Bridging the Chemical Profile and Biomedical Effects of Scutellaria edelbergii Essential Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Essential Oils Extraction
2.3. GC-MS Analysis and Compounds Identification
2.4. Antimicrobial Screening
2.4.1. Antibacterial Assay
2.4.2. Antifungal Assay
2.5. Antioxidant Activity
2.5.1. DPPH Assay
2.5.2. ABTS Assay
2.6. Approval of Experimental Animals
2.7. Analgesic Activity
2.8. Anti-Inflammatory Activity
2.9. Computational Analysis
2.9.1. Construction of Chemical Compounds Database
2.9.2. Selection of Target Protein
2.9.3. Molecular Docking and Interactions Investigation
2.9.4. Molecular Dynamics Simulations
2.9.5. Binding Free Energy (BFE) Estimation
2.9.6. In Silico Pharmacokinetic/ADMET Profile Calculations
2.10. Statistical Analysis
The equation can be explained as:
1 = Denote the concentration of the inhibitor.
Indicates the inhibitor’s reaction.
HillSlope indicates the steepness of the curve.
3. Result and Discussion
3.1. GC-MS Analysis
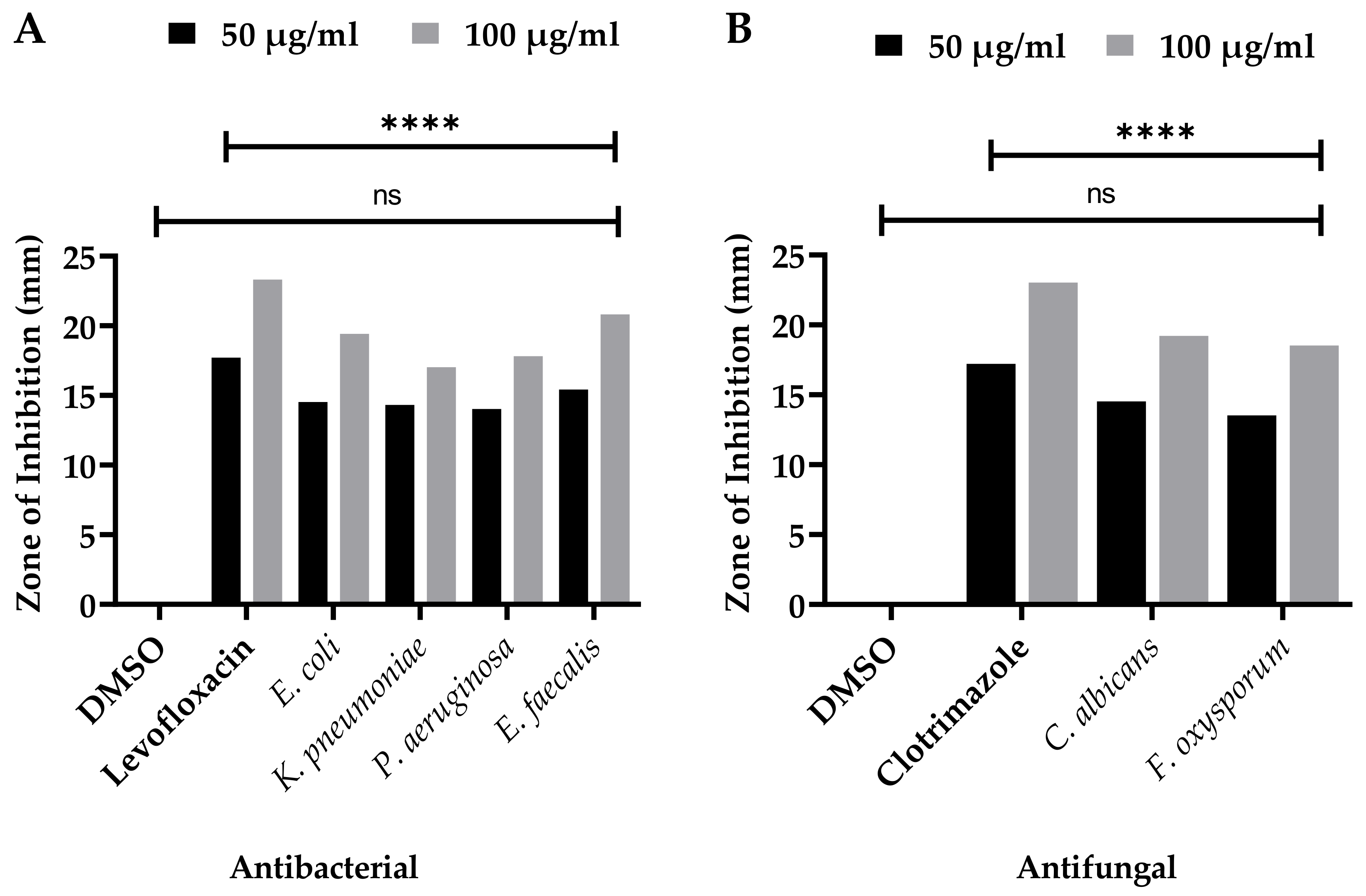
3.2. Antibacterial Capacities
3.3. Antifungal Significance
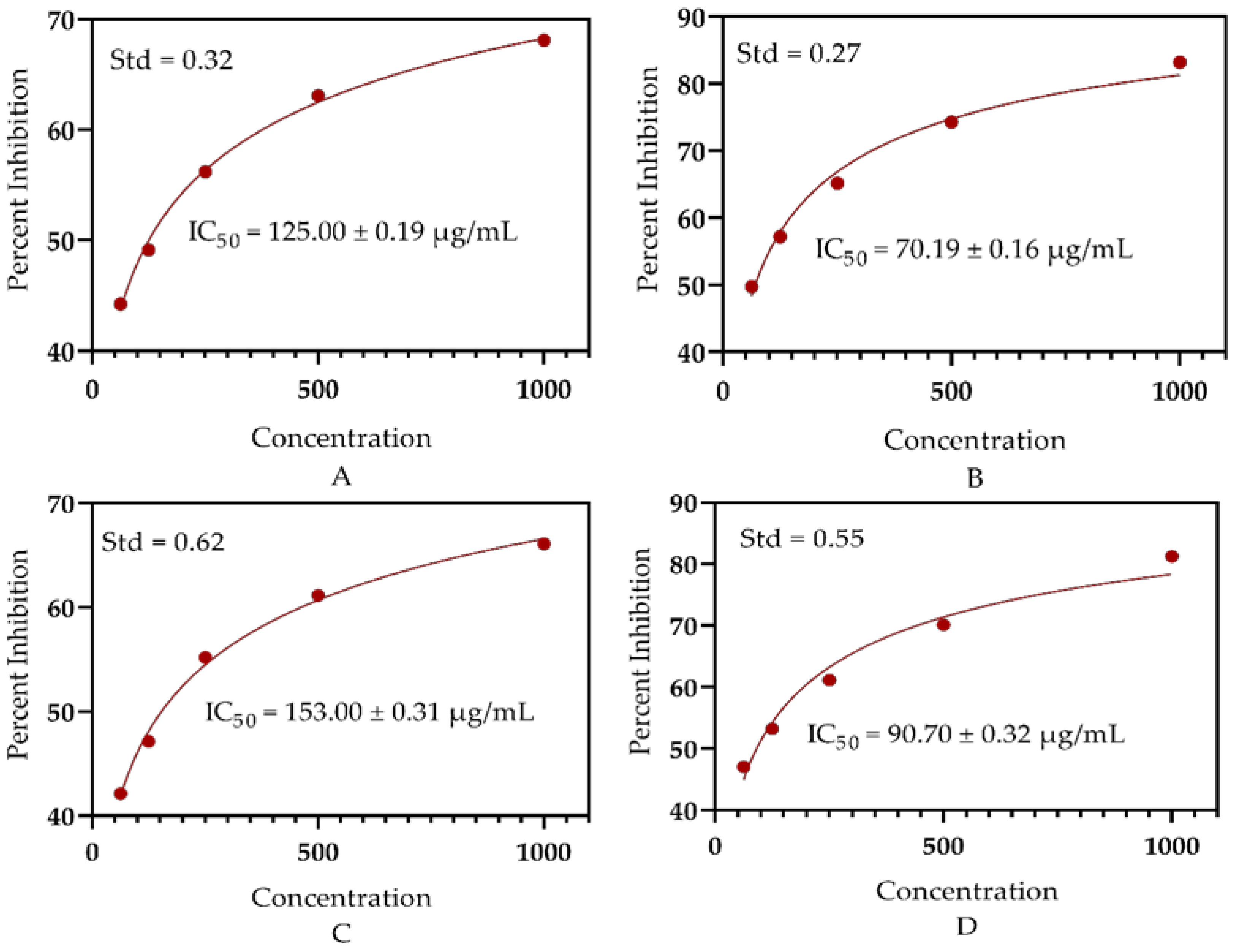
3.4. Antioxidant Significance
3.5. Analgesic Potential
3.6. Anti-Inflammatory Capabilities
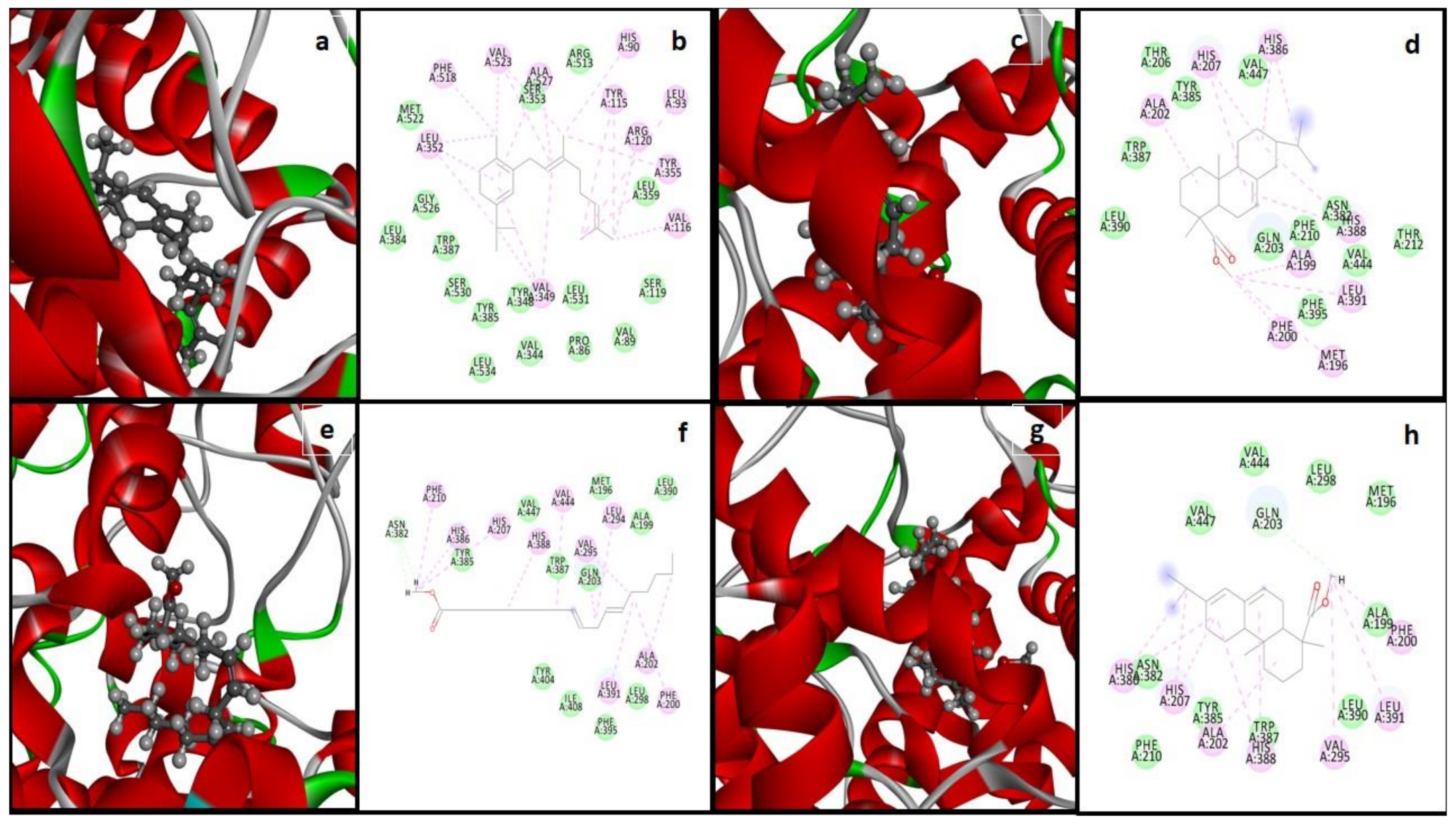
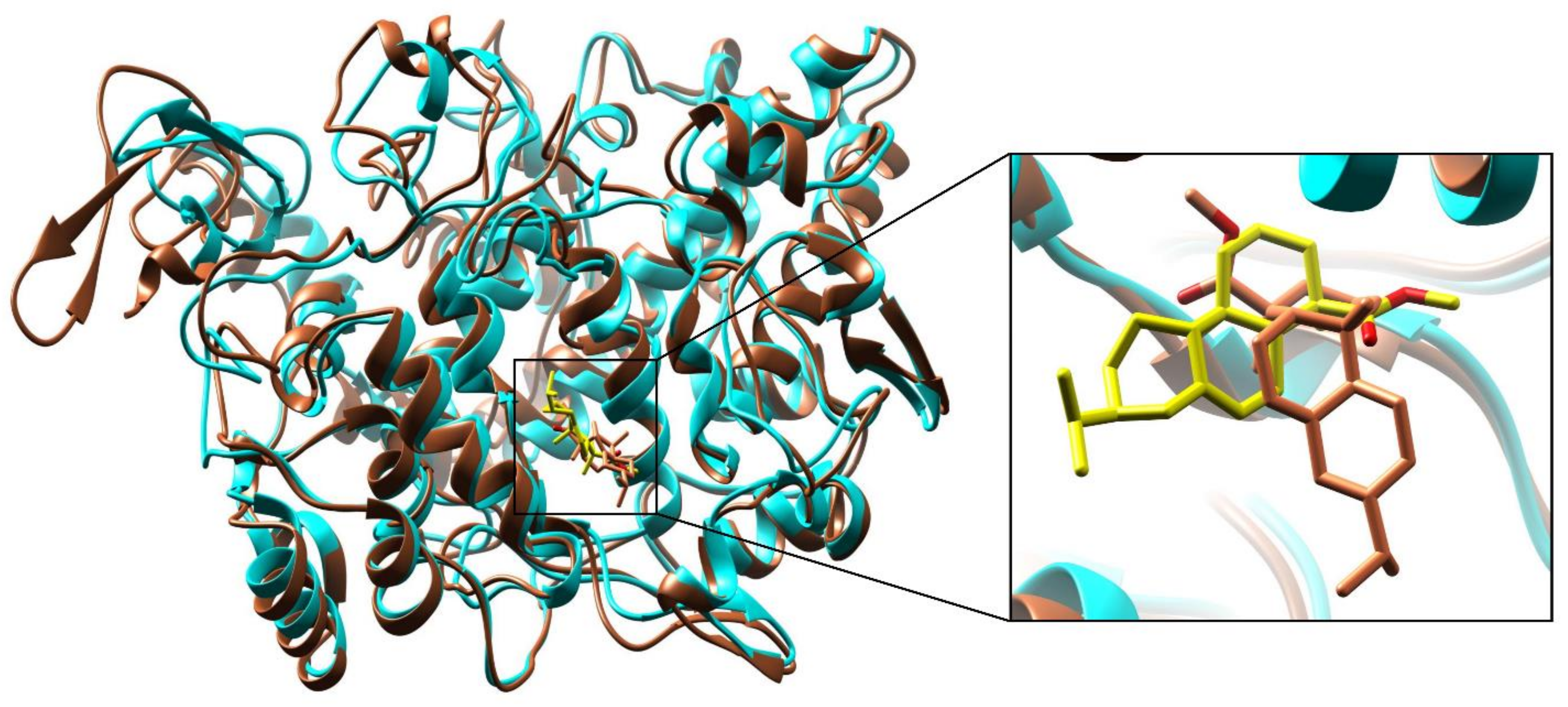
3.7. Molecular Docking and Interactions Analysis
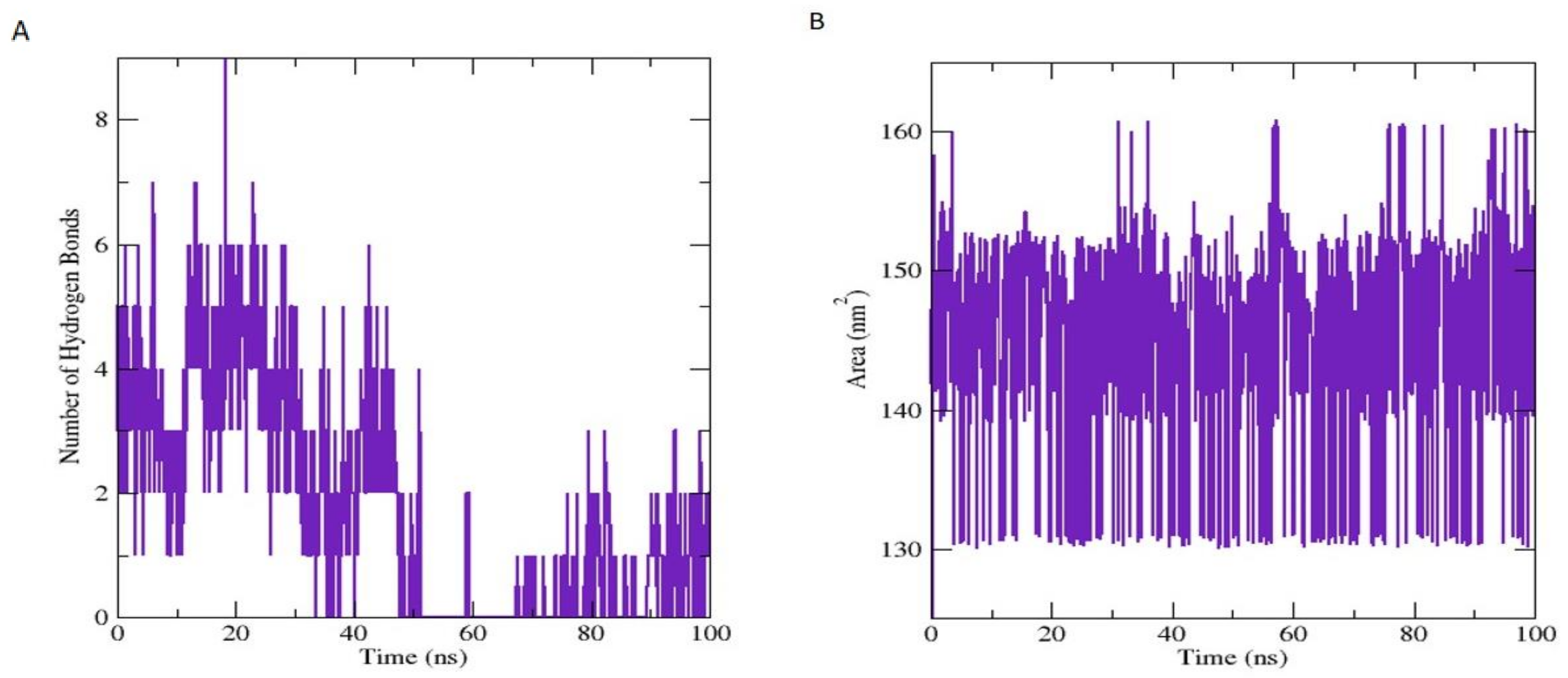
3.7.1. Molecular Dynamic Simulation Analysis
3.7.2. Binding Free Energies (BFE) Calculations (MM-GBSA/MM-PBSA)
3.7.3. In Silico Pharmacokinetic/ADMET Profile Calculations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, M.; Murad, W.; Ur Rehman, N.; Halim, S.A.; Ahmed, M.; Rehman, H.; Zahoor, M.; Mubin, S.; Khan, A.; Nassan, M.A. Biomedical applications of Scutellaria edelbergii Rech. f.: In vitro and in vivo approach. Molecules 2021, 26, 3740. [Google Scholar] [CrossRef]
- Shah, M.; Mubin, S.; Tagde, P.; Ullah, O.; Rahman, M.; Al-Harrasi, A.; Rehman, N.U.; Murad, W. Phytochemical Profiling and Bio-Potentiality of Genus Scutellaria: Biomedical Approach. Biomolecules 2022, 12, 936. [Google Scholar] [CrossRef]
- Cazella, L.N.; Glamoclija, J.; Soković, M.; Gonçalves, J.E.; Linde, G.A.; Colauto, N.B.; Gazim, Z.C. Antimicrobial activity of essential oil of Baccharis dracunculifolia DC (Asteraceae) aerial parts at flowering period. Front. Plant Sci. 2019, 10, 27. [Google Scholar] [CrossRef]
- Chebbac, K.; Moussaoui, A.E.; Bourhia, M.; Salamatullah, A.M.; Alzahrani, A.; Guemmouh, R. Chemical analysis and antioxidant and antimicrobial activity of essential oils from Artemisia negrei L. Against drug-resistant microbes Evid. Based Complement. Alternat. Med. 2021, 2021, 5902851. [Google Scholar]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- De Cássia da Silveira e Sá, R.; Lima, T.C.; da Nobrega, F.R.; de Brito, A.E.M.; de Sousa, D.P. Analgesic-like activity of essential oil constituents: An update. Int. J. Mol. Sci. 2017, 18, 2392. [Google Scholar] [CrossRef]
- Scuteri, D.; Hamamura, K.; Sakurada, T.; Watanabe, C.; Sakurada, S.; Morrone, L.A.; Rombolà, L.; Tonin, P.; Bagetta, G.; Corasaniti, M.T. Efficacy of essential oils in pain: A systematic review and meta-analysis of preclinical evidence. Front. Pharmacol. 2021, 12, 640128. [Google Scholar] [CrossRef]
- Abdollahi, A.; Koohpayeh, S.; Najafipoor, S.; Mansoori, Y.; Abdollahi, S.; Jaafari, S. Evaluation of drug Resistance and Staphylococcal cassette chromosome (SCCmec) types among methicillin-Resistant Staphylococcus aureus (MRSA). Alborz Univ. Med. J. 2012, 1, 47–52. [Google Scholar] [CrossRef][Green Version]
- Shah, M.; Murad, W.; Mubin, S.; Ullah, O.; Rehman, N.U.; Rahman, M. Multiple health benefits of curcumin and its therapeutic potential. Environ. Sci. Pollut. Res. 2022, 29, 43732–43744. [Google Scholar] [CrossRef]
- Jideani, A.I.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Ndoye Foe, F.M.-C.; Tchinang, T.F.K.; Nyegue, A.M.; Abdou, J.-P.; Yaya, A.J.G.; Tchinda, A.T.; Essame, J.-L.O.; Etoa, F.-X. Chemical composition, in vitro antioxidant and anti-inflammatory properties of essential oils of four dietary and medicinal plants from Cameroon. BMC Complement. Altern. Med. 2016, 16, 117. [Google Scholar] [CrossRef]
- Kabir, M.T.; Rahman, M.H.; Shah, M.; Jamiruddin, M.R.; Basak, D.; Al-Harrasi, A.; Bhatia, S.; Ashraf, G.M.; Najda, A.; El-Kott, A.F. Therapeutic promise of carotenoids as antioxidants and anti-inflammatory agents in neurodegenerative disorders. Biomed. Pharmacother. 2022, 146, 112610. [Google Scholar] [CrossRef] [PubMed]
- Ismael, J.; Dessalegn, E.; Fereja, W.M. In Vitro antioxidant and antibacterial activity of leaf extracts of Measa lanceolata. Int. J. Food Prop. 2021, 24, 702–712. [Google Scholar] [CrossRef]
- Basit, A.; Shutian, T.; Khan, A.; Khan, S.M.; Shahzad, R.; Khan, A.; Khan, S.; Khan, M. Anti-inflammatory and analgesic potential of leaf extract of Justicia adhatoda L.(Acanthaceae) in Carrageenan and Formalin-induced models by targeting oxidative stress. Biomed. Pharmacother. 2022, 153, 113322. [Google Scholar] [CrossRef] [PubMed]
- Sarmento-Neto, J.F.; Do Nascimento, L.G.; Felipe, C.F.B.; De Sousa, D.P. Analgesic potential of essential oils. Molecules 2015, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, D.P. Analgesic-like activity of essential oils constituents. Molecules 2011, 16, 2233–2252. [Google Scholar] [CrossRef]
- Liaqat, I.; Riaz, N.; Saleem, Q.-u.-A.; Tahir, H.M.; Arshad, M.; Arshad, N. Toxicological evaluation of essential oils from some plants of Rutaceae family. Evid. Based Complement. Alternat. Med. 2018, 2018, 4394687. [Google Scholar] [CrossRef]
- Kamte, S.L.N.; Ranjbarian, F.; Cianfaglione, K.; Sut, S.; Dall’Acqua, S.; Bruno, M.; Afshar, F.H.; Iannarelli, R.; Benelli, G.; Cappellacci, L. Identification of highly effective antitrypanosomal compounds in essential oils from the Apiaceae family. Ecotoxicol. Environ. Saf. 2018, 156, 154–165. [Google Scholar] [CrossRef]
- Chopra, H.; Dey, P.S.; Das, D.; Bhattacharya, T.; Shah, M.; Mubin, S.; Maishu, S.P.; Akter, R.; Rahman, M.H.; Karthika, C. Curcumin nanoparticles as promising therapeutic agents for drug targets. Molecules 2021, 26, 4998. [Google Scholar] [CrossRef]
- Zuzarte, M.; Sousa, C.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. The Anti-Inflammatory Response of Lavandula luisieri and Lavandula pedunculata Essential Oils. Plants 2022, 11, 370. [Google Scholar] [CrossRef]
- Cantino, P.; Harley, R.; Wagstaff, S. Genera of Labiatae: Status and classification. Adv.in Labiate Sci. 1992, 11, 511–522. [Google Scholar]
- Ghavam, M.; Manca, M.L.; Manconi, M.; Bacchetta, G. Chemical composition and antimicrobial activity of essential oils obtained from leaves and flowers of Salvia hydrangea DC. ex Benth. Sci. Rep. 2020, 10, 15647. [Google Scholar] [CrossRef] [PubMed]
- Zahra, G.; Khadijeh, B.; Hossein, D.; Ali, S. Essential oil composition of two Scutellaria species from Iran. J. Trad. Chinese Med. Sci. 2019, 6, 244–253. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Sharopov, F.; Satyal, P.; Azimova, S.S.; Wink, M. Composition of the essential oils of three Uzbek Scutellaria species (Lamiaceae) and their antioxidant activities. Nat. Prod. Res. 2017, 31, 1172–1176. [Google Scholar] [CrossRef]
- Georgieva, Y.; Katsarova, M.; Stoyanov, P.; Mladenov, R.; Denev, P.; Teneva, D.; Plotnikov, E.; Bozov, P.; Dimitrova, S. Metabolite profile and antioxidant activity of some species of Genus Scutellaria growing in Bulgaria. Plants 2020, 10, 45. [Google Scholar] [CrossRef]
- Shah, M.; Rahman, H.; Khan, A.; Bibi, S.; Ullah, O.; Ullah, S.; Ur Rehman, N.; Murad, W.; Al-Harrasi, A. Identification of α-Glucosidase Inhibitors from Scutellaria edelbergii: ESI-LC-MS and Computational Approach. Molecules 2022, 27, 1322. [Google Scholar] [CrossRef]
- Utegenova, G.A.; Pallister, K.B.; Kushnarenko, S.V.; Özek, G.; Özek, T.; Abidkulova, K.T.; Kirpotina, L.N.; Schepetkin, I.A.; Quinn, M.T.; Voyich, J.M. Chemical composition and antibacterial activity of essential oils from Ferula L. species against methicillin-resistant Staphylococcus aureus. Molecules 2018, 23, 1679. [Google Scholar] [CrossRef]
- Surendran, S.; Qassadi, F.; Surendran, G.; Lilley, D.; Heinrich, M. Myrcene—what are the potential health benefits of this flavouring and aroma agent? Front. Nutr. 2021, 400, 1–14. [Google Scholar] [CrossRef]
- Aati, H.; El-Gamal, A.; Kayser, O. Chemical composition and biological activity of the essential oil from the root of Jatropha pelargoniifolia Courb. native to Saudi Arabia. Saudi Pharm. J. 2019, 27, 88–95. [Google Scholar] [CrossRef]
- Rehman, N.U.; Shah, M.; Ullah, S.; Khan, M.; Khan, A.; Ullah, O.; Hussain, J.; Al-Harrasi, A. Enzymes Inhibition and Antioxidant Potential of Medicinal Plants Growing in Oman. BioMed Res. Int. 2022, 2022, 7880387. [Google Scholar] [CrossRef]
- Shah, M.; Murad, W.; Ur Rehman, N.; Mubin, S.; Al-Sabahi, J.N.; Ahmad, M.; Zahoor, M.; Ullah, O.; Waqas, M.; Ullah, S. GC-MS analysis and Biomedical therapy of oil from n-hexane fraction of Scutellaria edelbergii Rech. f.: In vitro, in vivo, and in silico approach. Molecules 2021, 26, 7676. [Google Scholar] [CrossRef] [PubMed]
- Rehman, N.U.; Alsabahi, J.N.; Alam, T.; Khan, A.; Rafiq, K.; Khan, M.; Al-Harrasi, A. Chemical constituents and carbonic anhydrase II activity of essential oil of Acridocarpus orientalis A. Juss. in comparison with stem and leaves. J. Essent. Oil Bear. Plants 2021, 24, 68–74. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Mondal, M.; Hossain, M.S.; Das, N.; Khalipha, A.B.R.; Sarkar, A.P.; Islam, M.T.; Smrity, S.Z.; Biswas, S.; Kundu, S.K. Phytochemical screening and evaluation of pharmacological activity of leaf Methanolic extract of Colocasia affinis Schott. Clin. Phytoscience 2019, 5, 8. [Google Scholar] [CrossRef]
- Vane, J.R.; Botting, R.M. Anti-inflammatory drugs and their mechanism of action. Inflam. Res. 1998, 47, 78–87. [Google Scholar] [CrossRef]
- Bibi, S.; Sakata, K. Current status of computer-aided drug design for type 2 diabetes. Curr. Comput. Aided Drug Des. 2016, 12, 167–177. [Google Scholar] [CrossRef]
- Bibi, S.; Hasan, M.M.; Wang, Y.-B.; Papadakos, S.P.; Yu, H. Cordycepin as a Promising Inhibitor of SARS-CoV-2 RNA dependent RNA polymerase (RdRp). Curr. Med. Chem. 2022, 29, 152–162. [Google Scholar] [CrossRef]
- Milne, G.W. Software Review of ChemBioDraw 12.0; CambridgeSoft, 100 CambridgePark Drive; ACS Publications: Cambridge, MA, USA, 2010. [Google Scholar]
- Bondhon, T.; Fatima, A.; Jannat, K.; Hasan, A.; Jahan, R.; Nissapatorn, V.; Wiart, C.; Pereira, M.; Rahmatullah, M. In silico screening of Allium cepa phytochemicals for their binding abilities to SARS and SARS-CoV-2 3C-like protease and COVID-19 human receptor ACE-2. Trop. Biomed. 2021, 38, 214–221. [Google Scholar]
- Orlando, B.J.; Malkowski, M.G. Crystal structure of rofecoxib bound to human cyclooxygenase-2. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2016, 72, 772–776. [Google Scholar] [CrossRef]
- Claria, J. Cyclooxygenase-2 biology. Curr. Pharm. Des. 2003, 9, 2177–2190. [Google Scholar] [CrossRef]
- Discovery Studio. Accelrys; BIOVIA: Cambridge, UK, 2008. [Google Scholar]
- Katsila, T.; Spyroulias, G.A.; Patrinos, G.P.; Matsoukas, M.-T. Computational approaches in target identification and drug discovery. Comput. Struct. Biotechnol. J. 2016, 14, 177–184. [Google Scholar] [CrossRef]
- Vilar, S.; Cozza, G.; Moro, S. Medicinal chemistry and the molecular operating environment (MOE): Application of QSAR and molecular docking to drug discovery. Curr. Top. Med. Chem. 2008, 8, 1555–1572. [Google Scholar] [CrossRef]
- Aldeghi, M.; Heifetz, A.; Bodkin, M.J.; Knapp, S.; Biggin, P.C. Accurate calculation of the absolute free energy of binding for drug molecules. Chem. Sci. 2016, 7, 207–218. [Google Scholar] [CrossRef]
- Ahmad, S.; Ranaghan, K.E.; Azam, S.S. Combating tigecycline resistant Acinetobacter baumannii: A leap forward towards multi-epitope based vaccine discovery. Eur. J. Pharm. Sci. 2019, 132, 1–17. [Google Scholar] [CrossRef]
- Lee, T.-S.; Allen, B.K.; Giese, T.J.; Guo, Z.; Li, P.; Lin, C.; McGee, T.D., Jr.; Pearlman, D.A.; Radak, B.K.; Tao, Y. Alchemical binding free energy calculations in AMBER20: Advances and best practices for drug discovery. J. Chem. Inf. Model. 2020, 60, 5595–5623. [Google Scholar] [CrossRef]
- Bergonzo, C.; Cheatham III, T.E. Improved force field parameters lead to a better description of RNA structure. J. Chem. Theory Comput. 2015, 11, 3969–3972. [Google Scholar] [CrossRef]
- Kräutler, V.; Van Gunsteren, W.F.; Hünenberger, P.H. A fast Shake algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J. Comput. Chem. 2001, 22, 501–508. [Google Scholar] [CrossRef]
- Roe, D.R.; Cheatham, T.E., III. PTRAJ and CPPTRAJ: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. iLOGP: A simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An open-source program for chemistry aware data visualization and analysis. J. Chem. Inf. Mod. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- Khan, M.S.; Mehmood, B.; Yousafi, Q.; Bibi, S.; Fazal, S.; Saleem, S.; Sajid, M.W.; Ihsan, A.; Azhar, M.; Kamal, M.A. Molecular docking studies reveal rhein from rhubarb (rheum rhabarbarum) as a putative inhibitor of ATP-binding cassette super-family G member 2. Med. Chem. 2021, 17, 273–288. [Google Scholar] [CrossRef]
- Bekana, D.; Kebede, T.; Assefa, M.; Kassa, H. Comparative phytochemical analyses of resins of Boswellia species (B. papyrifera (Del.) Hochst., B. neglecta S. Moore, and B. rivae Engl.) from northwestern, southern, and southeastern Ethiopia. Int. Sch. Res. Not. 2014, 2014, 374678. [Google Scholar] [CrossRef]
- Kurkcuoglu, M.; Yildiz, G.; Kose, Y.B. Essential Oil Composition of Two Scutellaria species from Tokat, Turkey. J. Turkish Chem. Soc. Sec. A Chem. 2019, 6, 115–118. [Google Scholar] [CrossRef][Green Version]
- Cicek, M.; Demirci, B.; Yilmaz, G.; Baser, K.H.C. Essential oil composition of three species of Scutellaria from Turkey. Nat. Prod. Res. 2011, 25, 1720–1726. [Google Scholar] [CrossRef]
- Formisano, C.; Rigano, D.; Senatore, F.; Piozzi, F.; Arnold, N.A. Analysis of essential oils from Scutellaria orientalis ssp. alpina and S. utriculata by GC and GC-MS. Nat. Prod. Commun. 2011, 6, 919–932. [Google Scholar] [CrossRef]
- Hussain, H.; Al-Harrasi, A.; Al-Rawahi, A.; Hussain, J. Chemistry and biology of essential oils of genus Boswellia. Evid. Based Complement. Alternat. Med. 2013, 2013, 140509. [Google Scholar] [CrossRef]
- Mahmood, A.; Ahmed, R.; Kosar, S. Phytochemical screening and biological activities of the oil components of Prunus domestica Linn. J. Saudi Chem. Soc. 2009, 13, 273–277. [Google Scholar] [CrossRef]
- Kasaian, J.; Alesheikh, P.; Mohammadi, A. Chemical compositions and biological activities of Scutellaria genus essential oils (lamiaceae). Jundishapur J. Nat. Pharm. Prod. 2020, 15, 62279. [Google Scholar] [CrossRef]
- Yilmaz, G.; Cice, M.; Demirci, B.; Baser, K.H.C. Composition of the Essential Oils of Scutellaria galericulata and S. tortumensis from Turkey. Nat. Volat. Essent. Oils 2019, 6, 1–7. [Google Scholar]
- Lawson, S.K.; Satyal, P.; Setzer, W.N. Phytochemical analysis of the essential oils from aerial parts of four Scutellaria “Skullcap” species cultivated in south Alabama: Scutellaria baicalensis Georgi, S. barbata D. Don, S. incana Biehler, and S. lateriflora L. Nat. Prod. Commun. 2021, 16, 193–259. [Google Scholar] [CrossRef]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.-G. Plant essential oils as active antimicrobial agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Legault, J.; Pichette, A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef]
- Malti, C.E.W.; Baccati, C.; Mariani, M.; Hassani, F.; Babali, B.; Atik-Bekkara, F.; Paoli, M.; Maury, J.; Tomi, F.; Bekhechi, C. Biological activities and chemical composition of Santolina africana Jord. et Fourr. aerial part essential oil from Algeria: Occurrence of polyacetylene derivatives. Molecules 2019, 24, 204. [Google Scholar] [CrossRef]
- Adnan, M.; Nazim Uddin Chy, M.; Mostafa Kamal, A.; Azad, M.O.K.; Paul, A.; Uddin, S.B.; Barlow, J.W.; Faruque, M.O.; Park, C.H.; Cho, D.H. Investigation of the biological activities and characterization of bioactive constituents of Ophiorrhiza rugosa var. prostrata (D. Don) & Mondal leaves through in vivo, in vitro, and in silico approaches. Molecules 2019, 24, 1367. [Google Scholar]
- Sonigra, P.; Meena, M. Metabolic profile, bioactivities, and variations in the chemical constituents of essential oils of the Ferula genus (Apiaceae). Front. Pharmacol. 2021, 11, 608649. [Google Scholar] [CrossRef]
- Gonzalez, M.A. Synthetic derivatives of aromatic abietane diterpenoids and their biological activities. Eur. J. Med. Chem. 2014, 87, 834–842. [Google Scholar] [CrossRef]
- Da Silva Rivas, A.C.; Lopes, P.M.; de Azevedo Barros, M.M.; Costa Machado, D.C.; Alviano, C.S.; Alviano, D.S. Biological activities of α-pinene and β-pinene enantiomers. Molecules 2012, 17, 6305–6316. [Google Scholar]
- Darwish, R.S.; Shawky, E.; El Naggar, E.M.B.; Hammoda, H.M.; Harraz, F.M. Evaluation of the effect of seasonal variation and organ selection on the chemical composition and antimicrobial activity of the essential oil of oriental-cedar (Platyclaudus orientalis (L.) Franco). J. Essent. Oil Res. 2021, 33, 69–79. [Google Scholar] [CrossRef]
- Ambrosio, C.M.; Diaz-Arenas, G.L.; Agudelo, L.P.; Stashenko, E.; Contreras-Castillo, C.J.; Da Gloria, E.M. Chemical composition and antibacterial and antioxidant activity of a citrus essential oil and its fractions. Molecules 2021, 26, 2888. [Google Scholar] [CrossRef]
- Popovici, R.A.; Vaduva, D.; Pinzaru, I.; Dehelean, C.A.; Farcas, C.G.; Coricovac, D.; Danciu, C.; Popescu, I.; Alexa, E.; Lazureanu, V. A comparative study on the biological activity of essential oil and total hydro-alcoholic extract of Satureja hortensis L. Exp. Ther. Med. 2019, 18, 932–942. [Google Scholar] [CrossRef]
- Skaltsa, H.D.; Lazari, D.M.; Kyriazopoulos, P.; Golegou, S.; Triantaphyllidis, S.; Sokovic, M.; Kypriotakis, Z. Composition and antimicrobial activity of the essential oils of Scutellaria sieberia Benth. and Scutellaria rupestris Boiss. et Heldr. ssp. adenotricha (Boiss. et Heldr.) Greuter et Burdet from Greece. J. Essent. Oil Res. 2005, 17, 232–235. [Google Scholar] [CrossRef]
- Skaltsa, H.D.; Lazari, D.M.; Mavromati, A.S.; Tiligada, E.A.; Constantinidis, T.A. Composition and antimicrobial activity of the essential oil of Scutellaria albida ssp. albida from Greece. Planta Med. 2000, 66, 672–674. [Google Scholar] [CrossRef]
- Bogdan, M.A.; Bungau, S.; Tit, D.M.; Zaha, D.C.; Nechifor, A.C.; Behl, T.; Chambre, D.; Lupitu, A.I.; Copolovici, L.; Copolovici, D.M. Chemical profile, antioxidant capacity, and antimicrobial activity of essential oils extracted from three different varieties (Moldoveanca 4, Vis Magic 10, and Alba 7) of Lavandula angustifolia. Molecules 2021, 26, 4381. [Google Scholar] [CrossRef]
- Moisa, C.; Lupitu, A.; Pop, G.; Chambre, D.R.; Copolovici, L.; Cioca, G.; Bungau, S.; Copolovici, D.M. Variation of the chemical composition of Thymus vulgaris essential oils by phenological stages. Rev. Chim. 2019, 70, 633–637. [Google Scholar] [CrossRef]
- Zhang, J.-H.; Sun, H.-L.; Chen, S.-Y.; Zeng, L.; Wang, T.-T. Anti-fungal activity, mechanism studies on α-Phellandrene and Nonanal against Penicillium cyclopium. Bot. Stud. 2017, 58, 13. [Google Scholar] [CrossRef]
- Popova, V.; Ivanova, T.; Stoyanova, A.; Nikolova, V.; Hristeva, T.; Gochev, V.; Yonchev, Y.; Nikolov, N.; Zheljazkov, V.D. Terpenoids in the essential oil and concentrated aromatic products obtained from Nicotiana glutinosa L. leaves. Molecules 2019, 25, 30. [Google Scholar] [CrossRef]
- Xu, C.; Zhao, S.; Li, M.; Dai, Y.; Tan, L.; Liu, Y. Chemical composition, antimicrobial and antioxidant activities of essential oil from flue-cured tobacco flower bud. Biotech. Biotechnol. Equip. 2016, 30, 1026–1030. [Google Scholar] [CrossRef]
- Burcova, Z.; Kreps, F.; Greifová, M.; Jablonsky, M.; Haz, A.; Schmidt, S.; Surina, I. Antibacterial and antifungal activity of phytosterols and methyl dehydroabietate of Norway spruce bark extracts. J. Biotechnol. 2018, 282, 18–24. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; Catalan, C.; De Lampasona, M. Studies on essential oils, Part 42: Chemical, antifungal, antioxidant and sprout suppressant studies on ginger essential oil and its oleoresin. Flavour Fragr. J. 2005, 20, 1–6. [Google Scholar] [CrossRef]
- Yu, J.; Lei, J.; Yu, H.; Cai, X.; Zou, G. Chemical composition and antimicrobial activity of the essential oil of Scutellaria barbata. Phytochemistry 2004, 65, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Han, C.; Gao, T.; Shao, H. Chemical composition, phytotoxic and antimicrobial activities of the essential oil of Scutellaria strigillosa Hemsley. J. Essent. Oil Bear. Plants 2016, 19, 664–670. [Google Scholar] [CrossRef]
- Ullah, O.; Shah, M.; Rehman, N.U.; Ullah, S.; Al-Sabahi, J.N.; Alam, T.; Khan, A.; Khan, N.A.; Rafiq, N.; Bilal, S.; et al. Aroma Profile and Biological Effects of Ochradenus arabicus Essential Oils: A Comparative Study of Stem, Flowers, and Leaves. Molecules 2022, 27, 5197. [Google Scholar] [CrossRef] [PubMed]
- Mot, M.-D.; Gavrilaș, S.; Lupitu, A.I.; Moisa, C.; Chambre, D.; Tit, D.M.; Bogdan, M.A.; Bodescu, A.-M.; Copolovici, L.; Copolovici, D.M.; et al. Salvia officinalis L. Essential Oil: Characterization, Antioxidant Properties, and the Effects of Aromatherapy in Adult Patients. Antioxidants 2022, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otrisal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M. Statistical analysis of the relationship between antioxidant activity and the structure of flavonoid compounds. Rev. Chim. 2019, 70, 3103–3107. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Viljoen, A.M. Linalool–A review of a biologically active compound of commercial importance. Nat. Prod. Commun. 2008, 3, 193–327. [Google Scholar] [CrossRef]
- Ghasemi, G.; Fattahi, M.; Alirezalu, A.; Ghosta, Y. Antioxidant and antifungal activities of a new chemovar of cumin (Cuminum cyminum L.). Food Sci. Biotechnol. 2019, 28, 669–677. [Google Scholar] [CrossRef]
- Khalilov, L.; Paramonov, E.; Khalilova, A.; Odinokov, V.; Muldashev, A.; Baltaev, U.; Dzhemilev, U. Identification and biological activity of volatile organic compounds emitted by plants and insects. IV. Composition of vapor isolated from certain species of Artemisia plants. Chem. Nat. Compd. 2001, 37, 339–342. [Google Scholar] [CrossRef]
- Uritu, C.M.; Mihai, C.T.; Stanciu, G.-D.; Dodi, G.; Alexa-Stratulat, T.; Luca, A.; Leon-Constantin, M.-M.; Stefanescu, R.; Bild, V.; Melnic, S. Medicinal plants of the family Lamiaceae in pain therapy: A review. Pain Res. Manag. 2018, 2018, 7801543. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Y.-Y.; Wang, X.-Y.; Liu, J.-T.; Huang, L.-Q.; Peng, C.-S. GC-MS analysis and analgesic activity of essential oil from fresh rhizoma of Cyperus rotundus. J. Chinese Med. Mat. 2011, 34, 1225–1229. [Google Scholar]
- Mishra, D.; Bisht, G.; Mazumdar, P.M.; Sah, S.P. Chemical composition and analgesic activity of Senecio rufinervis essential oil. Pharm. Biol. 2010, 48, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Hameed, I.H.; Altameme, H.J.; Mohammed, G.J. Evaluation of antifungal and antibacterial activity and analysis of bioactive phytochemical compounds of Cinnamomum zeylanicum (Cinnamon bark) using gas chromatography-mass spectrometry. Orien. J. Chem. 2016, 32, 1769. [Google Scholar] [CrossRef]
- Liang, J.; Huang, B.; Wang, G. Chemical composition, antinociceptive and anti-inflammatory properties of essential oil from the roots of Illicium lanceolatum. Nat. Prod. Res. 2012, 26, 1712–1714. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, V.; Sajjadi, S.E.; Zomorodkia, M. Antinociceptive and anti-inflammatory activities of Bunium persicum essential oil, hydroalcoholic and polyphenolic extracts in animal models. Pharm. Biol. 2011, 49, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.; Jeferson, F.; Santos, C.; Silveira, E.; Rao, V. Antinociceptive effect of leaf essential oil from Croton sonderianus in mice. Life Sci. 2005, 77, 2953–2963. [Google Scholar] [CrossRef] [PubMed]
- Colares, A.V.; Almeida-Souza, F.; Taniwaki, N.N.; Souza, C.d.S.F.; da Costa, J.G.M.; Calabrese, K.d.S.; Abreu-Silva, A.L. In vitro antileishmanial activity of essential oil of Vanillosmopsis arborea (Asteraceae) baker. Evid. Based Complement. Alternat. Med. 2013, 2013, 727042. [Google Scholar] [CrossRef]
- Mogosan, C.; Vostinaru, O.; Oprean, R.; Heghes, C.; Filip, L.; Balica, G.; Moldovan, R.I. A comparative analysis of the chemical composition, anti-inflammatory, and antinociceptive effects of the essential oils from three species of Mentha cultivated in Romania. Molecules 2017, 22, 263. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Darwish, N.H.; Sudha, T.; Bahlouli, S.; Kellou, D.; Benelmouffok, A.B.; Chader, H.; Rajabi, M.; Benali, Y.; Mousa, S.A. In vitro antifungal and topical anti-inflammatory properties of essential oil from wild-growing Thymus vulgaris (Lamiaceae) used for medicinal purposes in Algeria: A new source of carvacrol. Sci. Pharm. 2020, 88, 33. [Google Scholar] [CrossRef]
- Dragomanova, S.; Tancheva, L.; Georgieva, M. A review: Biological activity of myrtenal and some myrtenal-containing medicinal plant essential oils. Scripta Sci. Pharm. 2018, 5, 22–33. [Google Scholar] [CrossRef]
- Adeosun, T.E.; Ogunwande, I.A.; Avoseh, O.N.; Raji, I.P.; Lawal, O.A. Composition and anti-inflammatory activity of essential oil of Jatropha curcas. Nat. Prod. Commun. 2017, 12, 439–440. [Google Scholar] [CrossRef]
- Apel, M.A.; Lima, M.E.; Sobral, M.; Young, M.C.M.; Cordeiro, I.; Schapoval, E.E.; Henriques, A.T.; Moreno, P.R.H. Anti-inflammatory activity of essential oil from leaves of Myrciaria tenella and Calycorectes sellowianus. Pharm. Biol. 2010, 48, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Asl, N.; Mirzayi, S.; Mahnam, K.; Sepehri, S. Identification of COX-2 inhibitors via structure-based virtual screening and molecular dynamics simulation. J. Mol. Graph. Mod. 2018, 83, 138–152. [Google Scholar] [CrossRef]
- Lobanov, M.Y.; Bogatyreva, N.; Galzitskaya, O. Radius of gyration as an indicator of protein structure compactness. Mol. Biol. 2008, 42, 623–628. [Google Scholar] [CrossRef]
- Raniolo, S.; Limongelli, V. Ligand binding free-energy calculations with funnel metadynamics. Nat. Protoc. 2020, 15, 2837–2866. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Elguero, J.; Mó, O.; Yáñez, M.; Del Bene, J.E. Are resonance-assisted hydrogen bonds ‘resonance assisted’? A theoretical NMR study. Chem. Phys. Lett. 2005, 411, 411–415. [Google Scholar] [CrossRef]
- Ismail, S.; Ahmad, S.; Azam, S.S. Immunoinformatics characterization of SARS-CoV-2 spike glycoprotein for prioritization of epitope based multivalent peptide vaccine. J. Mol. Liq. 2020, 314, 113612. [Google Scholar] [CrossRef]
- Chandrasekaran, B.; Abed, S.N.; Al-Attraqchi, O.; Kuche, K.; Tekade, R.K. Computer-aided prediction of pharmacokinetic (ADMET) properties. In Dosage Form Design Parameters; Elsevier: Amsterdam, Netherlands, 2018; pp. 731–755. [Google Scholar]
- Sudha, K.N.; Shakira, M.; Prasanthi, P.; Sarika, N.; Kumar, C.N.; Babu, P.A. Virtual screening for novel COX-2 inhibitors using the ZINC database. Bioinformation 2008, 2, 325. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Dis. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Kimura, T.; Higaki, K. Gastrointestinal transit and drug absorption. Biol. Pharm. Bull. 2002, 25, 149–164. [Google Scholar] [CrossRef]
- Mayhan, W.G. Regulation of blood–brain barrier permeability. Microcirculation 2001, 8, 89–104. [Google Scholar] [PubMed]
- Alonso, C.; Carrer, V.; Espinosa, S.; Zanuy, M.; Cordoba, M.; Vidal, B.; Domínguez, M.; Godessart, N.; Coderch, L.; Pont, M. Prediction of the skin permeability of topical drugs using in silico and in vitro models. Eur. J. Pharm. Sci. 2019, 136, 104945. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Sakata, K. An integrated computational approach for plant-based protein tyrosine phosphatase non-receptor type 1 inhibitors. Curr. Comput. Aided Drug Des. 2017, 13, 319–335. [Google Scholar] [CrossRef] [PubMed]
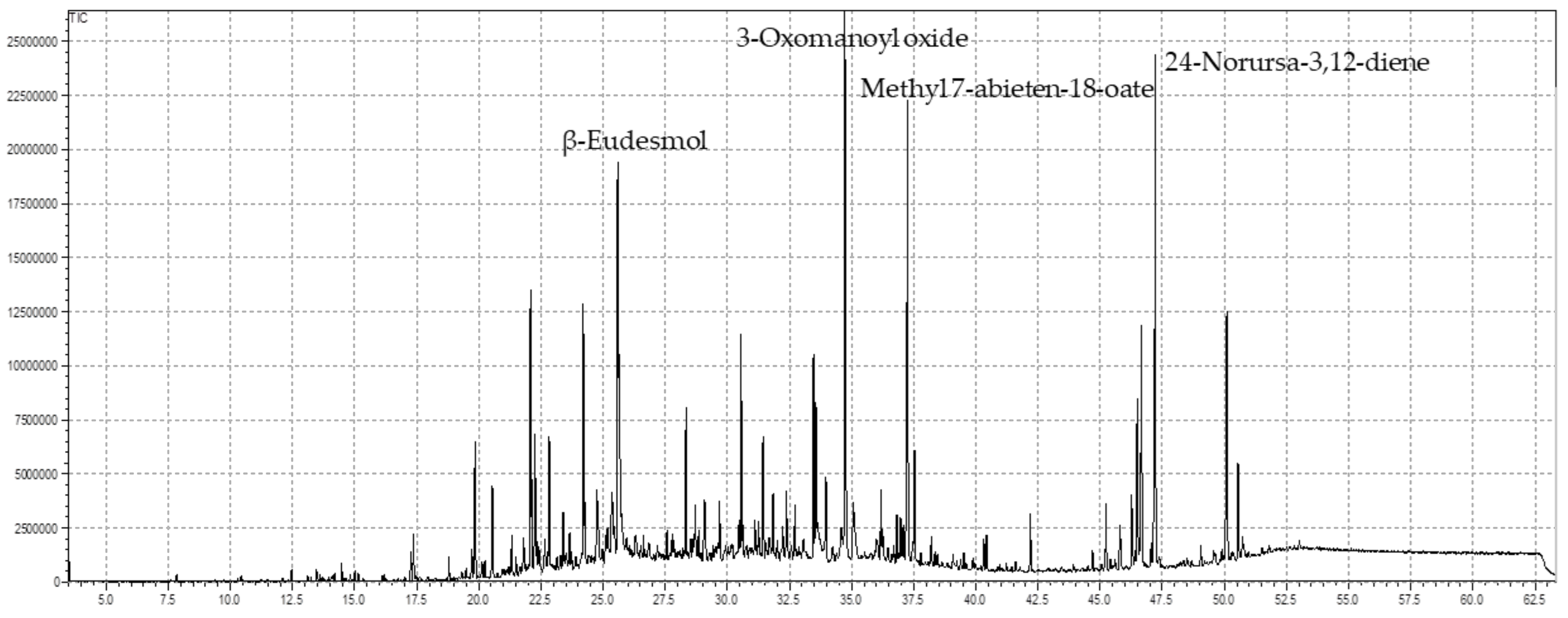


| C. No | Name of the Compounds | Rt | Contents (%) | RIRep. | RIcalc. |
|---|---|---|---|---|---|
| 1 | α-Pinene | 7.83 | 0.08 | 931 | 942 |
| 2 | β-Pinene | 8.91 | 0.02 | 943 | 982 |
| 3 | β-Myrcene | 9.37 | 0.03 | 958 | 999 |
| 4 | α-Pellandrene | 9.75 | 0.04 | 964 | 1013 |
| 5 | Sabinene | 10.43 | 0.10 | 969 | 1021 |
| 6 | psi-Limonene | 10.48 | 0.02 | 992 | 998 |
| 7 | (+)-4-Carene | 11.24 | 0.03 | 1017 | 1026 |
| 8 | Linalool | 12.31 | 0.02 | 1081 | 1104 |
| 9 | Nonanal | 12.46 | 0.14 | 1080 | 1110 |
| 10 | α-Campholenal | 13.10 | 0.08 | 1102 | 1134 |
| 11 | L-Pinocarveol | 13.47 | 0.15 | 1143 | 1147 |
| 12 | cis-Verbenol | 13.61 | 0.09 | 1131 | 1153 |
| 13 | α-Phellandren-8-ol | 14.19 | 0.09 | 1148 | 1174 |
| 14 | Terpinen-4-ol | 14.48 | 0.24 | 1160 | 1185 |
| 15 | α-Terpineol | 14.82 | 0.10 | 1172 | 1198 |
| 16 | Myrtenal | 15.01 | 0.13 | 1175 | 1205 |
| 17 | Cuminal | 16.11 | 0.08 | 1214 | 1248 |
| 18 | Carvone | 16.21 | 0.08 | 1229 | 1251 |
| 19 | Bornyl acetate | 17.26 | 0.59 | 1273 | 1292 |
| 20 | Phenol,2-methyl-5-(1methylethyl)- | 17.36 | 0.60 | 1278 | 1296 |
| 21 | α-Terpinyl acetate | 18.79 | 0.62 | 1322 | 1354 |
| 22 | (-)-β-Bourbonene | 19.71 | 0.46 | 1386 | 1392 |
| 23 | β-Elemene | 19.84 | 2.09 | 1398 | 1401 |
| 24 | Caryophyllene | 20.54 | 2.30 | 1421 | 1428 |
| 25 | Humulene | 21.32 | 0.40 | 1454 | 1462 |
| 26 | gamma-muurolene | 21.79 | 0.46 | 1471 | 1483 |
| 27 | β-eudesmene | 22.07 | 4.10 | 1478 | 1494 |
| 28 | α-Selinene | 22.26 | 1.83 | 1500 | 1503 |
| 29 | Cadina-1(10), 4-diene | 22.83 | 1.90 | 1514 | 1529 |
| 30 | Elemol | 23.38 | 0.93 | 1535 | 1554 |
| 31 | Caryophyllene oxide | 24.20 | 3.94 | 1575 | 1591 |
| 32 | γ-Eudesmole | 24.793 | 0.54 | 1627 | 1619 |
| 33 | tau-Cadinol | 25.35 | 0.60 | 1628 | 1646 |
| 34 | β-Eudesmol | 25.58 | 6.39 | 1644 | 1658 |
| 35 | tau-Muurolol | 25.63 | 4.43 | 1628 | 1660 |
| 36 | Ar-Turmerone | 25.77 | 0.19 | 1638 | 1667 |
| 37 | α-Phellandrene, dimer | 28.33 | 1.52 | 1801 | 1811 |
| 38 | Linalyl phenylacetate | 29.08 | 1.51 | 1945 | 1953 |
| 39 | Thunbergen | 29.70 | 1.74 | 1934 | 1942 |
| 40 | p-Camphorene | 30.47 | 2.47 | 1977 | 1986 |
| 41 | Geranyl.alpha-terpinene | 31.10 | 1.72 | 1962 | 1973 |
| 42 | Thunbergol | 31.83 | 0.50 | 2073 | 2082 |
| 43 | Verticiol | 32.38 | 0.96 | 2106 | 2118 |
| 44 | Linoleic acid, methyl ester | 33.46 | 4.12 | 2071 | 2080 |
| 45 | 3-Oxomanoyl oxide | 33.96 | 10.09 | 2133 | 2140 |
| 46 | Methyl pimar-8-en-18-oate | 36.97 | 1.40 | 2231 | 2297 |
| 47 | Methyl 7-abieten-18-oate | 37.25 | 7.02 | 2164 | 2178 |
| 48 | Methyl dehydroabietate | 37.53 | 2.79 | 2293 | 2335 |
| 49 | Methyl abietate | 38.20 | 1.36 | 2339 | 2379 |
| 50 | 24-Norursa-3,9(11),12-triene | 46.50 | 3.70 | 3042 | 3057 |
| 51 | 24-Norursa-3,12-diene | 47.21 | 8.05 | 3105 | 3062 |
| 52 | 24-Norursa-3,12-dien-11-one | 50.09 | 6.68 | 3351 | 3308 |
| Identified compounds | 89.52 |
| Treatment | Dose Conc. | No. of Writhes Mean ± SEM | % Reduction in Writhes after 45 min |
|---|---|---|---|
| Acetic acid | 1 mL | 27.6 ± 0.03 | |
| Normal saline | 1 mL | 27.3 ± 0.05 | - |
| Aspirin | 1 mL | 9.8 ± 0.02 | 64.49 |
| SEEO | 25 (mg/kg) | 18.4 ± 0.02 ** | 33.33 |
| 50 | 15.7 ± 0.03 ** | 43.11 | |
| 100 | 12.5 ± 0.05 ** | 54.71 |
| Changes in Paw Diameter in Swiss Albino Mice (Mean ± SEM) | ||||||
|---|---|---|---|---|---|---|
| Samples Used | Dose Conc. | 1 h | 2 h | 3 h | Av. Paw Diameter | % Inhibition |
| Carrageenan | 1 mL | 1.21 ± 0.03 | 1.42 ± 0.03 | 1.74 ± 0.05 | 1.45 ± 0.03 | |
| NS | 1 mL | 1.18 ± 0.02 | 1.40 ± 0.04 | 1.71 ± 0.02 | 1.43 ± 0.03 | - |
| Standard | 50 (mg/kg) | 0.49 ± 0.02 | 0.42 ± 0.03 | 0.33 ± 0.01 | 0.41 ± 0.02 | 71.72 |
| SEEO | 25 | 0.73 ± 0.04 | 0.68 ± 0.02 | 0.63 ± 0.03 | 0.68 ± 0.03 * | 53.10 |
| 50 | 0.67 ± 0.01 | 0.62 ± 0.03 | 0.56 ± 0.02 | 0.61 ± 0.04 * | 57.93 | |
| 100 | 0.58 ± 0.06 | 0.53 ± 0.04 | 0.46 ± 0.02 | 0.52 ± 0.03 * | 64.13 | |
| Numbering | Score | RMSD | Numbering | Score | RMSD |
|---|---|---|---|---|---|
| 1 | −5.323 | 1.2178 | 27 | −6.234 | 0.651 |
| 2 | −5.392 | 1.44 | 28 | −6.198 | 3.501 |
| 3 | −5.421 | 1.0412 | 29 | −6.144 | 2.123 |
| 4 | −5.422 | 1.042 | 30 | −6.543 | 1.577 |
| 5 | −5.364 | 1.4674 | 31 | −5.922 | 2.437 |
| 6 | −5.319 | 1.3645 | 32 | −6.477 | 0.716 |
| 7 | −5.311 | 0.6295 | 33 | −6.054 | 1.429 |
| 8 | −5.691 | 1.9427 | 34 | −6.032 | 1.647 |
| 9 | −5.758 | 1.0724 | 35 | −6.503 | 1.481 |
| 10 | −5.550 | 1.1354 | 36 | −6.248 | 1.524 |
| 11 | −5.677 | 1.1992 | 37 | −7.130 | 2.209 |
| 12 | −5.566 | 0.8491 | 38 | −7.043 | 2.447 |
| 13 | −5.490 | 0.5436 | 39 | −6.715 | 0.929 |
| 14 | −5.564 | 2.2633 | 40 | −6.711 | 1.964 |
| 15 | −5.536 | 0.4417 | 41 | −7.949 | 0.639 |
| 16 | −5.565 | 0.4645 | 42 | −6.640 | 2.445 |
| 17 | −5.160 | 1.2301 | 43 | −6.237 | 2.991 |
| 18 | −5.529 | 0.7864 | 44 | −7.626 | 1.845 |
| 19 | −6.139 | 2.7774 | 45 | −6.808 | 1.376 |
| 20 | −5.323 | 0.8127 | 46 | −6.731 | 1.114 |
| 21 | −6.572 | 1.2823 | 47 | −7.870 | 1.920 |
| 22 | −6.329 | 2.1625 | 48 | −6.802 | 2.784 |
| 23 | −6.006 | 1.2073 | 49 | −7.422 | 2.663 |
| 24 | −5.9901 | 1.3014 | 50 | −6.3748 | 4.1046 |
| 25 | −5.9146 | 2.6874 | 51 | −6.315 | 2.3077 |
| 26 | −6.3482 | 0.9651 | 52 | −6.6796 | 3.2421 |
| Numbering | Dock Score (kcal/mol) | Functional Residues | Binding Interactions |
|---|---|---|---|
| 41 | −7.9497 | PRO86 (A), VAL89 (A), HIS90 (A), LEU93 (A), TYR115 (A), VAL116 (A), SER119 (A), ARG120 (A), VAL344 (A), TYR348 (A), LEU352 (A), SER353 (A), TYR355 (A), LEU359 (A), LEU384 (A), TYR385 (A), TRP387 (A), ARG513 (A), PHE518 (A), MET522 (A), VAL523 (A), VAL523 (A), GLY526 (A), ALA527 (A), SER530 (A), LEU531 (A), LEU534 (A). | Van der Waals, Carbon-hydrogen bond, Alkyl, Pi-Alkyl |
| 47 | −7.8704 | MET196 (A), ALA199 (A), PHE200 (A), ALA202 (A), GLN203 (A), THR206 (A), HIS207 (A), PHE210 (A), THR212 (A), ASN382 (A), TYR385 (A), HIS386 (A), TRP387 (A), HIS388 (A), LEU390 (A), LEU391 (A), PHE395 (A), VAL444 (A), VAL447 (A). | Van der Waals, Carbon-hydrogen bond, Alkyl, Pi-Alkyl |
| 44 | −7.6261 | MET196 (A), ALA199 (A), PHE200 (A), ALA202 (A), GLN203 (A), HIS207 (A), PHE210 (A), LEU294 (A), VAL295 (A), LEU298 (A), LEU390 (A), LEU291 (A), ASN382 (A), TYR385 (A), HIS386 (A), TRP387 (A), HIS388 (A), LEU390 (A), PHE395 (A), TYR404 (A), ILE408 (A), VAL444 (A), VAL447 (A). | Van der Waals, Carbon-hydrogen bond, Alkyl, Pi-Alkyl |
| 49 | −7.4221 | MET196 (A), ALA199 (A), PHE200 (A), ALA202 (A), GLN203 (A), HIS207 (A), PHE210 (A), VAL295 (A), LEU298 (A), ASN382 (A), TYR385 (A), HIS386 (A), TRP387 (A), HIS388 (A), LEU390 (A), LEU391(A), VAL444 (A), VAL447 (A). | Van der Waals, Carbon-hydrogen bond, Alkyl, Pi-Alkyl |
| MM/GBSA Model | MM/PBSA Model | ||||||
|---|---|---|---|---|---|---|---|
| Energy (E) Component | Average Values | Standard Deviation Values | Standard Error of Mean Values | Energy (E) Components | Average Values | Standard Deviation Values | Standard Error of Mean Values |
| Van der Waals | −60.8915 | 2.3527 | 0.2469 | Van der Waals | −60.2943 | 4.3561 | 0.4356 |
| EEL | −32.3777 | 5.9753 | 0.7345 | EEL | −32.1854 | 5.9826 | 0.7432 |
| EGB | 46.3402 | 4.5936 | 0.5086 | EPB | 55.1001 | 5.1322 | 0.4423 |
| ESURF | −6.0429 | 0.1611 | 0.0163 | ENPOLAR | −4.6961 | 0.1102 | 0.0110 |
| EDISPER | 0 | 0 | 0 | ||||
| ΔG gas | −92.2470 | 6.5432 | 0.6949 | ΔG gas | −92.2092 | 6.3912 | 0.6391 |
| ΔG solv | 40.6321 | 4.6615 | 0.4661 | ΔG solv | 50.4040 | 5.0137 | 0.5014 |
| Δtotal | −50.9841 | 4.1324 | 0.4319 | ΔTOTAL | −42.4442 | 5.2303 | 0.5140 |
| Descriptors | Methyl 7-Abieten-18-Oate (47) |
|---|---|
| Formula | C21H34O2 |
| Molecular weight | 318.49 g/mol |
| Number of rotatable bonds | 3 |
| Number of hydrogen bond acceptors | 2 |
| Number of hydrogen bond donors | 0 |
| Molar refractivity | 97.01 |
| Total polar surface area | 26.30 Å2 |
| Lipophilicity (Log P) | 4.71 |
| Water Solubility (Log S) | −4.46 |
| Solubility Class | Moderately soluble |
| Drug-pharmacokinetic parameters | |
| Gastrointestinal absorption | Yes |
| Blood-brain barrier permeability | Yes |
| P-glycoprotein substrate | No |
| CYP1A2 inhibitor | No |
| CYP2C19 inhibitor | Yes |
| CYP2C9 inhibitor | Yes |
| CYP2D6 inhibitor | No |
| CYP3A4 inhibitor | No |
| Log Kp (skin permeation) | −4.15 cm/s |
| Drug-like characteristics | |
| Lipinski rule | Acceptable |
| Veber rule | Acceptable |
| Drug-likeness | Yes |
| Drug-likeness score | 0.55 |
| Parameters of medicinal chemistry | |
| PAINS alert | None |
| Brenk alert | 1 alert: isolated alkene |
| Synthetic accessibility score | 4.69 |
| Toxicity | None |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, M.; Bibi, S.; Kamal, Z.; Al-Sabahi, J.N.; Alam, T.; Ullah, O.; Murad, W.; Rehman, N.U.; Al-Harrasi, A. Bridging the Chemical Profile and Biomedical Effects of Scutellaria edelbergii Essential Oils. Antioxidants 2022, 11, 1723. https://doi.org/10.3390/antiox11091723
Shah M, Bibi S, Kamal Z, Al-Sabahi JN, Alam T, Ullah O, Murad W, Rehman NU, Al-Harrasi A. Bridging the Chemical Profile and Biomedical Effects of Scutellaria edelbergii Essential Oils. Antioxidants. 2022; 11(9):1723. https://doi.org/10.3390/antiox11091723
Chicago/Turabian StyleShah, Muddaser, Shabana Bibi, Zul Kamal, Jamal Nasser Al-Sabahi, Tanveer Alam, Obaid Ullah, Waheed Murad, Najeeb Ur Rehman, and Ahmed Al-Harrasi. 2022. "Bridging the Chemical Profile and Biomedical Effects of Scutellaria edelbergii Essential Oils" Antioxidants 11, no. 9: 1723. https://doi.org/10.3390/antiox11091723
APA StyleShah, M., Bibi, S., Kamal, Z., Al-Sabahi, J. N., Alam, T., Ullah, O., Murad, W., Rehman, N. U., & Al-Harrasi, A. (2022). Bridging the Chemical Profile and Biomedical Effects of Scutellaria edelbergii Essential Oils. Antioxidants, 11(9), 1723. https://doi.org/10.3390/antiox11091723









