMPs [65].
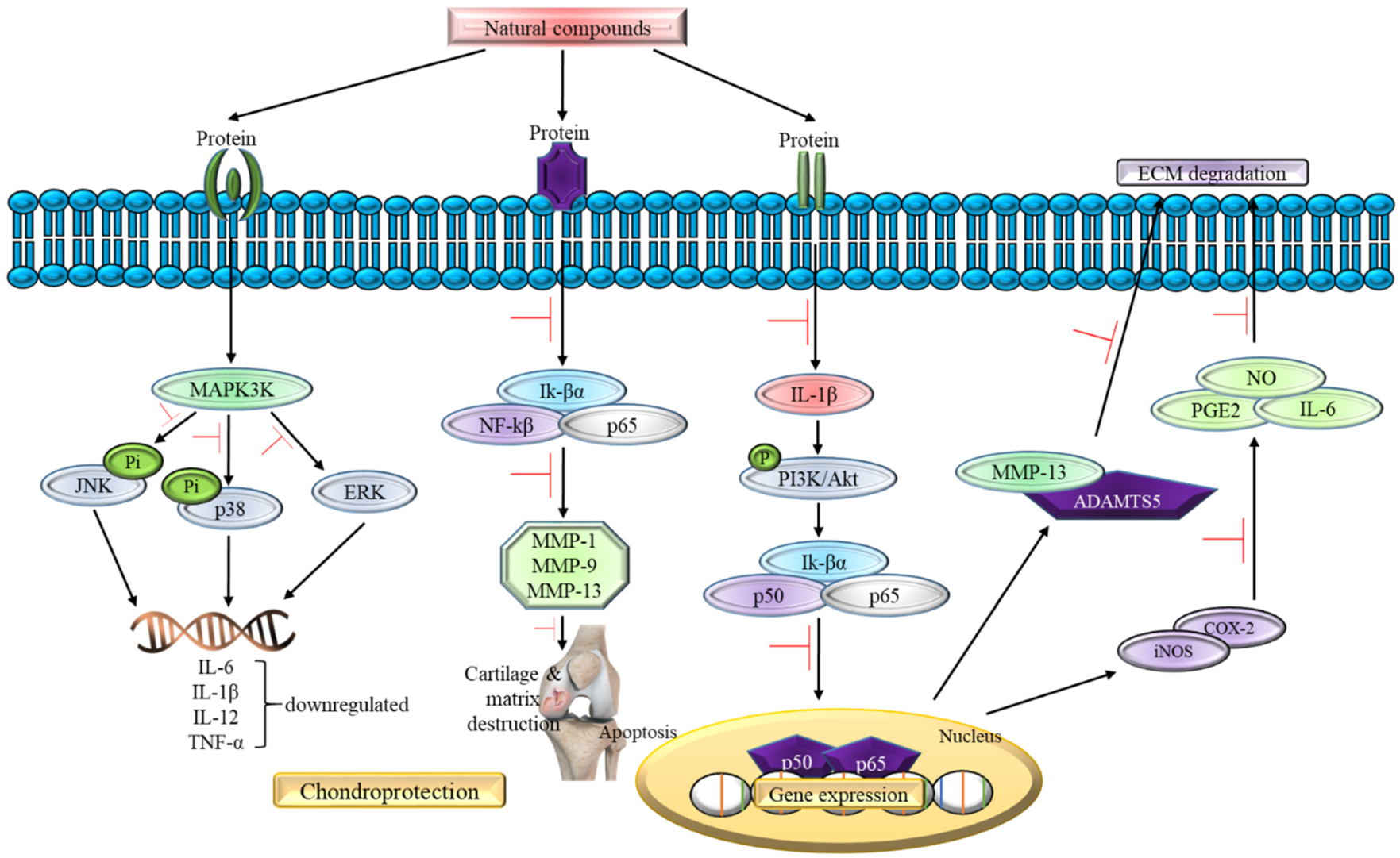
In addition, 20 studies reviewed in this article focus on the in vivo study as shown in Table 2 using the osteoarthritis-induced rat model. The data analysis shows no contraindication observed in the natural compounds-exposed rats and only positive outcome has been documented so far. For instance, all 20 studies supported the claim that natural compounds enhance chondroprotective action regardless of different downstream pathways of oxidative stress signaling pathway involvement. For instance, oral administration of natural compounds (galangin and propolis) is able to reverse articular degradation as early as 14 days [51,52], while a reduction in MMP-2 activity is recorded within 5 h of intraperitoneal injection of Cryptolepis buchanani [66]. Five studies show a reduction in ROS, lipid peroxidation, and inflammatory factors such as IL-1β, IL-6 and TNF-α, which in turn mitigates OA [51,52,54,67,68]. One in vivo study states that there is a gradual decrease in GAG and hydroxyproline levels in chondrocytes treated with natural compounds [52]. In the clinical setting, GAG and hydroxyproline could reflect the range of cartilage damage and a reduced level indicates a positive outcome in the treated rats. In summary, all in vivo studies show prevention in the articular degradation at the end phase of their study, thus supporting the data obtained in the in vitro experiments. Figure 4 summarizes the effect of natural compounds on the inflammatory pathway in OA.
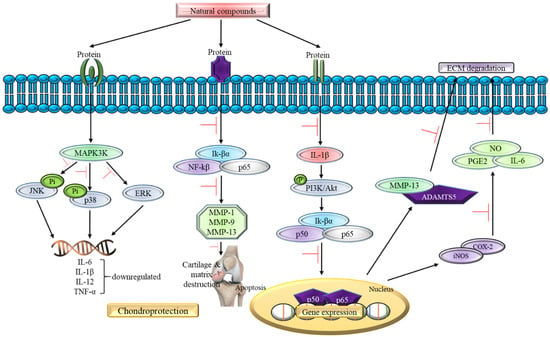
Figure 4.
The role of natural compounds in the inflammatory pathway of OA.
Role of Natural Compounds in Oxidative Stress Signaling Pathway in Osteoarthritis
6. Role of Natural Compounds on Oxidative Stress Signaling in OA
6.1. Aconitum carmichaelii Debx
Aconitum carmichaelii Debx. belongs to Aconitum genus and Ranunculaceae family. Europe, North America, and Asia are among the temperate zones of the northern hemisphere where they can be found [109]. Since ancient times, the ‘Chuan Wu’, the mother roots of A. carmichaelii is used to treat rheumatism and pains [110]. The effect of the oxidative stress signaling of A. carmichaelii on OA has been proven through several in vitro and in vivo studies. In accordance with the study by Tong et al. (2014) [81] on intra-articular mono-iodoacetate (MIA)-induced OA rat model and MIA-treated rat chondrocytes, the detoxicated ‘Fuzi’ is able to promote the proliferation of chondrocytes and suppress chondrocytes damage by MIA in vitro and show chondroprotective activity in vivo by preventing abnormalities in the subchondral bone, thus preserving the morphology of joint and density of the bone [81,111].
This result is also supported by Zhang et al. (2020) [67] who carry out the study by using processed A. carmichaelii Debx. lateral root with peel or also known as ‘Hei-Shun-Pian’ (Hsp). This detoxified Hsp (dHsp) can significantly reduce joint pain and also protect the articular cartilage from degeneration. According to the result of an in vivo experiment, dHsp can restore the MIA-induced upregulation of collagen type X, MMP2, SOX-5, ADAMTS4, ADAMTS5, and ADAMTS9 genes expression. A similar result was shown in an in vitro experiment using TNF-α-treated rat articular chondrocytes by increasing the aggrecan messenger ribonucleic acid (mRNA) level and reducing the MMP1 and ADAMTS9 mRNA expressions. This study also indicates that the benzoylaconitine and benzoylhypaconite in dHsp play roles in this chondroprotective effect [67]. Collectively, these results suggest that the extract of A. carmichaelii may be a potential antioxidant agent for treating OA.
6.2. Anthriscus sylvestris (L.) Hoffm
Anthriscus sylvestris (L.) Hoffm, also known as cow parsley or wild chervil, is from the family of the Apiaceae (Umbelliferae). It is widely distributed around the world, such as roadsides and shrubbery, but mainly in northern temperate zones and at high altitudes in the tropical jungle [112]. This plant is proven to play a vital role in oxidative stress signaling in OA in various studies. For instance, a positive outcome is seen on IL-1β-treated rat chondrocytes and destabilization of the medial meniscus (DMM) surgery-induced OA rat model. The leaves extract from A. sylvestris has shown the ability to repress nitrite, PGE2, iNOS, and COX-2 genes expression and also the expression and protein level of matrix-degrading enzymes (MMP3, MMP13, ADAMTS4) induced by IL-1β. The chondrocytes treated with plant extract also prevent collagen type II, aggrecan, and proteoglycan degradation. The IκBα, ERK, JNK, and p38 phosphorylation is also being suppressed by the A. sylvestris aqueous extract and causes inactivation of NF-κβ and MAPK signaling pathways [113].
Moreover, the rat model treated with the plant extract showed lesser cartilage destruction and proteoglycan degradation compared to the OA group [54]. Cynaroside has been identified as one of the key chemicals that possess anti-inflammatory in the extract of A. sylvestris and is believed to play a major role in the chondroprotective effect of A. sylvestris. This statement is supported by another study by Lee et al. in 2020. Cynaroside is able to suppress the expression of catabolic factors including nitrite, iNOS, ROS, PGE2, COX-2, MMP1, MMP3, MMP13, and ADAMTS4, as well as prevent aggrecan and collagen type II degradation. In addition, it can also inhibit MAPK and NF-κβ pathways, which play a role during inflammation. The ex vivo results revealed that the production of nitrite, PGE2, iNOS, COX-2, ADAMTS4, and MMP13 can be inhibited by cynaroside, which is consistent with the results obtained via an in vitro study. Besides that, it can also protect the proteoglycan from degradation [55,95]. The results obtained provide a promising candidate for the alleviation of OA focusing on the oxidative stress signaling pathway.
6.3. Artemisia argyi H. Lév. and Vaniot
Artemisia argyi (A. argyi) is a creeping rhizome herbaceous perennial plant from Artemisia L. genus, Asteraceae family. This plant is known as ‘aiye’ in China and ‘gaiyou’ in Japan. It is indigenous to China, Japan, and the former Soviet Union’s far eastern regions. A. argyi has been shown to have antioxidant and anti-inflammatory characteristics [114]. The role of chondroprotective of A. argyi in oxidative stress signaling was proven through an in vitro, ex vivo, and in vivo experiment by Lee et al. (2020) using seomae mugwort, A. argyi from Korean native. The in vitro experiment revealed that the extract is able to suppress the MMP3, MMP13, ADAMTS4, and ADAMTS5 expressions and also reduce the activity of aggrecanase induced by IL-1β, IL-6, and TNF-α. The extract of seomae mugwort also showed its ability in preventing the degradation of sulfate proteoglycan in IL-1β-treated cartilage explants. The extract suppresses the destruction of cartilage and reduces the thickness of subchondral bone in the in vivo experiment of the DMM-induced OA model [95].
The results from high-performance liquid chromatography (HPLC) reported that the extract of A. argyi consists high amount of jaceoside and this compound is able to reduce the expression of MMP3, MMP13, ADAMTS4, and ADAMTS5. Both the extract and jaceosidin are able to block the degradation of IκBα and NF-κβ activation. However, the suppression of JNK phosphorylation was only seen in the experiments which used seomae mugwort extract. These findings indicate the chondroprotective properties of the A. argyi extract and jaceosidin, which is the active component, as a potential candidate for managing OA [95].
6.4. Edible Bird’s Nest (EBN)
Edible bird’s nest is a dried viscous discharge of male swiftlets from several swiftlet species’ salivary glands including the Aerodramus and Collocalia (Apodidae) genus during the breeding season [115]. Chua et al. (2013) have demonstrated the chondroprotective properties of the extract of EBN by targeting oxidative stress in OA. This extract was able to suppress the expression of catabolic genes of MMP1, MMP3, IL-1, IL-6, IL-8, COX-2, and iNOS. The chondrocytes when cultured with EBN extract also showed a significant reduction in PGE2. The gene expression of collagen type II, aggrecan, and SOX-9 increased in the chondrocytes culture supplemented with EBN extract. The production of sulfated glycosaminoglycan (sGAG) was also elevated in the assessment of anabolic activity [79]. The result concluded that the extract of EBN possesses antioxidant characteristics and can be a potential agent for OA treatment.
6.5. Stichopus chloronotus
The Stichopus chloronotus (S. chloronotus) aqueous extract (SCAE) has been proven to show a chondroprotective effect on OA articular chondrocytes by targeting the oxidative stress signaling pathway. Thus, SCAE increased the expression of collagen type II, aggrecan, and SOX-9, which is the marker specific for cartilage. Moreover, it successfully suppresses the expression of IL-1, IL-6, IL-8, MMP1, MMP3, MMP13, COX-2, iNOS, proteinase-activated receptor 2 (PAR-2), and collagen type I. This extract is also able to reduce the PGE2 and NO production and increases the production of sGAG. This result hence suggests that SCAE is a good anti-oxidative, anti-inflammatory, and pro-chondrogenic agent [93]. Incongruent with Mou et al. (2018) [116], S. chloronotus consists of fucosylated chondroitin sulfate (fCS), a polysaccharide with high sulfate content, that has numerous biological properties including anti-inflammatory and antioxidant properties [116,117]. Chondroitin sulfate is a natural glycosaminoglycan, which is an essential proteoglycan component found abundantly in the cartilage and ECM [118]. The use of fCS on OA patients resulted in slow OA progression, increase cartilage regeneration, and anti-inflammatory activity [119,120,121]. This further supports S. chloronotus as a potential antioxidant agent to treat OA.
6.6. Galangin
Galangin, also known as norizalpinin, is a 3,5,7-trihydroxyflavone with a molecular formula of C15H10O5 and a molecular weight of 270.24 g/mol. It can be found abundantly in propolis, honey, the root of Alpinia officinarum, and the aerial parts of Helichrysum aureonitens [122]. The chondroprotective ability of galangin in oxidative stress signaling mechanism has been demonstrated by several studies. In the study by Huang et al. (2021), the in vitro experiment results showed that galangin can reduce the catabolic factors and inflammatory cytokines expression including iNOS, COX-2, MMP1, MMP3, MMP13, and ADAMTS5 and also prevent aggrecan and collagen type II degradation induced by IL-1β. Furthermore, galangin also restricts the Akt, IKKα/β, IκBα, and p65 phosphorylation that play roles in the activation of Akt and NF-κβ signaling. In the anterior cruciate ligament transection (ACLT) rat model, injection of galangin intra-articulary can prevent deterioration of cartilage [50].
Su et al. (2021) supported the therapeutic effect of galangin on OA using an MIA-induced OA rat model. The rat model receiving galangin supplementation showed a significantly reduced level of urinary collagen type II, lipid peroxidation, ROS, IL-1β, IL-6, and TNF-α. Additionally, the level of catalase, superoxide dismutase (SOD), glutathione peroxidase (GPx), and reduced glutathione (GSH) is being upregulated showing that galangin possesses an antioxidant effect. Galangin also inhibits cartilage breakdown as the mRNA and protein expression of collagen type II are reduced after treatment [51].
The use of galangin as an antioxidant agent is also being supported by a study by El-Ghazaly et al. (2011) using aqueous extract of propolis (AEP) as galangin is the major component in propolis. The use of AEP as the treatment in Freud’s adjuvant injected rat model showed reduced cartilage degradation. The reduction of sGAG and hydroxyproline level is being prevented by AEP and the cartilage oligomeric matrix protein (COMP) further support these results. In addition, the level of TNF-α is being reduced and the level of NO and oxidative stress biomarkers such as GSH and malondialdehyde (MDA) is maintained by the AEP [52]. All results obtained above proved that galangin can be a promising novel antioxidant agent in treating OA.
6.7. Astaxanthin
Astaxanthin (3,3′-dihydroxy-β,β’-carotene-4,4′-dione), a red xanthophyll carotenoid with a molecular weight of 596.8 g/mol, is a secondary metabolite that gives a variety of marine animals and microorganisms their red-orange hue. Astaxanthin showed its chondroprotective properties in several in vitro and in vivo experiments by targeting the oxidative stress signaling pathway. For instance, in an in vitro experiment by Chen et al. (2013) on IL-1β-treated human chondrocytes, astaxanthin inhibits MMP1, MMP3, and MMP13 expressions. It also blocks p38 and ERK1/2 phosphorylation and the degradation of Iκβα that are responsible for the activation of MAPK and NF-κβ signaling pathways [53]. Sun et al. (2019) also suggest that astaxanthin can protect against OA. In the in vitro study, astaxanthin reverses the changes caused by IL-1β, TNF-α, and tert-butyl hydroperoxide (TBHP) by reducing the level of MMP3, MMP13, and ADAMTS5 [59].
Furthermore, a study by Sun et al. (2019) [59] proves that this carotenoid is able to upregulate the Nrf-2 signaling and the downstream chondroprotective genes including HO-1 and reduced NQO1. The results also showed that astaxanthin reduced the level of iNOS and COX-2 protein upregulation by IL-1β and inhibit MAPK signaling by blocking the ERK and JNK phosphorylation. Furthermore, it also minimized the degradation of ECM and apoptosis stimulated by TNF-α by preventing activation of NF-κβ signaling through inhibiting phosphorylation of p65 and IκBα. The chondrocytes treated with astaxanthin also showed downregulation of TBHP-induced increase in ROS production that causes cell apoptosis. The apoptosis has been prevented as the increase in B-cell lymphoma 2 (Bcl-2)-associated X protein (BAX) and cleaved-caspase 3 and decrease in Bcl-2 protein level caused by OA inducing agents have been reversed. An in vivo model showed that astaxanthin successfully attenuated the breakdown of cartilage by showing less proteoglycan loss and cartilage erosion. These suggested that astaxanthin could protect the cartilage against OA, thus implying that it could be a promising therapeutic antioxidant agent for OA [59].
6.8. Berberine
Berberine (BBR) is a quaternary ammonium salt, with a molecular weight of 336.36 g/mol and a molecular formula of C20H18NO4, produced from isoquinoline alkaloid. The chondroprotective effects of BBR in the oxidative stress signaling pathway have been proven in several studies through in vitro and in vivo experiments. In the in vitro experiment using connective tissue growth factor (CNN2)-induced IL-1β expression treated human synovial fibroblast, the BBR reduces the expression of IL-1β that play a role in inflammation. Moreover, the generation of ROS is reduced, as well as the phosphorylation of apoptosis signal-regulating kinase 1 (ASK1), p38, JNK, and p65 [56]. While in another in vitro test, the sGAG release and NO production are inhibited by BBR in IL-1β-treated rat articular chondrocytes and cartilage explant. In addition, the MMP1, MMP3, and MMP13 productions are being suppressed by BBR together with the increase in tissue inhibitors of metalloproteinases-1 (TIMP-1). A decrease in the severity of cartilage lesions is also being observed in the BBR treatment group [75].
Liu et al. (2015) also showed that BBR is able to inhibit the progression of OA in a collagenase-induced OA (CIOA) rat model. The rat treated with BBR shows a significant reduction in swelling, width, and stiffness of the knee and BBR also lowered the level of IL-1β in the serum. Moreover, the Safranin O staining showed that BBR can help preserve the proteoglycan compared with the control group [56]. For the in vivo work by Hu et al. (2011), the chondroprotective effect of BBR in the oxidative stress metabolites is in a dose-dependent manner. The expression of MMP1, MMP3, and MMP13 is being reduced and the TIMP-1 mRNA level is being upregulated in the BBR treatment group [75]. These results suggest that BBR may become one of the novel therapeutic strategies for OA management by focusing on the antioxidant mechanism.
6.9. Sesamin
The seed of sesame (Sesamum indicum L.) is one of the traditional health foods that is being widely utilized in East Asia a long time ago, but its role in OA is just recently being explored. As stated in the study by Phitak et al. (2012), the cartilage explants treated with sesamin showed reduced release of sGAG, hydroxyproline, and uronic acid (UA) indicating that sesamin can prevent the breakdown of proteoglycan. In addition, the expression and activity of MMP1, MMP3, and MMP13 are downregulated while p38 and JNK signaling pathways are being inhibited [78]. Similar results were found in a study by Kong et al. (2016) using human chondrocytes; however, this study focuses on the NF-κβ signaling pathway. The activation of the NF-κβ signaling pathway is being blocked as the sesamin inhibits the phosphorylation of p65 and IκBα, which play an important role in NF-κβ signaling. Furthermore, the sesamin showed its ability in protecting chondrocytes by enhancing the Nrf-2 and HO-1 expressions [87]. In the OA rat model, sesamin is able to reverse the pathological changes induced by papain. It reduces the disorganisation of cartilage and increases the thickness of cartilage. Aside from that, sesamin not only prevents the loss of collagen type II and proteoglycan but also increases their formation [78].
6.10. Wogonin
Wogonin (5,7-dihydroxy-8-methoxyflavone) is a natural flavonoid derived from the root and entire Scutellaria baicalensis, the leaves of Andrographis paniculata (Burm.f.) Nees, and stems of Anodendron affine (Hook. Arn.) Druce. It is well known for its antioxidant and anti-inflammation properties, particularly in metabolites of ROS [90,123,124]. This was proven through in vitro and in vivo studies by Khan et al. (2017) [90,123]. The researcher demonstrated the effects of wogonin on IL-1β-stimulated OA human chondrocytes and cartilage explants. Wogonin significantly inhibits the production, gene expression, and activity of inflammatory mediators, oxidative stress, and MMP including IL-6, iNOS, NO, COX-2, PGE2, ROS, MMP3, MMP9, MMP13, and ADAMTS4. Furthermore, it also inhibits the collagenase activity and reverses the suppression of collagen type II and aggrecan expressions induced by IL-1β.
In the cartilage explants, wogonin significantly blocks the IL-1β-induced release of collagen type II and sGAG depending on the dosage used and it is also able to prevent proteoglycan loss from the cartilage. From the results above, wogonin is proven to exhibit a protective ability toward chondrocytes and cartilage. Apart from that, wogonin can enhance the expression of Nrf-2-dependent antioxidants and various chondroprotective enzymes including SOD-2, NQO1, glutamate-cysteine ligase catalytic subunit (GCLC), and HO-1. This further confirms that wogonin exerts chondroprotective properties [90,123]. In the study by Park et al. (2015), wogonin successfully inhibits gene expression and the production of MMP1, MMP3, MMP13, and ADAMTS4. Chondrocytes treated with wogonin showed an increase in the expression of collagen type II. In an in vivo model, a decrease in the MMP3 production was observed in the OA rat model [85]. These studies suggest that wogonin may be a promising antioxidant candidate for OA treatment.
7. Catabolic and Anabolic Effect of Natural Compounds in OA
The usage of certain natural compounds for treating OA inhibits catabolic and anabolic factors. For instance, as tabulated in Table 1, edible bird’s nest [79] and genistein [91] from soybean are able to inhibit catabolic factors from 24 h up to 7 days in an in vitro testing. It mainly focuses on the inhibition of catabolic factors such as nitrite, iNOS, ROS, PGE2, COX-2, MMP1, MMP2, MMP3, MMP9, MMP13, ADAMTS4, IL-1, IL-6, and IL-8 while cynaroside [55] is able to inhibit both catabolic and anabolic factors including collage type II and aggrecan when being exposed to OA-induced chondrocytes. Inhibition of iNOS and nitrite prevents damage to cartilages by hindering the activity of MMP [35] while suppression of PGE2 restores homeostasis in the bone [125]. Similarly, the inhibition of COX-2 modulates controlled apoptosis and proliferation and reduces the release of PGE2 in the articular cartilage thus leading to healthy chondrocytes [126]. MMPs usually have a distinct role in the pathology of osteoarthritis and all types of MMPs have their specialized catalytic domain. They usually stay inactive in the presence of N-terminal pro-peptide. Upon activation, proteolytic from the pro-peptides will be removed from its cellular sites in accordance with the proteinase. All types of MMPs have their specific substrate cleavage that has a role in the progression of any condition [127]. For instance, inhibition of catabolic factors such as MMP1 and MMP13 or MMP 9 (inducible type of MMP) prevents the cleavage of the triple helix of collagen thereby preventing the damage to interstitial collagens in the arthritic joints [128]. Meanwhile, MMP2 has the potential to inhibit inflammatory factors or stimulate the anti-inflammatory factors that contribute to the progression of OA [129].
Furthermore, the function of ADAMTS4 is to mediate the degradation of aggrecan independently without the involvement of MMPs in the cartilages [130]. Therefore, the inhibition of ADAMTS4 by the natural compounds ensures a cartilage protective mechanism. In contrast, elevation in the pro-inflammatory cytokines such as IL-1, IL-6, and IL-8 is able to trigger degradation in the ECM, loss of collagen, as well as sulfated proteoglycans. Such alteration is closely associated with the inflammatory process for OA progression [131]. However, natural compounds such as edible bird’s nest [79] and genistein [91] have the ability to inhibit such catabolic factors, thus preventing the worsening of OA pathology.
8. Conclusions
In conclusion, this review recapitulated the effect of natural compounds in treating OA pathology focusing on the oxidative stress signaling pathway. In short, there are various downstream mediators—such as MAPK, NF-κβ, PI3K/Akt, and JNK—that are involved in the oxidative stress signaling pathway of OA progression. There are various studies which suggest that the extracts or components from natural products exhibit chondroprotective properties. This study highlights the importance and the potential of natural products in becoming candidates as antioxidant agents for OA. The articles included in this review specifically concentrate on the therapeutic effect of selected natural compounds that inhibit catabolic and anabolic factors in OA. As such, this review could provide insight for future researchers to deepen their research on treating OA or in developing a new therapeutic drug based on the natural compounds focusing on oxidative stress signaling mechanism.
Author Contributions
Conceptualization, Y.T.L., A.U., M.D.Y. and M.H.M.Y.; Methodology, Y.T.L.; Software, Y.T.L.; Validation, A.U., M.D.Y. and M.H.M.Y.; Formal analysis, Y.T.L.; Investigation, Y.T.L.; Resources, Y.T.L., A.U., M.D.Y. and M.H.M.Y.; Data curation, M.D.Y.; Writing—original draft preparation, Y.T.L. and M.H.M.Y.; Writing—review and editing, M.H.M.Y.; Visualization, Y.T.L.; Supervision, A.U., M.D.Y. and M.H.M.Y.; Project administration, M.H.M.Y.; Funding acquisition, M.H.M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Faculty of Medicine, Universiti Kebangsaan Malaysia (UKM).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2020, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Aiello, F.C.; Szychlinska, M.A.; Rosa, M.D.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst Century: Risk Factors and Behaviours that Influence Disease Onset and Progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef]
- Hamood, R.; Tirosh, M.; Fallach, N.; Chodick, G.; Eisenberg, E.; Lubovsky, O. Prevalence and Incidence of Osteoarthritis: A Population-Based Retrospective Cohort Study. J. Clin. Med. 2021, 10, 4282. [Google Scholar] [CrossRef]
- Pérez-Lozano, M.L.; Cesaro, A.; Mazor, M.; Esteve, E.; Berteina-Raboin, S.; Best, T.M.; Lespessailles, E.; Toumi, H. Emerging Natural-Product-Based Treatments for the Management of Osteoarthritis. Antioxidants 2021, 10, 265. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage–bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Azzini, G.O.M.; Santos, G.S.; Visoni, S.B.C.; Azzini, V.O.M.; dos Santos, R.G.; Huber, S.C.; Lana, J.F. Metabolic syndrome and subchondral bone alterations: The rise of osteoarthritis—A review. J. Clin. Orthop. Trauma 2020, 11, S849–S855. [Google Scholar] [CrossRef] [PubMed]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef]
- Yu, S.P.; Hunter, D.J. Managing osteoarthritis. Aust. Prescr. 2015, 38, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Uchôa, M.; Constantino, G. Viscosupplementation. Rev. Bras. Ortop. 2012, 47, 160–164. [Google Scholar] [CrossRef]
- De l’Escalopier, N.; Anract, P.; Biau, D. Surgical treatments for osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 227–233. [Google Scholar] [CrossRef]
- Patrignani, P.; Tacconelli, S.; Bruno, A.; Sostres, C.; Lanas, A. Managing the adverse effects of nonsteroidal anti-inflammatory drugs. Expert Rev. Clin. Pharmacol. 2011, 4, 605–621. [Google Scholar] [CrossRef] [PubMed]
- Sukhikh, S.; Noskova, S.; Ivanova, S.; Ulrikh, E.; Izgaryshev, A.; Babich, O. Chondroprotection and Molecular Mechanism of Action of Phytonutraceuticals on Osteoarthritis. Molecules 2021, 26, 2391. [Google Scholar] [CrossRef] [PubMed]
- Sukhikh, S.; Babich, O.; Prosekov, A.; Patyukov, N.; Ivanova, S. Future of Chondroprotectors in the Treatment of Degenerative Processes of Connective Tissue. Pharmaceuticals 2020, 13, 220. [Google Scholar] [CrossRef]
- Hafsi, K.; McKay, J.; Li, J.; Lana, J.F.; Macedo, A.; Santos, G.S.; Murrell, W.D. Nutritional, metabolic and genetic considerations to optimise regenerative medicine outcome for knee osteoarthritis. J. Clin. Orthop. Trauma 2018, 10, 2–8. [Google Scholar] [CrossRef]
- Jayakumar, T.; Bhavan, P.S.; Sheu, J.R. Molecular Targets of Natural Products for Chondroprotection in Destructive Joint Diseases. Int. J. Mol. Sci. 2020, 21, 4931. [Google Scholar] [CrossRef]
- Man, G.S.; Mologhianu, G. Osteoarthritis pathogenesis—A complex process that involves the entire joint. J. Med. Life 2014, 7, 41. [Google Scholar]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef]
- Walimbe, T.; Panitch, A. Proteoglycans in biomedicine: Resurgence of an underexploited class of ECM molecules. Front. Pharmacol. 2019, 10, 1661. [Google Scholar] [CrossRef]
- Wang, M.; Peng, Z.; Vasilev, K.; Ketheesan, N. Investigation of Wear Particles Generated in Human Knee Joints Using Atomic Force Microscopy. Tribol. Lett. 2013, 51, 161–170. [Google Scholar] [CrossRef]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; Artis, D. Innate lymphoid cells in the initiation, regulation and resolution of inflammation. Nat. Med. 2015, 21, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Abu-Amer, Y.; O’Keefe, R.J.; McAlinden, A. Inflammation and epigenetic regulation in osteoarthritis. Connect. Tissue Res. 2016, 58, 49–63. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Med. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Chow, Y.Y.; Chin, K.Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Med. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef]
- Scanzello, C.R. Chemokines and inflammation in osteoarthritis: Insights from patients and animal models. J. Orthop. Res. 2017, 35, 735–739. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2010, 7, 33–42. [Google Scholar] [CrossRef]
- Ni, S.; Miao, K.; Zhou, X.; Xu, N.; Li, C.; Zhu, R.; Sun, R.; Wang, Y. The involvement of follistatin-like protein 1 in osteoarthritis by elevating NF-κB-mediated inflammatory cytokines and enhancing fibroblast like synoviocyte proliferation. Arthritis Res. Ther. 2015, 17, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigoglou, S.; Papavassiliou, A.G. The NF-κB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef] [PubMed]
- Viana, M.N.; Leiguez, E.; Gutiérrez, J.M.; Rucavado, A.; Markus, R.P.; Marçola, M.; Teixeira, C.; Fernandes, C.M. A representative metalloprotease induces PGE2 synthesis in fibroblast-like synoviocytes via the NF-κB/COX-2 pathway with amplification by IL-1β and the EP4 receptor. Sci. Rep. 2020, 10, 3269. [Google Scholar] [CrossRef] [PubMed]
- De Andrés, M.C.; Imagawa, K.; Hashimoto, K.; Gonzalez, A.; Roach, H.I.; Goldring, M.B.; Oreffo, R.O.C. Loss of methylation in CpG sites in the NF-κB enhancer elements of inducible nitric oxide synthase is responsible for gene induction in human articular chondrocytes. Arthritis Rheum. 2013, 65, 732–742. [Google Scholar] [CrossRef]
- Ahmad, N.; Ansari, M.Y.; Haqqi, T.M. Role of iNOS in osteoarthritis: Pathological and therapeutic aspects. J. Cell. Physiol. 2020, 235, 6366–6376. [Google Scholar] [CrossRef]
- Yunus, M.H.M.; Nordin, A.; Kamal, H. Pathophysiological Perspective of Osteoarthritis. Medicina 2020, 56, 614. [Google Scholar] [CrossRef]
- Xie, L.; Xie, H.; Chen, C.; Tao, Z.; Zhang, C.; Cai, L. Inhibiting the PI3K/AKT/NF-κB signal pathway with nobiletin for attenuating the development of osteoarthritis: In vitro and in vivo studies. Food Funct. 2019, 10, 2161–2175. [Google Scholar] [CrossRef]
- Chen, Y.; Shou, K.; Gong, C.; Yang, H.; Yang, Y.; Bao, T. Anti-Inflammatory Effect of Geniposide on Osteoarthritis by Suppressing the Activation of p38 MAPK Signaling Pathway. BioMed Res. Int. 2018, 2018, 8384576. [Google Scholar] [CrossRef]
- Keshet, Y.; Seger, R. The MAP kinase signaling cascades: A system of hundreds of components regulates a diverse array of physiological functions. Methods Mol. Biol. 2010, 661, 3–38. [Google Scholar] [CrossRef]
- Loeser, R.F.; Erickson, E.A.; Long, D.L. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 581–586. [Google Scholar] [CrossRef]
- Zahan, O.M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 22. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: Inhibition of IL-1β-induced NF-κB-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009, 11, R165. [Google Scholar] [CrossRef]
- Gilbert, S.J.; Bonnet, C.S.; Blain, E.J. Mechanical Cues: Bidirectional Reciprocity in the Extracellular Matrix Drives Mechano-Signalling in Articular Cartilage. Int. J. Mol. Sci. 2021, 22, 13595. [Google Scholar] [CrossRef]
- Na, J.Y.; Kim, S.; Song, K.; Lim, K.H.; Shin, G.W.; Kim, J.H.; Kim, B.; Kwon, Y.B.; Kwon, J. Anti-apoptotic Activity of Ginsenoside Rb1 in Hydrogen Peroxide-treated Chondrocytes: Stabilization of Mitochondria and the Inhibition of Caspase-3. J. Ginseng Res. 2012, 36, 247. [Google Scholar] [CrossRef]
- Marsden, V.S.; O’Connor, L.; O’Reilly, L.A.; Silke, J.; Metcalf, D.; Ekert, P.G.; Huang, D.C.S.; Cecconi, F.; Kuida, K.; Tomaselli, K.J.; et al. Apoptosis initiated by Bcl-2-regulated caspase activation independently of the cytochrome c/Apaf-1/caspase-9 apoptosome. Nature 2002, 419, 634–637. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, P.; Yao, T.; Ma, J.; Chen, Z.; Zhu, J.; Gong, Z.; Shen, S.; Fang, X. Novel role of circRSU1 in the progression of osteoarthritis by adjusting oxidative stress. Theranostics 2021, 11, 1900. [Google Scholar] [CrossRef]
- Huang, X.; Pei, W.; Ni, B.; Zhang, R.; You, H. Chondroprotective and antiarthritic effects of galangin in osteoarthritis: An in vitro and in vivo study. Eur. J. Pharmacol. 2021, 906, 174232. [Google Scholar] [CrossRef]
- Su, Y.; Shen, L.; Xue, J.; Zou, J.; Wan, D.; Shi, Z. Therapeutic evaluation of galangin on cartilage protection and analgesic activity in a rat model of osteoarthritis. Electron. J. Biotechnol. 2021, 53, 8–13. [Google Scholar] [CrossRef]
- El-Ghazaly, M.A.; Abd El-Naby, D.H.; Khayyal, M.T. The influence of irradiation on the potential chondroprotective effect of aqueous extract of propolis in rats. Int. J. Radiat. Biol. 2011, 87, 254–262. [Google Scholar] [CrossRef]
- Chen, W.P.; Xiong, Y.; Shi, Y.X.; Hu, P.F.; Bao, J.P.; Wu, L.D. Astaxanthin reduces matrix metalloproteinase expression in human chondrocytes. Int. Immunopharmacol. 2013, 19, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Moon, S.M.; Han, S.H.; Hwang, E.J.; Park, B.R.; Kim, J.S.; Kim, D.K.; Kim, C.S. Chondroprotective effects of aqueous extract of Anthriscus sylvestris leaves on osteoarthritis in vitro and in vivo through MAPKs and NF-κB signaling inhibition. Biomed. Pharmacother. 2018, 103, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Park, B.R.; Moon, S.M.; Hong, J.H.; Kim, D.K.; Kim, C.S. Chondroprotective Effect of Cynaroside in IL-1 β-Induced Primary Rat Chondrocytes and Organ Explants via NF-κ B and MAPK Signaling Inhibition. Oxid. Med. Cell. Longev. 2020, 2020, 9358080. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.C.; Lee, H.P.; Hung, C.Y.; Tsai, C.H.; Li, T.M.; Tang, C.H. Berberine attenuates CCN2-induced IL-1β expression and prevents cartilage degradation in a rat model of osteoarthritis. Toxicol. Appl. Pharmacol. 2015, 289, 20–29. [Google Scholar] [CrossRef]
- Wasik, U.; Milkiewicz, M.; Kempinska-Podhorodecka, A.; Milkiewicz, P. Protection against oxidative stress mediated by the Nrf2/Keap1 axis is impaired in Primary Biliary Cholangitis. Sci. Rep. 2017, 7, 44769. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Jiang, P.F.; Gao, Y.Z. Lutein prevents osteoarthritis through Nrf2 activation and downregulation of inflammation. Arch. Med. Sci. 2016, 14, 624. [Google Scholar] [CrossRef]
- Sun, K.; Luo, J.; Jing, X.; Guo, J.; Yao, X.; Hao, X.; Ye, Y.; Liang, S.; Lin, J.; Wang, G.; et al. Astaxanthin protects against osteoarthritis via Nrf2: A guardian of cartilage homeostasis. Aging 2019, 11, 10513–10531. [Google Scholar] [CrossRef]
- Sanada, Y.; Tan, S.J.O.; Adachi, N.; Miyaki, S. Pharmacological targeting of heme oxygenase-1 in osteoarthritis. Antioxidants 2021, 10, 419. [Google Scholar] [CrossRef]
- Drevet, S.; Gavazzi, G.; Grange, L.; Dupuy, C.; Lardy, B. Reactive oxygen species and NADPH oxidase 4 involvement in osteoarthritis. Exp. Gerontol. 2018, 111, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Kamrava, S.K.; Joghataei, M.T.; Darabi, R.; Shakeri-Zadeh, A.; Shahriari, M.; Reiter, R.J.; Ghaznavi, H.; Mehrzadi, S. Apoptosis signaling pathways in osteoarthritis and possible protective role of melatonin. J. Pineal Res. 2016, 61, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Leong, D.J.; Xu, L.; He, Z.; Wang, A.; Navati, M.; Kim, S.J.; Hirsh, D.M.; Hardin, J.A.; Cobelli, N.J.; et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016, 18, 128. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.J.; Choudhury, M.; Hanstein, R.; Hirsh, D.M.; Kim, J.J.; Majeska, R.J.; Schaffler, M.B.; Hardin, J.A.; Spray, D.C.; Goldring, M.B.; et al. Green tea polyphenol treatment is chondroprotective, anti-inflammatory and palliative in a mouse posttraumatic osteoarthritis model. Arthritis Res. Ther. 2014, 16, 508. [Google Scholar] [CrossRef]
- He, Z.; Leong, D.J.; Xu, L.; Hardin, J.A.; Majeska, R.J.; Schaffler, M.B.; Thi, M.M.; Yang, L.; Goldring, M.B.; Cobelli, N.J.; et al. CITED2 mediates the cross-talk between mechanical loading and IL-4 to promote chondroprotection. Ann. N. Y. Acad. Sci. 2019, 1442, 128–137. [Google Scholar] [CrossRef]
- Hanprasertpong, N.; Teekachunhatean, S.; Chaiwongsa, R.; Ongchai, S.; Kunanusorn, P.; Sangdee, C.; Panthong, A.; Bunteang, S.; Nathasaen, N.; Reutrakul, V. Analgesic, Anti-Inflammatory, and Chondroprotective Activities of Cryptolepis buchanani Extract: In Vitro and in Vivo Studies. BioMed Res. Int. 2014, 2014, 978582. [Google Scholar] [CrossRef]
- Zhang, L.; Li, T.; Wang, R.; Xu, J.; Zhou, L.; Yan, L.; Hu, Z.; Li, H.; Liu, F.; Du, W.; et al. Evaluation of Long-Time Decoction-Detoxicated Hei-Shun-Pian (Processed Aconitum carmichaeli Debeaux Lateral Root With Peel) for Its Acute Toxicity and Therapeutic Effect on Mono-Iodoacetate Induced Osteoarthritis. Front. Pharmacol. 2020, 11, 1053. [Google Scholar] [CrossRef]
- Jeong, J.H.; Moon, S.J.; Jhun, J.Y.; Yang, E.J.; Cho, M.L.; Min, J.K. Eupatilin exerts antinociceptive and chondroprotective properties in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. PLoS ONE 2015, 10, e0130882. [Google Scholar] [CrossRef]
- Miller, M.J.S.; Bobrowski, P.; Shukla, M.; Gupta, K.; Haqqi, T.M. Chondroprotective effects of a proanthocyanidin rich Amazonian genonutrient reflects direct inhibition of matrix metalloproteinases and upregulation of IGF-1 production by human chondrocytes. J. Inflamm. 2007, 4, 16. [Google Scholar] [CrossRef]
- Shakibaei, M.; John, T.; Seifarth, C.; Mobasheri, A. Resveratrol inhibits IL-1β-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann. N. Y. Acad. Sci. 2007, 1095, 554–563. [Google Scholar] [CrossRef]
- Sumantran, V.N.; Kulkarni, A.; Boddul, S.; Chinchwade, T.; Koppikar, S.J.; Harsulkar, A.; Patwardhan, B.; Chopra, A.; Wagh, U.V. Chondroprotective potential of root extracts of Withania somnifera in osteoarthritis. J. Biosci. 2007, 32, 299–307. [Google Scholar] [CrossRef]
- Sumantran, V.N.; Kulkarni, A.; Chandwaskar, R.; Harsulkar, A.; Patwardhan, B.; Chopra, A.; Wagh, U.V. Chondroprotective potential of fruit extracts of Phyllanthus emblica in osteoarthritis. Evidence-based Complement. Altern. Med. 2008, 5, 329–335. [Google Scholar] [CrossRef]
- Sanyachare, S.; Itghiarbha, A.; Kongtawele, P.; Meepowpan, P.; Nuntasaen, N.; Pompimon, W. A New Polyoxypregnane Glycoside from the Roots of Dregea volubilis (L.f) Benth. ex Hook. f and its Chondroprotective Effect. Am. J. Biochem. Biotechnol. 2009, 5, 202–209. [Google Scholar] [CrossRef]
- Liu, F.C.; Hung, L.F.; Wu, W.L.; Chang, D.M.; Huang, C.Y.; Lai, J.H.; Ho, L.J. Chondroprotective effects and mechanisms of resveratrol in advanced glycation end products-stimulated chondrocytes. Arthritis Res. Ther. 2010, 12, R167. [Google Scholar] [CrossRef]
- Hu, P.F.; Chen, W.P.; Tang, J.L.; Bao, J.P.; Wu, L.D. Protective effects of berberine in an experimental rat osteoarthritis model. Phyther. Res. 2011, 25, 878–885. [Google Scholar] [CrossRef]
- Rujirek, C.; Siriwan, O.; Siriwan, T.; Prachya, K.; Ampai, P.; Vichai, R. Chondroprotective potential of bioactive compounds of Zingiber cassumunar Roxb. against cytokine-induced cartilage degradation in explant culture. J. Med. Plants Res. 2012, 6, 5204–5213. [Google Scholar] [CrossRef]
- Itthiarbha, A.; Phitak, T.; Sanyacharernkul, S.; Pothacharoen, P.; Pompimon, W.; Kongtawelert, P. Polyoxypregnane glycoside from Dregea volubilis extract inhibits IL-1ß-induced expression of matrix metalloproteinase via activation of NF-kB in human chondrocytes. In Vitro Cell. Dev. Biol. Anim. 2012, 48, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Phitak, T.; Pothacharoen, P.; Settakorn, J.; Poompimol, W.; Caterson, B.; Kongtawelert, P. Chondroprotective and anti-inflammatory effects of sesamin. Phytochemistry 2012, 80, 77–88. [Google Scholar] [CrossRef]
- Chua, K.H.; Lee, T.H.; Nagandran, K.; Md Yahaya, N.H.; Lee, C.T.; Tjih, E.T.T.; Abdul Aziz, R. Edible Bird’s nest extract as a chondro-protective agent for human chondrocytes isolated from osteoarthritic knee: In vitro study. BMC Complement. Altern. Med. 2013, 13, 2–9. [Google Scholar] [CrossRef]
- Liu, L.; Gu, H.; Liu, H.; Jiao, Y.; Li, K.; Zhao, Y.; An, L.; Yang, J. Protective Effect of Resveratrol against IL-1β-Induced Inflammatory Response on Human Osteoarthritic Chondrocytes Partly via the TLR4/MyD88/NF-κB Signaling Pathway: An “in Vitro” Study. Int. J. Mol. Sci. 2014, 15, 6925–6940. [Google Scholar] [CrossRef]
- Tong, P.; Xu, S.; Cao, G.; Jin, W.; Guo, Y.; Cheng, Y.; Jin, H.; Shan, L.; Xiao, L. Chondroprotective activity of a detoxicated traditional Chinese medicine (Fuzi) of Aconitum carmichaeli Debx against severe-stage osteoarthritis model induced by mono-iodoacetate. J. Ethnopharmacol. 2013, 151, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chondroprotective activity of (+)-α-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Pradit, W.; Chomdej, S.; Nganvongpanit, K.; Ongchai, S. Chondroprotective potential of Phyllanthus amarus Schum. Thonn. in experimentally induced cartilage degradation in the explants culture model. In Vitro Cell. Dev. Biol.-Anim. 2014, 51, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Qu, T.B.; Zhai, K.; Ding, J.; Hai, Y.; Zhou, J.L. Gallic acid can play a chondroprotective role against AGE-induced osteoarthritis progression. J. Orthop. Sci. 2015, 20, 734–741. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, H.J.; Lee, D.Y.; Jo, H.S.; Jeong, J.H.; Kim, D.H.; Nam, D.C.; Lee, C.J.; Hwang, S.C. Chondroprotective Effects of Wogonin in Experimental Models of Osteoarthritis in vitro and in vivo. Biomol. Ther. 2015, 23, 442–448. [Google Scholar] [CrossRef]
- Buddhachat, K.; Chomdej, S.; Pradit, W.; Nganvongpanit, K.; Ongchai, S. In Vitro Chondroprotective Potential of Extracts Obtained from Various Phyllantus Species. Planta Med. 2016, 83, 87–96. [Google Scholar] [CrossRef]
- Kong, P.; Chen, G.; Jiang, A.; Wang, Y.; Song, C.; Zhuang, J.; Xi, C.; Wang, G.; Ji, Y.; Yan, J.; et al. Sesamin inhibits IL-1β stimulated inflammatory response in human osteoarthritis chondrocytes by activating Nrf2 signaling pathway. Oncotarget 2016, 7, 83720–83726. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.; Hu, B.; Zhang, M. Sirt1 regulates apoptosis and extracellular matrix degradation in resveratrol-treated osteoarthritis chondrocytes via the wnt/β-catenin signaling pathways. Exp. Ther. Med. 2017, 14, 5057–5062. [Google Scholar] [CrossRef]
- Gu, H.; Jiao, Y.; Yu, X.; Li, X.; Wang, W.; Ding, L.; Liu, L. Resveratrol inhibits the IL-1β-induced expression ofMMP-13 and IL-6 in human articular chondrocytes viaTLR4/MyD88-dependent and-independent signaling cascades. Int. J. Mol. Med. 2017, 39, 734–740. [Google Scholar] [CrossRef]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Devarapalli, P.; Haynie, S.; Haqqi, T.M. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free Radic. Biol. Med. 2017, 106, 288–301. [Google Scholar] [CrossRef]
- Liu, F.C.; Wang, C.C.; Lu, J.W.; Lee, C.H.; Chen, S.C.; Ho, Y.J.; Peng, Y.J. Chondroprotective effects of genistein against osteoarthritis induced joint inflammation. Nutrients 2019, 11, 1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ongchai, S.; Chokchaitaweesuk, C.; Kongdang, P.; Chomdej, S.; Buddhachat, K. In vitro chondroprotective potential of Senna alata and Senna tora in porcine cartilage explants and their species differentiation by DNA barcoding-high resolution melting (Bar-HRM) analysis. PLoS ONE 2019, 14, e0215664. [Google Scholar] [CrossRef]
- Mohd Heikal, M.Y.; Ahmad Nazrun, S.; Chua, K.H.; Norzana, A.G. Stichopus chloronotus aqueous extract as a chondroprotective agent for human chondrocytes isolated from osteoarthitis articular cartilage in vitro. Cytotechnology 2019, 71, 521–537. [Google Scholar] [CrossRef]
- D’Ascola, A.; Irrera, N.; Ettari, R.; Bitto, A.; Pallio, G.; Mannino, F.; Atteritano, M.; Campo, G.M.; Minutoli, L.; Arcoraci, V.; et al. Exploiting curcumin synergy with natural products using quantitative analysis of dose-effect relationships in an experimental in vitro model of osteoarthritis. Front. Pharmacol. 2019, 10, 1347. [Google Scholar] [CrossRef]
- Lee, H.; Jang, D.; Jeon, J.; Cho, C.; Choi, S.; Han, S.J.; Oh, E.; Nam, J.; Park, C.H.; Shin, Y.S.; et al. Seomae mugwort and jaceosidin attenuate osteoarthritic cartilage damage by blocking IκB degradation in mice. J. Cell. Mol. Med. 2020, 24, 8126–8137. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Zhang, R.; Wang, L.; Pan, Q.; Mao, Z.; Huang, X. Chondro-Protective Effects of Shikimic Acid on Osteoarthritis via Restoring Impaired Autophagy and Suppressing the MAPK/NF-κB Signaling Pathway. Front. Pharmacol. 2021, 12, 1702. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Brockmueller, A.; Mueller, A.L.; Shayan, P.; Shakibaei, M. Curcumin attenuates environment-derived osteoarthritis by Sox9/NF-kB signaling axis. Int. J. Mol. Sci. 2021, 22, 7645. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhai, Z.; Ying, H.; Lu, L.; Zhang, J.; Zeng, Y. Curcumin primed ADMSCs derived small extracellular vesicle exert enhanced protective effects on osteoarthritis by inhibiting oxidative stress and chondrocyte apoptosis. J. Nanobiotechnol. 2022, 20, 123. [Google Scholar] [CrossRef]
- Wang, J.; Gao, J.S.; Chen, J.W.; Li, F.; Tian, J. Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit. Rheumatol. Int. 2011, 32, 1541–1548. [Google Scholar] [CrossRef]
- Gu, H.; Li, K.; Li, X.; Yu, X.; Wang, W.; Ding, L.; Liu, L. Oral resveratrol prevents osteoarthritis progression in C57BL/6J mice fed a High-Fat Diet. Nutrients 2016, 8, 233. [Google Scholar] [CrossRef]
- Wei, Y.; Jia, J.; Jin, X.; Tong, W.; Tian, H. Resveratrol ameliorates inflammatory damage and protects against osteoarthritis in a rat model of osteoarthritis. Mol. Med. Rep. 2017, 17, 1493–1498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Cao, J.; Yang, E.; Liang, B.; Ding, J.; Liang, J.; Xu, J. Curcumin improves age-related and surgically induced osteoarthritis by promoting autophagy in mice. Biosci. Rep. 2017, 38, 20171691. [Google Scholar] [CrossRef]
- Jiang, M.; Li, X.; Yu, X.; Liu, X.; Xu, X.; He, J.; Gu, H.; Liu, L. Oral Administration of Resveratrol Alleviates Osteoarthritis Pathology in C57BL/6J Mice Model Induced by a High-Fat Diet. Mediat. Inflamm. 2017, 2017, 7659023. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.; Zhang, H.; Li, H.; Liu, J.; Zhang, F.; Jiang, T.; Jiang, S. Curcumin prevents osteoarthritis by inhibiting the activation of inflammasome NLRP3. J. Interf. Cytokine Res. 2017, 37, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; He, B.; Guo, J.; Li, S.; Wang, J. Involvement of TLR4 in the protective effect of intra-articular administration of curcumin on rat experimental osteoarthritis. Acta Cir. Bras. 2019, 34. [Google Scholar] [CrossRef]
- Feng, K.; Ge, Y.; Chen, Z.; Li, X.; Liu, Z.; Li, X.; Li, H.; Tang, T.; Yang, F.; Wang, X. Curcumin inhibits the PERK-eIF2α-CHOP pathway through promoting SIRT1 expression in oxidative stress-induced rat chondrocytes and ameliorates osteoarthritis progression in a rat model. Oxid. Med. Cell. Longev. 2019, 2019, 8574386. [Google Scholar] [CrossRef]
- Jiang, C.; Luo, P.; Li, X.; Liu, P.; Li, Y.; Xu, J. Nrf2/ARE is a key pathway for curcumin-mediated protection of TMJ chondrocytes from oxidative stress and inflammation. Cell Stress Chaperones 2020, 25, 395–406. [Google Scholar] [CrossRef]
- Susmiarsih, T.P.; Hadi, R.S.; Sofwan, A.; Razari, I. Effect of Green Tea Extract to the Degree of Knee Joint Damage and Nitric Oxide Levels in the Rabbit Osteoarthitis Model. In Proceedings of the 2019 International Conference on Science, Technology, and Humanity (ISETH), Surakarta, Indonesia, 3–4 December 2019; pp. 648–657. [Google Scholar]
- Xu, W.; Zhang, M.; Liu, H.; Wei, K.; He, M.; Li, X.; Hu, D.; Yang, S.; Zheng, Y. Antiviral activity of aconite alkaloids from Aconitum carmichaelii Debx. Nat. Prod. Res. 2017, 33, 1486–1490. [Google Scholar] [CrossRef]
- Liu, X.X.; Jian, X.X.; Cai, X.F.; Chao, R.B.; Chen, Q.H.; Chen, D.L.; Wang, X.L.; Wang, F.P. Cardioactive C19-Diterpenoid Alkaloids from the Lateral Roots of Aconitum carmichaeli “Fu Zi”. Chem. Pharm. Bull. 2012, 60, 144–149. [Google Scholar] [CrossRef]
- Shamsul, B.; Chowdhury, S.; Hamdan, M.Y.; Ruszymah, B.H.I. Effect of cell density on formation of three-dimensional cartilaginous constructs using fibrin human osteoarthritic chondrocytes. Indian J. Med. Res. 2019, 149, 649. [Google Scholar] [CrossRef]
- Lajayer, H.M.; Norouzi, R.; Shahi-Gharahlar, A. Essential oil components, phenolic content and antioxidant activity of Anthriscus cerefolium and Anthriscus sylvestris from Iran. J. Hortic. Postharvest Res. 2020, 3, 355–366. [Google Scholar] [CrossRef]
- Jang, G.; Lee, S.A.; Hong, J.H.; Park, B.R.; Kim, D.K.; Kim, C.S. Chondroprotective Effects of 4,5-Dicaffeoylquinic Acid in Osteoarthritis through NF-κB Signaling Inhibition. Antioxidants 2022, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef]
- Lee, T.H.; Wani, W.A.; Lee, C.H.; Cheng, K.K.; Shreaz, S.; Wong, S.; Hamdan, N.; Azmi, N.A. Edible Bird’s Nest: The Functional Values of the Prized Animal-Based Bioproduct From Southeast Asia—A Review. Front. Pharmacol. 2021, 12, 871. [Google Scholar] [CrossRef]
- Mou, J.; Li, Q.; Qi, X.; Yang, J. Structural comparison, antioxidant and anti-inflammatory properties of fucosylated chondroitin sulfate of three edible sea cucumbers. Carbohydr. Polym. 2018, 185, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Yin, H.; Qi, X.; Song, W.; Shi, W.; Mou, J.; Yang, J. Immunomodulatory effects of fucosylated chondroitin sulfate from Stichopus chloronotus on RAW 264.7 cells. Carbohydr. Polym. 2020, 251, 117088. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, M.; Jain, A.; Hurkat, P.; Jain, S.K. Chondroitin sulphate: A focus on osteoarthritis. Glycoconj. J. 2016, 33, 693–705. [Google Scholar] [CrossRef]
- Michel, B.A.; Stucki, G.; Frey, D.; De Vathaire, F.; Vignon, E.; Bruehlmann, P.; Uebelhart, D. Chondroitins 4 and 6 sulfate in osteoarthritis of the knee: A randomized, controlled trial. Arthritis Rheum. 2005, 52, 779–786. [Google Scholar] [CrossRef]
- Volpi, N. Analytical aspects of pharmaceutical grade chondroitin sulfates. J. Pharm. Sci. 2007, 96, 3168–3180. [Google Scholar] [CrossRef]
- Schiraldi, C.; Cimini, D.; Rosa, M. De Production of chondroitin sulfate and chondroitin. Appl. Microbiol. Biotechnol. 2010, 87, 1209–1220. [Google Scholar] [CrossRef]
- Mak, K.K.; Tan, J.J.; Marappan, P.; Balijepalli, M.K.; Choudhury, H.; Ramamurthy, S.; Pichika, M.R. Galangin’s potential as a functional food ingredient. J. Funct. Foods 2018, 46, 490–503. [Google Scholar] [CrossRef]
- Khan, N.M.; Ahmad, I.; Ansari, M.Y.; Haqqi, T.M. Wogonin, a natural flavonoid, intercalates with genomic DNA and exhibits protective effects in IL-1β stimulated osteoarthritis chondrocytes. Chem. Biol. Interact. 2017, 274, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Herrera-Bravo, J.; Salazar, L.A.; Shaheen, S.; Abdulmajid Ayatollahi, S.; Kobarfard, F.; Imran, M.; Imran, A.; Custódio, L.; Dolores López, M.; et al. The Therapeutic Potential of Wogonin Observed in Preclinical Studies. Evidence-based Complement. Altern. Med. 2021, 2021, 9935451. [Google Scholar] [CrossRef]
- Jiang, W.; Jin, Y.; Zhang, S.; Ding, Y.; Huo, K.; Yang, J.; Zhao, L.; Nian, B.; Zhong, T.P.; Lu, W.; et al. PGE2 activates EP4 in subchondral bone osteoclasts to regulate osteoarthritis. Bone Res. 2022, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, J.E.; Aspden, R.M. Cyclooxygenase inhibition lowers prostaglandin E2 release from articular cartilage and reduces apoptosis but not proteoglycan degradation following an impact load in vitro. Arthritis Res. Ther. 2007, 9, R129. [Google Scholar] [CrossRef] [PubMed]
- Murphy, G.; Lee, M.H. What are the roles of metalloproteinases in cartilage and bone damage? Ann. Rheum. Dis. 2005, 64, iv44–iv47. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Matsuda, H.; Tanioka, M.; Kuwabara, K.; Itohara, S.; Suzuki, R. The Role of Matrix Metalloproteinase-2 and Matrix Metalloproteinase-9 in Antibody-Induced Arthritis. J. Immunol. 2002, 169, 2643–2647. [Google Scholar] [CrossRef] [PubMed]
- Malfait, A.M.; Liu, R.Q.; Ijiri, K.; Komiya, S.; Tortorella, M.D. Inhibition of ADAM-TS4 and ADAM-TS5 prevents aggrecan degradation in osteoarthritic cartilage. J. Biol. Chem. 2002, 277, 22201–22208. [Google Scholar] [CrossRef]
- Akeson, G.; Malemud, C.J. A Role for Soluble IL-6 Receptor in Osteoarthritis. J. Funct. Morphol. Kinesiol. 2017, 2, 27. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).