The Effect of In Vitro Digestion on Matcha Tea (Camellia sinensis) Active Components and Antioxidant Activity
Abstract
:1. Introduction
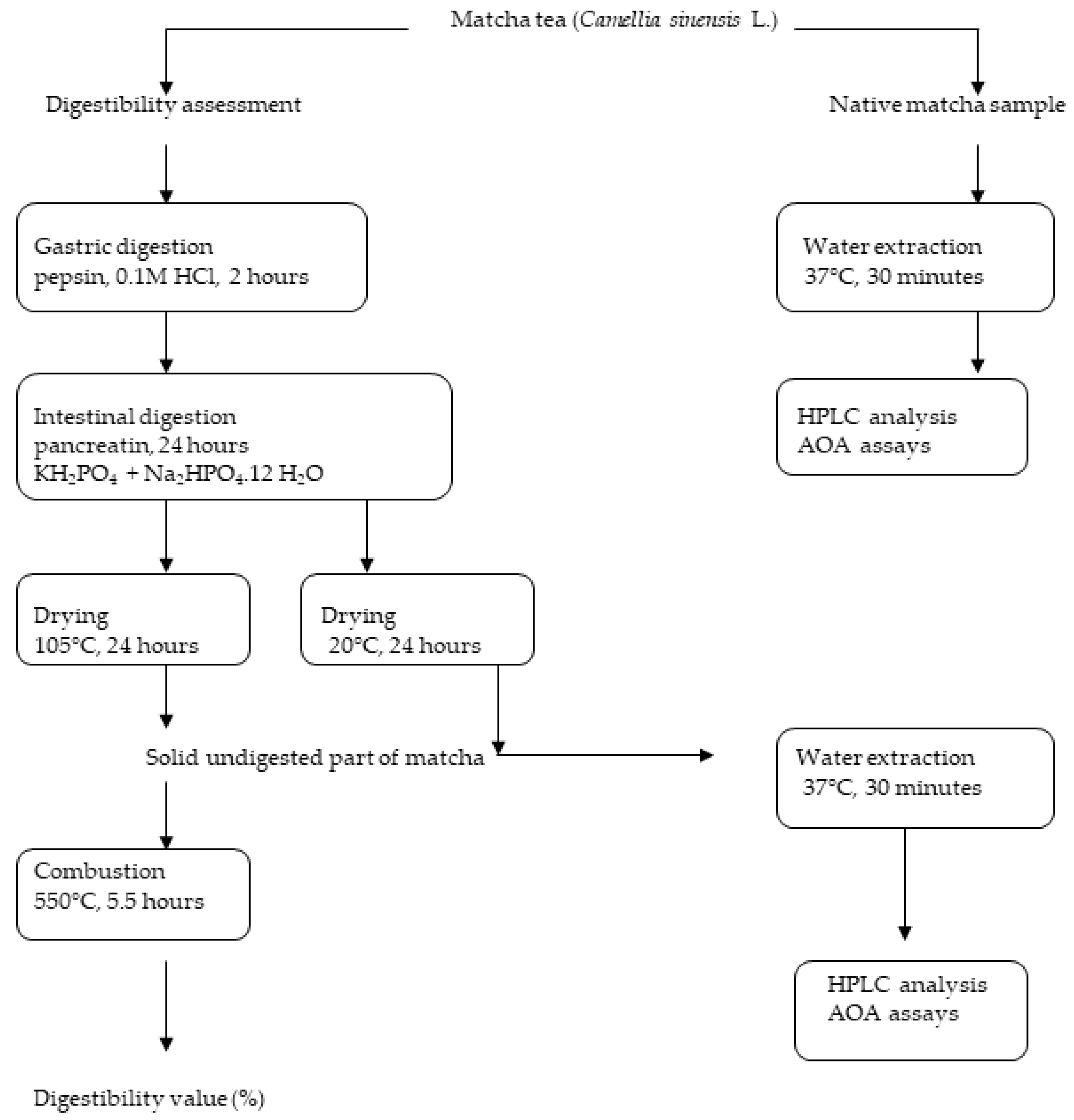
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Matcha Tea Samples
2.3. Sample Preparation
2.4. In Vitro Gastrointestinal Digestion
2.5. Determination of Phenolic Acids and Flavonoids Using HPLC
2.6. Determination of Xanthine Alkaloids and L-Theanine Using HPLC
2.7. Antioxidant Activity Measured Using ABTS and DPPH Radicals
2.8. Ferric Antioxidant Power Assay (FRAP)
2.9. Antioxidant Activity Determination by Using PCL
2.10. Scanning Electron Microscopy (SEM)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Dry Matter and Ash Contents and In Vitro Digestibility Values
3.2. Effect of In Vitro Digestibility on Phenolic Acids and Flavonoids of Matcha Tea
3.3. The Effect of In Vitro Digestibility on Xanthine Alkaloids and L-Theanine
3.4. Results of Antioxidant Activity Measurements
3.5. SEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reto, M.; Fugueira, M.E.; Filipe, H.M.; Almeida, C.M.M. Chemical composition of green tea (Camellia sinensis) infusions commercialized in Portugal. Plant Foods Hum. Nutr. 2007, 62, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Kochman, J.; Kwiatkowska, A.; Kałduńska, J.; Dec, K.; Kawczuga, D.; Janda, K. Antioxidant properties and nutritional composition of matcha green tea. Foods 2020, 9, 483. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, J.; Zhao, W.; Liu, R.; Zhang, L.; Kong, X. Chemical fingerprint analysis for quality control and identification of Ziyang green tea by HPLC. Food Chem. 2015, 171, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Yüksel, A.K.; Yüksel, M.; Şat, İ.G. Determination of certain physicochemical characteristics and sensory properties of green tea powder (matcha) added ice creams and detection of their organic acid and mineral contents. Gıda J. Food 2017, 42, 116–126. Available online: https://www.cabdirect.org/cabdirect/abstract/20173210303 (accessed on 25 October 2021).
- Das, P.R.; Kim, Y.; Hong, S.-J.; Eun, J.-B. Profiling of volatile and non-phenolic metabolites—Amino acids, organic acids, and sugars, of green tea extracts obtained by different extraction techniques. Food Chem. 2019, 296, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-Q.; Wang, J.-Q.; Chen, J.-X.; Wang, F.; Yin, J.-F.; Zeng, L.; Shi, J.; Xu, Y.-Q. Effect of baking on the flavour stability of green tea beverages. Food Chem. 2020, 331, 127258. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; et al. Bioavailability of tea catechins and its improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Kang, J.Y.; Park, S.K.; Han, H.J.; Lee, K.-Y.; Kim, A.-N.; Choi, S.-G.; Heo, H.J. Effect of storage temperature on the antioxidant activity and catechins stability of matcha (Camellia sinensis). Food Sci. Biotechnol. 2020, 29, 1261–1271. [Google Scholar] [CrossRef]
- Topuz, A.; Dinçer, G.; Torun, M.; Tontul, İ.; Şahin-Nadeem, H.; Haznedar, A.; Özdemir, F. Physico-chemical properties of Turkish green tea powder: Effects of shooting period, shading, and clone. Turk. J. Agric. For. 2014, 38, 233–241. [Google Scholar] [CrossRef]
- Sano, T.; Horie, H.; Matsunaga, A.; Hirono, Y. Effect of shading intensity on morphological and color traits and on chemical components of new tea (Camellia sinensis L.) shoots under direct covering cultivation. J. Sci. Food Agric. 2018, 98, 5666–5676. [Google Scholar] [CrossRef]
- Xia, Z.; Ni, Y.; Kokot, S. Simultaneous determination of caffeine, theophylline and theobromine in food samples by a kinetic spectrophotometric method. Food Chem. 2013, 141, 4087–4093. [Google Scholar] [CrossRef] [PubMed]
- Boros, K.; Jedlinszki, N.; Csupor, D. Theanine and caffeine content of infusions prepared from commercial tea samples. Pharmacogn. Mag. 2016, 12, 75–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikary, R.; Mandal, V. L-Theanine: A potential multifaceted natural bioactive amide as health supplement. Asian Pac. J. Trop. Biomed. 2017, 7, 842–848. [Google Scholar] [CrossRef]
- Azevedo, R.S.A.; Teixeira, B.S.; da Silva Sauthier, M.C.; Santana, M.V.A.; dos Santos, W.N.L.; de Andrade Santana, D. Multivariate analysis of the composition of bioactive in tea of the species Camellia sinensis. Food Chem. 2019, 273, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Swetha, M.P.; Radha, C.; Muthukumar, S.P. Bioaccessibility and bioavailability of Moringa oleifera seed flour polyphenols. J. Food Meas. Charact. 2018, 12, 1917–1926. [Google Scholar] [CrossRef]
- Yu, J.; Li, W.; You, B.; Yang, S.; Xian, W.; Deng, Y.; Huang, W.; Yang, R. Phenolic profiles, bioaccessibility and antioxidant activity of plum (Prunus salicina Lindl). Food Res. Int. 2021, 143, 110300. [Google Scholar] [CrossRef]
- ISO 1573; Tea. Determination of Loss in Mass at 103°C. Geneva; ISO: Geneva, Switzerland, 1980.
- ISO 1575; Tea. Determination of Total Ash. Geneva; ISO: Geneva, Switzerland, 1980.
- Koláčková, T.; Kolofiková, K.; Sytařová, I.; Snopek, L.; Sumczynski, D.; Orsavová, J. Matcha tea: Analysis of nutritional composition, phenolics and antioxidant activity. Plant Foods Hum. Nutr. 2020, 75, 48–53. [Google Scholar] [CrossRef]
- de Quirós, R.-B.; Lage-Yusty, M.A.; López-Hernández, J. Determination of phenolic compounds in macroalgae for human consumption. Food Chem. 2010, 121, 634–638. [Google Scholar] [CrossRef]
- Esposto, S.; Veneziani, G.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Sordini, B.; Daidone, L.; Gironi, G.; Servili, M. Chemical composition, antioxidant activity, and sensory characterization of commercial pomegranate juices. Antioxidants 2021, 10, 1381. [Google Scholar] [CrossRef]
- Besco, E.; Braccioli, E.; Vertuani, S.; Ziosi, P.; Brazzo, F.; Bruni, R. The use of photochemiluminiscence for the measurement of the integral antioxidant capacity of baobab products. Food Chem. 2007, 102, 1352–1356. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A.; Kobus-Cisowska, J.; Kmiecik, D.; Korczak, J.; Helak, B.; Dziedzic, K.; Górecka, D. Antioxidative potential, nutritional value and sensory profiles of confectionery fortified with green and yellow tea leaves (Camellia sinensis). Food Chem. 2016, 211, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Reg. No. 330; Ministry of Agriculture. Regulation for Tea, Coffee and Coffee Substitutes; Ministry of Agriculture: Prague, Czech Republic, 1997.
- ISO 11287; Green Tea. Definition and Basic Requirements; ISO: Geneva, Switzerland, 2011.
- Rehman, S.U.; Almas, K.; Shahzadi, N.; Bhatti, N.; Saleem, A. Effect of time and temperature on infusion of tannins from commercial brands of tea. Int. J. Agric. Biol. 2002, 4, 285–287. Available online: http://www.fspublishers.org/published_papers/46716_..pdf (accessed on 25 October 2021).
- Damiani, E.; Bacchetti, T.; Padella, L.; Tiano, L.; Carloni, P. Antioxidant activity of different white teas: Comparison of hot and cold tea infusions. J. Food Compos. Anal. 2014, 33, 59–66. [Google Scholar] [CrossRef]
- Song, R.; Kelman, D.; Johns, K.L.; Wright, A.D. Correlation between leaf age, shade levels, and characteristic beneficial natural constituents of tea (Camellia sinensis) grown in Hawaii. Food Chem. 2012, 133, 707–714. [Google Scholar] [CrossRef]
- Horie, H.; Ema, K.; Sumikawa, O. Chemical components of matcha and powdered green tea. J. Cook. Sci. Jpn. 2017, 50, 182–188. Available online: https://www.jstage.jst.go.jp/article/cookeryscience/50/5/50_182/_pdf (accessed on 15 September 2021).
- Sęczyk, Ł.; Sugier, D.; Świeca, M.; Gawlik-Dziki, U. The effect of in vitro digestion, food matrix, and hydrothermal treatment on the potential bioaccessibility of selected phenolic compounds. Food Chem. 2021, 344, 128581. [Google Scholar] [CrossRef]
- Drawbridge, P.C.; Apea-Bah, F.; Hornung, P.S.; Beta, T. Bioaccessibility of phenolic acids in Canadian hulled barley varieties. Food Chem. 2021, 358, 129905. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Qie, X.; Wu, Y.; Chen, Y.; Liu, C.; Zeng, M.; Qin, F.; Wang, Z.; Chen, J.; He, Z. Competitive interactions among tea catechins, proteins, and digestive enzymes modulate in vitro protein digestibility, catechin bioaccessibility, and antioxidant activity of milk tea beverage model systems. Food Res. Int. 2021, 140, 110050. [Google Scholar] [CrossRef]
- Venditti, E.; Bacchetti, T.; Tiano, L.; Carloni, P.; Greci, L.; Damiani, E. Hot vs. cold water steeping of different teas: Do they affect antioxidant activity? Food Chem. 2010, 119, 1597–1604. [Google Scholar] [CrossRef]
- Farooq, S.; Sehgal, A. Antioxidant activity of different forms of green tea: Loose leaf, bagged and matcha. Curr. Res. Nutr. Food Sci. 2018, 6, 35–40. [Google Scholar] [CrossRef]
- Friedman, M.; Kim, S.-Y.; Lee, S.-J.; Han, G.-P.; Han, J.-S.; Han, J.-S.; Lee, K.-R.; Kozukue, N. Distribution of catechins, theaflavins, caffeine, and theobromine in 77 teas consumed in the United States. J. Food Sci. 2005, 70, C550–C559. [Google Scholar] [CrossRef]
- de Paula Lima, J.; Farah, A. Methylxanthines in stimulant foods and beverages commonly consumed in Brazil. J. Food Compos. Anal. 2019, 78, 75–85. [Google Scholar] [CrossRef]
- Sharma, V.; Gulati, A.; Ravindranath, S.D.; Kumar, V. A simple and convenient method for analysis of tea biochemicals by reverse phase HPLC. J. Food Compos. Anal. 2005, 18, 583–594. [Google Scholar] [CrossRef]
- Heo, H.J.; Kim, Y.J.; Chung, D.; Kim, D.-O. Antioxidant capacities of individual and combined phenolics in a model system. Food. Chem. 2007, 104, 87–92. [Google Scholar] [CrossRef]
- Reber, J.D.; Eggett, D.L.; Parker, T.L. Antioxidant capacity interactions and a chemical/structural model of phenolic compounds found in strawberries. Int. J. Food Sci. Nutr. 2011, 62, 445–452. [Google Scholar] [CrossRef]
- Shu, Y.; Li, J.; Yang, X.; Dong, X.; Wang, X. Effect of particle size on the bioacessibility of polyphenols and polysaccharides in green tea powder and its antioxidant activity after simulated human digestion. J. Food Sci. Technol. 2019, 56, 1127–1133. [Google Scholar] [CrossRef]


| Values | Dry Matter (%) | Ash 1 (%) | Digestibility (%) |
|---|---|---|---|
| JT | 96.9 ± 0.3 a | 4.99 ± 0.05 a | 65.8 ± 0.3 a |
| AT | 96.2 ± 0.3 a | 4.85 ± 0.05 b | 64.9 ± 0.5 b |
| WT | 95.4 ± 0.4 b | 4.64 ± 0.05 c | 61.2 ± 0.4 c |
| Analytes | Native JT | Native AT | Native WT | Undigested JT | Undigested AT | Undigested WT | RP (%) |
|---|---|---|---|---|---|---|---|
| Phenolic acids (µg/g) | |||||||
| GA | 60.4 ± 2.0 a | 37.9 ± 0.3 b | 91.1 ± 1.0 c | 8.11 ± 0.20 A | 19.7 ± 0.5 B | 19.5 ± 0.5 B | 5–18 |
| PA | 43.2 ± 2.0 a | 54.1 ± 1.0 b | 24.9 ± 1.0 c | 0.25 ± 0.02 A | 0.31 ± 0.02 B | 0.12 ± 0.01 C | <1 |
| VA | 2.58 ± 0.10 a | 1.47 ± 0.05 b | 1.31 ± 0.03 c | 0.51 ± 0.02 A | 0.36 ± 0.02 B | 0.22 ± 0.01 C | 7–9 |
| SA | 4.34 ± 0.06 a | 1.98 ± 0.04 b | 4.36 ± 0.05 a | 1.38 ± 0.05 A | 1.24 ± 0.02 B | 1.29 ± 0.02 C | 11–22 |
| PAEE | 9.22 ± 0.30 a | 8.80 ± 0.12 b | 4.26 ± 0.13 c | 0.06 ± 0.01 A | 0.05 ± 0.01 A | 0.10 ± 0.01 B | <1 |
| EA | 236 ± 5 a | 184 ± 5 b | 181 ± 4 b | 452 ± 10 A | 401 ± 7 B | 359 ± 7 C | 66–80 |
| 4-hBA | 12.9 ± 0.1 a | 13.3 ± 0.2 b | 14.6 ± 0.2 c | 0.14 ± 0.01 A | 0.14 ± 0.01 A | 0.17 ± 0.01 B | <1 |
| trans-p-CA | 9.11 ± 0.04 a | 13.9 ± 0.3 b | 13.3 ± 0.2 c | 2.57 ± 0.10 A | 5.35 ± 0.20 B | 4.23 ± 0.10 C | 10–13 |
| CA | 2.31 ± 0.10 a | 2.46 ± 0.12 b | 4.09 ± 0.20 c | 5.12 ± 0.10 A | 5.26 ± 0.03 B | 7.17 ± 0.10 C | 68–76 |
| FA | 12.7 ± 0.2 a | 14.4 ± 0.2 b | 12.6 ± 0.3 a | 25.6 ± 0.5 A | 32.9 ± 0.6 B | 27.2 ± 0.2 C | 69–84 |
| SiA | 63.7 ± 1.1 b | 65.4 ± 2.0 b | 85.1 ± 2.5 a | 24.8 ± 0.2 B | 21.0 ± 0.1 C | 26.7 ± 0.3 A | 11–13 |
| ChA | 2310 ± 50 a | 2460 ± 40 b | 2090 ± 40 c | 512 ± 10 A | 526 ± 7 B | 517 ± 10 A | 7–10 |
| NeoChA | 27.0 ± 0.5 a | 12.5 ± 0.3 b | 14.4 ± 0.2 c | 0.60 ± 0.02 A | 0.12 ± 0.01 B | 0.52 ± 0.05 C | 1–3 |
| o-CA | 6.33 ± 0.20 a | 6.80 ± 0.03 b | 7.00 ± 0.10 b | ND | ND | ND | – |
| CiA | 67.4 ± 2.0 a | 23.6 ± 1.0 b | 60.7 ± 1.2 c | 26.5 ± 1.0 A | 12.5 ± 0.4 B | 35.2 ± 0.7 C | 14–23 |
| T-PAs | 2870 ± 50 a | 2900 ± 40 a | 2610 ± 40 b | 1060 ± 20 A | 1030 ± 10 B | 1000 ± 10 C | 12–15 |
| Flavonoids (µg/g) | |||||||
| EGC | 5390 ± 50 a | 4210 ± 40 b | 4850 ± 40 c | 15.0 ± 0.4 A | 12.6 ± 0.2 B | 14.1 ± 0.1 C | <1 |
| C | 10.2 ± 0.5 a | 23.1 ± 1.0 b | 17.8 ± 0.5 c | 1.01 ± 0.02 A | 1.30 ± 0.05 B | 1.95 ± 0.05 C | 2–4 |
| EC | 8940 ± 40 a | 14500 ± 100 b | 5140 ± 30 c | 650 ± 10 A | 320 ± 10 B | 160 ± 10 C | 1–3 |
| EGCG | 15300 ± 100 a | 7610 ± 50 b | 16900 ± 100 c | 51.6 ± 0.4 A | 28.9 ± 0.6 B | 44.8 ± 0.3 C | < 1 |
| ECG | 1640 ± 40 a | 1790 ± 40 b | 1520 ± 40 c | ND | ND | ND | – |
| R | 303 ± 10 a | 479 ± 20 b | 356 ± 15 c | 725 ± 10 A | 1060 ± 30 B | 746 ± 10 C | 78–82 |
| Q | 11.0 ± 0.3 a | 9.22 ± 0.12 b | 8.02 ± 0.20 c | 25.0 ± 0.5 A | 16.1 ± 0.3 B | 15.2 ± 0.3 C | 61–78 |
| T-FLs | 31600 ± 150 a | 28600 ± 150 b | 28800 ± 140 c | 1470 ± 10 A | 1440 ± 30 B | 982 ± 10 C | 19–41 |
| Total Phe | 34500 ± 150 a | 31500 ± 150 b | 31400 ± 100 c | 2530 ± 20 A | 2470 ± 30 B | 1980 ± 20 C | 16–38 |
| Compounds | Native JT | Native AT | Native WT | Undigested JT | Undigested AT | Undigested WT | RP (%) |
|---|---|---|---|---|---|---|---|
| (mg/g) | |||||||
| Caffeine | 16.1 ± 0.3 a | 14.1 ± 0.2 c | 15.3 ± 0.1 b | 5.26 ± 0.12 A | 3.66 ± 0.10 B | 4.99 ± 0.15 C | 9–13 |
| L-theanine | 9.85 ± 0.10 a | 4.22 ± 0.05 c | 4.46 ± 0.10 b | 0.15 ± 0.01 A | 0.09 ± 0.01 B | ND | <1 |
| Theobromine | 0.14 ± 0.02 c | 0.27 ± 0.02 a | 0.23 ± 0.02 b | ND | ND | ND | - |
| (µg/g) | |||||||
| Theophylline | 8.06 ± 0.15 c | 19.4 ± 0.2 a | 13.0 ± 0.2 b | ND | ND | ND | - |
| The ratio of Caffeine/ L-theanine | 1.63 | 3.34 | 3.43 | ||||
| Antioxidant Activity | Native JT | Native AT | Native WT | Undigested JT | Undigested AT | Undigested WT |
|---|---|---|---|---|---|---|
| ABTS (mg TE/g) | 187 ± 10 a | 249 ± 5 b | 305 ± 12 c | 10.5 ± 0.2 A − 94% | 3.82 ± 0.15 B − 98% | 13.4 ± 0.3 C − 96% |
| DPPH (mg TE/g) | 121 ± 4 a | 134 ± 5 b | 176 ± 10 c | 7.00 ± 0.20 A − 94% | 3.14 ± 0.05 B − 98% | 8.89 ± 0.20 C − 95% |
| FRAP (mg TE/g) | 102 ± 3 a | 125 ± 4 b | 162 ± 6 c | 5.16 ± 0.10 A − 95% | 4.33 ± 0.10 B − 97% | 6.12 ± 0.20 C − 96% |
| ACW (mg AAE/g) | 202 ± 7 a | 194 ± 6 b | 209 ± 7 c | 1.26 ± 0.03 A − 99% | 1.10 ± 0.01 B − 99% | 1.33 ± 0.03 C − 99% |
| ACW (mg TE/g) | 288 ± 9 a | 276 ± 7 b | 293 ± 10 a | 5.55 ± 0.15 A − 98% | 4.97 ± 0.12 B − 98% | 5.67 ± 0.14 A − 98% |
| ACL (mg TE/g) | 179 ± 5 a | 162 ± 7 b | 169 ± 6 c | 7.12 ± 0.20 A − 96% | 6.47 ± 0.20 B − 96% | 6.25 ± 0.30 C − 96% |
| IAC (mg TE/g) | 467 ± 10 a | 438 ± 10 b | 462 ± 12 c | 12.7 ± 0.3 A − 97% | 11.4 ± 0.2 B − 97% | 11.9 ± 0.3 C − 97% |
| Antioxidant Activity Values | |||||||
|---|---|---|---|---|---|---|---|
| r | ABTS | DPPH | FRAP | ACW | 1 ACW | ACL | IAC |
| Phenolics | |||||||
| GA | 0.5505 | 0.7882 | 0.6789 | 0.9919 | 0.9486 | 0.3272 | 0.7149 |
| PA | −0.5968 | −0.8217 | −0.7193 | −0.9832 | −0.9292 | −0.2733 | −0.6742 |
| VA | −0.9293 | −0.7628 | −0.8569 | −0.0774 | 0.1172 | 0.8587 | 0.5393 |
| SA | 0.0220 | 0.2982 | 0.1407 | 0.8880 | 0.9603 | 0.8066 | 0.9857 |
| PAEE | −0.8886 | −0.9885 | −0.9513 | −0.8030 | −0.6723 | 0.1770 | −0.2814 |
| EA | −0.9023 | −0.7175 | −0.8202 | −0.0101 | 0.1838 | 0.8913 | 0.5948 |
| 4-hBA | 0.9473 | 0.9998 | 0.9868 | 0.7045 | 0.5537 | −0.3226 | 0.1343 |
| trans-p-CA | 0.8200 | 0.5944 | 0.7160 | −0.1531 | −0.3416 | −0.9533 | −0.7179 |
| CA | 0.8884 | 0.9885 | 0.9512 | 0.8032 | 0.6726 | −0.1766 | 0.2817 |
| FA | −0.0201 | −0.3382 | −0.1823 | −0.9066 | −0.9712 | −0.7810 | −0.9777 |
| SiA | 0.8863 | 0.9878 | 0.9498 | 0.8059 | 0.6760 | −0.1722 | 0.2861 |
| ChA | −0.5672 | −0.8004 | −0.6935 | −0.9892 | −0.9420 | −0.3082 | −0.7007 |
| NeoChA | −0.8167 | −0.5898 | −0.7121 | 0.1586 | 0.3469 | 0.9550 | 0.7218 |
| o-CA | 0.9802 | 0.8658 | 0.9350 | 0.2537 | 0.0616 | −0.7537 | −0.3807 |
| CiA | −0.1710 | 0.1524 | −0.0086 | 0.8094 | 0.9078 | 0.8858 | 0.9998 |
| EGC | −0.4830 | −0.1782 | −0.3342 | 0.5737 | 0.7214 | 0.9887 | 0.9515 |
| C | 0.6096 | 0.3247 | 0.4726 | −0.4435 | −0.6087 | −1.000 | −0.8942 |
| EC | −0.3766 | −0.6526 | −0.5221 | −0.9976 | −0.9922 | −0.5057 | −0.8379 |
| EGCG | 0.1320 | 0.4415 | 0.2914 | 0.9482 | 0.9918 | 0.7060 | 0.9480 |
| ECG | −0.4170 | −0.6853 | −0.5592 | −0.9997 | −0.9857 | −0.4672 | −0.8130 |
| R | 0.3214 | 0.0024 | 0.1632 | −0.7088 | −0.8320 | −0.9469 | −0.9908 |
| Q | −0.9966 | −0.9182 | −0.9699 | −0.3646 | −0.1774 | 0.6721 | 0.2705 |
| Antioxidant Activity Values | |||||||
|---|---|---|---|---|---|---|---|
| r | ABTS | DPPH | FRAP | ACW | 1 ACW | ACL | IAC |
| Phenolics | |||||||
| GA | −0.2368 | −0.2088 | 0.0268 | −0.2350 | −0.3688 | −0.9662 | −0.9301 |
| PA | −0.9075 | −0.9192 | −0.9860 | −0.9082 | −0.8407 | 0.4397 | −0.1806 |
| VA | −0.2761 | −0.3035 | −0.5189 | −0.2778 | −0.1406 | 0.9669 | 0.6256 |
| SA | 0.5514 | 0.5273 | 0.3129 | 0.5499 | 0.6614 | 0.8221 | 0.9995 |
| PAEE | 0.8486 | 0.8634 | 0.9578 | 0.8496 | 0.7674 | −0.5473 | 0.0576 |
| EA | −0.2414 | −0.2691 | −0.4879 | −0.2431 | −0.1050 | 0.9754 | 0.6532 |
| 4-hBA | 0.7333 | 0.7525 | 0.8862 | 0.7346 | 0.6323 | −0.6956 | −0.1321 |
| trans-p-CA | −0.5940 | −0.5710 | −0.3621 | −0.5924 | −0.6993 | −0.7114 | −0.9998 |
| CA | 0.6904 | 0.7109 | 0.8562 | 0.6917 | 0.5838 | −0.7382 | −0.1924 |
| FA | −0.8729 | −0.8586 | −0.7140 | −0.8720 | −0.9320 | −0.4689 | −0.9022 |
| SiA | 0.9994 | 1.000 | 0.9732 | 0.9995 | 0.9852 | −0.0555 | 0.5491 |
| ChA | −0.7900 | −0.7721 | −0.6013 | −0.7895 | −0.8672 | −0.5963 | −0.9571 |
| NeoChA | 0.8979 | 0.8849 | 0.7507 | 0.8971 | 0.9501 | 0.4206 | 0.8776 |
| CiA | 0.9960 | 0.9981 | 0.9846 | 0.9961 | 0.9740 | −0.1114 | 0.5013 |
| EGC | 0.7777 | 0.7593 | 0.5851 | 0.7765 | 0.8571 | 0.6117 | 0.9622 |
| C | 0.4945 | 0.5192 | 0.7055 | 0.4960 | 0.3696 | −0.8797 | −0.4245 |
| EC | −0.1017 | −0.1303 | −0.3596 | −0.1035 | 0.0367 | 0.9968 | 0.7537 |
| EGCG | 0.8277 | 0.8277 | 0.6512 | 0.8267 | 0.8973 | 0.5431 | 0.9359 |
| R | −0.9374 | −0.9271 | −0.8130 | −0.9368 | −0.9766 | −0.3278 | −0.8253 |
| Q | 0.1404 | 0.1119 | −0.1247 | 0.1386 | 0.2758 | 0.9868 | 0.8896 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koláčková, T.; Sumczynski, D.; Minařík, A.; Yalçin, E.; Orsavová, J. The Effect of In Vitro Digestion on Matcha Tea (Camellia sinensis) Active Components and Antioxidant Activity. Antioxidants 2022, 11, 889. https://doi.org/10.3390/antiox11050889
Koláčková T, Sumczynski D, Minařík A, Yalçin E, Orsavová J. The Effect of In Vitro Digestion on Matcha Tea (Camellia sinensis) Active Components and Antioxidant Activity. Antioxidants. 2022; 11(5):889. https://doi.org/10.3390/antiox11050889
Chicago/Turabian StyleKoláčková, Tereza, Daniela Sumczynski, Antonín Minařík, Erkan Yalçin, and Jana Orsavová. 2022. "The Effect of In Vitro Digestion on Matcha Tea (Camellia sinensis) Active Components and Antioxidant Activity" Antioxidants 11, no. 5: 889. https://doi.org/10.3390/antiox11050889
APA StyleKoláčková, T., Sumczynski, D., Minařík, A., Yalçin, E., & Orsavová, J. (2022). The Effect of In Vitro Digestion on Matcha Tea (Camellia sinensis) Active Components and Antioxidant Activity. Antioxidants, 11(5), 889. https://doi.org/10.3390/antiox11050889






