Isolation, Characterization, Antioxidant Activity, Metal-Chelating Activity, and Protein-Precipitating Capacity of Condensed Tannins from Plum (Prunus salicina) Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Estimation of Total Phenolics
2.3. In Vitro Antioxidant Activity
2.4. NMR Analysis
2.5. MALDI-TOF/MS Analysis
2.6. Fluorescence Quenching Assay
2.6.1. Fluorescence Spectra
2.6.2. Data Processing
2.7. UV-Vis Spectra Study
3. Results and Discussion
3.1. Quantification of the Total Phenolic Content
3.2. Antioxidant Activities of the PCT
3.3. NMR Analysis
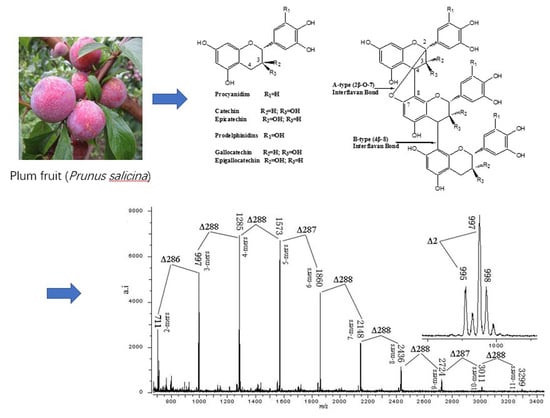
3.4. MALDI-TOF/MS Analyses
3.5. Quenching of the PCT Fluorescence Spectrum by Metal Ions
3.6. Protein-Precipitating Capacity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choi, I.H.; Kim, D.H.; Amanullah, S.M.; Kim, S.C. Nutritional characterization of tannin rich chestnut (Castanea) and its meal for pig. J. Appl. Anim. Res. 2016, 44, 258–262. [Google Scholar] [CrossRef]
- Hemingway, R.W.; Karchesy, J.J. Chemistry and Significance of Condensed Tannins; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Brandão, E.; Silva, M.S.; García-Estévez, I.; Williams, P.; Mateus, N.; Doco, T.; Freitas, V.D.; Soares, S. The role of wine polysaccharides on salivary protein-tannin interaction: A molecular approach. Carbohydr. Polym. 2018, 177, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Abu Zarin, M.; Wan, H.Y.; Isha, A.; Armania, N. Antioxidant, antimicrobial and cytotoxic potential of condensed tannins from Leucaena leucocephala hybrid-Rendang. Food Sci. Hum. Wellness 2016, 5, 65–75. [Google Scholar] [CrossRef]
- Zeng, X.; Du, Z.; Sheng, Z.; Jiang, W. Characterization of the interactions between banana condensed tannins and biologically important metal ions (Cu2+, Zn2+ and Fe2+). Food Res. Int. 2019, 123, 518–528. [Google Scholar] [CrossRef]
- Zheng, J.; Mao, X.; Ling, J.; He, Q.; Quan, J. Low serum levels of zinc, copper, and iron as risk factors for osteoporosis: A meta-analysis. Biol. Trace Elem. Res. 2014, 160, 15–23. [Google Scholar] [CrossRef]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary copper and human health: Current evidence and unresolved issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef]
- Gujar, V.; Pundge, V.; Ottoor, D. Interaction of antihypertensive drug amiloride with metal ions in micellar medium using fluorescence spectroscopy. J. Lumin. 2015, 161, 87–94. [Google Scholar] [CrossRef]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In vitro antioxidant versus metal ion chelating properties of flavonoids: A structure-activity investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds–nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Gonzalez, J.M.; Halvorson, J.J.; Hagerman, A.E. Metal mobilization in soil by two structurally defined polyphenols. Chemosphere 2013, 90, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Dobreva, M.A.; Frazier, R.A.; Mueller-Harvey, I.; Clifton, L.A.; Gea, A.; Green, R.J. Binding of pentagalloyl glucose to two globular proteins occurs via multiple surface sites. Biomacromolecules 2011, 12, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Karonen, M.; Oraviita, M.; Mueller-Harvey, I.; Salminen, J.P.; Green, R.J. Binding of an oligomeric ellagitannin series to bovine serum albumin (BSA): Analysis by isothermal titration calorimetry (ITC). J. Agric. Food Chem. 2015, 63, 10647–10654. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hagerman, A.E. Interactions between plasma proteins and naturally occurring polyphenols. Curr. Drug Metab. 2013, 14, 432–445. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Wu, G.; Zhang, H.; Gu, L.; Wang, L.; Qian, H.; Qi, X. Mitigation effects of proanthocyanidins with different structures on acrylamide formation in chemical and fried potato crisp models. Food Chem. 2018, 250, 98–104. [Google Scholar] [CrossRef]
- Song, W.; Zhu, X.F.; Ding, X.D.; Yang, H.B.; Qin, S.T.; Chen, H.; Wei, S.D. Structural features, antioxidant and tyrosinase inhibitory activities of proanthocyanidins in leaves of two tea cultivars. Int. J. Food Prop. 2017, 20, 1348–1358. [Google Scholar] [CrossRef]
- Basanta, M.F.; Rizzo, S.A.; Szerman, N.; Vaudagna, S.R.; Descalzo, A.M.; Gerschenson, L.N.; Perez, C.D.; Rojas, A.M. Plum (Prunus salicina) peel and pulp microparticles as natural antioxidant additives in breast chicken patties. Food Res. Int. 2018, 106, 1086–1094. [Google Scholar] [CrossRef]
- de Camargo, A.C.; Regitano-d’Arce, M.A.B.; Rasera, G.B.; Canniatti-Brazaca, S.G.; do Prado-Silva, L.; Alvarenga, V.O.; Sant’Ana, A.S.; Shahidi, F. Phenolic acids and flavonoids of peanut by-products: Antioxidant capacity and antimicrobial effects. Food Chem. 2017, 237, 538–544. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry 2000, 54, 173–181. [Google Scholar] [CrossRef]
- Taylor, A.W.; Barofsky, E.; Kennedy, J.A.; Deinzer, M.L. Hop (Humulus lupulus L.) Proanthocyanidins characterized by mass spectrometry, acid catalysis, and gel permeation chromatography. J. Agric. Food Chem. 2003, 51, 4101–4110. [Google Scholar] [CrossRef]
- Monagas, M.; Quintanilla-López, J.E.; Gómez-Cordovés, C.; Bartolomé, B.; Lebrón-Aguilar, R. MALDI-TOF MS analysis of plant proanthocyanidins. J. Pharm. Biomed. Anal. 2010, 51, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Price, M.L.; Butler, L.G. Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain. J. Agric. Food Chem. 1977, 25, 1268–1273. [Google Scholar] [CrossRef]
- Ruan, Z.P.; Zhang, L.L.; Lin, Y.M. Evaluation of the antioxidant activity of Syzygium cumini leaves. Molecules 2008, 13, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Atere, T.G.; Akinloye, O.A.; Ugbaja, R.N.; Ojo, D.A.; Dealtry, G. In vitro antioxidant capacity and free radical scavenging evaluation of standardized extract of Costus afer leaf. Food Sci. Hum. Wellness 2018, 7, 266–272. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Lin, Y.M. Antioxidant tannins from Syzygium cumini fruit. Afr. J. Biotechnol. 2009, 8, 2301–2309. [Google Scholar]
- Zhang, L.L.; Lin, Y.M. HPLC, NMR and MALDI-TOF MS analysis of condensed tannins from Lithocarpus glaber leaves with potent free radical scavenging activity. Molecules 2008, 13, 2986–2997. [Google Scholar] [CrossRef]
- Zhang, L.L.; Sahu, I.D.; Xu, M.; Wang, Y.M.; Hu, X.Y. Effect of metal ions on the binding reaction of (−)-epigallocatechin gallate to β-lactoglobulin. Food Chem. 2017, 221, 1923–1929. [Google Scholar] [CrossRef]
- Huang, Y.; Cui, L.J.; Wang, J.M.; Huo, K.; Chen, C.; Zhan, W.H.; Wang, Y.L. Interaction of aconitine with bovine serum albumin and effect of atropine sulphate and glycyrrhizic acid on the binding. J. Lumin. 2012, 132, 357–361. [Google Scholar] [CrossRef]
- Redondo, L.M.; Chacana, P.A.; Dominguez, J.E.; Miyakawa, M.E.F. Perspectives in the use of tannins as alternative to antimicrobial growth promoter factors in poultry. Front. Microbiol. 2014, 5, 118–123. [Google Scholar] [CrossRef]
- Naczk, M.; Oickle, D.; Pink, D.; Shahidi, F. Protein precipitating capacity of crude canola tannins: Effect of pH, tannin, and protein concentrations. J. Agric. Food Chem. 1996, 44, 2144–2148. [Google Scholar] [CrossRef]
- Basanta, M.F.; Marin, A.; De Leo, S.A.; Gerschenson, L.N.; Erlejman, A.G.; Tomás-Barberán, F.A.; Rojas, A.M. Antioxidant Japanese plum (Prunus salicina) microparticles with potential for food preservation. J. Funct. Foods 2016, 24, 287–296. [Google Scholar] [CrossRef]
- Behrens, A.; Maie, N.; Knicker, H.; Kögel-Knabner, I. MALDI-TOF mass spectrometry and PSD fragmentation as means for the analysis of condensed tannins in plant leaves and needles. Phytochemistry 2003, 62, 1159–1170. [Google Scholar] [CrossRef]
- Howell, A.B.; Reed, J.D.; Krueger, C.G.; Winterbottom, R.; Cunningham, D.G.; Leahy, M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry 2005, 66, 2281–2291. [Google Scholar] [CrossRef] [PubMed]
- Senter, S.D.; Forbus, W.R., Jr.; Okie, W.R. Variations in proanthocyanidins of japanese-type plums during maturation and storage. J. Sci. Food Agric. 1992, 60, 11–14. [Google Scholar] [CrossRef]
- Zhang, L.L.; Xu, M.; Wang, Y.M.; Hu, X.Y. The determining of complexation reaction between Sorghum bicolor Moench proanthocyanidin and metal ions by method of fluorescence quenching. J. For. Eng. 2016, 1, 58–63. [Google Scholar]
- Lakowicz, J. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Naczk, M.; Amarowicz, R.; Zadernowski, R.; Shahidi, F. Protein precipitating capacity of condensed tannins of beach pea, canola hulls, evening primrose and faba bean. Food Chem. 2001, 73, 467–471. [Google Scholar] [CrossRef]





| Polymer | No. of A-Type b Bonds | No. of B-Type Bonds | Calculated [M+Cs]+ | Observed [M+Cs]+ |
|---|---|---|---|---|
| Dimer | 0 | 1 | 711 | 711 |
| Trimer | 1 | 1 | 997 | 997 |
| 2 | 0 | 995 | 995 | |
| Tetramer | 1 | 2 | 1285 | 1285 |
| 2 | 1 | 1283 | 1283 | |
| Pentamer | 1 | 3 | 1573 | 1573 |
| 2 | 2 | 1571 | 1571 | |
| Hexamer | 1 | 4 | 1861 | 1860 |
| 2 | 3 | 1859 | 1858 | |
| Heptamer | 1 | 5 | 2149 | 2148 |
| 2 | 4 | 2147 | 2146 | |
| Octamer | 1 | 6 | 2437 | 2436 |
| 2 | 5 | 2435 | 2435 | |
| Nonamer | 1 | 7 | 2725 | 2724 |
| 2 | 6 | 2723 | 2722 | |
| Decamer | 2 | 7 | 3011 | 3011 |
| 3 | 6 | 3009 | 3009 | |
| Undecamer | 2 | 8 | 3299 | 3299 |
| 3 | 7 | 3297 | 3297 | |
| Dodecamer | 2 | 9 | 3587 | 3586 |
| Tridecamer | 2 | 10 | 3875 | 3875 |
| Concentration (μM) | Ka (×104 L/mol) | R | |
|---|---|---|---|
| Zn2+ a | <66.23 | 1.34 | 0.992 |
| Cu2+ a | <66.23 | 1.00 | 0.999 |
| Al3+ a | <33.22 | 7.65 | 0.999 |
| Fe2+ b | <39.84 | 1.15 | 0.985 |
| Fe3+ c | <33.22 | 6.48 | 0.989 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, H.; Tang, L.; Hu, X.; Xu, M. Isolation, Characterization, Antioxidant Activity, Metal-Chelating Activity, and Protein-Precipitating Capacity of Condensed Tannins from Plum (Prunus salicina) Fruit. Antioxidants 2022, 11, 714. https://doi.org/10.3390/antiox11040714
Zhang L, Zhang H, Tang L, Hu X, Xu M. Isolation, Characterization, Antioxidant Activity, Metal-Chelating Activity, and Protein-Precipitating Capacity of Condensed Tannins from Plum (Prunus salicina) Fruit. Antioxidants. 2022; 11(4):714. https://doi.org/10.3390/antiox11040714
Chicago/Turabian StyleZhang, Liangliang, He Zhang, Lihua Tang, Xinyu Hu, and Man Xu. 2022. "Isolation, Characterization, Antioxidant Activity, Metal-Chelating Activity, and Protein-Precipitating Capacity of Condensed Tannins from Plum (Prunus salicina) Fruit" Antioxidants 11, no. 4: 714. https://doi.org/10.3390/antiox11040714
APA StyleZhang, L., Zhang, H., Tang, L., Hu, X., & Xu, M. (2022). Isolation, Characterization, Antioxidant Activity, Metal-Chelating Activity, and Protein-Precipitating Capacity of Condensed Tannins from Plum (Prunus salicina) Fruit. Antioxidants, 11(4), 714. https://doi.org/10.3390/antiox11040714